
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Marine ecology progress series 353:275
Vol. 353: 275–288, 2008
MARINE ECOLOGY PROGRESS SERIES
Published January 17
doi: 10.3354/meps07164
Mar Ecol Prog Ser
Summer spatial distribution of cetaceans in
the Strait of Gibraltar in relation to
the oceanographic context
Renaud de Stephanis1, 2,*, Thomas Cornulier3, 4, Philippe Verborgh1, 2,
Juanma Salazar Sierra1, 2, Neus Pérez Gimeno2, 5, Christophe Guinet3
1CIRCE, Conservation Information and Research on Cetaceans, C/Cabeza de Manzaneda 3, Algeciras-Pelayo, 11390 Cadiz, Spain
2Sociedad Española de Cetáceos C/Nalon 16, La Berzosa, Madrid, Spain
3Centre d'Études Biologiques de Chizé, CNRS UPR 1934, 79 360 Villiers en Bois, France
4School of Biological Sciences, Zoology Building, Tillydrone Avenue, University of Aberdeen, Aberdeen AB24 2TZ, UK
5Laboratorio de Ingeniería Acústica de la Universidad de Cádiz (LAV), CASEM, Río de San Pedro S/N, Puerto Real, Cádiz, Spain
ABSTRACT: The Strait of Gibraltar, the only passage between the Mediterranean Sea and theAtlantic Ocean, and characterised by a surface inflow of Atlantic waters and a deep outflow ofMediterranean waters, is inhabited by a large number of cetacean species. The present study focuseson the occurrence and the spatial distribution of cetacean species within the strait in relation tooceanographic features. Shipboard visual surveys were conducted during the summers of 2001 to2004, covering 4926 km. A total of 616 sightings of 7 cetacean species were made. The spatial distri-butions of 6 species (short-beaked common dolphins
Delphinus delphis, striped dolphins
Stenellacoeruleoalba, bottlenose dolphins
Tursiops truncatus, long-finned pilot whales
Globicephala melas,sperm whales
Physeter macrocephalus and killer whales
Orcinus orca) were examined with respectto depth and slope. The analyses indicate that these species could be ordered into 3 groups. A firstgroup, with a northward tendency, is composed of common and striped dolphins. Due to its at-sealocation and feeding habits, this group is likely to feed on mesopelagic fishes or squids associatedwith the surface Atlantic waters. The second group, constituted by bottlenose dolphins, long-finnedpilot whales and sperm whales, is mainly found over the deep waters of the central part of the strait.
While the foraging ecology of bottlenose dolphins is still unclear, both sperm whales and pilot whalesare most likely to feed on squids occurring in deep Mediterranean waters. The third group, formedby killer whales
Orcinus orca, was associated with blue fin tuna
Thunnus thynnus fisheries in thesouthwestern part of the strait.
KEY WORDS: Cetacean · Strait of Gibraltar · Spatial distribution · Feeding ecology · Fisheries interaction
Resale or republication not permitted without written consent of the publisher
and not the Strait of Gibraltar itself (Aguilar 2006). Asurvey conducted from ferries navigating between
Cetacean distribution and abundance in the Strait of
Spain and Morocco from March to May 1999 found
Gibraltar is poorly described and limited to a few
that the main species encountered in the eastern part
sources. One of the latter relies on data from commer-
of the strait were the striped dolphin
Stenella
cial whaling activities, which took place between 1921
coeruleoalba, short-beaked common dolphin
Delphi-
and 1959 from shore-based whaling stations located in
nus delphis and, occasionally, bottlenose dolphin
Tur-
Getares (Spain) and Benzou (Morocco) and from fac-
siops truncatus, long-finned pilot whales
Globicephala
tory ships (Aguilar 2006). But these data describe the
melas and sperm whales
Physeter macrocephalus
situation in the Atlantic areas off the Strait of Gibraltar,
(Roussel 1999). Finally, studies from the Spanish drift-
Inter-Research 2008 · www.int-res.com
Mar Ecol Prog Ser 353: 275–288, 2008
net fishery operating until 1994 in the strait also
6°W in the central part of the strait (Kinder et al. 1988).
revealed the presence of common dolphin and striped
This Atlantic–Mediterranean water interface is consid-
dolphin (Silvani et al. 1999). In 2005, a study provided
ered to be a biogeographic boundary (Sanjuán et al.
evidence of the presence of these species in the
1994). Nevertheless, there is substantial transport of
Alborán Sea (Cañadas et al. 2005). However, the rela-
organisms across this ecotone. Most of the plankton
tive density and the spatial distribution of the cetacean
biomass is transported into the Mediterranean Sea by
species encountered within the Strait of Gibraltar
Atlantic waters. Reul et al. (2002) estimated that 5570 t
remain unknown.
C d–1, dominated by autotrophic nanoplankton (42%)
The Strait of Gibraltar is the narrow and shallow con-
and heterotrophic bacteria (37%), is transported
nection between the Mediterranean Sea and the
towards the Mediterranean Sea, while 1140 t C of het-
Atlantic Ocean (Fig. 1). The water circulation in the
erotrophic organisms (89%) is exported daily towards
strait is characterised by: (1) a surface inflow of
the Atlantic by the deep Mediterranean outflow.
Atlantic waters, which is driven by the excess of evap-
The highest biomass concentrations were observed
oration over precipitation in this basin, and (2) a deep
in the northern part of the strait, where enriched
outflow of dense Mediterranean water (Lacombe &
Atlantic shelf waters circulate (Van Geen & Boyle
Richez 1982). The strait is also characterised by mixing
1988). However, due to higher current velocities, most
processes through pulsed upwelling induced by the
of the biomass import took place in the central and
tides and constrained by the bathymetry of the area
southern parts of the strait, where we would expect to
(Echevarría et al. 2002).
find high concentrations of cetaceans, as ecological
The interface between the Atlantic surface waters
studies of apex predators at sea indicate that their dis-
and the deep Mediterranean waters generally takes
tribution and abundance can often be related to
place at a depth between 50 and 200 m, depending on
oceanographic features and marine productivity.
the geographic location and intensity of the tidal flows.
Bathymetry plays an important role on prey distribu-
The boundary between Atlantic waters and Mediter-
tion (Gil de Sola 1993). This role can be direct, as on
ranean waters becomes deeper from the Spanish coast
demersal prey, for which the distribution can often be
to the Moroccan coast (north to south) (Reul et al. 2002)
related to topographic features such as depth and
and from the Atlantic to the Mediterranean (east to
slope. For pelagic cetacean prey species, such as fishes
west), from approximately 100 m at 5° 20' W to 300 m at
or cephalopods, bathymetric features could act indi-
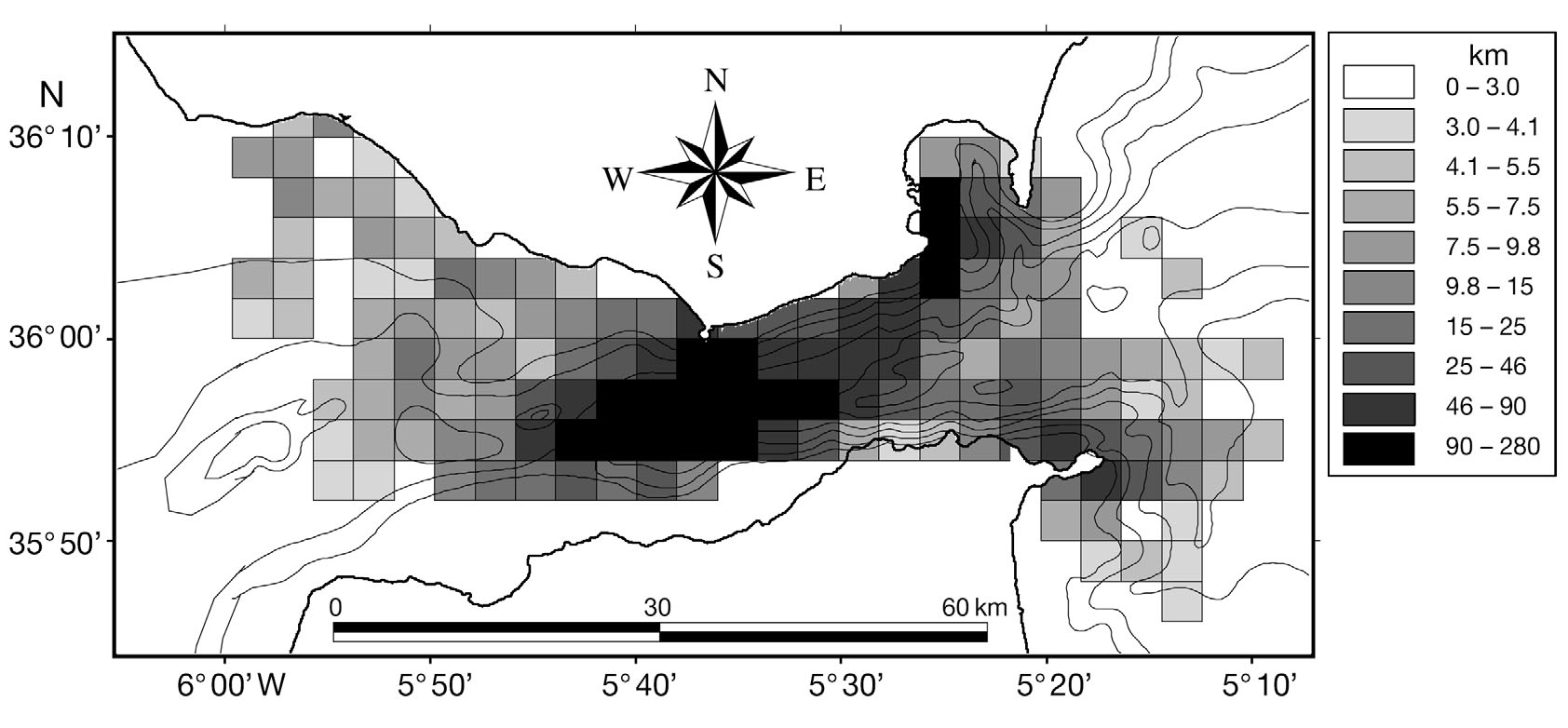
Fig. 1. Study area and bathymetry of the Strait of Gibraltar
de Stephanis et al.: Summer distribution of cetaceans in the Strait of Gibraltar
rectly through topographically induced vertical (up-
MATERIALS AND METHODS
welling) and horizontal (currents) circulation, both ofwhich can stimulate the primary and secondary pro-
Study area and surveys. The study area encom-
duction, but could also act directly on the spatial distri-
passes the Strait of Gibraltar and its contiguous waters,
bution of prey species through transport and/or aggre-
between 5 and 6°W, including Spanish and Moroccan
gating effects (Davis et al. 1998, Cañadas et al. 2005).
waters. The Strait of Gibraltar (Fig. 1) is nearly 60 km
Modelling the relationships between cetacean distri-
long. Its western border is located between Cape
bution and environmental factors is a complicated task
Trafalgar (Europe) and Cape Espartel (Africa), 44 km
for several reasons. For instance, relationships be-
apart. The strait then narrows to the east to reach a
tween cetacean abundance and habitat features may
minimum width of 14 km between Tarifa (Europe) and
be non-linear. To overcome this problem, many
Punta Cires (Africa). Its eastern border is located
cetacean distribution studies have used generalised
between Gibraltar and Punta Almina (Africa), 23 km
additive models (GAMs) that can fit non-parametric
apart. The bathymetry of the strait is characterised by
smoothing functions of the data (see e.g. Forney 2000,
a west to east canyon, with shallower waters (200 to
Redfern et al. 2006, Williams et al. 2006).
300 m) found on the Atlantic side and deeper waters
More importantly, cetacean surveys rarely follow
(800 to 1000 m) on the Mediterranean side (Fig. 1).
systematic designs in space and time, due to specific
Survey transects were conducted from the CIRCE
logistical constraints, especially when conducted from
(Conservation, Information and Research on Ceta-
opportunistic platforms (Williams et al. 2006). There-
ceans) research motorboat ‘Elsa' (11 m length), sam-
fore, sampling effort is typically heterogeneous and
pling the study area throughout the months of July,
needs to be carefully accounted for (Redfern et al.
August and September between 2001 and 2004. Tran-
2006). Additionally, encounter rates with cetaceans are
sects were conducted without any pre-defined track
often very low, typically resulting in sparse data that
for each of these surveys, but they were designed to
are difficult to analyse, both in terms of abundance and
cover the whole range of bathymetry in the strait
probability of sighting. The problem is even more
every month by crossing the isobaths perpendicularly.
acute with species living in (potentially large) groups
While carrying out the surveys it was attempted to
that tend to generate extremely skewed or over-
maximise the presence at sea, with restrictions due
dispersed distributions of counts for which standard
to the maritime traffic present in the area and the
(e.g. binomial, Poisson, or log-normal) statistical mod-
meteorological conditions. The sampling strategy was
els may not be suitable.
constant throughout the survey period. The area
Additionally, animal distribution data are autocor-
was surveyed at an average speed of 5.6 knots
related, especially for organisms like cetaceans, which
(9.8 km h–1). Searching effort stopped when a group
live in schools. Accounting for spatial autocorrelation is
of cetaceans was approached and started again when
vital when modelling the relations between animal
the sighting was ended, with a return to the course
distribution and the environment (Keitt et al. 2002).
Although this restraint is recognised in cetacean litera-
The observers were placed on an observation plat-
ture, a recent review of this field suggests that very
form, 4 m above the sea level. Two trained observers
few, if any, studies have attempted to include spatial
occupied the observation lookout post in 1 h shifts dur-
correlation in cetacean–habitat models (Redfern et
ing daylight, with visibility over 3 nautical mile
(5.6 km), assisted with 8 × 50 binoculars, covering 180°
In the present paper we propose to deal with these
ahead of the vessel. Sighting effort was measured as
issues within a single model-based approach. In accor-
the number of kilometres travelled with adequate
dance with, e.g., Williams et al. (2006), we use GAMs,
sighting conditions (i.e. with a sea state Douglas of < 4
while paying careful attention to controlling for error
and 2 observers at the lookout post. The Douglas sea
dispersion by using quasi-binomial methods (for pres-
state estimates the sea's roughness for navigation.)
ence/absence data). In addition, we show that spatial
The geographic position of the ship was recorded
autocorrelation can be included explicitly in order to
every minute on the ship's computer from a GPS
select more parsimonious cetacean–habitat models.
(global positioning system) navigation system logger
Our study focuses on the summer cetacean distribu-
using the IFAW (International Fund for Animal Wel-
tions in the Strait of Gibraltar with the aims: (1) to esti-
fare) Data Logging Software Logger 2000, Version 2.20
mate the relative abundance of cetacean species, (2) to
investigate how their distribution is related to the
?oid=25739). Data concerning the location and time of
bathymetric features of the Strait of Gibraltar and (3) to
a sighting and the species, number of individuals and
examine the inter-specific spatial association in the
behaviour were recorded along with ancillary environ-
Strait of Gibraltar in summer.
mental data (sea state, wind speed, visibility). These
Mar Ecol Prog Ser 353: 275–288, 2008
environmental data were also taken every 20 min, and
100, where Ind is the number of individuals of a given
at every course change of the boat.
species observed versus effort in the research area,
According to the Sociedad Española de Cetaceos
including only sightings when the animals were
(SEC 1999), a sighting was defined as a group of ani-
approached, and Eff is the distance (km) covered ver-
mals of the same species seen at the same time, show-
ing similar behaviour and for which the maximum dis-
Spatial distribution and bathymetry. The encounter
tance between 2 individuals was <1000 m. When a
rate for each species was calculated for each quadrat
group of animals was first seen, the location of the ship,
using the number of sightings per species per 100 km
and the distance and bearing of the cetaceans were
searched, pooled over the 4 yr of the study. Only sight-
recorded, to be able to localise the animals when
ings for which approach was established (i.e. the ani-
approaching them. The location of the animals was
mals' location was within a 100 m radius of the boat's
also recorded when they had been approached by the
GPS position) were used for these analyses because
vessel (i.e. <100 m away). Eighty-eight groups of
some species, like sperm whales, are visible several
cetaceans were sighted, but not all were approached
kilometres away from the survey track and conversely
(see Table 1). The observation effort did not take into
could not be located precisely.
account the kilometres sailed while tracking the
Two bathymetric features were assessed: the depth
and the slope. Mean depth and slope were calculated
The study area was divided into quadrats, with a cell
for each quadrat. Depth was obtained from the
resolution of 2 min latitude (3704 m) by 2 min longitude
ETOPO2v2 global elevation data set (2' × 2'); mean
(3006 m) (Fig. 2). This scale was used to be able to com-
depth (range from 0 to 850 m) was calculated for each
pare these results with results gathered in future
quadrat. The bathymetric gradient was defined as the
research programs carried out by members of the
maximum slope around each pixel from the local
Spanish Cetacean Society. The distance in kilometres
slopes in x and y. Only neighbours above, below, left
searched in each quadrat was calculated using a Geo-
and right of the pixel are accounted for in this ‘rook's
graphic Information System: Arc View 3.2 from ESRI
case procedure', and the largest value was kept. The
and its extension Animal Movement (Hooge & Eichen-
algorithm IDRISI Module Surface Analysis computes
laub 2000). Only cells with sighting effort of at least
the percent slope for each pixel using the ‘tangent'
3 km were used for the analysis (Fig. 2).
trigonometric function. Slope values were expressed in
Presence of cetaceans and relative abundance. Two
metres per kilometre and ranged from a minimum of 0
parameters were defined to quantify cetacean relative
to a maximum of 240 m km–1. Analyses of distribution
abundance. (1) The encounter rate (ER) is the number
of cetaceans according to depth and slope were made
of sightings of a given species per 100 km and is
based on a continuous depth and slope data set (i.e. the
defined as: ER = (Sigh/Eff) × 100, where Sigh is the
mean values of quadrats), but depth and slope were
number of sightings made of a species versus effort in
ranked into subjective depth and slope categories to
the research area, including all sightings whether the
provide a pertinent ecological context for the interpre-
animals were approached or not, and Eff is the dis-
tation of the results (Figs. 3 & 4). The depth was cate-
tance (km) covered versus effort. (2) The abundance
gorised according to the diving capabilities of the
rate (AR) (ind. km–1) was defined as: AI = (Ind/Eff) ×
cetaceans observed in this study: 0 to 200 m (reachable
Fig. 2. Distribution of the observa-tion effort in kilometres spent by
quadrat over the study area
de Stephanis et al.: Summer distribution of cetaceans in the Strait of Gibraltar
Delphinus delphis
Orcinus orca
Fig. 3. Distribution of encounter rate (bars) and effort in kilometres (r) in relation to depth
Delphinus delphis
Orcinus orca
Fig. 4. Distribution of encounter rate (bars) and effort (r) in relation to slope
by all species), 200 to 600 m (typical depth of long-
ture in the model. The 2 covariates used were the con-
finned pilot whales) and > 600 m (reachable mostly by
tinuous measures of depth and slope, which were sig-
sperm whales). The slope was categorised following
nificantly correlated in this data set (n = 141, r = 0.23,
the categories of Piatt et al. (2006) as gentle (0 to 80 m
p = 0.005). However, this correlation was sufficiently
km–1), moderate (80 to 160 m km–1) and steep (160
low to justify including both covariates in the models.
to 240 m km–1).
The degree of smoothing for the non-linear terms was
The relationships between species probability of
selected as part of the model fitting procedure using
presence, encounter rates and environmental variables
the default generalised cross-validation method (GCV)
were investigated using generalized additive mixed
implemented in the ‘gamm' function (Wood 2006). As
effects models (GAMMs), using the ‘gamm' function of
for correlation structure, we used both the exponential
the ‘mgcv' library (Wood 2006) in R 2.4.1 (R Develop-
model [the correlation between 2 points the distance r
ment Core Team 2006). Smooth terms were used in
apart is modelled as: (1 – n) × exp(–r/d), where d is the
order to allow for non-linear responses to the covari-
range and n is the nugget effect, i.e. the correlation at
ates, and spatial autocorrelation was accounted for by
distance 0 and r is the Euclidian distance], and the
including a spherical or exponential correlation struc-
spherical model {correlation is modelled as: (1 – n) × [1
Mar Ecol Prog Ser 353: 275–288, 2008
– 1.5(r /d) + 0.5(r /d)3], and takes the value 0 if r × d}, as
using 2 indices of the frequency of co-occurrence: the
implemented by the ‘corExp' and ‘corSpher' functions
half-weight association index and the simple ratio
of the ‘nlme' library for mixed-effect models in R (Pin-
association index (Ginsberg & Young 1992). However,
heiro & Bates 2000). The range is the distance until 2
as the inferences drawn were the same for the 2
observations are correlated. Beyond this distance, they
indices, only values of the half-weight association
are considered independent. The nugget is the corre-
index will be presented. Species present in the same
lation at a distance near zero. Variograms were esti-
quadrat were considered associated for this quadrat.
mated using defaults of the function, i.e. using 50 dis-
To illustrate the association patterns of the species,
tance lags covering the whole range of distances in the
average-linkage cluster analyses (Manly 1994) were
study area. The spherical and exponential models are
constructed (see Fig. 12). We used permutation tests
among the most versatile and commonly used correlo-
(Bejder et al. 1998) to test whether the association pat-
gram models and proved satisfactory in all the situa-
terns of the observed species were different from what
tions we encountered; therefore, no other model was
might be expected at random. An observed standard
tried. The choice between the 2 types of correlation
deviation of the pairwise association indices that is sig-
structures was based on minimising the deviance of
nificantly larger than those from permuted data sets is
the models and by visually checking the variogram.
taken as evidence that species share or avoid the
When the exponential and spherical models gave
quadrates with other species (Bejder et al. 1998). All
indistinguishable results, the spherical was chosen
quadrats were included; 20 000 permutations were
because of its range parameter (i.e. the distance
generated for each test; and to ensure that p-values
beyond which 2 observations are independent) has a
were stable, 6 runs of the permutation test were gener-
more intuitive interpretation. We did not attempt to
ated using the simple ratio and half-weight association
include any anisotropy in the spatial correlation struc-
indices in 3 runs each.
ture because: (1) anisotropic correlograms are not cur-
Boat distribution. The distribution of blue fin tuna
rently implemented in the ‘nlme' library (Pinheiro &
Thunnus thynnus fishing boats was assessed to test if it
Bates 2000) used for the spatial component of the
was related to the distribution of cetacean species.
GAMMs and (2) it is reasonable to assume that the
Every 20 min, a sampling station was established
only likely source of anisotropy in this data set would
during the surveys, and tuna boats fishing within
be related to the east –west orientation of the Strait of
1 mile were counted. If there was a doubt regarding
Gibraltar's topography, which is already accounted for
the distance, a Radar JRC 1000 was used to estimate
by our covariates (depth and slope).
the distance of the boats. The type of fishing boat was
For all species, a large number of grid cells had ER
confirmed using binoculars. The mean number of tuna
equal to zero. Quasi-binomial GAMM was used after
boats was then calculated in each quadrat where
converting into presence/absence data. Quasi-bino-
at least 5 sampling stations had been conducted
mial models are logistic regressions (binomial error)
(results see Fig. 11).
allowing for under- or over-dispersion. Because ERwas more variable in less intensively surveyed cells,observation effort per cell (in km) was included as a
covariate, assuming that the probability of sighting aspecies in a given cell is proportional to the observa-
Search effort in the research area
tion effort. Model selection was based on a forwardselection approach. Covariates were retained in the
A total of 6332 km of transect were covered in the
model when the associated coefficient was signifi-
research area during the months of July, August and
cantly different from zero.
September 2001 to 2004. Of those, 4926 km were sur-
For the species with the largest number of sightings
veyed during the effort transects. They encompassed
(common dolphin and striped dolphin), we repeated
150 quadrats, which represent 1670 km2 (Fig. 2).
the models on a yearly basis in order to check if therelation to environmental covariates differed from oneyear to another. For the other species, the numbers of
Cetacean presence and relative abundance
quadrats with recorded presence were too few peryear to expect reliable results: bottlenose dolphin
A total of 606 sightings of 7 species were recorded (in
(maximum number of quadrats in which presence was
24 of which the species could not be identified) within
recorded in one given year = 8), pilot whale (12), killer
the research area on effort, and were thus used for the
whale (4) and sperm whale (11).
calculation of the general ER. In 518 sightings (in 4 of
Overlap in distribution. The strength of the spatial
which the species could not be identified), the ceta-
relationships between pairs of species was represented
ceans were approached, and these sightings were
de Stephanis et al.: Summer distribution of cetaceans in the Strait of Gibraltar
used to calculate the ER and AR in each quadrat
linear effect of observation effort and slope on the
(Table 1). Mean numbers of individuals in the groups
dolphin's probability of presence in a cell, and no
for each species are given in Table 1. Fig. 2 shows the
significant effect of the depth (Table 2). We repeated
distribution of the encounter rates with respect to the
the quasi-binomial models on a yearly basis, assuming
depth. In terms of sightings, the most commonly
a linear effect of each covariate in order to obtain
observed species were the sperm whale Physeter
parametric coefficients. Depth did not appear sig-
macrocephalus and the long-finned pilot whales Glo-
nificant in any year, and the slope had a significant
bicephala melas. The less commonly observed species
positive effect in 2001 (0.011 ± 0.004, p = 0.01,
were the fin whale Balaenoptera physalus, with only 3
Napproached = 32, Nsurveyed = 119), 2002 (0.030 ± 0.011,
sightings, and the killer whale Orcinus orca (Table 1).
p = 0.005, Napproached = 19, Nsurveyed = 80), but not in 2003
When the mean number of individuals present within a
(0.012 ± 0.012, p = 0.32, Napproached = 6, Nsurveyed = 81) or
group is taken into account, the most abundant species
2004 (–0.001 ± 0.014, p = 0.93, Napproached = 4, Nsurveyed =
were the striped dolphin Stenella coeruleoalba and the
47), which is most likely a result of insufficient sta-
common dolphin Delphinus delphis, while the less
tistical power.
commonly seen species were the fin whale and the
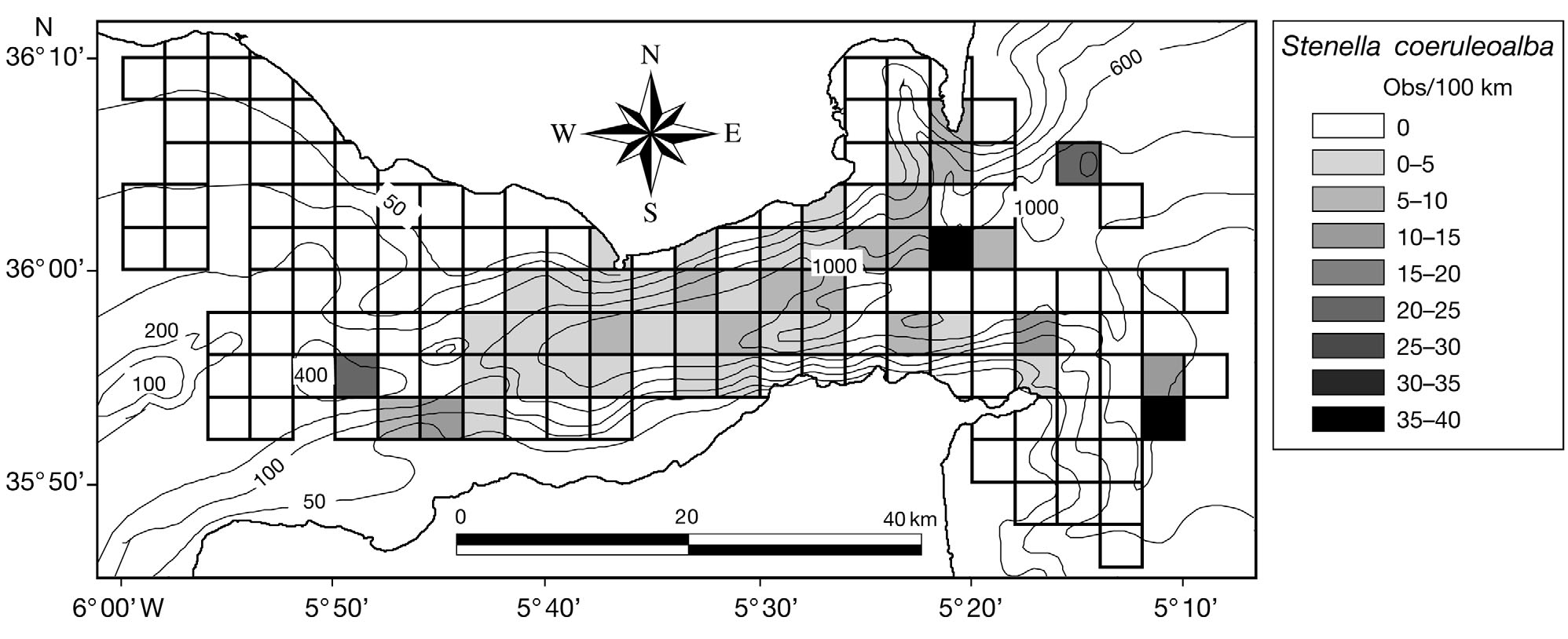
Striped dolphins Stenella coeruleoalba were ob-
sperm whale (Table 1).
served in 32.7% of the quadrats sampled (Fig. 6). Thespecies presence was positively and linearly related tothe observation effort, the depth and the slope of the
Distribution in relation to bathymetric features
sea bottom (suggesting that striped dolphins occur inthe deepest and steepest parts of the strait) (Table 2).
Due to their low ER, fin whales were excluded from
The quasi-binomial models were fitted again on a
these analyses. The distribution of the ER, with respect
yearly basis, assuming, in turn, a linear effect of depth
to the effort in each interval of depth and slope for the
and slope. Depth had a significant positive effect on
6 most commonly seen species of cetaceans is shown in
the probability of presence in 2001 (0.004 ± 0.001, p =
Figs. 3 & 4. The spatial distribution of their ER is
0.0003, Napproached = 32, Nsurveyed = 119) and 2004 (0.006
presented in Figs. 5 to 10. The analyses of the spatial
± 0.003, p = 0.039, Napproached = 9, Nsurveyed = 47), but
distribution of cetacean species within the strait are
not in 2002 (–0.001 ± 0.002, p = 0.60, Napproached = 24,
summarised in Table 2.
Nsurveyed = 80) or 2003 (–0.0003 ± 0.002, p = 0.88,
Common dolphins Delphinus delphis were observed
Napproached = 8, Nsurveyed = 81). The slope had a signifi-
in 31.3% of the quadrats sampled and were more
cant positive effect in 2001 (0.015 ± 0.004, p = 0.0007),
likely to be encountered in the northern part of the
but not in 2002 (0.011 ± 0.007, p = 0.105), 2003 (–0.001
Strait of Gibraltar (Fig. 5). There was a clear positive
± 0.009, p = 0.89), or 2004 (0.018 ± 0.011, p = 0.10).
Table 1. Number of sightings, mean group size, standard deviation (SD), encounter rate (ER) and abundance index (AI) calcu-lated in relation to the observation effort (i.e. 4926 km) over the study area in summer. Asterisks indicate species that were in-volved in multi-species sightings (number of multi-species sightings are given in brackets). Common and striped dolphins wereseen together on 18 occasions. Pilot whales were seen together with sperm whales on 5 occasions and with bottlenose dolphins on
groups approached
Delphinus delphis
Bottlenose dolphin*
Long-finned pilot whale*
Orcinus orca
Mar Ecol Prog Ser 353: 275–288, 2008
Table 2. Generalised additive mixed models of the presence/absence data of the 6 odontocete species commonly encounteredin the Strait of Gibraltar in relation to the observation effort and bathymetry. The ‘shape/edf' column indicates the direction(positive or negative) and the equivalent degrees of freedom (edf) of the relationship. Edf of 1 indicates linear relationships,
whereas values above indicate non-linear effects. Spatial autocorrelation ranges are given in kilometres
structure (range)
Delphinus delphis
Exponential (1.3)
Bottlenose dolphin
Long-finned pilot whale
Orcinus orca
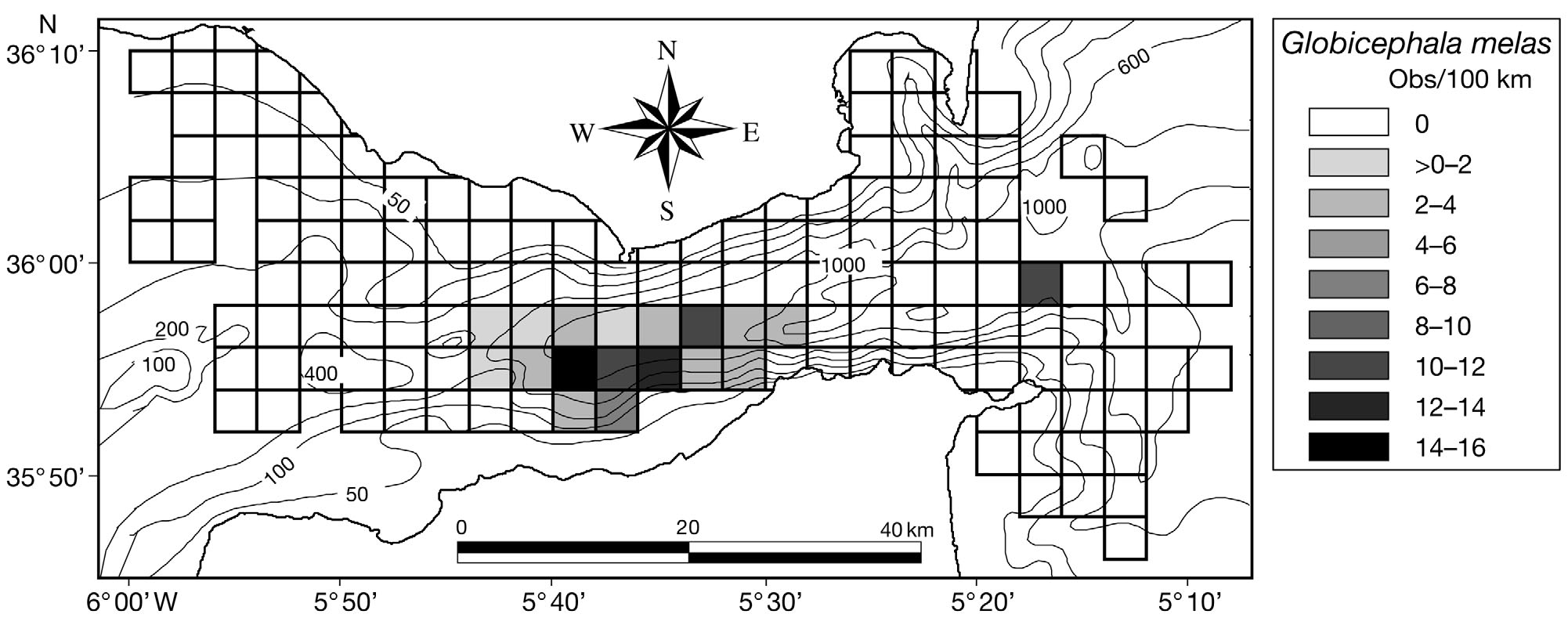
Bottlenose dolphins Tursiops truncatus were en-
Pilot whales Globicephala melas and sperm whales
countered in 10.0% of the quadrats sampled (Fig. 7).
Physeter macrocephalus were encountered in 12.0
Their presence was related to observation effort, and
and 5.3%, respectively, of the quadrats sampled. The
positively associated to steeper sea bottoms (linear
distribution of both species was confined to the
relationship, see Table 2). Bottlenose dolphins also
southern parts of the study area (Figs. 8 & 9). Pilot
tended to be found in the deeper areas, although
whale data were too sparse to fit complex models
the relationship was not or marginally significant
accounting for the spatial autocorrelation of the data
(p = 0.058).
(spatial models had very poor convergence and
Fig. 5. Delphinus delphis. Distribution of encounter rates of common dolphins over the study area during this study
de Stephanis et al.: Summer distribution of cetaceans in the Strait of Gibraltar
Fig. 6. Stenella coeruleoalba. Distribution of encounter rates of striped dolphins over the study area during this study
Fig. 7. Tursiops truncatus. Distribution of encounter rates of bottlenose dolphins over the study area during this study
Fig. 8. Globicephala melas. Distribution of encounter rates of long-finned pilot whales over the study area during this study
Mar Ecol Prog Ser 353: 275–288, 2008
Fig. 9. Physeter macrocephalus. Distribution of encounter rates of sperm whales over the study area during this study
residual diagnostics). Nevertheless, assuming spa-
Spatial association–segregation between cetacean
tially independent data, we found a non-linear effect
of depth on pilot whale presence (Table 2). Pilotwhales were less likely to be encountered in areas
The standard deviations of the observed pairwise as-
shallower than 300 m depth, and showed similar
sociation indices were significantly higher than those
preference for all depths between 300 and 800 m.
from permuted data (simple ratio index: p < 0.001; half-
Sperm whale presence was positively and linearly
weight index: p < 0.001), so the null hypothesis of a
correlated to depth (Table 2).
random association in the space of species could be re-
Killer whales Orcinus orca occurred in 7.2% of the
jected. The cluster diagram (Fig. 12) and visual obser-
quadrats sampled. They were encountered in the shal-
vation of the distribution maps (Figs. 5 to 10) show that
lower waters of the south-western part of the research
3 main groups of cetaceans can be distinguished. (1)
area (Fig. 10). However, there was no statistically sig-
Common dolphins and striped dolphins tended to be
nificant relationship between killer whale presence
spatially associated with each other. The overlap be-
and depth or slope, probably owing to their low
tween common dolphins and, to a lesser extent, be-
encounter rate. However, visual comparison of killer
tween striped dolphins and the other cetacean species
whale sightings (Fig. 10) and tuna fishery (Fig. 11)
was limited. (2) Bottlenose dolphins, long-finned pilot
shows an unambiguously strong match.
whales and sperm whales shared a large part of their
Fig. 10. Orcinus orca. Distribution of encounter rates of killer whales over the study area during this study
de Stephanis et al.: Summer distribution of cetaceans in the Strait of Gibraltar
Fig. 11. Distribution of sightings of tuna boats in the Strait of Gibraltar established from 2008 sampling stations (see ‘Materials
this study. Most of these species areabundant, reaching an ER of between291.14 and 3.23 ind. per 100 km ofeffort for striped dolphins Stenellacoeruleoalba and killer whales Orcinusorca, respectively. Seven of the 9 spe-cies of cetaceans regularly seen in theMediterranean Sea (Reeves & Notar-bartolo di Sciara 2006) have beendescribed in the Strait of Gibraltar. Onemay suggest that the relatively highdiversity of cetacean species observedat the entrance of the MediterraneanSea could be related to a large number
Fig. 12. Average-linkage cluster analyses of association (half-weight associ-
of cetaceans transiting in and out of the
ation index) between the different cetacean species in the Strait of Gibraltar
Mediterranean Sea. However, long-
(cophenetic correlation coefficient = 0.90)
term, photo-identification work indi-cates that individual sperm whales Phy-
habitat within the strait. (3) The smallest overlap with
seter macrocephalus, pilot whales Globicephala melas,
any other cetacean species was observed for killer
bottlenose dolphins Tursiops truncatus, killer whales
whales. All species but fin and killer whales were in-
O. orca and common dolphins Delphinus delphis at
volved in multi-species sightings (Table 1). A total of
least are resident in the strait in summer (de Stephanis
15% of the sightings of the striped dolphins was seen
unpubl. data), while the status of striped dolphins S.
with common dolphins, and 14% of the sightings of
coeruleoalba needs to be studied. Reul et al. (2002)
common dolphins was seen with striped dolphins; 11
estimated that 5570 t C d–1 were transported towards
and 4% of the sightings of long-finned pilot whales
the Mediterranean Sea, while 1140 t C were exported
were seen with bottlenose dolphins and sperm whales,
daily towards the Atlantic by the deep Mediterranean
respectively, representing 22 and 4% of the sightings of
outflow. The strait is characterised by mixing processes
bottlenose dolphins and sperm whales, respectively.
through a pulsed upwelling induced by the tides andconstrained by the strait's bathymetry (Echevarría etal. 2002). These phenomena are reflected in the boil-
ing-water phenomena, occurring close to the KamaraRidge, and that produce vertical advection and mixing
The Strait of Gibraltar is characterised by 7 species of
processes (Bruno et al. 2002). The area is then a highly
cetaceans, which were observed within the scope of
productive area, and the most likely hypothesis to
Mar Ecol Prog Ser 353: 275–288, 2008
explain the high density of cetaceans is prey availabil-
cephalopods feeding on zooplankton are also sup-
ity being enhanced within the Strait of Gibraltar.
ported. Diving studies of common dolphins and 2
In this study, data were pooled over 4 yr in order to
Stenella species have shown that most dives are shal-
produce statistically robust habitat –cetacean models.
lower than 150 m (Davis et al. 1996). This suggests that
As a consequence, we only examined the static deter-
both common dolphins and striped dolphins are likely
minants of cetacean distribution, i.e. bathymetric
to restrict most, if not all, of their feeding activity to the
characteristics, which do not change from one year to
layer of inflowing Atlantic waters. Furthermore the
another. In this context, pooling the distribution data
Atlantic–Mediterranean water interface may act as a
over several years should have reduced the noise due
boundary for their fish and squid prey and, as this
to unknown environmental fluctuations and provided
interface is shallower in the northern part of the strait,
more robust relationships with bathymetry. In fact,
it may enhance their foraging success in this area.
where the analyses could be run on individual years,
Within the second group of cetaceans, pilot whales
unstable results emerged, which could be attributed
and sperm whales are known to be mainly squid eaters
to insufficient data available. With temporally ‘aver-
(Desportes & Mouritsen 1993, Gannon et al. 1997,
aged' data, the spatial correlations we detect are
Santos et al. 1999) and deep divers (Watkins et al.
unlikely caused by short-term spatial interactions such
1993, Baird et al. 2002). Thus, the spatial summer
as conspecific attraction, but may rather reflect
distribution of these 2 species within the strait is likely
the spatial structures generated by un-modelled envi-
to be indicative of the distribution of larger squid
ronmental factors.
species encountered in the strait. However, it is
Three distinct groups of species were identified
unclear why bottlenose dolphins, which are mainly
according to their distribution within the strait. Com-
piscivorous (Barros & Odell 1990, Gannier 1995),
mon and striped dolphins had a larger, broader distrib-
tend to be spatially associated with sperm whales and
ution and a preference for the northern part of the
pilot whales.
study area, being mainly concentrated in deep waters
Several diving studies on sperm whales have shown
and along the north edge of the northern channel of
that they dive regularly to depths >1000 m (Watkins et
the Strait of Gibraltar (Figs. 5 & 6). Bottlenose dolphins,
al. 1993), and, thus, they are able to reach the sea floor
pilot whales and sperm whales shared a large part of
throughout the study area. Interestingly, the ER of
their foraging habitat, and were mainly found over
sperm whale is higher just west of the Central Ridge,
deep waters, over the main channel of the Strait of
which separates the northern and main channels. The
Gibraltar (Figs. 7 to 9). The third group included a sin-
other area of higher ER of sperm whales is found east
gle species: the killer whales, which were more com-
of the Kamara Ridge. This suggests that sperm whales
monly seen in the western part of the study area. The
may forage in the deepest areas of the central strait.
differences in the spatial distribution between these 3
Pilot whales are also known to be relatively deep
groups are likely related to their respective foraging
divers, reaching depths between 200 and 600 m, with a
ecology and, in particular, the fact that they are forag-
maximum recorded depth of 828 m in the Ligurian Sea
ing in different water masses.
(Baird et al. 2002). Pilot whales feed mainly on neritic
There is little information on the diet of these species
and oceanic squids and to a lesser extent on fishes that
in the study area. According to other studies, both
are most common at depths between 100 and 1000 m
common and striped dolphins appear to be opportunis-
(Desportes & Mouritsen 1993, Gannon et al. 1997).
tic feeders (Young & Cockcroft 1994, Gannier 1995),
However, these prey exhibit vertical movements that
targeting mainly small neritic fishes and cephalopods.
may allow the pilot whales to catch them at night,
The small meso-pelagic cephalopods and myctophids
when they are closer to the surface, while they may be
tend to be higher diet components for the striped dol-
inaccessible during the day (Desportes & Mouritsen
phins (Blanco et al. 1995, Santos et al. 1996) than for
1993, Baird et al. 2002). The spatial distribution in sum-
the common dolphins (Young & Cockcroft 1994, Santos
mer of long-finned pilot whales and sperm whales
et al. 1996). These differences may contribute to the
indicates that both species are likely to exploit
occurrence of striped dolphins over deeper waters
squids — and possibly fishes — associated with the
compared to common dolphins. Interestingly, both
deep outflow of Mediterranean waters.
species are more abundant in the northern part of the
Bottlenose dolphins present a similar distribution to
strait, where enriched Atlantic Spanish shelf waters
sperm whales. However, their dives are usually
are circulating (Van Geen & Boyle 1988) and where
between 10 and 50 m (Hastie et al. 2006). Therefore,
plankton biomass concentration is higher and current
they are likely to restrict their feeding to the surface
velocities are lower than in the central and southern
Atlantic water inflow. Bottlenose dolphins are oppor-
parts of the strait (Reul et al. 2002). These ecological
tunistic feeders and are considered to have a diet
conditions may be more favourable for fishes, but small
mainly based on demersal prey (Barros & Odell 1990,
de Stephanis et al.: Summer distribution of cetaceans in the Strait of Gibraltar
Gannier 1995), which seems unlikely in our study
is too small to observe non-linear relationships such as
because of their distribution over very deep waters,
thresholds or mid-range optima. However, there is lit-
where pelagic feeding appears to be the only possible
tle doubt that careful treatment of error dispersion and
foraging strategy. As we lack information on their diet
spatial autocorrelation were the main factors responsi-
over the study area, we are unable to determine if they
ble for the parsimony of our models. Both overdisper-
are feeding mainly on the pelagic prey associated with
sion and spatial autocorrelation tend to inflate Type I
the deep Mediterranean outflow migrating to the sur-
error, that is, make unimportant covariates appear
face at night, or if they feed on the pelagic prey associ-
unduly significant (Keitt et al. 2002, Redfern et al.
ated with the Atlantic water inflow or both.
2006). In the case of GAMs, this also tends to exagger-
Killer whale distribution was the most distinctive
ate the non-linearity of the relationships with covari-
when compared to other cetacean species occurring
ates. In fact, experiences during the development of
within the strait. Killer whales were seen interacting
our models confirmed that in this data set, overly com-
with the drop line fishery for bluefin tuna migrating
plex additive terms were very easy to obtain when fail-
out of the Mediterranean Sea after the completion of
ing to account for one or both of these features. As a
breeding. The summer killer whale distribution is spa-
consequence, we concur with Redfern et al. (2006) in
tially closely associated with the location of that fish-
that the error structure and non-independence of
ery, which is concentrated east of the Kamara Ridge,
the data (e.g. spatial autocorrelation) should be sys-
for the Moroccan fleet, and in the pass between Monte
tematically controlled in habitat –cetacean studies.
Seco and Monte Tartesos, for the Spanish fleet.
The frequency of fin whale sightings in summer is
Acknowledgements. This work was co-funded by CIRCE
very low and consistent with recent results indicating
from 2001 to 2004, the Autonomous City of Ceuta in 2001 and
that most of the fin whale observations take place from
the Life Nature Project (LIFE02NAT/E/8610) between 2002and 2004. Special thanks are due to P. Sanchez from the
fall to spring (de Stephanis unpubl. data). Further-
‘Centre Mediterrani d'Investigacions Marines i Ambientals
more, the catches reported by Aguilar (2006) were
(CSIC)' in Barcelona and Y. Cherel from CEBC-CNRS for
made in the Atlantic Ocean (Gulf of Cádiz), between
their comments on squid distribution in the Alborán Sea and
32 and 37° N, and not in the Strait of Gibraltar itself, so
the Gulf of Cadiz. We are also very grateful to the CIRCE staff
a high presence of this species in the study area was
and assistants in the field, P. Gozalbes, E. Pubill, R. Estebanand M. Fernández Casado. Thanks also to A. Cañadas, E.
not expected.
Urquiola, D. Desmonts, A. Aguilar, Y. Yaget and R. Sagarmi-
Research into producing reliable variance estimates
naga for their help, support and comments. Many thanks also
for spatial model predictions has been identified as a
to all the whale watching companies present in the strait,
priority for cetacean distribution studies (Williams et
Tumares S.L., Whale watch España, Aventura Marina and
al. 2006). One of the conditions for this is that the vari-
FIRMM for their comments on this manuscript and their help
ance in the data themselves is properly modelled.
at sea. This research was conducted using software Logger2000 developed by the International Fund for Animal Welfare
The present study shows that it is possible to esti-
(IFAW) to promote benign and non-invasive research.
mate habitat –cetacean models that account for the un-der- or over-dispersion of errors, as well as their spatialautocorrelation. However, in some cases, models could
not be estimated, or could only be after some simplifi-cation (e.g. not including spatial autocorrelation, see
Aguilar A (2006) Catches of fin whales around the Iberian
Peninsula: statistics and sources. Joint NAMMCO/IWC
pilot whale or sperm whale models in Table 2). Like-
Scientific workshop on the catch history, stock structure
wise, reducing the amount of data by fitting models for
and abundance of North Atlantic fin whales. Document
individual years often resulted in unreliable models
SC/14/FW/17-SC/M06/FW17, International Whaling
(e.g. fitting algorithms did not converge or residuals
Commission, Reykjavik
Baird RW, Borsani JF, Hanson MB, Tyack PL (2002) Diving
grossly violated normality or homoscedasticity as-
and night-time behaviour of long-finned pilot whales in
sumptions). This suggests that despite the intensive
the Ligurian Sea. Mar Ecol Prog Ser 237:301–305
observation effort and the relatively large number of
Barros NB, Odell DK (1990) Food habits of bottlenose dol-
sightings, our data set is near the minimum required to
phins in the southeastern United States. In: Leatherwood
estimate robust habitat –cetacean associations.
S, Reeves RR (eds) The bottlenose dolphin. AcademicPress, San Diego, CA, p 309–328
Interestingly, most of the relationships we found
Bejder L, Fletcher D, Bräger S (1998) A method for testing asso-
between cetacean presence (or abundance) and
ciation patterns of social animals. Anim Behav 56:719–725
covariates were linear or had very simple non-linear
Blanco C, Aznar J, Raga JA (1995) Cephalopods in the diet of
shapes (e.g. pilot whale), in contrast with many other
the striped dolphin Stenella coeruleoalba from the west-ern Mediterranean during an epizootic in 1990. J Zool
studies using GAMs (e.g. Forney 2000, Ferguson et al.
Lond 237:151–158
2006, Williams et al. 2006). One possible explanation is
Bruno M, Alonso J, Cózar A, Vidal J, Echevarría F, Ruiz J,
that the range of depths found in the Strait of Gibraltar
Ruiz-Cañavate A, Gómez F (2002) The boiling water phe-
Mar Ecol Prog Ser 353: 275–288, 2008
nomena at Camarinal Sill, the Strait of Gibraltar. Deep-
Manly BFJ (1994) Multivariate statistical methods: a primer.
Sea Res II 49(19):4097–4113
Chapman & Hall, New York
Cañadas A, Sagarminaga R, de Stephanis R, Urquiola E,
Piatt JF, Wetzel J, Bell K, DeGange AR and 5 others (2006)
Hammond P (2005) Habitat preference modelling as a
Predictable hotspots and foraging habitat of the endan-
conservation tool: proposals for marine protected areas for
gered short-tailed albatross (Phoebastria albatrus) in the
cetaceans in southern Spanish waters. Aquat Conserv 15:
North Pacific: implications for conservation. Deep-Sea Res
Part II 53:370–386
Davis RW, Worthy GAJ, Wursig B, Lynn SK, Townsend FI
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and
(1996) Diving behavior and at sea movements of an
S-PLUS. Springer Verlag, New York
Atlantic spotted dolphin in the Gulf of Mexico. Mar
R Development Core Team (2006) R: a language and environ-
Mamm Sci 12:569–581
ment for statistical computing. R Foundation for Statistical
Davis RW, Fargion GS, May N, Leming TD, Baumgartner M,
Computing, Vienna. Available at www.R-project.org
Evans WE, Hansen LJ, Mullin K (1998) Physical habitat of
Redfern JV, Ferguson MC, Becker EA, Hyrenbach KD and 15
cetacean along the continental slope in the north-central
others (2006) Techniques for cetacean-habitat modeling.
and western Gulf of Mexico. Mar Mamm Sci 14(3):
Mar Ecol Prog Ser 310:271–295
Reeves R, Notarbartolo di Sciara G (eds) (2006) The status and
Desportes G, Mouritsen R (1993) Preliminary results on the
distribution of cetaceans in the Black Sea and Mediter-
diet of long-finned pilot whales off the Faroe Islands. Rep
ranean Sea. IUCN Centre for Mediterranean Cooperation,
Int Whal Comm Spec Issue 14:305–324
Echevarría F, García Lafuente J, Bruno M, Gorsky G and 15
Reul A, Vargas JM, Jiménez-Gómez F, Echevarría F, Garcia-
others (2002) Physical–biological coupling in the Strait of
lafuente J, Rodriguez J (2002) Exchange of planktonic bio-
Gibraltar. Deep-Sea Res II 49:4115–4130
mass through the Strait of Gibralatar in late summer con-
Ferguson MC, Barlow J, Fiedler P, Reilly SB, Gerrodette T
ditions. Deep-Sea Res Part II 49:4131–4144
(2006) Spatial models of delphinid (family Delphinidae)
Roussel E (1999) Les cétacés dans la partie orientale du
encounter rate and group size in the eastern tropical
Détroit de Gibraltar au printemps: indications d'écologie.
Pacific Ocean. Ecol Model 193:645–662
Master thesis, Ecole Pratique des Hautes Etudes, Mont-
Forney KA (2000) Environmental models of cetacean abun-
dance: reducing uncertainty in population trends. Biol
Sanjuán A, Zapata C, Álvarez G (1994) Mytilus galloprovin-
Conserv 14:1271–1286
cialis and M. edulis on the coasts of the Iberian Peninsula.
Gannier A (1995) Les cétacés de Méditerranée nord-occiden-
Mar Ecol Prog Ser 113:131–146
tale: estimation de leur abondance et mise en relation de
Santos MB, Pierce GJ, López A, Barreiro A, Guerra A (1996)
la variation saisonnière de leur distribution avec l'écologie
Diets of small cetaceans stranded NW Spain. ICES Comm
du milieu. Master thesis, Ecole Pratique des Hautes
Etudes, Montpellier
Santos MB, Pierce GJ, Boyle PR, Reid RJ and 7 others (1999)
Gannon DP, Read AJ, Craddock JE, Fristrup KM, Nicolas JR
Stomach contents of sperm whales Physeter macro-
(1997) Feeding ecology of long-finned pilot whales Globi-
cephalus stranded in the North Sea 1990–1996. Mar Ecol
cephala melas in the western North Atlantic. Mar Ecol
Prog Ser 183:281–294
Prog Ser 148:1–10
SEC (Sociedad Española de Cetáceos) (1999) Recopilación,
Gil de Sola L (1993) Las pesquerías demersales del Mar de
análisis, valoración y elaboración de protocolos sobre
Alborán (sur-Mediterráneo Ibérico), evolución en los últi-
las labores de observación, asistencia a varamientos y
mos decenios. Inf Tec Inst Esp Oceanogr 142:1–179
recuperación de mamíferos y tortugas marinas de
Ginsberg JR, Young TP (1992) Measuring association
las aguas españolas. Technical Report Sociedad Española
between individuals or groups in behavioural studies.
de Cetáceos, Secretaria General de Medio Ambiente,
Anim Behav 44:377–379
Ministerio de Medio Ambiente Español, Madrid
Hastie GD, Wilson B, Thompson PM (2006) Diving deep in a
Silvani L, Gazo M, Aguilar A (1999) Spanish driftnet fishing
foraging hotspot: acoustic insights into bottlenose dolphin
and incidental catches in the western Mediterranean. Biol
dive depths and feeding behaviour. Mar Biol 148:
Conserv 90:79–85
Van Geen A, Boyle E (1988) Atlantic water masses in the
Hooge PN, Eichenlaub B (2000) Animal movement extension
Strait of Gibraltar: inversion of trace metal data. In:
to Arcview, Ver 2.0. Alaska Science Center — Biological
Almazan JL, Bryden H, Kinder T, Parrilla G (eds) Semi-
Science Office, U.S. Geological Survey, Anchorage, AK.
nario sobre la oceanografía física del Estrecho de Gibral-
Available at www.absc.usgs.gov/glba/ gistools/index.htm
tar. SECEG, Madrid, p 68–81
Watkins WA, Daher MA, Fristrup KM, Howald TJ, Nortobar-
Keitt TH, Bjornstad ON, Dixon PM, Citron-Pousty S (2002)
tolo di Sciara G (1993) Sperm whales tagged with
Accounting for spatial pattern when modelling organ-
transponders and tracked underwater by sonar. Mar
ism–environment interactions. Ecography 25:616–625
Mamm Sci 9:55–67
Kinder TH, Parrilla G, Bray NA, Burns DA (1988) The hydro-
Williams R, Hedley SL, Hammond PS (2006) Modelling distri-
graphic structure of the Strait of Gibraltar. In: Almazan JL,
bution and abundance of Antarctic baleen whales using
Bryden H, Kinder T, Parrilla G (eds) Seminario sobre la
ships of opportunity. Ecol Soc 11(1):1
oceanografía física del Estrecho de Gibraltar, Madrid,
Wood SN (2006) Generalized additive models: an introduction
with R. Chapman and Hall/CRC, Boca Raton, FL
Lacombe H, Richez C (1982) The regime of the Strait of
Young DD, Cockcroft VG (1994) Diet of common dolphins (Del-
Gibraltar. In: Nihoul JCJ (ed) Hydrodynamics of semi-
phinus delphis) off the south-east coast of southern Africa:
enclosed seas. Elsevier, Amsterdam, p 13–73
Opportunism or specialization? J Zool Lond 234:41–53
Editorial responsibility: Otto Kinne (Editor-in-Chief),
Submitted: December 31, 2006; Accepted: July 18, 2007
Proofs received from author(s): January 3, 2008
Source: http://circe.info/files/deStephanis2008.pdf
RINGWORM INFORMATION AND CONTROL MEASURES What is ringworm? Ringworm is a common skin infection caused by a fungus. Ringworm may affect the skin on the body, scalp, groin area (jock itch), feet (athlete's foot) or nails. The infection is not related to an infestation of worms. Ringworm occurs when a particular fungus grows and multiples anywhere on the body. Ringworm can affect anyone at any time due to the microscopic organisms that live off the dead outer layer of skin. Symptoms may not appear for 10-14 days after contact. How is ringworm detected? Ringworm is detected primarily based on the appearance of the skin. A scraping or culture of the affected area may be done by your doctor. Ringworm is recognized by:
Urea Nitrogen (BUN) Intended Use STANDARD (25 MG/DL) For in vitro diagnostic use in the quantitative colorimetric determination of A solution containing urea equivalent to 25 mg/dl with preservative. Avoid urea nitrogen in serum. contamination. Store at 2-8°C. Exercise the normal precautions required for the handling of al laboratory reagents. Pipetting by mouth is not recommended.







