Outer and inside cover
Syllabus: Bachelor of Pharmacy OPJS UNIVERSITY, CHURU OPJS UNIVERSITY, CHURU (RAJASTHAN) SYLLABUS BACHELORS IN PHARMACY (B. Pharma) School of Pharmacy OPJS UNIVERSITY, CHURU (RAJASTHAN) Syllabus: Bachelor of Pharmacy


Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.





2-AR Agonist and 1-AR Blocker in Treatment of DCM
Survivors at 12 mo
β +*** - p<0.001 vs nT; ††† - p<0.001 vs 1
Fig. 2. Average MI size in untreated and treated rats estimated by monthly Echo (left) or on the basis of histological measurements (right) and
presented as percentage of LV. Top, all animals; bottom, animals that survived 12 months after the MI induction. ⴱⴱⴱ, p ⬍ 0.001 versus nT; †††, p ⬍
0.001 versus 1-ACEi (pairwise comparison, group ⫻ time interaction with Bonferroni's correction).
riod. The top panels represent average measurements from
group, however, was significantly less and did not differ
all animals at each time point, whereas lower panels repre-
statistically from nT.
sent only animals that survived to the end of the study. Both
The Echo-calculated LVM (Fig. 4A) increased in all MI
the top and bottom panels reflect similar patterns. In nT
groups compared with SH, and that increase in LVM was
animals, the LV volume monotonically increased by 90 and
reduced by all treatment modalities; however, the differences
120% for EDV and ESV, respectively, whereas EF declined
with nT were statistically significant in 1-ACEi group only
by 50%. At the end of the observation period, EDV in nT
(p ⬍ 0.05; see Supplemental Table 1). Posterior wall thick-
animals averaged 1170 ⫾ 75 l, ESV was 1028 ⫾ 83 l, and
ness (Fig. 4B) became significantly reduced in all MI groups
EF was 12.5 ⫾ 1.8%. For comparison (data are not shown),
compared with SH. Compared with the nT group, the thin-
the LV EDV in SH animals at the end of observation was only
ning of posterior wall was attenuated in all treatment
506 ⫾ 13 l, ESV was 242 ⫾ 10 l, and EF was 52 ⫾ 1.9%.
groups, but only in the 1-2⫹ group was this attenuation
The expansion of LV volumes was significantly attenuated in
statistically significant (p ⬍ 0.02).
all treatment groups compared with nT (p ⬍ 0.002; see Sup-
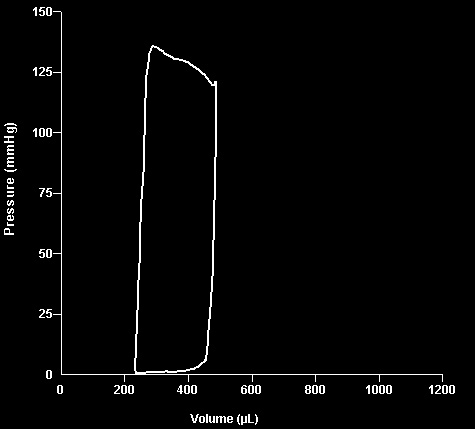
Hemodynamics. Figure 5 presents representative pres-
plemental Table 1). However, during the first 6 months of
sure-volume loops recorded from SH, nT, and different treat-
treatment LV expansion in the 1-ACEi group was similar to
ment groups at the end of the study. Compared with SH, a
that in the nT group. Statistical analyses with post hoc
substantial rightward shift accompanied by reduction of
comparisons conducted for the first 6 months revealed that
stroke volume and end-systolic pressure, characteristic for
both EDV and ESV in 1-ACEi group did not differ from nT,
CHF, was observed in the nT group. All treatment groups
whereas in 1-2⫹ and 1-2⫹ACEi groups, the beneficial
showed significant improvement in cardiac indices, and pres-
difference from nT was significant for ESV (p ⬍ 0.05) and
sure-volume loop recorded from the rat from  - ⫹ACEi
approached significance for EDV (p ⬍ 0.07). During the 12
group most closely approached the parameters of SH.
months of the study, the decline in EF was significantly
Table 2 lists the results of the pressure-volume loop anal-
attenuated, compared with the nT, in group with 2-AR
yses in randomly selected subsets of rats that survived 12-
agonist (p ⬍ 0.005); the preservation of EF in the 1-ACEi
months observation, i.e., 12.5 months after induction of MI. A
Ahmet et al.
End- Diastolic Volume
End- Systolic Volume
Survivors at 12 mo 500
Time (month)
* - p<0.002 vs nT; † - p<0.05 vs β1- ACEi
Fig. 3. LV end-diastolic volume (left), end-systolic volume (middle), and ejection fraction (right) in untreated and treated rats estimated by bimonthly
Echo during 12 months after induction of MI. Top, all animals; bottom, only animals that survived 12 months after induction of MI. ⴱⴱ, p ⬍ 0.002
versus nT; †, p ⬍ 0.05 versus 1-ACEi (pairwise comparison, group ⫻ time interaction with Bonferroni's correction).
comparison of hemodynamic indices of SH and nT rats
mass; thinning of posterior MI-free wall; progressive func-
clearly indicates an advanced stage of CHF in nT rats: the LV
tional decline; and, of course, substantial mortality. All of
is greatly dilated, cardiac output and stroke volume are re-
these characteristics have been described and well docu-
duced by 50%, and EF is reduced 4-fold. Furthermore, a
mented previously (Preffer and Braunwald, 1990; Goldman
greater than 5-fold reduction in PRSW indicates a pro-
and Raya, 1995; Krzemin
´ ski et al., 2008).
nounced systolic pump dysfunction; and a 3.5-fold elevation
We have been committed to testing different therapeutic
in Eed reflects the increased diastolic stiffness of myocar-
regimens in the DCM models (Ahmet et al., 2004, 2005,
dium. All three treatment groups showed a significant im-
2008). Initially, we had tested the effects of selective phar-
provement of hemodynamic indices compared with the nT
macological stimulation of 2-AR alone, or in combination
group. However, the 1-2⫹ and 1-2⫹ACEi were more
with 1-AR blocker in this in vivo rat model of postmyo-
effective than 1-ACEi in attenuation of LV volume expan-
cardial infarction DCM. The effects of 6 weeks of treatment
sion and improvement of EF.
with a 2-AR agonist, fenoterol, alone (Ahmet et al., 2004)or in combination with a 1-AR blocker, metoprolol (Ahmet
et al., 2005), were compared with metoprolol monotherapy.
Treatments were started 2 weeks after coronary ligation.
The rat model of permanent coronary ligation is the oldest
and one of the most established models of chronic heart
The progression of LV remodeling and MI expansion was
failure (Selye et al., 1960). Although the ischemic-reperfusion
monitored by serial echocardiography. At the endpoint of
(temporary coronary occlusion) model more relevantly re-
the study, cardiac function was analyzed by pressure-vol-
flects the modern clinical situation of restoration of coronary
ume loop measurements, and hearts were evaluated his-
flow, the permanent occlusion model is better suited to study
tologically. In these short-term, 6-week studies the effec-
the time course of infarct expansion, remodeling, perfor-
tiveness of a 2-AR agonist and the combination of a 2-AR
mance decline, and morphological changes. It also produces a
agonist plus a 1-AR blocker was similar, and both signif-
more uniform pathology; therefore, it is a more suitable for
icantly exceeded the effectiveness of the 1-AR blocker as
assessment of interventional strategies for DCM. In our ex-
a monotherapy in attenuation of LV dilatation and func-
periment, we observed in untreated rats all aspects charac-
tional decline. Both treatments that included 2-AR stim-
teristic of post-MI development of DCM: uniform MI size;
ulation also reduced myocardial apoptosis and arrested
progressive MI expansion; LV dilatation; increase of LV
the MI expansion. Moreover, we did not observe any
2-AR Agonist and 1-AR Blocker in Treatment of DCM
Left Ventricular Mass
Posterior Wall Thickness
Fig. 5. Representative pressure-volume loops of SH, nT, and three
treated groups recorded at the end of the study.
significantly attenuated in animals treated with the combi-
nation of both drugs during the first 6 months of treatment.
MI expansion was completely prevented only in animalstreated with the drug combination; the LV functional decline
was significantly attenuated during the entire year. Theepisodes of arrhythmic events were also lowest in combined
treatment group. Furthermore, a reduction of cardiac 1-ARdensity and a reduction in chronotropic and contractile re-
sponses to 2-AR-specific stimulation in the absence of areduction of 2-AR density that occurred in untreated ani-
mals were precluded in rats receiving combined therapy.
This explained the additional observation of the study inwhich the beneficial effect of 2-AR agonist as monotherapy
lasted only for the first 2 months after initiation of treat-ment, whereas the combination with a 1-AR blocker ex-
* - p<0.05 vs SH; † - p<0.05 vs nT
tended the therapeutic effectiveness throughout all 12
Fig. 4. LV mass (A) and posterior wall thickness (B) in SH, nT, and three
months of observation.
treated groups estimated by bimonthly Echo during 12 months after
Thus, the effects of 2 AR agonist treatment in the post-MI
induction of MI in all animals. ⴱ, p ⬍ 0.05 versus SH; †, p ⬍ 0.05 versus
model of DCM for only 6 weeks of treatment (8 weeks post-
nT (pairwise comparison, group ⫻ time interaction with Bonferroni'scorrection).
MI) showed its high therapeutic effectiveness. When we ex-tended the treatment period to 12 months, it became appar-
increase in the number of arrhythmic events during 2-AR
ent that the effect of 2 AR agonist monotherapy waned after
2 months. The long-term effectiveness of 2-AR agonist ther-
These studies, however, were of a relatively short duration
apy was possible only in combination with 1-AR blocker to
(6 weeks). In the next experiment in the same model, we
prevent 2-AR tachyphylaxis and reduce apoptosis. The use
compared the effects of long-term, combined therapy with a
of 2-AR agonist in combination with 1-AR blocker also
1-AR blocker, metoprolol, plus a 2-AR agonist, fenoterol,
resulted in preservation of 1-AR density and responsiveness
and either therapy alone for 12 months with survival as a
of 2-AR to stimulation, in which reduction was observed in
primary outcome (Ahmet et al., 2008). As in the short-term
DCM rats (Ahmet et al., 2008). The fact that the treatment
studies, therapy was started 2 weeks after permanent liga-
duration in the present study lasted for 12 months is a very
tion of the left descending coronary artery. Cardiac remodel-
important feature of the present study.
ing, MI expansion, and LV function were assessed by serial
The present study extended the series of our previous
echocardiography and compared with untreated animals. A
reports (Ahmet et al., 2004, 2005, 2008) demonstrating ther-
mortality of 67% observed at the end of 1-year observation in
apeutic effectiveness of combined therapy of a 1-AR blocker
untreated animals was reduced to 33% in the animals
and a 1-AR agonist in the rat model of post-MI DCM. In this
treated with the combination of 1-AR blocker and 2-AR
study, our goal was to specifically compare the beneficial
agonist. Progressive cardiac remodeling observed in un-
effects of combined -AR therapy with a combination treat-
treated rats or in rats treated with 1-AR blocker alone was
ment of a 1-AR blocker and an ACE inhibitor, a standard
Ahmet et al.
TABLE 2Hemodynamic indices in SH rats and treated and untreated rats 12.5 months after MI
SH (n ⫽ 5)
nT (n ⫽ 3)
1-ACEi (n ⫽ 6)
1-2⫹ (n ⫽ 11)
1-2⫹ACEi (n ⫽ 8)
197 ⫾ 13*a
201 ⫾ 11*a
963 ⫾ 35*a
828 ⫾ 47*a
195 ⫾ 12*a
20 ⫾ 1*a
102 ⫾ 6a
3.2 ⫾ 0.4a
2.4 ⫾ 0.3a
(⫹)dP/dt (mm Hg/s)
4649 ⫾ 448*a
4943 ⫾ 337*a
(⫺)dP/dt (mm Hg/s)
4046 ⫾ 307*a
49.4 ⫾ 5.1*a
48.9 ⫾ 3.3*a
36.6 ⫾ 3.0*a,b
Ees (mm Hg/ l)
Eed (103 mm Hg/ l)
dP/dt-EDV (mm Hg/s/ l)
17.4 ⫾ 1.4a
CO, cardiac output; EDP, end-diastolic pressure; ESP, end-systolic pressure; HR, heart rate; SV, stroke volume.
* p ⬍ 0.05 vs. SH.
a p ⬍ 0.05 vs. nT.
b p ⬍ 0.05 vs. 1-2⫹.
and widely used regimen in clinical practice. Beneficial ef-
activity (Ma et al., 2001; Watanabe et al., 2004; Brower et al.,
fects of 1-AR blocker-ACE inhibitor combination in experi-
2007). The mechanisms of cardioprotection by 2-AR agonist
mental model of post-MI CHF had been reported previously
have been investigated in many studies on isolated cardio-
¨ gel et al., 1999; Fedorov et al., 2006). In this 1-year-long
myocytes that discovered its marked antiapoptotic effects
study, we demonstrated that DCM rats treated with com-
related to activation of G signaling (Communal et al., 1999;
bined 1-AR blocker-2-AR agonist therapy showed 37% in-
Chesley et al., 2000; Zhu et al., 2001; Shizukuda and But-
crease in survival compared with untreated rats (triple com-
trick, 2002; Xiao et al., 2004). We believe that this antiapop-
bination of 1-AR blockade-ACEi-2-AR agonist showed 25%
totic effect of 2-AR stimulation was responsible for preven-
improvement)—a survival benefit that did not exceed a stan-
tion of cell loss and thus for attenuation of posterior wall
dard therapy (an improvement of 22%) but was at least equal
thinning in double and triple therapy groups of the present
to it. However, with respect to cardiac remodeling and MI
experiment. Because triple therapy (1-AR blocker ⫹ 2-AR
expansion, the addition of 2-AR agonist to a therapy (1-
agonist ⫹ ACE inhibitor) was not more effective in our ex-
2⫹ or 1-2⫹ACEi) clearly exceeded the effectiveness of
periment than double therapy with 1-AR blocker ⫹ 2-AR
1-AR blocker-ACE inhibitor combination. Specifically, al-
agonist, it is necessary to conclude that the primary mecha-
though the 1-AR blocker-ACE inhibitor combination did
nism responsible for the superior attenuation of LV remod-
attenuate the infarct expansion observed in untreated ani-
eling in the present experiment is a mechanism of double
mals, combined -AR therapy actually arrested the MI ex-
action of 1-AR blocker and 2-AR agonist. This mechanism
pansion, revealing a statistically significant improvement
was elucidated in our previous study (Ahmet et al., 2008),
over the 1-AR blocker-ACE inhibitor combination. Although
i.e., preservation of 1-AR density and responsiveness to
standard 1-AR blocker-ACE inhibitor combination attenu-
2-AR stimulation. Although coupling of -AR subtypes to
ated LV expansion, the addition of a 2-AR agonist made the
specific signaling pathways can be species specific and vary
treatment significantly more effective during the first 6
from mouse to rat to humans (Port and Bristow, 2001), the
months of treatment. Furthermore, the combinations includ-
effectiveness of therapies that are now clinically accepted for
ing a 2-AR agonist were twice as effective as 1-AR blocker-
humans has been demonstrated in animal experimental
ACE inhibitor combination in attenuating the functional de-cline. In addition, although a standard therapy was effective
models. Moreover, successful use of 2-AR agonist clen-
in attenuating the LV mass increase, the -AR combined
buterol in conjunction with left ventricular assist device to
treatment was effective in attenuating the thinning of pos-
help to attenuate myocardial atrophy before heart transplan-
terior wall, which has been interpreted to reflect myocyte cell
tation (Hon and Yacoub, 2003) suggests that the translation
death (Anversa et al., 1998). It is important to note that the
of current finding to clinical practice seems rational.
addition of 2-AR agonist to the standard therapy of 1-AR
In summary, the therapeutic utility of combined treatment
blocker and ACE inhibitor significantly increased the effec-
with 1-AR blocker and 2-AR agonist has been demon-
tiveness of a standard therapy, with respect to LV remodel-
strated in our previous studies in the rat model of post-MI
ing and MI expansion; the triple therapy was not more effec-
DCM. In the present preclinical study, in the same experi-
tive than the 1-AR blocker-2-AR agonist combination.
mental model, we showed that inclusion of 2-AR agonist in
The mechanisms of therapeutic effectiveness of ACE inhib-
treatment regimen as double (1-AR blocker-2-AR agonist)
itors in the CHF have been well characterized previously
or triple (1-AR blocker-2-AR agonist-ACEi) therapy ex-
(Wollert and Drexler, 1999; Anand and Florea, 2008; Werner
ceeds therapeutic effectiveness of combination of 1-AR
et al., 2008) and encompass numerous signaling pathways
blocker and ACE inhibitor. Thus, the 1-AR blocker-2-AR
from blocking the angiotensin II to suppression of transform-
agonist combination alone or in combination with ACEi war-
ing growth factor- to inhibition of matrix metalloproteinase
rants detailed clinical investigation as a treatment for CHF.
2-AR Agonist and 1-AR Blocker in Treatment of DCM
Ma M, Watanabe K, Wahed MI, Inoue M, Sekiguchi T, Kouda T, Ohta Y, Nakazawa
M, Yoshida Y, Yamamoto T, et al. (2001) Inhibition of progression of heart failure
We are grateful to Dr. Samer Najjar for encouraging us to under-
and expression of TGF-beta 1 mRNA in rats with heart failure by the ACE
take this study and to Shannon Marshall and Tina Turner for tech-
inhibitor quinapril. J Cardiovasc Pharmacol 38 (Suppl 1):S51–S54.
nical assistance.
Mann DL, Kent RL, Parsons B, and Cooper G 4th (1992) Adrenergic effects on the
biology of the adult mammalian cardiocyte. Circulation 85:790 – 804.
Martin RM, Dunn NR, Freemantle SN, and Mann RD (1998) Risk of non-fatal
cardiac failure and ischaemic heart disease with long acting beta 2 agonists.
Ahmet I, Krawczyk M, Heller P, Moon C, Lakatta EG, and Talan MI (2004) Bene-
Thorax 53:558 –562.
ficial effects of chronic pharmacological manipulation of beta-adrenoreceptor sub-
Mazza A, Tikhonoff V, Casiglia E, and Pessina AC (2005) Predictors of congestive
type signaling in rodent dilated ischemic cardiomyopathy. Circulation 110:1083–
heart failure mortality in elderly people from the general population. Int Heart J
46:419 – 431.
Ahmet I, Krawczyk M, Zhu W, Woo AY, Morrell C, Poosala S, Xiao RP, Lakatta EG,
Pfeffer JM, Pfeffer MA, and Braunwald E (1987) Hemodynamic benefits and pro-
and Talan MI (2008) Cardioprotective and survival benefits of long-term combined
longed survival with long-term captopril therapy in rats with myocardial infarc-
therapy with 2 adrenoreceptor (AR) agonist and beta1 AR blocker in dilated
tion and heart failure. Circulation 75:I149 –I155.
cardiomyopathy postmyocardial infarction. J Pharmacol Exp Ther 325:491– 499.
Pfeffer MA and Braunwald E (1990) Ventricular remodeling after myocardial infarc-
Ahmet I, Lakatta EG, and Talan MI (2005) Pharmacological stimulation of beta2-
tion. Experimental observations and clinical implications. Circulation 81:1161–
adrenergic receptors (beta2AR) enhances therapeutic effectiveness of beta1AR
blockade in rodent dilated ischemic cardiomyopathy. Heart Fail Rev 10:289 –296.
Anand IS and Florea VG (2008) Traditional and novel approaches to management of
Pfeffer MA, Braunwald E, Moye´ LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR,
heart failure: successes and failures. Cardiol Clin 26:59 –72, vi.
Geltman EM, Goldman S, and Flaker GC (1992) Effect of captopril on mortality
Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, and Kajstura J (1998) Apoptosis and
and morbidity in patients with left ventricular dysfunction after myocardial in-
myocardial infarction. Basic Res Cardiol 93 (Suppl 3):8 –12.
farction. Results of the survival and ventricular enlargement trial. The SAVE
Brodde OE, Michel MC, and Zerkowski HR (1995) Signal transduction mechanisms
Investigators. N Engl J Med 327:669 – 677.
controlling cardiac contractility and their alterations in chronic heart failure.
Pfeffer MA, Pfeffer JM, Steinberg C, and Finn P (1985) Survival after an experi-
Cardiovasc Res 30:570 –584.
mental myocardial infarction: beneficial effects of long-term therapy with capto-
Brower GL, Levick SP, and Janicki JS (2007) Inhibition of matrix metalloproteinase
pril. Circulation 72:406 – 412.
activity by ACE inhibitors prevents left ventricular remodeling in a rat model of
Pearce N, Crane J, Burgess C, Beasley R, and Jackson R (1989) Fenoterol and
heart failure. Am J Physiol Heart Circ Physiol 292:H3057–H3064.
asthma mortality. Lancet 1:1196 –1197.
Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, and Crow MT
Port JD and Bristow MR (2001) Altered beta-adrenergic receptor gene regulation
(2000) The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac
and signaling in chronic heart failure. J Mol Cell Cardiol 33:887–905.
myocytes through G. (i)-dependent coupling to phosphatidylinositol 3⬘-kinase. Circ
Selye H, Bajusz E, Grasso S, and Mendell P (1960) Simple techniques for the surgical
occlusion of coronary vessels in the rat. Angiology 11:398 – 407.
Communal C, Singh K, Sawyer DB, and Colucci WS (1999) Opposing effects of
Shizukuda Y and Buttrick PM (2002) Subtype specific roles of -adrenergic receptors
beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis: role of a
in apoptosis of adult rat ventricular myocytes. J Mol Cell Cardiol 34:823– 831.
pertussis toxin-sensitive G protein. Circulation 100:2210 –2222.
The AIRE Study Investigators (1993) Effect of ramipril on mortality and morbidity
CONSENSUS Trial Study Group (1987) Effects of enalapril on mortality in severe
of survivors of acute myocardial infarction with clinical evidence of heart failure.
congestive heart failure. Results of the Cooperative North Scandinavian Enalapril
Lancet 342:821– 828.
Survival Study (CONSENSUS). N Engl J Med 316:1429 –1435.
The CIBIS II Investigators (1999) The Cardiac Insufficiency Bisoprolol Study II
Fedorov VN, Sal'nikov EV, Sidorov AV, Bogatushin AV, and Fateev MM (2006)
(CIBIS-II): a randomised trial. Lancet 353:9 –13.
Survival of rats with experimental chronic heart failure depending on pharmaco-
The MERIT-HF Investigators (1999) Effect of metoprolol CR/XL in chronic heart
dynamic and pharmacokinetic parameters of angiotensin-converting enzyme in-
failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart
hibitor and beta-adrenoreceptor blockers. Bull Exp Biol Med 141:40 – 43.
Failure (MERIT-HF). Lancet 353:2001–2007.
Goldman S and Raya TE (1995) Rat infarct model of myocardial infarction and heart
The SOLVD Investigators (1991) Effect of enalapril on survival in patients with
failure. J Card Fail 1:169 –177.
reduced left ventricular ejection fractions and congestive heart failure. N Engl
Hall SA, Cigarroa CG, Marcoux L, Risser RC, Grayburn PA, and Eichhorn EJ (1995)
J Med 325:293–302.
Time course of improvement in left ventricular function, mass and geometry in
Watanabe K, Ma M, Wen J, Tachikawa H, Kodama M, Aizawa Y, Yamaguchi K, and
patients with congestive heart failure treated with beta-adrenergic blockade. J Am
Takahashi T (2004) Comparative effects of quinapril with enalapril in rats with
Coll Cardiol 25:1154 –1161.
heart failure. Pharmacology 71:157–161.
Heart Failure Society Of America (2006) HFSA 2006 Comprehensive Heart Failure
Werner C, Baumha¨kel M, Teo KK, Schmieder R, Mann J, Unger T, Yusuf S, and
Practice Guideline. J Card Fail 12:e1– e2.
Bo¨hm M (2008) RAS blockade with ARB and ACE inhibitors: current perspective
Hochman JS and Bulkley BH (1982) Expansion of acute myocardial infarction: an
on rationale and patient selection. Clin Res Cardiol 97:418 – 431.
experimental study. Circulation 65:1446 –1450.
Wollert KC and Drexler H (1999) The renin-angiotensin system and experimental
Hon JK and Yacoub MH (2003) Bridge to recovery with the use of left ventricular
heart failure. Cardiovasc Res 43:838 – 849.
assist device and clenbuterol. Ann Thorac Surg 75 (6 Suppl):S36 –S41.
Xiao RP, Zhu W, Zheng M, Chakir K, Bond R, Lakatta EG, and Cheng H (2004)
¨ gel S, Horn M, de Groot M, Remkes H, Dienesch C, Hu K, Ertl G, and Neubauer
Subtype-specific -adrenoceptor signaling pathways in the heart and their poten-
S (1999) Effects of ACE inhibition and beta-receptor blockade on energy metabo-
lism in rats postmyocardial infarction. Am J Physiol 277:H2167–H2175.
tial clinical implications. Trends Pharmacol Sci 25:358 –365.
Køber L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, Videbaek
Zhu W-Z, Zheng M, Koch WJ, Lefkowitz RJ, Kobilka BK, and Xiao R-P (2001) Dual
J, Cole DS, Auclert L, and Pauly NC (1995) A clinical trial of the angiotensin-
modulation of cell survival and cell death by 2-adrenergic signaling in adult
converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunc-
mouse cardiac myocytes. Proc Natl Acad Sci U S A 98:1607–1612.
tion after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study
Group. N Engl J Med 333:1670 –1676.
Address correspondence to: Dr. Mark I. Talan, National Institute on Aging,
´ ski JK, Grzyb J, and Porc M (2008) Widespread myocardial
Intramural Research Program, Gerontology Research Center, 5600 Nathan
remodeling after acute infarction in rat. Feature for heart failure progression. Vasc
Shock Dr., Baltimore, MD 21224-6825. E-mail: [email protected]
Pharmacol 48:100 –108.
Source: http://evergreen.loyola.edu/chm/www/Publications/otherpub/JPET2009.pdf
Syllabus: Bachelor of Pharmacy OPJS UNIVERSITY, CHURU OPJS UNIVERSITY, CHURU (RAJASTHAN) SYLLABUS BACHELORS IN PHARMACY (B. Pharma) School of Pharmacy OPJS UNIVERSITY, CHURU (RAJASTHAN) Syllabus: Bachelor of Pharmacy
SÍNTESIS INFORMATIVA DÍA 15-10-15 LA PRESENTE SÍNTESIS CONTIENE INFORMACIÓN RELEVADA Y EXTRAÍDA DE LOS DISTINTOS MEDIOS, TAL COMO LA REFLEJAN LOS MISMOS. STJ ordena cubrir tratamiento a una paciente La medida alcanza a una obra social y a una prepaga. Jueces revocaron un fallo de primera instancia. Una obra social y una prepaga deberán cubrir parcialmente el tratamiento con medicamentos de una paciente que presentó un recurso de amparo a la Justicia, el cual inicialmente había sido rechazado por un magistrado cuyo fallo fue recientemente revocado por el Superior Tribunal de Justicia. El máximo cuerpo judicial en pleno ordenó a la Obra Social de los Serenos de Buques y a la firma Swiss Medical SA que brinden a la accionante la cobertura del 70 por ciento de los medicamentos Deltisona B 40 mg y Entocort 3 mg, prescriptos por su médico, en razón de tratarse de fármacos de uso habitual destinados al tratamiento de una patología crónica prevalente, conocida como enfermedad de Crohn.