
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Effects of a ginger extract on knee pain in patients with osteoarthritis
ARTHRITIS & RHEUMATISM
Vol. 44, No. 11, November 2001, pp 2531–2538
2001, American College of Rheumatology
Published by Wiley-Liss, Inc.
Effects of a Ginger Extract on Knee Pain in
Patients With Osteoarthritis
R. D. Altman1 and K. C. Marcussen2
Objective. To evaluate the efficacy and safety of a
the 2 groups. Patients receiving ginger extract experi-
standardized and highly concentrated extract of 2 gin-
enced more gastrointestinal (GI) adverse events than
ger species, Zingiber officinale and Alpinia galanga
did the placebo group (59 patients versus 21 patients).
(EV.EXT 77), in patients with osteoarthritis (OA) of the
GI adverse events were mostly mild.
Conclusion. A highly purified and standardized
Methods. Two hundred sixty-one patients with
ginger extract had a statistically significant effect on
OA of the knee and moderate-to-severe pain were en-
reducing symptoms of OA of the knee. This effect was
rolled in a randomized, double-blind, placebo-
moderate. There was a good safety profile, with mostly
controlled, multicenter, parallel-group, 6-week study.
mild GI adverse events in the ginger extract group.
After washout, patients received ginger extract or pla-
cebo twice daily, with acetaminophen allowed as rescue
Present-day therapy for osteoarthritis (OA) of
medication. The primary efficacy variable was the pro-
the knee is directed at symptoms, since there is no
portion of responders experiencing a reduction in "knee
established disease-modifying therapy. Treatment pro-
pain on standing," using an intent-to-treat analysis. A
grams involve a combination of nonpharmacologic and
responder was defined by a reduction in pain of >
15
pharmacologic measures, utilizing a combination of an-
mm on a visual analog scale.
algesia, antiinflammatory, and intraarticular programs
Results. In the 247 evaluable patients, the per-
(1–3). If these are unsuccessful, a variety of surgical
centage of responders experiencing a reduction in knee
interventions are appropriate. Since none of the medic-
pain on standing was superior in the ginger extract
inal programs consistently provides adequate relief of
group compared with the control group (63% versus
pain, yet has attendant risk, the search continues for
50%; P ⴝ
0.048). Analysis of the secondary efficacy
agents that might provide improvement in symptoms
variables revealed a consistently greater response in the
with minimal risk. While scientists have turned to the
ginger extract group compared with the control group,
investigation of newly discovered pharmaceuticals, many
when analyzing mean values: reduction in knee pain on
patients have turned to herbal and other remedies that
standing (24.5 mm versus 16.4 mm; P ⴝ
0.005), reduc-
have not been adequately studied.
tion in knee pain after walking 50 feet (15.1 mm versus
The purpose of the present study was to test an
8.7 mm; P ⴝ
0.016), and reduction in the Western
extract of
Zingiber officinale Roscoe and
Alpinia galanga
Ontario and McMaster Universities osteoarthritis com-
posite index (12.9 mm versus 9.0 mm; P ⴝ
0.087).
Linnaeus Willdenow (both are of the Zingiberaceae
Change in global status and reduction in intake of
family, commonly called "gingers"). The Zingiberaceae
rescue medication were numerically greater in the gin-
family consists of 49 genera and 1,300 species, of which
ger extract group. Change in quality of life was equal in
there are 80–90 species of
Zingiber and 250 species of
Alpinia (4). The subspecies used in the tested extract
Supported by Gra¨ngeMatic Ltd, Dublin, Ireland.
were selected after analysis and testing of ⬎100 varieties
1R. D. Altman, MD: Miami Veterans Affairs Medical Center
(species and subspecies) of Zingiberaceae for antiin-
and University of Miami, Miami, Florida; 2K. C. Marcussen, MD:
flammatory effects, by in vivo assays and using animal
Narayana Research, Winter, Wisconsin.
Address correspondence and reprint requests to K. C. Mar-
models. The species selected by this process were grown
cussen, MD, Narayana Research, W 7041 Olmstead Road, Winter, WI
and harvested under controlled conditions.
Ginger is a very popular spice and the world
Submitted for publication August 23, 2000; accepted in
revised form April 11, 2001.
production is estimated at 100,000 tons annually, of
ALTMAN AND MARCUSSEN
which 80% is grown in China (5). Ginger also has a long
search based on the traditional use of the gingers has led
tradition of medicinal use and has been used as an
to the development of a patented ginger extract
antiinflammatory agent for musculoskeletal diseases,
(EV.EXT 77). In vitro experiments have shown that the
including rheumatism, in Ayurvedic and Chinese medi-
combined extract also inhibits the production of tumor
cine for more than 2,500 years (6,7). The German
necrosis factor ␣ (TNF␣) through inhibition of gene
Commission E Monographs contains reviews of drugs,
expression in human OA synoviocytes and chondrocytes
including herbal drugs, for quality, safety, and effective-
ness. As a result of this review of more than 300 herbs by
In this study, we have evaluated the safety and
an expert committee under the German Federal Insti-
efficacy of the extract in a double-blind, placebo-
tute for Drugs and Medical Devices, many herbs have
controlled study with intent-to-treat (ITT) analysis.
been excluded from sales in Germany. The Monographs
lists ginger for use in dyspepsia and prevention of
motion sickness (8). In the standard German text,
PATIENTS AND METHODS
Hager's Handbuch der Pharmazeutischen Praxis, ginger is
Study design. The study was a 6-week, double-blind,
listed as being used against nervousness, chronic inflam-
placebo-controlled, parallel-group trial performed at 10 clini-
mation of the intestine, coughing, conditions of the
cal centers in the US. It was designed according to guidelines
urinary tract and lower abdomen, rheumatism, and a
on conduct of clinical trials as reported by the Osteoarthritis
sore throat (9).
Research Society International (22) and as outlined in the
International Conference on Harmonisation clinical practice
Pharmacologically, ginger, similar to other plants,
guidelines (23). The protocol followed the 1975 Declaration of
is a very complex mixture of compounds.
Zingiber offi-
Helsinki as revised in 1983, with institutional review board
cinale contains several hundred known constituents (10),
approval, and all patients provided their oral and written
among them gingeroles, beta-carotene, capsaicin, caffeic
informed consent. Patients were centrally randomized to re-
acid, and curcumin. In addition, salicylate has been
ceive treatment by a computer-generated allocation schedule,
balanced by center, and both the investigators and the patients
found in ginger in amounts of 4.5 mg/100 gm fresh root
were blinded to treatment assignment.
(11). This would correspond to ⬍1 mg salicylate in 1
Patients. Patients had OA of the knee by the American
capsule of the presently tested ginger extract. The
College of Rheumatology classification criteria using the deci-
actions and especially the interactions of these ingredi-
sion tree format that includes radiographs (24). The radio-
ents have not been (and probably can not be easily)
graphic changes had to include at least osteophytes and
evaluated. Various powders, formulations, and extracts
correspond to OA grades 2, 3, or 4 by the Kellgren and
Lawrence criteria (25).
have, however, been commercially used and tested, both
Admission criteria included the presence of knee pain
in vitro and in vivo, in animal models. In these models,
on standing that had to be between 40 mm and 90 mm on a
ginger has been shown to act as a dual inhibitor of both
100-mm visual analog scale (VAS) during the preceding 24
cyclooxygenase (COX) and lipooxygenase (12), to in-
hours. This was assessed after a 1-week washout period. Both
hibit leukotriene synthesis (13), and to reduce
men and women ⱖ18 years old were included. Pain had to be
of a degree so that it could be tolerated with alleviation using
caregeenan-induced rat-paw edema (14,15), an animal
acetaminophen as an escape medication for 6 weeks. Prior
model of inflammation.
treatment for OA was not a requirement. Patients with any of
Another related plant, galanga, commonly called
the following were excluded: rheumatoid arthritis, fibromyal-
greater galanga, is also widely used as a spice in the East
gia, gout, recurrent or active pseudogout, cancer or other
and as a remedy for various ailments. It has an antiin-
serious disease, signs or history of liver or kidney failure,
asthma requiring treatment with steroids, treatment with oral
flammatory action through inhibition of prostaglandin
corticosteroids within the prior 4 weeks, intraarticular knee
synthesis (16), and has traditionally been used for rheu-
depo-corticosteroids within the previous 3 months, intraartic-
matic conditions in South East Asian medicine (17). The
ular hyaluronate within the previous 6 months, prior treatment
volatile oil of
Alpinia galanga L., which can be obtained
with immunosuppressive drugs such as gold or penicillamine,
by steam distillation of the rhizome, is a complex mixture
arthroscopy of the target joint within the previous year,
significant injury to the target joint within the previous 6
containing 1,8-cineol and 1⬘-acetoxychavicol acetate
months, other investigational drugs within the previous 1
which has antifungal (18) and antitumor (19) activity.
month, fever ⬎38°C at screening, and allergy to acetamino-
The German Commission E Monographs lists the use of
phen or ginger.
Alpinia officinarum, which is closely related to
Alpinia
After screening, patients entered a 1-week "washout"
galanga, for dyspepsia and loss of appetite. The US Food
for antiinflammatory and analgesic medications, during which
they were allowed to take acetaminophen as needed up to 4
and Drug Administration lists ginger and
Alpinia offici-
gm/day. Aspirin for anticoagulation up to 325 mg daily was
narum as "generally regarded as safe" (20). New re-
allowed throughout the study.
GINGER EXTRACT IN OA OF THE KNEE
If patients were determined to be eligible for the study,
coded according to World Health Organization adverse reac-
a baseline assessment of pain was performed after washout of
tion terminology (28). The adverse events were analyzed by
medications that would affect the arthritis and prior to ran-
preferred terms and by system organ classes.
domization. Each center was block-randomized with 130 pa-
Statistical analysis. Blinding was maintained until the
tients receiving ginger extract and 131 patients receiving
final database was cleaned and locked. However, there was an
interim analysis of 116 patients that was performed at a
Treatment. During the 6-week treatment period, pa-
significance level of 0.01% by an independent statistician. The
tients ingested 1 capsule twice daily, morning and evening.
results were disclosed to the sponsor only. Neither the inves-
Each capsule contained 255 mg of EV.EXT 77, extracted from
tigators nor the clinical research organization monitoring the
2,500–4,000 mg of dried ginger rhizomes and 500–1,500 mg of
study were aware of the results.
dried galanga rhizomes and produced according to good
Sample-size calculation was based on results of an
manufacturing practice (Eurovita Holding, Karlslunde, Den-
unpublished clinical trial using a ginger extract. Statistical
mark). Matching placebo capsules contained coconut oil. To
evaluation was performed using SAS (SAS Institute, Cary,
minimize a possible pungent sensation, patients were in-
NC). The statistical analysis was performed using analysis of
structed to swallow the whole (intact) capsule with a glassful of
covariance for analysis of means, with baseline scores, center,
water at the time of a meal.
sex, treatment-by-center interaction, and age as the covariates.
Acetaminophen was permitted as a rescue medication.
Chi-square tests were used for analysis of responders, Stu-
Patients were instructed to take the rescue medication only
dent's
t-test to analyze intake of rescue medication, and
when needed, to a maximum dosage of 2 tablets 4 times daily,
Fisher's exact test for comparing incidence of adverse events
i.e., 4 gm/day.
between groups. Except for the analysis of intake of rescue
Drug accountability was calculated by pill count for
medication, the ITT last observation carried forward method
both the study treatment and the rescue medication.
was used. All analyses were performed 2-sided, with a mini-
Assessments. The OA knee deemed to be more symp-
tomatic was defined as the target joint by the investigator, and
mum significance level of 5%.
the knee-specific pain was assessed for this joint. The primary
efficacy parameter was the proportion of responders experi-
encing at least a 15-mm reduction in pain between baseline and
the final visit for knee pain on standing during the preceding 12
Patients. There was no clinically relevant differ-
hours, as measured by a 100-mm VAS. Pain on standing is a
ence in the demographics between the 2 treatment
validated measure of pain and coincides with question 5 of the
Western Ontario and McMaster Universities (WOMAC) OA
groups (Table 1). The patients were predominantly
composite index (26). At the time of the design of this study,
women and predominantly white. Patients in both study
the full WOMAC index was not generally accepted as a
groups were generally overweight, since the average
primary efficacy variable in clinical trials of OA of the knee.
body mass index was ⬎30 kg/m2 (range 18–65 kg/m2).
Secondary efficacy measures that were used to com-
All patients with at least 1 visit after the baseline
pare the 2 study groups were as follows: 1) average improve-
ment in pain on standing, as measured by a 100-mm VAS; 2)
evaluation were included in the ITT analysis. Fourteen
consumption of rescue medication; 3) WOMAC index as
patients, 8 receiving placebo and 6 receiving ginger
measured by VAS, with one end of the scale being "no
extract, discontinued the trial before completing any
pain/stiffness/difficulty" and the other end, "extreme pain/
evaluation of efficacy. Among the patients in the pla-
stiffness/difficulty" (the total score was calculated as the mean
response); 4) patient assessment of global status, in which the
cebo group who discontinued, 3 dropped out due to
question, "Given all the ways your osteoarthritis affects you,
adverse events, 4 were lost to followup, and 1 withdrew
how have you been doing the last 24 hours?" was evaluated on
consent. Among the patients receiving ginger extract
a 5-point Likert scale (1 ⫽ very poor, 2 ⫽ poor, 3 ⫽ average,
who discontinued, 3 dropped out due to adverse events
4 ⫽ good, 5 ⫽ very good); 5) quality of life assessment using
and 3 were lost to followup. Thus, the modified ITT
the Short Form 12 (SF-12), which asks questions regarding the
patient's condition during the preceding 4 weeks (27); and 6)
analysis included the 247 patients (95% of the total
pain in the knee after walking 50 feet, recorded immediately
enrolled) who completed any postbaseline efficacy eval-
after walking and measured by a 100-mm VAS.
uation. A total of 194 patients (74%) completed the
Efficacy and safety assessments were performed at
study without protocol violations. Fifty-seven patients
baseline and after 2 and 6 weeks of treatment. The SF-12 was
administered at screening and after 6 weeks of treatment only.
discontinued prematurely (22% of the randomized pop-
Safety was assessed via open-ended questions concerning
ulation) (Table 2). The overall withdrawal rate was 28%
changes in the patients' health at each visit, supported by
in the ginger extract group and 16% among those
patients' responses on diary cards. For all adverse events, the
receiving placebo. The withdrawal rate due to adverse
onset, duration, and intensity (mild, moderate, or severe) of
events was 13% in the ginger extract group and 5% in
the event, as well as the action taken and outcome, were
recorded. The relationship between an adverse event and the
the placebo group. There were no followup data avail-
study medication was assessed, by the investigator, as none,
able for the patients who withdrew from the study
remote, possible, probable, or definite. Adverse events were
ALTMAN AND MARCUSSEN
Table 1. Demographic characteristics of study population*
Age, mean ⫾ SD years
Body mass index, mean ⫾ SD kg/m2
Diagnosed OA, mean ⫾ SD years
Radiographic classification of
* OA ⫽ osteoarthritis.
† By the Kellgren and Lawrence criteria (25).
Compliance. Compliance was calculated from the
extract group (78 of 130, or 60%) than in the placebo
amount of study medication (number of capsules) re-
group (62 of 131, or 47%) (P ⫽ 0.040). The analysis of
turned and the number of empty slots in the blister
means for pain on standing showed that the ginger
cards. Compliance was 98 ⫾ 12% (mean ⫾ SD) for the
extract group improved an average 8.1 mm more than
ginger extract group and 98 ⫾ 18% for the placebo
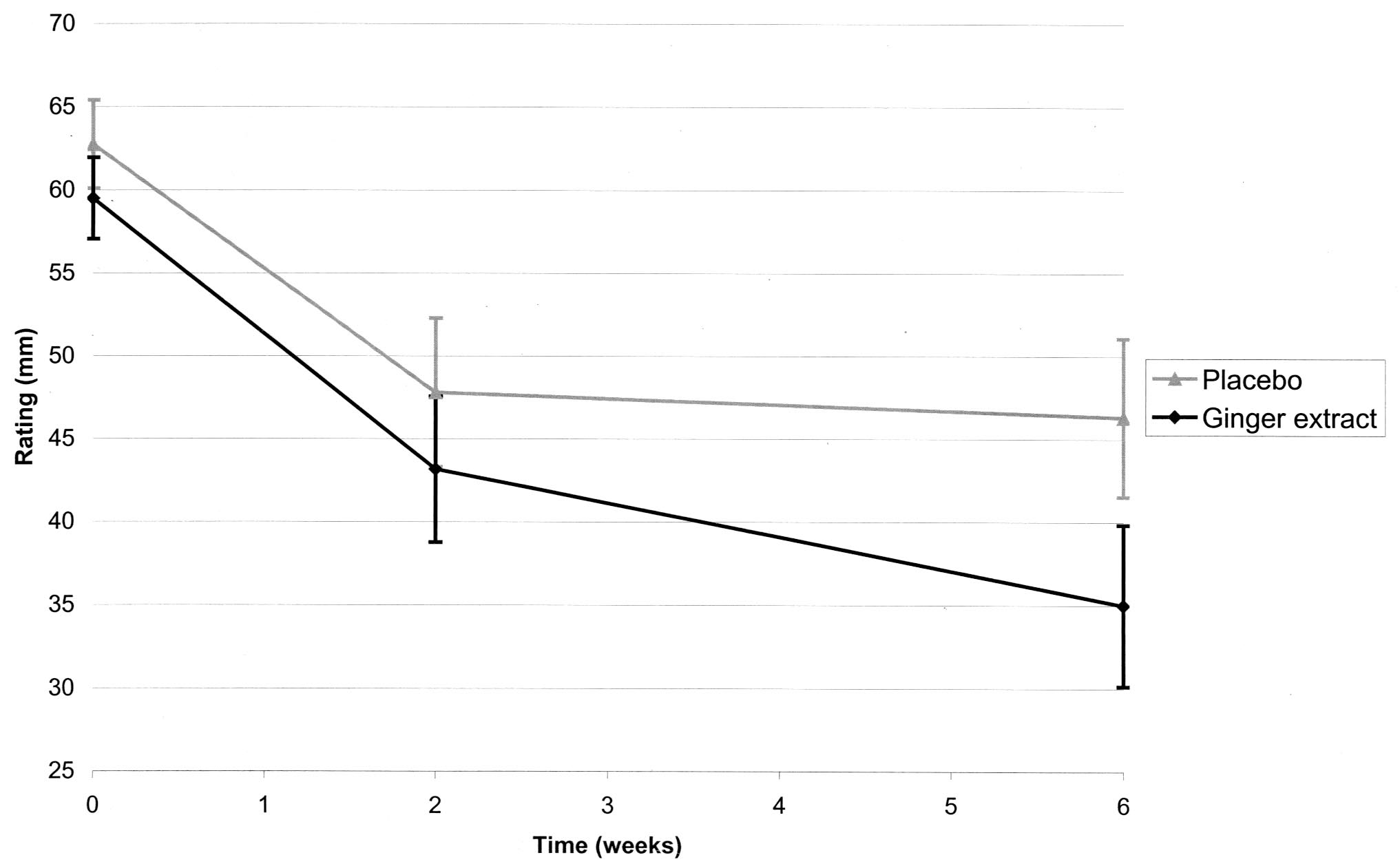
did the placebo group (P ⫽ 0.005) (Figure 1).
A subset analysis was performed for increased
Primary efficacy variable: pain on standing. Pain
responder levels. For ⱖ20-mm improvement in pain on
on standing after 6 weeks of treatment showed improve-
standing, the ginger extract group showed a response
ment in both treatment groups. However, as the primary
superior to that of the placebo group (n ⫽ 73 [59%]
efficacy parameter, there was a higher percentage of
versus n ⫽ 56 [46%]; P ⫽ 0.036). For a ⱖ25-mm
responders (improvement ⱖ15 mm on the VAS pain
improvement, the ginger extract group again displayed a
scale) in the ginger extract group (n ⫽ 78 [63%]) than in
the placebo group (n ⫽ 62 [50%]; P ⫽ 0.048). An ITT
analysis of all patients enrolled, regardless of whether
they underwent any postbaseline efficacy evaluation,
also showed a higher rate of responders in the ginger
Table 2. Discontinuations among the randomized population*
Primary reason for
early termination
Perceived lack of efficacy
Intercurrent illness
Figure 1. Knee pain on standing as measured by 100-mm visual
analog scale after 2 and 6 weeks in patients with osteoarthritis
receiving placebo (n ⫽ 123) or ginger extract (n ⫽ 124), in the
* Values are the number of patients.
intent-to-treat analysis. Bars show the mean pain rating (in mm) and
† P ⫽ 0.025 versus placebo.
95% confidence intervals.
GINGER EXTRACT IN OA OF THE KNEE
Table 3. Results of secondary parameters in the intent-to-treat analysis
Placebo (n ⫽ 123)*
Ginger extract (n ⫽ 124)*
Pain after walking 50 feet
* Numbers of patients vary between 121 and 124 at the single visits, and for quality of life (QOL), between 111 and 114.
† Western Ontario and McMaster Universities osteoarthritis index (WOMAC) consists of 24 questions, assessed on 100-mm visual analog scale,
analyzed in 3 subscales as the average score for 5 questions on pain, 2 questions on stiffness, and 17 questions on function. The total score is
calculated as the mean score for all 24 questions.
‡ The Short Form 12 (SF-12) consists of 12 questions that are combined into 8 scales, which are summarized in the physical and mental component
summaries shown here.
superior response compared with that of the placebo
Figure 2 shows the response on the individual questions
group (n ⫽ 65 [52%] versus n ⫽ 48 [39%]; P ⫽ 0.035).
of the WOMAC questionnaire, with responses to ques-
In an analysis of the patients who completed the
tions 6, 7, 11, 14, and 15 showing a significant improve-
study per protocol and experienced ⱖ15-mm improve-
ment among patients receiving the ginger extract. Im-
ment in pain on standing, results were similar to those of
provement in patient global status was numerically
the ITT analysis, although the difference between the 2
better in the ginger extract group and was statistically
treatment groups was smaller. The ginger extract group
superior in a per protocol analysis (P ⫽ 0.042). There
showed a response that was numerically superior (60 of
was no difference in the SF-12 score, since there was
92, or 65%) to that of the placebo group (54 of 102, or
little change from baseline in either group. Acetamino-
53%) (P ⫽ 0.083). In other parameters, significant
phen use was equal in the 2 study groups (mean ⫾ SD
improvements comparable with those in the ITT analysis
number of tablets daily 2.0 ⫾ 1.9 in the ginger extract
group and 2.2 ⫾ 2.0 in the placebo group).
Secondary efficacy variables. The results of the
Analysis of individual variables showed no effect
secondary parameters were consistent with the findings
of age (⬎65 years versus ⬍65 years), sex, center, or
with the primary parameter (Table 3). Pain after walking
treatment-per-center interaction on the efficacy para-
also demonstrated a significant improvement in the
meters. This analysis did show a difference in the
ginger extract group compared with the placebo group.
baseline scores, especially in global status, with the
The change in total WOMAC score was numerically
placebo group having the worse scores. This difference
superior in the ginger extract group versus the placebo
cannot be explained, but it was adjusted for through the
group, with the greatest improvement seen in stiffness.
analysis of covariance.
ALTMAN AND MARCUSSEN
There was concern that the adverse events might
affect the blinding of treatment status. Therefore, we
examined the percentage of responders for pain on
standing in the ginger extract group in the presence or
absence of GI adverse events. There were 65% respond-
ers in the presence of dyspepsia, eructation, or nausea,
and 62% responders in the absence of these adverse GI
events (P ⫽ 0.85). Through this analysis, the adverse
events were not found to significantly affect the outcome
of the study.
Patients were informed about the possible pun-
gency upon entering the study. Experience of the pun-
gent taste was captured as adverse events to an extent,
Figure 2. Mean change from baseline to the fourth visit in each
which may explain the incidence of these events. Still,
functional measure of the Western Ontario and McMaster Universi-ties osteoarthritis index for the 2 treatment groups, in the intent-to-
the possibility exists that some subjects were not truly
treat analysis. Bars show the mean and standard error.
blinded due to the pungency of the ginger extract.
Adverse events. There were 314 adverse events
reported on diary cards and by questioning. Seventy-six
In a 1999 Gallup questionnaire among Ameri-
patients (59%) receiving ginger extract experienced 202
cans with arthritis, 28% thought that herbals have a role
adverse events. Forty-nine patients (37%) receiving pla-
in the treatment of arthritis, and 17% believed that
cebo experienced 112 adverse events. Only 1 group of
herbals have a preventative role (29). In a 1997 US
adverse events showed a significant difference between
survey among 2,055 people, 27% of those with arthritis
the treatment groups: gastrointestinal (GI) adverse
had used an alternative treatment for the disease within
events were more common in the ginger extract group
the last year (30). Herbal remedies and other nutraceu-
(116 events in 59 patients [45%]) compared with the
ticals or botanicals are thus increasingly used by both the
placebo group (28 events in 21 patients [16%]).
healthy and the sick. Unfortunately, few of the remedies
None of the GI adverse events were considered
have been tested for efficacy and safety in well-designed
serious by the investigators; 70% were reported as mild,
clinical trials.
24% moderate, and 6% severe. When analyzing the
In order to address this issue, in a 6-week,
events by preferred terms, the only events seen signifi-
randomized clinical trial using ITT analysis in patients
cantly more often in the ginger extract group were
with OA of the knee, treatment with a ginger and
eructation, dyspepsia, and nausea. Words used by the
galanga extract (EV.EXT 77) demonstrated a reduction
patients included burping, belching, bad taste in the
in knee pain on standing when compared with patients
mouth, stomach upset, heartburn, and a burning sensa-
receiving placebo. Additional analyses of the primary
tion in the stomach. To examine whether preexisting
efficacy variable as well as changes in the WOMAC
conditions had any influence on this response, the
index and global status were consistent with the results
patients' medical history was related to the adverse
of the primary efficacy variable. In this short-term study,
events. Thirty-six patients in each treatment group had a
there was no essential difference in the ginger and
previous diagnosis of reflux disease, dyspepsia, ulcer,
placebo groups for quality of life (measured by the
heartburn, gastritis, or hiatus hernia. Of these, 4 patients
SF-12) or consumption of rescue analgesia (acetamino-
(11%) in the placebo group and 10 (28%) receiving
phen). The treatment group also had an increase in GI
ginger extract had at least 1 of the adverse events, including
adverse events.
dyspepsia, eructation, or nausea; it was concluded that
The benefits found in this trial are consistent with
there was no connection to previous conditions.
the results described in the few existing reports in the
There was no statistically significant difference
literature. Three published studies on the use of ginger
between the number of severe adverse events in the 2
in arthritis have been identified. Two were collections of
treatment groups. One serious adverse event occurred in
anecdotal reports (31,32). In the larger cohort, involving
the study, a myocardial infarction in a patient receiving
56 patients with rheumatic disorders, more than 75%
experienced relief of pain and swelling after an average
GINGER EXTRACT IN OA OF THE KNEE
dosage of 3 gm raw ginger per day for periods varying
or bleeding after intake of ginger despite widespread use
between 3 months and 2 years (32). A randomized
of ginger throughout the world. Surprisingly, both ginger
clinical trial included 67 patients, of whom 56 were able
(38) and galanga (39) have been shown to protect
to be evaluated (33). This was a 3-way, crossover study
against ulcers in animal studies. The lack of severe GI
comparing ibuprofen, ginger extract, and placebo. The
adverse events seen in this study is consistent with the
ranking of efficacy was ibuprofen ⬎ ginger extract ⬎
observations in the above-mentioned studies as well as in
placebo for VAS scores on pain and the Lequesne index,
studies on other uses of ginger: seasickness (40), post-
but no significant difference was seen when comparing
operative antiemetic (41,42), and vertigo (43).
ginger extract and placebo directly. Exploratory testing
A warning has been reported on the possible
of the first period of treatment (before crossover) was
effect of ginger on bleeding time (44). In vitro studies
performed and this showed a better effect of both
have shown that ginger inhibits thromboxane synthesis
ibuprofen and ginger extract compared with that of
and thereby platelet aggregation (45). In humans, an ex
placebo (P ⬍ 0.05 by chi-square test).
In the WOMAC subgroups in the present study,
vivo study tested a single dose of 2 gm dried ginger (46).
the greatest improvement was seen in stiffness. The
Another 3-way crossover study compared the oral intake
WOMAC index is described as being more sensitive to
of 15 gm raw ginger/day, 40 gm cooked stem ginger/day,
change in pain, followed by stiffness and function (34).
and placebo for 2 weeks in 18 healthy volunteers (47).
Further investigation into the effects of ginger on stiff-
None of the tested ginger preparations produced any
ness appears warranted, since this may indicate a differ-
significant change in thromboxane synthesis. We could
ent mechanism of action than most other OA remedies.
find no published data on adverse events connected with
This was a short-term study. At 6 weeks, the
coagulation with ginger.
placebo effect appeared to fade, whereas the group
The average body mass index for this study
treated with ginger extract continued to improve.
population was high. Patients were enrolled without
Longer-term studies are needed.
weight restrictions and may constitute a typical OA
Although the COX-2–specific inhibitors have less
population in the US. The dosing of the ginger extract
GI adverse effects than do nonselective nonsteroidal
given was empirically based on the 1–2 capsules per day
antiinflammatory drugs (NSAIDs), their overall safety
that is typically consumed in Europe. In retrospect,
versus placebo is not entirely known, and there are no
there may be concern that the US patients may have
studies comparing COX-2–specific inhibitors with the
been underdosed. Without a dose-finding study, it is
ginger extract. Both nonselective NSAIDs and COX-2–
uncertain if a higher dose would have a better effect.
specific inhibitors have potential renal adverse effects
In conclusion, this study showed that a highly
(35) not described with the ginger extracts.
purified ginger extract has demonstrated a statistical
Some of the patients reported mild GI side
effect of reducing pain in patients with OA of the knee.
effects in the form of dyspepsia, eructation, and nausea.
There was a good safety profile with mostly mild GI side
These may be caused by the pungent taste of the ginger
effects. Long-term effects bear further investigation.
extract. Adverse events for NSAIDs can be classified
into 3 categories (36): 1) "nuisance" symptoms, such as
heartburn, nausea, dyspepsia, and abdominal pain; 2)
mucosal lesions; and 3) serious GI complications, such
We thank Dr. Fong Wang Clow for preparing the
as bleeding and perforation. On average, 10–12% of
statistical plan and Rebecca Hoagland for performing the
patients will experience dyspepsia while taking a nonse-
statistical analysis. In addition to Dr. Altman, contributing
lective NSAID, sometimes leading to death (36,37).
investigators were as follows: Neal Birnbaum, MD, Pacific
Rheumatology Associates, San Francisco, California; Lon Bla-
Because ginger inhibits prostaglandin synthesis, there is
ser, MD, Marshfield Clinic, Marshfield, Wisconsin; Jacque
the potential for GI ulceration. However, the effect of
Caldwell, MD, Halifax Clinical Research Institute, Daytona
NSAIDs on the inflammatory process is mainly caused
Beach, Florida; Guy Fiocco, MD, Gundersen Lutheran Clinic,
by inhibition of prostaglandin synthesis. Contrary to this,
La Crosse, Wisconsin; Elie Gertner, MD, Regions Hospital, St.
the ginger extract is a complex mixture that reduces
Paul, Minnesota; Larry Gilderman, MD, University Clinical
inflammation through inhibition of prostaglandin syn-
Research, Pembroke Pines, Florida; Robert Leff, MD, Duluth
Clinic, Duluth, Minnesota; Howard Offenberg, MD, Halifax
thesis, inhibition of lipooxygenase (13), and reduced
Clinical Research Institute, New Smyrna Beach, Florida; and
production of TNF␣ (21).
Albert Razetti, MD, University Clinical Research, Deland,
We could find no data indicating mucosal lesions
ALTMAN AND MARCUSSEN
al. Development of criteria for the classification and reporting of
osteoarthritis: classification of osteoarthritis of the knee. Arthritis
1. Hochberg MC, Altman RD, Brandt KD, Clark BM, Dieppe PA,
Griffin MR, et al. Guidelines for the medical management of
25. Kellgren JH, Lawrence JS. Radiological assessment of osteoarthri-
osteoarthritis: part II. osteoarthritis of the knee. Arthritis Rheum
tis. Ann Rheum Dis 1957;16:494–501.
26. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW.
2. Lozada CJ, Altman RD. Management of osteoarthritis. In: Koop-
Validation study of WOMAC: a health status instrument for
man WJ, editor. Arthritis and allied conditions: a textbook of
measuring clinically important patient relevant outcomes to anti-
rheumatology. 14th ed. Baltimore (MD): Lippincott, Williams &
rheumatic drug therapy in patients with osteoarthritis of the hip or
Wilkins; 2001. p. 2246–63.
knee. J Rheumatol 1988;15:1833–40.
3. Pendleton A, Arden N, Dougados M, Doherty M, Bannwarth B,
27. Ware JE, Kosinski MA, Keller SD. A 12-item Short Form Health
Bijlsma JWJ, et al. EULAR recommendations for therapy of knee
Survey. Med Care 1996;34:220–33.
osteoarthritis: report of a task force of the Standing Committee for
28. Adverse reaction terminology. Uppsala: Uppsala Monitoring Cen-
International Clinical Studies Including Therapeutic Trials (ES-
CISITO). Ann Rheum Dis 2000;59:936–44.
29. The 1999 Gallup Trend Study of Attitudes Toward and Use of
4. Trease GE, Evans WC, editors. Pharmacognosy. 12th ed. London
Herbal Supplements. Princeton, NJ: Multi-sponsor Surveys Inc.;
(UK): Bailliere Tindall; 1983.
5. Langner ES, Griefenberg S, Gruenwald J. Ingwer: eine Heil-
30. Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Rompay
pflanze mit Geschichte [Ginger: a medicinal plant with a history].
M, et al. Trends in alternative medicine use in the United States,
Balance Z Prax Wiss Komplementarer Ther 1997;1:5–16.
1990–1997. JAMA 1998;280:1569–75.
6. Awang D. Ginger. Can Pharm J 1992:309–11.
31. Srivastava KC, Mustafa T. Ginger (Zingiber officinale) and rheu-
7. Tschirch A. Handbuch der Pharmakognosie [A handbook of
matic disorders. Med Hypotheses 1989;29:25–8.
pharmacognosy]. Leipzig: Verlag CH Tauchnitz; 1917.
32. Srivastava KC, Mustafa T. Ginger (Zingiber officinale) in rheu-
8. Blumenthal M, Busse WR, Goldberg A, Gruenwald J, Hall T,
matism and musculoskeletal disorders. Med Hypotheses 1992;39:
Riggins W, et al, editors. German Commission E Monographs.
Austin (TX): American Botanical Council; 1998.
33. Bliddal H, Rosetzsky A, Schlichting P, Weidner MS, Anderson
9. Blaschek W, Hager H, Ha¨nsel R, Wolf HU, Surmann P, Nyrnberg
LA, Ibfelt H, et al. A randomized, placebo-controlled, cross-over
E, et al. Hager's Handbuch der pharmazeutischen Praxis. 5th rev.
study of ginger extracts and ibuprofen in osteoarthritis. Osteoar-
ed. Berlin-New York: Springer Verlag; 1990.
thritis Cartilage 2000;8:9–12.
10. Schulick P. Ginger, common spice and wonder drug. 3rd ed.
34. Bellamy N. WOMAC user's guide. London (Ont): Victoria Hos-
Brattleboro (VT): Herbal Free Press Ltd; 1996.
pital Corporation; 1996.
11. Swain AR, Duton SP, Truswell AS. Salicylates in foods. J Am Diet
35. Brater DC. Effects of nonsteroidal anti-inflammatory drugs on
renal function: focus on cyclooxygenase-2-selective inhibition. Am
12. Mustafa T, Srivastava KC, Jensen KB. Drug development: report
J Med 1999;107:65–71.
9. Pharmacology of ginger, Zingiber officinale. J Drug Dev
36. Bjorkman DJ. Nonsteroidal anti-inflammatory drug-induced gas-
trointestinal injury. Am J Med 1996;101 Suppl 1A:25–32.
13. Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sandawa U. Inhibition
37. Singh G, Triadafilopoulos G. Epidemiology of NSAID induced
of prostaglandin and leukotriene biosynthesis by gingeroles and
gastrointestinal complications. J Rheumatol 1999;26:18–24.
diarylheptanoids. Chem Pharm Bull (Tokyo) 1992;40:187–91.
38. Al-Yahya MA, Rafatullah S, Mossa JS, Ageel AM, Parmar NS,
14. Mascolo N, Jain R, Jain SC, Capasso F. Ethnopharmacological
Tariq M. Gastroprotective activity of ginger zingiber officinal
investigation of Ginger (Zingiber officinale). J Ethnopharmacol
rosc., in albino rats. Am J Chin Med 1989;17:51–6.
39. Mitsui S, Kobayash S, Nagahori H, Ariba O. Constituents from
15. Jana U, Chattopadhayay RN, Shaw BP. Preliminary studies on
seeds of Alpinia galanga Wild, and their anti-ulcer activities. Chem
anti-inflammatory activity of Zingiber officinale Rosc., Vitex
Pharm Bull (Tokyo) 1976;24:2377–82.
negundo Linn. and Tinospora cordifolia (Willid) miers in albino
40. Groentved A, Brask T, Kambskard J, Hentzer E. Ginger root
rats. Indian J Pharmacol 1999;31:232–3.
against seasickness. Acta Otolaryngol 1998;105:45–9.
16. Rhizoma galangae. Ko¨ln: Bundesanzeiger [Federal German Legal
41. Bone ME, Wilkinson DJ, Young JR, McNeil J. Ginger root—a
Gazette]; 1990 Mar 13. Commission E monograph No.: 173.
new antiemetic: the effect of ginger root on postoperative nausea
17. Perry L, Metzger J. Medicinal plants of east and southeast Asia:
and vomiting after major gynecological surgery. Anaesthesia 1990;
attributed properties and uses. Cambridge (MA): MIT Press; 1980.
18. Janssen AM, Scheffer JJC. Acetoxychavicol acetate, an antifungal
42. Philips S. Zingiber officinale (ginger)—an antiemetic for day case
component of Alpinia galanga. Planta Med 1985;6:507–11.
surgery. Anaesthesia 1993;48:715–7.
19. Itokawa H, Morita H, Sumitomo T, Totsuka N, Takeya K.
43. Groentved A, Hentzer E. Vertigo-reducing effect of ginger root. J
Antitumor principals from Alpinia galanga. Planta Med 1987;53:
Otorhinolaryngol Relat Spec 1986;48:282–6.
44. Miller LG. Herbal medicinals: selected clinical considerations
20. Substances generally regarded as safe, 21 CFR Section 182.10,
focusing on known or potential drug-herb interactions. Arch
182.20. Washington (DC): GPO; 1998.
Intern Med 1998;158:2200–11.
21. Frondoza CG, Frazier C, Polotsky A, Lahiji K, Hungerford DS,
45. Srivastava KC. Effects of aqueous extracts of onion, garlic and
Weidner MS. Inhibition of chondrocyte and synoviocyte TNF␣
ginger on platelet aggregation and metabolism of arachidonic acid
expression by hydroxy-alcoxy-phenyl compounds (HAPC) [ab-
in the blood vascular system: in vitro study. Prostaglandins Leukot
stract]. Trans Orthop Res Soc 2000:1038.
22. Altman RD, Brandt K, Hochberg M, Moskowitz R. Design and
46. Lumb AB. Effect of dried ginger on human platelet function.
conduct of clinical trials in patients with osteoarthritis. Osteoar-
Thromb Haemost 1994;71:110–1.
thritis Cartilage 1996;4:217–43.
47. Janssen PLTMK, Meyboom S, van Staveren WA, de Vegt F,
23. International Conference on Harmonisation. Good clinical prac-
Katan MB. Consumption of ginger (Zingiber officinale Roscoe)
tice. Geneva: ICH Secretariat; 1996 May 1. Efficacy Topic: E6.
does not affect ex vivo platelet thromboxane production in hu-
24. Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et
mans. Eur J Clin Nutr 1996;50:772–4.
Source: http://www.im-care.cn/UpLoadFiles/%E4%B8%93%E9%A2%98%E6%96%87%E7%8C%AE/%E6%B2%BB%E5%85%B3%E8%8A%82%E7%82%8E/12%E7%94%9F%E5%A7%9C%E6%8F%90%E5%8F%96%E7%89%A9%E5%AF%B9%E9%AA%A8%E5%85%B3%E8%8A%82%E7%82%8E%E6%82%A3%E8%80%85%E7%9A%84%E4%BD%9C%E7%94%A8.pdf
Egg Thaw Cycle Orientation Visit us online at www.NYUFertilityCenter.org Copyright 2008 – 2013 NYU Fertility Center – rev. 06/05/2013 Meet Our Physicians Dr. Frederick Licciardi Dr. James Grifo Dr. Nicole Noyes Dr. Alan Berkeley Dr. Lisa Kump-Checchio Dr. M. Elizabeth Fino Dr. David Keefe
The Medication Therapy Management approach is a progressive model of prescription utilization and consumer centered purchase options. The program offers the consumer a variety of options for prescription access and out of pocket cost management. Over the Counter, generic, best brand, non-best brand, and cost share prescriptions are available. Step therapy, prior authorization management tools are incorporated into the program to assist in managing prescription costs; evidence based prescription approaches with clinical excellence as an outcome objective. Enclosed are the prescription resources to assist in effective prescription purchasing.





