
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Biospring.com.vn


Author's personal copy
Food Microbiology 28 (2011) 214e220
Contents lists available at ScienceDirect
Food Microbiology
Bacillus probiotics
Simon M. Cutting*
School of Biological Sciences, Royal Holloway University of London, Egham, Surrey TW20 0EX, UK
Bacterial spore formers are being used as probiotic supplements for use in animal feeds, for human
Available online 24 March 2010
dietary supplements as well as in registered medicines. Their heat stability and ability to survive thegastric barrier makes them attractive as food additives and this use is now being taken forward. While
often considered soil organisms this conception is misplaced and Bacilli should be considered as gut
commensals. This review summarises the current use of Bacillus species as probiotics, their safety, mode
of action as well as their commercial applications.
Ó 2010 Elsevier Ltd. All rights reserved.
1. Bacterial spores
2. The use of Bacillus as probiotics
Bacterial spores are produced in nature as a means to survive
Probiotics are live microbes, which when administered in
extreme environmental conditions enabling long-term survival in
adequate amounts confer a health benefit to the host (Araya et al.,
conditions that could otherwise kill vegetative bacteria (Nicholson
2002). Bacillus species have been used as probiotics for at least 50
et al., 2000). The decision to sporulate is very much dependant
years with the Italian product known as EnterogerminaÒ registered
upon the decline in nutrients in the immediate vicinity of the live
1958 in Italy as an OTC medicinal supplement. The scientific
cell. Sensing this, the bacterium enters an irreversible program of
interest in Bacillus species as probiotics though, has only occurred
development that results in the production of a spore some 8 h later
in the last 15 years and three principal reviews have covered the
(Fig. 1) (Errington, 2003). Intrinsic to survival is the structure of the
field (Hong et al., 2005; Mazza, 1994; Sanders et al., 2003). Of the
bacterial endospore, that contains, at its core, a condensed and
species that have been most extensively examined these are
inactive chromosome. Additional layers surround the spore,
Bacillus subtilis, Bacillus clausii, Bacillus cereus, Bacillus coagulans
including a peptidoglycan-rich cortex and one or more layers of
and Bacillus licheniformis. Spores being heat-stable have a number
proteinaceous material referred to as the spore coat (Henriques and
of advantages over other non-spore formers such as Lactobacillus
Moran, 2007). Together these protect the spore from UV radiation,
spp., namely, that the product can be stored at room temperature in
extremes of heat (typically up to 80e85 �C in most species),
a desiccated form without any deleterious effect on viability. A
exposure to solvents, hydrogen peroxide and enzymes such as
second advantage is that the spore is capable of surviving the low
lysozyme (Nicholson et al., 2000). The spore itself, is dehydrated
pH of the gastric barrier (Barbosa et al., 2005; Spinosa et al., 2000)
and if exposed to appropriate nutrients will germinate, a process
which is not the case for all species of Lactobacillus (Tuohy et al.,
taking just a few minutes, allowing water to enter the spore,
2007) so in principle a specified dose of spores can be stored
breakage and removal of the spore coats, and outgrowth and
indefinitely without refrigeration and the entire dose of ingested
resumption of vegetative cell growth (Fig. 1) (Moir, 2006).
bacteria will reach the small intestine intact.
Depending on species spores are spherical or ellipsoidal in shape,
Spore probiotics are being used extensively in humans as die-
between 0.8 and 1.4 mm in length, have a negative surface charge
tary supplements (Table 1), in animals as growth promoters and
competitive exclusion agents (Table 2) and lastly in aquaculture for
commonly fall under two genera, Bacillus and the strictly anaerobic
enhancing the growth and disease-resistance of cultured shrimps,
Clostridia although a surprisingly large number of other, lesser-
most notably the Black Tiger shrimp (Penaeus monodon) (Table 3).
known, genera include spore formers.
This review will focus primarily on the use of spore products forhuman use. Interestingly, a number of Bacillus products are licensedas medicinal supplements. Rather than describing specific productsa short summary of the major Bacillus species used in commercial
* Tel.: þ44 (0) 1784 443760; fax: þ44 (0) 1784 414224.
E-mail address: [email protected]
products will be summarised.
0740-0020/$ e see front matter Ó 2010 Elsevier Ltd. All rights reserved.
doi:10.1016/j.fm.2010.03.007
Author's personal copy
S.M. Cutting / Food Microbiology 28 (2011) 214e220
Vegetative Cell Growth
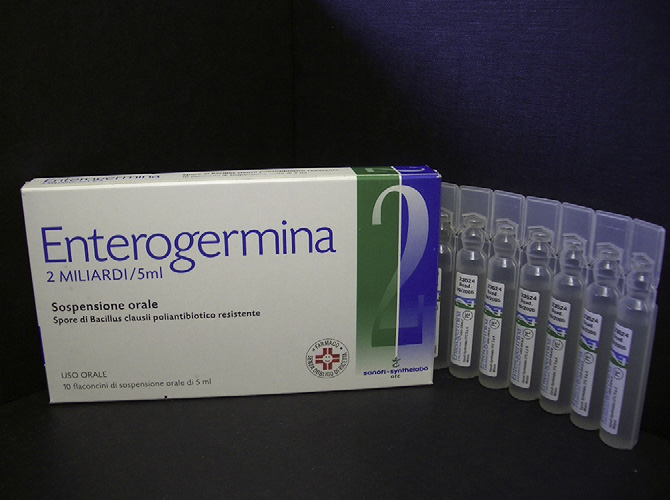
e3 vials are taken each day with the aim of pre-
venting infantile diarrhoea (Figs. 2e4). The suspension of spores inwater is thought to enhance delivery of spores to the mucosa anddemonstrates the versatility of spore formulations. The product
carries four antibiotic resistant strains of B. clausii that are recom-
mended for use with antibiotics (Coppi et al., 1985; Green et al.,1999; Senesi et al., 2001). The four strains are each derived from
ATCC 9799, a penicillin-resistant strain originally designated asB. subtilis. Through a multi-step process strains resistant to novo-
biocin þ rifampin (strain N/R), chloramphenicol (strain O/C),
streptomycin þ neomycin (strain SIN) and tetracycline (strain T)have been obtained (Ciffo, 1984; Mazza, 1994). Interestingly, theseB. clausii strains also carry resistance to a number of other antibi-otics including erythromycin, cephalosproins and cycloserine,
Fig. 1. The sporulation life cycle. A schematic showing the opposed life cycles of
kanamycin, tobramycin, and amikacin (Mazza et al., 1992). It has
bacterial spore formers. Under conditions of nutrient starvation the growing, vegeta-tive cell (VC) will undergo a series of morphological changes that create a forespore (F)
now been demonstrated that the resistance genes within these
within the mother cell (MC) of the sporangium. After approximately 8 h the spore (S) is
B. clausii strains are stable and are unable to transfer (Bozdogan
released by lysis of the MC.
et al., 2004; Mazza, 1983; Mazza et al., 1992).
Although the initial scientific studies used to register this
product in 1958 are obscure clinical trials have subsequently been
performed demonstrating efficacy, although a number of thesetrials lack completeness in terms of controls. Of note are clinical
B. clausii spores are used in the product EnterogerminaÒ which
studies assessing the effect of Enterogermina modulating the
is registered as an OTC medicinal supplement. Unlike most pro-
immune responses in allergic children with recurrent respiratory
biotic formulations that are supplied in tablet or capsule form the
infections (Ciprandi et al., 2004, 2005a,b). After administration of
Enterogermina product carries, spores (2 � 109) suspended in 5 ml
the probiotic nasal symptoms and eosinophil counts in allergic
Table 1Bacillus probiotics for human use.a
Produced by Marion Merrell (Levallois-Perret, France)
Capsule carrying 1 � 109 spores of Bacillus cereus strain IP5832b
but also by Hoechst and then Aventis Pharma following
(ATCC 14893) [n.b., originally deposited as B. subtilis.
merger acquisitions. Also cited as being produced byCasella-Med, Cologne, Germany
Protexin Health Care
B. subtilis is one component of 14 strains carried in this UK probiotic
(1) Biofarm, Dniepropetrovsk, Ukraine
BiosporinÒ is a mixture of two strains of living antagonistic bacteriaB. subtilis 2335 (sometimes referred to as B. subtilis 3) and B. licheniformis2336 (ratio is 3:1). Originally isolated from animal fodder.
(2) Garars, Russia
There are a number of versions of this products produced in differentcountries including a recombinant form, Subalin.
Geyer Medicamentos S.A. Porto Alegre, RS, Brazil
B. cereus strain GM Suspension of 106 spores ml�1.
Binex Co. Ltd., Busan, S. Korea
Tablet carrying spores (1.7 � 107) of B. polyfermenticus SCD.c
BioProgress SpA, Anagni, Italy
Vial carrying 1 � 109 spores of Bacillus clausii in suspension, labelled as
carrying B. subtilis. No longer marketed.
Sanofi Winthrop SpA, Milan, Italy
Vial (5 ml) carrying 1 � 106 spores of B. clausii in suspension. At least four
different strains of B. clausii present and product originally labelled ascarrying B. subtilis.
Flora-Balance, Montana, USA
Capsules labelled as carrying Bacillus laterosporus BODc but containing
Brevobacillus laterosporus BOD.
Ganeden Biotech Inc., Ohio, USA
B. coagulans GanedenBC30
This is a patented strain that has GRAS approval in the USA.
Istituto Biochimico Italiano SpA, Milan, Italy
Capsule carrying spores of B. subtilis labelled as carrying 2 � 109 spores ofLactobacillus sporogenes.c
Sabinsa Corp., Piscataway, NJ, USA
Labelled as Lactobacillus sporogenesc but contains B. coagulans 6e15 � 109 g�1.
Hanmi Pharmaceutical Co. Ltd., Beijing, China
B. subtilis strain RO179 (at 108 g�1) in combination with Enterococcus faecium.
Nature's First Food
Nature's First Law, San Diego, CA, USA
42 species listed as probiotics including: B. subtilis, B. polymyxa,c B. pumilus
and B. laterosporus.c
Newpharma S.r.l., Milan, Italy
Mixture of lactic acid bacteria inc. L. acidophilus, B. bifidum and L. sporogenes.cL. sporogenes at 3.3 � 105 CFU g�1 whose valid name is B. coagulans and ismislabelled as a strain of B. subtilis.
B. subtilis.
Palm Beach, Florida, USA.
www.gardenoflife.com/
a This list is likely incomplete and excludes Vietnamese products that are shown in Table 4.
b Contains the same strain used in the now discontinued animal feed product Paciflor.
c Not recognised as a Bacillus species (www.bacterio.cict.fr).
Author's personal copy
S.M. Cutting / Food Microbiology 28 (2011) 214e220
Table 2Bacillus probiotics for veterinary use.a
Alpharma Inc., Melbourne, Australia
B. licheniformis (NCTC 13123) at 109�1010 spores kg�1. This is a
non-bacitracin producing strain. Not licensed in the EU.
Poultry, calves and swine
Provita Eurotech Ltd., Omagh,
Listed as containing spores of B. licheniformis (1.6 � 109 CFU g�1) and
Northern Ireland, UK
B. subtilis (1.6 � 109 CFU g�1).
Piglets,a Chickens,
Christian Hansen Hoersholm,
Mixture (1/1) of B. licheniformis (DSM 5749) and B. subtilis (DSM 5750)
turkeys for fatteningc
at 1.6 � 109 CFU g�1 of each bacterium. EU approved.a
Esporafeed PlusÒ
Norel, S.A. Madrid, Spain
1 � 109 B. cereus (CECT 953). Not licensed in the EU.
Poultry, calves and swine
Pharmed Medicare, Bangalore, India
Labelled as Lactobacillus sporogenesb but contains B. coagulans.
Poultry, calves and swine
Sanofi Sante Nutrition Animale, France
2 strains of B. clausii (CNCM MA23/3V and CNCM MA66/4M). Not licensedin the EU.
Calves, poultry, rabbits and
Asahi Vet S.A., Tokyo (Head Off.), Japan
B. cereus var. toyoi (NCIMB-40112/CNCM-1012) at a minimum
swine. Possible use also for
concentration of 1 � 1010 CFU g�1 mixed with maize flour (4% by weight)
and calcium carbonate (90% by weight). Licensed in the EU.a
a Authorised for unlimited use by the EU.
b Not recognised as a Bacillus species (www.bacterio.cict.fr).
children were significantly reduced. In these studies a Th1
it is being used in a number of products such as Sustenex and is also
(T-helper 1) bias was observed showing that ingestion of Enter-
being incorporated into foods where spores can survive the mild
ogermina could enhance the cellular immunity in allergic children
heat-treatments used to sterilise foods. A recently published
who normally carry a Th2 bias. These studies have been supported
randomized, double-blind, placebo-controlled, parallel-design, has
by later studies by Marseglia et al. (2007) who have examined the
shown significant effects of B. coagulans as an adjunct therapy for
duration and rate of respiratory infections in 40 children (mean age
relieving symptoms of rheumatoid arthritis (Mandel et al., 2010).
4.3 � 1.5 years). After administration of Enterogermina for 90 days
Other than this the value of B. coagulans as a probiotic has, however,
they observed a decrease in the duration of respiratory infection,
recently been questioned (Drago and De Vecchi, 2009) and
but not the frequency of infection. Other clinical trials have
undoubtedly, further scientific evidence supporting the efficacy of
examined the positive effect of Enterogermina on the side effects of
this species is required.
antibiotic-based Helicobacter pylori therapy (Nista et al., 2004), andon urinary tract infections (Fiorini et al., 1985).
2.3. B. subtilis and B. licheniformis
The product was originally labelled as carrying spores of
B. subtilis but subsequent studies have identified the species as
B. subtilis has been extensively studied at a genetic and physi-
B. clausii (Green et al., 1999; Senesi et al., 2001). This product is not
ological level. Numerous probiotic products are labelled as carrying
specifically referred to as a probiotic but claims to enhance the
B. subtilis and in part, this probably results historically from
body's immune system following germination of the spores in the
a carelessness in assuming that most aerobic spore formers are
small intestine.
B. subtilis. Accordingly, numerous products claiming to carryB. subtilis have been shown to carry other species (see Table 1 and
2.2. B. coagulans
Table 4). However, B. subtilis var. Natto is worthy of comment. Thisbacterium is used in the fermentation of soybeans that is used to
This species is often labelled, incorrectly, as Lactobacillus spor-
prepare the Japanese staple known as Natto. Natto carries as many
ogenes which is an unrecognised species name. The origin of this
as 108 viable spores per gram of product and for decades health
species for use in probiotics stems from India where a number of
benefits have been associated with consumption of Natto including
manufacturers produce B. coagulans as a food ingredient for export
stimulation of the immune system (Hosoi and Kiuchi, 2004). A
and relabelling in Europe and the US. B. coagulans secretes
serine protease known as Nattokinase is secreted from vegetative
a bacteriocin, Coagulin, which has activity against a broad spectrum
cells of B. subtilis var. Natto and has been shown to reduce blood
of enteric microbes (Hyronimus et al., 1998). Recently one strain,
clotting by fibrinolysis (Sumi et al., 1987, 1995). There are several
labelled as GanedenBC30 has been granted self-affirmed GRAS
important points here, firstly, the serine protease that is named
status by the FDA in the US. Marketed by Ganeden, as GanedenBC30
Nattokinase is in fact produced by all strains of B. subtilis but in the
Table 3Bacillus probiotics for aquaculture.a
Sino-Aqua Corp., Kaohsiung, Taiwan
B. subtilis strains Wu-S and Wu-T at 108 CFU g�1,
product also contains Lactobacillus and Saccharomyces spp.
Microbial Solutions, Johannesburg, South Africa and
Mixture of: B. megaterium, B. licheniformis, Paenibacillus polymyxa
Advanced Microbial Systems, Shakopee, MN, USA
and two strains of B. subtilis.
Cargill, Animal Nutrition Division
Undefined Bacillus species.
Sino-Aqua company Kaohsiung, Taiwan
Carries four strains of B. subtilis.
Sanocare Sanolife Sanoguard
INVE Technologies nv Dendermonde, Belgium
Various Bacillus species.
a This shows just a selection of registered products from international companies. In shrimp-producing countries the number of ‘local' products is substantial, for example,
in Vietnam over 30 different products are sold.

Author's personal copy
S.M. Cutting / Food Microbiology 28 (2011) 214e220
Fig. 2. EnterogerminaÒ. This is a licensed OTC product containing 2 � 109 of GMP-produced spores of B. clausii in 5 ml of water. 2e3 vials are consumed per day to helpprevent gastroenteritis in infants and children.
Natto strain it is produced at high levels. Second, it cannot be ruledout that health benefits ascribed to Natto require consumption ofboth soybeans and bacteria, rather than just the bacterium. In anyevent, Nattokinase has GRAS status as an enzyme produced froma bacterium in the US and is purified and sold as a health supple-ment worldwide. In poultry studies controlled trials have shownthat oral administration of B. subtilis spores reduce infection bySalmonella enterica serotype Enteritidis, Clostridium perfringens andEscherichia coli O78:K80 (La Ragione et al., 2001; La Ragione andWoodward, 2003).
Fig. 4. Natto. Natto is normally consumed as a fermented soybean product either hot
B. subtilis and B. licheniformis are used together in two products,
or cold. In this example it is sold as a snack with dried soybeans coated with a fine
Biosporin and BioPlusÒ 2B. BioPlusÒ 2B is used in animal feed while
white powder of B. subtilis var. Natto, the active ingredient required for the taste andtexture of Natto.
Biosporin is licensed as a medicine in the Ukraine and Russia.
Biosporin is sold in glass vials that must be reconstituted in waterbefore consumption. The two Bacillus strains, B. subtilis 2335 and
H. pylori (Pinchuk et al., 2001). In the case of BioPlusÒ 2B this animal
B. licheniformis 2336 are well characterised and a number of clinical
feed product has also been extensively studied with numerous
studies have been used to demonstrate probiotic effects although
efficacy studies focused on the suppression of gastrointestinal
none been performed with the rigour of a full clinical trial (Bilev,
pathogens completed resulting in the registration of this product as
2002; Osipova et al., 2003, 2005; Sorokulova, 1997; Sorokulova
a feed supplement in Europe (SCAN, 2000b). It remains unclear
et al., 1997). Interestingly, B. subtilis 2335 has been shown to
whether there is any added benefit in the combined use of the two
produce the antibiotic Amicoumacin with in vitro activity against
B. cereus is a known human pathogen that is the cause of mild
food poisoning due to the production of up to three enterotoxinsand one emetic toxin (Stenfors Arnesen et al., 2008). Not all strainsof B. cereus carry enterotoxin genes yet a number of B. cereus pro-biotics have been shown to carry the enterotoxin genes (Hoa et al.,2000) and one product, Paciflor, used in animal feed has beenwithdrawn from use in the EU (SCAN, 2001a). Despite this B. cereusproducts are still being used for example, ToyerocinÒ, an animalfeed product is registered for use in Europe (SCAN, 2001b) andBactisubtilÒ as a registered medicinal supplement for human use.
Interestingly, the strain of B. cereus used in BactisubtilÒ known asIP5832 is the same as that in the withdrawn animal productPaciflorÒ.
3. How do spore probiotics work?
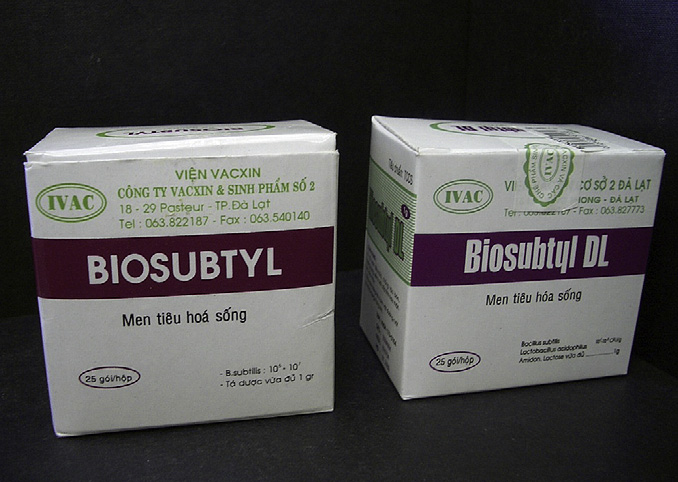
Fig. 3. Biosubtyl and Biosubtyl DL. Typical Vietnamese products, in this case, Biosubtyl
Bacillus species are often considered soil organisms since spores
that carries spores of B. cereus IP5832 and Biosubtyl DL carrying a mixture of B. cereus
they can readily be retrieved from soil. However, attempting to
IP5832 and Lactobacillus acidophilus. Neither product is labelled properly nor carriesthe stated dose.
isolate vegetative bacteria from soil is more problematic and it now
Author's personal copy
S.M. Cutting / Food Microbiology 28 (2011) 214e220
Table 4Vietnamese Bacillus OTC products licensed for human use.
Viet-Duc Pharmaceutical Co. Ltd., Hanoi
Labelled as containing B. subtilis, L. acidophilus, S. faecalis but B. subtilis isB. cereus at 107 g�1.
Tediphar Corporation (TEDIPHARCO), Ho Chi Minh City, Vietnam
Sachet (1 g) carrying 107�108 spores of B. subtilis.
Bidiphar. Binh Dinh Pharmaceutical and Medical Equipment
Labelled sachets carrying 1 � 106 spores of B. cereus but mislabelled as
Company, 498 Nguyen Thai Hoc, Qui Nhon, Vietnam
B. subtilis.
Biophar Company, Da lat, Vietnam
Sachet (1 g) carrying 106�107 of B. cereus spores mixed with tapioca.
Product labelled as B. subtilis. The strain is closely related by 16S rRNAanalysis to IP 5832 used in BactisubtilÒ.
IVAC, 18 Le Hong Phong, Da Lat, Vietnam
Sachets (1 g) carrying 107�108 CFU of B. subtilis andLactobacillus acidophilus.
Biosubtyl I and II
Biophar Company, Nha Trang, Vietnam
Sachet (1 g) carrying 106�107 of B. pumilus spores mixed with tapioca.
Product labelled as B. subtilis.
Pasteur Institute of Ho Chi Minh City, Vietnam
Sachets (1 g) carrying 108 spores of B. subtilis.
Mekophar, Pharmaceutical Factory No. 24, Ho Chi Minh City,
Capsule carrying 106�107 spores of a B. cereus species termed
B. cereus var. vietnami. Product labelled as carrying B. subtilis.
ILdong Pharm Co., Ltd., 60-1, SinKeonji-Dong, Ansung-Si,
Each gram of granules contains:
Kyong Ki-Do, Korea
Lactobacillus sporogenes 5.0 � 107 cfuClostridium butyricum 1.0 � 107 cfuBacillus subtilis 3.0 � 106Thiamine Nitrate 0.3 mgRiboflavin 0.2 mgAscorbic Acid 5.0 mgNicotinamide 0.1 mgDibasic calcium photphate 20.0 mgDried yeast 50.0 mg
ILdong Pharm Co., Ltd., 60-1, SinKeonji-Dong, Ansung-Si,
Each gram of granules contains:
Kyong Ki-Do, Korea
Lactobacillus sporogenes 5.0 � 107 cfuClostridium butyricum 1.0 � 107 cfuBacillus subtilis 3.0 � 106Thiamine Nitrate 0.3 mgRiboflavin 0.2 mgAscorbic Acid 5.0 mgNicotinamide 0.1 mgDibasic calcium phosphate 20.0 mgDried yeast 50.0 mg
seems likely that spores are designed to survive transit across the
B. subtilis spores were shown to suppress infection with pathogenic
gastric barrier of animals that ingest them. This view originates
S. enterica (La Ragione and Woodward, 2003), C. perfringens
from studies that show that a percentage (>10%) of an inoculum of
(La Ragione and Woodward, 2003) and E. coli (La Ragione et al.,
B. subtilis spores can germinate in the small intestine, grow and
2001). A mouse model has been used to show suppression of Cit-
proliferate and then re-sporulate (Hoa et al., 2001; Tam et al., 2006).
robacter rodentium (a model for the traveller's diarrhoea pathogen,
Peristalsis ensures that spores are shed in faeces resulting in their
ETEC) by administration of B. subtilis spores (D'Arienzo et al., 2006).
accumulation in the soil. An intestinal habitat of spore formershelps explain why spores can be found in the gut of insects, animals
and humans (Barbosa et al., 2005; Fakhry et al., 2008; Hong et al.,2009a). Recent work has shown that Bacilli can readily be
Two spore formers, Bacillus anthracis and B. cereus are known as
obtained from the human GI-tract using analysis of both biopsies
human pathogens. The former requires no elaboration while the
and faeces (Fakhry et al., 2008; Hong et al., 2009a). In the latter,
use of B. cereus appears to be a cause for concern on a case-by-case
Bacillus spores can be found at levels of approximately 104 spores/g
basis. The safety of Bacillus species has been extensively reviewed
of faeces which is several logs higher than can reasonably be pre-
elsewhere (de Boer and Diderichsen, 1991; Ishibashi and Yamazaki,
dicted from food intake alone (Hong et al., 2009b).
2001; Logan, 2004; Osipova et al., 1998; Sanders et al., 2003; SCAN,
Numerous studies have shown that germinating spores can
2000a) and most incidences of illness associated with Bacillus
elicit potent immune responses in the GI-tract of mouse models
appear to result for opportunistic infections or miss-diagnosis.
and this immune stimulation may be the underlying reason why
Extensive animal studies including acute and sub-chronic toxicity
spores exert a probiotic effect (Hong et al., submitted for
testing as well as in vitro studies have now been performed on
publication). One of the most informative, yet least recognised
a number of species, including B. subtilis var. Natto (Hong et al.,
studies was one examining the effect of orally administered
2008), Bacillus indicus (Hong et al., 2008), B. coagulans (Endres
bacteria on the development of the gut-associated lymphoid tissue
et al., 2009) and B. subtilis 2335 (Sorokulova et al., 2008) and
(GALT) in infant rabbits (Rhee et al., 2004). In these studies
B. licheniformis 2336 (Sorokulova et al., 2008). All appear to show no
B. subtilis was shown to be of greater importance than other
indicators of adverse effects.
commensal bacteria in GALT development. Of course, other prop-erties such as the secretion of antimicrobials such as Coagulin,
5. Approved products in Europe and the USA
Amicoumacin and Subtilisin may also further provide a probioticeffect by suppressing growth of competing microbes as well as
Bacillus products that have been formally approved in the West
enteric pathogens. Studies showing efficacy are less easy to distil
are few. Numerous authors routinely cite B. subtilis as having GRAS
yet a few convincing examples are as follows. In a poultry model
(Generally Regarded as Safe) status but this is incorrect. Nattokinase,
Author's personal copy
S.M. Cutting / Food Microbiology 28 (2011) 214e220
the proteolytic enzyme that is purified from B. subtilis var. Natto does
spores could be added to beverages and foods yet retain their
carry GRAS status as a microbially produced enzyme but not the
probiotic properties. Indeed, such probiotic foods have already
bacterium. In 2008 B. coagulans strain GanedenBC30 was the first
entered the market with "Activate Muffins" containing Gane-
Bacillus strain to be given self-affirmed GRAS approval. In Europe, for
denBC30 launched by Isabella's Health Bakery in the USA in 2008.
approval, for use as a supplement a case must be made based on prioruse. The application is first made by authorities in the host country
and then assessed by an EU committee. To date B. subtilis has beenapproved for use as a supplement in Italy and the UK. B. clausii that is
The use of Bacillus species as probiotic dietary supplements is
used in the medicinal OTC product EnterogerminaÒ and B. cereus
expanding rapidly with increasing number of studies demon-
IP5832 (BactisubtilÒ) are registered as medicines with specific claims
strating immune stimulation, antimicrobial activities and compet-
regarding the prevention of childhood diarrhoea and, as a medicine,
itive exclusion. The single and most important advantage of these
are not marketed under the probiotic label.
products is that they can be produced easily and the stability of thefinished product can be assured, further they can be incorporated
6. The Vietnamese market
into everyday foods. Studies are showing that these bacteria areable to grow within the intestinal tract and possibly be considered
In SE Asia, notably, Vietnam, where no concept of dietary
temporary residents. This is important because it shows that these
supplements exists, Bacillus products are licensed with the Ministry
bacteria are not foreigners but rather may exert a unique symbiotic
of Health as medicinal supplements (Table 4) with claims ranging
relationship with their host.
from prevention of rotavirus infection (infant diarrhoea) and foodpoisoning to immune stimulation. It is unclear whether theirapproval requires formal clinical trials but in any event these
products are easily obtained and often used as the first line ofdefence against enteric infections both prophylactically but more
This article was based in part on a publication in Nutrafoods
often therapeutically. The use of Bacillus probiotics in Vietnam is
(2009 Vol. 8:7e14). Research in the laboratory of SMC and TCD is
more developed than in any other country and the reason for this is
supported by an EU 7th FP grant, KBBE-2007-207948.
unclear. There is also intense interest in using heat-stable Bacillusspores in aquaculture and it is not uncommon for shrimp farms to
use products produced for human use.
Agnew, M.D., Koval, S.F., Jarrell, K.F., 1995. Isolation and characterisation of novel
7. Recent innovations: functional foods
alkaliphiles from bauxite-processing waste and description of Bacillus vedderisp. nov., a new obligate alkaliphile. Syst. Appl. Microbiol. 18, 221e230.
Araya, M., Morelli, L., Reid, G., Sanders, M.E., Stanton, C., 2002. Joint FAO/WHO
In recent work pigmented Bacillus species have been charac-
Working Group Report on Guidelines for the Evaluation of Probiotics in Food
terised and the pigment has been shown to be due one or more
carotenoids (Duc et al., 2006; Khaneja et al., 2009). These carotenoids
Barbosa, T.M., Serra, C.R., La Ragione, R.M., Woodward, M.J., Henriques, A.O., 2005.
Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl. Environ.
have been shown to carry anti-oxidant activity in vitro and thus could
Microbiol. 71, 968e978.
be of nutritional value (SM Cutting; unpublished data). Yellow,
Bilev, A.E., 2002. Comparative evaluation of probiotic activity in respect to in vitro
orange, red and pink Bacillus species can be easily obtained from soil,
pneumotropic bacteria and pharmacodynamics of biosporin-strain producers inpatients with chronic obstructive pulmonary diseases. Voen. Med. Zh. 323, 54e57.
river and pond sediments as well as from the intestinal tracts of
Bozdogan, B., Galopin, S., Leclereq, R., 2004. Characterization of a new erm-related
animals (Hong et al., 2009a; Yoon et al., 2001, 2005). This includes
macrolide resistance gene present in probiotic strains of Bacillus clausii. Appl.
a red pigmented Bacillus megaterium (Mitchell et al., 1986) a pink
Environ. Microbiol. 70, 280e284.
pigment found in some isolates of Bacillus firmus (Pane et al., 1996)
Ciffo, F., 1984. Determination of the spectrum of antibiotic resistance of the Bacillus
subtilis strains of Enterogermina. Chemioterapia 3, 45e52.
and red pigment found in Bacillus atrophaeus (Fritze and Pukall, 2001;
Ciprandi, G., Tosca, M.A., Milanese, M., Caligo, G., Ricca, V., 2004. Cytokines evalu-
Nakamura, 1989). A variable yellow-orange pigmentation has
ation in nasal lavage of allergic children after Bacillus clausii administration:
been found in a number of species including, B. indicus (Suresh et al.,
a pilot study. Pediatr. Allergy Immunol. 15, 148e151.
Ciprandi, G., Vizzaccaro, A., Cirillo, I., Tosca, M.A., 2005a. Bacillus clausii effects in
2004), Bacillus cibi (Yoon et al., 2005), Bacillus vedderi (Agnew et al.,
children with allergic rhinitis. Allergy 60, 702e703.
1995), Bacillus jeogali (Yoon et al., 2001), Bacillus okuhidensis (Li
Ciprandi, G., Vizzaccaro, A., Cirillo, I., Tosca, M.A., 2005b. Bacillus clausii exerts
et al., 2002), Bacillus clarkii (Nielsen et al., 1995), Bacillus pseudo-
immuno-modulatory activity in allergic subjects: a pilot study. Eur. Ann. Allergy
Clin. Immunol. 37, 129e134.
rmus (Nielsen et al., 1995) and B. firmus (Ruger and Koploy, 1980).
Coppi, F., Ruoppolo, M., Mandressi, A., Bellorofonte, C., Gonnella, G., Trinchieri, A., 1985.
The carotenoids are found in the vegetative cell as well as in the spore
Results of treatment with Bacillus subtilis spores (Enterogermina) after antibiotic
and they help protect spores from UV radiation (Khaneja et al., 2009).
therapy in 95 patients with infection calculosis. Chemioterapia 4, 467e470.
D'Arienzo, R., Maurano, F., Mazzarella, G., Luongo, D., Stefanile, R., Ricca, E., Rossi, M.,
It is no surprise that Bacillus species found in aquatic environments
2006. Bacillus subtilis spores reduce susceptibility to Citrobacter rodentium-
and the animals that inhabit these environments are often rich in
mediated enteropathy in a mouse model. Res. Microbiol. 157, 891e897.
carotenoids. Carotenoids are of nutritional value and used as dietary
de Boer, A.S., Diderichsen, B., 1991. On the safety of Bacillus subtilis and B. amylo-
supplements. When used as supplements the recommended daily
liquefaciens: a review. Appl. Microbiol. Biotechnol. 36, 1e4.
Drago, L., De Vecchi, E., 2009. Should Lactobacillus sporogenes and Bacillus coagulans
allowance of carotenoids is often quite high (e.g., 800 mg/day for b-
have a future? J. Chemother. 21, 371e377.
carotene). The reason for this is that carotenoids are rapidly degraded
Duc, L.H., Fraser, P., Cutting, S.M., 2006. Carotenoids present in halotolerant Bacillus
in the stomach which raises questions over their nutritional value.
spore formers. FEMS Microbiol. Lett. 255, 215e224.
Endres, J.R., Clewell, A., Jade, K.A., Farber, T., Hauswirth, J., Schauss, A.G., 2009. Safety
Spore carotenoids though appear to be gastric stable and studies
assessment of a proprietary preparation of a novel probiotic, Bacillus coagulans,
currently in progress are designed to establish the uptake of spore
as a food ingredient. Food Chem. Toxicol.
carotenoids using in vitro and in vivo models (SM Cutting, unpub-
Errington, J., 2003. Regulation of endospore formation in Bacillus subtilis. Nat. Rev.
Microbiol. 1, 117e126.
lished data). It is apparent that carotenoid-rich spores could be used
Fakhry, S., Sorrentini, I., Ricca, E., De Felice, M., Baccigalupi, L., 2008. Characteriza-
commercially as dietary supplements providing a source of carot-
tion of spore forming Bacilli isolated from the human gastrointestinal tract.
enoids as well as conferring probiotic properties.
J. Appl. Microbiol. 105, 2178e2186.
Fiorini, G., Cimminiello, C., Chianese, R., Visconti, G.P., Cova, D., Uberti, T., Gibelli, A.,
A further development with spore probiotics is that they can
1985. Bacillus subtilis selectively stimulates the synthesis of membrane bound
survive mild heat-treatments used to sterilise food. In principle,
and secreted IgA. Chemioterapia 4, 310e312.
Author's personal copy
S.M. Cutting / Food Microbiology 28 (2011) 214e220
Fritze, D., Pukall, R., 2001. Reclassification of bioindicator strains Bacillus subtilis
Osipova, I.G., Sorokulova, I.B., Tereshkina, N.V., Grigor'eva, L.V., 1998. Safety of
DSM 675 and Bacillus subtilis DSM 2277 as Bacillus atrophaeus. Int. J. Syst. Evol.
bacteria of the genus Bacillus, forming the base of some probiotics. Zh Mikrobiol
Microbiol. 51, 35e37.
Epidemiol. Immunobiol 6, 68e70.
Green, D.H., Wakeley, P.R., Page, A., Barnes, A., Baccigalupi, L., Ricca, E., Cutting, S.M.,1999.
Osipova, I.G., Makhailova, N.A., Sorokulova, I.B., Vasil'eva, E.A., Gaiderov, A.A., 2003.
Characterization of two Bacillus probiotics. Appl. Environ. Microbiol. 65, 4288e4291.
Spore probiotics. Zh. Mikrobiol. Epidemiol. Immunobiol., 113e119.
Henriques, A.O., Moran Jr., C.P., 2007. Structure, assembly, and function of the spore
Osipova, I.G., Sorokulova, I.B., Vasil'eva, E.A., Budanova, E.V., 2005. Pre-clinical trials
surface layers. Annu. Rev. Microbiol. 61, 555e588.
of new spore probiotics. Vestn. Ross. Akad. Med. Nauk, 36e40.
Hoa, N.T., Baccigalupi, L., Huxham, A., Smertenko, A., Van, P.H., Ammendola, S.,
Pane, L., Radin, L., Franconi, G., Carli, A., 1996. The carotenoid pigments of a marine
Ricca, E., Cutting, S.M., 2000. Characterization of Bacillus species used for oral
Bacillus firmus strain. Boll. Soc. Ital Biol. Sper. 72, 303e308.
bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl.
Pinchuk, I.V., Bressollier, P., Verneuil, B., Fenet, B., Sorokulova, I.B., Megraud, F.,
Environ. Microbiol. 66, 5241e5247.
Urdaci, M.C., 2001. In vitro anti-Helicobacter pylori activity of the probiotic strain
Hoa, T.T., Duc, L.H., Isticato, R., Baccigalupi, L., Ricca, E., Van, P.H., Cutting, S.M., 2001.
Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Che-
Fate and dissemination of Bacillus subtilis spores in a murine model. Appl.
mother. 45, 3156e3161.
Environ. Microbiol. 67, 3819e3823.
Rhee, K.J., Sethupathi, P., Driks, A., Lanning, D.K., Knight, K.L., 2004. Role of
Hong, H.A., Duc le, H., Cutting, S.M., 2005. The use of bacterial spore formers as
commensal bacteria in development of gut-associated lymphoid tissues and
probiotics. FEMS Microbiol. Rev. 29, 813e835.
preimmune antibody repertoire. J. Immunol. 172, 1118e1124.
Hong, H.A., Huang, J.-M., Khaneja, R., Hiep, L.V., Urdaci, M.C., Cutting, S.M., 2008.
Ruger, H.-J., Koploy, J.A.C., 1980. DNA base composition of halophilic and non-
The safety of Bacillus subtilis and Bacillus indicus as food probiotics. J. Appl.
halophilkic Bacillus firmus strains of marine origin. Microb. Ecol. 6, 141e146.
Microbiol. 105, 510e520.
Sanders, M.E., Morelli, L., Tompkins, T.A., 2003. Sporeformers as human probiotics:
Hong, H.A., Khaneja, R., Tam, N.M., Cazzato, A., Tan, S., Urdaci, M., Brisson, A.,
Bacillus, Sporolactobacillus, and Brevibacillus. Compr. Rev. Food Sci. Food Saf. 2,
Gasbarrini, A., Barnes, I., Cutting, S.M., 2009a. Bacillus subtilis isolated from the
human gastrointestinal tract. Res. Microbiol. 160, 134e143.
SCAN, 2000a. Opinion of the Scientific Committee on Animal Nutrition on the
Hong, H.A., To, E., Fakhry, S., Baccigalupi, L., Ricca, E., Cutting, S.M., 2009b. Defining
Safety of the Use of Bacillus Species in Animal Nutrition. European Commission,
the natural habitat of Bacillus spore-formers. Res. Microbiol.
Health and Consumer Protection Directorate-General. (SCAN) Scientific
Hong, A.H., Duc, H.L., Cutting, S.M. Immunogenicity and intracellular fate of Bacillus
Committee on Animal Nutrition.
subtilis spores. Microbiology, submitted for publication.
SCAN, 2000b. Report of the Scientific Committee on Animal Nutrition on Product
Hosoi, T., Kiuchi, K., 2004. Production and probiotic effects of Natto. In: Ricca, E.,
BioPlus 2BÒ for Use as Feed Additive. European Commission, Health and
Henriques, A.O., Cutting, S.M. (Eds.), Horizon Bioscience, pp. 143e154.
Consumer Protection Directorate-General. (SCAN) Scientific Committee on
Wymondham, UK.
Animal Nutrition.
Hyronimus, B., Le Marrec, C., Urdaci, M.C., 1998. Coagulin, a bacteriocin-like inhibi-
SCAN, 2001a. Assessment by the Scientific Committee on Animal Nutrition of the
tory substance produced by Bacillus coagulans I4. J. Appl. Microbiol. 85, 42e50.
Safety of Product PaciflorÒ for Use as Feed Additive. European Commission,
Ishibashi, N., Yamazaki, S., 2001. Probiotics and safety. Am. J. Clin. Nutr. 73,
Health and Consumer Protection Directorate-General. (SCAN) Scientific
Committee on Animal Nutrition.
Khaneja, R., Perez-Fons, L., Fakhry, S., Baccigalupi, L., Steiger, S., To, E., Sandmann, G.,
SCAN, 2001b. Report of the Scientific Committee on Animal Nutrition on Product
Dong, T.C., Ricca, E., Fraser, P.D., Cutting, S.M., 2009. Carotenoids found in
ToyocerinÒ for Use as Feed Additive. European Commission, Health and
Bacillus. J. Appl. Microbiol.
Consumer Protection Directorate-General. (SCAN) Scientific Committee on
La Ragione, R.M., Casula, G., Cutting, S.M., Woodward, M., 2001. Bacillus subtilis
Animal Nutrition.
spores competitively exclude Escherichia coli 070:K80 in poultry. Vet. Microbiol.
Senesi, S., Celandroni, F., Tavanti, A., Ghelardi, E., 2001. Molecular characterization
79, 133e142.
and identification of Bacillus clausii strains marketed for use in oral bacter-
La Ragione, R.M., Woodward, M.J., 2003. Competitive exclusion by Bacillus subtilis
iotherapy. Appl. Environ. Microbiol. 67, 834e839.
spores of Salmonella enterica serotype Enteritidis and Clostridium perfringens in
Sorokulova, I.B., 1997. A comparative study of the biological properties of Biosporin
young chickens. Vet. Microbiol. 94, 245e256.
and other commercial Bacillus-based preparations. Mikrobiol. Zh. 59, 43e49.
Li, Z., Kawamura, Y., Shida, O., Yamagata, S., Deguchi, T., Ezaki, T., 2002. Bacillus
Sorokulova, I.B., Beliavskaia, V.A., Masycheva, V.A., Smirnov, V.V., 1997. Recombinant
okuhidensis sp. nov., isolated from the Okuhida spa area of Japan. Int. J. Syst.
probiotics: problems and prospects of their use for medicine and veterinary
Evol. Microbiol. 52, 1205e1209.
practice. Vestn. Ross. Akad. Med. Nauk, 46e49.
Logan, N.A., 2004. Safety of aerobic endospore-forming bacteria. In: Ricca, E.,
Sorokulova, I.B., Pinchuk, I.V., Denayrolles, M., Osipova, I.G., Huang, J.M.,
Henriques, A.O., Cutting, S.M. (Eds.), Bacterial Spore Formers: Probiotics and
Cutting, S.M., Urdaci, M.C., 2008. The safety of two Bacillus probiotic strains for
Emerging Applications. Horizon Bioscience, pp. 93e106. Norfolk, UK.
human use. Dig. Dis. Sci. 53, 954e963.
Mandel, D.R., Eichas, K., Holmes, J., 2010. Bacillus coagulans: a viable adjunct therapy
Spinosa, M.R., Braccini, T., Ricca, E., De Felice, M., Morelli, L., Pozzi, G., Oggioni, M.R.,
for relieving symptoms of rheumatoid arthritis according to a randomized,
2000. On the fate of ingested Bacillus spores. Res. Microbiol. 151, 361e368.
controlled trial. BMC Complement. Altern. Med. 10, 1.
Stenfors Arnesen, L.P., Fagerlund, A., Granum, P.E., 2008. From soil to gut: Bacillus
Marseglia, G.L., Tosca, M., Cirillo, I., Licari, A., Leone, M., Marseglia, A.,
cereus and its food poisoning toxins. FEMS Microbiol. Rev. 32, 579e606.
Castellazzi, A.M., Ciprandi, G., 2007. Efficacy of Bacillus clausii spores in the
Sumi, H., Hamada, H., Tsushima, H., Mihara, H., Muraki, H., 1987. A novel fibrinolytic
prevention of recurrent respiratory infections in children: a pilot study. Ther.
enzyme (nattokinase) in the vegetable cheese Natto; a typical and popular
Clin. Risk Manag. 3, 13e17.
soybean food in the Japanese diet. Experientia 43, 1110e1111.
Mazza, G., 1983. Genetic studies on the transfer of antibiotic resistance genes in
Sumi, H., Yatagai, C., Wada, H., Yoshida, E., Maruyama, M., 1995. Effect of Bacillus
Bacillus subtilis strains. Chemioterapia 2, 64e72.
natto-fermented product (BIOZYME) on blood alcohol, aldehyde concentrations
Mazza, P., 1994. The use of Bacillus subtilis as an antidiarrhoeal microorganism. Boll.
after whisky drinking in human volunteers, and acute toxicity of acetaldehyde
Chim. Farm. 133, 3e18.
in mice. Arukoru Kenkyuto Yakubutsu Ison 30, 69e79.
Mazza, P., Zani, F., Martelli, P., 1992. Studies on the antibiotic resistance of Bacillus
Suresh, K., Prabagaran, S.R., Sengupta, S., Shivaji, S., 2004. Bacillus indicus sp. nov., an
subtilis strains used in oral bacteriotherapy. Boll. Chim. Farm. 131, 401e408.
arsenic-resistant bacterium isolated from an aquifer in West Bengal, India. Int.
Mitchell, C., Iyer, S., Skomurski, J.F., Vary, J.C., 1986. Red pigment in Bacillus mega-
J. Syst. Evol. Microbiol. 54, 1369e1375.
terium spores. Appl. Environ. Microbiol. 52, 64e67.
Tam, N.K., Uyen, N.Q., Hong, H.A., Duc le, H., Hoa, T.T., Serra, C.R., Henriques, A.O.,
Moir, A., 2006. How do spores germinate? J. Appl. Microbiol. 101, 526e530.
Cutting, S.M., 2006. The intestinal life cycle of Bacillus subtilis and close rela-
Nakamura, L.K., 1989. Taxonomic relationship of black-pigmented Bacillus subtilis strains
tives. J. Bacteriol. 188, 2692e2700.
and a proposal for Bacillus atrophaeus sp. nov. Int. J. Syst. Bacteriol. 39, 295e300.
Tuohy, K.M., Pinart-Gilberga, M., Jones, M., Hoyles, L., McCartney, A.L., Gibson, G.R.,
Nicholson, W.J., Munakata, N., Horneck, G., Melosh, H.J., Setlow, P., 2000. Resistance
2007. Survivability of a probiotic Lactobacillus casei in the gastrointestinal tract
of Bacillus endospores to extreme terrestrial and extraterrestrial environments.
of healthy human volunteers and its impact on the faecal microflora. J. Appl.
Microbiol. Mol. Biol. Rev. 64, 548e572.
Microbiol. 102, 1026e1032.
Nielsen, P., Fritze, D., Priest, F.G., 1995. Phenetic diversity of alkaliphilic Bacillus
Yoon, J.H., Kang, S.S., Lee, K.C., Kho, Y.H., Choi, S.H., Kang, K.H., Park, Y.H., 2001.
strains: proposal for nine new species. Microbiology 141, 1745e1761.
Bacillus jeotgali sp. nov., isolated from jeotgal, Korean traditional fermented
Nista, E.C., Candelli, M., Cremonini, F., Cazzato, I.A., Zocco, M.A., Franceschi, F.,
seafood. Int. J. Syst. Evol. Microbiol. 51, 1087e1092.
Cammarota, G., Gasbarrini, G., Gasbarrini, A., 2004. Bacillus clausii therapy to
Yoon, J.H., Lee, C.H., Oh, T.K., 2005. Bacillus cibi sp. nov., isolated from jeotgal,
reduce side-effects of anti-Helicobacter pylori treatment: randomized, double-
a traditional Korean fermented seafood. Int. J. Syst. Evol. Microbiol. 55,
blind, placebo controlled trial. Aliment. Pharmacol. Ther. 20, 1181e1188.
Source: http://biospring.com.vn/wp-content/uploads/2015/06/Review-Bacillus-probiotics-Cutting.pdf
Vol. 3 - No 1 - June 2011 CRCHUM — Research Centre - University of Montreal Hospital Centre CRCHUM: at the forefront of clinical research "Being able to ask questions and improve treatments lies at the heart of the mission of a tertiary and quaternary care hospital like the CHUM," notes Jacques Turgeon, Director of the CHUM's research centre (CRCHUM). In other words, research is an essential ingredient of health care.
LA RESPONSABILIDAD SOCIAL DE LAS EMPRESAS EN EL COMBATE A LA CONTRALORÍA GENERAL DE LA UNIÓN – CGU INSTITUTO ETHOS DE EMPRESAS Y RESPONSABILIDAD SOCIAL GRUPO DE TRABAJO DEL PACTO EMPRESARIAL POR LA INTEGRIDAD CONTRA LA CORRUPCIÓN CONTRALORÍA GENERAL DE LA UNIÓN Jorge Hage Sobrinho Ministro-Jefe de la Contraloría General de la Unión









