
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Equievo.pl


This article appeared in a journal published by Elsevier. The attached
copy is furnished to the author for internal non-commercial research
and education use, including for instruction at the authors institution
and sharing with colleagues.
Other uses, including reproduction and distribution, or selling or
licensing copies, or posting to personal, institutional or third party
websites are prohibited.
In most cases authors are permitted to post their version of the
article (e.g. in Word or Tex form) to their personal website or
institutional repository. Authors requiring further information
regarding Elsevier's archiving and manuscript policies are
encouraged to visit:
Author's personal copy
Journal of Equine Veterinary Science 32 (2012) 542-551
Journal of Equine Veterinary Science
Original Research
Effects of b-Hydroxy-b-Methylbutyrate and g-Oryzanol on BloodBiochemical Markers in Exercising Thoroughbred Race Horses
Piotr Ostaszewski PhD, DVM a, Agnieszka Kowalska MSc a, Ewa Szarska PhD b,Piotr Szpota�
nski DVM c, Anna Cywinska PhD, DVM d, Bo _zena Ba1asi�
Tomasz Sadkowski PhD, DVM a
a Faculty of Veterinary Medicine, Department of Physiological Science, Warsaw University of Life ScienceseSGGW, Poland
b The General Karol Kaczkowski Military Institute of Hygiene and Epidemiology, Warsaw, Polandc Equine Veterinary Clinic, Łasieczniki, Poland
d Faculty of Veterinary Medicine, Department of Preclinical Sciences, Warsaw University of Life Sciences, Poland
In both the horse and the man, nutritional ergogenic aids have been used to improve
Received 22 June 2011
physical ability in conjunction with an appropriate training regimen. Although training
Received in revised form
increases physical condition, the ease of taking a nutritional additive to improve training
results explains the demand for supplementation, which may increase mechanical energy
Accepted 20 January 2012
of work, delay onset of fatigue, or improve neuromuscular coordination. The purpose
Available online 6 March 2012
of this study was to determine the effects of oral supplementation of b-hydroxy-b-methylbutyrate (HMB) and g-oryzanol (GO) on indices of exercise-induced muscle damage
in Thoroughbred race horses. In this 32-week study, the horses were assigned to either
a placebo, GO (3.0 g/d), HMB (15 g/d), or GO and HMB treatment groups. The supplements
were administered for the first 16 weeks of the study during the training period before the
racing season began. Blood samples were taken at baseline, and then during training,
before exercise, immediately after exercise, and 30 minutes after exercise. Heart rate and
Biochemical markers
speed were monitored in each exercise session. Hematocrit, glucose, lactate (LA), creatinephosphokinase, and aspartate aminotransferase were measured before and after eachexercise session. Analysis of variance showed a significantly greater increase in post-exercise creatine kinase activity in placebo-supplemented group than in the other treat-ment groups, both in the training period and during the racing seasons (P < .05). Blood LAwas higher immediately after exercise in the placebo group compared with the supple-mented groups. In conclusion, supplementation with HMB and GO resulted in decreasedcreatine kinase and LA after exercise. These findings support the hypothesis that HMB andGO supplementation helps to prevent exercise-induced muscle damage.
Ó 2012 Elsevier Inc. All rights reserved.
endogenously in small amounts and has been shown toimprove gains in strength and lean body mass in humans
b-Hydroxy-b-methylbutyrate (HMB) and g-oryzanol
when associated with resistance training [1,2]. The efficacy
(GO) are supplements used to enhance the effects of
of HMB has been demonstrated in pathological conditions,
training in exercising humans, dogs, and horses. A metab-
where it has been reported to reduce muscle wasting
olite of the amino acid leucine, HMB is produced
associated with AIDS, trauma, and cancer cachexia [3-5].
More recently, HMB has been shown to decrease protein
Corresponding author at: Piotr Ostaszewski, PhD, DVM, Faculty of
degradation and increase protein synthesis [6]. In decreasing
Veterinary Medicine, Department of Physiological Science, Warsaw
muscle damage, HMB may also provide a source of cytosolic
University of Life Sciences-SGGW, ul. Nowoursynowska 159, 02-776
HMG-coenzyme A for cholesterol synthesis and increase
Warsaw, Poland.
E-mail address: [email protected] (P. Ostaszewski).
the availability of cholesterol for cell membrane synthesis.
0737-0806/$ - see front matter Ó 2012 Elsevier Inc. All rights reserved.
doi:10.1016/j.jevs.2012.01.002
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
This may result in an overall reinforcement of the sarco-
lemma as well as the provision of valuable substrate for its
Analyzed composition and calculated energy density of hay andconcentrates
repair following muscle damage or injurious exercise [7].
This is evidenced by studies demonstrating that HMB leads
to decreased markers of muscle damage following mechan-
ically strenuous exercise, including lower activity of creatine
DM, % of fresh matter
kinase (CK), lactate dehydrogenase, and a decrease in muscle
Crude protein, % of DM
protein breakdown as indicated by serum 3-methylhistidine,
Crude fat, % of DM
Crude fiber, % of DM
a direct marker of muscle protein degradation [8-10]. Other
Crude ash, % of DM
studies have shown that HMB reduces cancer-induced
Net energy, MJ/kg DM
muscle weight loss through attenuation of the ubiquitin-
DM, dry matter.
proteasome proteolytic pathway [11], suggesting that HMBfunctions predominantly as an anticatabolic, rather thananabolic compound. However, a recent study has shown that
520 � 50 kg were studied at the Sluzewiec Racetrack
HMB supplementation induces muscle hypertrophy in the
training center (Warsaw, Poland). The horses were
extensor digitorum longus and soleus muscles in rats via
privately owned, and the experimental design and all
mammalian target of rapamycin pathway [12], in addition to
procedures were approved by the Ethical Committee in
attenuating the depression in protein synthesis induced by
Warsaw and by the owners of horses. The horses were
the proteolysis-inducing factor [6]. In geldings fed an alfalfa-
selected on the basis of a clinical examination and hema-
based supplement containing 10 g of HMB per day during
tological analysis, and horses with pathological conditions
6 weeks of low to moderate-intensity training followed by
were excluded. The horses were also chosen on the basis of
6 weeks of high-intensity training, HMB supplementation
similar racing performance as recorded from the previous
resulted in a 10% improvement in treadmill endurance [13].
year's racing records. During a preliminary period of 2
This was followed by a study in racing Thoroughbreds
weeks, all the horses were acclimated to a basal diet and
where HMB-supplemented horses had reduced serum CK,
were individually housed on straw in box stalls under
maintained body weight better, needed less recovery time
identical conditions. Each horse consumed 5 kg of oats, 1 kg
between races, and had a better win rate. These effects were
of a complete high-quality commercial feed (daily ration)
most likely through a decrease in training and race-related
distributed in three feedings, and 6 kg of hay that was
muscle damage and increased aerobic ability, which
administered daily ad libitum. The concentrate/forage ratio
allowed for a quicker recovery after racing.
was 50/50. Table 1 shows the composition of the feedstuffs.
GO is a mixture of ferulic acid esters of sterol and tri-
Total net energy intake in the daily ration was 135 MJ/
terpene alcohols extracted from rice bran, and is known to
be a powerful inhibitor of iron-driven hydroxyl radicalformation; it has also been reported to possess antioxidantactivity in stabilizing lipids [14]. Because GO is insoluble in
2.2. Supplement and Placebo: Composition and
water, a GO emulsion is used in supplementing humans,
dogs, and horses. There are few studies in the peer-reviewed literature on GO, despite its apparent use as an
The HMB alfalfa-based supplement (150 g/horse/d) was
ergogenic aid. One study looked at resistance-weight-
distributed in a pelleted form twice daily and provided for
trained male athletes supplemented with 500 mg/d GO or
a total of 15 g of CaHMB (Metabolic Technologies Inc, Ames,
a placebo [15]. However, this study failed to show an effect
IA). The placebo for this treatment consisted of 150 g of the
of GO on training performance.
pelleted supplement matrix (dehydrated alfalfa meal, cane
Thoroughbred horses undergo intensive training start-
molasses, soy oil, soy lecithin, caramel flavor) but did not
ing at a young age. The results of this training and their race
contain HMB. GO was purchased from Oryza oil & Fat
performance may be improved by the use of dietary
chemical Co., Ltd (Aichi, Japan) as a crystalline powder.
supplementation. The use of supplementation may not only
Three grams of GO was suspended extempore in 20 mL of
improve performance but also improve muscle recovery
rice bran oil and was top-dressed on the morning
after a race or heavy training period. Therefore, the main
concentrate (once a day). The placebo for this treatment
objective of this study was to evaluate the effect of dietary
consisted of 20 mL of regular cooking oil and was also given
supplementation of HMB and GO on exercise parameters in
as a top-dress with the morning concentrate.
horses trained from winter break to their maximal physical
A groom verified the consumption of both the supple-
performance at the start of the racing season. Our hypoth-
ments and the placebo. All treatments were palatable and
esis is that one or both supplements, either alone or in
consumed by the horses, and no adverse reactions were
combination, will decrease muscle damage and improve
reported. The riders and trainers, as well as the technicians
recovery, and thus improve overall performance.
performing blood sample analyses, were blinded to thetreatment allocation.
2. Material and Methods
2.3. Experimental Design
After the 2-week acclimation period, four homogenous
Twenty-four Thoroughbred racehorses ranging in age
groups of six horses were chosen, with three mares and
from 3-6 years (12 mares and 12 stallions) and weighing
three stallions of similar age assigned within each group.
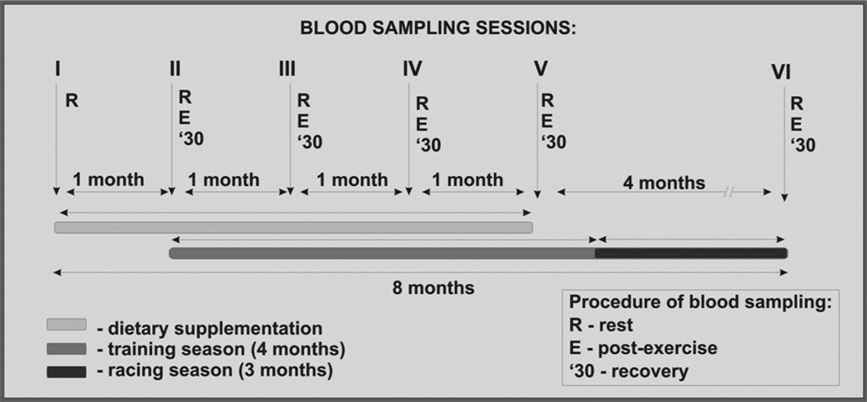
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
Fig. 1. Experimental design and sampling schedule.
The horses were fed the same diet as during acclimation,
was excluded after exercise session III and one horse from
and the supplement regimens were begun. The placebo
HMB group after exercise session IV due to lameness.
group consumed 150 g of pelleted matrix without HMB,and 20 mL of cooking oil; the GO group consumed 150 g of
2.4. Blood Sample Collection and Processing
pelleted matrix without HMB, and 3 g of GO suspended in20 mL of rice oil; the HMB group consumed 150 g of pel-
Peripheral venous blood samples were taken by
leted HMB supplement and 20 mL of cooking oil; and
external jugular venipuncture every 4-week intervals, with
the GO/HMB group consumed 150 g of pelleted HMB
the final sampling at 16 weeks while the horses were in the
supplement and 3 g of GO suspended in 20 mL of rice oil.
racing season. The first session had only one blood sample
Oral administration of selected supplements was initiated
taken in the morning, whereas the remaining sessions
4 weeks before the beginning of training season and las-
consisted of three samples taken at the following intervals:
ted for 16 weeks. The blood samples were taken between
Rerest (before exercise), Eeexercise (after exercise), and
February and September, and the exercise data were
'30ehalf an hour later after being in an exercise walker
collected between March and September.
(recovery). The blood samples were aspirated into 20-mL
Four weeks after horses were assigned to dietary
syringes and immediately transferred into sterile ethyl-
treatments, they began a conditioning program as shown in
enediaminetetraacetic acid tubes for hematological tests
Fig. 1. During the entire training season, the horses were
and into plain tubes for serum analyses. Glucose and lactate
exercised 6 d/wk on a training course with a sand footing.
(LA) concentrations were determined immediately by
The same exercise intensity was repeated every day. For the
ejecting a drop of full blood onto single-use Accu-Chek
first 3 weeks, the conditioning program consisted of 10
Active and BM-LA test strips (Roche Diagnostics Corp.
minutes of walking, 15 minutes of trotting at 250 m/min,
Indianapolis). The ethylenediaminetetraacetic acid-treated
and 4 minutes of cantering at 600 m/min, followed by 30
blood samples were kept in refrigerator (4�C) and analyzed
minutes of exercise on a horse walker. During the following
within 6 hours after collection. Hematocrit (Hct) was
weeks, only time and intensity of the exercise were
counted with an automated hematology analyzer (Abacus
changed so that at week 5, galloping time consisted of 5
Diatron, Hungary). The samples taken for serum were
minutes of cantering at 500 m/min followed by 1 minute of
promptly centrifuged, and the serum samples immediately
galloping at about 750 m/min. Starting week 8, the inten-
frozen and kept at �20�C until analyzed. The serum
sity was again increased to 5 minutes of cantering at 550 m/
samples were analyzed for creatine phosphokinase (CK)
min, followed by 1 minute of galloping at 850 m/min. This
and aspartate aminotransferase (AST) activity by a kinetic
higher intensity conditioning program was continued until
method, using a reagent kit (Pointe Scientific, Inc., Canton).
the beginning of the racing season (end of June). After theconditioning program, all horses participated in the races
2.5. Statistical Analysis
for the next 12 weeks. During this time (racing season), thehorses were also exercised, but with the intensity modified
Results are expressed as means � standard errors of
to the racing schedule. Exercise during the racing season
mean. Exercise session II (n ¼ 6 for each group) was
was designed to maintain the horses' current condition.
considered as beginning of training, and the data from
Additional training was withheld on the day of the races.
exercise sessions III, IV, and V were pooled for each group
The training was monitored using heart rate (HR)
and referred to as the training season. The last exercise
monitors RS800 on Polar Equine Wearlink W.I.N.D. (Polar
session (VI) was referred to as the racing season. The data
Electro Oy, Finland) and G3 GPS. HR monitor consists of
were analyzed for differences between blood indices and
a receiver and two electrodes placed under the girth. Model
HRs at various stages using a repeated-measures analysis of
RS800 allows simultaneous control of the HR values and
variance, and pairwise comparisons were made using
speed of the horse. Twenty-two of the horses successfully
Tukey test. Probabilities of P < .05 were considered statis-
completed the entire study. One horse from the GO group
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
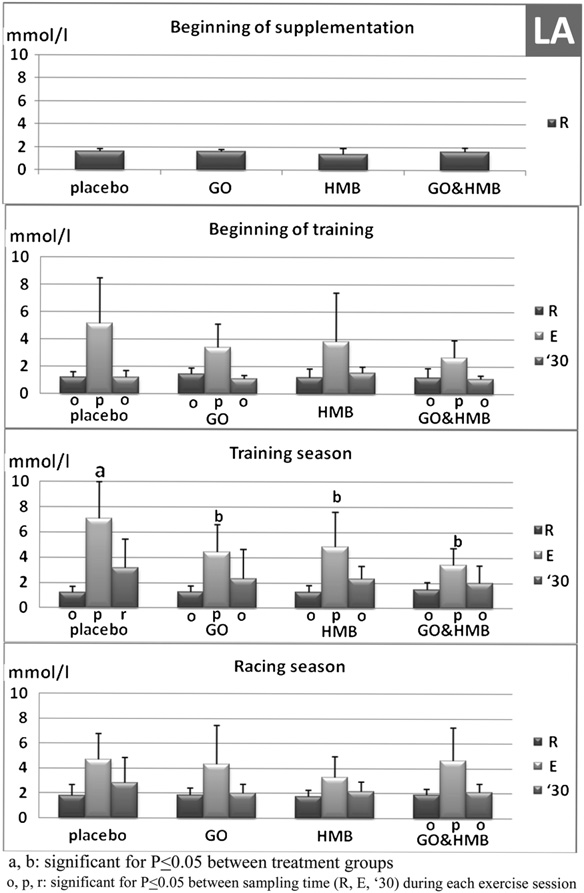
groups (Table 2). Hct was, on average, approximately 41% inall groups. Resting blood glucose and LA levels alsoremained unchanged during the study (Table 3, Fig. 3,respectively).
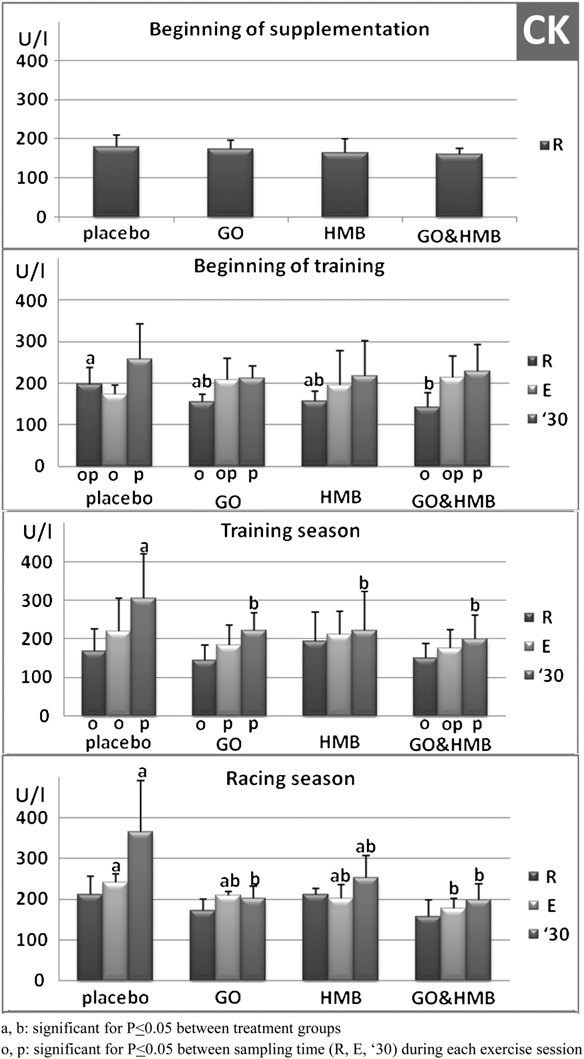
There were no differences in serum CK activity among
treatment groups at the beginning of the dietary supple-mentation (Fig. 4). However, after 4 weeks of training,resting serum CK was significantly (P < .05) decreased inthe GO þ HMB-supplemented horses compared with theplacebo-supplemented horses. During the remainder of thetraining and racing seasons, resting serum CK was notsignificantly different from pretraining values for any of thegroups.
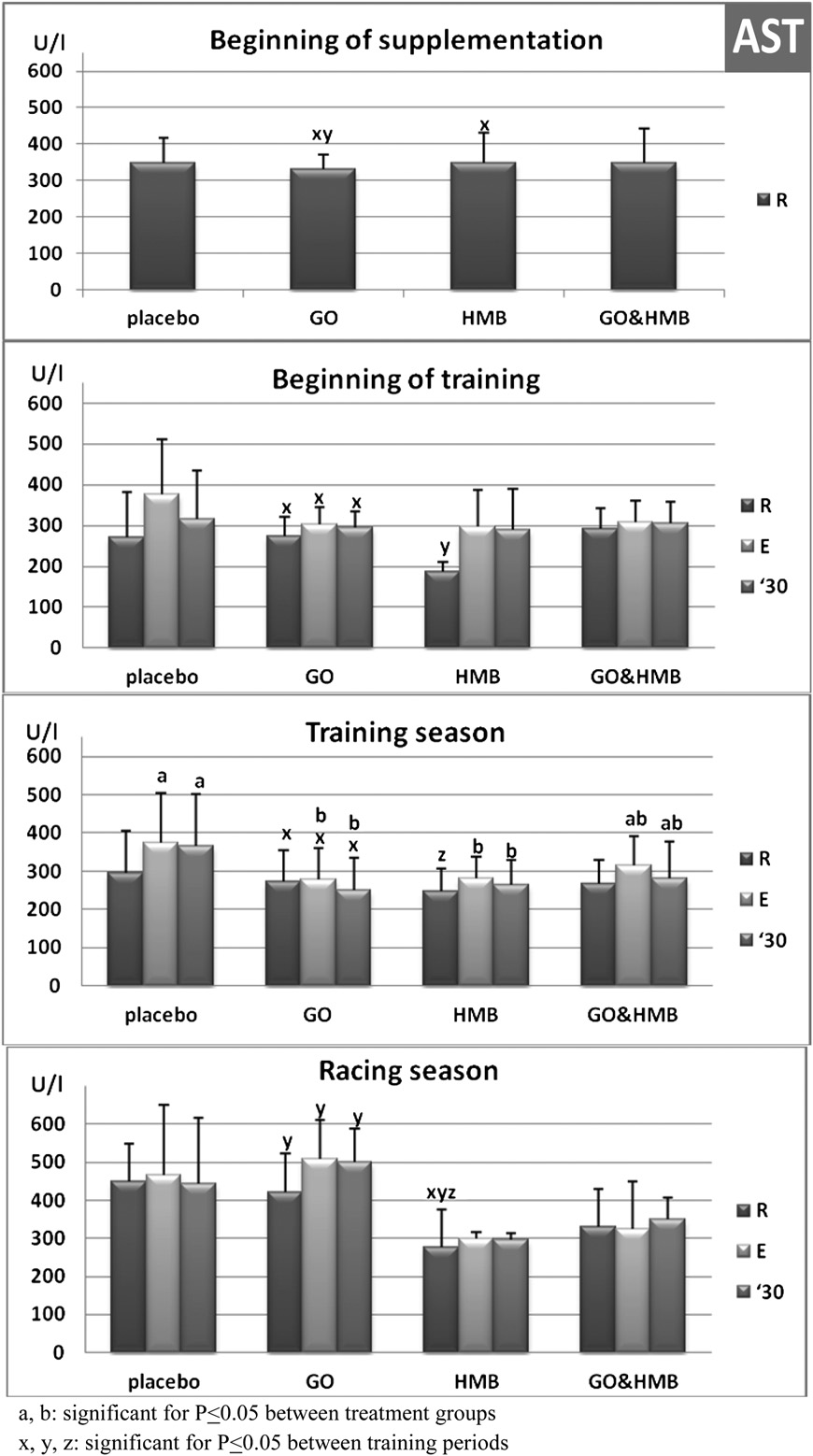
Resting AST activity was the same among the groups at
the beginning of an experiment (sampling session I, Fig. 5).
After 4 weeks of training, resting AST activity had decreasedin all groups, and a significant 30% decrease was observedin the HMB-supplemented horses (P < .05). During theremainder of the training season, pre-exercise AST activitywas still significantly lower in horses receiving HMB whencompared with the beginning of supplementation (P < .05).
During the racing season, pre-exercise AST activity wasincreased in the GO-supplemented horses when comparedwith the training season (P < .05).
3.3. Supplements Versus Placebo After Exercise
Exercise produced a significant increase in Hct levels in
all the groups (P < .05); however, no differences wereobserved between the placebo and the supplementedgroups (Table 2). On average, a 29% increase was observedcompared with the resting values at the beginning of die-tary supplementation, a 36% increase was observed duringtraining season, and a 29% increase was observed duringracing season. Exercise did not affect blood glucoseconcentration in any group except at the beginning oftraining (sampling session II), where horses from HMB-
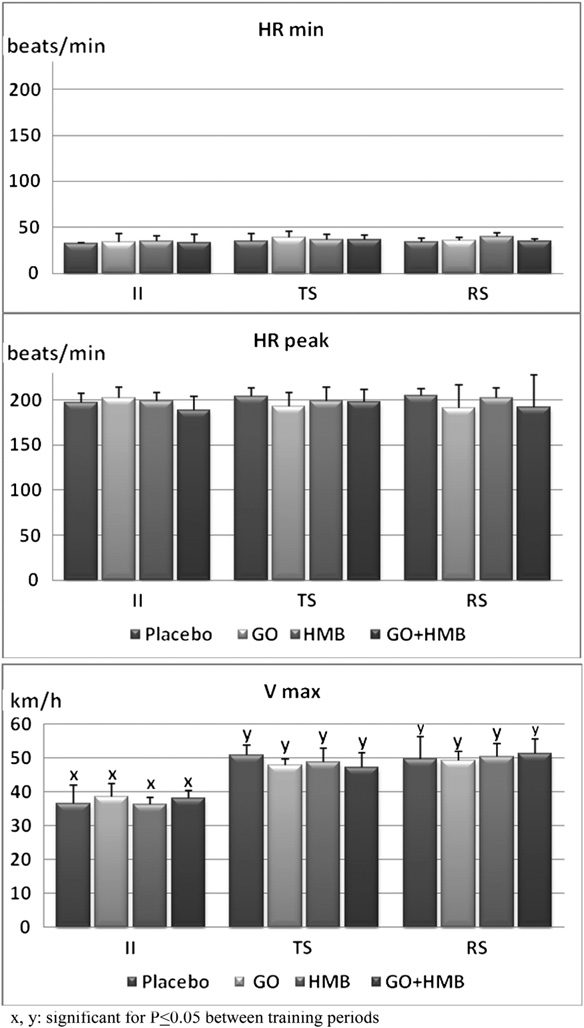
Fig. 2. Minimum and peak heart rate (beats/min) as well as maximumspeed (km/hr) determined in Thoroughbred racing horses at the beginning
of training (sampling session II; n ¼ 22), during the training season
glucose level (Table 3, P < .05). Thirty minutes after exer-
(sampling sessions III, IV, and V; n ¼ 66), and during the racing season
cise, the glucose level in the HMB-supplemented group
(sampling session VI; n ¼ 22). For 16 weeks, the horses were supplemented
was still significantly higher than in GO þ HMB group
with an oral placebo (n ¼ 6), g-oryzanol (GO, n ¼ 5), 3-hydroxy-3-
methylbutyrate (HMB, n ¼ 5), or GO þ HMB (n ¼ 6).
< .05). During the training season, exercise resulted in
a significant increase in glucose level only in HMB-
supplemented group (P < .05). Exercise also resulted ina significant increase in blood LA concentration in all
3.1. Exercise Monitoring
groups (Fig. 3) during the beginning of training (P < .05),except in the HMB-supplemented group. As the training
Resting and peak HR as well as maximum speed did not
season progressed, the exercise-related increase in LA
differ between placebo and experimental groups (Fig. 2).
became significant in the HMB-supplemented group
Resting HR during all exercise sessions was in the range of
(P < .05). In all horses, 30 minutes after exercise, blood LA
32-37 beats/min. Average peak HR was approximately 200
was only slightly elevated when compared with the cor-
beats/min for all exercised horses and was not affected by
responding pre-exercise values. During the training season
the length of training. Maximal speed increased from
(sampling sessions III, IV, and V), the postexercise (E)
average 38 km/hr at the beginning of training to about
increase in blood LA was significantly lower in GO, HMB,
50 km/hr at the end of the racing season. These data
and GO þ HMB groups when compared with the placebo
confirm that training exertion was similar and intense in all
group (P < .05). Exercise sessions performed at the
beginning of training, after 4 weeks of dietary supple-mentation, induced an increase in serum CK activity in all
3.2. Supplements Versus Placebo at Rest
groups. However, only in the GOþHMB supplementedgroup was the increase significantly different 30 minutes
After 4 weeks of dietary supplementation, no change in
following the end of exercise (P < .05). During the training
resting hematocrit (Hct) values was observed among the
season, a significant exercise-related increase in CK was
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
Table 2Hematocrit (%) determined in jugular venous blood of 22 horses at the beginning of training (sampling session II; n ¼ 22), during the training season(sampling sessions III, IV, and V; n ¼ 66), and during the racing season (sampling session VI; n ¼ 22) at rest before (R), immediately after exercise test (E), and30 minutes later ('30); for 16 weeks, horses were treated with an oral placebo (n ¼ 6), GO (n ¼ 5), HMB (n ¼ 5), and GO þ HMB (n ¼ 6)
Group Effect Exercise-Time Time Period
Nontraining Beginning of
Beginning of training R
42.36 � 4.38o 42.05 � 3.89o
44.45 � 5.12o n.s.
54.55 � 7.57p 54.62 � 6.13p
55.07 � 5.76p n.s.
'30 42.98 � 2.74o 44.60 � 4.55o
45.73 � 4.56o n.s.
42.06 � 2.74o 40.45 � 3.09o
42.58 � 4.63o n.s.
58.03 � 6.31p 55.28 � 5.23p
56.22 � 5.40p n.s.
'30 46.26 � 4.63r 44.93 � 3.26r
46.34 � 4.62o n.s.
44.00 � 3.96o n.s.
51.55 � 11.57 54.93 � 6.55p
58.32 � 7.02p n.s.
‘30 46.63 � 4.13
46.27 � 3.18op 46.75 � 4.83op 49.68 � 5.38o n.s.
GO, g-oryzanol; HMB, b-hydroxy-b-methylbutyrate.
Data are shown as means � SEM.
For general comparison, P values of group supplements or time effect are indicated in separate columns. Differences between the placebo and experimentaltreatment groups receiving supplements are significant (P � .05) if the first superscript is different (a, b, or c for within-line comparisons); differencesbetween sampling time (R, E, ‘30) during each exercise session are significant (P � .05) if the first superscript is different (o, p, or r for within columncomparisons); differences between training periods (beginning of training, training, and racing seasons) are significant (P � .05) if the first superscript isdifferent (x, y, or z for within column comparisons).
* Significant for P � .05; n.s., nonsignificant.
observed in the placebo, GO, and GO þ HMB groups
activity 30 minutes after exercise than the placebo-
(P < .05), but not in the HMB-treated horses. The placebo-
supplemented horses (sampling sessions III, IV, and V,
supplemented group had the largest postexercise increase,
Fig. 5). During the racing season, serum AST activity 30
45% above pre-exercise values (Fig. 4, P < .05), which
minutes after exercise was significantly higher in horses fed
was also significantly higher than the HMB- and GO-
GO when compared with the same horses during the
supplemented groups 30 minutes after exercise (P < .05).
training season (P < .05).
Additionally, during the racing season (sampling sessionVI), CK activity remained significantly greater than that of
the placebo-supplemented group 30 minutes after exercise(P < .05).
The present study was the first study to determine
Exercise did not significantly affect serum AST activity in
whether dietary supplementation with either HMB or GO
any treatment group; however, placebo-supplemented
alone, or in combination, would affect indirect markers of
horses tended to have increased postexercise AST activity
muscle damage and fatigue in Thoroughbred horses during
during the beginning of training as well as during a training
16 weeks of training in preparation for the racing season.
season (Fig. 5). During the training season, horses supple-
Currently, little data are available on the effects of these
mented with both GO and HMB had significantly lower AST
supplements in horses, with only one study describing the
Table 3Glucose (mmol/L) determined in jugular venous blood of 22 horses at the beginning of training (sampling session II; n ¼ 22), during the training season(sampling sessions III, IV, and V; n ¼ 66), and during the racing season (sampling session VI; n ¼ 22) at rest before (R), immediately after exercise test (E), aswell as 30 minutes later ('30); for 16 weeks, horses were treated with an oral placebo (n ¼ 6), GO (n ¼ 5), HMB (n ¼ 5), and GO þ HMB (n ¼ 6)
Beginning of training
'30 4.75 � 0.34ab 4.55 � 0.31ab 5.00 � 0.46a
4.71 � 0.51op 4.71 � 0.39
Data are shown as means � SEM.
For general comparison, P values of group supplements or time effect are indicated in separate columns. Differences between the placebo and experimentaltreatment groups receiving supplements are significant (P � .05) if the first superscript is different (a, b, or c for within-line comparisons); differencesbetween sampling time (R, E, '30) during each exercise session are significant (P � .05) if the first superscript is different (o, p, or r for within columncomparisons); differences between training periods (beginning of training, training, and racing seasons) are significant (P � .05) if the first superscript isdifferent (x, y, or z for within column comparisons).
* Significant for P � .05; n.s., nonsignificant.
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
Fig. 3. Blood lactate (mmol/L) in 22 horses at the beginning of training(sampling session II; n ¼ 22), during the training season (sampling sessionsIII, IV, and V; n ¼ 66), and during the racing season (sampling session VI; n ¼22) at rest before (R), immediately after exercise test (E), and 30 minuteslater ('30). For 16 weeks, the horses were treated with an oral placebo (n ¼6), GO (n ¼ 5), HMB (n ¼ 5), or GO þ HMB (n ¼ 6).
Fig. 4. Serum creatine phosphokinase (CK) activity in 22 horses at the
effect of HMB on the physiological response to exercise in
beginning of training (sampling session II; n ¼ 22), during the training
horses. Miller et al. [13] fed horses an alfalfa-based
season (sampling sessions III, IV, and V; n ¼ 66), and during the racing
supplement twice daily containing a daily dosage of 10 g
season (sampling session VI; n ¼ 22) at rest before (R), immediately after
of CaHMB. In our study, we administered 15 g of CaHMB
exercise test (E), and 30 minutes later ('30). For 16 weeks, the horses weretreated with an oral placebo (n ¼ 6), GO (n ¼ 5), HMB (n ¼ 5), or GO þ HMB
daily, split between the morning and evening rations. This
dosage provided w30 mg CaHMB/kg bw d�1, as the horsesweighed approximately 500 kg, and is closer to the rec-ommended dosage of 38 mg/kg bw d�1 for humans [1].
between training stress and recovery. If the next training
Because there were no data concerning the daily require-
session is applied without sufficient time for recovery,
ment for GO in horses, we decided to use 3 g of GO
decreases in the performance occur in the form of earlier
administered once daily. This was similar to the 500-mg
onset of fatigue within each session [16,17]. In our study,
GO/d dosage previously administered to humans [15].
the total length of the training program before competitionwas 16 weeks. This was longer than the 4-6 weeks rec-
4.1. Supplements Versus Placebo at Rest and After Exercise
ommended for horses aged >3 years [16], but we wanted toachieve a moderate pace in improvement in performance.
The theory of intense training is that a single exercise
The horses were exercised 6 d/wk, with the intensity of
session will lead to fatigue and cellular damage, which in
training increasing as the study progressed. The data
turn results in a short-term adaptive response [16]. When
indicated that our methods used for training the Thor-
exercise is performed regularly, and the training stimulus is
oughbreds under field conditions were satisfactory for
increased gradually, the adaptation that occurs during the
maximization of physiological adaptations within the
recovery period of a single exercise session leads to an
animal's body. HRs at rest ranged from 32 to 37 beats/min
overall improvement in performance. When training is too
and were similar to the values obtained by Harris et al. [18].
vigorous and/or rest periods between training sessions are
Peak HRs during cantering/galloping were approximately
too short, performance is reduced because of an imbalance
200 beats/min and remained the same during both the
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
Fig. 5. Serum aspartate aminotransferase (AST) activity in 22 horses at the beginning of training (sampling session II; n ¼ 22), during the training season(sampling sessions III, IV, and V; n ¼ 66), and during the racing season (sampling session VI; n ¼ 22) at rest before (R), immediately after exercise test (E), and 30minutes later ('30). For 16 weeks, the horses were treated with an oral placebo (n ¼ 6), GO (n ¼ 5), HMB (n ¼ 5), or GO þ HMB (n ¼ 6).
training and the racing seasons. At this value, most horses
accompanied by an increase in maximal speed, which in
are close to the point of onset of blood LA accumulation,
turn indicates a physiological adaptation to the exercise.
and it is suggested as a reference point for comparison of
Because the horses in the present study were in a familiar
cardiovascular capacity [19,20]. This sustained peak HR was
environment and were handled and ridden by the regular
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
full-time staff, the risk of excitement influencing the HR
using an internal conversion factor. The present study
was minimized.
showed a significant increase in blood LA at the beginning
In our experiment, increases in LA concentrations were
of exercise in all treatment groups except the groups sup-
evident after all training sessions. Thus, the intensity of
plemented with HMB. Throughout the training, post-
exercise during training sessions in our study was high and
exercise blood LA remained lower in GO, HMB, and GO þ
may be compared with the values reported in the literature
HMB groups when compared with the placebo group, and
[20,21]. This observation was confirmed by changes in Hct
continued to be lower during racing season. These data
levels, which reflect mobilization of splenic erythrocytes.
support the concept of a protective role of HMB and/or GO
Hct levels increased significantly after all training sessions
in counteracting the accumulation of LA; however, from the
and did not differ between horses from either the placebo
present study, it is impossible to tell whether this was the
or experimental groups. The extent of the increase in Hct
result of a concomitant reduction of intramuscular acidosis
values depends on exercise intensity and has a linear
or a shortage of fuel, especially depletion of glycogen stores.
relation to the speed of the exercise, up to a maximum of
In fact, 30 minutes after exercise, blood LA concentrations
60%-65% [22]. Our study saw similar significant exercise-
returned to near pre-exercise values in all horses indicating
related increases in Hct levels.
fast LA removal from blood.
Changes in blood glucose concentration are also
CK is a muscle-specific enzyme with a relatively short
dependent on the intensity of exercise. Although plasma
half-life in serum, that is, w2 hours. Increased serum CK
glucose concentration decreases during prolonged exercise
activity is used as an indicator of muscle damage or injury.
(>3 hours), studies with short intense exercise have shown
Resting plasma level in horses should not exceed 200 U/L.
both decreases and increases, depending on exercise
The CK activity increases and decreases through larger
intensity and training and feeding status of the horse [23].
ranges earlier at the beginning of training season, and
The exercise-associated increase in hepatic glucose output
tightens into a narrower range when the horse is attending
is mainly mediated through a decrease in the insulin:glu-
good fitness levels required for racing. Several studies have
cagon ratio, whereas the rate of uptake and utilization in
shown a direct relationship between the levels of HMB
exercising muscle is restrained by increases in circulating
achieved after supplementation and improved nitrogen
epinephrine resulting in an elevation in blood glucose
retention. In humans, Nissen et al. [8] demonstrated a dose-
concentration [24]. In our study, postexercise blood glucose
dependent response to oral administration of HMB given at
concentration remained within reference ranges in horses;
either 1.5 or 3 g/d, with the higher dosage resulting in
however, HMB supplementation significantly increased
a greater decrease in serum CK. Nissen et al. also observed
blood glucose concentration during the training and racing
a decrease in serum 3-methylhistidine, an indicator of
seasons. This may suggest a glucose-sparing effect of HMB.
muscle protein breakdown. Dietary supplementation of
Anaerobic energy production is essential for the production
3.0 g HMB/d in individuals undergoing intense endurance
of muscular tension when the demand for energy is greater
exercise resulted in decreased CK and LA dehydrogenase
than can be provided aerobically, or when oxygen is in
after a prolonged 20-km run [9]. In our studies, 15 g of
short supply. The largest source of anaerobic energy during
CaHMB/d was administered to horses and resulted in
the intensive exercise of short duration is from the
a significant attenuation of the exercise-related increase of
glycolytic pathway. Although the yield of adenosine-5'-
plasma CK, an indicator of an alteration in membrane
triphosphate from 1 mole of degraded glucose is only 2-3
permeability. Taken together, the aforementioned studies
moles, muscle has a high glycolytic capacity, and the two
indicate a benefit of having more HMB available to the
end products of the glycolytic reactions, pyruvate and
muscles during intense training in horses.
hydrogen, combine to form LA. After cessation of exercise,
AST has a much longer half-life than CK, approximately
the rate of oxygen consumption remains elevated, and
1 week, and therefore reflects muscle changes over several
blood LA concentration continues to increase [23]. There is
days or even weeks. The activity of AST in horses is much
a point where LA efflux mechanisms are probably saturated
higher than in other animals; AST is less specific for muscle
and rapid accumulation of intracellular LA leads to
than for CK, as AST is found in many tissues and organs.
muscular acidosis [25]. Further high-intensity contractions
Muscle use affects AST level, and an increase in plasma AST
cause loss of intracellular K with the accumulation of
activity has been observed in response to exercise [31]. This
extracellular Kþ, which is associated with muscle fatigue.
increase is related either to overt damage or to a change in
Therefore, LA itself cannot be considered as an indicator of
the muscle fiber membrane, causing a transient increase in
fatigue, as has been stated in some earlier studies [26]. LA,
permeability. Increased AST has been shown to occur
however, contributes to fatigue by increasing muscular
without any tissue destruction. Moreover, working horses
acidosis. Usually, the highest LA concentrations are seen
have approximately 60% higher AST activity compared with
2-10 minutes after exercise. Blood LA concentration has
horses that are at rest for several days [32]. At the begin-
been used as an indicator of training intensity and perfor-
ning of our study, resting serum AST activity was typical for
mance [27]. Blood LA assessment in the athletic horse is
race horses (300-360 U/L). Four weeks of dietary supple-
necessary to evaluate the onset of LA accumulation as an
mentation resulted in a significant decrease of resting AST
indicator of the aerobic capacity [28] and is useful for
activity in horses receiving HMB. We observed trends for
assessing fitness in equine athletes [29]. In equines, LA may
a postexercise increase in AST activity only in horses
be stored in red blood cells [30], and so measuring LA in
receiving the placebo supplement, which may indicate
the whole blood is necessary for determining total LA
exercise-associated minor muscle damage in that group. In
production. We used the Accusport technique to determine
contrast, a significant decrease in resting AST activity in
plasma LA, which was then converted to whole blood LA
horses fed HMB may suggest the potential effectiveness of
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
this leucine metabolite to reduce muscle damage or protein
Recently, Kreider et al. [36], in the official position
paper of the International Society of Sports Nutrition, rated
In our study, GO and HMB administered either together
HMB in the second highest category of possible effective
or separately did not counteract the postexercise increase
in plasma CK activity during the training season. This
enhancement in human athletes. Although HMB has gained
increase was, however, significantly less than in exercised
the reputation of an effective dietary supplement for
horses from placebo group. Similar differences were
training humans, more studies are needed to confirm the
observed during the racing season. Therefore, both GO and
effectiveness of its supplementation in sport horses. In the
HMB may attenuate muscle CK leakage; however, their
same paper, based on available literature, GO was classified
mechanism of action may be different. Oxidative stress is
as an apparently not effective dietary supplement.
a detrimental imbalance in the oxidativeeantioxidativesystem in cells and may damage DNA and cell membranes,
Increased oxidation during exercise may be related to
This field study, performed on 22 trained Thoroughbred
muscle enzyme leakage and microtrauma, hydration status,
horses, is the first showing that dietary supplementation
and animal welfare. Therefore, the use of GO as a potent
with GO and HMB may significantly improve training
antioxidant for human athletes as well as for horses is
results by decreasing muscle damage caused as a result of
widespread. So far, two studies in horses have failed to
the intensity of the training. The current study has shown
demonstrate any reduction in CK after supplementation
that GO does not significantly affect performance-related
with the antioxidants vitamin C [33] and a-tocopherol [34].
physiological parameters in training Thoroughbred race
Similarly, Piercy et al. [35] found no attenuation in the CK
horses; however, when GO is supplemented with HMB, the
increase in exercising sled dogs as a result of feeding an
training results in increased performance outcomes, such
antioxidant supplement containing vitamins C, E, and b-
as decreased muscle damage and improved recovery.
carotene. The results of the present study would be the first
Further studies investigating the effects of ergogenic
to indicate that GO, a powerful inhibitor of reactive oxygen
supplements in training and racing horses should be
species formation, may reinforce cell membrane and
conducted to validate the use of these ergogenic supple-
decrease its permeability with less CK released into the
ments in horses.
plasma. The results of the present study would seem to bein agreement with our observations that horses receiving
GO had significantly lower postexercise total antioxidantstatus and thiobarbituric acid reactive substance level than
The study was supported by the State Committee for
horses from other groups (unpublished data).
Scientific Research, Poland (grant number N N308 3076 33
In contrast to GO, HMB is not considered as an antiox-
for P.O.). The authors thank Mrs Ma1gorzata Podgurniak
idant but attenuates the loss of muscle mass and function
and Dr John C. Fuller for their assistance with the manu-
in various conditions such as resistance exercise training,
script preparation.
cancer, and AIDS [3,4,8]. It is also a substrate for cellmembrane cholesterol synthesis, and therefore HMB may
help stabilize cell membranes during intense exercise.
Based on our present study, we are unable to say whether
[1] Nissen SL, Sharp RL. Effect of dietary supplements on lean mass and
GO and HMB administered together had any additive effect.
strength gains with resistance exercise: a meta-analysis. J Appl
They both act through different mechanisms; however,
[2] Panthon LB, Rathmacher JA, Baier S, Nissen S. Nutritional supple-
average number of starts per horse during the racing
mentation of the leucine metabolite b-hydroxy-b-methylbutyrate
season amounted 6.8, 4.3, 6.4, and 8.3 for placebo, GO,
(hmb) during resistance training. Nutrition 2000;9:734-9.
HMB, and GO þ HMB treatments, respectively. These data
[3] Clark RH, Feleke G, Din M, Yasmin T, Singh G, Khan FA, et al.
immunodeficiency virus-
indicate that HMB/GO-supplemented horses maintained
associated wasting using b-hydroxy-b-methylbutyrate, glutamine
better condition during the racing season amounted and
and arginine: a randomized, double-blind, placebo-controlled study.
had an overall 45% win rate compared with 24% for HMB,
J Parenter Enteral Nutr 2000;24:133-9.
[4] Kuhls DA, Rathmacher JA, Musngi D, Frish DA, Nielson J, Barber A,
16% for GO, and 25% for placebo groups. Thus, horses
et al. b-hydroxy-b-methylbutyrate supplementation in critically ill
receiving both GO and HMB dietary supplements per-
trauma patients. J Trauma 2007;62:125-32.
formed better during the racing season.
[5] May PE, Barber A, D'Olimpio JT, Hourichane A, Abumrad NN.
Reversal of cancer-related wasting using oral supplementation with
A significant increase in AST activity was observed only
a combination of b-hydroxy-b-methylbutyrate, arginine and gluta-
in GO-supplemented horses during the racing season
mine. Am J Surg 2002;183:471-9.
compared with training season. In contrast, during training
[6] Eley HL, Russel ST, Baxter JH, Mukerji P, Tisdale J. Signaling pathways
and racing seasons, AST activity remained the same in the
initiated by b-hydroxy-b-methylbutyrate to attenuate the depres-sion of protein synthesis in skeletal muscle in response to cachectic
HMB and GO þ HMB-supplemented groups. AST is less
stimuli. Am J Physiol Endocrinol Metab 2007;293:E923-31.
specific for muscle, and its increase may indicate not only
[7] Nissen SL, Abumrad NN. Nutritional role of the leucine metabolite
muscle damage but also hepatocyte damage. If GO had an
b-hydroxy-b-methylbutyrate (HMB). J Nutr Biochem 1997;8:300-11.
[8] Nissen S, Sharp R, Ray M, Rathmacher JA, Rice J, Fuller JC Jr, et al.
adverse effect on the liver, our study showed that HMB may
Effect of leucine metabolite b-hydroxy-b-methylbutyrate (HMB) on
prevent that adverse side effect. Moreover, only HMB
muscle metabolism during resistance exercise training. J Appl
treatment resulted in a postexercise increase in blood
[9] Knitter AE, Panton L, Rathmacher JA, Petersen A, Sharp R. Effects of
glucose level, indicating glucose-sparing effect and possible
b-hydroxy-b-methylbutyrate on muscle damage after a prolonged
gluconeogenesis in the liver.
run. J Apply Physiol 2000;89:1340-4.
Author's personal copy
P. Ostaszewski et al. / Journal of Equine Veterinary Science 32 (2012) 542-551
[10] Van Someren K, Edwards A, Howatson G. Supplementation with
[23] Evans D. Exercise testing in the field. In: Hinchcliff K, Geor RJ,
beta-hydroxy-beta-methylbutyrate (HMB) and alpha-ketoisocaproic
Kaneps AJ, editors. Equine Exercise Physiology. Saunders Elsevier;
acid (KIC) reduces signs and symptoms of exercise-induced muscle
2008. p. 12-27.
damage in man. Int J Sport Nutr 2005;15:413-24.
[24] Geor RJ, Hinchcliff KW, Sams RA. b-Adrenergic blockade augments
[11] Smith HJ, Mukerji P, Tisdale MJ. Attenuation of proteasome-induced
glucose utilization in horses during graded exercise. J Appl Physiol
proteolysis in skeletal muscle by b-hydroxy-b-methylbutyrate in
cancer induced muscle loss. Cancer Res 2005;65:277-83.
[25] Hodgson DR, Rose RJ. Effects of a nine-month endurance training
[12] Pimentel GD, Rosa JC, Lira FS, Zanchi NE, Ropelle ER, Oyama LM,
programme on muscle composition in the horse. Vet Rec 1987;121:
et al. b-hydroxy-b-methylbutyrate supplementation stimulates
skeletal muscle hypertrophy in rats via the mTOR pathway. Nutr
[26] Katz A, Shalin K. Regulation of lactic acid production during exercise.
J Appl Physiol 1988;65:509-18.
[13] Miller P, Sandberg L, Fuller JC. The effect of supplemental b-hydroxy-
[27] Eto D, Yamano S, Mukai K, Shigura T, Nasu T, Tokuriki M, et al. Effect
b-methylbutyrate on training and racing thoroughbreds. Proc 17th
of high intensity training on anerobic capacity of middle gluteal
Assoc Equine Sport Med 1998;17:23-4.
muscle in thoroughbred horses. Res Vet Sci 2004;76:139-44.
[14] Kim JS, Godber JS, Prinaywiwatkul W. Restructured beef roasts
[28] Piccione G, Messina V, Casella S, Giannetto C, Caola G. Blood lactate
containing rice bran oil and fiber influences cholesterol oxidation
levels during exercise in athletic horses. Comp Clin Pathol 2010;19:
and nutritional profile. J Muscle Foods 2000;11:111-27.
[15] Fry AC, Bonner E, Lewis DL, Johnson RL, Stone MH, Kraemer WJ. The
[29] Williamson CC, James EA, James MP, May CD, Casey PJ. Horse plasma
effects of gamma-oryzanol supplementation during resistance
lactate determinations: comparison of wet and dry chemistry
exercise training. Int J Sport Nutr 1997;7:318-29.
methods and the effect of storage. Equine Vet J 1996;28:337-8.
[16] Rogers CW, Rivero JLL, van Breda E, Lindner A, van Oldruitenborgh-
[30] Vaihkonen LK, Hyyppa S, Poso AR. Factors affecting accumulation of
Oosterbaan MM. Describing workload and scientific information on
lactate in red blood cells. Equine Vet J Suppl 1999;30:443-7.
conditioning horses. Equine Comp Exerc Physiol 2007;4:1-6.
[31] O'Connor CI, Lawrence LM, Lawrence AC, Janicki KM, Warren LK,
[17] Van Ginneken MME. Adaptation of signal transduction and muscle
Hayes S. The effect of dietary fish supplementation on exercising
proteome in trained horses. PhD Thesis, 2006, The Netherlands,
horses. J Anim Sci 2004;82:2978-84.
University of Utrecht.
[32] Weigert P, Scheck K, Lemmer B, Noreisch W. Labordiagnostische
[18] Harris P, Marlin DJ, Davidson H, Rodgerson J, Gregory A, Harrison D.
Haflinger Pferden und Maultieren (Tragtiere der Buneswehr).
Practical assessment of heart rate response to exercise under
Enzymaktivitaten im Serum. Tierärztl. Praxis 1980;8:387.
field conditions. Equine and Comparative Exercise Physiol 2007;4:
[33] White A, Estrada M, Walker K, Wisnia P, Filgueria G, Valdes F, et al.
Role of exercise and ascorbate on plasma antioxidant capacity in
[19] Evans DL, Rainger JE, Hodgson DR, Eaton MD, Rose RJ. The effects of
Thoroughbred race horses. Comp Biochem Physiol 2001;128:99-104.
intensity and duration of training on blood lactate concentration
[34] Siciliano PD, Aparker AL, Lawrence LM. Effect of dietary vitamin
during and after exercise. Equine Vet J Suppl 1995;18:422-5.
supplementation on the integrity of skeletal muscle in exercised
[20] Inoue Y, Matsui A, Asai Y, Aoki F, Matsui T, Yano H. Effect of exercise
horses. J Anim Sci 1996;75:1553-60.
on iron metabolism in horses. Biol Trace Elem Res 2005;107:
[35] Piercy RJ, Hinchcliff KW, DiSilestro RA, Reinhart GA, Baskin CR,
Hayek MG, et al. Effect of dietary supplementation containing
[21] Schott HC, Hodgson DR, Bayly WM. Haematuria, pigmenturia and
antioxidants on attenuation of muscle damage in exercising sled
proteinuria in exercising horses. Equine Vet J 1995;27:67-72.
dogs. Am J Vet Res 2000;61:1438-45.
[22] Kingston JK. Hematologic and serum biochemical responses to
[36] Kreider RB, Wilborn CD, Taylor L, Campbell B, Almada AL, Collins R,
exercise and training. In: Hinchcliff K, Geor RJ, Kaneps AJ, editors.
et al. Exercise and sports nutrition review: research and recom-
Equine Exercise Physiology. Saunders Elsevier; 2008. p. 398-409.
mendations. J Int Soc Sports Nutr 2010;7:7.
Source: http://equievo.pl/wp-content/uploads/2014/12/Effects.pdf
PSYCHOTROPIC MEDICATIONS JUDICIAL REFERENCE GUIDE (Revised Edition 7/15/10) PSYCHOTROPIC MEDICATIONS JUDICIAL REFERENCE GUIDE FIRST EDITION THE STEERING COMMITTEE ON FAMILIES AND CHILDREN IN THE COURT Distributed by Florida Supreme Court 500 South Duval Street Tallahassee, FL 32399-1900 INTRODUCTION One of the toughest challenges facing our dependency courts is the mental health of our children. "In July 2003, the Florida Statewide Advocacy Council published a Red Item Report finding 55% of foster children…in the state of Florida had been put on powerful mind altering psychotropic drugs."1 In order to assist in this regard, the Psychotherapeutic Medication Subcommittee of the Steering Committee on Families and Children in the Court of the Supreme Court of Florida compiled this resource guide to help judges have a better understanding of psychotropic medications and their interaction with other drugs and with mental health disorders. Recently, the tragic case of Gabriel Myers in 2009 highlighted the fact that a number of child deaths were linked to the off label use of anti-psychotic medications. This is of special concern to Dependency Judges who are ultimately responsible for children in Florida's Foster Care system. The researchers used publically available data from the internet, FDA manufactures' published guidelines, publically available non-copyrighted articles and Dr. Brenda Thompson graciously prepared the Psychotropic Medication Chart. Special thanks to Dr. Brenda Thompson, the Honorable Herbert J. Baumann, the Honorable Ralph C. Stoddard, General Magistrate Tracy Ellis, Avron Bernstein, Selena Schoonover, Daniel Ringhoff, Jovasha Lang and to the Members of the Psychotherapeutic Medication Subcommittee.
Naremburn Mat ers June 2014 Vol.10, No.1 Circulation 3,000 Naremburn library grand reopening Thank-you to al who were able to join us at the of icial reopening celebration of the Naremburn Library and Community Centre on Saturday 3 May 2014. It was a fantastic day despite the cold and the poor weather at the beginning. There were activities for children, including face painting, story











