
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Aime07_final.dvi
Formalizing 'Living Guidelines' using LASSIE:
A Multi-step Information Extraction Method
Katharina Kaiser1 and Silvia Miksch12
1 Institute of Software Technology & Interactive Systems
Vienna University of Technology, Vienna, Austria
2 Department of Information and Knowledge Engineering
Danube University Krems, Krems, Austria
Abstract. Living guidelines are documents presenting up-to-date and state-of-
the-art knowledge to practitioners. To have guidelines implemented by computer-
support they firstly have to be formalized in a computer-interpretable form. Due
to the complexity of such formats the formalization process is challenging, but
burdensome and time-consuming.
The LASSIE methodology supports this task by formalizing guidelines in several
steps from the textual form to the guideline representation language Asbru using
a document-centric approach. LASSIE uses Information Extraction technique to
semi-automatically accomplish these steps.
We apply LASSIE to support the implementation of living guidelines. Based on
a living guideline published by the Scottish Intercollegiate Guidelines Network
(SIGN) we show that adaptations of previously formalized guidelines can be ac-
complished easily and fast. By using this new approach only new and changed
text parts have to be modeled. Furthermore, models can be inherited from previ-
ously modeled guideline versions that were added by domain experts.
The development process for a clinical practice guideline (CPG) takes at least two years.
Thus, CPGs can be out of date as soon as they are produced, as new research findingsare continuously published. To overcome this problem sometimes the shelf life for aguideline is identified; either by a date (e.g., this guideline will be reviewed in 2 years)or by a statement that the review date will be determined by the availability of newevidence (e.g., this guideline will be considered for review as new evidence becomesavailable). Alternatively, we can consider a new option – the living guideline. A livingguideline is one that remains under review on an ongoing basis, with updates publishedat set intervals (e.g., annually).
The review of the guideline (i.e., a new article in the specified field is available) may
have various characteristics. On the one hand it can add additional evidence and thusalter the evidence level of a recommendation. On the other hand it can lead to a newrecommendation or it may change an existing one. However, in the majority of casesonly small text parts are changed; often only the reference to the new article is added orto an obsolete article is removed.
Modeling CPGs in a computer-interpretable form is a prerequisite for various com-
puter applications to support their application. However, transforming guidelines in aformal guideline representation is a difficult task. In [1] and [2] we have proposed asemi-automatic methodology called LASSIE to model treatment processes in multiplesteps using Information Extraction (IE).
We will now show that we can use LASSIE to support the formalization of living
guidelines. Applying this method, which traces both the general formalization steps andthe changes to new versions has the potential to reduce the modeling effort. The ScottishIntercollegiate Guidelines Network (SIGN) has already published a living guideline[3]. Based on the documents provided we will show that adaptations of formalizedguidelines can be accomplished easily and fast.
In the next section we will discuss some work on guideline formalization tools and
guideline versioning methods. Afterwards we will give a short introduction in LASSIE.
In Section 4 we describe the adaptation of LASSIE for supporting living guidelinesfollowed by a case study illustrating our methodology. Section 6 summarizes our workand represents our conclusions.
Related Work
In this section, we present relevant work describing guideline formalization tools andapproaches for guideline versioning.
For formalizing clinical guidelines into a guideline representation language (see
[4] for an overview and comparison) various tools exist. We can classify such tools indocument-centric and model-centric tools.
Markup-based tools utilize a document-centric approach. Thereby, the original guide-line document is systematically marked-up by the user in order to generate a semi-formal model of the marked text part.
The
GEM Cutter [5] was one of the first exponents of this apporach transforming
guideline information into the GEM format [6].
Stepper [7] is a tool that formalizes theinitial text in multiple user-definable steps corresponding to interactive XML transfor-mations. The
Document Exploration and Linking Tool / Addons (DELT/A) [8] supportsthe translation of HTML documents into any XML language. It uses links between thetext part in the original document and its corresponding XML model. To generate aspecific model user-definable
macros can be used.
Uruz, part of the
Digital electronicGuideline Library (Degel) framework [9], is a web-based markup tool that supports in-dexing and markup using any hierarchical guideline-representation format. It enablesthe user to embed in the guideline document terms originating from standard vocabu-laries.
In model-centric approaches a conceptual model is formulated by domain experts. Therelationship between the model and the original document is only indirect.
AsbruView [10] uses graphical metaphors to represent Asbru plans.
AREZZO and
TALLIS [11] support the translation into PROforma using graphical symbols represent-ing the task types of the language.
Prot´eg´e [12] is a knowledge-acquisition tool thatsupports the translation into guideline representation languages EON, GLIF, and PRO-forma. It uses specific ontologies for these languages, whereas parts of the formalizationprocess can be accomplished with predefined graphical symbols. AREZZO, TALLIS,and Prot´eg´e offer a flowchart-based representation of the processes.
Unfortunately, guideline versioning has not been adequately addressed by now. Thereare two approaches dealing with versioning:
Peleg and Kantor [13] propose a model-centric approach for GLIF. Thereby, the
underlying GLIF ontology is extended by version information and a versioning toolwas developed that supports the creation of a new CPG model or the modification ofan existing one as well as the displaying of versions of a CPG model, highlighting thedifferences.
Seyfang et al. [14] describe the formalization of 'living guidelines' using a document-
centric approach. They start with an HTML version of the guideline and use differentintermediate representations to derive a formal model of the guideline. The first inter-mediate representation is MHB and the DELT/A tool is used to mark-up text chunks.
The original marked-up guideline document is then manually updated to the new ver-sion by highlighting both newly added and removed text fragments. Using the DELT/Atool the highlighted text fragments are selected to visualize the corresponding MHBchunk in order to make the necessary changes.
But still, using the mentioned tools the modeling process is complex and labor in-
tensive. Methods are needed to automate parts of the modeling task.
LASSIE – Modeling Treatment Processes Using Information
Extraction
Most guideline representation languages are very powerful and thus very complex. Theycan present a multitude of different information and data. We apply a multi-step trans-formation process that facilitates the formalization process by various intermediate rep-resentations (IRs) obtained in stepwise procedures.
Our multi-step transformation methodology, called LASSIE3, supports the document-
centric approach by marking the original guideline document and generating the partic-ular models for each marked text part. It is intended to be a semi-automatic approach.
This enables the user not only to correct the transformations, but also to augment themby implicit knowledge necessary for a subsequent execution. After each step the user isable to view the results using the DELT/A tool [8].
The benefits of the multi-step approach and in the following of the IRs are that IRs
(1) support a concise formalization process, (2) provide different formats and separate
3 modeLing treAtment proceSSes using Information Extraction
Representations Independent of
the Final Guideline Language
Clinical
Practice
Guideline
Fig. 1. Steps to (semi-)automatically gain an Asbru representation of CPGs. To gain process in-
formation from a CPG the first two steps are accomplished in order to have a representation
independent of the final guideline language.
views and procedures for various kinds of information, (3) specific heuristics for eachparticular kind of information can be applied, and (4) a simpler and more concise evalu-ation and tracing of each process step is accomplishable. The IRs are specific templatesused by IE methods to present the desired information. The IE methods use a termi-nology based on the Medical Subject Headings (MeSH)4 [15] and manually generatedextraction patterns.
CPGs present effective treatment processes. One challenge when authoring CPGs
is the detection of individual processes and their relations and dependencies. We cangenerate simple representations of treatment instructions (i.e., actions), which are in-dependent from the final guideline representation language. Based on this independentrepresentation we can transform the information in further steps into the guideline lan-guages. In [1] and [2] we have demonstrated that it is possible to formalize processesusing IE for modeling guidelines in Asbru (see Fig. 1).
Adaptation of LASSIE for 'Living Guidelines'
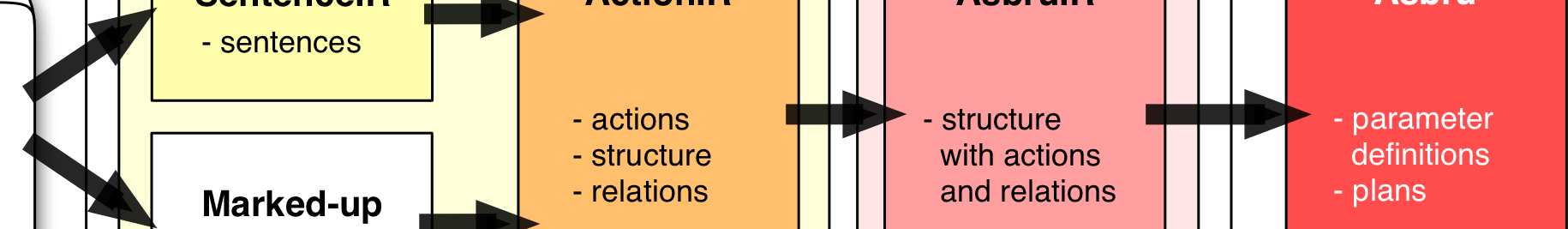
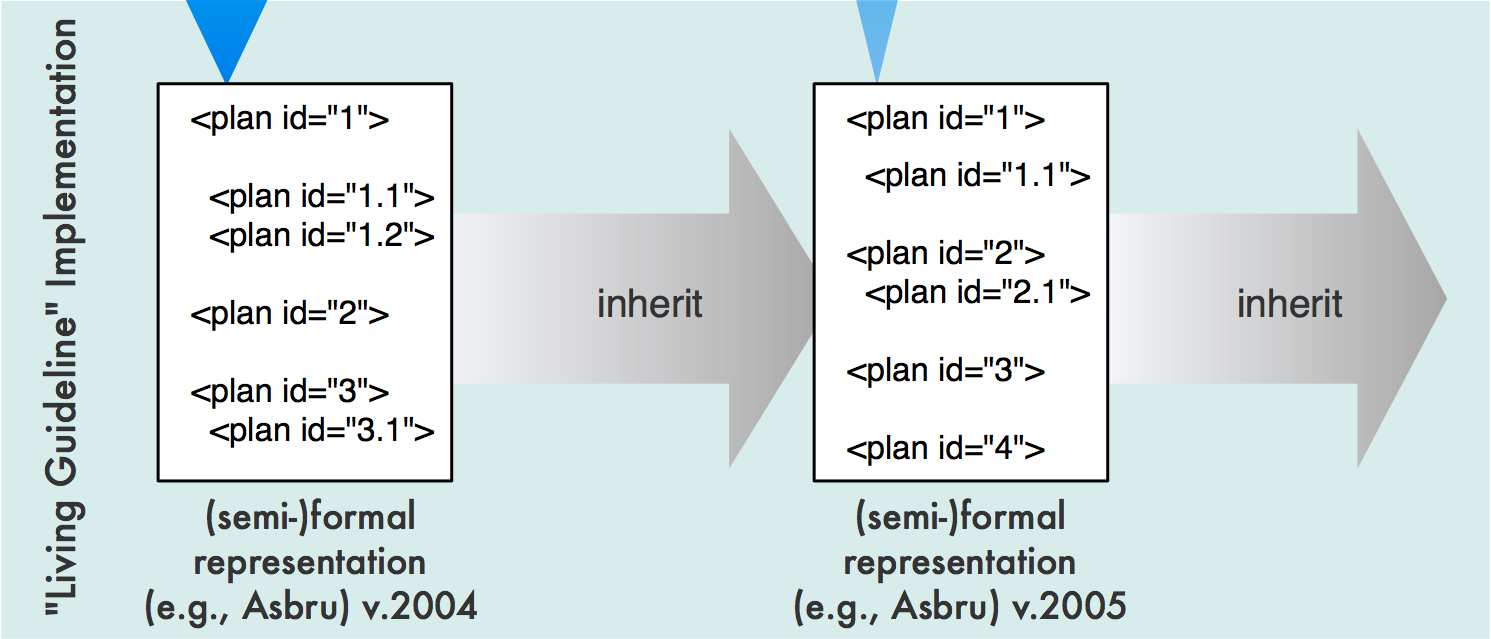
Using LASSIE a unique identifier (i.e., the DELT/A link) marks information trans-formed from one step to the next. We now apply LASSIE to support the formalizationof living guidelines. The document provide us the information that has changed: Adap-tations of every new revision are marked by arrows and highlighted in terms of color(or in different gray scales) (see Fig. 2).
We now propose a new method utilizing this information. Thereby, the new guide-
line is not going to be modeled from scratch, but already modeled parts from previousversions are inherited. Thus, only new text parts have to be modeled (see Fig. 3).



Fluticasone provides equal clinical activity to BDP and budesonide at half the dosage. The evidence that it causes fewer side-effects
at doses with equal clinical effect is limited.
Mometasone is a new inhaled steroid and the relatively limited number of studies suggests it is equivalent to twice the dose
of BDP-CFC. 521 The relative safety of mometasone is not fully established. Ciclesonide is a new inhaled steroid. Its efficacy and safety relative to other inhaled steroids has not been fully established.
4.2.4 OTHER PREVENTER THERAPIES
Inhaled steroids are the first choice preventer drug. Long-acting inhaled beta2 agonists should not be used without inhaled
corticosteroids. 529 Alternative, less effective preventer therapies in patients taking short-acting beta2 agonists alone are:
- Sodium cromoglicate is of some benefit in adults 170 and is effective in children aged 5-12 572
> 5-12 Evidence level 1+
- Nedocromil sodium is also of some benefit in adults and children >5 170, 519
> 5-12 Evidence level 1++ 5-12 Evidence level 1+
- There is no clear evidence of benefit with sodium cromoglicate in children aged <5 573
Leukotriene receptor antagonists have some beneficial clinical effect (and an effect on eosinophilic inflammation) 165, 666 ,172 >12 Evidence level 1++ 5-12 Evidence level 1++ <5 Evidence level 1++
Fig. 2. Excerpt of the 2005 version of the "living guideline" [3]. Adaptations of every new revision
are marked by arrows and highlighted in terms of color (or in different gray-scales).
As LASSIE is a multi-step methodology, we have to satisfy each step for the living
As the input of LASSIE's first step is the XHTML-conform guideline document, wehave to preprocess the document to get a unified document format. We accomplish thisby XSLT scripts, HTML Tidy5, and manual post-processing in order to obtain not onlya well-formed but also a hierarchically well-structured XHTML document.
Marking-up the New Guideline Version
LASSIE's first step is to detect relevant sentences and text parts in the guideline doc-ument. Text parts are thereby list entries that may not be complete sentences, but arereferred to as sentences in the remaining paper.
The output of LASSIE's first step are two files: (1) the marked-up guideline docu-
ment, where relevant sentences are marked and tagged by a DELT/A link, and (2) a filecontaining all relevant sentences and their corresponding DELT/A links.
We use these files of the previous guideline version to detect unchanged relevant
sentences in the new guideline version. We parse the new guideline document and

A national clinical guideline
British Thoracic Society
Scottish Intercollegiate G uidelines N etwork
<plan id="1.1">
<plan id="1.1">
<plan id="1.2">
<plan id="2.1">
<plan id="3.1">
<plan id="1">
<plan id="1">
<plan id="1.1">
<plan id="1.1">
<plan id="1.2">
<plan id="2"> <plan id="2.1">
<plan id="2">
<plan id="3">
<plan id="3"> <plan id="3.1">
<plan id="4">
"Living Guideline" Im
(e.g., Asbru) v.2004
(e.g., Asbru) v.2005
Fig. 3. Formalizing a living guideline using LASSIE. The documents provide us the information
that has changed. After comparing the new documents with the previous ones we are able to adapt
the former formalized documents using LASSIE.
search for each sentence marked-up in the previous version. Thereby, we have to con-sider not only equal sentences but also equal contexts of them. This is necessary as amarked-up sentence can appear repeatedly in the document and we have to assign thecorrect DELT/A link in the new document. For each sentence in the new guideline thatis marked as updated as a part or whole we apply step 1 of the LASSIE methodology(see [2] for details) in order to detect relevant sentences for further processing. Relevantsentences of the old guideline version that are not found in the new document can beseen as removed.
For each sentence of the new guideline that has been marked as relevant by the
LASSIE methodology we assign also a version id. Furthermore, we have to be aware tonot assign an obsolete DELT/A link to a new sentence.
Thus, we obtain the new marked-up guideline version and are now able to extract the
processes in order to gain a representation independent of the final guideline language.
After this step the user is also able to view the resulting files with the DELT/A tool andmake corrections.
Further Transformation of the Extracted Information
After obtaining the new marked-up guideline document we can proceed with the subse-quent steps coming up with LASSIE. That means, we can inherit models of subsequentrepresentations that correspond with text parts that were not changed in the new guide-line version. For new or changed models the particular processing step of LASSIE isapplied. For instance, to detect processes we proceed as following:
Within the next step of the LASSIE methodology relevant sentences are structured
and relationships between sentences are found. The output of this step is a representa-tion (ActionIR) containing actions, relations controlling the process flow between theseactions, and the structure illustrating the hierarchy and nesting of groups of actions.
An action contains the action sentence, possible assigned annotation sentences,
treatment instruments, information about the dosage, duration or iteration of a drugadministration, and conditions. If the action is part of a selection, it is given a selectionid. DELT/A links are inherited from the SentenceIR representation in order to providethe traceability of the process.
In order to obtain actions from our new version of the marked-up guideline, we can
inherit action and annotation sentences from the previous ActionIR version. Further-more, new relevant sentences of the current guideline version are classified in actionand annotation sentences. When sentences are classified as annotation they must beassigned an action sentence. If an action and its assigned annotation sentences werenot changed in the new version, the complete action node is inherited to the new Ac-tionIR representation. Otherwise, the action node and its additional information has tobe generated by LASSIE. Additionally, a version id is assigned for these new nodes.
Likewise, we are able to inherit relations between actions nodes if none of the both ac-tion nodes has changed. Otherwise, we have to detect new relations using LASSIE. Thethird part of the ActionIR representation, the structure of the actions, is then generatedby LASSIE.
The output of this step is then a new version of the ActionIR representation, which
can be viewed with the DELT/A tool. Changed information is identifiable by the versionid. The user may then make corrections or add new information to the representation.
Case Study
We tested the applicability of our method to a real living guideline. Based on the Britishguideline on the management of asthma [3] from SIGN in its version of 2005 we gen-erated the previous guideline versions (i.e., from 2004) due to the non-availability ofthe old documents6. This was possible because SIGN offers a document which clearlydescribes every adaptation (i.e., change, adding, removal) of the text. For evaluatingthe method we only used Section 4 (Pharmacological Management) of the guideline.
It describes an important part of the asthma treatment and contains also updated textparts.
6 We were not able to receive the older guideline versions from SIGN.
Formalizing the Original Guideline Version
We preprocessed the old guideline document to comply our unified document format.
Starting with the old guideline document we used LASSIE to generate the particularmodels necessary for formalization in Asbru. We automatically generated the interme-diate representations and adapted them according to our needs. The document consistsof 509 sentences. 139 of them were classified as relevant for further processing.
Formalizing the New Guideline Version
The next step was to model the new guideline version using our new method. There-fore, we prototypically implemented our method to automate this task and adapted ourimplementation of LASSIE to enable the processing of living guidelines.
Preprocessing. We preprocessed the new guideline document in order to gain a unified
document format complying the XHTML format.
Markup of new guideline version. Afterwards, we automatically searched for un-
changed sentences that were marked in the previous guideline version and added the
corresponding DELT/A links into the current document. Now, we were able to have
LASSIE check the adapted sentences for relevancy. The new version of Section 4 con-
sists of 515 sentences. We were able to inherit 133 sentences of the old version, which
means that six relevant sentences were either changed or removed in the new version.
13 updated or new sentences were found and checked with LASSIE, which classified
ten as relevant. The new relevant sentences were marked and assigned a new DELT/A
link as well as a version id.
Action generation and further transformations. Within the next step the new sen-
tences were classified in action or annotation sentences. The latter are then assigned to
action sentences. We received five action sentences and five annotation sentences. Four
of the annotation sentences were assigned to two previously available action sentences;
one to a new action sentence. Thus, the remaining unchanged action models were in-
herited from the previous version.
The same procedure is done for all subsequent steps in an analogous manner.
Our study shows that using a document-centric approach – LASSIE with the DELT/Atool – offers distinct benefits in modeling living guidelines. A fast adaptation of thenew document is possible. As in living guidelines there will not be radical changesfrom one version to the succeeding version, inheriting of previous models is a simple,time-saving, but effective method for modeling computer-supported guidelines. Also,in the intermediate representations the new models are marked by their version ids to
enable a prompt identification. Thus, the user is able to perform adaptations quickly andconveniently.
A limitation of our methodology is that minor changes in the text may result in
applying a new relevance check, sentence classification, action generation, and so on,which will require an evaluation by a human afterwards. In methods described in Sec-tion 2.3 such minor changes may be checked and accomplished by a human user moreefficiently.
Furthermore, we have to mention that the IRs do not contain the models of all ver-
sions, only the actual ones. Thus, it is not possible to have one file for all versions, butone file for each version of a representation.
Living guidelines are documents presenting up-to-date and state-of-the-art knowledgeto practitioners. To support their application they have to be brought in a computer-interpretable form, which is a difficult task.
We propose a method applicable on documents previously being formalized using
a document-centric approach. Thereby, the guideline document is marked-up and cor-responding formal models are generated. Our method utilizes these links between thetextual document and the formal models. It inherits formalized models of the previousguideline version by re-linking them to their corresponding text parts in the new guide-line version. Only changed or added texts have to be analyzed and modeled. The for-malization task is thereby done using the LASSIE methodology. It is a semi-automaticapproach using IE and various intermediate representations to model different kinds ofinformation in various granularities. Our case study showed that the modelling effortcan be reduced considerably by applying our LASSIE methodology.
By re-using previously formalized models of guidelines we are able to quickly and
effectively formalize new guideline versions.
Acknowledgements. This work is supported by "Fonds zur F¨orderung der wissen-
schaftlichen Forschung FWF" (Austrian Science Fund), grant L290-N04.
1. Kaiser, K., Akkaya, C., Miksch, S.: How can information extraction ease formalizing treat-
ment processes in clinical practice guidelines? A method and its evaluation. Artificial Intel-
ligence in Medicine 39(2) (2007) 151–163
2. Kaiser, K., Miksch, S.: Modeling treatment processes using information extraction. In
Yoshida, H., Jain, A., Ichalkaranje, A., Jain, L.C., Ichalkaranje, N., eds.: Advanced Com-putational Intelligence Paradigms in Healthcare – 1. Volume 48 of Studies in ComputationalIntelligence (SCI). Springer Verlag (2007) 189–224
3. Scottish Intercollegiate Guidelines Network (SIGN), British Thoracic Society: British guide-
line on the management of asthma. a clinical national guideline. Scottish IntercollegiateGuidelines Network (SIGN) (November 2005)
4. Peleg, M., Tu, S.W., Bury, J., Ciccarese, P., Fox, J., Greenes, R.A., Hall, R., Johnson, P.D.,
Jones, N., Kumar, A., Miksch, S., Quaglini, S., Seyfang, A., Shortliffe, E.H., Stefanelli, M.:
Comparing computer-interpretable guideline models: A case-study approach. Journal of the
American Medical Informatics Association (JAMIA) 10(1) (Jan-Feb 2003) 52–68
5. Polvani, K.A., Agrawal, A., Karras, B., Deshpande, A., Shiffman, R.: GEM Cutter Manual.
Yale Center for Medical Informatics, New Haven, CT. (2000)
6. Shiffman, R.N., Karras, B.T., Agrawal, A., Chen, R., Marenco, L., Nath, S.: GEM: a proposal
for a more comprehensive guideline document model using XML. Journal of the American
Medical Informatics Association (JAMIA) 7(5) (2000) 488–498
7. R˚uˇziˇcka, M., Sv´atek, V.: Mark-up based analysis of narrative guidelines with the Step-
per tool. In Kaiser, K., Miksch, S., Tu, S.W., eds.: Computer-based Support for ClinicalGuidelines and Protocols. Proceedings of the Symposium on Computerized Guidelines andProtocols (CGP 2004). Volume 101 of Studies in Health Technology and Informatics., Am-sterdam, NL, IOS Press (2004) 132–136
8. Votruba, P., Miksch, S., Kosara, R.: Facilitating knowledge maintenance of clinical guide-
lines and protocols. In Fieschi, M., Coiera, E., Li, Y.C.J., eds.: Proceedings from the Medinfo2004 World Congress on Medical Informatics, AMIA, IOS Press (2004) 57–61
9. Shahar, Y., Young, O., Shalom, E., Mayaffit, A., Moskovitch, R., Hessing, A., Galperin, M.:
DEGEL: A hybrid, multiple-ontology framework for specification and retrieval of clinicalguidelines. In Dojat, M., Keravnou, E., Barahona, P., eds.: Proceedings of the 9th Confer-ence on Artificial Intelligence in Medicine in Europe, AIME 2003. Volume 2780 of LNAI.,Protaras, Cyprus, Springer Verlag (2003) 122–131
10. Kosara, R., Miksch, S.: Metaphors of Movement: A Visualization and User Interface for
Time-Oriented, Skeletal Plans. Artificial Intelligence in Medicine, Special Issue: Information
Visualization in Medicine 22(2) (May 2001) 111–131
11. Steele, R., Fox, J.: Tallis PROforma Primer – Introduction to PROforma Language and Soft-
ware with Worked Examples. Technical report, Advanced Computation Laboratory, CancerResearch, London, UK (2002)
12. Gennari, J.H., Musen, M.A., Fergerson, R.W., Grosso, W.E., Crub´ezy, M., Eriksson, H., Noy,
N.F., Tu, S.W.: The Evolution of Prot´eg´e: An Environment for Knowledge-based Systems
Development. International Journal of Human Computer Studies 58(1) (2003) 89–123
13. Peleg, M., Kantor, R.: Approaches for guideline versioning using GLIF. In Musen, M.A.,
ed.: Proceedings of the 2003 American Medical Informatics Association (AMIA) AnnualSymposium, Washington, DC, American Medical Informatics Association (Nov. 2003) 509–513
14. Seyfang, A., Martinez-Salvador, B., Serban, R., Wittenberg, J., Rosenbrand, K., ten Teije, A.,
van Harmelen, F., Marcos, M., Miksch, S.: Maintaining formal models of living guidelinesefficiently. In Bellazzi, R., Abu-Hanna, A., Hunter, J., eds.: Proc. of the 11th Conference onArtificial Intelligence in Medicine (AIME'07), Springer Verlag (2007)
15. National Library of Medicine: Medical Subject Headings. The Library (updated annually)
Source: http://www.cvast.tuwien.ac.at/sites/default/files/publications/PDF/2007/aime_2007/kaiser_lassie/final/aime07_final.pdf
VETERINARY SURGEONS STRAVEN RD. VET CENTRE Dr. MICHAEL J. AVERILL, B.V.Sc (Dist) THE STRAVE ROAD 8 STRAVEN RD, RICCARTON Dr .EVE ALLELY B.V.Sc Dr. LINDA SORENSEN DVM VETERI ARY Dr. CHANTAL MORETON B.V.Sc Dr. FRANCESCA MATTHEWS B.V.Sc PO Box 8169, RICCARTON PH. (03) 348 9728 FAX (03) 348 8012 CE TRE (LTD)
Ital J Gastroenterol l993; 25:174-178 ORIGINAL ARTICLES A randomized controlled trial of a new PEG-electrolyte solutioncompared with a standard preparation for colonoscopy OA PAOLUZI, MARIA CARLA DI PAOLO, F RICCI, C PASQUALI, S ZARUG, F DE LIBERO, P PAOLUZICattedra di Gastroenterologia, Istituto di Il Clinica Medica, Università "La Sapienza", Roma, Italy










