
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Khat
34th ECDD 2006/4.4 khat
Assessment of khat (
Catha edulis Forsk)
Substance Identification
A. International Nonproprietary Name (INN): - B. Chemical Abstract Service (CAS) Registry Number: - C. Other Names: qat, q'at, kat, kath, gat, chat, tschat (Ethiopia), miraa (Kenya), murungu; the
dried leaves of khat are known as Abyssinian tea or Arabian tea;
D. Trade Names: - E. Identification Characteristics: Khat refers to the leaves and the young shoots of the plant
Catha edulis Forsk, a species belonging to the plant family Celastraceae.
F. WHO Review History: The WHO Expert Committee on Drug Dependence (ECDD) and
other UN bodies reviewed khat at earlier occasions. The Advisory Committee on the Traffic in Opium and Other Dangerous Drugs of the League of Nations discussed on the subject in 1933, but no action was taken. On request of the Commission on Narcotics Drugs (CND), the Expert Committee on Addiction-Producing Drugs (as the ECDD was named at the time) reported in its 12th report (1962) that clarification on the chemical and pharmacological identification of the active principles of khat was needed before a sound medical appraisal of the chronic use of khat could be made [1]. No referral was made to a study on the effects. In its 13th report (1964) the committee studied a report by the Secretariat on the medical aspects of the habitual chewing of khat leaves. It concluded "that the problems connected with khat and with the amphetamines should be considered in the same light because of the similarity of their medical effects, even though there are quantitative differences and specific socio-economic features; this is all the more desirable since the problems with respect to khat are confined at present to a few countries in one region" [2]. In 1971 CND recommended again to ECOSOC to ask WHO to review khat, and requested that the UN Narcotics Laboratory should undertake research on the chemistry of khat and its components. These studies were reported in a series of internal UN documents from 1974 through 1978 [3]. In 1978 an Expert group financed by the UN Fund for Drug Abuse Control convened to consider the botany and chemistry of khat [4]. However, in the reports of ECDD no review of khat could be found after 1971. In 1983 an international conference on khat took place in Madagascar [5]. In 2002 the 33rd ECDD pre-reviewed khat and concluded that there was sufficient information on khat to justify a critical review [6].
Chemistry
The environment and climate conditions determine the chemical profile of khat leaves. In the Yemen Arab Republic, about 44 different types of khat exist originating from different geographic areas of the country [7,8]. Its taste varies from one kind to another and depends on
34th ECDD 2006/4.4 khat the tannic acid content. Khat leaves have an astringent taste and have an aromatic odour. The young leaves are slightly sweet. Many different compounds are found in khat including alkaloids, terpenoids, flavonoids, sterols, glycosides, tannins, amino acids, vitamins and minerals [9-11]. The phenylalkylamines and the cathedulins are the major alkaloids. The cathedulins are based on a poly-hydroxylated sesquiterpene skeleton and are basically polyesters of euonyminol. Recently, 62 different cathedulins from fresh khat leaves were characterized [12]. The khat phenylalkylamines comprise cathinone [S-(–)-cathinone], and the two diastereoisomers cathine [1S,2S-(+)-norpseudoephedrine or (+)-norpseudoephedrine] and norephedrine [1R,2S-(–)-norephedrine]. These compounds are structurally related to amphetamine and noradrenaline. The plant contains the (–)-enantiomer of cathinone only; the (+)-enantiomer is not found [11]. Thus, the naturally occurring S-(–)-cathinone has the same absolute configuration as S-(+)-amphetamine. Cathinone is mainly found in the young leaves and shoots. During maturation, cathinone is metabolised to cathine [(+)-norpseudoephedrine] and (-)-norephedrine. The leaves contain [(+)-norpseudoephedrine] and (-)-norephedrine in a ratio of approximately 4:1 [11]. Other phenylalkylamine alkaloids found in khat leaves are the phenylpentenylamines merucathinone, pseudomerucathine and merucathine. These seem to contribute less to the stimulant effects of khat [10,13,14] and will not be reviewed here. Cathinone is unstable and undergoes decomposition reactions after harvesting and during drying or extraction of the plant material [10,11,15,16]. Decomposition leads to a ‘dimer' (3,6-dimethyl-2,5-diphenylpyrazine) and possibly to smaller fragments. Both the dimer and phenylpropanedione have been isolated from khat extracts [15]. As cathinone is presumably the main psychoactive component of khat, this explains why fresh leaves are preferred and why khat is wrapped up in banana leaves to preserve freshness. The phenylalkylamine content of khat leaves varies within wide limits. Fresh khat from different origin contained on the average 36 mg cathinone, 120 mg cathine, and 8 mg norephedrine per 100 gram of leaves [7]. Toennes et al. (2003) found 114 mg cathinone, 83 mg cathine and 44 mg norephedrine in 100 gram of khat leaves confiscated at Frankfurt airport [17]. Widler et al. (1994) found 102 mg cathinone, 86 mg cathine and 47 mg norephedrine in 100 gram of fresh leaves from Kenya confiscated at Geneva Airport [18]. Al-Motarreb et al. (2002) reported higher levels of cathinone in fresh leaves: 78 – 343 mg/100 gram [8]. Khat leaves also contain considerable amounts of tannins (up to 10% in dried material) and flavonoids [8,19]. A.
Chemical Names:
cathinone: S-(–)-cathinone; S-(–)-α-aminopropiophenone; (S)-2-
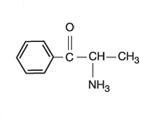
34th ECDD 2006/4.4 khat B.
Chemical Structures:
Chemical Formulas: cathinone: C9H11NO
cathine and norephedrine: C9H13NO
Molecular Weights: cathinone:
cathine and norephedrine: 151.2
Stereoisomers: Cathinone refers to the naturally occurring S-(–)-cathinone;
cathine to the naturally occurring 1S,2S-(+)-norpseudoephedrine; and norephedrine to the naturally occurring 1R,2S-(–)-norephedrine.
General pharmacology
Khat contains many different compounds and therefore khat chewing may have many different effects. The major effects include those on the gastro-intestinal system and on the nervous system. Constipation, urine retention and acute cardiovascular effects may be regarded as autonomic (peripheral) nervous system effects; increased alertness, dependence, tolerance and psychiatric symptoms as effects on the central nervous system. As cathinone, and to a lesser extent cathine, are held responsible for the effects of khat on the nervous system, the effects of the many other constituents of the khat plant are frequently overlooked. As a consequence, much research has been focused on the pharmacological effects of cathinone and cathine, and much less on the other constituents of khat. Because of the large number of different compounds in khat, it is not feasible to include all effects of all components of khat. As this report is submitted to the Expert Committee on Drug Dependence of the WHO, it focuses on the psychoactive properties of khat and the main psychoactive compounds, cathinone and cathine, found in khat. In addition, the main health effects of chronic khat chewing are described.
34th ECDD 2006/4.4 khat
Animal Studies
Behavioral effects Rats fed C. edulis material (extract or whole) show increased locomotor activity and reduced weight gain [20]. Retardation of growth rate was considered to be due to decreased absorption of food and not due to decreased food consumption. In pregnant rats, khat reduces food consumption and maternal weight gain, and also lowers the food efficiency index [21]. Many reports have since confirmed the enhanced locomotor activity. In addition, khat extracts and (–)-cathinone produce stereotyped behavior, self-administration and anorectic effects in animal species [15,22-26]. Qualitatively, this behavior is similar to that evoked by amphetamine [S-(+)-amphetamine] [27,28]. Both khat extract and (–)-cathinone enhance baseline aggressive behavior of isolated rats [29]. Furthermore, (–)-cathinone is capable of producing conditioned place-preference in rats at the dose (1.6 mg/kg) that produces increased locomotor activity, thus showing the rewarding effect of the drug [30,31]. A lower dose of cathinone (0.2 mg/kg) that did not increase locomotion, also failed to show conditioned place preference. Cathinone is also able to act as a discriminative stimulus in a food-reinforced operant task [32]. (–)-Cathinone appears to have stronger effects than cathine [(+)-norpseudoephedrine] and norephedrine [(–)-norephedrine] [11,27]. For example, it was 7-10 times more potent than cathine on a behavioral measure of food intake [33]. Compared to cathine, cathinone also has a more rapid onset of action, which agrees with its higher lipophilic character facilitating entry into the central nervous system, and a shorter duration of action, which agrees with the rapid metabolism of cathinone [11,27,33]. Dopaminergic antagonists (e.g. haloperidol) and dopamine release inhibitors are able to partially block the activity-enhancing properties of (–)-cathinone [23,34], but this has not been confirmed in another study [35]. Generally, cathinone is not considered a direct dopamine agonist but rather a presynaptic releaser and re-uptake inhibitor of dopamine [10]. (–)-Cathinone also releases radioactivity from rat striatal tissue pre-labelled with 3H-serotonin, similar to (+)-amphetamine although one-third as potent [36]. Apparently, (–)-cathinone shares important effects of (+)-amphetamine on neurotransmission. Further evidence for serotonergic involvement is given in a recent study in which both khat extract and cathinone produced a significant depletion of serotonin and its metabolite 5-hydroxyindoleacetic acid in both the anterior and posterior striatum [29]. Locomotor sensitisation and deficits in prepulse inhibition (PPI) induced by psychostimulants are two paradigms that have been widely studied as animal behavioral models of amphetamine psychosis. Repeated oral administration of a standardised C. edulis extract (containing a dose of 1 mg cathinone per kg body weight) or (–)-cathinone (1.5 mg/kg) to rats induced a strong locomotor sensitisation and led to a gradual deficit in prepulse inhibition [37,38]. The behavioral sensitisation was long-lasting and persisted after cessation of the treatments, comparable to amphetamine-induced sensitisation. Clozapine, an atypical antipsychotic agent, was able to reverse this behavioral sensitisation and the PPI deficits induced by C. edulis extract or cathinone [38]. These results may support the reports on khat-induced psychosis in humans. Neurotransmitter level analyses showed a significant increase in the level of dopamine in the prefrontal cortex (p<0.05). There was also a significant decrease in the level of serotonin in the nucleus accumbens (p < 0.05) and its metabolite 5-hydroxyindoleacetic acid in the prefrontal cortex (p<0.01). In the remaining regions (anterior and posterior striatum) no significant changes were found.
34th ECDD 2006/4.4 khat Cardiovascular effects Cathinone has vasoconstrictor activity in isolated perfused hearts from guinea pigs [39]. The effect was unlikely to be due to an indirect action by release of noradrenaline from sympathetic nerve endings or due to a direct action on alpha1-adrenoreceptors. (–)-Cathinone is able to potentiate noradrenaline-evoked contractions of the rat right ventricle [40] and to inhibit the uptake of noradrenaline into ventricular slices by a mechanism involving competitive blockade of the noradrenaline transporter [41]. The vasoconstrictor activity of cathinone explains the increase in blood pressure seen in humans [42] and in animals [43], and might be related to the increased incidence of myocardial infarction occurring during khat sessions, i.e. during the khat-effective period [44], and associated with heavy khat chewing [45].
Effects on the adrenocortical function In rabbits, a khat extract given orally for 30 successive days induced a decrease in adrenal cholesterol, glycogen, ascorbic acid and an increase in adrenal phosphorylase activity, serum free fatty acids and urinary 17-hydroxycorticosteroids [46]. These results have been interpreted as a stimulating effect of khat on adrenocortical function. This effect was also seen after oral administration of cathinone and cathine (6.5 mg/kg).
Effects on the reproductive system Animal data are conflicting. Treatment of male mice with a khat extract over a period of 6 weeks produced a dose-dependent reduction in fertility rate in female mice in the first week after the 6-week khat treatment [47]. In cathinone-treated rats, a significant decrease in sperm count and motility, and an increase in the number of abnormal sperm cells were found [48]. Histopathological examination of the testes revealed degeneration of interstitial tissue, cellular infiltration and atrophy of Sertoli and Leydig cells in cathinone-treated animals. Cathinone also produced a significant decrease in plasma testosterone levels of the rats. Although both enantiomers of cathinone produced deleterious effects on male reproductive system, (–)-cathinone was found to be more toxic [48]. In contrast, rabbits fed khat for three months had an increased rate of spermatogenesis and the Leydig cells were in good condition [49]. In male adult olive baboon, crude khat extract (equivalent to 250 g leaves and shoots) given orally once a week during 2 months produced an increase in plasma testosteron levels and a decrease in the plasma levels of prolactin and cortisol [50]. The testosteron results are in contrast with earlier observations in humans [11] and rats [48]. In biopsies taken 1 month after the last khat administration, no histopathological changes were found in the testis, epididymis, liver, kidney and pituitary gland of the animals. This contrasts with results of cathinone on rabbit liver, which showed increasing chronic inflammation with porto-portal fibrosis in the tissue sections obtained from animals treated with both 20% and 30% C. edulis [51]. The doses and administration regimens were different and this may explain the differences. Khat given to pregnant guinea pigs reduces placental blood flow [52] and produces growth retardation in the offspring [53].
Studies in Humans
The main effects of khat chewing are on the central and peripheral nervous system, and on the oro-gastro-intestinal system.
Subjective effects Khat chewing induces a state of euphoria and elation with feelings of increased alertness and arousal. This is followed by a stage of vivid discussions, loquacity and an excited mood.
34th ECDD 2006/4.4 khat Thinking is characterised by a flight of ideas but without the ability to concentrate. However, at the end of a khat session the user may experience depressive mood, irritability, anorexia and difficulty to sleep [8,10]. Lethargy and a sleepy state follow the next morning. In a Yemen study with adult healthy volunteers, functional mood disturbances were reported during khat sessions (Hospital Anxiety and Depression scale). The effect on anxiety and depression was temporary and had disappeared the next day [54]. Many Yemenite users, however, believe that khat chewing improves their sexual desire and excitement [8]. Khat chewing induced anorexia and insomnia (delayed bedtime) resulting in late wake-up next morning and low work performance the next day [19]. In the study of Toennes et al. [17] subjects reported subjective feelings of alertness and being ‘energetic'. They did not report any severe adverse reactions. The chewing dose was 0.6 gram of khat leaves per kg of body weight resulting in a mean absorption dose of 45 mg of cathinone. This is about one-half to one-fourth of the regular khat dose chewed in sessions.
Effects on the urinary bladder Khat induces a fall in average and maximum urine flow rate in healthy men [19,55]. The urinary effects are probably mediated through stimulation of alpha1-adrenergic receptors by cathinone. This is indicated by the complete blockage of this effect by indoramin, a selective antagonist of alpha1-adrenergic receptors [55].
Effects on the gal bladder Khat chewing has no clinically significant effect on gal bladder motility [56].
Cardiovascular effects Khat chewing induces small and transient rises in blood pressure and heart rate [8,57-61]. Cathinone (0.5 mg base/kg of body weight) has similar effects coinciding with the presence of cathinone in blood plasma [42,62]. These effects could be blocked by the beta1-adrenoreceptor blocker atenolol, but not by the alpha1-adrenorecptor blocker indoramin, indicating mediation through stimulation of beta1-adrenoreceptors [59]. In a pharmacokinetic study, diastolic and systolic blood pressures were elevated for about 3 hours after chewing [17]. The rise of blood pressure already started before the rise of alkaloid plasma concentrations, indicating an initial study engagement effect. The dose used was about one quarter (0.6 g/kg) of a traditional khat session dose and chewing was for 1 hour. This resulted in a mean oral dose of 45 mg cathinone. This rather low dose did not affect heart rate, pupil size and reaction to light, and it did not induce rotary nystagmus or impairment of reaction. All participants reported the personal feeling of being alert and ‘energetic'. An impairment of other psychophysical functions could not be objectified [17]. In another study, diastolic and systolic blood pressure, mean arterial blood pressure, and heart rate were raised during the 3 hours of khat chewing and during the following hour [61].
Effects on the adrenocortical function Nencini et al. (1984) found that khat and cathinone increase adrenocorticotrophic hormone levels in humans [63].
Toxicology, including adverse reactions in humans
Khat use affects cardiovascular, digestive, respiratory, endocrine, and genito-urinary systems. In addition, it affects the nervous system and can induce paranoid psychosis and hypomanic illness with grandiose delusions [64]. The effects on the nervous system resemble those of amphetamine with differences being quantitative rather than qualitative [9,19,65-68].
34th ECDD 2006/4.4 khat The main toxic effects include increased blood pressure, tachycardia, insomnia, anorexia, constipation, general malaise, irritability, migraine and impaired sexual potency in men [10]. Mild depressive reactions have been reported during khat withdrawal or at the end of a khat session [11,54,69]. Frequent use of high doses may evoke psychotic reactions. Biochemically, khat leaves decreased plasma cholesterol, glucose and triglycerides in rabbits [70], and increased plasma alkaline phosphatase and alanine aminotransferase in white rabbits [49]. Histopathological signs of congestion of the central liver veins were observed with acute hepatocellular damage and regeneration. In addition, some kidney lesions were seen with the presence of fat droplets in the upper cortical tubules, acute cellular swelling, hyaline tubules, and acute tubular nephrosis. Spleen was not affected and the histoarchitecture of the testes and cauda epididymis was normal showing, however, increased rate of spermatogenesis. The amount of khat consumed by the rabbits cannot be evaluated from the details given. The authors reported that, in general, the activity and the behaviour of the animals were observed to be normal [49]. Acute effects include: ·
relief of fatigue, increased alertness, reduced sleepiness
mild euphoria and excitement; improved ability to communicate, loquacity
tachycardia, hypertension
moderate hyperthermia
mydriasis, blurred vision
anorexia, dry mouth
constipation (supposedly due to tannins, but amphetamines may also cause constipation)
psychotic reactions at high doses
irritability and depressive reactions at the end of a khat session
lethargy and sleepy state (next morning)
Long-term effects include: ·
psychotic reactions after chronic use; depressive reactions
irritative disorders of the upper gastro-intestinal tract (gastritis, enteritis)
cardiovascular disorders
impaired male sexual function, spermatorrhoea, impotence
periodontal disease, mucosal lesions (keratosis)
Adverse effects of khat may be summarised according to the system involved. Table 1 is from [9] with modifications and updated. Table 1. Reported and suggested adverse effects of khat in man [9] Cardiovascular system
tachycardia, palpitations, hypertension, arrhythmias, vasoconstriction, myocard infarction, cerebral hemorrhage, pulmonary edema
Respiratory system
tachypnoea, bronchitis
Gastro-intestinal system
dry mouth, polydipsia, dental caries, periodontal disease, chronic gastritis, constipation, hemorrhoids, paralytic ileus, weight loss, duodenal ulcer, upper gastro-intestinal malignancy
Hepatobiliary system
fibrosis, cirrhosis
34th ECDD 2006/4.4 khat Genito-urinary system
urinary retention, spermatorrhoea, spermatozoa malformations, impotence, libido change
Obstetric effects
low birth weight, stillbirths, impaired lactation
Metabolic and endocrine effects hyperthermia, perspiration, hyperglycaemia Ocular effects
blurred vision, mydriasis
Central nervous system
dizziness, impaired cognitive functioning, fine tremor, insomnia, headaches
Psychiatric effects
lethargy, irritability, anorexia, psychotic reactions, depressive reactions, hypnagogic hallucinations
Khat-induced psychosis
Khat chewing can induce two kinds of psychotic reactions. First, a manic illness with grandiose delusions and second, a paranoid or schizophreniform psychosis with persecutory delusions associated with mainly auditory hallucinations, fear and anxiety, resembling amphetamine psychosis [69,71-76]. Both reactions are exceptional and associated with chewing large amounts of khat [77,78]. Symptoms rapidly abate when khat is withdrawn [72,79,80]. In fact, khat withdrawal consistently appears to be an effective treatment of khat psychosis and anti-psychotics are usually not needed for full remission [69,78,79]. Nevertheless, in most cases described in the literature antipsychotic medication has been used to alleviate the symptoms. Khat psychosis, however, is an infrequent phenomenon, probably due to the physical limits of the amount of khat leaves that can be chewed [65,66,81]. Khat psychosis may be accompanied by depressive symptoms and sometimes by violent reactions [72]. It has been argued that khat chewing might exacerbate symptoms in patients with pre-existing psychiatric disorder [54]. Recently, a large study in the city of Hargeisa, Northwest Somalia, revealed a relationship between khat consumption and onset of psychotic reactions [82]. The results indicated that not khat consumption per se but rather early onset and excessive khat chewing were related to psychotic symptoms. In most cases a pattern of binge chewing (more than two ‘bundles' per day) preceded the onset of psychotic symptoms. Dhapdole and Omolo [77] studied psychiatric morbidity among khat users. In moderate users there was no excess morbidity. Chewing more than two bundles per day was associated with increased psychiatric morbidity. Case reports confirm that adverse effects occur at high doses of khat [76,83,84]. Cases in the UK were characterized by the solitary use of khat by young individuals and these may represent a vulnerable group [69]. This may explain contradictory results sometimes obtained. For example, in a large survey among Yemenite khat users the incidence of adverse psychological symptoms was not greater than in non-users [85]. In fact, there was a negative association between the incidence of phobic symptoms and khat use. On the other hand, a 34-year-old Somali woman living in the UK attempted suicide during a khat-induced paranoid psychosis [76].
Hypnagogic hallucinations
Hypnagogic hallucinations have been reported in chronic khat users [86]. These consist of continuous visual and/or auditory dreamlike experiences that accompany daily life and are not related to khat sessions. Patients may consider them as normal and do not usually report these hallucinations unless specifically asked about.
34th ECDD 2006/4.4 khat
Impairment of cognitive functions
Adverse effects of khat chewing include impairment of perceptual-visual memory and decision-speed cognitive functions [87]. This study was carried out in flight attendants during a standard aviation medical examination. Toennes and Kauert (2004) investigated plasma khat alkaloid concentrations in 19 cases suspected of driving under the influence of drugs. In all cases, cathinone or cathine was found in blood and urine, but an association between alkaloid concentrations and impaired driving could not be established. Nevertheless, the authors concluded that chronic khat use might lead to a marked deterioration of psychophysical functions [88].
Neurological complications
One case history of severe leukoencephalopathy associated with khat misuse has been reported [89]. EEG and MRI findings indicated progressive leukoencephalopathy but the link with khat use is not proven (coinciding).
Cardiovascular complications
An increased incidence of acute myocardial infarction presenting between 2 pm and midnight, i.e. occurring during khat sessions, has been found [44]. Recently, it has been reported that khat chewing is associated with acute myocardial infarction [45,90]. The authors concluded that khat chewing is an independent dose-related risk factor for the development of acute myocardial infarction with heavy chewers having a 39-fold increased risk [45]. Two cases of pregnant patients with chest pain, sinus tachycardia and hypertension have been described [91,92]. As khat intake was unknown at admission, the physicians were faced with a diagnostic dilemma. Only after repeated questioning, it appeared that the women had used khat in familial gatherings and that the symptoms had developed after khat use only. Khat chewing has also been reported to be a significant risk factor for acute cerebral infarction [93]. The prevalence of high blood pressure was significantly higher in the patient group than in the control group and this higher prevalence was associated with khat chewing. Another cardiovascular complication of khat chewing is the higher incidence of hemorrhoids and hemorrhoidectomy found in chronic khat chewers (62% and 45%) as compared to non-khat users (4% and 0.5%) [94].
Pronounced hyperthermia has been observed in rabbits treated with 24 mg/kg (–)-cathinone (two out of three animals died). When the dose was reduced to 16 mg/kg, behavioral symptoms were less pronounced and the hyperthermic response was less (1.9 °C rise) [95]. This response to (–)-cathinone was blocked by haloperidol and strongly inhibited by pimozide, two antagonists of amphetamine hyperthermia. The effect of (–)-cathinone on body temperature shares a phenomenon with the effect of (+)-amphetamine and 3,4-methylenedioxymethamphetamine (MDMA, XTC): hyperthermia at room temperatures and above, but hypothermia in animals kept below room temperature [11].
Oral and gastro-intestinal complications
As a consequence of its mode of consumption khat affects the oral cavity and the digestive tract [66]. A high frequency of periodontal disease has been suggested as well as gastritis [96] and chronic recurrent subluxation and dislocation of the temperomandibular joint [97]. Epidemiological studies, however, have yielded conflicting results. Several studies indicated no such detrimental effects of khat chewing and suggested beneficial effects on the
34th ECDD 2006/4.4 khat periodontium [98,99]. Another study could not show a significant role of khat chewing and suggested bad oral hygiene as a major factor in periodontal disease [100]. No significant association could be found between khat chewing and oral leukoplakia in a Kenyan study [101]. In a recent study, the authors concluded that khat chewing does not seem to increase the colonization of gingival plaque and that, in stead, khat chewing might induce a microbial profile compatible with gingival health [102]. Recently, oral keratotic lesions at the site of chewing [103] and plasma cell gingivitis (allergic reaction to khat) [104] have been reported. The tannins present in khat leaves are held responsible for the gastritis that has been observed [65,72].
Makki (1975) stressed the importance of khat when she found that most of the oral squamous cell carcinomas of her study patients were located in the buccal mucosa and lateral sides of the tongue, which comes into direct contact with the khat during chewing [105]. In a survey that reviewed cancers for the past two years in the Asir region of Saudi Arabia, 28 head and neck cancer patients were found [106]. Ten of these presented with a history of khat chewing. All were non-smoking chewers and all of them had used khat over a period of 25 years or longer. Eight of these ten presented with oral cancers. In some cases the malignant lesion occurred at exactly the same site where the khat bolus was held. The authors concluded that a strong correlation between khat chewing and oral cancer existed. In another study performed in Yemen, 30 of 36 patients suffering from squamous cell carcinoma (in the oral cavity: 17; oropharynx: 1; nasopharynx: 15; larynx: 3) were habitual khat chewers from childhood [107]. The authors considered khat as an important contributing factor. It was reported that 50% of khat chewers develop oral mucosal keratosis [98]. Keratosis of the oral buccal mucosa is considered as a pre-cancerous lesion that may develop into oral cancer [108]. Recently, Ali et al. (2004) reported that 22.4% of khat chewers had oral keratotic white lesions at the site of khat chewing, while only 0.6% of non-chewers had white lesions in the oral cavity [103]. The prevalence of these lesions and its severity increased with frequency and duration of khat use. In human leukaemia cell lines and in human peripheral blood leucocytes, khat extract, cathinone and cathine produced a rapid and synchronized cell death with all the morphological and biochemical features of apoptotic cell death [109].
Reproductive system
Detailed studies on the effects of khat on human reproduction are lacking. However, the available data suggest that chronic use may cause spermatorrhoe and may lead to decreased sexual functioning and impotence [65,110]. In chronic chewers, sperm count, sperm volume and sperm motility were decreased [111,112]. Deformed spermatozoa (65% of total) have been found in Yemenite daily khat users, with different patterns including head and flagella malformations in complete spermatozoa, aflagellate heads, headless flagella, and multiple heads and flagella [111]. In pregnant women, khat consumption may have detrimental effects on uteri-placental blood flow and as a consequence, on foetal growth and development [110]. Lower mean birth weights have been reported in khat-chewing mothers compared to non-using mothers indicating an association between khat chewing and decreased birth weight [113].
34th ECDD 2006/4.4 khat
Genotoxicity and teratogenic effects
Orally administered khat extract induced dominant lethal mutations in mice [47], chromosomal aberrations in sperm cells in mice [114], and teratogenic effects in rats [21]. With the micronucleus test to determine genetic damage, an 8-fold increase in micronucleated buccal mucosa cells was seen among khat chewing individuals living in the area of the horn of Africa [115]. Khat consumption did not lead to a detectable elevation of micronucleated bladder mucosa cells. Among heavy khat chewers, 81% of the micronuclei had a centromere signal indicating that khat is aneuploidogenic. The effect of khat, tobacco and alcohol was found to be additive. These results suggest that khat consumption, especially when accompanied by alcohol and tobacco consumption might be a potential cause of oral malignancy [115].
The euphoric effects of khat start after about 1 hour of chewing. Blood levels of cathinone start to rise within 1 hour and peak plasma levels are obtained 1.5 – 3.5 hours after the onset of chewing [116]. Maximum plasma levels in this study ranged from 41-141 ng/ml (mean 83 ng/ml) after a 1-hour chewing dose of 60 g fresh khat leaves per subject (cathinone: 0.8 – 1 mg/kg body weight). Cathinone was barely detectable at 0.5 h and 7.5 h, and not detectable any more after 24 hours. Six drug-naive male volunteers received a single khat dose of 54 – 71 gram fresh leaves, corresponding to 0.8 mg cathinone per kg body weight. Alkaloid-free khat, prepared by gentle extraction of the alkaloid fraction with 0.1 M hydrochloric acid, was used as the placebo. Chewing was for 1 hour. Maximal plasma concentrations of cathinone (127 ± 53 ng/ml) were attained after 127 ± 30 minutes (values given as the mean ± S.D.). The area under the plasma concentration-time curve from 0 to 9 hours was 415 ± 207 ng/ml.hr, and the terminal elimination half-life was 260 ± 102 minutes [18]. Comparing these values with those from an earlier study in which pure S-(–)-cathinone was given to subjects, shows that the time period for reaching the maximal plasma concentration was significantly shorter (72 ± 33 minutes) after administration of pure S-(–)-cathinone. The other values did not differ significantly [42]. In humans, after oral administration of synthesized cathinone (isomers, racemate) 22-52% was recovered in 24 h urine samples mainly as the aminoalcohols norephedrine and norpseudoephedrine. The main metabolite of S-(–)-cathinone was identified as R,S-(–)-norephedrine and the main metabolite of R-(+)-cathinone as R,R-(–)-norpseudoephedrine. Both aminoalcohols are apparently formed by a stereospecific keto reduction [117]. Metabolism is rapid and occurs already during first passage through the liver. Only 2% of administered cathinone was found unchanged in the urine [10,117]. The rate of metabolism is close to the rate of absorption, which limits cathinone blood levels reachable by chewing [9]. In humans, norephedrine and norpseudoephedrine are slowly absorbed and excreted almost unchanged in urine [11,118]. Recently, a pharmacokinetic study was performed with 4 volunteers and a dose of 0.6 gram of khat leaves per kg body weight, i.e. one fourth of a traditional khat session dose [17]. A two-compartment kinetic model with a two-segment absorption could describe the plasma concentration-time data with a tlag1 (first compartment) of 0.1-0.2 h and a tlag2 (second compartment) of more than 1.2 h. The oral mucosa was seen as the first absorption segment, where the major proportion of cathinone and cathine is absorbed (mean ± S.D.: 59 ± 21% for cathinone; 84 ± 6% for cathine). On the average, peak plasma levels were obtained after 2.3 h for cathinone, 2.6 h for cathine and 2.8 h for norephedrine. Chewing resulted in a high
34th ECDD 2006/4.4 khat extraction of the alkaloids with only 9.1 ± 4.2% remaining in the leaf residues. The mean ± S.D. khat dose was 0.63 ± 0.04 mg of cathinone, 0.45 ± 0.03 mg of cathine, and 0.26 ± 0.01 mg of norephedrine per kg of body weight. The mean ± S.D. terminal elimination half-life of cathinone was 1.5 ± 0.8 h and that of cathine 5.2 ± 3.4 h. The apparent volume of the central compartment for cathinone was 2.7 ± 1.6 L/kg and for cathine 0.7 ± 0.4 L/kg. The data obtained for norephedrine could be explained by the metabolism of cathinone into norephedrine [17]. It has been reported that khat chewing delays gastric emptying of a semi-solid meal [119]. It is not known whether this effect is caused by cathinone or by other khat constituents.
Dependence potential
According to [64], khat chewing may induce moderate but often persistent psychological dependence. Withdrawal symptoms after prolonged use are mild and may consist of lethargy, mild depression, slight trembling and recurrent bad dreams [64]. In fact, there are very few reports on khat dependence [120,121] and habitual users do not show serious problems when stopping use. Discontinuation results in improvement of sleep and appetite, and fewer constipation problems. Kennedy et al. (1984) concluded that, in general, remarkably few of the allegations regarding the direct effects of khat use on health were supported by his study [96].
Animal Studies
Self-administration Self-administration of khat has not been described but (–)-cathinone induces self-administration behavior in monkeys [22] and rats [122]. Prolonged use of psychostimulants has been suggested to induce long-lasting behavioral sensitisation involved in the acquisition and maintenance of addictive behavior. To evaluate the effect of repeated oral administration of C. edulis, male rats divided into five groups received: saline, (–)-cathinone (1.5 mg/kg), (+)-amphetamine (1.5 mg/kg) and standardized C. edulis extract (50 or 200 mg/kg) once daily for nine consecutive days and later challenged with the same psychostimulants after five days of abstinence. Behavioral activities were monitored using activity and sniffing boxes. The results demonstrated that the khat extracts induced a long-lasting behavioral sensitisation in rats, similar to cathinone and amphetamine [29]. Neurotransmitters analyses showed no significant changes in the basal levels of dopamine in most regions except that C. edulis extract (200 mg/kg) significantly reduced the level of dopamine and its metabolites dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) in the anterior caudate putamen (P < 0.05). In a study of cathinone versus cocaine self-administration and the effects of dopamine antagonists on cathinone self-administration in male rats it was found that the dose response curve for cathinone was shifted to the left of that for cocaine by a factor of approximately 2 [122]. Pretreatment with the dopamine D1-type receptor antagonist SCH 23390, at 10 mg/kg, significantly increased the number of infusions obtained. It was concluded that cathinone is a potent reinforcer in rats and a role was suggested for D1-type dopamine receptors in mediating its reinforcing effects [122]. In many other studies, similar results were obtained on the reinforcing effects of (–)-cathinone [22,31,123-127]. The (–)-isomer appeared to be more potent than the (+)-isomer [125]. Presynaptic dopamine release seemed to be involved [23,128].
34th ECDD 2006/4.4 khat Drug discrimination The conditioned place preference has been used as a parameter of dependence and abuse potential. Cathinone produces place preference in rats both by peripheral and by central administration [31]. Dopamine release inhibitors block this effect of cathinone [129].
Tolerance Rats previously trained to discriminate between 0.6 mg/kg S-(–)-cathinone and saline in a 2-lever, food-motivated operant task were administered S-(–)-cathinone at the same dose, every 8 hrs for 10 days. Results indicate that tolerance to the discriminative effects of S-(–)-cathinone can be produced within 10 days of chronic administration and recovery from this observed tolerance occurs within 15 days of cessation of chronic administration [130]. Tolerance and cross-tolerance between cathinone and cathine have been reported in many studies [131-134] and these effects are probably mediated by presynaptic dopamine release [135].
Withdrawal Studies going into the estimation of withdrawal effects after khat use have not been found.
Human Studies
Several authors have argued that regular consumption of khat seriously affects the social and economic life of the user [136-138]. The medical problems that arise from khat consumption are partly due to the sympathomimetic effects of the drug and partly to its effect on mental health. An effect of khat was observed in the Addiction Research Center Inventory (ARCI) scales Abuse Potential (p < 0.01), Motor Stimulation (p < 0.02), Amphetamine-Like Effect (p < 0.005), and Stimulation-Euphoria (p < 0.005), as well as in the visual analog scales Excited-Calm (p < 0.001) and Energetic-Lethargic (p < 0.001). These results provided evidence for the amphetamine-like stimulatory effects of khat leaves. These effects were similar to those observed after cathinone, 0.5 mg/kg body weight, although peak plasma concentrations of cathinone after khat were delayed [18,42]. Although cathinone is assumed to be the dependence-producing constituent, khat has low abuse potential in humans and khat dependence is mild. It is associated with consuming khat on a daily basis [10]. Mild craving and tolerance to the effects of khat do exist but there is no definite withdrawal syndrome. A habitual user may feel hot, lethargic and gripped with the desire to chew khat in the first two days [8]. During sleep nightmares are common but these stop after a few nights. 2 Cases (a 20-yr-old woman and a 26-yr-old man) with khat dependence have been described in the literature [121]. They were successfully treated with bromocriptine. The psychiatric manifestations (hyperactivity, mania, pressured speech) were similar to those induced by other stimulants such as amphetamine and cocaine [121].
Tolerance Tolerance is difficult to evaluate because chewing sets an upper limit to the amount of khat that can be consumed. Nevertheless, a certain degree of tolerance seems to develop to the increases in blood pressure, heart rate, respiratory rate and body temperature, and to the insomnia [11,63,66,139].
Withdrawal A khat withdrawal syndrome has not been described. Discontinuation after prolonged use may produce symptoms such as slight trembling, loss of energy, lethargy and depressive feelings but these symptoms are mild and resolve in short time [66]. In one study, 0.6% of khat
34th ECDD 2006/4.4 khat chewers reported to take khat because of dependence [140]. No signs of khat dependence were found in a survey held in Kenya among outpatients attending rural and urban health centres [141]. Stopping of khat chewing generally improves sleep, appetite, and constipation problems [8,139]. Due to its self-limiting way of administration, khat was not classified as an inevitably dependence producing drug [65,142].
Epidemiology of use and abuse, with an estimate of the abuse potential
Khat leaves are chewed habitually in the south-western part of the Arabian Peninsula and in the East African countries between Sudan and Madagascar, namely Djibouti, Ethiopia, Somalia, Kenya, Tanzania and Uganda. In addition to the reported health problems, the regular consumption of khat is associated with a variety of social and economic problems affecting the consumers and their families. The impact of khat chewing in Yemen is considerable. It is deep-rooted in the Yemenite society where khat is consumed in social gatherings with family and friends while holding conversations, smoking cigarettes and drinking tea and soft drinks. In Yemen khat users, mostly male, meet after noon and start masticating the leaves thoroughly one by one. In other countries, it is less predominantly a male habit and there is less social pressure to participate in khat sessions [66]. Much time is spent on buying and chewing khat leaves, which affects working hours and time with family [8]. For some, the daily cost of the khat habit exceeds their expenditure on food for their families. In 1992, an anti-khat association was formed to draw the nation's attention to the deleterious effects of khat chewing and to discourage its use. Usually, a person consumes 100-200 gram of the leaves. Young leaves are preferred because these have the highest stimulant activity. In 1990, khat consumption was estimated at about 5 million portions per day [42]. In the last decades, the khat ‘habit' has spread to other African countries and to Europe, to Australia and to the United States. In Europe, Australia and the United States, khat use is seen amongst immigrants from Yemen, Somalia and Ethiopia [108,143,144]. Some authors estimate that 10 million people chew khat worldwide [84]. A large survey study was carried out in three urban and three rural areas in Yemen. The sample constituted of 800 Yemenite adults (15-76 years), both male and female, with overrepresentation of students, state employees and housewives. The cross-sectional survey was undertaken with face-to-face interviews and no preset selection criteria regarding profession, socio-economic status, age or gender. The Symptoms Checklist-90 (SCL-90) was used containing 90 items, which cover nine scales of the following domains: somatisation, depression, anxiety, phobia, hostility, interpersonal sensitivity, obsessive-compulsive, hostility, interpersonal sensitivity, paranoia and psychoticism. At least one lifetime episode of khat use was reported in 81.6% of men and 43.3% of women. Male users tended to use more frequently. Khat use was not associated with adverse psychological symptoms [85]. In South-western Uganda, use and perception of khat was studied among 130 students, 35 law enforcement officials and 16 transporters [145]. In this sample, 32% had experience with chewing khat and 20% were still using khat. The authors concluded that knowledge of khat is becoming widespread in Uganda and that its consumption is increasing especially among youths and young adults. Khat is freely available in Ethiopia and is a highly valued export commodity in that country. The number of khat chewers has significantly increased in this country and khat consumption has become popular in all segments of the Ethiopian population [146]. Previously, khat was mainly cultivated in the eastern part of Ethiopia. Nowadays, it is grown in all parts of the
34th ECDD 2006/4.4 khat country. The Ethiopian authorities have recommended the banning of khat consumption from schools and workplace [146]. In a house-to-house survey held in 1997, 1200 adults from a rural Ethiopian community were interviewed to determine the prevalence of khat chewing and to find associations with health, social functioning, and economic well being [147]. Prevalence of khat use was 32%. Significant associations of khat use were found with physical illness, injuries, undernutrition, and mental distress. Mental distress was higher among frequent and daily users and among those who chewed khat for more than two years. In addition, sleep disturbances were significantly higher among khat users than among non-users. Social functioning, economic well-being, and problem drinking were not associated with khat use [147]. Provincial studies suggested a prevalence rate among males of between 50% and 75%, with some authors suggesting higher rates based on those of neighbouring countries. A prevalence of khat use of 44% was found in an Ethiopian sample of 25 juvenile delinquents drawn from the national Remand Home in Addis Ababa [148]. As the only such institution in the country, it provided a sample of respondents from all over Ethiopia. The relatively low prevalence of 44% suggested that khat is not associated with crime as previously thought. Low levels of other socially unacceptable habits were discovered, such as high levels of social deprivation, low parental education and widespread family separation. A study of rural Yemenite communities in Israel has reported a usage rate of 39% among 15- to 65-year-old males [149]. The prevalence and socio-demographic correlates of khat use have been determined in a large house-to-house survey carried out in the rural Ethiopian community of Butajira [140]. Over 10 thousand residents aged 15 years and above were interviewed. Of these, 58% were female and 74% were Muslim. Lifetime experience of khat chewing was 55.7% and the prevalence of current use was 50%. Among current chewers, 17.4% reported taking khat on a daily basis. Various reasons were given for chewing khat. Eighty percent of the chewers used it to obtain maximum concentration during prayer. There was a strong association of Muslim religion, smoking, and high educational level with daily khat chewing. The authors concluded that khat chewing affects a majority of the adult population of the Butajira district, in particular the most educated and most productive age group [140]. Among Somali refugees in the UK, war-related experiences, occupational status before migration and current khat use were found to be risk factors for anxiety, depression, suicidal thinking and symptoms of psychosis [150]. This study showed a higher prevalence of khat use in men (63%) than in women (17%). Examined patterns of use, perceptions, associated effects and problems of using khat in a sample of 94 Somalis (aged 11-26 yrs) in Sheffield, UK. A questionnaire regarding the use and perceptions of using khat was developed and administered. Findings indicate that khat chewing has a social dimension, occupies a significant proportion of one's time and may be associated with other drugs. Most respondents considered khat to be a problem among Somalis with some negative health and social effects, but they rationalized khat use citing personal pressures, socio-cultural and emotional problems faced as a result of dislocation from their home country, and need for recreation. Social intervention including counselling, health education and advice about khat was seen as necessary. It is suggested that excessive khat consumption among Somalis in UK should be seen in the wider context of a people dislocated from their country of origin as facilitating a deviant pattern of drug abuse [151]. Griffiths et al. (1997) studied patterns of khat use among 207 Somalis (aged 18-78 yrs) living in London [144]. Seventy-eight percent had used khat at some point in their lives and 67% reported khat use in the week prior to the interview. The majority (76%) used more khat in the
34th ECDD 2006/4.4 khat UK than in Somali. Some users reported moderate dependence. A minority reported severe problems. Adverse psychological effects included sleep problems, anxiety and depression. Medical problems associated with khat use were rare. The authors concluded that khat users who continue to use this drug when it is transplanted from a traditional context might experience difficulties. On the other hand, khat use can be seen as playing a positive role in supporting the cultural identity of the Somalian community [144]. In a recent study of khat use among Somalis in four English cities, 38% of the overall sample (602 interviews) had ever used khat in their lifetime and 34% had used it in the month prior to the interview [152]. The average frequency of khat use in this study was three days a week with 10% of last-month users chewing khat on a daily basis. Sleeping problems were reported by 65% (8% severe, 23% moderate, 34% mild), 51% (2% severe, 18% moderate, 14% mild) reported loss of appetite, and 44% (11% severe, 11% moderate, 22% mild) reported feeling the urge to chew khat. Out of 67 countries that answered a questionnaire sent out by WHO in October 2005, 9 countries responded that abuse in their country exists. In 25 countries it does not exist and the remainder have no knowledge of abuse in their countries or do not know. Out of the 9 countries with abuse only Kenya has a high prevalence, estimating the percentage using khat to 20 %. The other countries report that (ab-)use is confined to certain ethnic groups, in most countries to Somali, and often to adult men, although there is a tendency in several countries for use among women and adolescents. In Denmark it is 30-40 % (1350 people), in Sweden almost 100 % of the adult men (2000 - 3000 people). Except for Kenya, that reports that use of khat "is associated with dependence" (which is understood: with dependence of other substances), there are no reports that the situation is severe. In Sweden a project "to map and change the situation" started in 3 cities, after Somali women asked attention for problem. Here, one early observation was that the Somalis today are more worried that the younger generation will be influenced by the Swedish drug customs among young people then the khat abuse among compatriots. Only one case of emergency after use of khat was reported in the United States of America between 1995 and 2002 (DAWN) and no reports between 2001 and 2003 (TESS).
Nature and magnitude of public health problems
See the previous section 7 for a description of nature of public health problems. Socio-cultural and socio-economic problems are associated with chronic khat use. No quantitative data are available.
International control
Khat is not under international control at present, but, two substances that are usually present in khat, cathine and cathinone are, since in the early 1980s all amphetamine-like substances were placed group wise under international control. (22nd report, ECDD, TRS 729, 1985) Cathinone was included in Schedule I of the UN Convention on Psychotropic Substances in 1988 [18,58], and cathine was included in Schedule III of this Convention then.
10. National control
34th ECDD 2006/4.4 khat According to the literature selected for this review, khat is illegal in France and Switzerland but legal, at the time of writing, in the USA, the UK, the Netherlands and in most African countries [142]. Today it is illegal in the USA if it contains cathinone (schedule I) or cathine (schedule IV) (Answer to questionnaire). Other countries that prohibited khat are Sweden (1998), Eritrea (1993), Finland and Jordan. In Australia only licenced persons can import khat for personal use only up to a maximum of 5 kgs (answers to questionnaire).
11. Therapeutic and industrial use
None industrial use is reported. In the Kenyan region where the plant originates from, the use is reported among the Meru tribe for the treatment of erectile dysfunction, malaria, influenza, vomiting and headache (annex to a questionnaire).
12. Production, consumption and international trade
Khat is an evergreen plant grown by grafting and cultivated as a bush or small tree. It is mainly found in Ethiopia, Yemen, Somali, Sudan, Madagascar and South Africa, but it is also seen in Turkestan and Afghanistan [9]. It grows at altitudes of 1500-2500 meters and it usually reaches a height of 3-8 meters. Under optimal conditions the trees may reach 15-20 meters. The tree requires about 10 years to attain maturity, but the leaves and shoots are already harvested after 3-4 years [8,153]. Khat is harvested throughout the year. A tree gives two crops a year. The effects of khat are obtained by chewing the tender leaves and shoots. Fresh leaves are preferred as the psychoactive properties decrease after harvesting, in particular during drying of the plant material. This is caused by the decomposition of cathinone into an inactive ‘dimer' [15]. Immigrants have spread the use of the khat habit to Europe and the USA. Improved road and air transport have facilitated the wider distribution to other countries. Khat is harvested in the early morning hours and sold at markets in late morning. To preserve its freshness, khat is wrapped in banana leaves and sold as bundles (30-40 cm) of twigs, stems and leaves. In the UK, khat is not illegal and it has been estimated that about 7000 kg of khat pass through Heathrow Airport each week from where it is distributed into the UK and into other European countries [9].
13. Illicit manufacture and illicit traffic, and related information
The countries that prohibited khat report some seizures, the highest being Sweden with 9000 kgs yearly. There was an increasing tendency, but this might have flattened by now. (Answers to the questionnaire). In the Annual Reports Questionnaire (ARQ) for 2003 submitted by Governments to UNODC, 16 Governments reported seizures of khat in 2003 (Belgium, Canada, Eritrea, Finland, France, Germany, Ireland, Italy, New Zealand, Norway, South Africa, Sweden, Tanzania, United Arab Emirates, United States of America and Zambia). The largest seizures were reported by Canada (25 tones), Tanzania (12 tones), Sweden (8.9 tones), Germany (5.4 tones) and Norway (4.8 tones).
34th ECDD 2006/4.4 khat In the Annual Reports Questionnaire (ARQ) for 2004 submitted by Governments to UNODC, 12 Governments reported seizures of khat in 2004 (Bahrain, Belgium, Canada, France, Finland, Italy, FYR of Macedonia, Norway, Switzerland, United Arab Emirates, United States of America and Zambia). The largest seizures were reported by the United States (almost 50 tones), followed by Canada (13.5 tones), Norway (3.7 tones), Finland (2.1 tones) and Belgium (almost 2.1 tones).
14. Current international controls in place and their impact
Khat is currently not under international control.
15. References
Expert Committee on Addiction-Producing Drugs. World Health Organ Tech Rep Ser 1962;229:9.
WHO Expert Committee on Addiction-Producing Drugs. World Health Organ Tech Rep Ser 1964;273.
Schorno HX, Steinegger E. The phenylalkylamines of Catha edulis Forsk. The absolute configuration of cathinone. 1978;IX:1-11. UN Narcotics Laboratory Studies on the chemical composition of khat.
The Botany and Chemistry of Khat. Report of an Expert Group. 1979;GE. 79-10365:1-11. UN Narcotics Laboratory.
Baasher T, Sadoun R. The epidemiology of khat. 1983:1-29.
WHO Expert Committee on Drug Dependence. World Health Organ Tech Rep Ser 2003;915:i-26, back.
Geisshusler S, Brenneisen R. The content of psychoactive phenylpropyl and phenylpentenyl khatamines in Catha edulis Forsk. of different origin. J Ethnopharmacol 1987;19:269-277.
Al Motarreb A, Baker K, Broadley KJ. Khat: pharmacological and medical aspects and its social use in Yemen. Phytother Res 2002;16:403-413.
Cox G, Rampes H. Adverse effects of khat: A review. Adv Psychiatr Treatm 2003;9:456-463.
Nencini P, Ahmed AM. Khat consumption: a pharmacological review. Drug Alcohol Depend 1989;23:19-29.
Kalix P, Braenden O. Pharmacological aspects of the chewing of khat leaves. Pharmacol Rev 1985;37:149-164.
Kite GC, Ismail M, Simmonds MS, Houghton PJ. Use of doubly protonated molecules in the analysis of cathedulins in crude extracts of khat (Catha edulis) by liquid chromatography/serial mass spectrometry. Rapid Commun Mass Spectrom 2003;17:1553-1564.
Kalix P, Geisshusler S, Brenneisen R. The effect of phenylpentenyl-khatamines on the release of radioactivity from rat striatal tissue prelabelled with [3H]dopamine. J Pharm Pharmacol 1987;39:135-137.
Kalix P, Geisshusler S, Brenneisen R. Differential effect of phenylpropyl- and phenylpentenyl-khatamines on the release of radioactivity from rabbit atria prelabelled with 3H-noradrenaline. Pharm Acta Helv 1987;62:332-334.
Review of the pharmacology of khat. Report of a WHO advisory group. Bull Narc 1980;32:83-93.
Brenneisen R, Geisshusler S. Psychotropic drugs. III. Analytical and chemical aspects of Catha edulis Forsk. Pharm Acta Helv 1985;60:290-301.
34th ECDD 2006/4.4 khat 17.
Toennes SW, Harder S, Schramm M, Niess C, Kauert GF. Pharmacokinetics of cathinone, cathine and norephedrine after the chewing of khat leaves. Br J Clin Pharmacol 2003;56:125-130.
Widler P, Mathys K, Brenneisen R, Kalix P, Fisch HU. Pharmacodynamics and pharmacokinetics of khat: a controlled study. Clin Pharmacol Ther 1994;55:556-562.
Hassan NAGM, Gunaid AA, El Khally FMY, Murray-Lyon IM. The subjective effects of chewing Qat leaves in human volunteers. Ann Saudi Med 2002;22:34-37.
Maitai CK. The toxicity of the plant Catha edulis in rats. Toxicon 1977;15:363-366.
Islam MW, al Shabanah OA, al Harbi MM, al Gharably NM. Evaluation of teratogenic potential of khat (Catha edulis Forsk.) in rats. Drug Chem Toxicol 1994;17:51-68.
Yanagita T. Studies on cathinones: cardiovascular and behavioral effects in rats and self-administration experiment in rhesus monkeys. NIDA Res Monogr 1979;27:326-327.
Schechter MD. Dopaminergic mediation of a behavioral effect of l-cathinone. Pharmacol Biochem Behav 1986;25:337-340.
Gordon TL, Meehan SM, Schechter MD. Differential effects of nicotine but not cathinone on motor activity of P and NP rats. Pharmacol Biochem Behav 1993;44:657-659.
Calcagnetti DJ, Schechter MD. Increases in the locomotor activity of rats after intracerebral administration of cathinone. Brain Res Bull 1992;29:843-846.
Kalix P. Hypermotility of the amphetamine type induced by a constituent of khat leaves. Br J Pharmacol 1980;68:11-13.
Zelger JL, Schorno HX, Carlini EA. Behavioural effects of cathinone, an amine obtained from Catha edulis Forsk.: comparisons with amphetamine, norpseudoephedrine, apomorphine and nomifensine. Bull Narc 1980;32:67-81.
Goudie AJ. Comparative effects of cathinone and amphetamine on fixed-interval operant responding: a rate-dependency analysis. Pharmacol Biochem Behav 1985;23:355-365.
Banjaw MY, Miczek K, Schmidt WJ. Repeated Catha edulis oral administration enhances the baseline aggressive behavior in isolated rats. J Neural Transm 2005.
Calcagnetti DJ, Schechter MD. Reducing the time needed to conduct conditioned place preference testing. Prog Neuropsychopharmacol Biol Psychiatry 1992;16:969-976.
Schechter MD, Meehan SM. Conditioned place preference produced by the psychostimulant cathinone. Eur J Pharmacol 1993;232:135-138.
Schechter MD, Glennon RA. Cathinone, cocaine and methamphetamine: similarity of behavioral effects. Pharmacol Biochem Behav 1985;22:913-916.
Peterson DW, Maitai CK, Sparber SB. Relative potencies of two phenylalkylamines found in the abused plant Catha edulis, khat. Life Sci 1980;27:2143-2147.
Calcagnetti DJ, Schechter MD. Psychostimulant-induced activity is attenuated by two putative dopamine release inhibitors. Pharmacol Biochem Behav 1992;43:1023-1031.
Huang D, Wilson MC. Comparative discriminative stimulus properties of dl-cathinone, d-amphetamine, and cocaine in rats. Pharmacol Biochem Behav 1986;24:205-210.
34th ECDD 2006/4.4 khat 36.
Kalix P. Effect of the alkaloid (-)-cathinone on the release of radioactivity from rat striatal tissue prelabelled with 3H-serotonin. Neuropsychobiology 1984;12:127-129.
Banjaw MY, Schmidt WJ. Behavioural sensitisation following repeated intermittent oral administration of Catha edulis in rats. Behav Brain Res 2005;156:181-189.
Banjaw MY, Fendt M, Schmidt WJ. Clozapine attenuates the locomotor sensitisation and the prepulse inhibition deficit induced by a repeated oral administration of Catha edulis extract and cathinone in rats. Behav Brain Res 2005;160:365-373.
Al Motarreb AL, Broadley KJ. Coronary and aortic vasoconstriction by cathinone, the active constituent of khat. Auton Autacoid Pharmacol 2003;23:319-326.
Cleary L, Buber R, Docherty JR. Effects of amphetamine derivatives and cathinone on noradrenaline-evoked contractions of rat right ventricle. Eur J Pharmacol 2002;451:303-308.
Cleary L, Docherty JR. Actions of amphetamine derivatives and cathinone at the noradrenaline transporter. Eur J Pharmacol 2003;476:31-34.
Brenneisen R, Fisch HU, Koelbing U, Geisshusler S, Kalix P. Amphetamine-like effects in humans of the khat alkaloid cathinone. Br J Clin Pharmacol 1990;30:825-828.
Kohli JD, Goldberg LI. Cardiovascular effects of (-)-cathinone in the anaesthetized dog: comparison with (+)-amphetamine. J Pharm Pharmacol 1982;34:338-340.
Al Motarreb A, Al Kebsi M, Al Adhi B, Broadley KJ. Khat chewing and acute myocardial infarction. Heart 2002;87:279-280.
Al Motarreb A, Briancon S, Al Jaber N, Al Adhi B, Al Jailani F, Salek MS, Broadley KJ. Khat chewing is a risk factor for acute myocardial infarction: a case-control study. Br J Clin Pharmacol 2005;59:574-581.
Ahmed MB, el Qirbi AB. Biochemical effects of Catha edulis, cathine and cathinone on adrenocortical functions. J Ethnopharmacol 1993;39:213-216.
Tariq M, Qureshi S, Ageel AM, al Meshal IA. The induction of dominant lethal mutations upon chronic administration of khat (Catha edulis) in albino mice. Toxicol Lett 1990;50:349-353.
Islam MW, Tariq M, Ageel AM, el Feraly FS, al Meshal IA, Ashraf I. An evaluation of the male reproductive toxicity of cathinone. Toxicology 1990;60:223-234.
Al Mamary M, Al Habori M, Al Aghbari AM, Baker MM. Investigation into the toxicological effects of Catha edulis leaves: a short term study in animals. Phytother Res 2002;16:127-132.
Mwenda JM, Owuor RA, Kyama CM, Wango EO, M'arimi M, Langat DK. Khat (Catha edulis) up-regulates testosterone and decreases prolactin and cortisol levels in the baboon. J Ethnopharmacol 2005.
Al Habori M, Al Aghbari A, Al Mamary M, Baker M. Toxicological evaluation of Catha edulis leaves: a long term feeding experiment in animals. J Ethnopharmacol 2002;83:209-217.
Jansson T, Kristiansson B, Qirbi A. Effect of khat on uteroplacental blood flow in awake, chronically catheterized, late-pregnant guinea pigs. J Ethnopharmacol 1988;23:19-26.
Jansson T, Kristiansson B, Qirbi A. Effect of khat on maternal food intake, maternal weight gain and fetal growth in the late-pregnant guinea pig. J Ethnopharmacol 1988;23:11-17.
Hassan NA, Gunaid AA, El Khally FM, Murray-Lyon IM. The effect of chewing Khat leaves on human mood. Saudi Med J 2002;23:850-853.
34th ECDD 2006/4.4 khat 55.
Nasher AA, Qirbi AA, Ghafoor MA, Catterall A, Thompson A, Ramsay JW, Murray-Lyon IM. Khat chewing and bladder neck dysfunction. A randomized controlled trial of alpha 1-adrenergic blockade. Br J Urol 1995;75:597-598.
Murugan N, Burkhill G, Williams SG, Padley SP, Murray-Lyon IM. The effect of khat chewing on gallbladder motility in a group of volunteers. J Ethnopharmacol 2003;86:225-227.
Kalix P, Brenneisen R, Koelbing U, Fisch HU, Mathys K. [Khat, a herbal drug with amphetamine properties]. Schweiz Med Wochenschr 1991;121:1561-1566.
Kalix P. Cathinone, a natural amphetamine. Pharmacol Toxicol 1992;70:77-86.
Hassan NA, Gunaid AA, El Khally FM, Al Noami MY, Murray-Lyon IM. Khat chewing and arterial blood pressure. A randomized controlled clinical trial of alpha-1 and selective beta-1 adrenoceptor blockade. Saudi Med J 2005;26:537-541.
Nencini P, Ahmed AM, Elmi AS. Subjective effects of khat chewing in humans. Drug Alcohol Depend 1986;18:97-105.
Hassan NA, Gunaid AA, Abdo-Rabbo AA, Abdel-Kader ZY, al Mansoob MA, Awad AY, Murray-Lyon IM. The effect of Qat chewing on blood pressure and heart rate in healthy volunteers. Trop Doct 2000;30:107-108.
Kalix P, Geisshusler S, Brenneisen R, Koelbing U, Fisch HU. Cathinone, a phenylpropylamine alkaolid from khat leaves that has amphetamine effects in humans. NIDA Res Monogr 1991;105:289-290.
Nencini P, Ahmed AM, Amiconi G, Elmi AS. Tolerance develops to sympathetic effects of khat in humans. Pharmacology 1984;28:150-154.
Kalix P. Khat: a plant with amphetamine effects. J Subst Abuse Treat 1988;5:163-169.
Halbach H. Medical aspects of the chewing of khat leaves. Bull World Health Organ 1972;47:21-29.
Kalix P. Pharmacological properties of the stimulant khat. Pharmacol Ther 1990;48:397-416.
Tariq M, Al Meshal I, Al Saleh A. Toxicity studies on Catha edulis. Dev Toxicol Environ Sci 1983;11:337-340.
Dhaifalah I, Santavy J. Khat habit and its health effect. A natural amphetamine. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2004;148:11-15.
Pantelis C, Hindler CG, Taylor JC. Khat, toxic reactions to this substance, its similarities to amphetamine, and the implications of treatment for such patients. J Subst Abuse Treat 1989;6:205-206.
Al Habori M, Al Mamary M. Long-term feeding effects of Catha edulis leaves on blood constituents in animals. Phytomedicine 2004;11:639-644.
Dhadphale M, Mengech A, Chege SW. Miraa (catha edulis) as a cause of psychosis. East Afr Med J 1981;58:130-135.
Pantelis C, Hindler CG, Taylor JC. Use and abuse of khat (Catha edulis): a review of the distribution, pharmacology, side effects and a description of psychosis attributed to khat chewing. Psychol Med 1989;19:657-668.
Gough SP, Cookson IB. Khat-induced schizophreniform psychosis in UK. Lancet 1984;i:455.
McLaren P. Khat psychosis. Br J Psychiatry 1987;150:712-713.
34th ECDD 2006/4.4 khat 75.
Yousef G, Huq Z, Lambert T. Khat chewing as a cause of psychosis. Br J Hosp Med 1995;54:322-326.
Critchlow S, Seifert R. Khat-induced paranoid psychosis. Br J Psychiatry 1987;150:247-249.
Dhadphale M, Omolo OE. Psychiatric morbidity among khat chewers. East Afr Med J 1988;65:355-359.
Jager AD, Sireling L. Natural history of Khat psychosis. Aust N Z J Psychiatry 1994;28:331-332.
Nielen RJ, van der Heijden FM, Tuinier S, Verhoeven WM. Khat and mushrooms associated with psychosis. World J Biol Psychiatry 2004;5:49-53.
Giannini AJ, Castellani S. A manic-like psychosis due to khat (Catha edulis Forsk.). J Toxicol Clin Toxicol 1982;19:455-459.
Kalix P. Khat: scientific knowledge and policy issues. Br J Addict 1987;82:47-53.
Odenwald M, Neuner F, Schauer M, Elbert T, Catani C, Lingenfelder B, Hinkel H, Hafner H, Rockstroh B. Khat use as risk factor for psychotic disorders: a cross-sectional and case-control study in Somalia. BMC Med 2005;3:5.
Alem A, Shibre T. Khat induced psychosis and its medico-legal implication: a case report. Ethiop Med J 1997;35:137-139.
Stefan J, Mathew B. Khat chewing: an emerging drug concern in Australia? Aust N Z J Psychiatry 2005;39:842-843.
Numan N. Exploration of adverse psychological symptoms in Yemeni khat users by the Symptoms Checklist-90 (SCL-90). Addiction 2004;99:61-65.
Granek M, Shalev A, Weingarten AM. Khat-induced hypnagogic hallucinations. Acta Psychiatr Scand 1988;78:458-461.
Khattab NY, Amer G. Undetected neuropsychophysiological sequelae of khat chewing in standard aviation medical examination. Aviat Space Environ Med 1995;66:739-744.
Toennes SW, Kauert GF. Driving under the influence of khat--alkaloid concentrations and observations in forensic cases. Forensic Sci Int 2004;140:85-90.
Morrish PK, Nicolaou N, Brakkenberg P, Smith PE. Leukoencephalopathy associated with khat misuse. J Neurol Neurosurg Psychiatry 1999;67:556.
Alkadi HO, Noman MA, Al Thobhani AK, Al Mekhlafi FS, Raja'a YA. Clinical and experimental evaluation of the effect of Khat-induced myocardial infarction. Saudi Med J 2002;23:1195-1198.
Kuczkowski KM. Re: cathinone: a new differential in the diagnosis of pregnancy induced hypertension. East Afr Med J 2004;81:436.
Kuczkowski KM. Herbal ecstasy: cardiovascular complications of khat chewing in pregnancy. Acta Anaesthesiol Belg 2005;56:19-21.
Mujlli HM, Bo X, Zhang L. The effect of Khat (Catha edulis) on acute cerebral infarction. Neurosciences 2005;10:219-222.
Al Hadrani AM. Khat induced hemorrhoidal disease in Yemen. Saudi Med J 2000;21:475-477.
Kalix P. Hyperthermic response to (-)-cathinone, an alkaloid of Catha edulis (khat). J Pharm Pharmacol 1980;32:662-663.
34th ECDD 2006/4.4 khat 96.
Kennedy JG, Teague J, Rokaw W, Cooney E. A medical evaluation of the use of qat in North Yemen. Soc Sci Med 1983;17:783-793.
Kummoona R. Surgical reconstruction of the temporomandibular joint for chronic subluxation and dislocation. Int J Oral Maxillofac Surg 2001;30:344-348.
Hill CM, Gibson A. The oral and dental effects of q'at chewing. Oral Surg Oral Med Oral Pathol 1987;63:433-436.
Jorgensen E, Kaimenyi JT. The status of periodontal health and oral hygiene of Miraa (catha edulis) chewers. East Afr Med J 1990;67:585-590.
100. Mengel R, Eigenbrodt M, Schunemann T, Flores-de-Jacoby L. Periodontal status of a subject sample of
Yemen. J Clin Periodontol 1996;23:437-443.
101. Macigo FG, Mwaniki DL, Guthua SW. The association between oral leukoplakia and use of tobacco,
alcohol and khat based on relative risks assessment in Kenya. Eur J Oral Sci 1995;103:268-273.
102. Al Hebshi NN, Skaug N. Effect of khat chewing on 14 selected periodontal bacteria in sub- and
supragingival plaque of a young male population. Oral Microbiol Immunol 2005;20:141-146.
103. Ali AA, Al Sharabi AK, Aguirre JM, Nahas R. A study of 342 oral keratotic white lesions induced by qat
chewing among 2500 Yemeni. J Oral Pathol Med 2004;33:368-372.
104. Marker P, Krogdahl A. Plasma cell gingivitis apparently related to the use of khat: report of a case. Br
Dent J 2002;192:311-313.
105. Makki I. Oral carcinomas and their relationship to khat and shamma abuses. 1975. Germany: The
University of Heidelberg.
106. Soufi HE, Kameswaran M, Malatani T. Khat and oral cancer. J Laryngol Otol 1991;105:643-645.
107. Nasr AH, Khatri ML. Head and neck squamous cell carcinoma in Hajjah, Yemen. Saudi Med J
2000;21:565-568.
108. Goldenberg D, Lee J, Koch WM, Kim MM, Trink B, Sidransky D, Moon CS. Habitual risk factors for
head and neck cancer. Otolaryngol Head Neck Surg 2004;131:986-993.
109. Dimba EA, Gjertsen BT, Bredholt T, Fossan KO, Costea DE, Francis GW, Johannessen AC, Vintermyr
OK. Khat (Catha edulis)-induced apoptosis is inhibited by antagonists of caspase-1 and -8 in human leukaemia cells. Br J Cancer 2004;91:1726-1734.
110. Mwenda JM, Arimi MM, Kyama MC, Langat DK. Effects of khat (Catha edulis) consumption on
reproductive functions: a review. East Afr Med J 2003;80:318-323.
111. el Shoura SM, Abdel AM, Ali ME, el Said MM, Ali KZ, Kemeir MA, Raoof AM, Allam M, Elmalik EM.
Deleterious effects of khat addiction on semen parameters and sperm ultrastructure. Hum Reprod 1995;10:2295-2300.
112. Hakim LY. Influence of khat on seminal fluid among presumed infertile couples. East Afr Med J
113. Abdul GN, Eriksson M, Kristiansson B, Qirbi A. The influence of khat-chewing on birth-weight in full-
term infants. Soc Sci Med 1987;24:625-627.
114. Qureshi S, Tariq M, Parmar NS, al Meshal IA. Cytological effects of khat (Catha edulis) in somatic and
male germ cells of mice. Drug Chem Toxicol 1988;11:151-165.
34th ECDD 2006/4.4 khat 115. Kassie F, Darroudi F, Kundi M, Schulte-Hermann R, Knasmuller S. Khat (Catha edulis) consumption
causes genotoxic effects in humans. Int J Cancer 2001;92:329-332.
116. Halket JM, Karasu Z, Murray-Lyon IM. Plasma cathinone levels following chewing khat leaves (Catha
edulis Forsk.). J Ethnopharmacol 1995;49:111-113.
117. Brenneisen R, Geisshusler S, Schorno X. Metabolism of cathinone to (-)-norephedrine and (-)-
norpseudoephedrine. J Pharm Pharmacol 1986;38:298-300.
118. Maitai CK, Mugera GM. Excretion of the active principle of of Catha edulis (Miraa) in human urine. J
Pharm Sci 1975;64:702-703.
119. Heymann TD, Bhupulan A, Zureikat NE, Bomanji J, Drinkwater C, Giles P, Murray-Lyon IM. Khat
chewing delays gastric emptying of a semi-solid meal. Aliment Pharmacol Ther 1995;9:81-83.
120. Patel NB. Mechanism of action of cathinone: the active ingredient of khat (Catha edulis). East Afr Med J
2000;77:329-332.
121. Giannini AJ, Miller NS, Turner CE. Treatment of khat addiction. J Subst Abuse Treat 1992;9:379-382.
122. Gosnell BA, Yracheta JM, Bell SM, Lane KE. Intravenous self-administration of cathinone by rats. Behav
Pharmacol 1996;7:526-531.
123. Schechter MD, McBurney D. Effect of repeated administrations upon cathinone discrimination and
conditioned place preference. Gen Pharmacol 1991;22:779-782.
124. Schechter MD. Effect of learned behavior upon conditioned place preference to cathinone. Pharmacol
Biochem Behav 1991;38:7-11.
125. Yanagita T. Intravenous self-administration of (-)-cathinone and 2-amino-1-(2,5-dimethoxy-4-
methyl)phenylpropane in rhesus monkeys. Drug Alcohol Depend 1986;17:135-141.
126. Woolverton WL, Johanson CE. Preference in rhesus monkeys given a choice between cocaine and d,l-
cathinone. J Exp Anal Behav 1984;41:35-43.
127. Johanson CE, Schuster CR. A comparison of the behavioral effects of l- and dl-cathinone and d-
amphetamine. J Pharmacol Exp Ther 1981;219:355-362.
128. Schechter MD. Rats become acutely tolerant to cathine after amphetamine or cathinone administration.
Psychopharmacology (Berl) 1990;101:126-131.
129. Calcagnetti DJ, Schechter MD. Place preference for the psychostimulant cathinone is blocked by
pretreatment with a dopamine release inhibitor. Prog Neuropsychopharmacol Biol Psychiatry 1993;17:637-649.
130. Schechter MD. Induction of and recovery from tolerance to the discriminative stimulus properties of l-
cathinone. Pharmacol Biochem Behav 1986;25:13-16.
131. Knoll J. Studies on the central effects of (-)cathinone. NIDA Res Monogr 1979;27:322-323.
132. Foltin RW, Schuster CR. Behavioral tolerance and cross-tolerance to dl-cathinone and d-amphetamine in
rats. J Pharmacol Exp Ther 1982;222:126-131.
133. Foltin RW, Woolverton WL, Schuster CR. Effects of psychomotor stimulants, alone and in pairs, on milk
drinking in the rat after intraperitoneal and intragastric administration. J Pharmacol Exp Ther 1983;226:411-418.
34th ECDD 2006/4.4 khat 134. Zelger JL, Carlini EA. Anorexigenic effects of two amines obtained from Catha edulis Forsk. (Khat) in
rats. Pharmacol Biochem Behav 1980;12:701-705.
135. Schechter MD. Dopaminergic nature of acute cathine tolerance. Pharmacol Biochem Behav 1990;36:817-
136. Balint GA, Ghebrekidan H, Balint EE. Catha edulis, an international socio-medical problem with
considerable pharmacological implications. East Afr Med J 1991;68:555-561.
137. Balint GA, Balint EE. On the medico-social aspects of khat (Catha edulis) chewing habit. Hum
Psychopharmacol 1994;9:125-128.
138. Kalix P. Khat, an amphetamine-like stimulant. J Psychoactive Drugs 1994;26:69-74.
139. Luqman W, Danowski TS. The use of khat (Catha edulis) in Yemen. Social and medical observations.
Ann Intern Med 1976;85:246-249.
140. Alem A, Kebede D, Kullgren G. The prevalence and socio-demographic correlates of khat chewing in
Butajira, Ethiopia. Acta Psychiatr Scand Suppl 1999;397:84-91.
141. Othieno CJ, Kathuku DM, Ndetei DM. Substance abuse in outpatients attending rural and urban health
centres in Kenya. East Afr Med J 2000;77:592-595.
142. Adam F, Hasselot N. [Khat: from traditional usage to risk of drug addiction]. Med Trop (Mars)
1994;54:141-144.
143. Browne DL. Qat use in New York City. NIDA Res Monogr 1991;105:464-465.
144. Griffiths P, Gossop M, Wickenden S, Dunworth J, Harris K, Lloyd C. A transcultural pattern of drug use:
qat (khat) in the UK. Br J Psychiatry 1997;170:281-284.
145. Ihunwo AO, Kayanja FI, Amadi-Ihunwo UB. Use and perception of the psychostimulant, khat (catha
edulis) among three occupational groups in south western Uganda. East Afr Med J 2004;81:468-473.
146. Selassie SG, Gebre A. Rapid assessment of drug abuse in Ethiopia. Bull Narc 1996;48:53-63.
147. Belew M, Kebede D, Kassaye M, Enquoselassie F. The magnitude of khat use and its association with
health, nutrition and socio-economic status. Ethiop Med J 2000;38:11-26.
148. Metekie D en Hughes BM. A brief survey of khat use among juvenile delinquents in Addis Ababa,
Ethiopia. Ir J Psychol 2001;22:51-58.
149. Litman A, Levav I, Saltz-Rennert H, Maoz B. The use of khat. An epidemiological study in two Yemenite
villages in Israel. Cult Med Psychiatry 1986;10:389-396.
150. Bhui K, Abdi A, Abdi M, Pereira S, Dualeh M, Robertson D, Sathyamoorthy G, Ismail H. Traumatic
events, migration characteristics and psychiatric symptoms among Somali refugees--preliminary communication. Soc Psychiatry Psychiatr Epidemiol 2003;38:35-43.
151. Nabuzoka D en Badhadhe FA. Use and perceptions of khat among young Somalis in a UK city. Addiction
Res 2000;8:5-26.
152. Patel SL, Wright S,.Gammampila A. Khat use among Somalis in four English cities. 2005. Home Office
Online Report 47/05.
153. Nordal A. Khat: pharmacognostical aspects. Bull Narc 1980;32:51-64.
Source: http://eurad.net/filestore/PDF/WHO_expert_KhatCritReview.pdf
Ionic Liquids in Photopolymerizable Holographic Materials Hechun Lin and Peter W. de Oliveira INM – Leibniz Institute for New Materails, Campus D 2 2, Saarbruecken, 1. Introduction A variety of materials have been used to record hologram, such as silver halide emulsions, hardened dichromated gelatin, ferroelectric crystals, photochromics, photoresist, photodichroics and photopolymerizable materials [1-3]. Photopolymerizable holographic materials due to their low cost and dry processing have attracted great interest in academics and industry. They have broad applications in holographic memories, recording media, LCD displays, helmet-mounted display, optical interconnects, waveguide couples, holographic diffusers, laser eye protection devices, automotive lighting, and security holograms. The photopolymerizable holographic composite contains mainly a matrix binder, a photopolymerizable monomer, an initiator system, a plasticizer and additives [4-17]. Due to the inter diffusion of the unpolymerized monomers in a holographic film, areas with high and low refractive index are formed during the irradiation with an interference pattern. Many photopolymer systems have been developed including binary photopolymer composites, organic-inorganic nanocomposites, a hybrid organic-inorganic host consisting of porous glass, and a system using monomers capable of cationic ring-opening polymerization. The addition of a plasticizer or an additive can increase the refractive index modulation and the final diffraction efficiency. Monroe et al. reported that tri(2-ethylhexyl)phosphate, glyceryl tributyrate, polyethylene glycol or functional polyethylene glycol etc. as plasticizers may increase the refractive index modulation [18]. Frank recommended photopolymerizable compositions with triglycerides as additives, which provide a stable holographic material with high refractive index modulation [19]. Tucker et al. used trithiocarbonate as additive to increase the diffraction efficiency, uniformity and reproducibility in the formation of electrically switchable holographic gratings [20]. Finally, one publication reports about an additive to improve the sensitivity of photopolymerizable hologram material [21]. Ionic liquids are organic salts that are liquid at ambient temperatures, preferably at room temperature. They are nonvolatile, thermally and chemically stable, highly polar liquids, high ionic conductivity, large electrochemical window and ease of solubilization of a large organic molecules and transition metal complexes [22-25]. Applications of ionic liquids include their use in synthesis, catalysis, separation, electrochemistry, electrolytes, lubrication, biomass processing, drug delivery and others. The cations of ionic liquids are
Fichas Internacionales de Seguridad Química BENZO [a] ANTRACENO ICSC: 0385 BENZO a ANTRACENO 1,2-Benzoantraceno 2,3-Benzofenantreno Masa molecular: 228.3 Nº CAS 56-55-3 Nº RTECS CV9275000 Nº ICSC 0385 Nº NU 2811 PELIGROS/ SINTOMAS PRIMEROS AUXILIOS/ PELIGRO/ PREVENCION





