
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Fubio.chfiz.pub.ro
Scientific Report
Regarding implementation of the project "Elaboration and characterisation of
functional biopolymers for photonic and electronic applications"
Financial Contract 279/7.10.2011,
January – December 2013
According to the work plan during January-December 2013 were performed
activities related to the spectral characterisation of the synthesised biopolymers,
nonlinear optical properties of the thin films and electrochemical characterization of the
membranes prepared.
In this regard were prepared and studied new membranes based on DNA
membranes in which has been added glycerin as plasticizer. To increase the
conductivity of these membranes they were doped with different chromophores: Blue
Nile, Prussian Blue, lithium perchlorate in various concentrations.
Hydrophilic / hydrophobic character of these membranes membranes has been
studied using the contact angle method. The results showed that the hydrophobicity of
the membrane depends on the dopant used, and its concentration (Fig. 1)
Fig. 1 Contact angle dependence on dopant type and concentration
The obtained membranes were chracterized by UV-Vis spectrometry, FTIR,
fluorescence and THG measurements (membranes doped with Disperse Orange 3).
In order to determine the conductivity of the membrane was measured their
impedance. The ionic conductivity of the DNA biomembrane doped with different
molecules was determined using electrochemical impedance spectroscopy (EIS).
Electrochemical measurements were performed at free potential and the frequency
1000-100000 Hz with an amplitude of 10mV using PGSTE AUTOLAB 302N with
appropriate software.
The measurements were carried out also depending on the temperature (20; 30;
40; 50; 60; 70; 80; 90; 100 ° C). The conductivity was calculated using Eq. 1, where -
the conductivity is expressed in Scm-1 - membrane thickness, expressed in μm; S is the
area of contact between the membrane and the electrode, expressed in cm2, Z -
impedance of the membrane, expressed in .
Table 1 shows the results for a membrane containing DNA, glycerol and Nile
Blue, and in Fig. 2 are given the Arrhenius plots.
As it can be seen from Figure 2 all of the DNA membranes doped with Nile Blue
present at room temperature conductivity between 10‐9 and 10-8 Scm-1. These values
increase with increasing temperature up to about 10-5 Scm-1 at 70 °C. The high
conductivity values obtained for temperatures greater than 70 ° C are probably due to
the distortion of the DNA. The two chains linked by purine and pyrimidine bases in the
double helix structure of the macromolecules of DNA are partially separated as a result
of the breaking of hydrogen bonds, thus free molecules of guanine and adenine appear
and therefore charge carriers appear due to delocalization of electrons of nucleobase.
It is also observed in Figure 2 that the highest conductivity is obtained for the
membrane containing 2% Blue Nile. Higher dopant concentrations led to a lower
conductivity probably due to the formation of chromophore aggregates on the surface
of DNA that have the effect of reducing the number of charge carriers.
Table 1 - Example of results obtained for a membrane containing DNA, Glycerine and Nile Blue
Figure 2 - Arrhenius plot of conductivity for DNA - Glycerine - Blue Nile membranes
Figure 3 shows the effect of glycerol and chromophore on the DNA based
membrane conductivity. It may be noted that the addition of glycerin leads to increased
conductivity. A concentration of 1% of Nile Blue determine a decrease of the
conductivity due to the fact that ionic mobility is low, while a concentration of 1% of
lithium perchlorate led to an increase of an order of magnitude conductivity. In the
latter case the energy required for the ions migration is lower because the mobile
charges are numerous favouring the increasing of the conductivity.
Figure 3 - The influence of the glycerol and the nature of dye on the conductivity of DNA
membranes (room temperature)
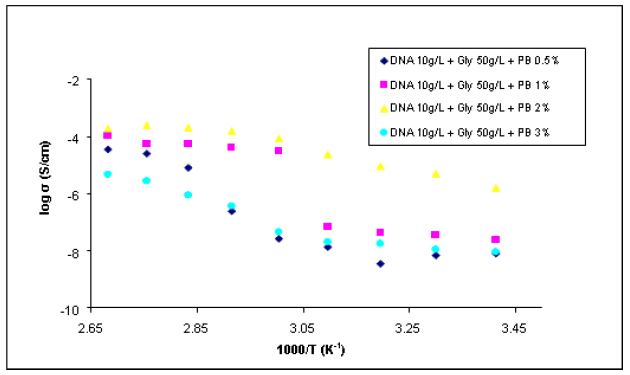
Figure 4 shows the conductivity variation with temperature for membranes
doped with Prussian Blue (Arrhenius dependencies). It is observed an increasing of the
conductivity by two orders of magnitude comparing to the membranes doped Blue Nile.
In this case the best results in terms of conductivity have been obtained for membranes
with 2% of Prussian Blue. It is observed a similar behaviour of membranes at
temperatures above 70 ° C
DNA based ionic electrolytes doped with lithium perchlorate present
conductivity higher than those obtained for membranes doped with Blue Nile, but lower
than those obtained for membranes doped with Prussian Blue. As with the other two
cases, the conductivity of the membrane increases with temperature increasing (Fig. 5).
It has been observed that in some cases there is a decrease in the conductivity at
temperatures above 80 ° C due to evaporation of glycerol used as plasticizers and for
increasing the conductivity. Increasing the concentration of lithium perchlorate has a
positive effect on the ionic conductivity which can be explained by increasing the
number of the mobile ionic species. However, the increase in conductivity is limited by
the number of sites of solvation that is sufficient to cause dissolution of the salt. If the
number of sites solvation is lower and the salt concentration is high, then ion
compounds are formed or the recrystallization occurs, leading to decreased ion
mobility. In the case of membranes tested the presence of glycerine causes a higher
mobility of ions through a better lithium ion solvation. The addition of greater amounts
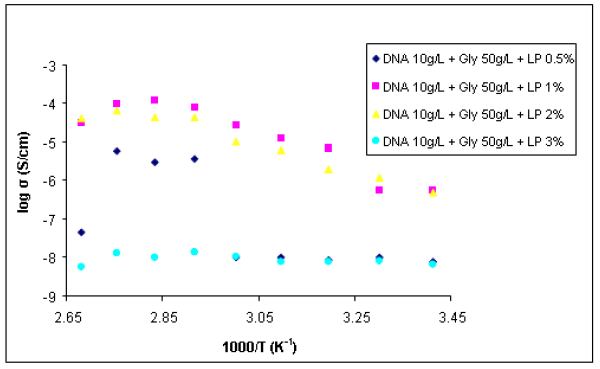
of lithium perchlorate causes a decrease in conductivity due to the formation of
Figure 4 - Arrhenius dependence for the membranes doped with Prussian Blue
Figure 5 - Arrhenius dependence for DNA-based membranes doped with lithium
All membranes synthesized were characterized by UV – VIS spectrometry,
fluorescence, FTIR, conductivity and contact angle measurements.
UV - VIS spectroscopy revealed peaks corresponding to the chromophores
colour. Measurements of the fluorescence as expected did not provide conclusive
results because of quenching fluorescence (high chromophore concentration due
membrane thickness)
Conductivity measurements showed that optimum chromophore concentration
for Nile Blue and Prussian Blue is 2%, while for the lithium perchlorate a concentration
of 1% is considered optimal.
The synthesised membranes have hydrophobic character, the highest
hydrophobic character showed membranes doped with Prussian Blue.
Corroborating the results obtained it can be concluded that it is preferable for
electrochromic devices to use the chromophore Prussian Blue.
Further research will be performed in order to check the possibility to use these
membranes for electrochromic devices such as "Smart Windows".
The research results obtained were published in two articles in ISI journals. Also
part of the research results were presented at international conferences and workshops
in the form of plenary conferences (1), invited conference (2) and oral contributions (5).
Project Director,
Conf. Habil. Dr. Ing. Ileana Rău
Source: http://fubio.chfiz.pub.ro/Raport%202013%20-%20rezumat.pdf
How Does Psychotherapy Influence Personality?A Theoretical Integration John D. MayerUniversity of New Hampshire A given type of psychotherapy (e.g., psychodynamic) is associated with aset of specific change techniques (e.g., interpreting defenses, identifyingrelationship themes). Different change techniques can be conceived of asinfluencing different parts of personality (e.g., interpreting defense increasesconscious awareness). An integrated model of personality is presented.Then, change techniques from different theoretical perspectives are assignedby judges to areas of personality the techniques are believed to influence.The results suggest that specific change techniques can be reliably sortedinto the areas of personality. Thinking across theoretical perspectives leadsto important new opportunities for assessment, therapy outcome research,and communication with patients concerning personality change. ©2004Wiley Periodicals, Inc. J Clin Psychol 60: 1291–1315, 2004.
Society for Industrial and Applied Mathematics3600 Market Street, 6th Floor • Philadelphia, PA 19104-2688 USAwww.siam.org • [email protected] 5/7/2012 1:41:13 PM The cover image illustrates the results of a fluid flow calculation over an airplane. For this design, there is little flow separation occurring on the wing except near the wingtips and near the side of the body. In addition, the flowfield streamlines from the nacelle up over the wing show vortex shedding from "chines" which are structures mounted on the nacelles that are specifically designed and optimized to shed this vortex. Without them, installing the nacelle forward of the wing as in this design would compromise both the efficiency of the wing as well as its maximum lift capability. See [Konigs 2005]






