
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Antibethera.com
Gastrointestinal Safety and Anti-Inflammatory Effects of a Hydrogen
Sulfide–Releasing Diclofenac Derivative in the Rat
JOHN L. WALLACE,* GIUSEPPE CALIENDO,‡ VINCENZO SANTAGADA,‡ GIUSEPPE CIRINO,§ and STEFANO FIORUCCI*,储
*Inflammation Research Network, University of Calgary, Calgary, Alberta, Canada; ‡Departments of Medicinal Chemistry and §Experimental Pharmacology, Universityof Naples, Naples, Italy; and 储
Department of Gastroenterology and Hepatology, University of Perugia, Perugia, Italy
Background & Aims: Gastrointestinal damage caused
and patients. Attempts to design NSAIDs that do not
by nonsteroidal anti-inflammatory drugs (NSAIDs) re-
cause gastrointestinal damage still face the challenge of
mains a significant clinical problem. Hydrogen makes
overcoming the detrimental effects of suppression of
an important contribution to mucosal defense, and
prostaglandin (PG) synthesis while maintaining the ben-
NSAIDs can suppress its synthesis. In this study, we
eficial effects of these drugs, which are also related to
evaluated the gastrointestinal safety and anti-inflamma-
inhibition of the COX
tory effects of a novel "HS-NSAID" (ATB-337) that con-
Nitric oxide (NO)–releasing NSAIDs were first de-
sists of diclofenac linked to a hydrogen sulfide–releas-
scribed in and have since been assessed in several
ing moiety. Methods: The gastrointestinal injury–
clinical The principle behind these compounds
inducing effects of single or repeated administration of
was that the slow release of NO would compensate, in
diclofenac versus ATB-337 were compared in rats, as
terms of mucosal defense, for the inhibition of PG syn-
were their effects on prostaglandin synthesis and cyclo-
thesis in the Hydrogen sulfide, like NO, is a
oxygenase-1 and -2 activities. The ability of these drugs
gaseous mediator now recognized as making important
to reduce carrageenan-induced paw edema and to elicit
contributions to several physiologic functions, including
leukocyte adherence to the vascular endothelium (intra-
many in the gastrointestinal tract and Recently, we
vital microscopy) were also examined in rats. Results:
reported that H2S is produced by the gastric mucosa and
Diclofenac (10 –50
mol/kg) dose-dependently dam-
makes a significant contribution to mucosal
aged the stomach, while ATB-337 did not. Repeated
Interestingly, the expression of one of the key enzymes
administration of diclofenac caused extensive small in-
responsible for endogenous H2S synthesis is inhibited by
testinal damage and reduced hematocrit by 50%. ATB-
a number of NSAIDs. Moreover, administration of H2S
337 induced >90% less intestinal damage and had no
donors reduced the severity of NSAID-induced gastric
effect on hematocrit. Diclofenac, but not ATB-337, ele-
damage in rats. These observations raised the possibility
vated gastric granulocyte infiltration and expression of
that an H2S-releasing derivative of an NSAID may exhibit
tumor necrosis factor ␣
, lymphocyte function–associ-
reduced gastric toxicity.
ated antigen 1, and intercellular adhesion molecule 1.
We have also reported that H2S exhibits anti-inflam-
ATB-337 inhibited cycloxygenase-1 and cyclooxygen-
matory Endogenous H2S production appears to
ase-2 activity as effectively as diclofenac. ATB-337 did
down-regulate leukocyte adherence to the vascular endo-
not induce leukocyte adherence, whereas diclofenac did,
thelium, a key early event in inflammation. Administra-
and was more potent at reducing paw edema.
tion of H2S donors could suppress leukocyte adherence
Conclusions: An HS-NSAID spares the gastric mucosa
stimulated by aspirin or by a proinflammatory peptide.
of injury despite markedly suppressing prostaglandin
Moreover, H2S donors reduced edema formation in the
synthesis. This effect may be related to hydrogen sul-
rat paw following injection of carrageenan. These obser-
fide–mediated inhibition of tumor necrosis factor–␣
vations therefore suggest that drugs releasing H2S may
expression and of the leukocyte adherence to vascular
exhibit anti-inflammatory activity.
endothelium normally induced by cyclooxygenase in-
In the present study, we have characterized the effects
of a novel NSAID (ATB-337), formed by linking diclofe-
Abbreviations used in this paper: CBS, cystathionine -synthase;
he use of nonsteroidal anti-inflammatory drugs
COX, cyclooxygenase; CSE, cystathionine ␥-lyase; HS-NSAID, hydrogen
(NSAIDs) continues to be associated with an unac-
sulfide–releasing nonsteroidal anti-inflammatory drug; ICAM, intercel-
ceptable risk for gastrointestinal ulceration and bleeding,
lular adhesion molecule; LFA, lymphocyte function–associated anti-
even after the introduction of selective cyclooxygenase
gen; L-NAME,
N-nitro-L-arginine methyl ester; TNF, tumor necrosis fac-
(COX)-2 inhibitors. Other adverse effects of NSAIDs,
2007 by the AGA Institute
including those associated with the renal and cardiovas-
cular systems, are of growing concern to practitioners
GASTROENTEROLOGY Vol. 132, No. 1
hours after administration of the drugs or vehicle for
measurement of plasma H2S concentrations, as describedSamples of the stomach were excised 3
hours after drug/vehicle administration for measurement
of myeloperoxidase using a commercially avail-
able spectrophotometric kit. Myeloperoxidase is an en-
zyme found primarily in the azurophilic granules of theneutrophils and therefore has been used extensively as a
Figure 1. Structure of ATB-337.
biochemical marker of the granulocyte infiltration intovarious tissues, including the gastrointestinal tract. Ad-ditional samples of the stomach were excised for deter-
nac to an H2S-releasing moiety We compared
mination of expression of messenger RNA (mRNA) for
the effects of this novel NSAID with diclofenac in terms
cystathionine ␥-lyase (CSE), tumor necrosis factor
of ulcerogenic effects, ability to suppress gastric PG syn-
(TNF)-␣, intercellular adhesion molecule (ICAM)-1, and
thesis, ability to induce small intestinal injury, effects on
lymphocyte function–associated antigen (LFA)-1, by
COX-1 and COX-2 activity in vivo, and acute anti-inflam-
quantitative reverse-transcription polymerase chain reac-
matory effects. Our results suggest that the linking of an
tion, as described in detail The primers used
H2S-releasing moiety to NSAIDs, forming an "HS-
were as follows (sense and antisense, respectively): glyc-
NSAID," is a rational approach to the development of
eraldehyde-3-phosphate dehydrogenase, ATGACTCTAC-
TNF-␣, TGATCCGAGATGTGGAACTG, and CGAG-
Materials and Methods
Male Wistar rats weighing 175–200 g were ob-
LFA-1, GTCATGGAGTGTGGCATCTG and TCACTTT-
tained from Charles River Breeding Farms (Montreal,
GTTGGGGATGTCA. Similar experiments were performed
Quebec, Canada) and were housed in the Animal Care
in which gastric tissue was excised at 1 hour or 3 hours after
Facility at the University of Calgary. The rats were fed
drug/vehicle administration and expression of mRNA for
standard laboratory chow and tap water. All experimental
the 2 key enzymes in endogenous H2S synthesis (cystathi-
protocols were approved by the Animal Care Committee
onine -synthase [CBS] and CSE) was determined by quan-
at the University of Calgary, and the experiments were
titative reverse-transcription polymerase chain reaction. The
performed in accordance with the guidelines of the Ca-
primers used were as follows (sense and antisense, respec-
nadian Council on Animal Care. Unless otherwise noted,
tively): CBS, CCAGGACTTGGAGGTACAGC and TCG-
in all experiments described in the following text, the
GCACTGTGTGGTAATGT; CSE GTATTGAGGCACCAA-
sample size in each group was at least 5.
CAGGT and GTTGGGTTTGTGGGTGTTTC.
In other experiments, rats were treated with diclofenac
Effects of NSAIDs on Gastrointestinal
alone or together with the H2S-releasing moiety of ATB-
Mucosal Integrity and PG Synthesis
Rats were deprived of food for 18 –20 hours, with
These experiments would allow us to determine if the
free access to drinking water, and were then treated orally
separate but concomitant administration of the 2 moi-
with diclofenac (10 –50 mol/kg), ATB-337 (equimolar
eties of ATB-337 would provide the same degree of gas-
doses), or vehicle (1% carboxymethylcellulose). Three
tric safety as the intact compound.
hours later, the rats were killed for blind assessment of
We also examined the effects of ATB-337 versus di-
gastric The lengths (in millimeters) of all hem-
clofenac in rats in circumstances in which mucosal de-
orrhagic lesions were measured with digital calipers, and
fense was diminished. Specifically, we treated rats orally
the "gastric damage score" was calculated for each stom-
with an inhibitor of NO synthase (
N-nitro-L-arginine
ach by summing these values. After scoring the damage,
methyl ester [L-NAME]; 15 mg/kg) or with aspirin (10
a sample of the corpus region of each stomach was fixed
mg/kg) 30 minutes before oral administration of vehicle,
in neutral buffered formalin for subsequent histologic
diclofenac, or ATB-337 (both drugs at 27 mol/kg).
assessment. Other tissue samples from each stomach
To compare the effect of ATB-337 with that of diclofe-
were processed, as described previously, for measurement
nac in terms of capacity to produce small intestinal
of PGE2 synthesis using a specific enzyme-linked immu-
damage, groups of 5 rats each (not fasted) were given one
of these drugs at a dose of 50 mol/kg 3 times at 12-hour
Another experiment was performed in which groups of
intervals. The rats were killed 24 hours after the final
5 rats each were fasted overnight and then treated orally
administration of the test drugs. The small intestine was
with vehicle, diclofenac, or ATB-337 (both drugs at 50
excised, and the extent of small intestinal hemorrhagic
mol/kg). Blood samples were drawn from a tail vein 3
damage was quantified by measuring the lengths of le-
H2S–RELEASING NSAID
sions in millimeters and then summing these to give a
injected subcutaneously on the back of the rat on the
damage score for each The observer also made note
first day. Two days later, another 10 mL of air was
of the distribution of the lesions and their depth. Before
injected at the same site. On the fifth day after the first
the initial administration of one of the test drugs and
injection, a further 10 mL of air was injected into the
immediately before the rats were killed, a blood sample
pouch. Twenty-four hours later, zymosan (1 mL of a 1%
was collected from a tail vein for measurement of hemat-
wt/vol solution in sterile saline) was injected into the air
ocrit as a surrogate marker of
pouch. All of the injections were performed after the ratshad been anesthetized with 5% (vol/vol) halothane. Six
Gastric Acid Secretion
hours after the zymosan injection, the rats were anesthe-
Gastric-sparing effects of ATB-337 might be due
tized with 5% (vol/vol) halothane and the pouch was
to suppression of gastric acid secretion. To test this
carefully opened by a small incision. The exudate was
hypothesis, groups of 5 rats each were given vehicle,
collected and transferred to a sterile tube. The volume of
diclofenac, or ATB-337 orally (50 mol/kg for both
the exudate was measured gravimetrically, and the exu-
drugs). Thirty minutes later, the rats were anesthetized
dates were then stored at –20°C until such time as PGE2
with halothane and a laparotomy was performed. The
concentrations were measured using a specific enzyme-
pylorus was ligated, and the abdomen was sutured
linked immunosorbent
closed. Three hours later, the rats were anesthetized again
To assess the effects of the test drugs on COX-1 activ-
and the stomach was excised with care taken not to lose
ity in vivo, a sample of blood was drawn from the inferior
the contents. The volume of fluid in the stomach was
vena cava. One milliliter was transferred to a glass tube
determined gravimetrically, and the mount of titratable
and allowed to clot at 37°C for 45 minutes. The samples
acidity was determined using a Brinkmann (Rexdale, On-
were then centrifuged for 10 minutes at 3,000
g, after
tario, Canada) automated titrator.
which the serum was decanted and frozen at ⫺20°C until
such time as the concentrations of thromboxane B2 were
measured by specific enzyme-linked immunosorbent as-
Adhesion of leukocytes to the vascular endothe-
Virtually all of the thromboxane B2 produced by
lium has been shown to be an important step in the
clotting whole blood is synthesized via COX-1 in plate-
pathogenesis of NSAID-induced gastric
The ability of ATB-337, versus diclofenac, to cause leu-kocyte adherence was examined using intravital micros-
Effects on Human Platelet Function
copy, as described in detail Briefly, rats
The effects of diclofenac and ATB-337 on human
were deprived of food for 18 –20 hours and anesthetized
platelet aggregation and thromboxane synthesis were ex-
with sodium pentobarbital (65 mg/kg intraperitoneally).
amined. Blood from 3 healthy donors was aliquoted into
A postcapillary mesenteric venule with a diameter of
Eppendorf tubes containing one of the test drugs (1 or 10
25–30 m was positioned under the objective of a mi-
mol/L) or vehicle and incubated for 30 minutes at
croscope. Following a 15-minute equilibration period,
37°C. Thirty minutes later, indomethacin (10 mol/L)
the rats were randomly assigned to receive vehicle, di-
was added to each sample to prevent further thrombox-
clofenac (50 mol/kg), or ATB-337 (50 mol/kg). Images
ane synthesis. The samples were centrifuged (9000
g) for 3
of the mesenteric microcirculation were recorded over a
minutes and the plasma was transferred to another tube
5-minute period every 15 minutes for 60 minutes for
and frozen at ⫺20°C until the assay for thromboxane B2
blind quantification of leukocyte adherence. Basal leuko-
was performed. Other blood samples were used to pre-
cyte adherence was measured over a 5-minute period
pare platelet-rich plasma, as described Ali-
immediately before administration of vehicle, diclofenac,
quots (0.5 mL) of platelet-rich plasma were incubated
or ATB-337. Leukocyte adherence was quantified from
with diclofenac or ATB-337 (1 or 10 mol/L) or with
the videotaped images by an observer blind as to the
vehicle for 30 minutes at 37°C. Aliquots of the platelet
treatments the rats had received. A leukocyte was consid-
suspensions were then added to the cuvette of a Chro-
ered as adherent if it remained stationary for at least 30
nolog (Buffalo, NY) platelet aggregometer. Three min-
utes later, arachidonic acid (0.5 mol/L) was added to theplatelet suspension and aggregation was monitored for 5
In Vivo COX-1 and COX-2 Activity
minutes. Results are expressed as the extent of aggrega-
The effects of the novel NSAID on COX-2 activity
tion as a percent of maximum possible aggregation.
in vivo were assessed using the air pouch model inInjection of zymosan into a preformed air
Carrageenan-Induced Paw Edema
pouch results in a substantial increase in PGE2 synthesis,
Rats were deprived of food, but not water, for
which occurs almost exclusively via COX-2. This was
18 –22 hours. Groups of 5 rats each were treated orally
confirmed in the present study using the highly selective
with vehicle, diclofenac (3–30 mol/kg), or ATB-337 (10
COX-2 inhibitor Briefly, 20 mL of air was
mol/kg). Thirty minutes later, the volume of the left
GASTROENTEROLOGY Vol. 132, No. 1
hind paw was measured using a Ugo Basile hydroplethys-mometer (Stoelting, Chicago, IL). Immediately thereafter,the rats were then anesthetized with halothane andlambda carrageenan (100 L; 1% wt/vol) was injectedinto the footpad. The volume of the injected paw wasmeasured hourly thereafter for 5 hours. All measure-ments of paw volume were performed by an individualunaware of the treatments the rats had received.
H2S Release
Release of H2S from ATB-337 and its H2S-donat-
ing moiety, ADT-OH, was examined using an in vitro
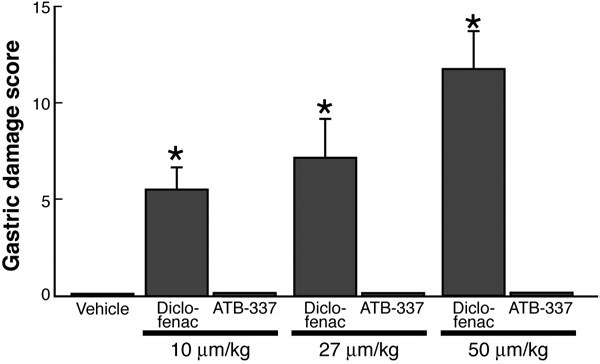
Figure 2. Diclofenac, but not ATB-337, dose-dependently induced
system that was recently described in Briefly, this
hemorrhagic gastric erosion formation in the rat stomach. Each group
method involves incubation of the test compounds (10
consisted of 5 rats. Results are shown as the mean ⫾ SEM. *P ⬍ .05 vs
mmol/L) in polyethylene glycol and 100 mmol/L potas-
the vehicle-treated group.
sium phosphate buffer (pH 7.4). Incubations were per-formed in this buffer alone or in the presence of 10%(wt/vol) rat liver homogenate and 2 mmol/L pyridoxal
ATB-337 consists of a molecule of diclofenac linked
5=-phosphate for 30 minutes. The generation of H2S was
through an ester bond to an H2S-releasing moiety (ADT-
detected via a sulfide-sensitive electrode, as previously
OH). We examined whether or not separate, concomitant
administration of the 2 moieties of ATB-337 would result
in the same lack of gastric damage as seen with ATB-337.
As shown in coadministration of diclofenac and
All data are expressed as mean ⫾ SEM. Groups of
ADT-OH resulted in hemorrhagic gastric damage of sim-
data were compared using a one-way analysis of variance
ilar severity to that seen with diclofenac alone, while
followed by a Dunnett's multiple comparison test. An
ATB-337 did not produce damage. Moreover, the lack of
associated probability (P value) of less than 5% was con-
gastric damage observed with ATB-337 was not attribut-
able to a diminished ability of this compound to sup-press gastric PG synthesis. ATB-337 and diclofenac (given
with or without ADT-OH) suppressed gastric PG synthe-
sis by more than 90%.
acetic acid 4-(5-thioxo-5H-1,2-dithiol-3-yl)-phenyl 2-(2-
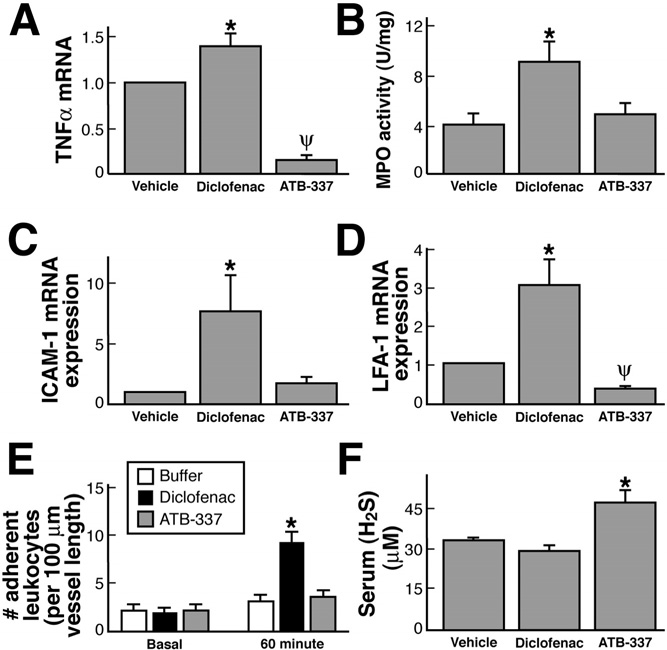
Administration of diclofenac (50 mol/kg) resulted,
within 3 hours, in significant increases in expression of
by Antibe Therapeutics Inc (Toronto, Ontario, Canada).
mRNA for TNF-␣ ) and in tissue granulocyte
Diclofenac sodium, L-NAME, and aspirin were obtained
levels, as measured by myeloperoxidase activity
from Sigma Chemical Co (St Louis, MO). Lumiracoxib
). Consistent with the latter, there was also a signifi-
was obtained from SynphaBase (Zurich, Switzerland).
cant increase in the expression of the endothelial and
The kits for measuring myeloperoxidase activity were
leukocyte adhesion molecules, ICAM-1 and LFA-1, re-
obtained from CytoStore (Calgary, Alberta, Canada). The
spectively and D). Such increases were not
enzyme-linked immunosorbent assay kits for PGE2 and
observed in the group treated with an equimolar dose of
thromboxane B2 were obtained from Cedarlane Labora-
tories (Hornby, Ontario, Canada). All other materials
NSAIDs and selective COX-2 inhibitors have been
were obtained from Fisher Scientific Co (Edmonton, Al-
shown previously to induce adherence of leukocytes to
berta, Canada).
the vascular endothelium, and this contributes signifi-cantly to their ability to induce gastric We
therefore compared diclofenac and ATB-337 with respectto their ability to trigger leukocyte adherence in postcap-
illary mesenteric venules in the rat. Basal levels of adher-
Oral administration of diclofenac resulted in the
ence, per 100-m vessel length, averaged 2.1 ⫾ 0.7 (n ⫽
development of hemorrhagic erosions in the rat stomach,
5), and this did not change significantly over the course
which increased in severity with the dose In
of 60 minutes of superfusion of the vessels with buffer
contrast, oral administration of ATB-337 at doses
). However, in rats given diclofenac (50 mol/
equimolar to those of diclofenac did not produce any
kg), leukocyte adherence increased significantly over the
hemorrhagic erosions. Blind histologic evaluation of the
course of the 1-hour study. In contrast, administration of
gastric tissue from these rats confirmed the absence of
ATB-337 (50 mol/kg) had no significant effect on leu-
any damage to the mucosal tissue.
kocyte adherence ).
H2S–RELEASING NSAID
rhagic erosion and ulcer formation in the small intestineThese lesions were found principally in thejejunum and to a lesser extent the ileum. Extensive bleed-ing was evident. While perforations were not observed,some of the lesions clearly penetrated to the muscularis.
The rats treated with diclofenac also exhibited a pro-found decrease in hematocrit, presumably a reflection ofsignificant intestinal bleeding. In contrast, administra-tion of an equimolar dose of ATB-337 produced ⬎90%less small intestinal damage and had no significant effecton the hematocrit. The lesions observed in rats treatedwith ATB-337 were confined to the jejunum and weresmall (1–2 mm2) and superficial.
Anti-Inflammatory and Antiplatelet Effects
ATB-337 exhibited a similar ability to suppress
COX-1 and COX-2 activity as diclofenac. In a rat airpouch model, in which inflammation is induced by zy-mosan, the large increase in PGE2 synthesis occurs al-most exclusively via COX-2. Lumiracoxib, at a dose (10mg/kg) that had no effect on whole blood thromboxanesynthesis, inhibited PGE2 synthesis by ⬎90% (from 25.5
Figure 3. ATB-337 did not induce the formation of hemorrhagic ero-
⫾ 3.0 ng/mL of exudate to 2.1 ⫾ 0.1 ng/mL). At a dose
sions in the stomach (A) despite profoundly suppressing gastric pros-taglandin synthesis (B). Coadministration of ADT-OH (the H
moiety of ATB-337) and diclofenac did not affect the severity of gastricdamage produced as compared with diclofenac alone. In these exper-
iments, equimolar doses (27 mol/kg) of diclofenac, ATB-337, andADT-OH were administered orally and the stomach was examined 3hours later. Each bar represents the mean ⫾ SEM, and each groupconsisted of 5– 6 rats. *P ⬍ .05 vs the vehicle-treated group.
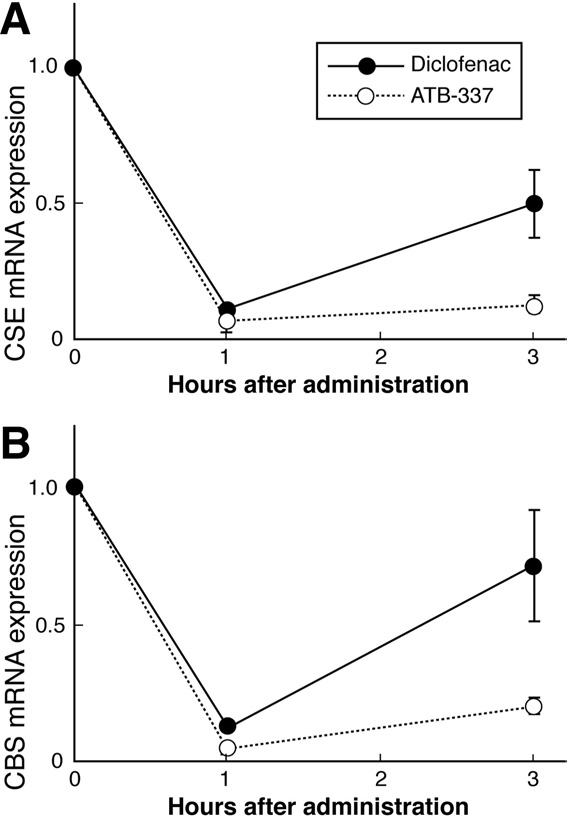
Plasma levels of H2S were significantly increased (by
⬃40%) 3 hours after oral administration of ATB-337 butunchanged in rats treated with diclofenac ). Incontrast, expression in the stomach of CSE and CBS, thekey enzymes for H2S synthesis, was suppressed by bothdiclofenac and ATB-337, although the magnitude of sup-pression by the latter was greater
Inhibitors of gastric acid secretion can reduce the se-
verity of NSAID-induced gastric mucosal injury. If ATB-337 were to markedly diminish gastric acid secretion,such an effect could contribute to the reduced mucosalinjury observed in rats given this compound. As shown inneither the volume of gastric juice produced overa 3-hour period nor the concentration of acid within thegastric juice was significantly affected by treatment with
Figure 4. Effects of diclofenac and ATB-337 (each at 50 mol/kg) on
diclofenac or ATB-337.
gastric expression of mRNA for (A) TNF-␣, (C) ICAM-1, and (D) LFA-1
The gastric safety of ATB-337 was also evident when it
and on (B) gastric myeloperoxidase activity, (E) leukocyte adherence tothe vascular endothelium of mesenteric venules, and (F) serum H
was administered to rats that had been pretreated with
concentrations. Results are shown as the mean ⫾ SEM of 5 rats per
aspirin or with an inhibitor of NO synthesis (L-NAME).
group. For the mRNA expression, the data are shown relative to ex-
As shown in ATB-337 at a dose of 50 mol/kg
pression in the vehicle-treated group (densitometric analysis of the gels
did not produce significant gastric damage even when
was performed, with normalization to expression of glyceraldehyde-3-
given following one of these inhibitors.
phosphate dehydrogenase). In the studies shown in E, leukocyte ad-herence was measured before (basal) and 60 minutes after intragastric
ATB-337 was also much better tolerated in the small
administration of vehicle, diclofenac, or ATB-337 (50 mol/kg for both
intestine than diclofenac. Administration of diclofenac 3
drugs). *P ⬍ .05 comparing the ATB-337 group with the diclofenac
times at 12-hour intervals resulted in extensive hemor-
group; ⌿P ⬍ .05 comparing the ATB-337 group with the vehicle group.
GASTROENTEROLOGY Vol. 132, No. 1
Table 2. Effects of L-NAME and Aspirin on Gastric Damaging
Effects of Diclofenac and ATB-337
Gastric Damage Score
NOTE. Results are expressed as the mean ⫾ SEM for 5 rats pergroup. Neither aspirin (10 mg/kg) nor L-NAME (15 mg/kg) significantlyincreased the extent of gastric damage induced by diclofenac orATB-337. The vehicle for aspirin was 1% carboxymethylcellulose,while the vehicle for L-NAME was 0.9% saline. Both diclofenac andATB-337 were administered orally at 50 mol/kg.
Again, we observed comparable inhibitory effects of di-clofenac and ATB-337
The effects of ATB-337 on human platelet function
were also examined. As in the rat, ATB-337 produced
comparable inhibition of thromboxane synthesis as wasseen with diclofenac Moreover, ATB-337 sup-
pressed arachidonic acid–induced human platelet aggre-
Figure 5. Gastric expression of mRNA for the 2 principal enzymes in
endogenous H2S synthesis: (A) CSE and (B) CBS. Expression was
measured by quantitative reverse-transcription polymerase chain reac-
tion 1 and 3 hours after oral administration to rats of diclofenac or
ATB-337, both at 50 mol/kg. Results are shown as the mean ⫾ SEM
for 5 rats per group. Both drugs caused a significant decrease in CSE
and CBS expression at 1 hour (P ⬍ .05). CSE expression remained
significantly suppressed with both drugs at 3 hours, but the suppres-
sion was greater with ATB-337 (P ⬍ .05). In the case of CBS expres-
sion, the suppressive effect of diclofenac was no longer significant at 3
hours, while ATB-337 still exhibited a marked suppressive effect.
of 1 mol/kg, both diclofenac and ATB-337 suppressedCOX-2 activity in this model by ⬃50% At adose of 10 mol/kg, both diclofenac and ATB-337 sup-pressed COX-2 activity by ⬎95%
COX-1 activity was assessed by measuring thrombox-
ane synthesis in blood collected from these rats at thesame time that the inflammatory exudates were collected.
Table 1. Effects of Diclofenac and ATB-337 on Gastric Acid
Secretion in Rats
Acid Secretion (mEq/h)
Figure 6. (A) Small intestinal damage and (B) change in hematocrit
following treatment with diclofenac or ATB-337. Oral administration of
the drugs (each at 50 mol/kg) was performed 48, 36, and 24 hours
before assessment of small intestinal damage. Blood samples were
NOTE. Each group consisted of 5 rats. Results are expressed as the
taken at the beginning and end of the study for measurement of hemat-
mean ⫾ SEM. The pylorus was ligated 30 minutes after oral admin-
ocrit. ATB-337 induced significantly less (*P ⬍ .05) intestinal damage
istration of the test drugs or vehicle, and gastric juice was collected 3
than diclofenac. Diclofenac, but not ATB-337, caused a significant de-
hours later. Neither the volume of secretion nor the quantity of acid
crease in hematocrit. Each group consisted of 5 rats, and the bars show
secretion differed significantly among the groups.
the mean ⫾ SEM.
H2S–RELEASING NSAID
Figure 8. Injection of carrageenan into the hind paw of a rat resulted in
significant edema formation, as measured by an increase in paw vol-
ume. Pretreatment with diclofenac resulted in a dose-dependent inhi-
bition of the edema formation. Pretreatment with ATB-337 at 10
mol/kg produced a reduction in edema formation similar to that seenwith diclofenac at 30 mol/kg. Both drugs were administered orally 30minutes before injection of carrageenan. Each group consisted of 5– 6
rats. Data are presented as the mean ⫾ SEM.
Figure 7. Inhibition of (A) COX-2 and (B) COX-1 activity in an in vivo
inflammation model. Injection of 1% zymosan into a preformed air
with edema formation Pretreatment with di-
pouch in the rat results in an acute inflammatory reaction accompanied
clofenac resulted in a dose-dependent reduction of the
by COX-2– dependent PGE2 production. Diclofenac and ATB-337 (ei-
increase in paw volume. Pretreatment with ATB-337 at a
ther at 1 or 10 mol/kg) produced comparable suppression of COX-1and COX-2 activity. Each group consisted of 5 rats, with the mean ⫾
dose of 10 mol/kg produced a reduction of paw swell-
SEM shown. *P ⬍ .05, **P ⬍ .01, ***P ⬍ .001 vs the vehicle ⫹ zymosan
ing similar to that produced by a 30 mol/kg dose of
gation to the same extent as equimolar concentrations of
When incubated in buffer, there was negligible
Injection of carrageenan into a hind footpad of the rat
2S from ADT-OH, the H2S-releasing moiety
of ATB-337 However, ATB-337 generated ⬃12
resulted in a marked increase in paw volume, consistent
Table 3. Effects of Diclofenac and ATB-337 on Human
Platelet Function
Diclofenac (1 mol/L)
71.9 ⫾ 7.2a
ATB-337 (1 mol/L)
86.5 ⫾ 6.3a
Diclofenac (10 mol/L)
28.9 ⫾ 4.1a
ATB-337 (10 mol/L)
36.5 ⫾ 4.0a
NOTE. For thromboxane synthesis, whole blood was allowed to stand
Figure 9. Generation of H2S from ATB-337 and its H2S-donating moi-
at 37°C for 30 minutes. For aggregation, platelet-rich plasma was
ety (ADT-OH) when incubated in phosphate buffer (pH 7.4) or in a rat
stimulated with arachidonic acid (0.5 mol/L). The results (mean ⫾
liver homogenate (see Methods for more details). Both ADT-OH and
SEM) are expressed as a percent of maximal aggregation. Samples
ATB-337 released significantly more H2S when incubated in the ho-
from 3 donors were tested in each assay, repeated in triplicate.
mogenate as compared to the buffer (*P ⬍ .05). ATB-337 released
Effects of diclofenac and ATB-337 did not differ significantly from one
significantly more H2S than ADT-OH when incubated in either medium
another for any given concentration.
(⌿P ⬍ .05). Incubation of diclofenac in buffer or in homogenate did not
aP ⬍ .05 vs the vehicle-treated group.
result in any detectable H2S generation (not shown).
GASTROENTEROLOGY Vol. 132, No. 1
nmol/min of H2S when it was incubated in buffer. When
Previous studies have highlighted the important con-
ADT-OH was added to a liver homogenate, we could
tribution of TNF-␣ to the generation of gastric mucosal
detect H2S release. However, the levels of H2S generated
injury following NSAID administration to
when ATB-337 was incubated with the liver homogenate
In the present study, we observed a significant elevation
were ⬃3-fold greater than those from ADT-OH.
of gastric TNF-␣ expression. Whether or not H2S candirectly suppress TNF-␣ expression is not clear, but it is
noteworthy that we have previously observed reduced
The pathogenesis of NSAID-induced gastroenter-
expression of mRNA for TNF-␣ and several other cyto-
opathy remains incompletely understood. It is clear that
kines in the colon of rats following treatment with an
inhibition of the production of endogenous mediators
H2S-releasing derivative of We speculated
that contribute to mucosal defense is a major contribut-
that those effects may be attributable to inhibition of
ing The importance of PGs as mediators of mu-
nuclear factor B activation by the H2S-releasing moiety
cosal defense has been recognized for more than 30 years,
of that compound. Whether or not suppression of nu-
while the importance of NO in this regard became clear
clear factor B could contribute to the gastric safety of
in the past 2 Recent studies have highlighted
ATB-337 has not yet been examined.
the contribution of H
In addition to sparing the gastric mucosa, repeated
2S to gastric mucosal defense, as
well as the ability of NSAIDs to suppress endogenous
administration of ATB-337 was found to produce mark-
edly less intestinal damage (⬎90% reduction) than that
2S In the present study, we have shown that
modification of a commonly used NSAID (diclofenac)
observed with an equimolar dose of diclofenac. A marked
such that it generates H
decrease in hematocrit was observed in the diclofenac-
2S resulted in a substantial re-
duction in its ability to induce gastrointestinal damage
treated group, likely a result of the extensive intestinal
and bleeding, while not diminishing its ability to inhibit
bleeding that was clearly evident at the time of necropsy.
PG synthesis or reduce edema formation. Indeed, the H
Further evidence for the relative safety of ATB-337 was
release from ATB-337 may even enhance its anti-inflam-
the absence of any change in hematocrit during the
matory activity relative to diclofenac, consistent with
period of treatment. These observations are particularly
recently reported effects of other H
noteworthy considering the increasing recognition of the
NSAIDs were recently shown to inhibit H
ability of NSAIDs to produce significant bleeding from
tion by the gastric This may be an additional
the NSAIDs are also capable of impairing
mechanism through which these commonly used drugs
platelet aggregation and therefore blood clotting. These
induce damage to the mucosa, that is, by suppressing the
effects are directly related to the ability of NSAIDs to
synthesis of 2 key mediators of mucosal defense (PGs and
suppress platelet thromboxane synthesis, which occurs
via COX-1. We compared the effects of diclofenac and
2S). Previous studies from our laboratory showed that
the reduction of gastric blood flow and the induction of
ATB-337 on human platelet thromboxane synthesis and
leukocyte adherence to the vascular endothelium could
aggregation (induced by arachidonate) and found that
be significantly attenuated through administration of
the 2 drugs behaved similarly to one another. This is
agents that spontaneously release H
congruent with our observations in rat studies that the 2
2S in solution (NaHS
drugs exhibit comparable inhibitory activity on COX-1.
2The ability of ATB-337 to spare the gastro-
intestinal mucosa of injury may therefore be related to
H2S and NO share many actions and may even
the generation of H
counter-regulate production of one We ex-
2S from this compound and its sub-
sequent effects on the gastrointestinal microcirculation.
amined the possibility that ATB-337 would no longer
In particular, it was noteworthy that ATB-337 did not
exhibit gastric safety in a circumstance of concomitant
stimulate leukocyte adherence to the vascular endothe-
suppression of both NO and PG synthesis. However,
lium of postcapillary mesenteric venules, in contrast to
suppression of NO synthesis with L-NAME did not im-
the effects of diclofenac. Consistent with this finding,
pair the ability of ATB-337 to spare the gastric mucosa of
ATB-337 did not cause a significant increase in gastric
injury. We also examined the possibility that gastric
granulocyte infiltration (myeloperoxidase activity) or ex-
safety of ATB-337 was related to suppression of gastric
pression of leukocyte (LFA-1) or endothelial (ICAM-1)
acid secretion, because proton pump inhibitors have been
adhesion molecules, as observed with diclofenac in the
shown to significantly reduce the severity of NSAID-
present study, and as previously observed with other
induced However, neither ATB-337 nor
We recently showed that suppression of endog-
diclofenac significantly affected acid secretion in the rat
enous H2S synthesis with -cyanoalanine resulted in a
over a 3-hour period after their administration (the same
marked increase in leukocyte-endothelium adherence,
time frame within which we observed lesion formation in
consistent with a physiologic role of H2S in modulating
This hypothesis is further supported by
One of the more surprising findings in this study
the observation that suppression of endogenous H2S
was that administration of ADT-OH, the H2S-releasing
synthesis exacerbated carrageenan-induced paw
moiety of did not protect the stomach
H2S–RELEASING NSAID
against the damaging effects of diclofenac. However,
pression of COX-2 in the and similarly
the data on H2S generation in vitro offer a possible
there is evidence that NO can reduce expression of in-
explanation for this observation. In terms of H2S re-
lease, ADT-OH and ATB-337 behave quite differently.
While gastrointestinal safety of NSAIDs is a major
ADT-OH did not generate detectable H2S when incu-
limitation to the use of this class of drugs, increasingly
bated in buffer, while ATB-337 did. Moreover, the
there is concern about their cardiovascular adverse ef-
amount of H2S generated when ATB-337 was incu-
fects, particularly since the withdrawal of rofecoxib
bated in a liver homogenate was about 3 times that
(Vioxx; Merck & Co, Inc, Whitehouse Station, NJ) from
generated from ADT-OH in the same conditions. Thus,
worldwide markets. Whether or not HS-NSAIDs will ex-
it is possible that the enhanced generation of H2S from
hibit reduced cardiovascular toxicity has not yet been
ATB-337 as compared with ADT-OH could account for
examined. Unlike conventional and selec-
the gastric safety of the former. However, this requires
tive COX-2 ATB-337 did not induce leuko-
cyte adherence to the vascular endothelium. Leukocyte
In the studies performed in the air pouch model, in
adherence to endothelial cells can be an important com-
which inflammation was induced by injection of zymo-
ponent of thrombosis and of atherosclerosis. Also, given
san, ATB-337 reduced inflammatory PGE
2S is a potent it is possible that its
effectively as diclofenac. A highly selective COX-2 inhib-
release from HS-NSAIDs may attenuate any hypertensive
itor, lumiracoxib, almost completely suppressed PGE
effects associated with the action of the NSAID compo-
synthesis in this model, confirming that this model is
nent of the compounds, as was observed with NO-releas-
appropriate for estimating the ability of drugs to inhibit
ing NSAIDs in animal Clearly, this is an
COX-2 in vivo. ATB-337 also exhibited similar COX se-
exciting aspect of the potential utility of HS-NSAIDs that
lectivity to diclofenac, because the 2 drugs suppressed
warrants further study.
COX-1 activity (whole blood thromboxane synthesis) to
In summary, the results presented herein show that
the same extent. Given these observations and the known
an H2S-releasing derivative of an NSAID (ATB-337)
importance of PGs to edema formation, it is not surpris-
exhibits greatly reduced gastrointestinal-damaging ef-
ing that ATB-337 would exhibit anti-inflammatory ef-
fects as compared with the parent drug, while still
fects in the carrageenan-induced paw edema model sim-
suppressing mucosal PG synthesis. The anti-inflamma-
ilar to those of diclofenac. On the other hand, the ability
tory effects of the HS-NSAID were comparable or even
of ATB-337, at a dose of 10 mol/kg, to suppress edema
improved over those of the parent NSAID, possibly
formation as effectively as diclofenac at 3 times this dose
attributable to the anti-inflammatory effects of H2S.
indicated an increase in potency of the HS-NSAID. This
The lack of an effect of ATB-337 in terms of inducing
may be attributable to the recently reported ability of H
leukocyte adherence to the vascular endothelium and
to reduce edema While analgesic effects of
inducing TNF-␣ expression, in contrast to diclofenac
ATB-337 have not yet been characterized, it is noteworthy
and other NSAIDs, may contribute to the gastrointes-
tinal-sparing attributes of this compound. While the
2S donors, including a derivative of mesalamine
containing the same H
conclusions that we can draw regarding HS-NSAIDs
2S-releasing moiety as ATB-337,
were recently shown to significantly reduce visceral pain
are limited by the relatively short period of exposure of
rats to ATB-337 in this study, the results are neverthe-
less an early indication that the coupling of an H
2S synthesis occurs principally via the
actions of 2 enzymes: CSE and CBS. We previously re-
releasing moiety to conventional or COX-2–selective
ported that a number of NSAIDs were capable of reduc-
NSAIDs is an attractive approach to reducing toxicity
ing the expression of CSE and the generation of H
and enhancing efficacy.
from gastric tissue in In the present study, bothdiclofenac and ATB-337 markedly reduced gastric expres-
sion of both CSE and CBS. Interestingly, the expression
1. Wallace JL. Nonsteroidal anti-inflammatory drugs and gastroen-
of these enzymes following diclofenac administration
teropathy: the second hundred years. Gastroenterology 1997;
recovered toward control levels within the 3-hour period
112:1000 –1016.
of the study, but this was not the case in the rats treated
2. Wallace JL, Reuter B, Cicala C, McKnight W, Grisham MB, Cirino
G. Novel nonsteroidal anti-inflammatory drug derivatives with
with ATB-337. It is possible that H2S generation from
markedly reduced ulcerogenic properties in the rat. Gastroenter-
ATB-337 down-regulated the expression of these en-
zymes, thereby prolonging the suppression of expression
3. Fiorucci S, Santucci L, Wallace JL, Sardina M, Romano M, del
over that induced by the diclofenac component of this
Soldato P, Morelli A. Interaction of a selective cyclooxygen-
compound. The notion that H
ase-2 inhibitor with aspirin and NO-releasing aspirin in the
2S can down-regulate ex-
human gastric mucosa. Proc Natl Acad Sci U S A 2003;100:
pression of enzymes responsible for H2S synthesis is
purely speculation at this point; however, it is notewor-
4. Hawkey CJ, Jones JI, Atherton CT, Skelly MM, Bebb JR, Fagerholm
thy that PGs have been suggested to down-regulate ex-
U, Jonzon B, Karlsson P, Bjarnason IT. Gastrointestinal safety of
GASTROENTEROLOGY Vol. 132, No. 1
AZD3582, a cyclooxygenase inhibiting nitric oxide donator: proof
21. Ubuka T, Abe T, Kajikawa R, Morino K. Determination of hydrogen
of concept study in humans. Gut 2003;52:1537–1542.
sulfide and acid-labile sulfur in animal tissues by gas chromatog-
5. Schnitzer TJ, Kivitz AJ, Lipetz RS, Sanders N, Hee A. Comparison
raphy and ion chromatography. J Chromatogr B Biomed Sci Appl
of the COX-inhibiting nitric oxide donator AZD3582 and rofecoxib
in treating the signs and symptoms of osteoarthritis of the knee.
22. Khan SU, Morris GF, Hidiroglou M. Rapid estimation of sulfide in
Arthritis Rheum 2005;53:827– 837.
rumen and blood with a sulfide-specific ion electrode. Microchem
6. Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of
J 1980;25:388 –395.
hydrogen sulfide in the gastrointestinal tract and liver. Gastroen-
23. Wallace JL., McKnight W, Reuter BK, Vergnolle N. NSAID-induced
terology 2006;131:259 –271.
gastric damage in rats: requirement for inhibition of both cyclo-
7. Fiorucci S, Antonelli E, Distrutti E, Rizzo G, Mencarelli A, Orlandi
oxygenase 1 and 2. Gastroenterology 2000;119:706 –714.
S, Zanardo R, Renga B, Di Sante M, Morelli A, Cirino G, Wallace
24. Wallace JL, Miller MJ. Nitric oxide in mucosal defense: a little
JL. Inhibition of hydrogen sulfide generation contributes to gastric
goes a long way. Gastroenterology 2000;119:512–520.
injury caused by anti-inflammatory nonsteroidal drugs. Gastroen-
25. Santucci L, Fiorucci S, Giansanti M, Brunori PM, Di Matteo FM,
terology 2005;129:1210 –1224.
Morelli A. Pentoxifylline prevents indomethacin induced acute
8. Zanardo RC, Brancaleone V, Distrutti E, Fiorucci S, Cirino G,
gastric mucosal damage in rats: role of tumour necrosis factor
Wallace JL. Hydrogen sulfide is an endogenous modulator of
alpha. Gut 1994;35:909 –915.
leukocyte-mediated inflammation. FASEB J 2006;20:2118 –
26. Appleyard CB, McCafferty DM, Tigley AW, Swain MG, Wallace JL.
Tumor necrosis factor mediation of NSAID-induced gastric dam-
9. Wallace JL, Bak A, McKnight W, Asfaha S, Sharkey KA, Mac-
age: role of leukocyte adherence. Am J Physiol 1996;270:G42–
Naughton WK. Cyclooxygenase 1 contributes to inflammatory
responses in rats and mice: implications for gastrointestinal
27. Fiorucci S, Distrutti E, Mencarelli A, Renga B, Cirino G, Wallace
JL. Enhanced anti-inflammatory activity of hydrogen sulfide re-
10. Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Russo G,
leasing derivative of mesalamine in rodent models of colitis
Caliendo G, Santagada V, Cirino G, Wallace JL, Fiorucci S. 5-Ami-
involves direct inhibition of Nf-KB (abstr). Gastroenterology 2006;
ester (ATB-429), a hydrogen sulfide-releasing derivative of me-
28. Laine L, Connors LG, Reicin A, Hawkey CJ, Burgos-Vargas R,
salamine, exerts antinociceptive effects in a model of postinflam-
Schnitzer TJ, Yu Q, Bombardier C. Serious lower gastrointestinal
matory hypersensitivity. J Pharmacol Exp Ther 2006;319:447–
clinical events with nonselective NSAID or coxib use. Gastroen-
terology 2003;124:288 –292.
11. Morris GP, Beck PL, Herridge MS, Depew WT, Szewczyk MR,
29. Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen
Wallace JL. Hapten-induced model of chronic inflammation and
sulfide as an endogenous smooth muscle relaxant in synergy
ulceration in the rat colon. Gastroenterology 1989;96:795– 803.
with nitric oxide. Biochem Biophys Res Commun 1997;237:527–
12. Reuter BK, Davies NM, Wallace JL. Nonsteroidal anti-inflamma-
tory drug-enteropathy in rats: role of permeability, bacteria and
30. Zhao W, Wang R. H S-induced vasorelaxation and underlying
cellular and molecular mechanisms. Am J Physiol 2002;283:
H474 –H480.
13. Wallace JL, Arfors KE, McKnight GW. A monoclonal antibody
31. Regula J, Butruk E, Dekkers CP, de Boer SY, Raps D, Simon L,
against the CD18 leukocyte adhesion molecule prevents indo-
Terjung A, Thomas KB, Luhmann R, Fischer R. Prevention of
methacin-induced gastric damage in the rabbit. Gastroenterology
NSAID-associated gastrointestinal lesions: a comparison study
1991;100:878 – 883.
pantoprazole versus omeprazole. Am J Gastroenterol 2006;101:
14. Wallace JL, McKnight W, Miyasaka M, Tamatani T, Paulson J,
Anderson DC, Granger DN, Kubes P. Role of endothelial adhesion
32. Christen MO. Anetole dithiolenthione: biochemical consider-
molecules in NSAID-induced gastric mucosal injury. Am J Physiol
ations. Methods Enzymol 1995;252:316 –323.
33. Davies NM, Sharkey KA, Asfaha S, MacNaughton WK, Wallace JL.
15. Edwards JCW, Sedgwick AD, Willoughby DA. The formation of a
Aspirin induces a rapid up-regulation of cyclooxygenase-2 expres-
structure with features of synovial lining by subcutaneous injec-
sion in the rat stomach. Aliment Pharmacol Ther 1997;11:1101–
tion of air: an in vivo tissue culture system. J Pathol 1981;134:
34. Takeuchi K, Tanaka A, Ohno R, Yokota A. Role of COX inhibition
16. Sedgwick AD, Moore AR, Al-Duaij AY, Edwards JC, Willoughby DA.
in pathogenesis of NSAID-induced small intestinal damage.
Studies into the influence of carrageenan-induced inflammation
J Physiol Pharmacol 2003;54(Suppl 4):165–182.
on articular cartilage degradation using implantation into air
35. Cirino G, Wheeler-Jones CP, Wallace JL, Del Soldato P, Baydoun
pouches. Br J Exp Pathol 1985;66:445– 453.
AR. Inhibition of inducible nitric oxide synthase expression by
17. Wallace JL, Chapman K, McKnight W. Limited anti-inflammatory
novel nonsteroidal anti-inflammatory derivatives with gastrointes-
efficacy of cyclo-oxygenase-2 inhibition in carrageenan-airpouch
tinal-sparing properties. Br J Pharmacol 1996;117:1421–1426.
inflammation. Br J Pharmacol 1999;126:1200 –1204.
36. Asako H, Kubes P, Wallace J, Wolf RE, Granger DN. Modulation of
18. Rordorf CM, Choi L, Marshall P, Mangold JB. Clinical pharmacol-
leukocyte adhesion in rat mesenteric venules by aspirin and
ogy of lumiracoxib: a selective cyclo-oxygenase-2 inhibitor. Clin
salicylate. Gastroenterology 1992;103:146 –152.
37. Asako H, Kubes P, Wallace J, Gaginella T, Wolf RE, Granger DN.
19. Wallace JL, McKnight W, Del Soldato P, Baydoun AR, Cirino G.
Anti-thrombotic effects of a nitric oxide-releasing, gastric-sparing
venules: role of lipoxygenase products. Am J Physiol 1992;262:
aspirin derivative. J Clin Invest 1995;96:2711–2718.
20. Distrutti E, Sediari L, Mencarelli A, Renga B, Orlandi S, Antonelli
38. Wang R. Two's company, three's a crowd: can H2S be the third
E, Roviezzo F, Cirino G, Wallace JL, Fiorucci S. Evidence that
endogenous gaseous transmitter? FASEB J 2002;16:1792–
hydrogen sulfide exerts antinociceptive effects in the gastrointes-
tinal tract by activating K
channels. J Pharmacol Exp Ther
39. Muscara MN, Lovren F, McKnight W, Dicay M, del Soldato P,
Triggle CR, Wallace JL. Antihypertensive properties of a nitric
H2S–RELEASING NSAID
oxide-releasing naproxen derivative in two-kidney, one-clip rats.
Am J Physiol Heart Circ Physiol 2000;279:H528 –H535.
[email protected]; fax: (403) 270-3353.
40. Muscara MN, McKnight W, Lovren F, Triggle CR, Cirino G, Wallace
Supported by a grant from the Canadian Institutes of Health
JL. Vasorelaxant effects of a nitric oxide-releasing aspirin deriv-
Research. J.L.W. holds a Canada Research Chair and is supported
ative in normotensive and hypertensive rats. Br J Pharmacol
by an Alberta Heritage Foundation for Medical Research Senior
Scientist Award.
All authors of this paper hold shares in Antibe Therapeutics Inc.
Received August 13, 2006. Accepted September 28, 2006.
The authors thank Webb McKnight, Michael Dicay, Barbara Renga,
Address requests for reprints to: John L. Wallace, PhD, Department
Andrea Mencarelli, and Stefano Orlandi for their assistance in perform-
of Pharmacology and Therapeutics, University of Calgary, 3330 Hos-
ing these studies.
Source: http://www.antibethera.com/wordpress/wp-content/uploads/2015/12/Wallace-et-al-Gastro-2007-H2S-diclofenac.pdf
VETERINARY SURGEONS STRAVEN RD. VET CENTRE Dr. MICHAEL J. AVERILL, B.V.Sc (Dist) THE STRAVE ROAD 8 STRAVEN RD, RICCARTON Dr .EVE ALLELY B.V.Sc Dr. LINDA SORENSEN DVM VETERI ARY Dr. CHANTAL MORETON B.V.Sc Dr. FRANCESCA MATTHEWS B.V.Sc PO Box 8169, RICCARTON PH. (03) 348 9728 FAX (03) 348 8012 CE TRE (LTD)
Automated Dielectrophoretic Characterization of Mycobacterium smegmatisBenjamin G. Hawkins,† Chao Huang,† Srinitya Arasanipalai,‡ and Brian J. Kirby*,‡†Department of Biomedical Engineering, and ‡Sibley School of Mechanical and Aerospace Engineering,Cornell University, Ithaca, New York, United States We report the positive dielectrophoretic (pDEP) characterization of wild-type and ethambutol-treated Mycobacterium smegmatispopulations via automated pDEP cell trapping experiments. The automated technique was validated by measurements ofcarboxylate-modified polystyrene microspheres and Escherichia coli. The characterization of M. smegmatis identifies a key frequencyregime where the membrane-specific action of ethambutol leads to a change in the cellular dielectrophoretic response. This workrepresents the first such characterization of Mycobacteria and highlights the potential for DEP measurements to measure changes inmycobacterial membrane properties associated with chemical treatments or genetic mutation.







