
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
What can the basic neurosciences do for psychiatry?



PRESENTATION BY GUY GOODWIN
UN VIAGGIO DI 100 ANNI NELLA MENTE
A 100 YEAR JOURNEY THROUGH THE MIND
3 dicembre 2015 / December 3, 2015
Accademia Nazionale dei Lincei - Palazzina dell'Auditorio
Via della Lungara 230 - Roma
Kindly authorized by the Author.
This document is only for personal use. No part of it may be copied, made available in any way to third parties or used in any form, commercial or
not, without The European House-Ambrosetti's prior written consent.
Con il Patrocinio del Ministero della Salute
Under the Patronage of Ministry of Health
Major depression: What
Innovations in Treatment and
Therapies?
University of Oxford
UK/VOR/1506/0112dPrescribing information is available at the end of this presentation
Wellcome Trust, NIHR
AstraZeneca, BMS, Lundbeck, Medscape, Otsuka, Servier, Takeda
• Paid positions University of Oxford• Advisory boards
AstraZeneca, BMS, TevaLundbeck, Medscape, Otsuka, P1Vital, Servier, Sunovion, Takeda
• Multimodal, multifunctional antidepressant
– Vortioxetine (BRINTELLIX)
• Evidence for an independent effect on
executive function
– Experimental medicine in drug development
DSM-IV Major Depressive Episode
(1) depressed mood
(2) markedly diminished interest or pleasure
(3) significant weight loss
(4) insomnia or hypersomnia(5) psychomotor agitation or retardation
(6) fatigue or loss of energy(7) feelings of worthlessness or excessive or inappropriate guilt
(8) diminished ability to think or concentrate, or indecisiveness(9) recurrent thoughts of death, suicidal ideation etc
DSM-IV Major Depressive Episode
(1) depressed mood
(2) markedly diminished interest or pleasure
(3) significant weight loss
(4) insomnia or hypersomnia(5) psychomotor agitation or retardation
(6) fatigue or loss of energy(7) feelings of worthlessness or excessive or inappropriate guilt
(8) diminished ability to think or concentrate, or indecisiveness(9) recurrent thoughts of death, suicidal ideation etc
DSM-IV Major Depressive Episode
(1) depressed mood
(2) markedly diminished interest or pleasure
(3) significant weight loss
(4) insomnia or hypersomnia
(5) psychomotor agitation or retardation
(6) fatigue or loss of energy(7) feelings of worthlessness or excessive or inappropriate guilt
(8) diminished ability to think or concentrate, or
indecisiveness
(9) recurrent thoughts of death, suicidal ideation etc
Depression and Cognitive
• List learning in 2 groups
of depressed patients and controls
• Both endogenous and
neurotic groups impaired
• Other domains also
• Related to depression
Austin et al. 1992JAD, 25, 21–29. doi:10.1016/0165-0327(92)90089-O
Impaired Cognition
Journal of Affective Disorders, 25, 21–29. doi:10.1016/0165-0327(92)90089-O

Impaired Cognition
Journal of Affective Disorders, 25, 21–29. doi:10.1016/0165-0327(92)90089-O


Impaired Cognition
Journal of Affective Disorders, 25, 21–29. doi:10.1016/0165-0327(92)90089-O
Executive impairment in
patients with depression
Douglas KM et al. Br J Psychiatry
GMLT, Groton Maze Learning Test; ISLT, International Shopping List Task; SECT, Social Emotional Cognition Test; d, Cohen's d effect size
Is memory and executive
failure secondary to negative
Melancholic patients
Moffoot et al JAD 1994 32, 257-369
Moffoot et al JAD 1994 32, 257-369
Moffoot et al JAD 1994 32, 257-369
Digit symbol substitution test
• DSST – cue: digit-symbol pairs (e.g. 1/_,. 7/Λ,8/X,9/=)
Response: list of digits. Under each digit the subject should write down the corresponding symbol as fast as possible.
The challenge is to correct
emotional bias AND executive
Does vortioxetine (Brintellix)
Multimodal action
6 pharmacological targets and
2 modes of action
(receptor activity + reuptake
SERT, serotonin transporter;
Nutt DJ. J Psychopharmacol 2009;23:343-5;
Westrich L et al. Int J Psychiatry Clin Pract 2012;16(Suppl
Vortioxetine reverses memory deficits in serotonin-depleted
Low serotonin has a negative impact on memory
(novel object recognition test)
Control PCPA 0.0001
Vortioxetine dose (mg/kg)
Vortioxetine dose (mg/kg)
+p<0.05 vs control; **p<0-01 vs PCPA;
Pehrson AL et al. Poster NR4-34 presented
***p<0.001 vs PCPA; +++p<0.001 vs control
at the 165th APA Annual Meeting 2012
Is it antidepressant?
Is it pro-executive?
Vortioxetine: effective in short-term studies – change from
baseline in MADRS total score at Week 6/8
HLu 11492A (non-US)
HLu 11984A (non-US)
• Meta-analysis of the results
HLu 13267A (non-US)
of 11 short-term adult studies
confirmed the superiority of
vortioxetine compared to
placebo at doses of 5, 10
HLu 14122A (US and non-US)
TAK CCT-002 (non-US)
1. Alvarez E et al. 2012;
2. Baldwin DS et al. 2012;
3. Henigsberg N et al. 2012;
4. Boulenger JP et al. 2014;
5. Mahableshwarkar AR et al. APA 2013;
6. Jacobsen PL et al. APA 2013;
HLu 12541A (non-US and US)
7. McIntyre RS et al. 2014;
8. Vortioxetine EPAR;
9. Jain R et al. 2013;
10. Mahableshwarkar AR et al. 2013;
Purple line indicates the target of clinical relevance
11. Mahableshwarkar AR et al. APA 2013;
Difference to placebo
FAS, full analysis set; MMRM, mixed model for repeated measurements
12. Katona C et al. 2012
First signals of effect in executive
Randomised, double-blind, placebo-controlled study in elderly patients with recurrent MDD (vortioxetine, n=156; duloxetine, n = 151; placebo, n = 145)
Duloxetine was an active reference
Improvement in cognitive dysfunction was a pre-defined secondary outcome measure
Key inclusion criteria
Key exclusion criteria
Age range: ≥65 years
Any current psychiatric disease
Patients with MDE as primary
other than MDD (DSM-IV-TR)
diagnosis (DSM-IV-TR)
• With a current MDE ≥4 weeks• ≥1 MDE at age <60 years
score ≥26 at screening and baseline
DSM-IV-TR, Diagnostic and Statistical Manual for Mental Disorders, 4th Edition, Text Revision; MMSE, Mini Mental State Examination
Katona C et al. Int Clin Psychopharmacol 2012;27:215-23
First signal that vortioxetine improves cognitive
performance: secondary outcome from study in elderly
Vortioxetine improved (objective) cognitive performance in both DSST
and RAVLT – secondary end points, Week 8 (FAS, ANCOVA, Cohen's d)
*p<0.05, **p<0.01 vs placebo; nominal p values, n values are APTSRAVLT, Rey Auditory Verbal Learning Test; APTS, all patients treated set
Katona C et al. Int Clin Psychopharmacol 2012;27:215-23
Effective for depressive symptoms in elderly
patients with MDE
Mean change from baseline in HAM-D24 total score in elderly depressed
patients by visit (FAS, OC, MMRM; end point LOCF)
Vortioxetine 5 mg (n=155)
Duloxetine 60 mg (n=148)
*p<0.05; **p<0.01; ***p<0.001HAM-D24, Hamilton Depression Rating Scale 24-item version
Katona C et al. Int Clin Psychopharmacol 2012;27:215-23
Vortioxetine – a direct effect on cognitive
dysfunction in depression
Path analysis showing the treatment effect on cognitive performancea and
83.2% Direct effect
16.8% Indirect effect
aMeasured by DSST, Digit Symbol Substitution TestbMeasured by HAM-D24, Hamilton Depression Rating Scale 24-item version
Katona C et al. Int Clin Psychopharmacol 2012;27:215-23
Can we prove a direct executive
• Current depressive symptoms are a
– If changes in executive functiontion are due to
changes in mood, it is a pseudo-specific effect
• However, pro-executive effects will also be
relevant in healthy controls and remitted MDD
– Proof of concept
• Manuscript in submission
BOLD fMRI signals in subjects remitted from depression and
controls - Study design
Double-blind treatment period
Vortioxetine 20 mg/day
Previous MDE >2
Patients remitted from depression
Subjective reporting of
cognitive dysfunction
Vortioxetine 20 mg/day
Control individuals
No history of MDE25–55 years
No subjective reporting of
cognitive dysfunction
Safety Baseline-I
Baseline-III Follow-up
-42 to -11 -10 to -5
13 (or 14) 20–25
Primary end point:
BOLD fMRI signal in brain areas associated with executive function (working memory),
specifically the prefrontal cortex and anterior cingulate during performance of the N-back task
Number of patients: 24 per treatment arm fMRI and cognitive assessments at baseline and Week 2
Main Study Objectives
– to determine whether vortioxetine compared
to placebo in subjects remitted from
depression modulates BOLD signal in fMRI of
the brain areas associated with executive
function (working memory) during performance
of the N-back task
Main Study Objectives
– to determine whether vortioxetine compared
to placebo in subjects remitted from
depression modulates BOLD signal in fMRI of
the brain areas associated with executive
function (working memory) during performance
of the N-back task
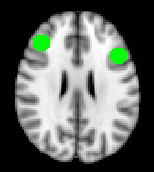
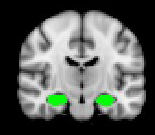
Regions of Interest (RoIs)
• Target regions of the brain which have previously
been shown to have altered activity in depression
Vortioxetine significantly reduces right dlPFC activity during
In regions previously
reported to be
hyperactive in
patients with
If we look here specifically
depression
vortioxetine reduces
neural activity while
performing the N-
back task
Unmedicated MDDv. CONTROLSN back task Hippocampus
Norbury et al. Psychological Medicine (2014), 44, 1197–1203
Vortioxetine significantly reduces left hippocampal
activity during the N-Back task (ROI analysis)
In regions previously
reported to be
hyperactive in patients
with depression
significantly reduces
neural activity while
performing the N-back
task in subjects
remitted from their
– Mike Browning
– Christina Kurre Olsen
• Multimodal, multifunctional antidepressant
– Vortioxetine (BRINTELLIX)
• Evidence for an independent effect on
executive function
– Experimental medicine in drug development
GMG is supported by a Senior Investigator
award from the National Institute for Health Research. The views expressed herein are
the author's and not necessarily those of the
National Health Service, the National Institute
for Health Research, or the Department of
Monitor patients for appearance of serotonin syndrome or neuroleptic
Brintellix® (vortioxetine) film-coated tablets
malignant syndrome, and discontinue if occurs.
Prescribing information: Please refer to the ful Summary of Product
Drug interactions: Alcoholic drinks not advisable. Vortioxetine is extensively
Characteristics (SPC) before prescribing, particularly in relation to side effects,
metabolised in the liver, primarily through oxidation catalysed by CYP2D6 and to
precautions and contraindications.
a minor extent CYP3A4/5 and CYP2C9. Potential for interactions with: MAOIs,
Presentation: Tablets containing 5, 10 or 20mg of vortioxetine (as the
MAO-A and MAO-B inhibitors; serotonergic medicines (e.g. triptans or tramadol);
St John's wort; products which may lower the seizure threshold, e.g
Indications: Treatment of major depressive episodes in adults.
antidepressants (tricyclic, SSRIs, SNRIs), neuroleptics (phenothiazines,
Dosage: 10mg once daily. Dose may be increased to a maximum of 20mg daily or
thioxanthenes and butyrophenones), mefloquine or bupropion. Depending on
reduced to 5mg if necessary. After depressive symptoms resolve, treatment for at
individual patient response, a lower dose of vortioxetine may be considered if
least 6 months is recommended.
strong CYP2D6 inhibitor (e.g. bupropion, quinidine, fluoxetine, paroxetine) is
Elderly (≥65 years): Initial dosage is 5mg once daily. Caution advised if using doses
added to vortioxetine treatment. Additional y these effects may be greater in
above 10mg daily as data are limited.
patients who are poor metabolisers of CYP2D6. A dose adjustment may be
Children and adolescents (<18 years): Not recommended as safety and efficacy not
considered if a broad cytochrome P450 inducer (e.g. rifampicin, carbamazepine,
phenytoin) is added to vortioxetine treatment.
Cytochrome P450 inhibitors and inducers: Consider dose reduction of vortioxetine
Adverse events: Adverse reactions were usual y mild or moderate, transient and
if a strong CYP2D6 inhibitor is added. Consider dose adjustment if a broad CYP450
occurred within the first two weeks of treatment. The fol owing adverse events
inducer is added to treatment.
were reported: Very common (>1/10 patients); nausea. Common (>1/100 <1/10);
Renal impairment: Exercise caution in severe impairment as data are limited in
abnormal dreams, dizziness, diarrhoea, constipation, vomiting, pruritus,
these patients.
including generalised pruritus. Uncommon (>1,000 <1/100); flushing, night
Hepatic impairment: Exercise caution in severe hepatic impairment as no data in
sweats. Unknown; serotonin syndrome. Sexual dysfunction: The 20mg dose of
these patients.
vortioxetine was associated with an increase in treatment-emergent sexual
Contraindications: Hypersensitivity to the active substance or any of the excipients.
dysfunction. Class effect: Studies in patients ≥50 years of age, show an increased
Concomitant use with non-selective, monoamine oxidase inhibitors (MAOIs) or
risk of bone fractures in patients receiving SSRIs and TCAs. Not known if relevant
selective MAO-A inhibitors (e.g. moclobemide).
to vortioxetine. Prescribers should consult the full SPC in relation to other side
Fertility, pregnancy and lactation: Do not use in pregnancy unless clinical y
necessary. Limited data on the use of vortioxetine in pregnant women. Animal
studies have shown reproductive toxicity. Use of SSRIs in pregnancy, particularly in
Overdose: Limited experience. Management consisting of treating clinical
late pregnancy, may increase the risk of persistent pulmonary hypertension in the
symptoms and relevant monitoring.
newborn (PPHN). It is expected that vortioxetine wil be excreted into human milk,
Legal category: POM. Brintel ix Tablets, blisters of:
and a risk to the suckling child cannot be excluded.
5mg (EU/1/13/891/002) 28 tablets, £27.72;
Fertility: Animal data showed no effect on fertility, sperm quality or mating
10mg (EU/1/13/891/010) 28 tablets, £27.72;
performance. Human case reports with some SSRIs have shown that an effect on
20mg (EU/1/13/891/028) 28 tablets, £27.72.
sperm quality is reversible. Impact on human fertility has not been observed so far.
Precautions: Use caution when driving a car or operating machinery. Closely
Further information available from: Lundbeck Limited, Building K1, Timbold
supervise patients, especial y those at high risk, for suicide-related behaviours
Drive, Kents Hil , Milton Keynes MK7 6BZ.
during first few weeks of treatment and during dose changes. Use with caution in
Tel: 01908 638972.
patients: at risk of hyponatraemia; with a history of mania/hypomania; undergoing
® Brintel ix is a Registered Trade Mark.
ECT; with unstable epilepsy (discontinue if seizures begin for the first time or
increase in frequency); with bleeding tendencies/disorders, taking anticoagulants
Date of last revision of PI: June 2015.
or medicines affecting platelet function; in patients on lithium or tryptophan.
Adverse events should be reported. Reporting forms and information can be found a Adverse events
should also be reported to Lundbeck Limited, Medical Information, on: 01908 638972.
Source: http://www.ambrosetti.eu/wp-content/uploads/Pres-GOODWIN-Lundbeck-2015.pdf
PORT COSTA SANITARY COMMISSION MINUTES OF REGULAR MEETING, MARCH 9, 2016 1. CALL TO ORDER: The meeting was called to order at 7:00 PM by Chairperson Surges. Present were Commissioners Cusack, Mann and alt. Barassi, along with Dept. Manager Barnhill and General Manager McDonald. Mr. Guarnieri arrived at 7:05 PM. Mr. English was absent. 2. AGENDA ORDER: There were no requests to hear agenda items out of order.
Author's personal copy Psychiatry Research 189 (2011) 62–66 Contents lists available at ScienceDirect Psychiatry Research Schizophrenia patients with predominantly positive symptoms have more disturbed sleep–wake cycles measured by actigraphy Pedro Afonso a,⁎, Sofia Brissos a, Maria Luísa Figueira b, Teresa Paiva ba Lisbon's Psychiatric Hospitalar Center (CHPL), Lisbon, Portugalb Hospital Santa Maria, Faculty of Medicine, University of Lisbon, (FMUL), Lisbon, Portugal












