
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Atomoxetine hydrochloride in the treatment of children and adolescents with attention-deficit/hyperactivity disorder and comorbid oppositional defiant disorder: a placebo-controlled italian study


European Neuropsychopharmacology (2009) 19, 822–834
Atomoxetine hydrochloride in the treatmentof children and adolescents withattention-deficit/hyperactivity disorder and comorbidoppositional defiant disorder: A placebo-controlledItalian study
Grazia Dell'Agnello a, Dino Maschietto b, Carmela Bravaccio c,Filippo Calamoneri d, Gabriele Masi e, Paolo Curatolo f, Dante Besana g,Francesca Mancini a, Andrea Rossi a, Lynne Poole h,Rodrigo Escobar i, Alessandro Zuddas j,⁎for the LYCY Study Group
a Medical Department, Eli Lilly Italia, Italyb Operative Unit of Child Neuropsychiatry, Azienda USL n 10 Veneto Orientale, San Donà di Piave, Venezia, Italyc Department of Pediatrics, University of Naples "Federico II", Italyd Clinic of Child Neuropsychiatry, University Policlinic of Messina, Italye Department of Child Neuropsychiatry, IRCCS Fondazione Stella Maris, Calambrone, Pisa, Italyf Department of Child Neuropsychiatry, Tor Vergata University of Rome, Italyg Operative Structure of Child Neuropsychiatry, Hospital of Alessandria, Italyh Eli Lilly and Co. UKi European Medical Department, Eli Lilly and Co. Alcobendas, Madrid, Spainj Department of Neuroscience, Section of Child Neuropsychiatry, University of Cagliari, Italy
Received 3 January 2009; received in revised form 2 July 2009; accepted 23 July 2009
Objective: The primary aim of this study was to assess the efficacy of atomoxetine in improving
hyperactivity disorder;
ADHD and ODD symptoms in paediatric patients with ADHD and comorbid oppositional defiant
Oppositional defiant
disorder (ODD), non-responders to previous psychological intervention with parent support.
Methods: This was a multicentre, randomised, placebo-controlled trial conducted in patientsaged 6–15 years, with ADHD and ODD diagnosed according to the DSM-IV criteria by a structured
⁎ Corresponding author. Centre for Pharmacological Therapies in Child and Adolescent Neuropsychiatry, Department of Neuroscience,
University of Cagliari, via Ospedale, 46, 09124 Cagliari, Italy. Tel.: +39 070 609 3509/3510; fax: +39 070 669591.
E-mail address: (A. Zuddas).
0924-977X/$ - see front matter 2009 Elsevier B.V. and ECNP. All rights reserved.
Treatment with atomoxetine of children and adolescents with ADHD and ODD
clinical interview (K-SADS-PL). Only subjects who are non-responders to a 6-week standardizedparent training were randomised to atomoxetine (up to 1.2 mg/kg/day) or placebo (in a 3:1 ratio)for the following 8-week double blind phase. Results: Only 2 of the 156 patients enrolled for theparent support phase (92.9% of males; mean age: 9.9 years), improved after the parent trainingprogram; 139 patients were randomised for entering in the study and 137 were eligible for efficacyanalysis. At the end of the randomised double blind phase, the mean changes in the Swanson,Nolan and Pelham Rating Scale-Revised (SNAP-IV) ADHD subscale were −8.1 ± 9.2 and −2.0 ± 4.7,respectively in the atomoxetine and in the placebo group (p b 0.001 between groups); changes inthe ODD subscale were −2.7 ± 4.1 and −0.3 ± 2.6, respectively in the two groups (p = 0.001 betweengroups). The CGI-ADHD-S score decreased in the atomoxetine group (median change at endpoint:−1.0) compared to no changes in the placebo group (pb0.001 between groups). Statisticallysignificant differences between groups, in favour of atomoxetine, were found in the CHIP-CEscores for risk avoidance domain, emotional comfort and individual risk avoidance subdomains.
An improvement in all the subscales of Conners Parents (CPRS-R:S) and Teacher (CTRS-R:S)subscales was observed with atomoxetine, except in the cognitive problems subscale in the CTRS-R:S. Only 3 patients treated with atomoxetine discontinued the study due to adverse events. Noclinically significant changes of body weight, height and vital signs were observed in both groups.
Conclusions: Treatment with atomoxetine of children and adolescents with ADHD and ODD, whodid not initially respond to parental support, was associated with improvements in symptoms ofADHD and ODD, and general health status. Atomoxetine was well tolerated.
2009 Elsevier B.V. and ECNP. All rights reserved.
Longitudinal data suggest that ADHD predicts academicoccupational dysfunction, earlier sexual intercourse, and
Attention-deficit/hyperactivity disorder (ADHD) is charac-
early parenthood (
terised by a persistent high level of hyperactive, inattentive
), whereas the presence of ODD may modulate persistence
and impulsive behaviour, which can be prevalent in both child
of associated disorders ) and may
and adolescent populations. Although information on preva-
predispose affected children to the subsequent development
lence and incidence of ADHD in Europe is scarce and depending
conduct disorders, delinquent behaviour and substance misuse
on the used definition, a range from 2 to 5% (for subjects
aged 6–16 years) has been reported in most of studies based on
the ICD-10 and DSM-IV diagnostic criteria, respectively
Clinical studies evaluating the effects of stimulants in
(A few different epidemiological studies
treating children with ADHD and comorbid ODD reported
conducted in Italy estimated a frequency of ADHD in the
inconclusive results. Some of these studies that investigate
paediatric population ranging from 4% to 7%
children with mental retardation, were conducted either
in laboratory conditions or very specific settings such as
gender ratio of 7:1 between males and females, aligned with
partial hospitalization programs or strictly academic situa-
data from international literature
). Factor analysis of ADHD and
), so that the generalization
oppositional defiant symptoms reports by both Italian parents
of their findings does not necessarily extend to the home
and teachers indicate a structure similar to that observed in
environment or more natural conditions. Others, without
the US and Northern Europe ).
specifically assessing oppositional-defiant symptoms,
ADHD may be associated with additional psychiatric
reported the efficacy of methylphenidate for CD symptoms
disorders such as mood and anxiety disorders and disruptive
in ADHD patients with comorbid CD, also indicating that
behaviour disorders. In particular, oppositional defiant disor-
the presence of a diagnosis of ODD or CD diminished the
der (ODD) is among the most common comorbid psychiatric
effect size of the drug
disorders in patients with ADHD, occurring in up to 67% of
). The Multicenter Treatment
clinically referred populations () and
Study of Children with ADHD (MTA study) showed that the
representing a serious clinical problem. ODD is characterized
presence of ODD did not alter the expected pattern of ADHD
by a pattern of developmentally inappropriate negativistic,
symptom response and suggested that ODD symptoms may
hostile and defiant behaviour causing clinically significant
show greater improvement with pharmacological treatment
impairment in social, familiar or academic functioning.
than with behavioural management (
Genetic, family environment and psychometric studies indi-
cate that they have separate aetiologies and pathophysio-
Atomoxetine hydrochloride is a potent inhibitor of the
logical mechanisms
presynaptic norepinephrine transporters, and has minimal
affinity for other neurotransmitter transporters or receptors.
ADHD combined with ODD tend to have more severe ADHD
In comparative placebo-controlled studies conducted in
symptoms, more peer problems, and more family distress
children, adolescents and adults, atomoxetine consistently
compared to children with ADHD alone ().
reduced symptoms of ADHD (
G. Dell'Agnello et al.
cant laboratory or ECG abnormalities; medical conditions likely to
and positive outcomes have also been reported in family and
increase sympathetic nervous system activity or regular intake of
social functioning of children and adolescents with ADHD and
sympathomimetic drugs; narrow-angle glaucoma; uncontrolled
in their quality of life
thyroid dysfunction; likelihood of start of structured psychotherapyat any time during the study; pregnant or breastfeeding females, or
Moreover, atomoxetine seems to
females at risk of pregnancy.
exert positive effects on additional psychiatric conditions,such as other disruptive behaviour disorders, mood andanxiety disorders, which are often associated with ADHD
2.2. Study design
(An analysis of data from placebo-controlled studies in
The entire study consisted of 4 study periods (
children and adolescents provided evidence on the efficacyof atomoxetine in reducing symptoms and improving social
a) Study period I (screening phase): this was a screening and
and family functioning in patients with ADHD and comorbid
assessment/evaluation period, ranging from 3 to 28 days, to
ensure eligibility for the study, and was started after parent's
ally, a recent review has shown that atomoxetine reduces
consent was obtained.
ADHD symptoms in both ODD-comorbid and non-comorbid
b) Study period II (open-label, parent support phase): during this
subjects to similar extents and that reduction in ODD
6-week phase, the investigators provided a standardized
symptoms is highly related to the magnitude of ADHD
management for the parental support. Parents received weeklyseries of advice on the management of the behavioural problems
response, indicating that the presence of ODD symptoms
of their children from qualified psychologists or child neuro-
does not affect the clinical effectiveness of atomoxetine in
psychiatrists, based on standardized procedures (
ADHD subjects Relapse of ADHD
With this program, parents were trained to provide clear,
symptoms is also not influenced by the presence of comorbid
consistent expectations, directions and limits to their children,
to use modification principles to reinforce positive behaviours
In a recent placebo-controlled study conducted in Europe
and to eliminate or reduce negative behaviours that create
and Australia on children with ADHD and comorbid ODD, no
problems for their children. Additionally, the training helped
significant differences between atomoxetine and placebo
parents to become able to assist their children in making friends
were found for ODD symptoms, although atomoxetine
and learning to work cooperatively with others. Response
significantly improved ADHD symptoms
criteria were defined as an improvement in CGI-S score of 2 ormore from baseline and at least a 30% decrease from baseline
suggesting that the social and cultural environment may
in 18 items of the ADHD subscale score of investigator-rated
play a role in modulating the atomoxetine effects on ODD
SNAP-IV. Only patients who did not reach both mentioned
criteria were randomised to the study period III.
The present study was carried out to evaluate the efficacy
c) Study period III (randomised, double blind, placebo-controlled
of atomoxetine in improving ADHD and ODD symptoms in an
phase). This was an 8-week period of double blind treatment. At
Italian population of children and adolescents initially not
the beginning of this period, patients who did not respond to the
responding to a parent training intervention, and to assess
6-week period of parent support were randomly assigned to
the extent of improvements in health status and level of
treatment with atomoxetine or placebo in a ratio of 3:1 (i.e. with
approximately 75% of patients receiving atomoxetine and 25% ofpatients receiving placebo). Patients randomised to atomoxetinewere titrated, in 7 days, from 0.5 mg/kg/day (dose ranging from
0.5 to 0.8 mg/kg/day) to the target dose of 1.2 mg/kg/day (rangefrom 1.0 to 1.4 mg/kg/day), to be administered for the first
2.1. Patient population
8 weeks of the study once daily in the morning. In case of onset offatigue or somnolence during the day, the investigator coulddecide to administer the dose in the evening.
The study group included patients of both sexes aged 6–15 years,
d) Study period IV (long-term, open-label extension phase). This was
with ADHD and ODD diagnosed according to the DSM-IV criteria. The
an optional, open-label, long-term extension phase for patients
Kiddie Schedule for Affective Disorders and Schizophrenia for School
who had completed study period III. At the end of the 8-week
Aged Children-Present and Lifetime Version (K-SADS-PL), a semi-
double blind period, all patients had the choice to receive open
structured diagnostic interview that includes supplements for
label atomoxetine treatment for a long-term period until the drug
affective disorders, anxiety, and behavioural disorders (including
became commercially available. During this phase information on
ADHD) was completed at screening for each subject.
efficacy, health outcomes and safety were collected.
To be eligible in the study, patients were required to have a score
of at least 1.5 SD above the age norm for the ADHD subscale of theSNAP-IV, a CGI-S ≥4 at both screening and baseline, a SNAP-IV ODD
The study periods I–III included 14 visits: visit 1 was the screening
subscale score of at least 15, and a normal intelligence, i.e. a score
visit, weekly visits were placed during the study period II (parent
of ≥70 on an Intelligence Quotient (IQ) test. Patients with any of
support phase, visits 2–8) and during the initial 4 weeks of the phase
the following conditions were excluded from study participation:
III (randomised double blind phase, visits 8–12); the remaining visits
body weight b20 kg; history of bipolar I or II disorder, or history of
(13 and 14) took place every 2 weeks. As study period IV is already
psychosis or pervasive development disorder; history of any seizure
ongoing, this article refers to data measured up at the end of the
disorder (other than febrile seizures) or past/concomitant intake of
randomised double blind phase.
anticonvulsants for seizure control; serious risk of suicide; history of
Antipsychotics, antidepressants, anticonvulsants, anorexics,
severe drug allergies; current or past (within 3 months) alcohol or
anticoagulant, benzodiazepines and monoamine oxidase inhibitors
drug abuse; clinically significant cardiovascular disease (including
were not permitted at any time during the study. Concomitant
hypertension) or other conditions that could be worsened by an
administration of CYP2D6 inhibitors was not permitted, and in any
increased heart rate or increased blood pressure; clinically signifi-
case they could be used only after consultation and permission of

Treatment with atomoxetine of children and adolescents with ADHD and ODD
Study design and study phases.
study staff physicians. Formal individual or family psychotherapy
rated instrument measures presence and severity of depression.
was excluded for the entire duration of the study.
A total score below 20 indicates an absence of depression, a scoreof 20–30 indicates borderline depression and a score of 40–60indicates moderate depression. The SCARED-Parent Version is a 41-
2.3. Outcome measures
item parent self-report questionnaire (),which measures symptoms of DSM-IV linked anxiety disorders in
Efficacy variables were measured at the start and at the end of
children, aimed at screening for panic disorder, general anxiety
period I–III. The primary efficacy measure for this study is the 18
disorder, separation anxiety disorder, social phobia, and the
items of the ADHD subscale score of the SNAP-IV. The SNAP-IV is a 26-
presence of a relevant simple phobia, and the school phobia in
item scale (0–3 score for each item) that includes 1 item for each of
clinical () and community samples (
the 18 symptoms contained in the DSM-IV diagnosis of ADHD and 1
item for each of the 8 symptoms contained in the DSM-IV diagnosis of
The Health Related Quality of Life (HRQOL) was measured by
ODD. The SNAP-IV is a widely used measure of the symptoms of ADHD
means of the Child Health and Illness Profile-Child Edition (CHIP-CE).
and ODD. It has been validated and normalized in a sample of school-
It is a 76-item parent-rated assessment of a child's health status and
aged children from the US (
level of functioning (and examines the following
The SNAP-IV yields scores in three domains: inattention (items 1–9),
domains and sub domains: satisfaction (satisfaction with health,
hyperactivity/impulsivity (items 10–18), and oppositional (items
satisfaction with self), comfort (physical comfort, emotional
19–26). The inattention, hyperactivity/impulsivity, and combined
comfort, limitation of activity), risk avoidance (individual risk
scores were considered as the primary efficacy measure while the
avoidance, threats to achievement, peer influences), resilience
ODD subscale score was considered a secondary measure in this
(family involvement, physical activity, social problem solving), and
achievement (academic performance, peer relations). Most of the
The CGI-S ) was used to assess the severity of the
items assess frequency of activities or feelings using a 5-point
patient's ADHD symptoms, in relation to clinician's total experience
response format.
of ADHD patients, on a 7-point scale (from 1 = normal, not ill at all, to
Adverse events were recorded at any time during the study. Body
7 = among the most extremely ill patients).
weight, height, heart rate and blood pressure were measured at
Other outcome measures of the study included the Conners'
screening and at any visit during the randomised phase.
Parent Rating Scale-Revised: Short Form (CPRS-R:S) and the Conners'Teacher Rating Scale-Revised: Short Form (CTRS-R:S). The CPRS-R:S) is a 27-item rating scale completed by the parents to
2.4. Statistical analysis
assess problem behaviours related to ADHD. The CTRS-R:S is a 28-item rating scale completed by a teacher to assess
The sample size was based on the primary outcome variable, i.e. the
problem behaviours related to ADHD in the school setting. Both
18 items of the ADHD subscale score of the investigator-rated SNAP-
scales include the oppositional, cognitive problems, hyperactivity
IV. Using an estimate of the common standard deviation of 13 points,
ADHD Index subscales. In the cases of administration outside of the
a sample of 130 patients (in a 3:1 ratio atomoxetine:placebo) gave
school sessions, the CTRS-R:S had to be administered at the earliest
about 80% power to detect a difference between groups of 8 points
next time point of school-time or at the last visit prior to the school
on the SNAP-IV, that can be considered as clinically significant. The
break in the final assessment.
sample size was determined using a two-sided test with alpha = 0.05
Patients' depression and anxiety were scored by means of the
and assumed that up to 10% of patients discontinued the study
Children's Depression Rating Scale-Revised (CDRS-R) and the Screen
without providing post-baseline efficacy data in the randomised
for Child Anxiety Related Emotional Disorders (SCARED)-Parent
Version, respectively. The CDRS-R ()
The analysis of primary and secondary efficacy endpoints, and of
was based on the Hamilton Depression Rating Scale (HAMD) for
vital signs, was carried out using an analysis of covariance (ANCOVA)
adults, but also includes questions about school. This clinician-
model on the last observation carried forward (LOCF) change from
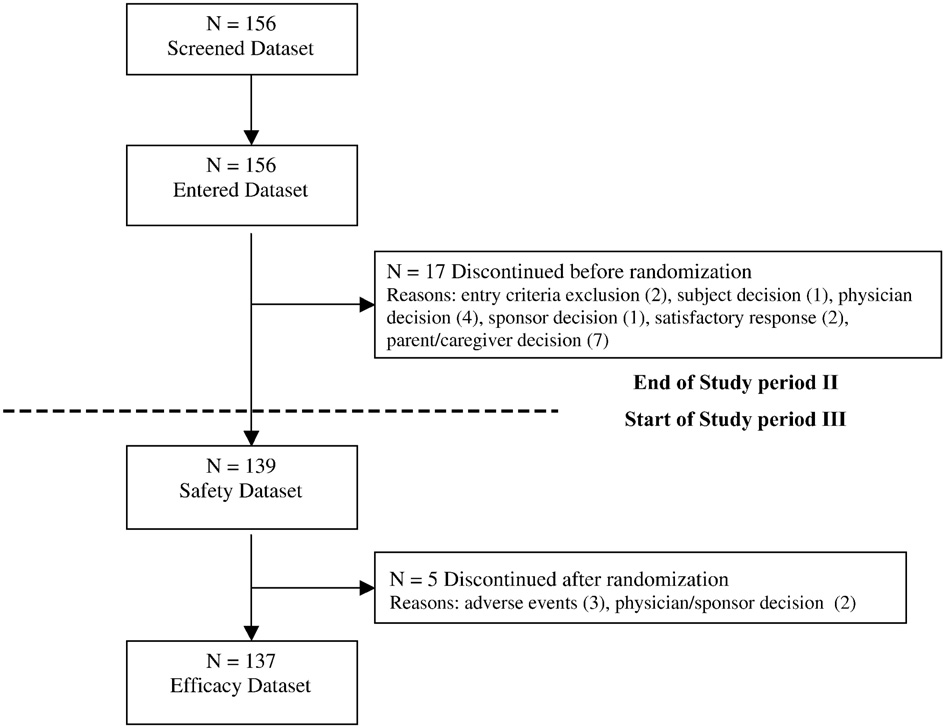
G. Dell'Agnello et al.
Disposition of patients in the study.
baseline to endpoint, in the double blind randomised phase of the
therapy. ADHD diagnosis and anxiety/affective diagnoses at
study. The baseline score was included in the model as one of the
baseline, according with DSM IV, are summarized in .
covariates. The results of the ADHD subscale were also expressed as
The mean (± standard deviation) starting atomoxetine dose
response rate, where response was defined as at least 25%, 30% or
was 0.61 ± 0.08 mg/kg/day (range 0.44–0.80) and was titrated
40% improvement (reduction) from the start to the end of the
to 1.10 ± 0.13 mg/kg/day (range 0.85–1.33) at the end of the
randomised treatment phase of the study.
randomised phase of the study, a dose slightly below the one
Raw scores of CHIP-CE were converted in T scores based on
established standardized scores (mean of a healthy population, 50;
recommended (SPC Strattera).
standard deviation from this mean, 10) ().
Adverse events were coded using the MedDRA dictionary. Events
were considered treatment emergent adverse events (TEAE) if they
started or worsened after the first intake of study medicationcompared to the pre-baseline period. Rates of patients with TEAE in
A slight non-significant decrease in all SNAP-IV subscales scores
the double blind phase of the study were compared between groups
was observed during the parent support phase: the mean scores
using the Fisher's exact test.
at the start and at the end of this phase were, respectively, 43.3 ±6.6 and 42.1 ± 6.9 for the ADHD subscale (i.e. the 18 items ofinattention, hyperactivity/impulsivity, and combined domains),
A total of 156 patients (mean age: 9.9 years, 92.9% males) werescreened and entered the parent support phase. The patients'
Demographic data by treatment group at baseline.
disposition is shown in Seventeen patients discontinuedthe study during the parent support phase, before randomization
(mainly due to patient/caregiver's or Investigator's decision).
All 139 remaining patients were analysed for safety and 137
Demographic data:
for efficacy (two did not have post-baseline data). In the
atomoxetine group, 5 patients discontinued the study during
mean ± SD (range)
the double blind randomised phase. Only two patients (1.3% of
screened) responded to the parent psychological intervention
and were not randomised.
Patients' demographics are shown in No statisti-
mean ± SD (range)
cally significant differences between groups were found at
baseline (visit 8). Previous psychotherapy (of any type) was
mean ± SD (range) (110–174)
used in 18 patients (17.1%) in the atomoxetine group and in 6
(18.8%) in the placebo group, while 21 (20.0%) and 4 (12.5%)
mean ± SD (range)
patients, respectively in the two groups, used previous drug
Treatment with atomoxetine of children and adolescents with ADHD and ODD
DSM-IV ADHD diagnosis and anxiety/affective
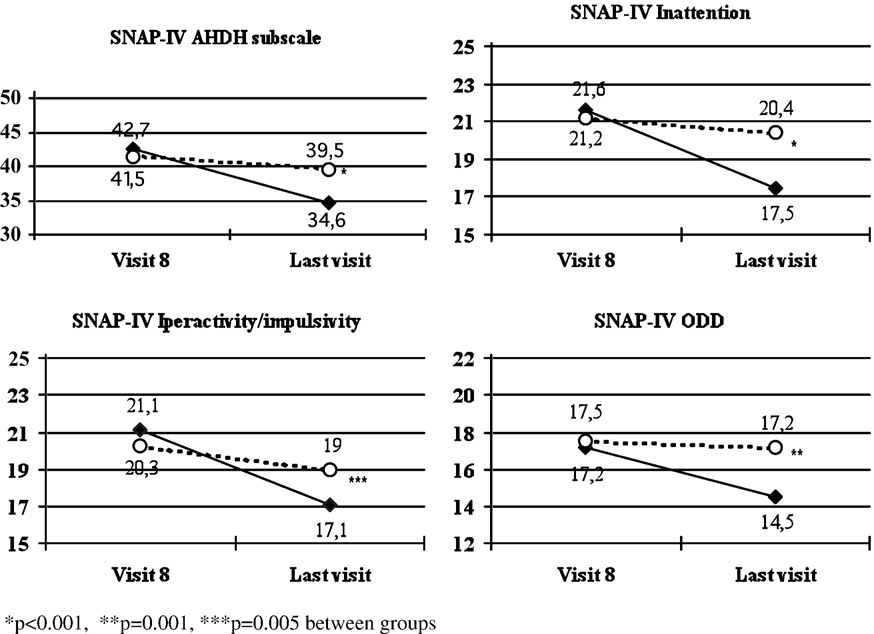
During the randomized phase, the mean changes from visit 8
diagnoses at baseline.
to the last visit in the ADHD subscale were −8.1 ± 9.2 and −2.0 ±4.7, respectively in the atomoxetine and in the placebo group
(p b 0.001 between groups). The corresponding changes in the
ODD subscale were −2.7 ± 4.1 and −0.3 ± 2.6, respectively in the
DSM-IV ADHD subtype
two groups (p = 0.001 between groups) (An analysis in
Inattentive, number (%)
response rate, defined as at least 25%, 30% or 40% improvement
Hyperactive, number (%)
(reduction) from visit 8 to the last visit in SNAP-IV ADHD
Combined, number (%)
subscale score, showed statistically significant differences
Age at onset of ADHD symptoms,
between groups, in favour of atomoxetine compared to
years, mean ± SD (range)
placebo, in 25% response (39.0% vs. 9.4%, respectively in thetwo groups, p = 0.001), in 30% response (31.4% vs. 6.3%,
Anxiety diagnoses from K-SADS:
p = 0.004) and in 40% response (18.1% vs. 3.1%, p = 0.043). A
Generalised anxiety disorders,
decrease of mean scores from visit 8 to the last visit was also
observed in any subscale for atomoxetine treated patients,
compared to no substantial changes with placebo (p b 0.001
between groups for inattention and p = 0.005 for hyperactivity/
Panic disorder, number (%)
impulsivity) ).
Separation anxiety disorder,
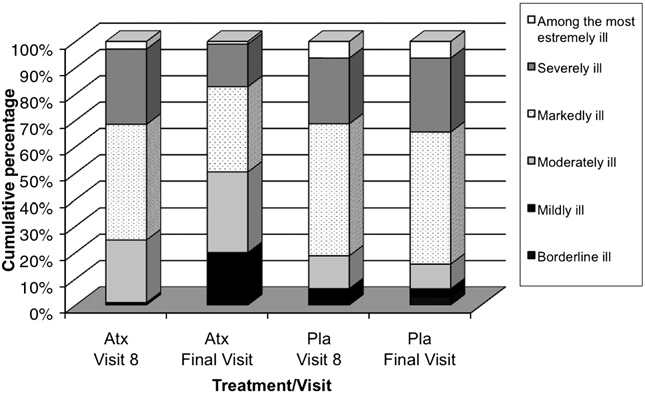
The median CGI-ADHD-S score did not change from the start to
the end (median score: 5.0 at both visits) of the parent support
Specific phobias, number (%)
phase. A statistically significant decrease in the atomoxetinegroup was observed during the randomised double-blind phase
Affective diagnoses from K-SADS:
(median change at endpoint: −1.0), compared to no changes in
Adjustment disorder, number (%)
the placebo group (p b 0.001 between groups). The cumulative
Dysthymia, number (%)
distribution of CGI-ADHD-S scores shows that patients in the
Major depressive disorders,
placebo group have a similar distribution between baseline and
last visit, while almost 50% of patients treated with atomoxetine
Seasonal pattern disorders,
were moderately ill or mildly ill at last visit
The results of the CPRS-R:S and the CTRS-R:S in the parent
Any other depressive disorders,
support phase showed small decreases from baseline in all
subscales (except for unchanged mean values in the cognitiveproblems subscale in the CTRS-R:S): the mean changes in theADHD index were −1.5 in the CPRS-R:S and −1.1 in the CTRS-R:S.
The results of the CPRS-R:S and the CTRS-R:S in the randomised
21.9 ± 3.3 and 21.3 ± 3.6 for the inattention subdomain, 21.4 ± 4.2
phase are summarised in An improvement in all CPRS-R:S
and 20.8 ± 4.3 for the hyperactivity/impulsivity subdomain, and
and CTRS-R:S subscales was observed following treatment with
18.1 ± 2.5 and 17.2 ± 3.3 for the ODD subdomain.
atomoxetine, except in the cognitive problems subscale in the
Results of the SNAP-IV subscales during the randomised double blind phase (atomoxetine: full diamonds; placebo: empty
circles). Values are mean scores.
G. Dell'Agnello et al.
(+ 3.3) and peer relations (+ 2.1). The comparisons betweengroups showed statistically significant differences, in favour ofatomoxetine, for risk avoidance domain (p = 0.013), and foremotional comfort (p = 0.007) and individual risk avoidance(p = 0.007) subdomains.
shows the TEAEs reported in at least 5% of patients in anygroup during the randomised double blind phase of the study. Themost commonly involved system organ classes by MedDRAdictionary were gastrointestinal disorders (mainly nausea,vomiting and abdominal pain), which were reported in 45.8%
Frequency of CGI-ADHD-S score by treatment group —
of patients in the atomoxetine group and 21.9% in the placebo
study period III.
group, and metabolism and nutrition disorders (anorexia/decreased appetite), which were reported in 43.0% and 9.4% ofpatients, respectively in the two groups.
CTRS-R:S. Statistically significant differences vs. placebo were
Almost all adverse events (except in 5 cases) were of mild or
found in all subscales of the CPRS-R:S and in the oppositional
moderate severity and only 3 patients treated with atomoxetine
subscale of the CTRS-R:S, while the p value was at the limit of
discontinued the study due to adverse events. No serious
significance level in the hyperactivity subscale and in the AHDH
adverse events were reported in both groups. No substantial
index of the CTRS-R:S.
changes of mean body weight and height were observed during
The mean total scores of CDRS-R and SCARED were below the
the parent support phase, while the results in the randomised
clinical threshold at baseline and did not change in the parent
phase showed a small increase (+ 0.5 kg) of body weight with
support phase: the mean changes from baseline to the end of
placebo and a small decrease (−1.2 kg) with atomoxetine
this phase were −0.6 ± 4.1 and −0.3 ± 7.5, respectively. The
(p b 0.001), as well as mean height increased slightly more
mean changes of CDRS-R total score from visit 8 to the last visit
markedly in the placebo group (+ 1.5 cm) than in the
were −0.5 ± 4.4 in the atomoxetine group and −0.1 ± 5.0 in the
atomoxetine group (+ 1.0 cm) (p = 0.021). The mean changes in
placebo group (p = 0.870 between groups). The corresponding
vital signs from visit 8 to the end of randomised phase in the
changes of SCARED were −2.1 ± 7.6 and −1.7 ± 6.5, respectively
atomoxetine group and in the placebo group were, respectively,
in the two groups (p = 0.836 between groups).
1.0 and 5.1 mmHg in systolic blood pressure (p = 0.482), −0.2
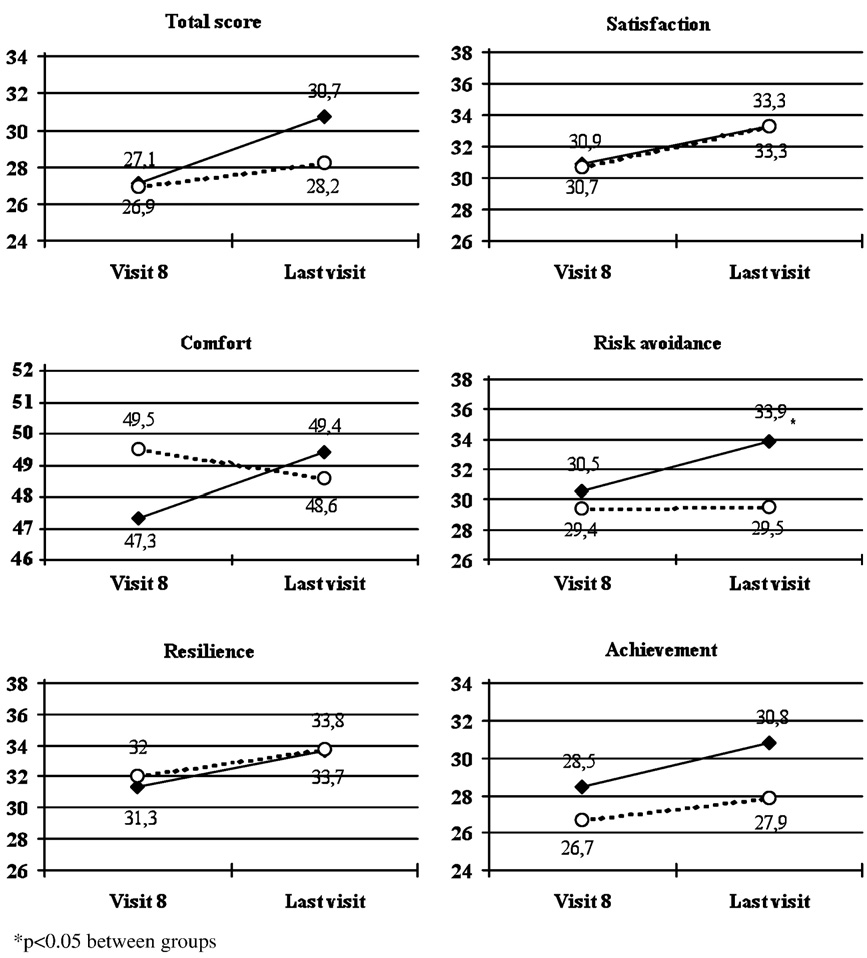
The mean CHIP-CE total, domain and subdomain scores did
and 2.3 mmHg in diastolic blood pressure (p = 0.557), and 3.7
not change during the parent support phase. shows the
and 1.5 bpm in heart rate (p = 0.312). No difference was ob-
CHIP-CE total and domain T scores during the randomised
served in body temperature, between study groups at any time
double blind phase. The mean changes of CHIP-CE total score
and at endpoint.
from visit 8 to the last visit were 3.6 with atomoxetine and 1.2with placebo (p = 0.071 between groups). Improvements in meanscores of all domains were observed in the atomoxetine group.
The results of CHIP-CE subdomains in the atomoxetine groupshowed marked improvements from visit 8 to the last visit in
ADHD treatment guidelines suggest that pharmaco-therapy
satisfaction with self (mean change: + 4.2), emotional comfort
should be used as part of a multi-modal treatment package
(+ 2.1), individual risk avoidance (+ 2.7), threats to achievement
including parent training, family or school interventions
Results of the CPRS-R:S and the CTRS-R:S in the randomised double blind phase.
Cognitive problems
Cognitive problems
Values are means ± standard deviation. p values refer to comparisons between groups in changes from visit 8 to the last visit.
Treatment with atomoxetine of children and adolescents with ADHD and ODD
CHIP-CE total and domains scores during the randomised double blind phase (atomoxetine: full diamonds; placebo: empty
circles). Values are mean scores.
and psychotherapy. In Europe, pharmaco-therapy is largely
significant compared to placebo not only in the primary
through psycho-stimulant medications such as methylpheni-
variable 18 items of the ADHD subscale score of the SNAP-IV,
date and dexamphetamine NICE,
but also in each of the three examined domains (inattention,
Although the Italian guidelines of diagnosis and treatment of
hyperactivity/impulsivity and oppositional). A significantly
ADHD recommend the use of pharmaco-therapy for children
higher number of patients on atomoxetine compared to
and adolescents with severe and disabling ADHD (SINPIA,
placebo showed various degrees of response to therapy,
), psychotherapy and psychosocial intervention is still
consistently with the improvement observed in the ADHD
the main (often the only) type of treatment, for both ADHD
and ODD. In this trial only subjects who failed to respond to a
It should be highlighted that, despite that the results did
6-week validated and standardized behavioural management
not show initial response to the psychoeducational phase,
training for the parents of all the eligible patients were
the duration of the parental support phase was relatively
randomised to atomoxetine or placebo.
short and, therefore, potential carry-on positive effects of
This study included only paediatric patients meeting
this training program effect might have been produced
criteria for ADHD and comorbid ODD, and used a wide
during the randomized phase of the study: an informal non-
spectrum of validated outcome measures, including ADHD
structured psychoeductional support to the parents was
symptoms, ODD symptoms, comorbid anxiety and depres-
provided during the pharmacological double blind phase of
sion, problem behaviours related to ADHD (including the
the study. In the present study, treatment with atomoxetine
school setting), and emotional and social well-being of the
or placebo could be considered as an ‘add-on' therapy given
patient and the family.
in combination with the psychoeducational support. This
The manualized psychological intervention with parental
particular design might explain the, minimal or no placebo
support resulted in a very low rate of response and the mean
effects observed in the present study. With this respect, the
values of all efficacy outcome measures of the study did not
results of a recently published study conducted in Europe and
vary in this phase accordingly. In non-responder patients,
Australia (in which treatment with
treatment with atomoxetine was associated with improve-
atomoxetine or placebo of children with ADHD and comorbid
ments of symptoms of both ADHD and ODD, which resulted
ODD was not preceded by psychotherapy, showed that ADHD
G. Dell'Agnello et al.
Treatment-emergent adverse events reported in at least 5% of patients in any group during the randomised double blind
phase of the study.
Atomoxetine (n = 107)
Abdominal pain upper
Decreased appetite
n = number of patients; p values refer to comparisons between groups.
symptoms in the atomoxetine arm improved in a similar
the tricyclics may be mediated by the improvement in
extent to that of the present trial, whereas no significant
symptoms of ADHD. On the other hand, Spencer et al.
differences between atomoxetine and placebo were found
showed that improvements by amphetamine salts on ODD
on ODD symptoms due to a placebo effect. Similarly, in a
subscale of the SNAP-IV can be measured, even in subjects
Northern American study (), atomoxetine
with pure ODD, suggesting that the improvement in symptoms
was effective for the treatment of ADHD symptoms but
of oppositionality may be independent of any change in
appeared to not significantly reduce oppositional symptoms
ADHD symptoms. Moreover, it has also been suggested that
compared to placebo; in another study
environment can also play a crucial role on the effects of ADHD
significant improvements vs. placebo in ADHD, ODD,
medication on ODD symptoms. showed
and quality-of-life measures in patients with concomitant
that methylphenidate appears to improve symptoms of hy-
ODD symptoms were obtained at a dose level (1.8 mg/kg/
peractivity and impulsivity in a traditional classroom setting
day) higher than that of the present trial. It should also be
without significant effects on symptoms of oppositionality.
considered that although severity of ADHD and ODD
Contrarily, in a structured program of therapeutic, educa-
symptoms was similar in all the studies published (Newcorn
tional, and recreational activities, subjects appeared to
et al., ; Kaplan et al., ; Bangs et al., ;
improve with methylphenidate treatment in both their
Biederman et al., and in the present study, patients
hyperactive/impulsive and oppositional symptoms. Taken
of the Newcorn study were older (mean age 11 compared 9 of
together, these findings further suggest the independence of
the other studies), with a higher proportion of inattentive
oppositional and hyperactive/impulsive symptoms to treat-
subtype (about 30% compared to the 10% of other studies)
ment, and the crucial effect that psychoeducation interven-
and with medication administered twice a day, in compari-
tion can play on clinical efficacy of ADHD medication on ODD
son to the single daily dose of the other studies.
In the present study, pre-selected children and adoles-
In the patients with dysthymia,
cents meeting the DSM-IV diagnostic criteria for ADHD (any
generalized or separation anxiety, showed a smaller reduc-
subtype) and ODD were shown to benefit from an 8-week
tion in ODD symptoms during atomoxetine treatment,
treatment with atomoxetine (and significantly compared
although excluding these patients from the overall LOCF
to a placebo control group) not only in ADHD, but also in
analysis had no effect because their small number. In the
oppositional symptoms. Additionally, the effects on ODD
present study, only few patients (approximately 20% of
symptoms were obtained at a mean atomoxetine dose (final
patients in total) presented evidence of comorbid anxiety or
dose: 1.10 ± 0.13 mg/kg/day) that approximates the mean
affective disorders, and the mean baseline scores of CDRS-R
dose used with success in ADHD symptoms in children
and SCARED were indicative of no or borderline symptoms in
and that is recommended
most of the participants. Consequently, the mean total scores
for the maintenance of symptoms' control
of both scales did change neither in the parent support phase,
nor in the randomised treatment phase in both groups.
Patients with ADHD and comorbid oppositional symptoms
The assessment of the problem behaviours related to
exhibit significantly greater ADHD symptom severity and social
ADHD, as measured by parents with the CPRS-R:S, showed that
dysfunction than ADHD patients without such comorbidity
treatment with atomoxetine was associated with improve-
A recent metanalysis (
ments in all subscales (oppositional, cognitive problems,
) suggests that much of the improvement in oppositional
hyperactivity and AHDH index) and significantly compared
symptoms by atomoxetine, pemoline, psychostimulants, or
to placebo. Similarly, patients treated with atomoxetine
Treatment with atomoxetine of children and adolescents with ADHD and ODD
experienced improvements of the problem behaviours related
down during the initial 6 months of treatment, but increases
to ADHD in the school environment (by teacher-rated CTRS-R:
to values close to those predicted by age and gender norms at
S), except in the cognitive problems subscale. Consistently
the end of the 2 years of observation ).
with previous published data these
The results of the open-label extension phase will provide
findings suggest that both parents and teachers are able to
further insight on the long-term safety of atomoxetine on
detect improvements in a wide spectrum of functional
growth and cardiovascular effects.
behaviours, including the school setting.
In conclusion, the results of this study indicate that, in
The mean CHIP-CE total score at the start of the ran-
children and adolescents with ADHD and ODD, who initially
domised phase was indicative of a moderate impairment of
did not respond to short term psychological intervention with
HRQOL, which was lower than that reported in another trial
parental support, the treatment with atomoxetine was
conducted in the UK that used the same scale
associated with improvements on symptoms of both ADHD
). Although this previous study (
and ODD measured by both parents and teachers, as well as
showed greater improvements than those observed in our
in specific aspects of quality of life and general health.
study, caution should be used in the interpretation of the
Atomoxetine exhibited a satisfactory safety profile, with
results due to the open-label design of this study and to
low incidence of dropouts and no serious adverse events
possible cross-cultural differences in the perception of
observed. The results in the open-label extension phase will
quality of life. Moreover, another analysis of effects of
assess whether the benefits observed after 8 weeks of
atomoxetine on HRQOL that used a different scale pointed
treatment are maintained in a long-term exposure to
into evidence that the likelihood of obtaining improvements
in physical functioning correlates with a worse baselineperception of HRQOL (The results of
Role of the funding source
our study showed that an HRQOL improvement was observedin some subdomains; other scores improved but did not reach
The study is fully sponsored by Eli Lilly Italy.
statistical significance maybe because of the small samplesize due to the study design based on SNAP-IV improvement.
Importantly, patients treated with atomexetine greatly
G. Dell'Agnello has written the study protocol, coordinated research
benefited in domains, such as risk avoidance or achievement,
activities and substantially contributed to data analisis and
which are considered as the most impacted ones in this
interpretation. D. Maschietto, A. Pascotto, F. Calamoneri, G. Masi,
patient population ).
P. Curatolo, D. Besana, have given their contribution to study design
The safety results of this study are consistent with those
and data collection. F. Mancini has coordinated study activities and
of previous reports. In all trials atomoxetine resulted safe
A. Rossi has contributed to data analysis and interpretation of the
and well tolerated, with discontinuations among children
results and coordinated publication's activities; L. Poole has
and adolescents due to adverse events typically less than 5%
contributed to data analysis and interpretation of the results. R.
() and with a spectrum of adverse
Escobar has contributed to study design, data interpretation and
events that are mostly mild to moderate and transient
general study coordination. A. Zuddas has substantially contributed
(; see also ). The typology of
to study design, data collection, analysis and interpretation andgeneral study coordination. All authors have critically revised the
adverse events most frequently reported in this study
article and approved the manuscript before submission.
(anorexia, somnolence and gastrointestinal complaints) andtheir severity, coupled with the lack of serious adverseevents, are in line with the known safety profile of
Conflict of interest
atomoxetine in children and adolescents over variable
G. Dell'Agnello, F. Mancini, A. Rossi are full time employees at Eli
extents of exposure
Lilly Italy. L. Poole and R. Escobar are full time employees at Eli Lilly
Atomoxetine has also been previously
& Co. D. Maschietto is a consultant for Eli Lilly and Shire, has
associated with mild increases in blood pressure and pulse
received research grants from Eli Lilly. C.Bravaccio has received
that plateau during treatment and resolve upon discontin-
research grants from Eli Lilly, Shire and Astra Zeneca. F.Calamoneri
uation The noradrenergic activity of
has received research grants from Eli Lilly. G. Masi is a consultant for
atomoxetine accounts for these effects. In this study, the
Eli Lilly and Shire, has received research grants from Eli Lilly, and hasbeen speaker for Eli Lilly and Sanofi-Aventis. D. Besana is a
mean blood pressure did not vary following treatment with
consultant for Eli Lilly, has received research grants from Eli Lilly
atomoxetine, while a small increase in heart rate from the
and Janssen Cilag and has been speaker for Eli Lilly. P. Curatolo is a
start to the end of the randomised phase has been reported
consultant for Eli Lilly, and has received research grants from
in patients receiving atomoxetine, however not significantly
Janssen Cilag and Eli Lilly. D. Besana is a consultant for Eli Lilly, has
compared to placebo.
received research grants from Eli Lilly and Janssen Cilag and has
A small decrease of body weight and a slightly lower
been a speaker for Eli Lilly. A. Zuddas has an advisory or consulting
height gain compared to placebo were observed in patients
relationship with Eli Lilly, Shire, UCB and Astra Zeneca, has received
receiving atomoxetine. This could be an expected finding
research grants from Eli Lilly and Shire, and has been a speaker for
based on previous experiences, which showed that atomox-
Eli Lilly and Sanofi-Aventis.
etine may have a modest initial effect on growth rates.
However, this apparent growth-lowering effect does not
persist on a long-term exposure: a pooled analysis of data onweight and height from 13 trials in children and adolescents
The authors thank Luca Cantini and Andrea Rossi for their support in
treated with atomoxetine at usual doses for at least 2 years
medical writing of this article, Pierluigi Crisà and Roberto Pino for
has shown that mean growth rates may moderately slow
their contribution in the study organization.
G. Dell'Agnello et al.
The LYCY study group is:
effects on quality of life and school functioning. Clin. Pediatr.
Dino Maschietto, Unità Operativa di Neuropsichiatria Infantile,
(Phila) 45, 819–827.
Azienda USL 10 Veneto orientale, San Donà di Piave, Venezia;
Bukstein, O.G., Kolko, D.J., 1998. Effects of methylphenidate on
Carmela Bravaccio, Departimento di Pediatria, Università di Napoli
aggressive urban children with attention-deficit hyperactivity
"Federico II", Napoli; Filippo Calamoneri, Clinica di Neuropsichiatria
disorder. J. Clin. Child. Psychol. 27, 340–351.
Infantile, Policlinico Universitario, Messina; Gabriele Masi, Divisione
Camerini, G., Coccia, M., Caffo, E., 1996. Il Disturbo da Deficit
di Neuropsichiatria Infantile, IRCCS Fondazione Stella Maris,
dell'Attenzione-Iperattività: analisi della frequenza in una
Calambrone, Pisa; Antonio Condini, Unità Operativa Autonoma di
popolazione scolastica attraverso questionari agli insegnanti.
Neuropsichiatria infanzia e adolescenza ULSS 16, Padova; Amerigo
Psichiatria. dell'infanzia. e. dell'adolescenza 63, 587–594.
Zanella, IRCCS Eugenio Medea, San Vito al Tagliamento, Pordenone;
Christman, A.K., Fermo, J.D., Markowitz, J.S., 2004. Atomoxetine, a
Giovanni Lanzi, Fondazione Mondino, Dipartimento di Clinica
novel treatment for attention-deficit–hyperactivity disorder.
Neurologica e Psichiatrica dell'Età Evolutiva, Pavia; Lucia Margari,
Pharmacotherapy 24, 1020–1036.
Clinica Neurologica 2°, Sezione di Neuropsichiatria Infantile,
Conners, C.K., 1997. Conners' Rating Scales-Revised, Technical
Policlinico di Bari, Bari; Paolo Curatolo, Neuropsichiatria Infantile,
Manual. MultiHealth Systems Inc, Toronto.
Policlinico di Tor Vergata, Clinica Sant'Alessandro, Roma; Edwige
Connor, D., Glatt, S.J., Lopez, I.D., Jackson, D., Melloni, R.H., 2002.
Veneselli, Ospedale Giannina Gaslini, Genova; Dante Besana,
Psychopharmacology and aggression: I: a meta-analysis of
Struttura Operativa Complessa di Neuropsichiatria Infantile, Ospe-
stimulant effects on overt/covert aggression-related behaviors
dale Infantile, Azienda Ospedaliera Nazionale di Alessandria,
in ADHD. J. Am. Acad. Child. Adolesc. Psychiatry 41, 253–262.
Alessandria; Anna Fabrizi, Dipartimento di Scienze Neurologiche e
Corman, S.L., Fedutes, B.A., Culley, C.M., 2004. Atomoxetine: the
Psichiatriche dell'Età Evolutiva, Università La Sapienza, Roma;
first nonstimulant for the management of attention-deficit/
Alessandro Zuddas, Clinica di Neuropsichiatria Infantile, Azienda
hyperactivity disorder. Am. J. Health. Syst. Pharm. 61, 2391–2399.
Ospedaliero-Universitaria di Cagliari, Cagliari.
Escobar, R., Soutullo, C.A., Hervas, A., Gastaminza, X., Polavieja,
P., Gilaberte, I., 2005. Worse quality of life in children newlydiagnosed with Attention Deficit/Hyperactivity Disorder (ADHD)
compared with asthmatic and healthy children. Pediatrics 116,e364–369.
Aman, M.G., Kern, R.A., Osborne, P., Tumuluru, R., Rojahn, J., del
Fergusson, D.M., Horwood, L.J., Lynskey, M.T., 1993. The effects of
Medico, V., 1997. Fenfluramine and methylphenidate in children
conduct disorder and attention deficit in middle childhood on
with mental retardation and borderline IQ: clinical effects. Am.
offending and scholastic ability at age 13. J. Child. Psychol.
J. Ment. Retard. 101, 521–534.
Psychiatry 34, 899–916.
Bangs, M.E., Emslie, G.J., Spencer, T.J., Ramsey, J.L., Carlson, C.,
Fergusson, D.M., Lynskey, M.T., Horwood, L.J., 1997. Attentional
Bartky, E.J., Busner, J., Duesenberg, D.A., Harshawat, P.,
difficulties in middle childhood and psychosocial outcomes in
Kaplan, S.L., Quintana, H., Allen, A.J., Sumner, C.R., 2007.
young adulthood. J. Child. Psychol. Psychiatry 38, 633–644.
Efficacy and safety of atomoxetine in adolescents with attention-
Gallucci, F., Bird, H.R., Berardi, C., Gallai, V., Pfanner, P.,
deficit/hyperactivity disorder and major depression. J. Child.
Weinberg, A., 1993. Symptoms of attention-deficit hyperactivity
Adolesc. Psychopharmacol. 17, 407–420.
disorder in an Italian school sample: findings of a pilot study. J.
Bangs, M.E., Hazell, P., Danckaerts, M., Hoare, P., Coghill, D.R.,
Am. Acad. Child. Adolesc. Psychiatry 32, 1051–1058.
Wehmeier, P.M., Williams, D.W., Moore, R.J., Levine, L., 2008.
Gaub, M., Carlson, C.L., 1997. Behavioral characteristics of DSM-IV
Atomoxetine for the treatment of attention-deficit/hyperactivity
ADHD subtypes in a school-based population. J. Abnorm. Child.
disorder and oppositional defiant disorder. Pediatrics 121, e314–320.
Psychol. 25, 103–111.
Barkley, R.A., Fischer, M., Smallish, L., Fletcher, K., 2004. Young
Geller, D., Donnelly, C., Lopez, F., Rubin, R., Newcorn, J., Sutton,
adult follow-up of hyperactive children: antisocial activities and
V., Bakken, R., Paczkowski, M., Kelsey, D., Sumner, C., 2007.
drug use. J. Child. Psychol. Psychiatry 45, 195–211.
Atomoxetine treatment for pediatric patients with attention-
Barkley, R.A., Fischer, M., Smallish, L., Fletcher, K., 2006. Young
deficit/hyperactivity disorder with comorbid anxiety disorder.
adult outcome of hyperactive children: adaptive functioning in
J. Am. Acad. Child. Adolesc. Psychiatry 46, 1119–1127.
major life activities. J. Am. Acad. Child. Adolesc. Psychiatry 45,
Guy, W., 1976. ECDEU assessment manual for psychopharmacology,
revised. Bethesda (MD), US Dept. of Health, Education and Welfare.
Biederman, J., Heiligenstein, J.H., Faries, D.E., Galil, N., Dittmann,
Hazell, P., Zhang, S., Wolanczyk, T., Barton, J., Johnson, M., Zuddas,
R., Emslie, G.J., Kratochvil, C.J., Laws, H.F., Schuh, K.J., 2002.
A., Danckaerts, M., Ladikos, A., Benn, D., Yoran-Hegesh, R.,
Efficacy of atomoxetine versus placebo in school-age girls with
Zeiner, P., Michelson, D., 2006. Comorbid oppositional defiant
attention-deficit/hyperactivity disorder. Pediatrics 110, e75.
disorder and the risk of relapse during 9 months of atomoxetine
Biederman, J., Spencer, T.J., Newcorn, J.H., Gao, H., Milton, D.R.,
treatment for attention-deficit/hyperactivity disorder. Eur.
Feldman, P.D., Witte, M.M., 2007. Effect of comorbid symptoms
Child. Adolesc. Psychiatry 15, 105–110.
of oppositional defiant disorder on responses to atomoxetine in
Hinshaw, S.P., Hencker, B., Whalen, C.K., Erhardt, D., Dunnington
children with ADHD: a meta-analysis of controlled clinical trial
Jr, R.E., 1989. Aggressive, prosocial and non-social behavior in
data. Psychopharmacology 190, 31–41.
hyperactive boys: dose effects of methylphenidate in naturalistic
Birmaher, B., Khetarpal, S., Brent, D., Cully, M., Balach, L., Kaufman,
settings. J. Consult. Clin. Psychol. 57, 636–643.
J., Neer, S.M., 1997. The Screen for Child Anxiety Related
Hinshaw, S.P., Heller, T., Mc Hale, J.P., 1992. Covert antisocial
Emotional Disorders (SCARED): scale construction and psycho-
behavior in boys with attention-deficit hyperactivity disorder:
metric characteristics. J. Am. Acad. Child. Adolesc. Psychiatry 36,
external validation and effects of methylphenidate. J. Consult.
Clin. Psychol. 60, 274–281.
Birmaher, B., Brent, D., Chiappetta, L., Bridge, J., Monga, S.,
Kaplan, S., Heiligenstein, J., West, S., Busner, J., Harder, D.,
Baugher, M., 1999. Psychometric properties of the Screen for
Dittmann, R., Casat, C., Wernicke, J.F., 2004. Efficacy and safety
Child Anxiety Related Emotional Disorders Scale (SCARED): a
of atomoxetine in childhood attention-deficit/hyperactivity
replication study. J. Am. Acad. Child. Adolesc. Psychiatry 38,
disorder with comorbid oppositional defiant disorder. J. Atten.
Disord 8, 45–52.
Brown, R.T., Perwien, A., Faries, D.E., Kratochvil, C.J., Vaughan, B.S.,
Kelsey, D.K., Sumner, C.R., Casat, C.D., Coury, D.L., Quintana, H.,
2006. Atomoxetine in the management of children with ADHD:
Saylor, K.E., Sutton, V.K., Gonzales, J., Malcolm, S.K., Schuh, K.J.,
Treatment with atomoxetine of children and adolescents with ADHD and ODD
Allen, A.J., 2004. Once-daily atomoxetine treatment for children
Perwien, A.R., Faries, D.E., Kratochvil, C.J., Sumner, C.R., Kelsey,
with attention-deficit/hyperactivity disorder, including an assess-
D.K., Allen, A.J., 2004. Improvement in health-related quality of
ment of evening and morning behavior: a double-blind, placebo-
life in children with ADHD: an analysis of placebo controlled
controlled trial. Pediatrics 114, 1–8.
studies of atomoxetine. J. Dev. Behav. Pediatr. 25, 264–271.
Kirley, A., Lowe, N., Mullins, C., McCarron, M., Daly, G., Waldman,
Perwien, A.R., Kratochvil, C.J., Faries, D.E., Vaughan, B.S., Spencer,
I., Fitzgerald, M., Gill, M., Hawi, Z., 2004. Phenotype studies of
T., Brown, R.T., 2006. Atomoxetine treatment in children and
the DRD4 gene polymorphisms in ADHD: association with
adolescents with attention-deficit hyperactivity disorder: what
oppositional defiant disorder and positive family history. Am. J.
are the long-term health-related quality-of-life outcomes?
Med. Genet. B. Neuropsychiatr. Genet 131, 38–42.
J. Child. Adolesc. Psychopharmacol. 16, 713–724.
Klein, R.G., Abikoff, H., Klass, E., Ganeles, D., Seese, L.M., Pollack,
Polanczyk, G., de Lima, M.S., Horta, B.L., Biederman, J., Rohde, L.A.,
S., 1997. Clinical efficacy of methylphenidate in conduct disorder
2007. The worldwide prevalence of ADHD: a systematic review and
with and without attention-deficit hyperactivity disorder. Arch.
metaregression analysis. Am. J. Psychiatry 164, 942–948.
Gen. Psychiatry 54, 1073–1080.
Poznanski, E., Mokros, H.B., 1999. Children's Depression Rating
Kolko, D.J., Bukstein, O.G., Barron, J., 1999. Methylphenidate and
Scale, Revised. Western Psychological Services, Los Angeles.
behavior modification in children with ADHD and comorbid ODD
Prasad, S., Harpin, V., Poole, L., Zeitlin, H., Jamdar, S., Puvanendran,
or CD: main and incremental effects across settings. J. Am. Acad.
K., The SUNBEAM Study Group, 2007. A multi-centre, randomised,
Child. Adolesc. Psychiatry 38, 578–586.
open-label study of atomoxetine compared with standard current
Kuhne, M., Schachar, R., Tannock, R., 1997. Impact of comorbid
therapy in UK children and adolescents with attention-deficit/
oppositional or conduct problems on attention-deficit hyper-
hyperactivity disorder (ADHD). Curr. Med. Res. Opin. 23, 379–394.
activity disorder. J. Am. Acad. Child. Adolesc. Psychiatry 36,
Ralston, S.J., Lorenzo, M.J., 2004. ADORE — Attention-Deficit
Hyperactivity Disorder Observational Research in Europe. Eur.
Lavigne, J.V., Cicchetti, C., Gibbons, R.D., Binns, H.J., Larsen, L.,
Child. Adolesc. Psychiatry. 13 (Suppl 1), 36–42.
De Vito, C., 2001. Oppositional defiant disorder with onset in
Riley, A.W., Forrest, C.B., Starfield, B., Karig, M., Ensminger, M.E.,
preschool years: longitudinal stability and pathways to other
1998. A taxonomy of adolescent health need: reliability and
disorders. J. Am. Acad. Child. Adolesc. Psychiatry 40,
validity of the adolescent health and illness profiles. Med. Care
36, 1237–1248.
Michelson, D., Faries, D., Wernicke, J., Kelsey, D., Kendrick, K.,
Riley, A.W., Forrest, C.B., Starfield, B., Rebok, G.W., Robertson, J.A.,
Sallee, F.R., Spencer, T., 2001. Atomoxetine in the treatment of
Green, B.F., 2004. The parent report form of the CHIP-Child
children and adolescents with attention-deficit/hyperactivity
Edition: reliability and validity. Med. Care 42, 210–220.
disorder: a randomized, placebo-controlled, dose–response
Satake, H., Yamashita, H., Yoshida, K., 2004. The family psychosocial
study. Pediatrics 108, E83.
characteristics of children with attention-deficit hyperactivity
Michelson, D., Allen, A.J., Busner, J., Casat, C., Dunn, D., Kratochvil,
disorder with or without oppositional or conduct problems in
C., Newcorn, J., Sallee, F.R., Sangal, R.B., Saylor, K., West, S.,
Japan. Child. Psychiatry Hum. Dev. 34, 219–235.
Kelsey, D., Wernicke, J., Trapp, N.J., Harder, D., 2002. Once-
Società Italiana di NeuroPsichiatria dell'Infanzia e dell'Adolescenza
daily atomoxetine treatment for children and adolescents with
(SINPIA), 2004. Linee guida per il disturbo da deficit attentivo
attention deficit hyperactivity disorder: a randomized, placebo-
con iperattività (ADHD) in età evolutiva. I: Diagnosi e terapia
controlled study. Am. J. Psychiatry 159, 1896–1901.
farmacologica. Giornale di Neuropsichiatria dell'età evolutiva 24
MTA Cooperative Group, 1999. Moderators and mediators of treat-
(Suppl. 1), 41–87.
ment response for children with attention-deficit/hyperactivity
Spencer, T., Biederman, J., Wilens, T., Prince, J., Hatch, M., Jones, J.,
disorder. Arch. Gen. Psychiatry 56, 1088–1096.
Harding, M., 1998. Effectiveness and tolerability of atomoxetine
Mugnaini, D., Masi, G., Brovedani, P., Chelazzi, C., Matas, M.,
in adults with attention deficit hyperactivity disorder. Am. J.
Romagnoli, C., Zuddas, A., 2006. Teacher reports of ADHD
Psychiatry 155, 693–695.
symptoms in Italian children at the end of first grade. Eur.
Spencer, T.J., Newcorn, J.H., Kratochvil, C.J., Ruff, D., Michelson,
Psychiatry 21, 419–426.
D., Biederman, J., 2005. Effects of atomoxetine on growth after
Muris, P., Merckelbach, H., Brakel, A., Mayer, B., Dongen, L., 1999.
2-year treatment among pediatric patients with attention-
The Revised Screen for Child Anxiety Related Emotional Disorders
deficit/hyperactivity disorder. Pediatrics 116, 74–80.
(SCARED-R): factor structure in normal children. Person. Indiv.
Spencer, T.J., Abikoff, H.B., Connor, D.F., Biederman, J., Pliszka, S.R.,
Diff. 26, 99–112.
Boellner, S., Read, S.C., Pratt, R., 2006. Efficacy and safety
National Institute for Clinical Excellence, 2000. Guidance on the use
of mixed amphetamine salts extended release (Adderall XR) in
of methylphenidate (ritalin, equasym) for attention deficit/
the management of oppositional defiant disorder with or without
hyperactivity disorder (ADHD) in childhood. Technology Appraisal,
comorbid attention-deficit/hyperactivity disorder in school-aged
Guidance No. 13.
children and adolescents: a 4-week, multicenter, randomized,
Newcorn, J.H., Spencer, T.J., Biederman, J., Milton, D.R.,
double-blind, parallel-group, placebo-controlled, forced-dose-es-
Michelson, D., 2005. Atomoxetine treatment in children and
calation study. Clin. Ther. 28, 402–418.
adolescents with attention-deficit/hyperactivity disorder and
Steinhausen, H.C., Novik, T.S., Baldursson, G., Curatolo, P., Lorenzo,
comorbid oppositional defiant disorder. J. Am. Acad. Child.
M.J., Rodrigues Pereira, R., Ralston, S.J., Rothenberger, A., 2006.
Adolesc. Psychiatry 44, 240–248.
Co-existing psychiatric problems in ADHD in the ADORE cohort. Eur.
Newcorn, J.H., Michelson, D., Kratochvil, C.J., Allen, A.J., Ruff, D.D.,
Child. Adolesc. Psychiatry 15 (suppl 1), 25–29.
Moore, R.J., Atomoxetine Low-dose Study Group, 2006. Low-dose
Strattera SPC (last updated on the eMC: 20/11/2008)
atomoxetine for maintenance treatment of attention-deficit/
hyperactivity disorder. Pediatrics 118, 1701–1706.
Oosterlaan, J., Scheres, A., Sergeant, J.A., 2005. Which executive
Swanson, J., 1992. School-Based Assessment and Interventions for
functioning deficits are associated with AD/HD, ODD/CD and
ADD. KC Publishing, Irvine, p. 184.
comorbid AD/HD+ODD/CD? J. Abnorm. Child. Psychol. 33, 69–85.
Taylor, E., Dopfner, M., Sergeant, J., Asherson, P., Banaschewski,
Pelham, W.E., Bender, M.E., Caddell, J., Booth, S., Moorer, S.H., 1985.
T., Buitelaar, J., Coghill, D., Danckaerts, M., Rothenberger, A.,
Methylphenidate and children with attention-deficit disorder: dose
Sonuga-Barke, E., Steinhausen, H.C., Zuddas, A., 2004. European
effects on classroom academic and social behavior. Arch. Gen.
clinical guidelines for hyperkinetic disorder — first upgrade. Eur.
Psychiatry 42, 948–952.
Child. Adolesc. Psychiatry 13 (Suppl 1), I7–30.
G. Dell'Agnello et al.
van Lier, P.A., van der Ende, J., Koot, H.M., Verhulst, F.C., 2007.
Wernicke, J.F., Kratochvil, C.J., 2002. Safety profile of atomoxetine
Which better predicts conduct problems? The relationship of
in the treatment of children and adolescents with ADHD. J. Clin.
trajectories of conduct problems with ODD and ADHD symptoms
Psychiatry 63 (Suppl 12), 50–55.
from childhood into adolescence. J. Child. Psychol. Psychiatry
Wernicke, J.F., Faries, D., Girod, D., Brown, J., Gao, H., Kelsey, D.,
48, 601–608.
Quintana, H., Lipetz, R., Michelson, D., Heiligenstein, J., 2003.
Vio, C., Offredi, G., Marzocchi, G.M., 1999. Il bambino con deficit di
Cardiovascular effects of atomoxetine in children, adolescents,
attenzione/iperattività. Trento, Edizioni Erickson.
and adults. Drug. Saf. 26, 729–740.
Weiss, M., Tannock, R., Kratochvil, C., Dunn, D., Velez-Borras, J.,
Zuddas, A., Marzocchi, G.M., Oosterlaan, J., Cavolina, P., Ancilletta,
Thomason, C., Tamura, R., Kelsey, D., Stevens, L., Allen, A.J.,
B., Sergeant, J., 2006. Factor structure and cultural factors of
2005. A randomized, placebo-controlled study of once-daily
disruptive behaviour disorders symptoms in Italian children. Eur.
atomoxetine in the school setting in children with ADHD. J. Am.
Psychiatry 21, 410–418.
Acad. Child. Adolesc. Psychiatry 44, 647–655.
Source: http://cp2012.it/corsi-e-convegni/schede-eventi/atti-convegno-epidemiologia/DellAgnello%20G_2009.pdf
This compilation is for general information only and not to be used for profit or a substitute for yourtraining. Consult a professional. Please respect IP & copyright Laws. Slight variations may occur for layout purposes.Errors and omissions are not intentional, Your Host & Hostess, Chief Dave "Passing Wind" & Faith "D'Sagwagon" Substances: World Anti-Doping Code:
Medicines and Illness Policy (Boarders) 2016-2017 The following protocol has been written using the guidelines from the Handbook of School Health, Boarding Schools National Minimum Standards (April 2015), Supporting students with medical conditions (DfES Sept 2014) We aim to provide guidelines for boarding & teaching staff who find themselves in a position of responsibility regarding the storage and administration of drugs. The aim of this policy is to protect the boarding & teaching staff against any unforeseen liability. Despite the fact that many medicines are available over the counter, the boarding staff are advised by the medical centre staff only to use those which have been prescribed by a doctor or those that have been sanctioned by the school doctor or nursing staff at the medical centre (List below). This is stressed in order to protect not only the students from any errors but to protect the staff. No child under the age of sixteen should be given medicines without their parents' consent. Each child must have a completed medical form prior to starting the school which includes a declaration giving permission for nursing staff, boarding staff or teaching staff to give appropriate treatment for minor problems using non-prescription medicines. This is also authorisation for housemistress or a member of staff to approve such medical treatment as is deemed necessary in an emergency. Parents have a clear responsibility to provide the school with written details of the medicines and medical needs of their daughters. They are also expected to inform the school of any changes as they arise. Please note that if a girl has been accepted into the school without prior notification of health problems that could significantly affect the management of their daughter in the school then the school has the right to review the continuation of the student in the school. When students start or return to school, all drugs and medicines must be given to the housemistress or nurse who will dispense them as prescribed. These medications should be patient named & listed in the British National Formulary and any foreign language must be translated into English. The school medical officer will treat and prescribe for students as necessary whilst the student is in their care as a boarder Sixth Form students (i.e. those over the age of 16) may give their own consent for medical treatment. Gillick competence is used in medical law to decide whether a child (16 years or younger) is able to consent to his or her own medical treatment, without the need for parental permission or knowledge. A child will be Gillick competent if he or she has sufficient understanding and intelligence to understand fully what is proposed. The Medical Centre St. Mary's Medical Centre is staffed by registered nurses (or qualified first aider in their absence) who are available to assist student, provide first aid and advice between the hours of 08.15 – 16.00 Monday to Friday term time only. Should you wish to contact the nurses directly please telephone 01223 224169 between these hours or email











