
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Endtb.org




expand new drug
markets for TB
endTB aims to find shorter, less toxic and more
effective treatments for multidrug-resistant TB
5 Approach
through access to new drugs, a clinical trial,
6 Countries
and advocacy at country and global levels.
7 Country requirements
Only 11% of multidrug-resistant tuberculosis (MDR-TB) patients get
Treating and closely monitoring a large cohort
successful treatment. In the absence of successful treatment, MDR-TB is
of patients with new TB drugs
transmitted among families and communities, and is often fatal.
13 Electronic Medical Records (EMR)
Of the 480,000 estimated new MDR-TB patients in 2014, only 123,000 (26%) were reported as diagnosed. 110,000 (23%) ever received appropriate treatment. Even fewer received treatment
14 Pharmacovigilance (PV)
with quality-assured drugs. Approximately 50% of those treated, or 11%, are expected to have
16 Output 2
successful outcomes.
Testing, novel, short, all-oral regimens
Access is limited for many reasons. A key contributor is the absence of an effective, user-
for MDR-TB
friendly treatment regimen. Moreover, existing structures do not facilitate development of
20 Output 3
Breaking down local barriers to accessing
MDR-TB treatment is extremely challenging to administer:
new drugs
➜ Treatment requires a combination of at least five drugs;
24 Output 4
➜ Among them, an intramuscular injection is required daily for eight months;
Support development of WHO guidelines for
Programmatic Management of Drug-resistant
➜ The remaining drugs are oral but some have to be taken twice daily, for a total
duration of 18-24 months;
27 endTB organization
➜ The treatment is extremely toxic and difficult to tolerate; completion demands daily
28 Annex I: abbreviations
support of treatment administration as well as nutritional, social, and economic support.
29 Annex II: endTB Project Timeline
Manufacturers are motivated to test drugs, not regimens. The objective of industry is to bring single anti-TB drugs to a paying market. Although two new drugs, bedaquiline and delamanid, have been brought to market recently, the manufacturers do not have an incentive to support development of optimal regimens including one or both of these drugs.
Without concerted, externally supported efforts to test the drugs in combination, such combinations will never be evaluated.
Major gaps in regimen development for MDR-TB remain. Existing trials aim mostly to identify
a single regimen, which invariably will not be adequate for all patients in all settings at all times. Few of these efforts combine new drugs in a single regimen.
endTB partners – Partners In Health (PIH), Médecins Sans
Frontières (MSF), and Interactive Research and Development (IRD)
This evidence gap leads to a market failure that prevents acceptance of new
drugs and regimens by National TB Programs (NTPs).
will implement the following activities:
Introduce new drugs
We will treat at least 2,600 TB patients with new drugs and regimens in 15 countries,
according to WHO guidance.
➜ Establish an evidence base for broader, safe use of new MDR-TB drugs:
Collection and analysis of data from this cohort of 2,600 patients will generate evidence
for the safe, effective use of these drugs.
➜ Address country-level barriers to enable access to new TB drugs:
In each country, we will meet government requirements to authorize the import
and use of these new drugs. We will work to incorporate their use in national guidelines,
informed by the evidence generated by the project.
Clinical trial
The trial will evaluate five new combinations of drugs to identify those that are as good or
better than the current regimen. 750 patients will be enrolled across trial sites, managed
NEW TB DRUGS ARE MORE
by PIH and MSF. Potential sites are: Georgia, Kazakhstan, Kyrgyzstan, Lesotho and Peru.
ACCESSIBLE AND USED TO THEIR
We will use the results to
Shape global policy and treatment practice
We will advocate for increased access to new drugs worldwide by disseminating and sharing
our data with NGOs, UN agencies, Ministries of Health, pharmaceutical companies and global
health donors.
Improve availability of anti-TB drugs
Using evidence from the regimens tested in our clinical trials, we will put pressure on manu-
facturers to supply adequate quantities of TB treatment drugs.
Negotiate fair TB drug pricing
The endTB partnership, will negotiate with suppliers to make new (and re-purposed) TB
drugs affordable.
endTB is implemented in 15 countries struggling with serious
endTB countries were selected on the following criteria:
MDR-TB epidemics. Thirteen rank among the 30 countries with the highest burdens of MDR-TB worldwide.
endTB activities are implemented through close collaboration between NTPs and other
national authorities and an NTP partner: PIH, MSF or IRD.
endTB partners have already been working with NTPs on compassionate use
treatment protocols, which include using delamanid and bedaquiline.
A formal NTP "Project Acceptance Form" was signed by the appropriate authorities.
Osh Oblast
endTB partners obtain separate approvals for the observational study and the clinical
trial preparation.
Countries implement the consent processes required for receipt of new drugs and
participation in studies.
Addis Ababa
MDR-TB regimens
Kanamycin Capreomycin
before and after endTB
Moxiflacin Levofloxacin
Observational study
Gatifloxacin Ethionamide Cycloserine Terizidone
endTB countries are home to a range of patients who face many challenges beyond MDR-TB
including extreme poverty, HIV, hepatitis C, alcohol and substance addiction, and social and
political disruption. Implementing endTB in these varied settings provides important infor-
Amoxicillin/Clavulanate
mation about the use of new drugs in the full range of patients afflicted by MDR-TB.
Imipenem/Cilastatin
1-3 priority regimens including new TB
Numerous non-standard regimens and
drugs that treat all forms of MDR-TB
different regimens for MDR-TB, pre-XDR-TB
Priority TB drugs for endTB
including pre-XDR-TB and XDR-TB
and XDR-TB Current list of TB drugs used in
MDR-TB treatment (22 different drugs).
5-8 priority TB drugs to treat MDR-TB
and closely monitoring
a large cohort of patients
with new TB drugs

Treating and closely monitoring a large cohort of
Evaluate MDR-TB patients for eligibility for new TB drugs
patients with new TB drugs
Inclusion criteria
Two types of patients will be considered for inclusion in the study
Approximately 2,600 patients will receive new TB drugs in the 15 endTB countries. The
project will meet all WHO conditions1 for the use of new TB drugs.
➜ newly diagnosed patients with MDR-TB
Procure new and companion TB drugs
patients whose MDR regimen is failing or who are experiencing excessive toxicity who
would benefit from a new regimen with new TB drugs.
endTB procurement will focus on five WHO Group 5 drugs2 in the table below:
Patients who have already started second-line MDR regimens4 without new drugs and are doing well will generally not be evaluated for initiation of new TB drugs.
TB Treatment Drug
Lab support
Second-line drug susceptibility testing (see text box on following page) will be important to
the evaluation of MDR-TB patients enrolled in endTB. As such, access to a quality-assured/
quality-controlled (QA/QC) laboratory will be needed at all sites. In sites where there is no such access, samples will be sent to a supra-national reference laboratory.
reatment guidelines
Output 1 will follow WHO TB treatment guidelines, as operationalized in the endTB Guide
for New TB Drugs. This document describes indications for the new TB drugs, developed
jointly by in-country and central experts. This guide will serve as a "how-to" manual to eva-
luate patients for new TB drugs specific to the site's individual MDR-TB program.
New drugs: Bdq and Dlm are considered to be "new" TB drugs as they have recently
been granted conditional approval for MDR-TB treatment by a stringent regulatory authority3.
Conduct an observational study
Using data from routine treatment and follow-up of MDR-TB patients receiving one of the
Repurposed drugs: Lzd, Cfz and Imp/Cln are considered TB "re-purposed" drugs. They
new anti-TB drugs, we will evaluate the safety and effectiveness of treatment with the new
have been developed to treat other diseases but are also used by clinicians to treat MDR-TB
drugs. A standard protocol will guide the collection of data for evaluation.
and extensively drug-resistant tuberculosis (XDR-TB). In countries where these drugs are not
yet approved, we will find the best ways to ensure these drugs can be procured and imported
Every patient who receives Bdq or Dlm at an endTB project site will be invited to participate
according to country regulations.
in the observational study. Inclusion requires signing of an informed consent form for study participation. Any patient who declines to participate in the observational study will receive
Patients enrolled in Output 1 will also need additional drugs for a complete treatment regi-
the same quality of care as study participants, but their data will not be included in the collec-
men; these companion drugs will be supplied by existing mechanisms.
tion or analysis.
Initiate and monitor MDR-TB treatment with new drugs
Enrollment totals per site: The endTB goal is to enroll a minimum of 2,600 patients on
1 - WHO conditions for the use of new TB drugs: 1) Proper patient inclusion; 2) Adherence to the principles of designing a WHO-recom-mended MDR-TB regimen; 3) Close monitoring of patients; 4) Active pharmacovigilance and proper management of adverse drug reactions;
MDR-TB treatment with new drugs. The country-specific targets (see map in Project Countries
and 5) Patient consent.
section) reflect that an estimated 30% of MDR-TB patients in each catchment area will meet
2 - Group 5 drugs are those on the list of drugs with limited data on efficacy and/or long-term safety in WHO guidelines for the programmatic management of MDR-TB.
3 -Bedaquiline by the U.S. Food and Drug Administration (FDA) in 2012 and European Medicines Agency (EMA) in 2013 ; and Delamanid by
4 - A second-line MDR regiment is one that is given when the initial MDR treatment regiment (also known as first-line therapy) doesn't
EMA in 2013.
work, or stops working.
the criteria for prescription of a new TB drug. Program capacity to enroll and closely monitor
Establish an endTB care management system/open-source
patients at each site was also considered.
electronic medical record and a pharmacovigilance system
Individual patient treatment decisions
Data management systems are an essential part of any MDR-TB program. Generally, they
Local physicians will prescribe the treatment regimen for each patient. Site physicians and
allow clinicians quick and secure access to a patient's complete health history without having
staff will receive training on the endTB Guide for New TB Drugs. The Guide will be updated
to locate the patient's paper chart; reduces medical errors by restricting entry to only plausible
on a regular basis throughout the duration of the project.
value ranges; can alert clinicians to missing data and abnormal or dangerous signals; provide indications that the patient is not responding to therapy, and can be used to compile aggregate
statistics and reports.
Each enrolled patient will be monitored according to the schedule outlined in the endTB Guide for New TB Drugs. Monitoring will measure response to treatment (e.g. through sputum
Existing data systems used by national TB programs to record and report information
culture) and screen for potential adverse events. Although the new drugs were found to be
about MDR-TB treatment cohorts are often very basic and seldom facilitate monitoring and
relatively safe in the trials leading to their approval, these trials were small and rare events
evaluation or data analysis. endTB will use its partners' extensive experience with electronic
may have been missed. Routine screening is therefore critical to identifying such events.
medical record (EMR) systems to improve clinical care and conduct operational research. (See next heading.)
Drug susceptibility testing
Drug susceptibility testing : Testing to determine which
Electronic Medical Records (EMR)
drugs are effective against the bacteria causing disease.
EMRs are generally used as an electronic data entry system for clinicians who are directly
managing patients. endTB will maximize the capabilities of its EMR system so that it meets
the needs of clinical, research, and reporting purposes. Once developed, this system will be
available for free to all other TB treatment sites and countries outside of the endTB program.
It will also allow national TB programs to continue the work after the endTB project ends.
Clinical decisions will rely on drug susceptibility testing (DST) for drugs that have a reliable, pre-established testing method at laboratories with a proper QA/QC system.
The endTB EMR system will include:
Projects will be asked to freeze baseline cultures from all Output 1 participants. For patients
➜ data capture
with positive cultures after four months of treatment, sites will be asked to ship baseline and
➜ secure storage of all study variables
a late positive culture to the Institute of Tropical Medicine (ITM) in Antwerp. ITM is a supra-national reference laboratory working to develop drug susceptibility testing for the new TB
➜ collection and storage of additional valuable clinical information for MDR-TB treatment
drugs, will be the primary reference lab for endTB.
➜ adverse event tracking (of key importance to pharmacovigilance component)
➜ a safety module that will facilitate reporting and interface with the dedicated
pharmacovigilance database.
Central PV Unit: A central endTB PV unit will serve both Outputs 1 and 2 for two primary
adverse drug reaction = any response to a drug which is
noxious and unintended, including when a drug is not
SAE reporting - SAEs and suspected unexpected serious adverse reactions (SUSAR)5
effective in its intended use
will be reported to the relevant national authorities, in accord with national regulations
concerning the timing and content of adverse event reporting. In addition, during
Output 2, staff will also report any adverse events to the endTB central PV unit.
Pharmacovigilance is a proactive, medically-driven, safety risk management system that
This unit will ensure the quality and completeness of the information and contact
focuses on adverse drug reactions. A good pharmacovigilance system requires training and a
the clinician for additional or clarifying information, when needed. This will facilitate
real-time communication system with the central endTB PV unit (see diagram below). While
standardized reporting of SAEs to all relevant authorities.
this means that staff have an increased workload, we believe it is essential for a responsible and responsive TB treatment program.
SAE database - SAE reports will be entered in a central PV database6 for immediate
Individual Case Safety Reports must be filed for serious adverse events (SAEs). The endTB
project pharmacovigilance component is diagrammed in below.
endTB Pharmacovigilance Reporting of serious
A set of endTB guides, tools and forms will be provided to all
collaborators to help standardize project operations:
program data
endTB Guide for New TB Drugs
endTB Pharmacovigilance guidelines
Immediate filling out of CSR
for serious adverse events or
Study participant consent forms
adverse events of interest
Standard clinical chart that includes enrollment and
National Ethics Board and
National Drug Authority
follow-up pharmacovigilance forms
(as per country protocols)
International IRB
RAPID DeClARATIoN
and Ethics board
Central PV UNIT
RAPID DeClARATIoN
Review & declaration of CSR
PIH Leadership Team and
Expert Group to Analyze
Validation of PV reports
endTB data
Coding PV database
5 - A suspected unexpected serious adverse reaction (SUSAR) is an adverse reaction that is both unexpected (not among the known
adverse reactions caused by the drug or treatment regimen so far) and also meets the definition of a Serious Adverse Event (SAE). The
Drug Company
term "adverse reaction" implies that a causal relationship between the event and the drug or treatment regimen is at least a reasonable
possibility.
6 - Database will utilize the Medical Dictionary for Regulatory Activities (MedDRA).

Output 2 Testing,
novel, short,
all-oral regimens
for MDR-TB

Testing, novel, short, all-oral regimens for MDR-TB
Several independent groups will provide formal external oversight of the study protocol,
Study design
implementation, operation and analysis:
Institutional Review Boards or Ethics Committees at Harvard Medical School, MSF,
This is a randomized, controlled, trial of five new, all, oral, 9-month regimens compared to the
ITM and in each country participating in Output 2
current standard of care. Randomization will be outcome adapted, rather than fixed. This means that the probability of being randomized to regimens changes as the outcomes are
Independent Scientific Advisory Committee (SAC)
reported: more patients will be assigned to regimens that are producing better outcomes.
Global Community Advisory Board, convened by the Treatment Action Group
Data Safety and Monitoring Board (DSMB)
Study treatment regimens
These groups will continue to assure the relevance of the trial for clinical care and policy, its
Each experimental regimen will contain at least one new drug, in combination with up to four
safety for participants, and that global standards for clinical research are met or exceeded.
companion drugs. The control regimen will be composed according to local interpretation of WHO guidance and may include a new drug if indicated.
Bedaquiline Delamanid
Standard of care control, composed according to WHo Guidelines, including the possible use of De or Be
Output 3 Breaking
down local barriers
to accessing new drugs
Breaking down local barriers to accessing new
endTB will produce its own TB-related progress report starting 2017. This dashboard report
will cover at least six endTB country data, including:
drug registration
Facilitate importation of new and re-purposed drugs in endTB
inclusion of new TB drugs in national guidelines
In most of the countries where endTB projects will be implemented, quality-assured Lzd, Cfz,
assessment of the use of, and access to, new technologies for diagnostics
Imp, Bdq and Dlm are neither marketed nor registered. endTB seeks to change this.
national and donor funding support to implement new testing and
treatment strategies.
In the short term, endTB will seek importation waivers (exceptions) to allow these drugs to
reach patients. This strategy has been used for other second-line TB drugs that have not been
The dashboard will be available on the endTB and MSF-AC websites.
registered in country; the process for seeking importation waivers is different in each country and depends on local laws.
The information provided on this dashboard will guide MSF-AC's ongoing work to improve transparency and accountability in the global rollout of new TB-drug use. This will provide MSF-
In the long term, endTB partners will negotiate three main issues with manufacturers:
AC and other advocacy organizations invaluable information about the pace of the rollout of new TB drugs in each country so they can adapt their strategy accordingly.
➜ Country-level, TB-specific registration of these products
➜ Affordable pricing to match MDR-TB-affected countries' resources
Work toward sustainable financing for transitioning to new TB
drugs and regimens in endTB countries
➜ Volume of each drug to be manufactured.
Each endTB site implementation plan will include:
Progress in these areas will alleviate importation constraints for endTB organizations and
Assistance to national TB programs to include new TB drug financing in existing
secure a sustainable supply beyond the project. These negotiations are part of a long-term
MDR-TB program budgets (including existing national budgets, Global
strategy that can be assisted by endTB and other partners and stakeholders, such as the WHO.
Fund budgets, etc.)
Update national TB guidelines in all endTB countries to include
Assistance in measuring funding gaps for MDR-TB programs
new TB drugs
Estimation of future new TB drug consumption to national TB programs
Most country TB treatment guidelines don't currently include new TB drugs, since some WHO
recommendations were only recently released (November, 2014). As such, clinicians at endTB
A smooth transition to new TB drugs and regimens (including monitoring &
sites will use the endTB Guide on New TB Drugs for guidance during the initial phase of the
evaluation and reporting) once the targeted number of endTB patients from
project (Output 1). endTB country staff will meet regularly with the national TB program in
Output 1 is reached.
each operating country. These meetings will allow them to provide technical assistance for
updating the national guidelines based on the evidence generated from the endTB cohort,
as well as continuing WHO updates.
Improve transparency and accountability of national and
nongovernmental TB programs aimed at accessing new TB drugs
endTB staff will formalize and present ongoing data on the progress countries are making
in increasing access to more effective TB drugs and regimens through continually updated
Web-based progress reports.
Output 4 Support development
of the WHO Programmatic
Management of Drug-resistant
Tuberculosis (PMDT) guidelines
for new TB drugs.
lessons learned from endTB sites8. MSF-AC will disseminate information related to patent
barriers on new TB drugs through an existing online patent opposition database. As part of its core work outside of this proposal, MSF-AC will engage in patent oppositions to remove
Support development of WHO guidelines for
secondary patents9, negotiate with manufacturers, and potentially work with key affected
Programmatic Management of Drug-resistant
countries to leverage flexible aspects of Trade-Related Aspects of Intellectual Property Rights
Disseminate endTB clinical and programmatic findings globally
The endTB leadership team is responsible for overseeing Outputs 1, 3 and 4.
The endTB team will regularly review the results from Output 1 and 2 and present this data,
Clinical trial (Output 2) activities across PIH and MSF will be linked via the endTB coordinating
as needed, to stakeholders such as the WHO, that would like to use it to develop TB treatment
Principal Investigators, Drs. Carole Mitnick (HMS) and Francis Varaine (MSF). The clinical trial
guidelines. For this purpose, the endTB website will share updates on endTB activities and
is sponsored by MSF.
provide access to endTB guidance on use of new TB drugs. In addition, tools for staff training,
pharmacovigilance, using the EMR, and the endTB Guide to Use of New TB Drugs manual
will all be available for download.
The full endTB team includes staff at PIH, MSF and IRD, as well as clinicians, researchers, epidemiologists, pharmacists and other experts drawn from leading institutions.
Collaborate with other groups promoting use of new TB drugs and
endTB will collaborate with other stakeholders to create a Network of Early Implementers
Dr. Aamir Khan (Interactive Research & Development)
of New TB Drugs under the Global Drug-resistant TB Initiative (GDI), hosted by WHO. Key
Dr. Michael Rich (Partners In Health)
stakeholders promoting uptake of new TB drugs include:
Dr. KJ Seung (Partners in Health)Dr. Francis Varaine (Médecins sans Frontières)
➜ WHO Task Force for New Drug Policy Development
Observational Study (Output 1) Principal Investigators
➜ Global Drug-resistant TB Initiative (GDI)
Dr. Helena Huerga (Epicentre)
➜ Bill & Melinda Gates Foundation
Dr. Uzma Khan (Interactive Research & Development)Dr. KJ Seung (Partners In Health)
➜ USAID (and USAID sponsored NGO's such as KNCV, URC and others)
Clinical Trial (Output 2) Principal Investigators
➜ Organizations doing research with new TB drugs (TB Alliance, Critical Path
to TB Drug Regimens [CPTR])
Dr. Carole Mitnick (Harvard Medical School)Dr. Francis Varaine (Médecins sans Frontières)
➜ Clinton Health Access Initiative (CHAI).
Clinical Trial (Output 2) Co-Investigators
The network will also be open to any national TB program or other stakeholder in any country that is treating TB with new drugs.
Elisabeth Baudin (Epicentre)Maryline Bonnet (Epicentre)
Disseminate market intelligence information7 for new TB drugs
Dr. Bouke de Jong (Antwerp Institute of Tropical Medicine)
and key companion TB drugs
Dr. Michael Rich (Partners In Health)Dr. Alex Telnov (Médecins sans Frontières)
MSF-AC will write and disseminate publications that report on DR-TB drug prices and other key barriers to scaling use of DR-TB drugs,focusing on the new TB drugs and highlighting
8 - For example, MD-TB Drugs Under The Microscope: http://www.msfaccess.org/content/dr-tb-drugs-under-microscope3rd-edition.
9 - "Secondary" patents are patents used for new developments or improvements of an initial, basic patent. They are used to extend the term of protection around the product of interest. When secondary patents are active, TB drugs can't be produced by other
7- Market intelligence is the information companies gather and analyze to make production decisions.
companies at cheaper prices.
Annex I: abbreviations
Annex II: endTB Project Timeline
AdAptAtion of guidelines
Democratic People's Republic of Korea
June 2015 to May 2016 (ongoing)
trAinings (research, clinical management, PV and EMR)
Drug Susceptibility Testing
June 2015 to September 2016 (ongoing)
Eugene Bell Foundation
protocol finAlized And submitted to
Electronic Medical Record
centrAl irbs And msf erb: August 2015 (completed)
Expand New Drug Markets for TB
enrolment of pAtients in the study
September 2015 to December 2017 (ongoing)
Imp Imipenem
pAtients on treAtment
Institutional Review Board
June 2015 to October 2018 (ongoing)
Interactive Research and Development
emr implementAtion January to March 2016 (ongoing)
Koninklijke Nederlandse Chemische Vereniging
development of reporting forms
And pv guidelines
April and May 2015 (completed)
MedDRA Medical Dictionary for Regulatory Activities
protocol finAlized And submitted to
centrAl irbsJune 2015 (completed)
Ministry of Health
site prepAredness
Médecins Sans Frontières
October to December 2015 (completed)
MSF-AC Médecins Sans Frontières Access Campaign
enrolment of pAtients
Nepal Anti-Tuberculosis Association
June 2016 ➤ 30 months
National Institutes for Health
National Tuberculosis Program
updAte of endtb guide on new tb drugs May 2016 (due to start)
Operational Centre Amsterdam/MSF Holland
lAunch of Kiit report
Operational Center Geneva/MSF Switzerland
January 2017 (due to start)
Operational Center Paris/MSF France
Partners In Health
meeting of A networK of eArly
Scientific Advisory Committee
December 2015 (completed)
Serious Adverse Event
Stringent Regulatory Authority
University Research Co., LLC
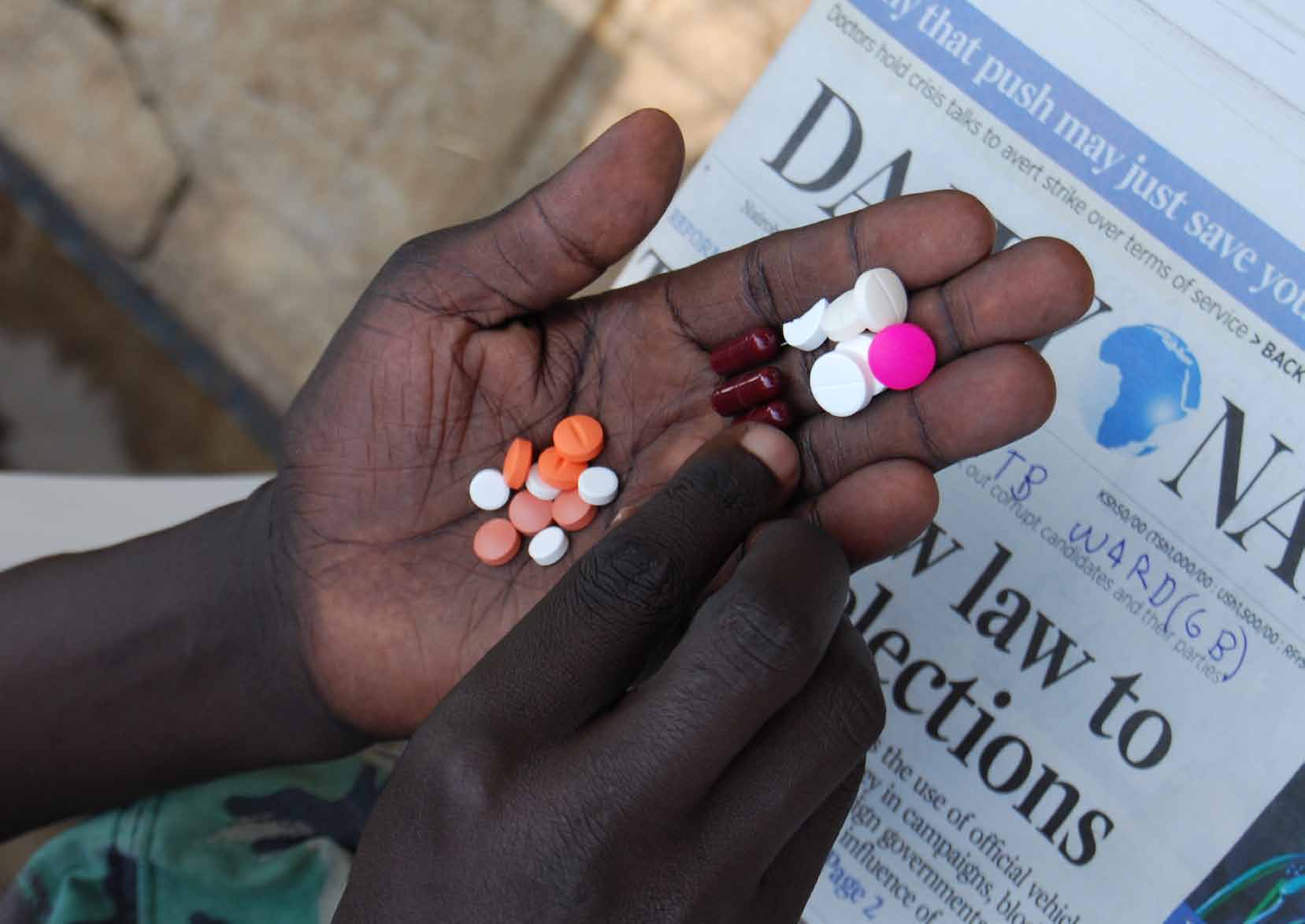
P. 8-9: Dickens, who has HIV and MDR-TB, sorts his daily dose of tablets. MDR-TB clinic, Homa Bay district hospital, Kenya.
United States Agency for International Development
(Photo by: Jane Linekar / Médecins Sans Frontières)
World Health Organization
P. 16-17: Mrs. Flor*, an XDR-TB patient, receives medication from Gaby Merlin Contreras, a PIH field technician, at Flor's home in the Ate District of Lima, Peru. *Name has been changed. (Photo by: William Castro Rodríguez / Partners In Health)
XDR-TB Extensively Drug-resistant Tuberculosis
P. 20-21: Daily dose of pills for Hekim, 40, a XDR-TB patient from Myanmar. (Photo by: Sami Silva / Médecins Sans Frontières)
P. 24-25: A female MDR-TB patient waiting at the TB area entrance at MSF's Myitkyina clinic, Myitkyina, Kachin State, Myanmar. (Photo by: Aye Pyae Sone / Médecins Sans Frontières)
Source: http://www.endtb.org/sites/default/files/2016-06/endTB%20project%20presentation.pdf
Clin Chem Lab Med 2012;50(1):5–21 © 2011 by Walter de Gruyter • Berlin • Boston. DOI 10.1515/CCLM.2011.822 Circulating levels of HER-2/neu oncoprotein in breast cancer Rafael Molina *, Jose M. Escudero, Montse Mu ñ oz, Introduction Jose M. Aug é and Xavier Filella HER-2/neu, also known as c-erbB-2/neu, is an oncogen
Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial François Chollet, Jean Tardy, Jean-François Albucher, Claire Thalamas, Emilie Berard, Catherine Lamy, Yannick Bejot, Sandrine Deltour, Assia Jaillard, Philippe Niclot, Benoit Guillon, Thierry Moulin, Philippe Marque, Jérémie Pariente, Catherine Arnaud, Isabelle Loubinoux












