
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Doi:10.1016/j.cell.2007.11.047
NFATc1 Balances Quiescence andProliferation of Skin Stem CellsValerie Horsley,Antonios O. Aliprantis,Lisa Polak,Laurie H. Glimcher,and Elaine Fuchs,1Howard Hughes Medical Institute, Laboratory of Mammalian Cell Biology and Development, The Rockefeller University,New York, NY 10065, USA2Department of Infectious Diseases and Immunology, Harvard School of Public Health, Boston, MA 02115, USA3Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA*Correspondence: DOI 10.1016/j.cell.2007.11.047
nals. Both signaling pathways contribute to the stabilization ofb-catenin (
Quiescent adult stem cells reside in specialized
), a transcription cofactor for the
niches where they become activated to proliferate
TCF/Lef1 family of DNA binding proteins (Acti-
and differentiate during tissue homeostasis and in-
vation of b-catenin/TCF/Lef1 target genes is required for bulge
jury. How stem cell quiescence is governed is poorly
stem cell activation and maintenance (
understood. We report here that NFATc1 is preferen-
tially expressed by hair follicle stem cells in their
That said, mice conditionally targeted for loss of
niche, where its expression is activated by BMP
BMPR1a () lose the ability of the bulge to
signaling upstream and it acts downstream to tran-
regulate quiescence. These data suggest that the mechanisms
scriptionally repress CDK4 and maintain stem cell
controlling stem cell behavior in the niche are complex.
quiescence. As stem cells become activated during
Microarray profiling has identified genes that are preferentially
hair growth, NFATc1 is downregulated, relieving
expressed in the bulge relative to the proliferative basal cells of
CDK4 repression and activating proliferation. When
calcineurin/NFATc1 signaling is suppressed, phar-
One of the upregulated genes in these profiles
macologically or via complete or conditional NFATc1
and also in an array distinguishing embryonic hair buds from
gene ablation, stem cells are activated prematurely,
epidermis (is the transcription factor nuclear
resulting in precocious follicular growth. Our findings
factor of activated T cells c1 (NFATc1). NFATc1 (also referred
may explain why patients receiving cyclosporine A
to as NFAT2) belongs to the NFAT family of transcription factorswhich consists of four calcium sensitive members, NFATc1-4,
for immunosuppressive therapy display excessive
that are broadly expressed in many different tissues and organs
hair growth, and unveil a functional role for cal-
(). The subcellular regulation of NFAT
cium-NFATc1-CDK4 circuitry in governing stem cell
transcription factors can influence their activity, and in many
cell types, NFAT proteins are phosphorylated and confinedto the cytoplasm under basal conditions. In response to in-
creases in intracellular calcium, the serine/threonine phospha-tase calcineurin becomes activated, dephosphorylating NFAT
The hair follicle (HF) is an excellent model for studying stem cell
proteins and allowing their nuclear translocation. Once in the
activity because it continuously proceeds through rounds of tis-
nucleus, NFAT proteins in association with other transcription
sue regeneration. This cyclic nature of HF formation requires
factors bind to consensus DNA sequences to regulate gene
a subset of stem cells within a specialized niche called the bulge,
located within the HF outer root sheath (ORS)
NFATc1's prominence as a HF stem cell signature gene is
intriguing, given that the immunosuppressant drug cyclosporine
Following embryonic HF morphogenesis and postnatal
A (CSA), which inhibits calcineurin upstream of NFAT, can induce
completion of the first round of hair growth (anagen), the cycling
hair growth in humans during organ transplantation (
portion of the follicle dies (catagen) regressing up to the bulge
region, where the HF then remains dormant during the resting
). In addition, hair growth is precociously activated in
calci-
phase (telogen) available online).
neurin B1 null skin ). A role for NFAT pro-
The mechanisms by which the bulge niche environment
teins has been postulated for the catagen (destructive) phase of
changes to induce new follicular growth are still unfolding.
the hair cycle (), but whether NFAT pro-
BMP inhibitory signals likely emanating from the dermal papillae
teins function in HF stem cells and if so how, remains unex-
(DP) at the base of the HF have been implicated, as have Wnt sig-
Cell
132, 299–310, January 25, 2008 ª2008 Elsevier Inc. 299
To date, mouse genetics have not revealed a role for NFATs in
Interestingly, in both embryonic and adult skin, NFATc1 pro-
skin. Epidermal mouse keratinocytes (MKs) in vivo and in vitro
tein and mRNA appeared to be specific for bulge cells (
can respond to CSA treatment by blocking nuclear localization
NFATc1 antibodies did not immunolabel cells in epidermis, se-
baceous glands or dermis (and 1L). Western anal-
and NFATs have been po-
ysis confirmed the absence of NFATc1 protein in epidermis
sitioned downstream of Notch signaling in cultured epidermal
(and real-time PCR of mRNAs isolated from FACS-
cells ). However, in contrast to
NFATc2
purified skin cell populations revealed high levels of
NFATc1
(
NFAT1) null mice, which are viable and fertile
mRNA in P4 ORS and adult a6(+)/CD34(+) bulge cells, in com-
),
NFATc1 null mice die early in
parison to background levels in P4 epidermal, DP, melanocyte
embryogenesis, and display defects in cardiac valve, bone and
(MC), dermal fibroblast (DF) and adult a6(+)/CD34(-) cells (
pancreatic development (
and1K).
NFATc1 mRNA expression in FACS-purified
a6(+)/CD34(+) bulge MKs was sequentially lost with proliferation
Here, we focused on addressing the function of NFATc1 in the
and passage in culture N). Appreciable
NFATc1 mRNA
skin. We show that NFATc1 is expressed exclusively in the bulge
was also not observed in cultured epidermal MKs (
region of the HF and using gain- and loss-of function studies, we
These data provide compelling evidence that NFATc1 expres-
identify an inhibitory role for NFATc1 in stem cell activation in the
sion in skin is specific for the HF bulge. The correlation between
HF. Moreover, we find that NFATc1-mediated quiescence in-
nuclear NFATc1 and
NFATc1 mRNA expression in the bulge also
volves transcriptional repression of the
cyclin dependent kinase
correlated with NFATc1's known ability to regulate its own tran-
4 gene encoding CDK4, which is required for progression
scription ().
through the G1/S phase of the cell cycle
Consistent with the slow-cycling nature of bulge cells, nuclear
Finally, we find that
NFATc1 gene expression is linked up-
NFATc1(+) cells did not colabel the proliferative cells marked by
stream to BMP signaling. Taken together, these findings provide
Ki67 during HF morphogenesis nor during anagen initiation fol-
significant new insights into how stem cell activation is orches-
lowing the first hair cycle G and S1J). In addition,
trated in the HF.
short pulses of BrdU did not label NFATc1(+) cells either duringthe first growth phase (data not shown) nor during the transitionto the second hair cycle However, when slow cy-
cling cells were labeled by expression of tetracycline-regulatableH2B-GFP and then chased for 4 weeks, NFATc1 was observed
NFATc1 Is Specific to the Quiescent Stem Cell
in the majority of label retaining cells (LRCs) I and
Niche of the Hair Follicle
S1J). Based upon these data, cells marked by nuclear NFATc1
To determine whether NFATc1 protein is expressed in the bulge
exhibit the characteristics of bulge LRCs.
region of the HF as suggested from our microarray analyses(), we analyzed its
Cyclosporine A Enhances Stem Cell Activity
expression by immunofluorescence microscopy and
in the Bulge Niche
). Nuclear NFATc1 was first detected during the late
CSA is known to stimulate precocious entry of telogen HFs into
stages of HF morphogenesis and S1C). As HFs ma-
anagen and is also
tured, their midsegments became marked by nuclear NFATc1(+)
known to inhibit NFAT activity
(NFATc1-expressing cells persisted not only through
These data led us to wonder whether CSA might act on bulge
the growing phase (anagen) of postnatal HFs, but also the resting
stem cells to influence the telogen to anagen transition and if
(telogen) phase and E).
To more precisely define the cells marked by nuclear NFATc1,
To evaluate whether CSA affects the proliferation of bulge cells
we compared the localization of NFATc1 with other proteins ex-
during anagen induction, we coadministered CSA and a short
pressed in the bulge. NFATc1 colocalized with CD34, which is
BrdU pulse during the 2nd telogen (As expected,
highly upregulated in quiescent bulge cells and has been used
within 5 days, the majority of CSA-treated HFs had precociously
as a marker for purifying these cells
entered anagen (B). Irrespective of whether the experi-
and 1I). NFATc1 also overlapped substantially with
ments were performed on HFs in their 1st or 2nd hair cycles,
Lhx2, required for HF stem cell maintenance (
most HFs of CSA-treated skin contained BrdU(+) cells in both
and 1I). In addition, NFATc1(+) cells partially colocal-
the bulge and the hair germ (B, 2C, and
ized with TCF3 and Sox9, transcription factors expressed by
By contrast, the majority of proliferation at the onset of a sponta-
bulge cells 1I).
neous anagen was in the hair germ, while only 10% of HFs
NFATc1 was not detected in the TCF3/Sox9-positive cells of
showed proliferative activity in the bulge 2C, and
the lower ORS, thought to be less quiescent stem cells which
A). This was consistent with prior studies showing
have exited the bulge (Real-time PCR of
that in normal HFs, most slow cycling bulge cells (marked by
fluorescence activated cell sorted (FACS) skin populations con-
pulse-chase with H2BGFP) do not lose their quiescent status
firmed the enrichment of
NFATc1 mRNAs in neonatal ORS
even in the anagen phase of a hair cycle
(and more specifically in the adult
Since most CSA-treated bulge cells incorporated BrdU over
(a6 integrin+/CD34+) bulge cells at both the 1st and 2nd telogen
multiple days, the effects could not be attributed to an accelera-
stages ; K).
tion in the timing at which an otherwise normal bulge undergoes
300 Cell
132, 299–310, January 25, 2008 ª2008 Elsevier Inc.
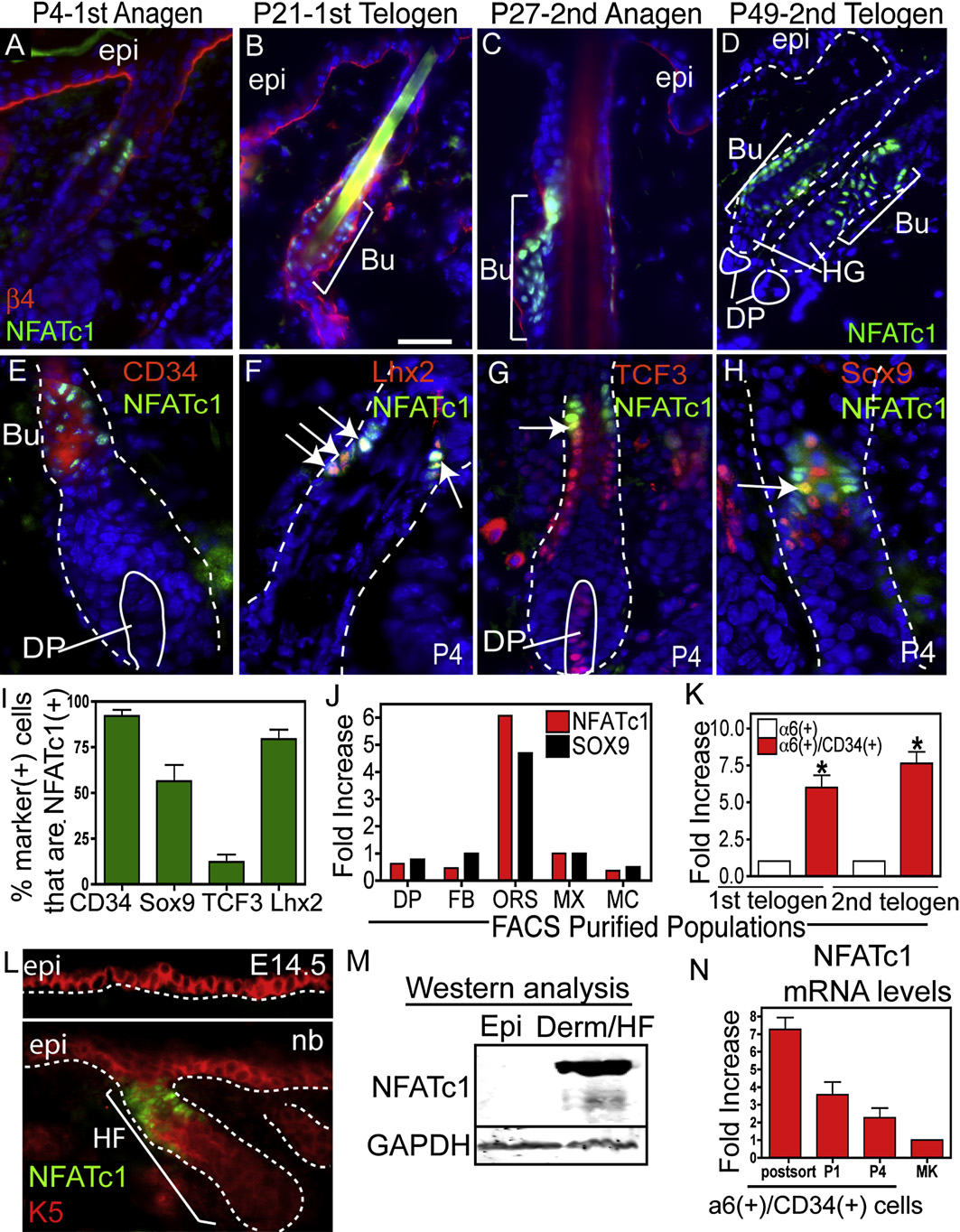
Figure 1. NFATc1: A Marker of Hair Follicle Stem Cells(A–D) NFATc1 is expressed the upper HF during anagen at P4 and P27 and at the base of the follicle during telogen (P21 and P49). b4 integrin marks the dermo-epidermal interface. (C–H) Immunohistochemistry showing NFATc1 colocalization with bulge cell markers, CD34, Lhx2, TCF3, and Sox9. Arrows denote exam-ples of coexpression. (I) Quantification of the percent of cells with bulge cell markers that colocalize with NFATc1. Data are mean ± SEM. N = 50–176 cells. (J andK) Real-time PCR analysis of NFATc1 and Sox9 mRNA in FACS isolated populations at P4 (J) and NFATc1 mRNA in the a6/CD34-positive bulge compartmentduring the 1st (P19) and 2nd (P49) telogen. (K) Data are mean ± SEM. N = 2 (J) and N = 3 (K) FACS isolated populations. Data are mean ± SEM. Asterisks indicatesignificance, p < 0.05. (L) Expression of NFATc1 in the keratin 5 (K5) (+) epidermis at E14.5 or newborn (nb). (M) Isolated P4 epidermis and the dermis (containingHFs) were subjected to western analysis. (N) Real-time PCR analysis of NFATc1 mRNA from FACS isolated a6(+)/CD34(+) bulge cells, after passage (P1, P4) andin epidermal keratinocytes (MK). Abbreviations are as follows: DP, dermal papillae; Bu, bulge; ORS, outer root sheath; epi, epidermis; derm, dermis; HG, hairgerm; FB, fibroblasts; MX, matrix; and MC, melanocytes. Dapi staining (blue) shows nuclear localization. The scale bar represents 30 mm.
Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc. 301
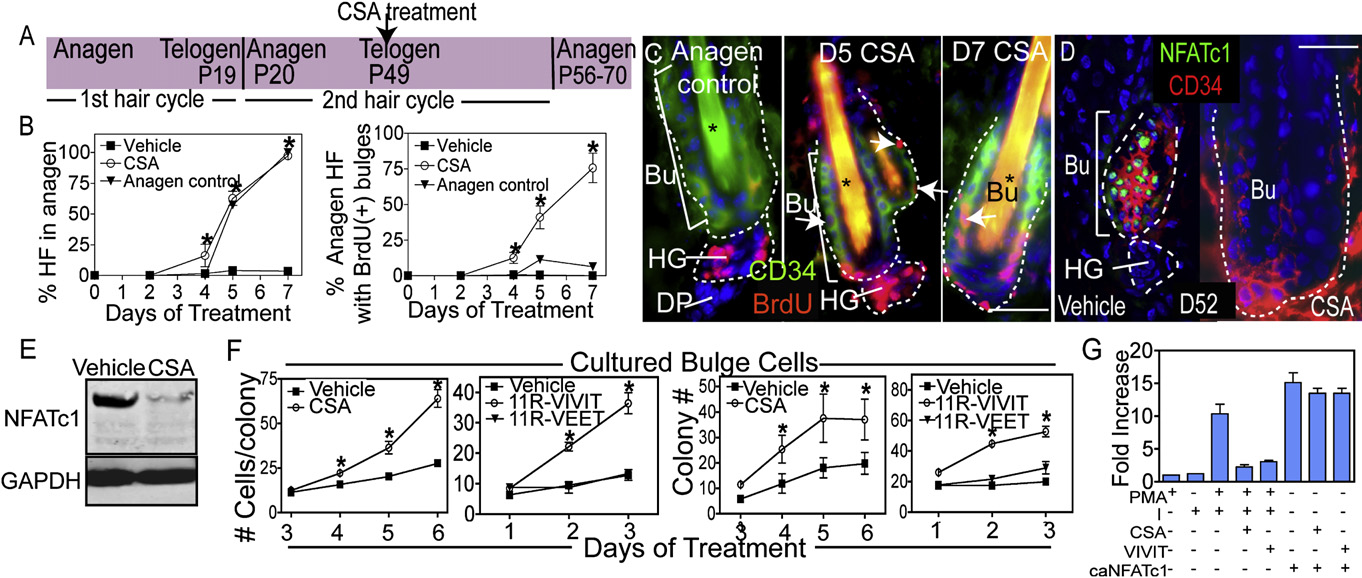
Figure 2. Impairing Calcineurin/NFAT Signaling Results in Hair Growth and Stem Cell Proliferation(A) Schematic illustrating the experimental design of CSA experiments.
(B and C) Quantification of anagen induction and BrdU incorporation in follicle stem cells of the bulge (Bu) with cyclosporine A (CSA) treatment. The anagencontrol used was the first hair cycle (P18–P25). Data are the mean ± SEM for 50–100 follicles for three individual mice for each time point. Asterisks in (C) indicatehair shaft autofluorescence.
(D and E) Immunohistochemistry and western analysis of NFATc1 expression following 3 day treatment with vehicle or CSA.
(F) Colony formation and cell number of bulge cells following treatment with vehicle, CSA, 11R-VIVIT, or 11R-VEET. N = 3 experiments with independent sortedpopulations.
(G) NFAT reporter activity in response to calcium ionophore (I), phorbol myristate acetate (PMA), caNFATc1, CSA, or 11R-VIVIT. Data are mean ± SEM. N = 3–5individual experiments for each treatment.
Asterisks indicate significance, p < 0.05. The scale bars represent 30 mm. Dapi staining (blue) shows nuclear localization. Abbreviations are as follows: HF, hairfollicle; Bu, bulge; HG, hair germ; and DP, dermal papillae.
a telogen to anagen transition (The effects of CSA
firmed by NFAT reporter gene assays in conjunction with the
also contrasted with hair depilation, another treatment which in-
calcium ionophore ionomycin (I) and phorbol myristate acetate
duces anagen. While depilation resulted in a short-lived burst of
(PMA), required together for full activation of NFAT transcrip-
proliferation within the bulge at D2, proliferation in the upper
tional activity, and a constitutively active form of NFATc1 (
ORS and surrounding epidermal cells also occurred
Taken together, these results suggest that calcium
We did not observe enhanced epidermal proliferation
signaling and calcineurin activity may also influence NFATc1's
with CSA treatment E). Consistent with CSA's ability to
localization in the bulge and its ability to activate target genes.
inhibit calcineurin, required for nuclear NFATc1 localization, CSAresulted in the disappearance of NFATc1 in the bulge D
NFATc1 Regulates Bulge Stem Cell Quiescence
and I). Nuclear NFATc1 was also lost when bulge cell prolifer-
To determine whether NFATc1 gene expression is essential for
ation and anagen were precociously induced by depilation
maintaining HF stem cell quiescence, we performed loss of func-
tion studies. Since most NFATc1 null embryos die between
To further explore the relation between calcineurin, NFAT ac-
E14.5 and E17.5 ), skins from NFATc1 null
tivity and bulge cell proliferation, we purified a6(+)/CD34(+) bulge
and control embryos were grafted onto the backs of Nude
cells from 1 month old K14-H2BGFP mice and cultured them in
mice to monitor their effects on HF morphogenesis and cycling.
the presence of CSA or cell-permeable VIVIT (11R-VIVIT), a spe-
HFs formed and underwent cycling normally, including reentry
cific NFAT inhibitor ).
into telogen in both WT and KO skin grafts (This
CSA or 11R-VIVIT-treated cultures formed larger colonies and
should not have been observed had NFATc1's effects been
with greater efficiency than vehicle or scrambled VIVIT (11R-
exerted on catagen, the hair cycle stage that
VEET) treated cells F and A). The proliferative effects
had postulated to be regulated in CSA-mediated inhibition
of CSA on primary bulge cells contrasted with CSA's growth
suppressive effects observed with cultures of either epidermal
To address whether loss of NFATc1 affects stem cell activa-
MKs (see also or a6(+)CD34(!) cells,
tion, we shaved or dyed hairs at D60 after skin engraftments dur-
containing primarily epidermal and non-bulge ORS cells
ing what is normally an extended telogen phase. By D75 when
and S3C). The ability of CSA and VIVIT to regulate calcium-
WT HFs were in telogen, NFATc1 null HFs had entered anagen,
sensitive calcineurin/NFAT activity in keratinocytes was con-
as visualized by growth of new (white) hairs A and 3B)
302 Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc.
and shedding of old (blue dyed) hairs (Histological
expressing cultures showed 50%–80% fewer BrdU-labeled
analyses confirmed that D60 HFs of both genotypes were in
(and S phase MKs (C).
telogen as indicated by the close proximity of DP to bulge
The effects of caNFATc1 appeared to be specific for the
(However, by D75 only KO HFs were in full anagen
reduction in cell cycling rather than an effect on terminal differen-
as indicated by the distance of the DP from the bulge due to
tiation or apoptosis. caNFATc1 did not appreciably induce termi-
new hair growth Taken together, these data indi-
nal differentiation markers transglutaminase (TGM) 1 and 3,
cated that the loss of NFATc1 affected hair cycling by shortening
filaggrin and loricrin, which are induced by elevated calcium
telogen and prompting precocious entry into anagen.
(nor did it increase the percentages of dead or apo-
To evaluate whether these aberrations in hair cycling reflect
ptotic cells in MK cultures (
a specific alteration in bulge stem cells, we conducted BrdUpulse-chase experiments. Even at P60, after WT and KO HFs
NFATc1 Represses the Expression of CDK4
had regressed and were morphologically in telogen, nearly half
in Hair Follicle Stem Cells
of the NFATc1 null bulge cells abnormally incorporated BrdU
The effects of NFATc1 on cell cycle kinetics both in vitro and
during short labeling periods, and most bulge cells failed to retain
in vivo suggested that NFATc1 may control key genes involved
label in BrdU-pulse chase experiments and
in promoting cell cycle progression. Of the cell cycle genes
A). Despite these marked changes in proliferative status
which are downregulated in the telogen bulge relative to pro-
within the bulge, CD34, Sox9, tenascin C and nuclear phos-
liferating progeny (
pho-Smad 1, 5, 8 (reflective of active BMP signaling) as well as
cyclin-dependent kinase 4 (CDK4) stood out.
activated caspase 3, indicative of apoptotic cells (data not
NFATc2 represses CDK4 promoter activity in T-lymphocytes
shown), appeared normal in the absence of NFATc1 (
(), and injection of a TAT-p16INK4a protein,
and B–S4D). In addition, hair shaft length was slightly
which inhibits CDK4/6, impairs hair growth in mice (
longer in KO follicles (but long-term grafts main-
tained hair growth Thus, the quiescent status and
By immunohistochemistry, CDK4 appeared to be upregulated
initiation of hair growth were selectively altered upon loss of
in the telogen to anagen transition of WT HFs, where it appeared
NFATc1, while stem cell self-renewal seemed to be maintained.
in a few cells at the base of the bulge, in the developing hair germ
Since hair cycling requires mesenchymal-epithelial interac-
and later in the matrix cells of the anagen hair bulb
tions, it was important to ablate NFATc1 specifically in the skin
The elevated CDK4 protein during HF stem cell activation was
epithelial cells. To this end, we bred NFATc1fl/fl mice (to be de-
reflected at the mRNA level, as judged by real-time PCR on
scribed further elsewhere) to transgenic mice expressing the
FACS-purified cell populations B). By contrast, NFATc1
Cre recombinase under the control of the keratinocyte-specific
null HFs displayed anti-CDK4 staining within the bulge region
K14 promoter, active by E15.5 ). NFATc1
even prior to visible signs of anagen progression in vivo
cKO mice developed normal HFs (F) which lacked
(C), and constitutively nuclear caNFATc1 in vitro elicited
detectable NFATc1 immunolabeling (In contrast to
a reduction in both CDK4 mRNA (and protein (
the calcineurin B1 cKO (), telogen follicles
The regulation of CDK4 expression was at the level of
were maintained in NFATc1 cKO mice However,
transcription, since NFATc1 repressed the activity of a luciferase
consistent with the phenotype of the complete NFATc1 null,
reporter driven by the wild-type CDK4 promoter but not the pro-
while hair germs of WT HFs were still in telogen, a majority of
moter harboring mutations in the NFAT binding sites
NFATc1 cKO HFs entered anagen at D56, as revealed by BrdU
labeling in hair germs (H and 3I) and the growth of
The elevation in CDK4 appeared to be specific for the bulge,
hairs after shaving Quantification documented that
as no change in CDK4 was noted in NFATc1 null epidermis
"75% of NFATc1 cKO bulges were BrdU-labeled within a
(The effects also appeared to be specific for
48 hr pulse H and 3I). FACS analysis further substanti-
CDK4, as other cell cycle regulators, including the closely related
ated the significant increase of S-phase bulge cells in NFATc1
homolog CDK6, did not exhibit this behavior (5D, and
cKO HFs I). This activation of bulge cell proliferation
5E). Importantly, the major downstream target of CDK4/6,
was specific as no effect on epidermal proliferation was noted
namely retinoblastoma protein (RB), was not phosphorylated in
with loss of NFATc1 (Together, these data suggest
caNFATc1-infected cells (E). Moreover, when a CDK4
that in the absence of NFATc1, the slow cycling properties are
transgene was coexpressed with caNFATc1, MK proliferation
selectively lost without perturbing other features of HF stem
and S phase progression were restored to WT levels
(G). Taken together, these findings indicated that the
To determine whether NFATc1 can confer slow cycling char-
suppression of CDK4 by NFATc1 was sufficient to inhibit G1/S
acteristics upon MKs, we used retroviral delivery to express
progression in quiescent stem cells.
a constitutively nuclear form of NFATc1 (caNFATc1; [
Finally, we addressed whether NFATc1 acts downstream of
]) in cultured epidermal MKs, which as shown
either of two endogenous signals, BMP and Wnt, known to pro-
in lack endogenous NFATc1. caNFATc1 markedly re-
mote the telogen to anagen transition. As shown in
duced the proliferation rate of MKs grown in low-calcium me-
and 6B, NFATc1 was still present and nuclear in bulge cells
dium, which is favorable to proliferation of WT cells A).
from mice expressing a constitutively stabilized b-catenin
The reduction in proliferation rate was confirmed by BrdU incor-
(DNbcatenin). The HFs of these mice precociously activate ana-
poration and FACS analyses of cell cycle profiles: caNFATc1-
gen, but the bulge stem cells return to quiescence (
Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc. 303
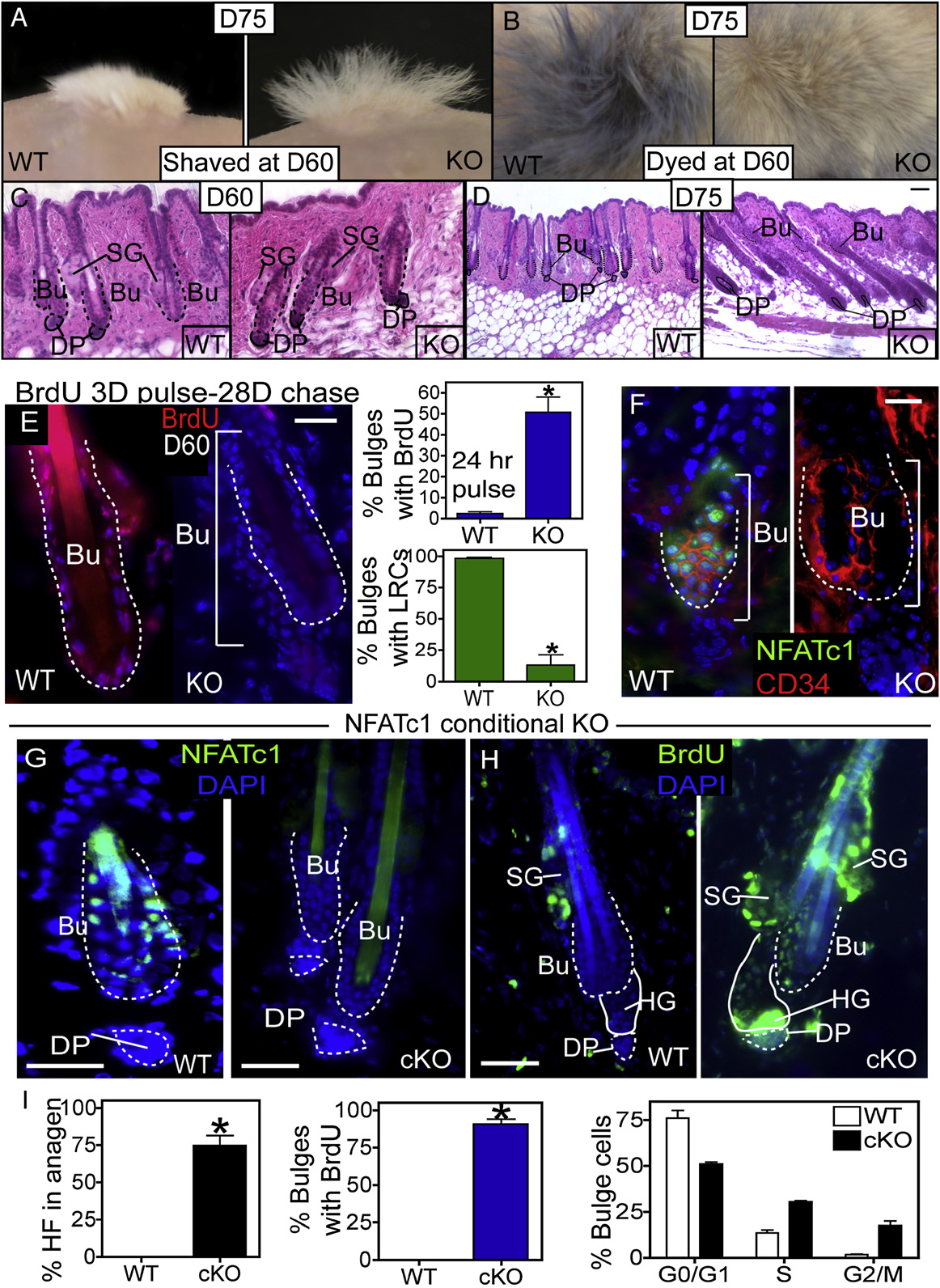
Figure 3. Full and Conditional NFATc1 Deletion Activates Precocious Follicular Growth and Enhanced Stem Cell Activity(A and B) When shaved or dyed blue during telogen (D60), the KO follicles regrow white hair (A and B) and shed their dyed hairs (B) by D75.
(C and D) Histological analysis of the WT and KO HFs at D60 (C) and D75 (D).
(E) Immunostaining of WT and KO follicles after BrdU label retaining experiments (BrdU pulse at D24–27 postgraft followed by 28 days of chase). Quantification ofbulge cell proliferation (24 hr BrdU pulse) and label retention at D60 in WT and NFATc1 null follicles. Data are mean ± SEM. N = 3 mice for each genotype.
304 Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc.
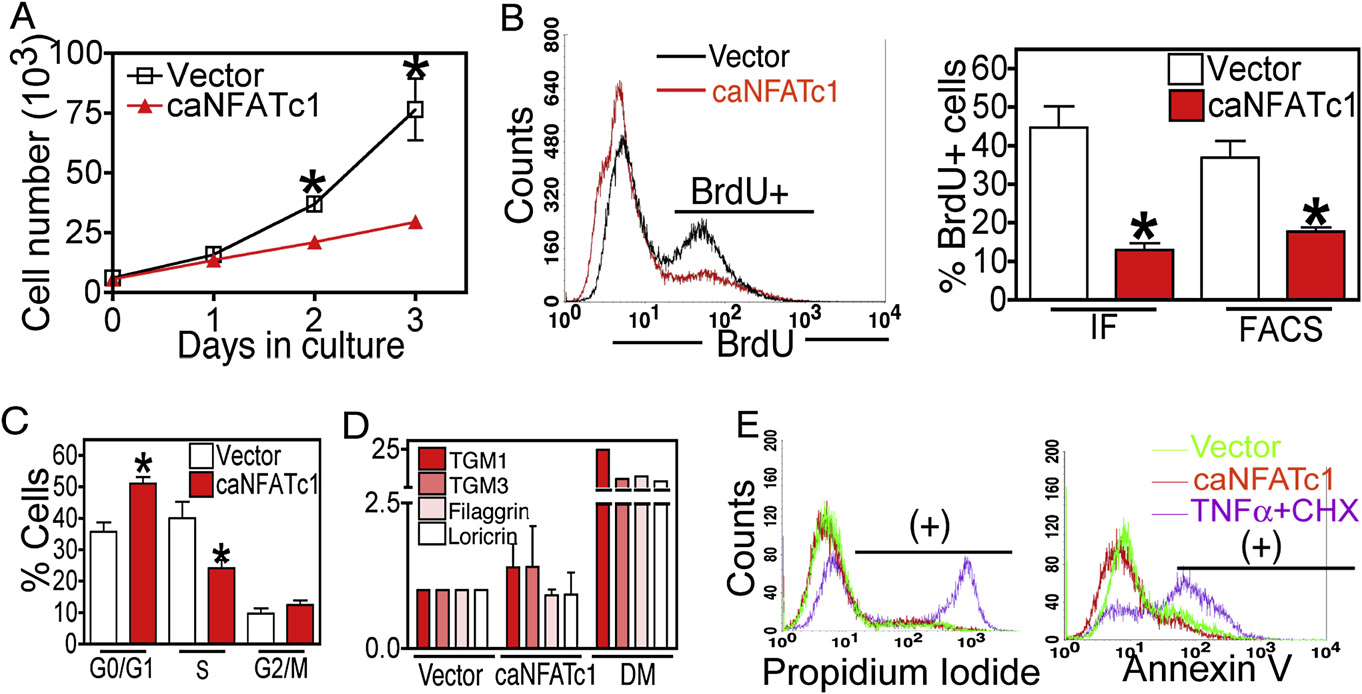
Figure 4. NFATc1 Inhibits Cellular Proliferation by Blocking G1/S Phase Progression(A) Retroviral expression of constitutively active NFATc1 (caNFATc1) represses cell proliferation in epidermal MKs. N = 3 individual experiments for each timepoint.
(B and C) Quantification of BrdU incorporation as analyzed by FACS or immunostaining (IF) (B) or combined with DNA content (C) in control or caNFATc1 infectedMKs. Representative FACS histogram plot of BrdU immunostaining is shown (left). Data are mean ± SEM. N = 3–4 individual experiments.
(D) Real-time PCR analysis of the epidermal terminal differentiation markers transglutaminase (TGM) 1 and 3, filaggrin, or loricrin in control, caNFATc1-infectedMKs, or cells in high calcium media (Diff. Media). Data are mean ± SEM. N = 3 individual experiments.
(E) FACS analysis of propidium iodide (dead cells) and Annexin V (apoptotic cells) in control, caNFATc1-expressing cells, or cells treated with TNFa (100 ng/ml)and cycloheximide (CHX) (5ug/ml). Data are mean ± SEM.
Asterisks indicate significance, p < 0.05.
enhanced > 100-fold by BMP4, and again these effects were ab-
addition, treatment of DNbcatenin mice with CSA resulted in
rogated by noggin F). Interestingly, despite these pro-
loss of NFATc1 expression, suggesting that its regulation was
found BMP-specific effects on NFATc1 gene expression, NFAT
not affected (data not shown). Interestingly, NFATc1 localization
reporter activity was only slightly upregulated by BMP4 treat-
was lost in HFs conditionally targeted for loss of the BMP recep-
ment and required calcium/CN activity G).
tor 1a (), required for BMPsignaling. This was true both for the presumptive bulge of new-
born HFs (and for 22 day-old HFs of BMPR1a cKOskin Importantly and in contrast to DNbcatenin,
Our data support a model whereby transcriptional repression of
BMPR1a ablation causes bulge cells to lose quiescence
CDK4 by NFATc1 in WT HFs maintains bulge stem cells in a qui-
escent state in all stages of the hair cycle Our studies
To address how BMP signaling might affect NFATc1, we first
in vitro and in vivo suggest that BMP signaling and calcineurin
tested whether exogeneous BMP could activate NFATc1 gene
activity are both required for NFATc1 expression. While BMP
expression in vitro. As shown in BMP4 increased
signaling affects NFATc1 gene expression, calcium signaling ac-
NFATc1 mRNA levels by 7- to 9-fold, and this effect was blocked
tivates NFAT's action as a transcription factor, suggesting that
by the BMP inhibitor, noggin. We next addressed whether
both of these upstream activators, BMP and calcium, impinge
BMP signaling acted directly upon the activity of the NFATc1
on NFATc1's role in the bulge. As cells are activated to proliferate
promoter (). NFATc1 promoter activity was
and form the new HF, nuclear NFATc1 and NFATc1 gene
(F) Immunostaining for NFATc1 and CD34 in WT and NFATc1 null follicles.
(G) Immunolocalization of NFATc1 in WT and NFATc1 cKO mice at P56.
(H) BrdU immunolabeling after a 48 hr pulse in WT and NFATc1 cKO follicles at P56. Data are mean ± SEM. N = 6 mice for WT and N = 5 mice for NFATc1 cKO.
(I) Quantification of BrdU incorporation and cell cycle analysis of a6(+)/CD34(+) bulge cells from WT and NFATc1 cKO mice. Data are mean ± SEM.
Asterisks indicate significance, p < 0.05. The scale bars represent 30 mm. Dapi staining (blue) shows nuclear localization. Abbreviations are as follows: Bu, bulge;SG, sebaceous gland; DP, dermal papillae; and HG, hair germ.
Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc. 305
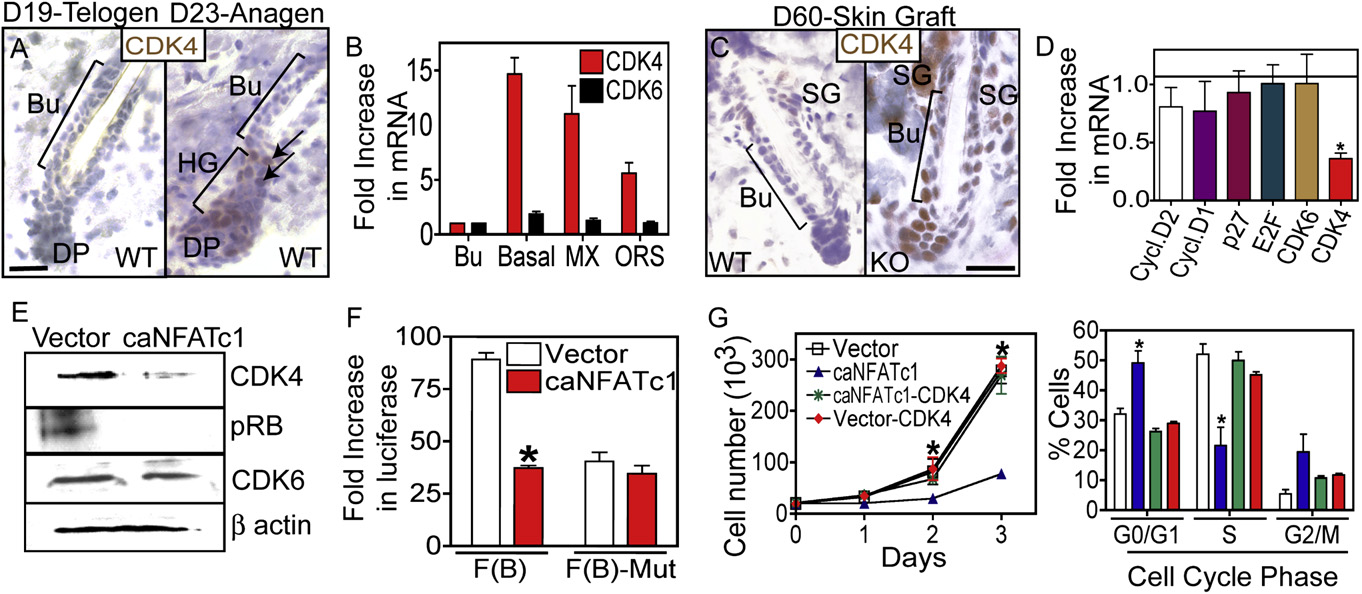
Figure 5. NFATc1 Acts by Repressing CDK4 Expression to Regulate G1/S Progression in Hair Follicle Stem Cells(A) Anti-CDK4 immunohistochemistry during the telogen (D19) to anagen (D23) transition. Hematoxylin counterstain.
(B) Real-time PCR analysis of CDK4 and CDK6 mRNA expression in bulge (Bu), all basal (a6+), ORS and matrix (MX) cell populations. Data are mean ± SEM. N = 3for each sample.
(C) Immunostaining of CDK4 in WT and NFATc1 null follicles.
(D) Expression of cell cycle regulatory genes in control or caNFATc1-expressing cells. Data are mean ± SEM. N = 3 individual experiments.
(E) Western analysis for CDK4, phosphorylated retinoblastoma protein (Rb), and CDK6 in control and caNFATc1 expressing keratinocytes (MKs).
(F) Reporter assays using the CDK4 promoter with NFAT sites (F[B]) or mutated NFAT sites (F[B]-mut) in conjuction with caNFATc1 in MKs. Data are mean ± SEM.
N = 4 individual experiments.
(G) Expression of CDK4 rescues MK growth and S phase progression of cells expressing caNFATc1. N = 3 individual experiments. Data are mean ± SEM.
Abbreviations are as follows: DP, dermal papillae; Bu, bulge; ORS, outer root sheath; SG, sebaceous gland; HG, hair germ; and MX, matrix. Asterisks indicatesignificance, p < 0.05. The scale bars represent 30 mm.
expression are downregulated and CDK4 expression is upregu-
postulated that inhibition of calcineurin by CSA may influence HF
lated. Finally, when NFATc1 activity is inhibited, either by CSA
regression during the catagen phase of the hair cycle by acting
treatment or conditional ablation of NFATc1, CDK4 levels are
on NFATc2 nuclear localization
elevated in the bulge cells and their slow cycling character is
Although NFATc2 null mice are viable and display no overt ab-
normalities on the hair coat
CSA has pleiotropic effects on hair growth, although most
), further studies will be required to determine the
studies have concentrated on the notion that CSA enhances hair
mechanisms by which calcineurin and CSA might affect other
growth by preventing the apoptosis that occurs in the catagen
substrates in the skin, including NFAT proteins.
phase of the hair cycle (
It has also been shown that in vitro, calcineurin/NFATc2
). Our studies confirm the ability of CSA
regulates terminal differentiation of epidermal MKs by acting
to precociously induce the telogen to anagen transition in resting
downstream of Notch signaling to activate the p21 promoter
HFs and now demonstrate that this transi-
(). NFATc1 did not appear to be expressed
tion is mediated at least in part by promoting proliferation within
in normal epidermis in vivo, nor did its loss result in any discern-
the bulge stem cell compartment. Furthermore, our data reveal
able defects in the epidermis. Moreover, in keratinocytes in vitro,
that NFATc1, previously unstudied in the skin, is likely responsi-
activated NFATc1 did not appear to affect terminal differentia-
ble for the effects of CSA on bulge stem cell activation. The stim-
tion. Thus, both in vivo and in vitro, NFATc1's function in bulge
ulation of bulge proliferation in response to CSA or loss of
cells was distinct from that previously attributed to other NFAT
NFATc1 contrasts with the normal behavior of bulge stem cells,
family members in epidermal cell cultures.
which typically are slow cycling even during the growing phase of
Our data suggest that calcium signaling may contribute to the
the hair cycle.
regulation of NFATc1 activity in the HF stem cell niche. Calcium
Calcineurin may have additional substrates in the skin that
is a well-known regulator of homeostasis and differentiation in
control catagen or HF retention, since NFATc1 null mice do not
the epidermis in vitro (
have defects in either process. Calcineurin B1 cKO mice display
and in vivo Intriguingly, the bulge signature
alopecia due to the inability to retain telogen HFs, but the down-
contains a number of genes encoding calcium binding proteins
stream targets of calcineurin that control this phenotype are un-
and ion channels (
known In addition, other groups have
Future studies will be necessary to dissect the
306 Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc.
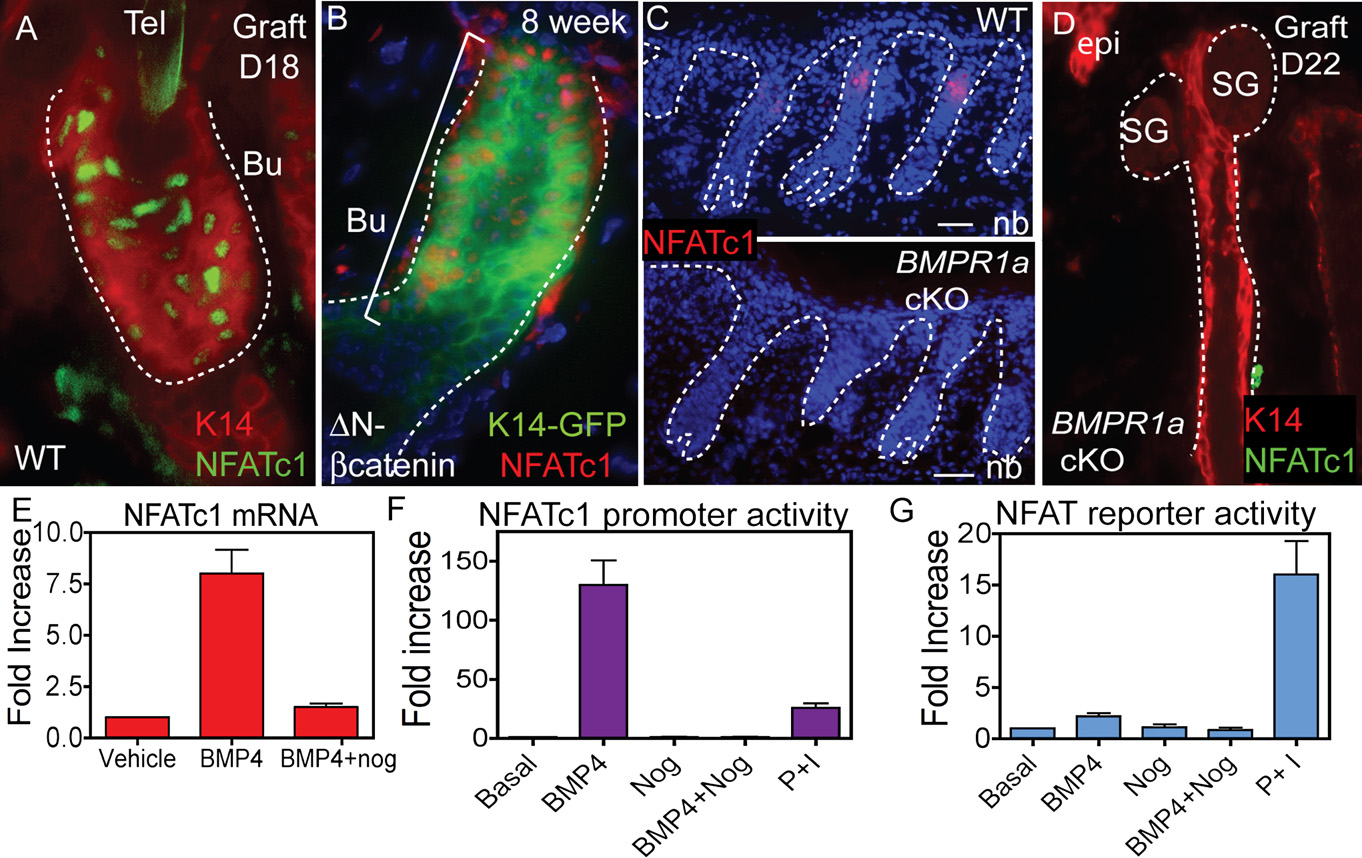
Figure 6. Expression of NFATc1 in Mutant Mouse Models with Altered Hair Follicle Stem Cell Biology(A–D) K14 expression (immunolocalization and K14-GFP) and NFATc1 immunolocalization are shown for grafted skin from WT (A), heterozygous stabilized b-cat-enin (DN) (B), and BMPR1a conditional knockout (cKO) mice (C and D).
(E) Real-time PCR of NFATc1 mRNA levels upon MK treatment with BMP4 and/or the BMP inhibitor, noggin. Data are mean ± SEM. N = 3 individual experiments.
(F) The activity of a 3.6 kb NFATc1 promoter construct following treatment of MKs with BMP4 and/or noggin or PMA (P) and ionomycin (I). Data are mean ± SEM.
N = 3 individual experiments.
(G) NFAT reporter activity upon BMP4, noggin or P/I treatment of MKs. Data are mean ± SEM. N = 3 individual experiments.
Dapi staining (blue) shows nuclear localization. Abbreviations are as follows: Bu, bulge; CN, calcineurin; DP, dermal papillae; HG, hair germ; and epi, epidermis.
mechanisms that regulate calcium signaling in HF stem cells to
In contrast to the effects of BMP signaling and NFATc1 on the
regulate their activity and fate.
slow-cycling properties of bulge stem cells, inhibition of Wnt sig-
Despite the identification of bulge stem cell populations based
naling seems to act primarily by repressing the differentiation lin-
on their relative quiescence (
eages afforded to them ().
Conversely, while both inhibition of BMP signaling and elevated
into how quiescence is controlled have only recently begun to
Wnt signaling seem to promote the telogen to anagen transition,
emerge. BMP signaling appears to participate in maintaining qui-
gene targeting and transgenic studies are suggestive that they
escence within the bulge, as conditional ablation of BMPR1a re-
activate proliferation and lineage commitment by different
sults in sustained activation of HF stem cells without apparent
loss of many stem cell features, including Sox9
). Our findings provide insights as to how BMP signaling
Our data suggest that part of BMPs role in the stem cell
may function in controlling the quiescent state of HF stem cells
niche may be to control NFATc1 expression. This is supported
and in their transition to an activated state at the beginning of
by the loss of NFATc1 in BMPR1a cKO skin as well as the marked
the new hair cycle.
elevation in NFATc1 promoter activity and mRNA expression in
It is intriguing that the epidermis seems to be largely unaf-
BMP4-treated MKs. In this regard, it is noteworthy that 10 puta-
fected irrespective of whether NFATc1, BMPR1a or b-catenin
tive SMAD sites reside within the NFATc1 promoter. While
is targeted for ablation. The cell lineage-specific differences in
NFATc1 is not likely the only downstream target of BMP signal-
stem cell regulation between the epidermis and HF may explain
ing, our findings suggest that NFATc1 acts downstream of
why in the epidermis, quiescence and homeostasis appear to be
BMPR1A signaling in the bulge, where it participates in stem
regulated by different mechanisms involving EGF receptor sig-
cell quiescence.
naling () and NFkB-mediated regulation
Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc. 307
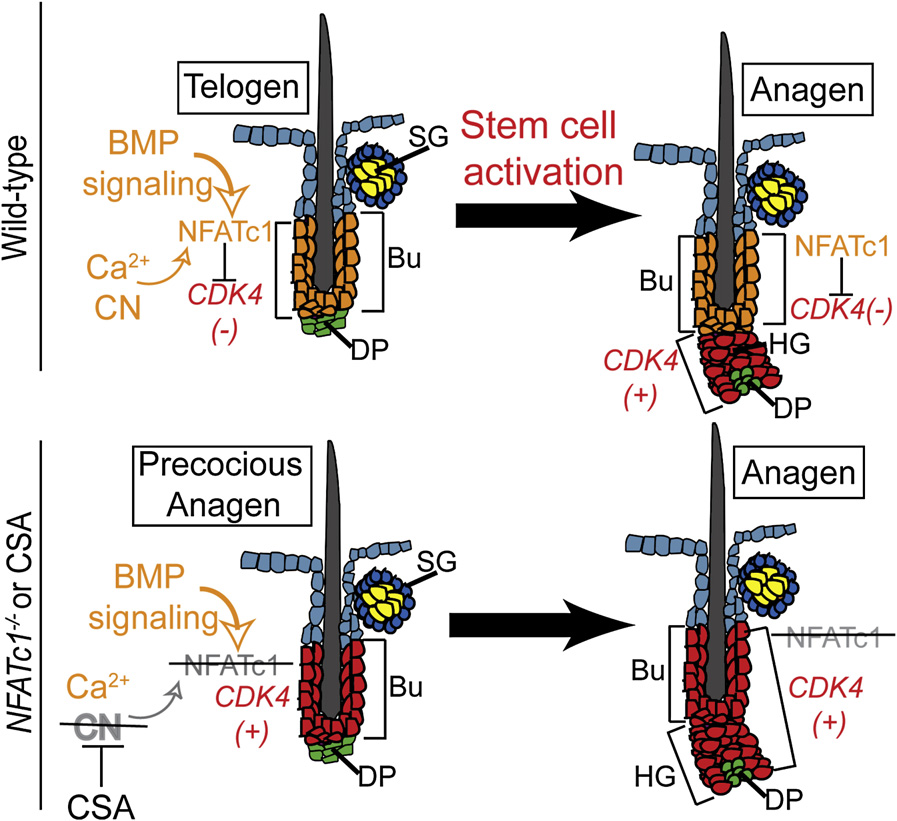
Figure 7. Model Describing the Role ofNFATc1 Signaling in Bulge Stem Cell Quies-cenceCalcium/calcineurin (CN) activity and BMP signal-ing are required to maintain NFATc1 expressionand activity, which transcriptionally repressesCDK4 gene expression. Upon activation of hairgrowth, BMP signaling is inhibited, leading toa loss in NFATc1 expression and relief of CDK4 re-pression. When CN signaling is blocked by CSA orloss of NFATc1 expression, CDK4 is expressedand precocious activation of the stem cells occurswithin the bulge.
thereafter either directly, or following a chase pe-riod. For in vivo experiments using CSA, micewere injected with 100 mg/kg CSA solution (Bed-ford Laboratories) intraperitoneally daily at P49for 6 days. Hair dye was used according to manu-facturer's directions (L'Oreal).
Retroviral Infection, Cell Culture, and FACSMSCV-GFP (Vector control), constitutively nuclearcaNFATc1, a NFAT reporter construct and CDK4retroviral vectors were previously described (Retrovirusproduction is described in the Growth assays using
of CDK4 Given that CSA affects prolifera-
infected cells were performed by trypsizing and counting cells with a Z1 Par-
tion, albeit differentially, in both epidermal and HF stem cells, it
ticle Coulter Counter at indicated intervals. Quantification of BrdU and cell cy-
will be interesting in the future to evaluate whether calcineurin
cle was performed using FACS as described previously ().
Differentiation of MKs was induced by adding MK growth media with 1.5 mM
signaling and other NFAT proteins play general roles in regulating
calcium. FACS analysis of apoptosis was performed via Annexin V (Invitrogen)
stem cell proliferation in other tissues and organs.
staining according to manufacturer's instructions. As a positive control for the
In closing, our data suggest that excessive hair growth in hu-
apoptosis experiments, cells were treated with TNFa (100 ng/ml) and cyclo-
man patients during immunosuppressive therapy involving CSA
heximide (5 mg/ml) for 24 hr prior to staining.
treatment might result from inhibition of NFATc1, which in turn
For experiments using cultured bulge stem cells, a6+ cells alone and a6+/
leads to CDK4 expression and HF stem cell activation. If the
CD34+ bulge cells were FACS purified as described )from K14-H2BGFP mice. Following FACS isolation, a minimum of 1 3 105 cells
mechanism we've uncovered in mouse skin occurs in human
were seeded onto mitomycin treated fibroblast feeder layers and cultured in
HFs, NFATc1 activation could have clinical significance by pro-
MK growth media with 0.3 mM calcium. After the first passage, the MKs
viding a possible target for controlling hair growth. Additionally,
were treated with vehicle, cyclosporine A (1 mM; Sigma), cell-permeable VIVIT
by specifically inhibiting or activating NFATc1 in skin, it may be
(11R-VIVIT; 1 mM; Calbiochem), or cell-permeable scrambled VIVIT, (11R-
possible to pharmacologically uncouple the immunosuppressive
VEET; generous gift from Masayuki Matsushita and Rockefeller Proteomics
and hair growth effects caused by general calcineurin inhibitors
Resource Center) () and analyzed for colony size (>5 cells)
or cell number for the indicated days. No toxicity was noted with the use ofVIVIT on MKs.
EXPERIMENTAL PROCEDURES
Reporter AssaysFor CDK4 and NFATc1 promoter reporter assays, MKs were grown to 30%–
Mice, Cyclosporine A Treatments, Grafts, and BrdU Incorporation
40% confluency before Fugene 6 (Roche) reagent-assisted transfections of
NFATc1 null, K14-H2BGFP, DN-b!catenin transgenic mice, and BMPR1a
cytomegalo virus–Renilla luciferase DNA (control) and the CDK4 or NFATc1
conditional null mice have been described previously (
promoter firefly luciferase constructs. The WT CDK4 promoter construct
A conditional al-
(F[B]) consisted of –404 to +43 ). This construct was used
lele of NFATc1 (NFATc1 fl) was generated by flanking exon 3 with loxP sites (to
to generate a mutated version of F(B) lacking a NFAT binding site using
be described further elsewhere). Exon 3 encodes the NFAT regulatory domain
the Quickchange Kit (Stratagene) using the complementary oligonucleotide,
and deletion results in a null allele and data not
F'-CCCGCCTCCCAGAGAGTGCGCGCCTCTTTGGC-30 (mutational changes are
shown). NFATc1fl/fl, K14-Cre negative littermates were used as WT controls
underlined). 48 hr after transfection, luciferase assays with the CDK4 promoter
for all experiments. Grafting of E15.5 skins was performed as described
were performed as described previously
For 5-Bromo-20-deoxyuridine (BrdU) (Sigma-Aldrich)
The NFATc1 promoter construct consisted of 3.7 kb upstream of the
pulse-chase experiments mice were injected intraper-
NFATc1 transcriptional start site 48 hr posttransfection,
itoneally with 50 mg/g BrdU (Sigma-Aldrich) and animals were then sacrificed
cells were treated with BMP4 (100 ng/ml; R&D Systems) and/or noggin
308 Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc.
(1000 ng/ml; R&D Systems) for 48 hr. 6 hr prior to performing the luciferase
Andl, T., Ahn, K., Kairo, A., Chu, E.Y., Wine-Lee, L., Reddy, S.T., Croft, N.J.,
assay, cells were treated with PMA (10 nM) and ionomycin (1 mm).
Cebra-Thomas, J.A., Metzger, D., Chambon, P., et al. (2004). Epithelial
For NFAT reporter assays, MKs were infected with retroviruses encoding the
Bmpr1a regulates differentiation and proliferation in postnatal hair follicles
NFAT responsive plasmid in control or caNFATc1 infected cells
and is essential for tooth development. Development 131, 2257–2268.
After 48 hr, PMA (10 nM), ionomycin (1 mM), VIVIT (1 mM) and CSA (1 mM)
Andl, T., Reddy, S.T., Gaddapara, T., and Millar, S.E. (2002). WNT signals are
were added to the cultures as indicated for 6 hr. PMA acts to induce AP-1 tran-
required for the initiation of hair follicle development. Dev. Cell 2, 643–653.
scription factors and ionomycin increases intracellular calcium, activates
Aramburu, J., Garcia-Cozar, F., Raghavan, A., Okamura, H., Rao, A., and Ho-
calcineurin, and leads to nuclear translocation of NFAT family members. Lucif-
gan, P.G. (1998). Selective inhibition of NFAT activation by a peptide spanning
erase assays were performed as described above.
the calcineurin targeting site of NFAT. Mol. Cell 1, 627–637.
Baksh, S., Widlund, H.R., Frazer-Abel, A.A., Du, J., Fosmire, S., Fisher, D.E.,
Histology and Immunofluorescence
DeCaprio, J.A., Modiano, J.F., and Burakoff, S.J. (2002). NFATc2-mediated re-
Skins were embedded in OCT, frozen, sectioned and fixed in 4% formalde-
pression of cyclin-dependent kinase 4 expression. Mol. Cell 10, 1071–1081.
hyde. For paraffin sections, skins were incubated in 4% formaldehyde at4#C overnight, dehydrated with a series of increasing concentrations of etha-
Blanpain, C., Lowry, W.E., Geoghegan, A., Polak, L., and Fuchs, E. (2004).
nol and xylene, and embedded in paraffin. Paraffin sections were rehydrated in
Self-renewal, multipotency, and the existence of two cell populations within
decreasing concentrations of ethanol and subjected to antigen unmasking in
an epithelial stem cell niche. Cell 118, 635–648.
10 mM Citrate (pH 6.0). For histological analysis, sections were stained with
Canning, M.T., Nay, S.L., Pena, A.V., and Yarosh, D.B. (2006). Calcineurin in-
hematoxylin and eosin. Sections were subjected to immunofluorescence mi-
hibitors reduce nuclear localization of transcription factor NFAT in UV-irradi-
croscopy. For immunohistochemistry, HRP conjugate secondary antibodies
ated keratinocytes and reduce DNA repair. J. Mol. Histol. 37, 285–291.
(Abs) were used followed by the HRP substrate, diaminobenzidine. When
Claudinot, S., Nicolas, M., Oshima, H., Rochat, A., and Barrandon, Y. (2005).
applicable, the MOM Basic Kit (Vector Laboratories) was used to prevent
Long-term renewal of hair follicles from clonogenic multipotent stem cells.
nonspecific binding of mouse monoclonal Abs. Antibodies and dilutions are
Proc. Natl. Acad. Sci. USA 102, 14677–14682.
Clevers, H. (2006). Wnt/beta-catenin signaling in development and disease.
For each type of analysis performed in this study, we assayed for hair cycle
Cell 127, 469–480.
by both histology and proliferation status as indicated by BrdU incorporationduring short pulses.
Clipstone, N.A., and Crabtree, G.R. (1992). Identification of calcineurin as a keysignalling enzyme in T-lymphocyte activation. Nature 357, 695–697.
Cotsarelis, G., Sun, T.T., and Lavker, R.M. (1990). Label-retaining cells reside
To determine significance between groups, comparisons were made using
in the bulge area of pilosebaceous unit: implications for follicular stem cells,
Student's t tests. Analyses of multiple groups were performed using One-
hair cycle, and skin carcinogenesis. Cell 61, 1329–1337.
Way ANOVA with Bonferroni's posttest with GraphPad Prism version for
Crabtree, G.R., and Olson, E.N. (2002). NFAT signaling: choreographing the
Macintosh (GraphPad Software). For all statistical tests, the 0.05 level of con-
social lives of cells. Cell Suppl. 109, S67–S79.
fidence was accepted for statistical significance.
de la Pompa, J.L., Timmerman, L.A., Takimoto, H., Yoshida, H., Elia, A.J.,Samper, E., Potter, J., Wakeham, A., Marengere, L., Langille, B.L., et al.
Supplemental Data
(1998). Role of the NF-ATc transcription factor in morphogenesis of cardiac
Supplemental Data include four figures and Supplemental Experimental Pro-
valves and septum. Nature 392, 182–186.
cedures and can be found with this article online at
Gafter-Gvili, A., Kalechman, Y., Sredni, B., Gal, R., and Gafter, U. (2004).
Cyclosporin A-induced hair growth in mice is associated with inhibition of hairfollicle regression. Arch. Dermatol. Res. 296, 265–269.
Gafter-Gvili, A., Sredni, B., Gal, R., Gafter, U., and Kalechman, Y. (2003). Cy-closporin A-induced hair growth in mice is associated with inhibition of calci-
We graciously thank the many colleagues who generously donated mice and
neurin-dependent activation of NFAT in follicular keratinocytes. Am. J. Physiol.
reagents. We thank our colleagues in the Fuchs lab for their valuable discus-
Cell Physiol. 284, C1593–C1603.
sions and advice, especially Michael Rendl, Hoang Nguyen, and XuanWang. A.O.A. is a recipient of the Abbott Scholar Award in Rheumatology Re-
Gat, U., DasGupta, R., Degenstein, L., and Fuchs, E. (1998). De Novo hair fol-
search and the American Society for Clinical Investigation Young Investigator
licle morphogenesis and hair tumors in mice expressing a truncated beta-cat-
Award. V.H. was a Robert Black Fellow of the Damon Runyon Cancer Re-
enin in skin. Cell 95, 605–614.
search Foundation (DRG-1802-04) and receives funding through a Pathway
Graef, I.A., Chen, F., Chen, L., Kuo, A., and Crabtree, G.R. (2001). Signals
to Independence Award from National Institutes of Health (K99-AR054775).
transduced by Ca(2+)/calcineurin and NFATc3/c4 pattern the developing vas-
E.F. is an Investigator of the Howard Hughes Medical Institute. This work
culature. Cell 105, 863–875.
was supported in part by RO1-AR31737 (E.F.) and AI31541 (L.H.G.) from NIH.
Heit, J.J., Apelqvist, A.A., Gu, X., Winslow, M.M., Neilson, J.R., Crabtree, G.R.,and Kim, S.K. (2006). Calcineurin/NFAT signalling regulates pancreatic beta-
Received: June 26, 2007
cell growth and function. Nature 443, 345–349.
Revised: September 28, 2007
Hennings, H., Holbrook, K., Steinert, P., and Yuspa, S. (1980). Growth and dif-
Accepted: November 26, 2007
ferentiation of mouse epidermal cells in culture: effects of extracellular cal-
Published: January 24, 2008
cium. Curr. Probl. Dermatol. 10, 3–25.
Hodge, M.R., Ranger, A.M., Charles de la Brousse, F., Hoey, T., Grusby, M.J.,and Glimcher, L.H. (1996). Hyperproliferation and dysregulation of IL-4 expres-
Abbott, K.L., Friday, B.B., Thaloor, D., Murphy, T.J., and Pavlath, G.K. (1998).
sion in NF-ATp-deficient mice. Immunity 4, 397–405.
Activation and cellular localization of the cyclosporine A-sensitive transcription
Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G., and Birchmeier, W. (2001).
factor NF-AT in skeletal muscle cells. Mol. Biol. Cell 9, 2905–2916.
beta-Catenin controls hair follicle morphogenesis and stem cell differentiation
Al-Daraji, W.I., Grant, K.R., Ryan, K., Saxton, A., and Reynolds, N.J. (2002). Lo-
in the skin. Cell 105, 533–545.
calization of calcineurin/NFAT in human skin and psoriasis and inhibition of cal-
Jensen, K.B., and Watt, F.M. (2006). Single-cell expression profiling of human
cineurin/NFAT activation in human keratinocytes by cyclosporin A. J. Invest.
epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell
Dermatol. 118, 779–788.
quiescence. Proc. Natl. Acad. Sci. USA 103, 11958–11963.
Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc. 309
Karashima, T., Hachisuka, H., and Sasai, Y. (1996). FK506 and cyclosporin A
Ranger, A.M., Grusby, M.J., Hodge, M.R., Gravallese, E.M., de la Brousse,
inhibit growth factor-stimulated human keratinocyte proliferation by blocking
F.C., Hoey, T., Mickanin, C., Baldwin, H.S., and Glimcher, L.H. (1998). The
cells in the G0/G1 phases of the cell cycle. J. Dermatol. Sci. 12, 246–254.
transcription factor NF-ATc is essential for cardiac valve formation. Nature
Kaufman, C.K., Sinha, S., Bolotin, D., Fan, J., and Fuchs, E. (2002). Dissection
of a complex enhancer element: maintenance of keratinocyte specificity but
Rendl, M., Lewis, L., and Fuchs, E. (2005). Molecular dissection of mesenchy-
loss of differentiation specificity. Mol. Cell. Biol. 22, 4293–4308.
mal-epithelial interactions in the hair follicle. PLoS Biol. 3, e331. 10.1371/journal.
Kobielak, K., Pasolli, H.A., Alonso, L., Polak, L., and Fuchs, E. (2003). Defining
BMP functions in the hair follicle by conditional ablation of BMP receptor IA.
Rhee, H., Polak, L., and Fuchs, E. (2006). Lhx2 maintains stem cell character in
J. Cell Biol. 163, 609–623.
hair follicles. Science 312, 1946–1949.
Kobielak, K., Stokes, N., de la Cruz, J., Polak, L., and Fuchs, E. (2007). Loss of
Santini, M.P., Talora, C., Seki, T., Bolgan, L., and Dotto, G.P. (2001). Cross talk
a quiescent niche but not follicle stem cells in the absence of bone morphoge-
among calcineurin, Sp1/Sp3, and NFAT in control of p21(WAF1/CIP1) expres-
netic protein signaling. Proc. Natl. Acad. Sci. USA 104, 10063–10068.
sion in keratinocyte differentiation. Proc. Natl. Acad. Sci. USA 98, 9575–9580.
Lo Celso, C., Prowse, D.M., and Watt, F.M. (2004). Transient activation of beta-
Sawada, M., Terada, N., Taniguchi, H., Tateishi, R., and Mori, Y. (1987). Cyclo-
catenin signalling in adult mouse epidermis is sufficient to induce new hair fol-
sporin A stimulates hair growth in nude mice. Lab. Invest. 56, 684–686.
licles but continuous activation is required to maintain hair follicle tumours.
Sherr, C.J., and Roberts, J.M. (2004). Living with or without cyclins and cyclin-
Development 131, 1787–1799.
dependent kinases. Genes Dev. 18, 2699–2711.
Lowry, W.E., Blanpain, C., Nowak, J.A., Guasch, G., Lewis, L., and Fuchs, E.
Taylor, G., Lehrer, M.S., Jensen, P.J., Sun, T.T., and Lavker, R.M. (2000).
(2005). Defining the impact of beta-catenin/Tcf transactivation on epithelial
Involvement of follicular stem cells in forming not only the follicle but also the
stem cells. Genes Dev. 19, 1596–1611.
epidermis. Cell 102, 451–461.
Mammucari, C., Tommasi di Vignano, A., Sharov, A.A., Neilson, J., Havrda,
Trempus, C.S., Morris, R.J., Bortner, C.D., Cotsarelis, G., Faircloth, R.S., Re-
M.C., Roop, D.R., Botchkarev, V.A., Crabtree, G.R., and Dotto, G.P. (2005). In-
ece, J.M., and Tennant, R.W. (2003). Enrichment for living murine keratino-
tegration of Notch 1 and calcineurin/NFAT signaling pathways in keratino-
cytes from the hair follicle bulge with the cell surface marker CD34. J. Invest.
cyte growth and differentiation control. Dev. Cell 8, 665–676.
Dermatol. 120, 501–511.
Matsushime, H., Ewen, M.E., Strom, D.K., Kato, J.Y., Hanks, S.K., Roussel,
Tumbar, T., Guasch, G., Greco, V., Blanpain, C., Lowry, W.E., Rendl, M., and
M.F., and Sherr, C.J. (1992). Identification and properties of an atypical cata-
Fuchs, E. (2004). Defining the epithelial stem cell niche in skin. Science 303,
lytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 71, 323–
van Mater, D., Kolligs, F.T., Dlugosz, A.A., and Fearon, E.R. (2003). Transient
Maurer, M., Handjiski, B., and Paus, R. (1997). Hair growth modulation by top-
activation of beta -catenin signaling in cutaneous keratinocytes is sufficient
ical immunophilin ligands: induction of anagen, inhibition of massive catagen
to trigger the active growth phase of the hair cycle in mice. Genes Dev. 17,
development, and relative protection from chemotherapy-induced alopecia.
Am. J. Pathol. 150, 1433–1441.
Vasioukhin, V., Degenstein, L., Wise, B., and Fuchs, E. (1999). The magical
Menon, G.K., Grayson, S., and Elias, P.M. (1985). Ionic calcium reservoirs in
touch: genome targeting in epidermal stem cells induced by tamoxifen appli-
mammalian epidermis: ultrastructural localization by ion-capture cytochemis-
cation to mouse skin. Proc. Natl. Acad. Sci. USA 96, 8551–8556.
try. J. Invest. Dermatol. 84, 508–512.
Vidal, V.P., Chaboissier, M.C., Lutzkendorf, S., Cotsarelis, G., Mill, P., Hui,
Merrill, B.J., Gat, U., DasGupta, R., and Fuchs, E. (2001). Tcf3 and Lef1 regu-
C.C., Ortonne, N., Ortonne, J.P., and Schedl, A. (2005). Sox9 is essential for
late lineage differentiation of multipotent stem cells in skin. Genes Dev. 15,
outer root sheath differentiation and the formation of the hair stem cell com-
partment. Curr. Biol. 15, 1340–1351.
Morris, R.J., Liu, Y., Marles, L., Yang, Z., Trempus, C., Li, S., Lin, J.S., Sawicki,
Winslow, M.M., Pan, M., Starbuck, M., Gallo, E.M., Deng, L., Karsenty, G., and
J.A., and Cotsarelis, G. (2004). Capturing and profiling adult hair follicle stem
Crabtree, G.R. (2006). Calcineurin/NFAT signaling in osteoblasts regulates
cells. Nat. Biotechnol. 22, 411–417.
bone mass. Dev. Cell 10, 771–782.
Neal, J.W., and Clipstone, N.A. (2003). A constitutively active NFATc1 mutant
Xanthoudakis, S., Viola, J.P., Shaw, K.T., Luo, C., Wallace, J.D., Bozza, P.T.,
induces a transformed phenotype in 3T3–L1 fibroblasts. J. Biol. Chem. 278,
Luk, D.C., Curran, T., and Rao, A. (1996). An enhanced immune response in
mice lacking the transcription factor NFAT1. Science 272, 892–895.
Nguyen, H., Rendl, M., and Fuchs, E. (2006). Tcf3 governs stem cell features
Yamamoto, S., and Kato, R. (1994). Hair growth-stimulating effects of cyclo-
and represses cell fate determination in skin. Cell 127, 171–183.
sporin A and FK506, potent immunosuppressants. J. Dermatol. Sci. Suppl.
Noguchi, H., Matsushita, M., Okitsu, T., Moriwaki, A., Tomizawa, K., Kang, S.,
Li, S.T., Kobayashi, N., Matsumoto, S., Tanaka, K., et al. (2004). A new cell-per-
Yarosh, D.B., Pena, A.V., Nay, S.L., Canning, M.T., and Brown, D.A. (2005).
meable peptide allows successful allogeneic islet transplantation in mice.
Calcineurin inhibitors decrease DNA repair and apoptosis in human keratino-
Nat. Med. 10, 305–309.
cytes following ultraviolet B irradiation. J. Invest. Dermatol. 125, 1020–1025.
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K., and Barrandon, Y. (2001).
Yu, B.D., Becker-Hapak, M., Snyder, E.L., Vooijs, M., Denicourt, C., and
Morphogenesis and renewal of hair follicles from adult multipotent stem cells.
Dowdy, S.F. (2003). Distinct and nonoverlapping roles for pRB and cyclin
Cell 104, 233–245.
D:cyclin-dependent kinases 4/6 activity in melanocyte survival. Proc. Natl.
Pan, M., Winslow, M.M., Chen, L., Kuo, A., Felsher, D., and Crabtree, G.R.
Acad. Sci. USA 100, 14881–14886.
(2007). Enhanced NFATc1 nuclear occupancy causes T cell activation inde-
Zhang, J., He, X.C., Tong, W.G., Johnson, T., Wiedemann, L.M., Mishina, Y.,
pendent of CD28 costimulation. J. Immunol. 178, 4315–4321.
Feng, J.Q., and Li, L. (2006). Bone morphogenetic protein signaling inhibits
Paus, R., Handjiski, B., Eichmuller, S., and Czarnetzki, B.M. (1994). Chemo-
hair follicle anagen induction by restricting epithelial stem/progenitor cell acti-
therapy-induced alopecia in mice. Induction by cyclophosphamide, inhibition
vation and expansion. Stem Cells 24, 2826–2839.
by cyclosporine A, and modulation by dexamethasone. Am. J. Pathol. 144,
Zhang, J.Y., Tao, S., Kimmel, R., and Khavari, P.A. (2005). CDK4 regulation by
TNFR1 and JNK is required for NF-kappaB-mediated epidermal growth con-
Pillai, S., Bikle, D.D., Mancianti, M.L., Cline, P., and Hincenbergs, M. (1990).
trol. J. Cell Biol. 168, 561–566.
Calcium regulation of growth and differentiation of normal human keratino-
Zhou, B., Cron, R.Q., Wu, B., Genin, A., Wang, Z., Liu, S., Robson, P., and
cytes: modulation of differentiation competence by stages of growth and ex-
Baldwin, H.S. (2002). Regulation of the murine Nfatc1 gene by NFATc2.
tracellular calcium. J. Cell. Physiol. 143, 294–302.
J. Biol. Chem. 277, 10704–10711.
310 Cell 132, 299–310, January 25, 2008 ª2008 Elsevier Inc.
Source: http://horsley.yale.edu/sites/default/files/files/cellhorsley.pdf
Generic PrescriptionsUnderstanding TherapeuticSubstitutionBy Claims and Benefits, Inc, A Noridian Company CBI UpFront Newsletter October 2006 Not every brand-name drug has a generic equivalent, "but that doesn't necessarilymean you are stuck using a pricier brand-name product," says Bethany Pfister, aclinical pharmacist with Prime Therapeutics, a regional pharmacy benefits manager.You may be a candidate for a generic alternative.
APPLIED AND ENVIRONMENTAL MICROBIOLOGY, May 1998, p. 1902–1909 Copyright © 1998, American Society for Microbiology Effect of Bacterial Distribution and Activity on Conjugal Gene Transfer on the Phylloplane of the Bush Bean BO NORMANDER,1 BJARKE B. CHRISTENSEN,2 SØREN MOLIN,2 AND NIELS KROER1* National Environmental Research Institute, Department of Marine Ecology and Microbiology, DK-4000 Roskilde,1 and











