
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Doi:10.1016/j.optm.2005.05.004
Non-ptotic ocular myasthenia gravis: a
common presentation of an uncommon
Jennifer Colavito, O.D.,a Jeffrey Cooper, O.D., M.S.,a,b andKenneth J. Ciuffreda, O.D., Ph.D.c
aPrivate practice, 539 Park Avenue, New York, New York; and bState University of New York, College of Optometry,
Department of Clinical Sciences and cDepartment of Visual Sciences, New York, New York
Background: Myasthenia gravis (MG) is an acquired auto-
immune disease of the neuromuscular junction which
Greek, meaning "gravely weak muscles," and was
causes rapid muscle fatigue and weakness. Two thirds ofall cases of myasthenia gravis (MG) initially manifest
first described by Sir Thomas Willis in It was
ptosis. In the absence of the characteristic variable ptosis,
later considered in detail by three German physicians—
MG can present a challenge to the clinician. This article
Goldflam, Erb, and Jolly—in 1890. Twenty years ago, an
will review the current diagnostic and management strat-
animal model was developed that led to a better physio-
egies for MG.
logically based understanding of the disease, and which
Case Reports: Five cases will be presented that did not
resulted in new treatment
initially present with ptosis. Each of these cases waspreviously misdiagnosed as a result of presentation ofatypical myasthenia gravis signs and symptoms. The first
The pathophysiology of the disorder requires an under-
two cases had signs and symptoms of a typical accom-
standing of the neuromuscular junction (NMJ), which is
modative/vergence anomaly. The others manifested dip-
the point at which the nerve fiber either synapses with or
lopia not normally associated with MG: one had a non-
terminates on a muscle fiber. When a nerve impulse is
comitant vertical deviation; another had a stable 6th nervepalsy; and the third had a basic esotropia.
initiated, it travels to the NMJ. Here the neurotransmitter
acetylcholine (Ach) is then released, passes through the
Conclusion: Although the hallmark findings of MG are ptosis
and eye muscle palsy with variability, MG may present
NMJ, and ultimately attaches to receptors on the muscle
without ptosis, affect nonstriated muscles, and/or mani-
surface, thereby resulting in muscular contraction. In MG,
fest either as a nonstrabismic vergence anomaly or as
antibodies erroneously destroy Ach receptor sites, which
comitant nonvariable strabismic deviation.
are located at the postsynaptic membrane of the NMJ. This
Key Words: Accommodative insufficiency, convergence insuf-
prevents Ach from binding to muscle cells, and hence
ficiency, diplopia, myasthenia gravis, oculomotor paresis,
inhibiting muscle contraction. (Current thinking is that T
cells derived from the thymus stimulate B cells to produce
the antibodies that react at the Ach The result is a
progressive muscle weakness that worsens with sustained
MG occurs in approximately 14 of every 100,000 people,
resulting in a prevalence of 36,000 cases in the U.S. It can
occur at any age, but has a bimodal distribution that affects
women below age 40 years and men more than 60 years of
MG is characterized by ptosis, facial weakness, dysar-
Colavito J, Cooper J, Ciuffreda KJ. Non-ptotic ocular myas-thenia gravis: a common presentation of an uncommon dis-
thria, dysphagia, and Among patients who mani-
ease. Optometry 2005;76:363-75.
fest ocular myasthenia gravis (OMG), generalized myasthenic
VOLUME 76 / NUMBER 7 / JULY 2005
symptoms will develop in more than 50% within
The first test involves "fatiguing" the extraocular
two Two thirds of all cases of MG initially
muscles in upgaze. The patient attempts to sus-
manifest ptosis and/or diplopia. A history of a
tain extreme upgaze for 30 seconds, then quickly
variable ptosis makes MG the most likely differen-
returns to primary position. Patients with OMG
tial diagnosis. However, when the patient mani-
often demonstrate either a lid-lag or an increase
fests diplopia, or with an accommodative/vergence
in ptosis (Darple's sign). This is repeated five
insufficiency, the diagnosis of MG becomes more
times, to initiate fatigue. Second, the patient is
told close his or her eyes, and then there is an
attempt to pry open the eyelids. In a non-OMG
The exact cause of this auto-immune disease is
patient, this will be difficult to perform. How-
unknown; it is thought there might be a genetic
ever, in the OMG patient, minimal resistance
and/or viral etiology. Thymus abnormalities are
will be found. Third, the eyebrow is lifted over
associated with MG, but the exact relationship is
the more ptotic eye. If a patient has OMG, the
uncertain. The thymus produces cells involved
contralateral eyelid will exhibit more ptosis,
in immune responses. Approximately 10% of
while in normal patients only a minimal change
will occur. These three tests are specific to the
patients with MG have a thymoma or tumor of
patient who manifests a ptosis.
the thymus, and 70% have hyperplasia of the
thymus, which is usually associated with active
Similar tests of ocular fatigue can be conducted
auto-immune disease. Since the thymus is the
while version, vergence, and/or accommodative
central organ for immunological self-tolerance, it
testing are performed. It may be necessary to
has been suggested that abnormalities of the
place a red lens over one eye to disrupt fusion
thymus may cause an immune-mediated attack
and to identify a suppressing eye. Accommoda-
on the Ach receptor in
tive fatigue may be determined with accommo-
dative facility testing (i.e., behind the phoropter
Patients with MG also have an increased preva-
while binocularly alternating the sphere power
lence of other auto-immune diseases, such as
by !1.50 D to produce large changes in accom-
rheumatoid arthritis, thyroid disease, and vita-
modative demand). In patients with MG, the
min B-12 deficiency. Symptoms of MG, which
initial response is often one of clarity. With
vary day-to-day, may be exacerbated by stress,
repeated changes, however, fatigue rapidly oc-
systemic illness, thyroid disease, pregnancy,
curs. Increased blur or diplopia on repetition is
menstruation, and certain
suggestive of In addition, monocular ac-
commodative amplitudes may be different be-
tween the eyes by at least a diopter, generally
lower in the second eye tested.
The diagnosis of OMG is usually made by a
combination of patient history, clinical findings,
If MG is still suspected, three simple tests may
and other diagnostic procedures. The first suspi-
be performed at home by the patient: photo
cion of OMG should come to mind during the
review, the ice and the sleep The
case history if the patient manifests symptoms of
photo review test requires looking at previous
pictures, then taking early morning and late
ptosis, diplopia, and/or blur, which increases
evening full-face pictures for three days. Lid
with use of the ocular muscles, or as the day
position and ocular alignment are noted. If a
progresses. Patients with generalized systemic
patient has OMG, there will be an increase in the
MG may also manifest fatigue of the face, neck,
ptosis and/or ocular deviation in the evening
and limbs, worsening with activity. Brushing
pictures, when greater fatigue would be ex-
one's teeth or combing hair may become prob-
pected to be present. The picture review process
lematic. Head droop and down-sloping of a smile
may be combined with the sleep and ice test
may be noted. Variability of symptoms should
described here.
further trigger suspicion. The gold standard for
diagnosis of OMG is the Tensilon test. However,
The ice-pack test has been shown to be both
there are a number of non-invasive tests that can
highly sensitive and specific for OMG in more
also be used to make the diagnosis; all are sim-
than 90% of the The palpebral fissure is
ple, inexpensive, and highly sensitive.
measured, and then an ice-pack is applied to the
VOLUME 76 / NUMBER 7 / JULY 2005
ptotic lid for a minimum of 3 minutes. An in-
If Tensilon testing is contraindicated or negative
crease in the palpebral fissure of 2 mm is con-
in highly suspected cases, there are several elec-
sidered a positive response. Non-myasthenic pa-
trodiagnostic tests that can be performed to sup-
tients do not demonstrate such a change. The
port a diagnosis of MG. Repetitive nerve stimu-
ice-pack test can also be performed to look for a
lation (RNS)—as the name suggests—involves
decrease in diplopia; however, the results may
repetitive electrical stimulation of the nerve
while recording the muscle A de-
crease in the fourth or fifth response by 10% of
The sleep test is also sensitive and specific for
the initial value is a positive finding. While this
MG. The patient sleeps for 30 minutes in the
test is highly specific for disorders of neuromus-
middle of the day or evening to ascertain if the
cular transmission (such as MG), it is not very
ptosis and/or diplopia decrease with rest. In our
sensitive. A negative result does not exclude the
practice, all three tests are combined at home.
diagnosis of MG. Additionally, it is much more
On one of the days the MG suspect performs a
sensitive for general MG (60% to 85%) than for
OMG (18% to 35%).
photo review, a 30-minutes nap is taken in the
evening with an ice bag placed on one eye.
Single-fiber electromyography (SFEMG) is the
Pictures are taken immediately on awakening.
most-sensitive clinical test of neuromuscular
The patient then brings the pictures to the doc-
SFEMG shows an increased
tor, who reviews them with magnification (a 20
activity in some muscles in almost all patients
D condensing lens), looking for signs of reduc-
with MG. Its sensitivity is 91% to 100% for
tion of ptosis and/or strabismus.
generalized MG, and 80% to 88% in patients
with OMG. Though the SFEMG is very sensitive
In addition, the patient with suspected MG
for MG and OMG, it is not very specific. The
should have a blood test performed to measure
SFEMG requires specialized equipment and re-
the level of serum anti-acetycholine receptor an-
lies heavily on the skill of the examiner, so is not
tibodies. The caveat to this test is that 20% of
as easily performed as the RNS test.
patients with general MG and 50% with ocular
MG will be sero-negative. Another more-specific
A CT or MRI of the thymus is necessary for
blood test can detect the presence of anti-striated
patients in whom OMG is diagnosed to rule out
muscle antibodies, which is positive in about
the presence of a thymoma. Lastly, all cases that
84% of patients with thymoma who are younger
manifest neurological signs such as ptosis and/or
than 40 years of age. In individuals more than 40
diplopia should have a threshold visual fields
years, anti-striated muscle antibodies can be
performed. (In the following cases presented,
found in MG without
Humphrey threshold 24–2 visual fields were
performed and were normal.)
If the diagnosis of MG is still suspected but uncon-
firmed, a Tensilon Test may be performed. Tensi-
lon (edrophonium chloride), an anti-cholinesterase
drug, inactivates the enzyme that breaks down
Treatment of OMG consists of one or more of
acetylcholine. This results in an excess of acetyl-
the following options: cholinesterase inhibitors,
thymectomy, plasmapheresis, corticosteroids,
choline in the neuromuscular junction, thus pro-
and/or other immunosuppressive drugs. Treat-
ducing transiently improved muscle function. Ten-
ment goals are individualized according to the
silon is injected at a rate of 2 cc every two minutes
severity of the disease and the patient's pre-
for 10 minutes, until 10 cc are injected. Immedi-
dicted tolerance to specific therapies. Untreated
ately following the injection, either the patient's
MG has a mortality rate of 25% to 31%, usually
ptosis or eye-muscle function will improve in a
due to respiratory muscle paralysis; however,
myasthenic patient. It should be noted that some
with current treatment, the mortality rate has
patients with MG will have a negative Tensilon
result. However, more than 90% of patients with
OMG will have a positive Tensilon test. (Atropine
Oral cholinesterase inhibitors such as pyridostig-
should be readily available in case of a hypersen-
mine bromide (Mestinon) are the first line of treat-
ment. They slow down the enzymatic destruction
VOLUME 76 / NUMBER 7 / JULY 2005
of Ach at the neuromuscular This
pressure and cataract formation should be mon-
allows the concentration of Ach to accumulate,
which, in turn, prolongs muscle contraction.
Cholinesterase inhibitors, the first line of treat-
Recently, other immunosuppresive drugs have
ment for OMG, may result in considerable
been used effectively to treat MG, such as Cy-
improvement in some patients, but little to no
closporine and Azathioprine. Cyclosporine is a
improvement in others. Generally speaking, cho-
fungal peptide with potent immunosuppressive
linesterase inhibitors work better in systemic MG.
It inhibits the T-lymphocyte-depen-
In some cases, they are ineffective because they
dent immune response in MG. Its maximum
may only reduce but not eliminate ocular misalign-
effectiveness occurs in six months, after which
ment, thereby causing diplopia to be more bother-
time the drug is tapered to achieve the minimal
some, or they may improve a severely ptotic eye-
effective dosage. Two of the most-serious side
lid, which can unmask diplopia. Because of their
effects of cyclosporine are hypertension and
high incidence of gastrointestinal side effects, they
nephrotoxicity. Azathioprine (Imuran™) may be
are not well-tolerated in the elderly population.
effective in those patients who do not respond to
Rarely, cholinergic medications may result in a
either Prednisone or The effect
cholinergic crisis.
of this drug, however, may take 6 to 8 months;
therefore, the two drugs might be used simulta-
A thymectomy is the surgical removal of the
neously. While the Prednisone is tapered, the
thymus gland. It is often performed on young
Azathioprine effect begins.
people early in the course of the The
surgery is performed in young patients with or
A new short-term treatment currently under in-
without a tumor. Unfortunately, patients more
vestigation uses intravenous human immune
than 60 years of age rarely show substantial
globulin It saturates the body with
improvement from thymectomy.
pooled gamma globulin antibodies derived from
many donors, which is thought to have a non-
Plasmapheresis, or plasma exchange, is used as a
specific suppressive effect on the immune sys-
temporary treatment for patients with sudden
tem. Improvement starts within a few days and
peaks in a few weeks.
worsening of Several liters of blood
are removed, the plasma cells are filtered out,
Recent studies by both Kupersmith et and
and then the red blood cells are returned with
Mee et alstrongly suggest that immunomodu-
artificial plasma, in an attempt to remove the
latory therapy (e.g., corticosteroids, azathio-
offending antibodies. Patients feel better for a
prine, thymectomy), significantly delays—or
few days after the procedure, but symptomatic
even prevents—generalization of the disease.
improvement only lasts several weeks.
Immunosuppressive therapy improves muscle
strength by suppressing the production of abnor-
mal antibodies. Oral corticosteroid therapy
The pathognomonic pattern of accommodative
(Prednisone) is typically prescribed in moderate-
fatigue in ocular myasthenia A 25-
to-severe cases that do not respond to cholines-
year-old female reported asthenopia after 5 min-
terase inhibitors and Significant
utes of near work; i.e., blurred vision, pulling
improvement occurs in more than 75% of the
sensations, and headaches. The symptoms had
cases. The patient is started on a high daily dose
been occurring for 12 years prior to the initial
of Prednisone (60 to 80 mg), which is then sys-
examination. Her hyperopia was corrected to
tematically reduced until the minimal effective
20/20 OU with spectacles. Extraocular muscle
dosage is reached. Long-term treatment may
movements were full and concomitant. Cover
cause either remission or significant improve-
testing revealed orthophoria at distance and 4"
ment in most patients in 1 to 4 months. How-
exophoria at near. The near point of conver-
ever, long-term use may predispose patients to
gence was 2/4". Suppression was noted on first-
significant problems such as hyperglycemia, os-
and second-degree targets, thus suggesting an
teoporosis, gastric ulcer disease, weight gain,
oculomotor anomaly of long duration. Vergence
and Cushing's syndrome. Ocularly, intraocular
amplitudes were reduced and without elicitation
VOLUME 76 / NUMBER 7 / JULY 2005
Case 2
Ocular myasthenia gravis masquerading as ac-
commodative and convergence insufficiency
A 25-year-old female optometry student came to us
with a history of ocular fatigue, associated with
near work, occurring over the preceding two
years. She had a childhood diagnosis of asthma for
which she was taking salmeterol xinafoate (Ser-
event, GlaxoWellcome, Reasearch Triangle Park,
North Carolina), triamcinolone acetonide (Asma-
cort, Rhone–Poulenc Rorer, Collegeville, Pennsyl-
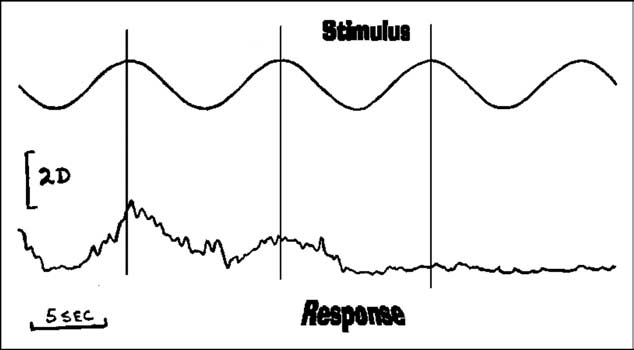
Figure 1 Accommodative findings of a patient with ocular myasthenia
gravis are presented. The top tracing depicts the sinusoidal
vania), and metaproterenol sulfate (Alupent,
stimulus and the bottom the response. Note, the initial re-
Boehringer Ingelheim, Ridgefield, Connecticut).
sponses were reasonability robust with a delayed latency.
Non-cycloplegic and cycloplegic refractions were
After a complete response, accommodative fatigue is evident,
identical (–0.75 sph 20/20 O.D.; –0.75 sph 20/20
becoming flat over a short period of time. (Reprinted withpermission of Binocul Vis Eye Muscle Surg Q. 1988:3 p.145).
At her initial vision examination, as a first-year
of blur (BO X/9/4 and BI X/8/5), but with asthe-
student, she reported difficulty focusing while
nopia induced on testing. Accommodative facil-
reading. This was verified with an abnormal
ity was initially normal, with ! 1.50 flippers
monocular and binocular ! 1.50 accommodative
performed both monocularly and binocularly at
flipper test result and reduced accommodative
40 cm, but rapidly showed fatigue with
amplitudes of 2 D sphere right eye and 0.5 D
sphere left eye. The age-related clinical norm
was 11 D. (By chance, this patient also partici-
These findings suggested an overall accommoda-
pated in a study in which dynamic accommoda-
tive and fusional-vergence insufficiency with as-
tive measurements were performed and were
sociated asthenopia. Vision therapy was begun,
found to be normal.) Phorometric findings re-
which included activities to improve smooth fu-
vealed 2" of left hyperphoria. Random-dot ste-
sional capabilities, step or jump ductions, and
reopsis was present, but reduced (660 sec arc),
accommodative facility and amplitude training.
thus indicating bifoveal fixation. All other ocular
Paradoxically, training increased her signs and
findings were normal. The record did not suggest
symptoms. Two months after the initial exami-
any treatment or further testing based on these
nation, a re-evaluation was performed that dem-
abnormal findings.
onstrated a variable extraocular muscle paresis
and mild ptosis with repeated eye movement
Two years later, the patient returned with simi-
lar symptoms of ocular fatigue, but now with the
additional symptom of occasional diplopia. Find-
A Tensilon test was performed, which was
ings again suggested an accommodative insuffi-
equivocal. However, single-fiber electomyogra-
ciency now associated with convergence insuffi-
phy tests were positive for MG. To document the
ciency. Both near point of convergence and
presence of an accommodative deficit objec-
fusional amplitudes were reduced. Interestingly,
tively, a high-speed infra-red optometer was
the initially measured hyperphoria of two years
used to measure accommodative responses dy-
earlier was not present. On the basis of these
namically to sinusoidally moving accommoda-
findings, weekly vision therapy was initiated to
tive targets. presents the recording.
improve both accommodative and fusional ver-
Initially, accommodation was robust and accu-
gence function. The therapy was designed to
rate. With repetition, effects of fatigue were ev-
improve static and dynamic accommodation,
ident. Accommodation initially displayed a lag,
vergence, and their interactions. Normally, vi-
and eventually showed fatigue to the point that
sion therapy is successful in improving accom-
no change in accommodation occurred in re-
modative and vergence function with concur-
sponse to the blur stimulation.
rent elimination of symptoms in more than 90%
VOLUME 76 / NUMBER 7 / JULY 2005
Figure 3 NRA and PRA measurements were performed morning, after-
noon, and early evening. This figure depicts NRA and PRA overtime. The NRA is normal and unchanged with Mestinon.
However, the PRA is reduced (normal for age -2.50) to zero
Figure 2 Accommodative amplitudes were measured using pushup
before Mestinon, but improves with Mestinon. (Reprinted with
methods on the right and left eye in the morning, afternoon,
permission of Neuro-Ophthalmol 2000;20:p8)
and early evening. It is readily apparent that amplitudedecreases from morning to evening. Also, note the discrep-ancy between the right and left eyes. (Reprinted with permis-sion of Neuro-Ophthalmol 2000;20:p8)
of patients with either convergence insufficiency
or various accommodative anomalies
In this case, however—and as in Case 2—vision
therapy produced a transient reduction in ac-
commodative and vergence function. The stu-
dent at this point contacted one of the authors
(JC), since her symptoms had not abated from
therapy. A review of her record by JC noted that
her pattern of fatigue occurring during therapy
morning, afternoon, and early evening. The phoria in-
was suggestive of MG. Ocular fatigue testing and
creases dramatically during the course of testing from 8"to 16". The phoria decreased after administration of
the sleep test were equivocal, and the ice test
Mestinon. (Reprinted with permission of Neuro-Ophthalmol
was negative. Approximately two months later,
a 3-mm ptosis of the left eye developed. She then
had a comprehensive neurological examination,
On the basis of these findings, the consulting
including magnetic resonance testing. The pa-
neurologist agreed with our suggestion of plac-
tient refused to have a Tensilon test because of
ing the patient on a diagnostic trial of Mestinon
her history of asthma. On initial testing, her
(Zeneca, Wilmington, Delaware) in an attempt to
accommodative and convergence findings were
mimic the results of Tensilon testing. We contin-
initially normal, but showed fatigue on repeti-
ued to measure accommodative and vergence
tion. There was also a subtle oculomotor non-
after the administration of Mestinon. It is clear
comitancy of 4" on extreme levoduction.
from the results that all accommodative/ver-
gence measurements exhibited significant reduc-
We measured accommodation (positive relative
tion from morning to evening. In addition, Mes-
accommodation, negative relative accommoda-
tinon clearly improved both accommodative and
tion and accommodative amplitudes); vergence
vergence amplitudes and facility. These findings
(positive fusional amplitudes and their respected
are diagnostic for MG—since Mestinon is similar
recoveries); and cover tests in the morning, af-
to Tensilon—except for having a longer duration
ternoon, and evening (see through
VOLUME 76 / NUMBER 7 / JULY 2005
Case 3
Ocular myasthenia gravis masquerading as a
non-comitant vertical muscle palsy. A 50-year-
old man was referred to author JC for eval-
uation of his recent-onset diplopia. The pa-
tient had a history of vertical diplopia for the
previous two months. The diplopia worsened
as the day progressed, and improved when
he tilted his head to the left. He had been
seen previously for this symptom of the dip-
lopia without resolution. Other than Crohn's
disease, all other history was unremarkable.
He mentioned he was going through a stress-
ful time in his personal life.
Figure 5 Convergence amplitudes (break) and recovery were measured
with the Risley prisms in the morning, afternoon, and early
Best-corrected vision with a low hyperopic cor-
evening. The convergence amplitude decreased dramatically
rection was 20/20 in each eye. Distance cover
from morning to evening from 15 to -8". One third of the
testing revealed a 3" left hypertropia in primary
decrease may be attributed to a change in phoria. Improve-
gaze, orthophoria in right gaze, and a 9" left
ment occurred with the administration of Mestinon. (Reprintedwith permission of Neuro-Ophthalmol 2000;20:p9)
hypertopia in left gaze. Diplopia was worse on
right head tilt. A Park's 3-Step Test indicated a
left inferior rectus (IR) palsy. (It should be noted
that isolated nontraumatic, noncongenital palsy
of the IR is very The patient's left supra-
duction was 5/3, and left infraduction was –1/–3.
This is consistent with a recent-onset deviation,
since there was no evidence of prism adaptation.
No ptosis was present. All other findings were
unremarkable. Due to the recent onset of his
symptoms, as well as the presentation of a non-
comitant vertical muscle deviation, a blood
work-up was ordered, which included Acetyl-
choline Receptor Antibody Titers, TSH, T3,T4
ESR, RPR, Lyme serology, and a fasting blood
sugar test. The patient was instructed to perform
Figure 6 Convergence amplitudes (break and recovery) in the evening are
presented by date. It is apparent that the amplitude varies daily,
a home sleep/ice pack test with picture review.
but is improved with the administration of Mestinon. (Reprinted
He was also prescribed a 3" Fresnel press-on
with permission of Neuro-Ophthalmol 2000;20:5-11)
prism base-down over the left eye, to alleviate
his diplopia.
Prior to taking Mestinon, the patient experi-
enced significant ocular and systemic fatigue
On a follow-up examination, the blood test re-
by 7:00 PM. After taking Mestinon (60 mg
sults were abnormal for the acetylcholine-recep-
daily), there was an immediate improvement
tor antibody level (i.e., 1.4 nmol/L—reference
in both accommodative and vergence find-
range is less than 0.7 nmol/L); Striated Muscle
ings, as well as a decrease in the ptosis. In
Antibody Titers (i.e., 1:160—reference range is
addition, she reported elimination of her
1:40); and TSH (i.e., 6.94 mIU/L—reference
general fatigue. Follow-up examination one
range, 0.40 to 5.50 mIU/L). All other blood find-
and a half years later substantiated these
ings were normal. The patient reported improve-
findings. During a short interval in which
ment of his diplopia after performing an ice-pack
she ran out of her medication, the ptosis and
test at home on two separate occasions. These
ocular and systemic fatigue all reappeared.
findings were consistent with acquired autoim-
She has since remained stable with a regimen
mune MG and thyroiditis. A CT scan of the
of 60-mg Mestinon twice a day.
thymus was ordered, and the patient was re-
VOLUME 76 / NUMBER 7 / JULY 2005
ferred to a neurologist who specialized in neuro-
3" exophoria at near. The ice pack test was
muscular disease, and to an endocrinologist.
equivocal in reducing the patient's diplopia.
Blood tests were negative. Due to the unex-
There was no evidence of a thymoma or thymic
plained hyperphoric deviation associated with
hyperplasia on a chest CT scan. A Tensilon test
the horizontal deviation, the patient was re-
was performed, which was positive. The patient
ferred to a neuro-ophthalmologist for evaluation.
was placed on a regimen of 40-mg Prednisone
The neuro-ophthalmologist wanted to rule out
every other day, later tapered by 5 mg per day.
MG by performing a Tensilon test. A diagnosis
After two weeks of treatment, the patient re-
of OMG was made on the basis of a positive
ported a decrease in the frequency and magni-
Tensilon test, and a diagnosis of hypothyroidism
tude of his diplopia. Subsequently, the patient
as indicated by a repeated blood test. The patient
moved out of state and was lost to followup.
was placed on a regimen of 60-mg Mestinon
q.i.d. for the OMG and Synthroid for the hypo-
thyroidism. On follow-up examination 3 months
later, the patient stated that her diplopia was no
Ocular myasthenia gravis masquerading as a
longer present with her current spectacles and
stable, longstanding 6th nerve palsy. A 66-year-
that she was doing well with current medica-
old woman came to us with a history of horizon-
tions. Cover testing with correction revealed or-
tal diplopia for the previous 5 years. She noted
thophoria at distance and near. The patient was
that the diplopia was worse when looking into
instructed to continue with her current specta-
the distance than at near. The patient was taking
cles and medication regimen to maintain her
hormone replacement therapy. She had a history
positive status.
of a surgical ptosis repair of her left eyelid two
years earlier for cosmetic reasons. The etiology
of the ptosis was not investigated at the time, nor
was the diplopia, despite numerous previous
Ocular myasthenia gravis masquerading as a
visits to eye doctors.
concomitant esotropia. A 22-year-old man with
best-corrected visual acuities of 20/20 OU was
With a slight hyperopic prescription, best cor-
referred to author JC for vision therapy. The
rected visual acuities were 20/25 in each eye.
referring optometrist noted a basic esotropia of
Distance cover test measured 10" left esotropia
recent onset. She had ordered an MRI, which
with 2" left hyperphoria, and the near cover test
was negative. At the initial examination, a 16"
measured 12" esophoria with 4" left hyperpho-
constant esotropia with 2" of hypertropia was
ria. Vertical vergence ranges were normal at
found at distance and near. Since the patient was
distance and near. Near vergence ranges were BI
leaving for college, he was prescribed a pris-
x/–4/–14 and BO x/25/12. All other findings were
matic correction and was referred for vision
within normal limits.
therapy in the city in which he attended college.
A few months later, he returned from college
The diagnosis of a longstanding 6th nerve palsy
and returned to JC for a followup. He stated that
was made, and the patient was given prism with
the prism correction initially eliminated the dip-
her prescription of #2.25 D –0.75 D $ 85 % 11 "
lopia, which lasted for only a short period of
BU % 31 " BO right eye and #1.25 D sph %
time. After the patient returned to college, the
11 " BD % 31 " BO left eye with an add of #2.75
optometrist—who initially provided the vision
D in a progressive addition lens design. Blood tests
therapy—advised the patient that the initial pris-
were ordered, including thyroid function tests
matic correction was incorrect.
(T3,T4, TSH) sed-rate (ESR), and Acetylcholine re-
ceptor antibody titers. Given the length of time the
On the patient's return to JC, the variability of
patient was experiencing symptoms, an MRI was
his measured deviation led us to suspect the
not immediately indicated.
possibility of MG. We performed an ice/sleep
test and ordered an Ach Receptor Antibody
On follow-up examination two weeks later, the
blood test. The ice/sleep test was positive for MG
patient stated that the diplopia was eliminated
(see A and B), while the antibody level
with the prism spectacles. Cover testing with
was negative. A subsequent cover testing re-
correction revealed 3" esophoria at distance and
vealed a concomitant 20" left esotropia at dis-
VOLUME 76 / NUMBER 7 / JULY 2005
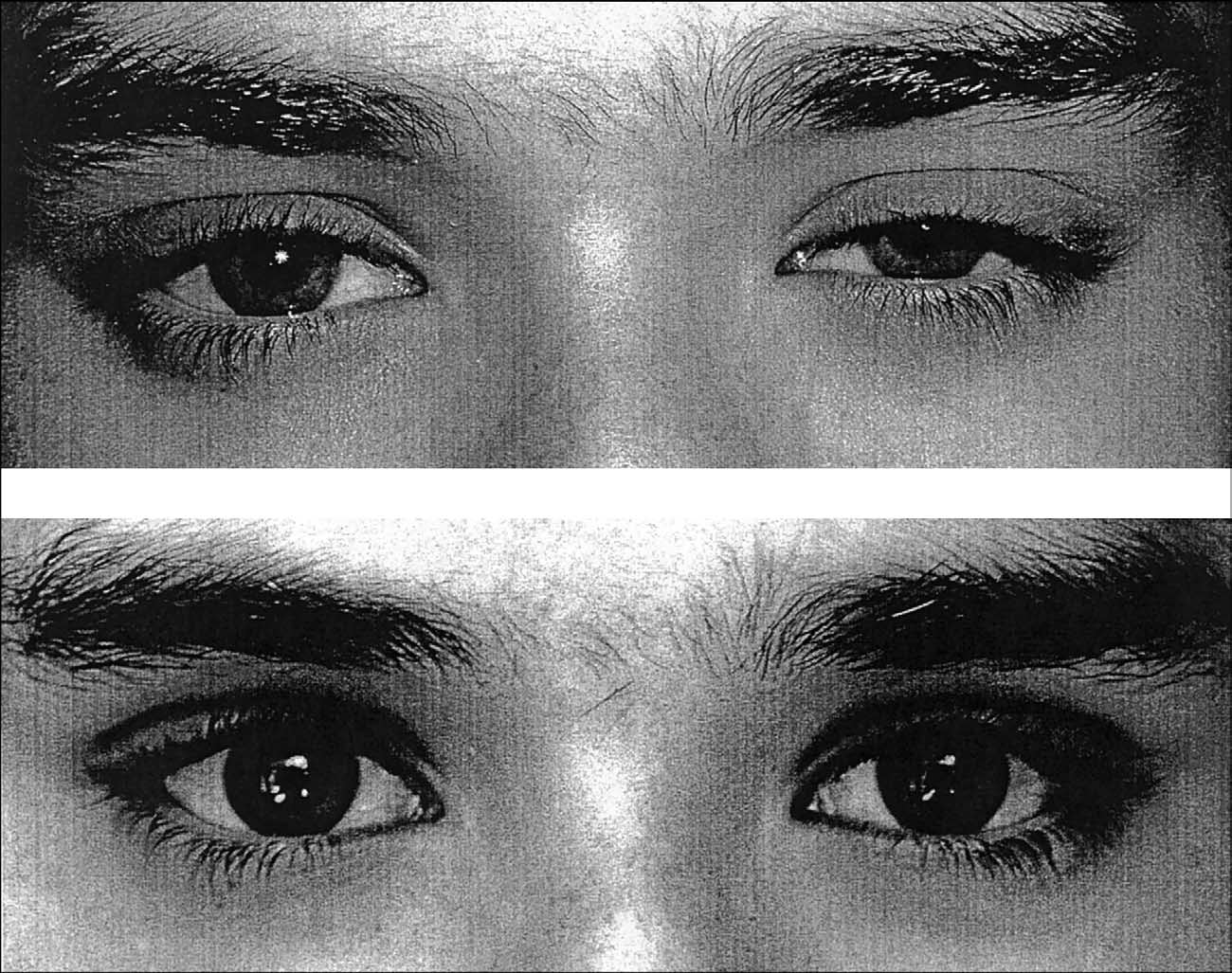
of his lids was noted.
tance and 10" of esophoria at near. Versions
The patient returned to us six months later,
were concomitant.
taking 60-mg Mestinon q.i.d. p.o. He reported
less ptosis with the medication; however, the
The patient had a neuro-ophthalmological
abdominal side effects forced him to discontinue
examination; nerve-conduction studies, and
the medication. Cover testing revealed a con-
EMG results, all of which were normal. The
comitant 16" esotropia at distance and or-
neuro-ophthalmologist concluded that OMG
thophoria at near. Accommodative amplitudes
was not present. However, a Tensilon test—
were 6.5 diopters in each eye. A thymectomec-
which we requested—was not performed at
tomy was performed; the patient discontinued
that time. We saw him again and noted the
Mestinon and was treated with 60-mg Pred-
variable esotropia. We conferred with a sec-
nisone, with some improvement. His deviation
ond neuro-ophthalmologist, who agreed with
stabilized and he was advised to wear a distance
our tentative diagnosis of MG. We referred
prism correction.
the patient back to the neuro-ophthalmolo-
gist located in his college town who subse-
quently noted a new ptosis and performed a
Tensilon test, which was positive. The pa-
MG usually manifests a recently acquired, vari-
tient was started on a regimen of 30-mg
able ptosis, greater in one eye than the other.
Mestinon q.i.d. p.o. and advised to have a
When this occurs, the diagnosis is straightfor-
chest CT scan.
ward and is readily confirmed by the gold stan-
VOLUME 76 / NUMBER 7 / JULY 2005
dard—the Tensilon test. Previous studies have
bell and demonstrated that accom-
suggested that 90% of all patients with myasthe-
modation may be involved in the MG syndrome.
nia gravis manifest a ptosis, and that a general-
We have presented and published two case stud-
ized MG will eventually develop (within two
ies that objectively demonstrate accommodative
years) in 50% of those who manifest an ocular
insufficiency as the presenting We be-
In dealing with relatively stable acquired
lieve that the paucity in reporting of accommo-
ptosis is more difficult to ascertain the origin;
dative anomalies is related to the lack of testing
however, even in those cases, MG must be con-
of accommodative amplitude, and/or facility of
sidered. We believe that patients manifest non-
accommodation. It is important that those pro-
ptotic signs of MG more frequently than previ-
viding vision therapy recognize the pattern of
ously reported. When the presenting sign is
accommodative findings associated with MG.
other than ptosis—such as an accommodative/
There are a few caveats in making the diagnosis
vergence insufficiency, or a concomitant or non-
comitant oculomotor paresis—then diagnosis of
of accommodative insufficiency secondary to
MG is much more elusive. For the patient with
MG, since these accommodative findings are
myasthenia gravis who manifests nonclassical
unique. First, asymmetrical accommodative am-
symptoms, MG may be overlooked by the
plitudes between the eyes are present. Second,
neuro-ophthalmolgists, ophthalmologists, op-
the amplitudes are usually reduced and will de-
tometrists, and primary care practitioners.
crease further on repeated testing. Third, accom-
modative facility as measured with ! 1.50 D
Perhaps the primary reason that patients who
lenses in the phoropter demonstrates fatigue
initially manifest MG-induced accommodative
with repeated testing. Fourth, the general accom-
and vergence deficits are not appropriately diag-
modative (and related vergence) findings fatigue
nosed is that the ophthalmic community is not
by the end of the day, and thus their respective
sensitive to the subtle initial signs and symptoms
amplitudes and/or facility will be less in the
of MG—i.e., asthenopia, blur, diplopia, and vi-
evening, as compared to the morning. Fifth, the
sual fatigue. The most-common finding of MG is
findings will vary from day to day. And sixth,
fatigability of either striated or non-striated ocu-
Tensilon or Tensilon equivalent medications
lar motor muscles—accommodation, vergence,
(such as Mestinon) will have a positive effect on
and levator palpebrae. This fatigability may oc-
cur with minimal usage: within minutes, from
morning to evening, and/or from day to day. No
After the classical sign of ptosis, the most com-
other condition manifests such variable findings.
monly described sign of MG is variable diplopia.
Thus, any patient suspected of having MG
The classical myasthenic patient will manifest a
should have morning baseline testing to estab-
non-comitant, oculo-motor paresis varying as the
lish lid position and accommodative/vergence
day progresses. This myasthenic patient will
functioning. Measurement should be repeated in
have motor fields that are not indicative of a
the evening to assess fatigability.
specific oculomotor paresis; variability is the
hallmark of MG. Like accommodation, in-
There are a variety of non-invasive, simple office
and home tests that we have discussed previously
creased variability is usually noted with re-
to support the diagnosis of MG (see . If
peated testing or time of day. The patient may
ptosis is present, the clinician should attempt to
manifest a subtle ptosis, so the external exami-
fatigue the orbicularis muscles. Also, 2.5% neo-
nation must be carefully performed. Fatigue,
synephrine may be used to differentiate senile
induced by repeated versions or vergence testing
ptosis from other forms of ptosis. The patient
with a prism bar, will result in increased diplo-
should be instructed to perform the photo test at
pia and/or a change in the cover test value.
home. If a patient has diplopia, atypical oculo-
Version and vergence testing may be enhanced
motor fatigue, and/or demonstrates a decrement
with the use of a red lens. The red lens serves
of findings during or immediately after vision
three functions: to disrupt binocularity, to elim-
therapy, MG should be suspected.
inate suppression, and to determine the diplopia
direction. The sleep/ice test usually results in
It has been assumed that MG only affects stri-
improvement of the diplopia, as noted in two of
ated muscles. As early as 1900, however, Camp-
VOLUME 76 / NUMBER 7 / JULY 2005
with a photo review, are sensitive and specific to myasthenia gravis.
Myasthenia may masquerade as a convergence
plained diplopia—particularly under the age of
insufficiency, a divergence insufficiency, or a
60 years—should include MG in the differential
concomitant hyperdeviation. Myasthenia both
diagnosis, even if another health care practitio-
follows and breaks the rules. Thus, any unex-
ner has dismissed the diagnosis of MG. Although
VOLUME 76 / NUMBER 7 / JULY 2005
four of the five cases had variable findings which
lead to a more-detailed investigation of possible
1. Conti–Fine BM, Protti MP, Belone M, et al. Myasthenia
MG, one did not. This patient was particularly
gravis and its experimental model. The immunobiology of
elusive, since the ptosis was previously elimi-
an autoimmune disease. Georgetown: Landes Bioscience
nated by surgery, and her deviation was con-
Publishers, 1997.
comitant and unchanging over a course of sev-
2. Porter NC, Salter BC. Ocular myasthenia gravis. Curr
Treat Options Neurol 2005;7:79-88.
3. Engel AG. Myasthenia gravis and myasthnic syn-
dromes. Ann Neurol 1984;16:519-34.
If the patient is still not confirmed as having MG
4. Sampson JA. Myasthenia gravis and myasthenic syn-
after simple, non-invasive office and home tests,
dromes. In: Walton J, ed. Disorders of voluntary muscle.
Boston: Churchill–Livingstone, 1981:585.
further testing is warranted. A blood workup
5. Kurland IT, Ater M. Current status in epidemiology and
should include a MG antibody test, thyroid test-
genetics of myasthenia gravis. In: Viets HR, ed. Myasthe-
ing, fasting blood sugar, Lyme titer, and ESR in
nia gravis: the Second International Symposium proceed-
those over 60 years of age. If intracranial disease
ings. Springfield, Ill.: Charles Thomas, 1961:307-36.
6. Somnier FE, Keiding N, Paulson OB. Epidemiology of
is suspected, an MRI with contrast should be
myasthenia gravis in Denmark: a longitudinal and com-
performed. The radiologist should be advised to
prehensive study. Arch Neurol 1991;48:733-9.
carefully examine the pathways of 3rd, 4th, or 6th
7. Kalb B, Matell G, Pirskanen R, et al. Epidemiology of
N from the eye to the midbrain, and the higher
myasthenia gravis a population-based study in Stock-
holm, Sweden. Neuroepidemiology 2002;21:221-5.
neurological centers if a gaze palsy is noted.
8. Kupersmith MJ, Latkany R. Development of general-
Lastly, a neuro-ophthalmological consult is in
ized disease at 2 years in patients with ocular myasthe-
order with Tensilon testing and single-fiber myo-
nia gravis. Arch Neurol 2003;60:243-8.
graphy to make the final differential diagnosis.
9. Evoli A, Minisci C, Schino D. Thymoma in patients with
MG: characteristics and long-term outcome. Neurology
After stabilization with medication, patients
10. Cooper J, Kruger P, Panariello G. The pathognomic
with MG should have a re-evaluation of their
pattern of accommodative fatigue in myasthenia gravis.
accommodative and vergence functions. Stable
Binoc Vis Eye Muscle Surg Q 1988;3:141-8.
11. Lertchavanakul A, Gamnerdsiri P. Ice test for ocular
MG patients who manifest accommodative and
myasthenia gravis. J Med Assoc Thai 2001;84:S131-6.
vergence defects may benefit from prismatic cor-
12. Golnik KC, Pena R, Lee AG, et al. The ice test in the
rection incorporated into a progressive lens. A
diagnosis of myasthenia gravis. Ophthalmology 1999;
progressive lens provides the advantage of com-
13. Kubis KC, Danesh–Meyer HV, Savino PJ, et al. The ice
pensating for the variable accommodative ampli-
test versus the rest test in myasthenia gravis. Ophthal-
tudes noted during the day and from day to day.
One must remember, for the young patient, that
14. Ellis FD, Hoyt CS, Ellis FJ, et al. Extraocular muscle
the add power should be determined monocu-
responses to orbital cooling (Ice Test) for ocular myas-
thenia gravis diagnosis. Am Assoc Pediatr Ophth Surg
larly before prism is determined (by relaxing
accommodation, there is a relaxation of conver-
15. Soliven BC, Lange DJ. Seronegative myasthenia gravis.
gence; i.e., increase in exophoria). The add,
which may be determined by conventional
16. Limburg PC, The TH. Anti-acetylcholine receptor anti-
bodies in myasthenia gravis. Part 1. Relation to clinical
means (i.e., cross cylinder, balancing PRA/NRA,
parameters in 250 patients. J Neurol Sci 1983;58:357-70.
etc.) should be prescribed monocularly to elim-
17. Skorin L. Myasthenia gravis. In: Onofrey BE. Clinical
inate asymmetry in accommodative findings.
optometric pharmacology and therapeutics. Philadel-
phia: JD Lippincott Company, 1991:1-17.
18. Howard JF, Sanders DB. The electrodiagnosis of myas-
In summary, MG should be considered when
thenia gravis and the Lambert–Eaton myasthenic syn-
examining the patient with unexplained ocular
drome. Neurol Clin North Am 1994;12:305-30.
and general fatigue, unexplained accommoda-
19. Weinberg DH, Rizzo JF, Hayes MT. Ocular myasthenia
tive fatigue or vergence fatigue, unexplained pto-
gravis: predictive values of single-fiber electromyogra-
phy. Muscle Nerve 1999;22:1222-7.
sis, or diplopia. There are a multitude of simple
20. Rivero A, Crovetto L. Single-fiber electromyography of
clinical tests that help make the diagnosis of this
extraocular muscles: a sensitive method for the diagnos-
often elusive disease. Early diagnosis and treat-
tic of ocular myasthenia gravis. Muscle Nerve 1995;18:
ment of MG is beneficial, to eliminate costly
21. Oosterhis HJ. The natural course of myasthenia gravis:
neurological testing and to improve the quality
a long-term follow-up study. J Neurol Neurosurg Psychi-
of life of our patients.
VOLUME 76 / NUMBER 7 / JULY 2005
22. Evoli A, Batocchi AP, Tonali P. A practical guide to the
31. Mee J, Paine M, Byrne E, et al. Immunotherapy of
recognition and management of mysthenia gravis.
ocular myasthenia gravis reduces conversion to gener-
alized myasthenia gravis. J Neuro-ophthalmol 2003;23:
23. Heitmiller RF. Myasthenia gravis: clinical features,
pathogenesis, evaluation, and medical management. Se-
32. Cooper J. Accommodative Dysfunction in Diagnosis and
min Thorac Cardiovasc Surg 1999;11:41-6.
Management of Vision Care. Ed J Amos. Butterworth.
24. Roberts PF, Venuta F, Redina E. Thymectomy in the
1987, pp 440-3.
treatment of ocular myasthenia gravis. J Thorac Cardio-
33. Cooper JS, Pollak GJ, Ciuffreda KJ. Accommodative and
vasc Surg 2001;122:562-8.
Vergence Findings in Ocular Myasthenia: A Case Anal-
25. Heitmiller RF. Myasthenia gravis: clinical features,
ysis. Neuro-Ophthalmol 2000;20:5-11.
pathogenesis, evaluation and medical management. Se-
34. Cooper J, Duckman R. Convergence Insufficiency: In-
min Thorac Cardiovasc Surg 1999;11:41-6.
cidence,diagnosis, and treatment. J Am Opt Assoc 1978;
26. Tackenberg B, Hemmer B, et al. Immunosuppressive
35. Von Norden GK.
treatment of ocular myasthenia gravis.
Binocular Vision and Ocualr Motlity:
BioDrugs 2001;
Theory and Management of Strabismus. CV Mosby. 1996,
27. Bonifati DM, Angelini C. Long-term cyclosporine treat-
36. Campbell H, Bramwell E. Myasthenia Gravis. Brain
ment in a group of severe myasthenia gravis patients.
J Neurol 1997;244:542-7.
28. Palace J, Newson-Davis J, Lecky B. A randomized dou-
ble-blind trial of prednisolone alone or with azathio-prine in myasthenia gravis. Myasthenia Gravis Study
Corresponding author:
Group. Neurology 1998;50:1778-83.
29. Jongen JL, van Doorn PA, Vander Meche FG. High-dose
Jeffrey Cooper, O.D., M.S.
intravenous immunoglobulin therapy for myasthenia
Dept. of Clinical Sciences
gravis. J Neurol 1998;245:26-31.
33 W. 42nd Street
30. Kupersmith MJ, Moster M, Bhuiyan S, et al. Beneficial
New York, New York 10036
effects of corticosteroids on ocular myasthenia gravis.
Arch Ophthalmol 1996;53:802-804.
VOLUME 76 / NUMBER 7 / JULY 2005
Source: http://coopereyecare.com/wp-content/uploads/2013/10/4Myasthenia-Gravis-Paper.pdf
Alcohol studies in translational models: behavioural consequences of adolescent exposure and novel approaches to reduce the propensity to relapse. The research described in this thesis was conducted at the department of Anatomy and Neurosciences, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands and at the department of Molecular and Cellular Neurobiology, Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University, Amsterdam, The Netherlands. My research was supported by ZONMW Topgrant 912-06-148. Cover: artwork by Martijn C.L. Van Roovert. Layout by Esger Brunner. Printed by GVO drukkers & vormgevers B.V. P+L ISBN: 978-90-6464-703-1 Copyright © J.A. Wouda ([email protected]), 2013. Al rights reserved. No part of this thesis may be reproduced, stored in a retrieval system, transmitted in any form without prior permission from the author.
The Journal of Neuroscience, August 1, 2002, 22(15):6321–6324 Caffeine Induces Dopamine and Glutamate Release in the Shell ofthe Nucleus Accumbens Marcello Solinas,1 Sergi Ferre´,1 Zhi-Bing You,2 Marzena Karcz-Kubicha,1 Patrizia Popoli,3 andSteven R. Goldberg1 Sections of 1Preclinical Pharmacology and 2Behavioral Neuroscience, Behavioral Neuroscience Branch, National Instituteon Drug Abuse, National Institutes of Health Intramural Research Program, Baltimore, Maryland 21224, and3Department of Pharmacology, Istituto Superiore di Sanita, 00161 Rome, Italy






