
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Effects of jsog-6 on protection against bone loss in ovariectomized mice through regulation of osteoblast differentiation and osteoclast formation
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184http://www.biomedcentral.com/1472-6882/14/184
Effects of JSOG-6 on protection against bone lossin ovariectomized mice through regulation ofosteoblast differentiation and osteoclast formation
Hwa-Jin Chung1, Lan Cho1, Joon-Shik Shin2, Jinho Lee2, In-Hyuk Ha2, Hyen Joo Park1 and Sang Kook Lee1*
Background: JSOG-6 is used as a traditional medicine to relieve the symptoms associated with inflammation,rheumatism, and osteoporosis in Korea. In the present study, we investigated the effects of JSOG-6 on bone lossprevention both in in vitro and in vivo as well as its underlying mechanism of action.
Methods: Protection against bone loss was assessed in an ovariectomized (OVX) mouse model. Bonemicroarchitecture was measured using a micro-computed tomography to detect the parameters of three-dimensionalstructure of a trabecular bone. Serum biomarkers were also evaluated in an OVX-induced model. Osteoclasts derivedfrom mouse bone marrow cells (BMCs) and osteoblastic MC3T3-E1 cells were also employed to investigate themechanism of action.
Results: Oral administration of JSOG-6 significantly increased the bone mineral density (BMD) of the femur in OVX micein vivo. Especially, the reduced Tb.No (trabecular bone number) in the OVX group was significantly recovered byJSOG-6 treatment. The serum levels of alkaline phosphatase (ALP), osteocalcin, C-terminal telopeptide, andtartrate-resistant acid phosphatase, biomarkers of bone resorption, were significantly elevated in OVX mice, butJSOG-6 effectively inhibited the increase in OVX mice. JSOG-6 was also found to enhance the osteoblasticdifferentiation and maturation with the increase of the density and ALP activity, a marker of osteoblasticdifferentiation, as well as calcium deposition, a marker of osteoblastic maturation in MC3T3-E1 cells. The effectsof JSOG-6 on osteoblastic differentiation were also associated in part with the increase of ALP and OPN mRNAexpressions and the decrease of RANKL mRNA expression in MC3T3-E1 cells.
Conclusions: The findings demonstrate that JSOG-6 induced protection against bone loss in OVX mice, and itsanti-osteoporotic property might be, in part, a function of the stimulation of osteoblast differentiation and theinhibition of osteoclast formation. These findings suggest that JSOG-6 might be an applicable therapeutictraditional medicine for the regulation of the osteoporotic response.
Keywords: JSOG-6, Ovariectomized mice, Bone loss, Osteoclast, Osteoblast
altered. Bone mass is regulated by continuous bone re-
Osteoporosis is an age-dependent metabolic bone disease
modeling through bone resorption and bone formation by
characterized by the decrease in bone mass, the deterior-
osteoclasts and osteoblasts, respectively . Indeed, bone
ation of bone tissue, and an increased risk of fractures
homeostasis depends on maintaining a balance between
[]. In the process of osteoporosis, the bone mineral
the activities of bone-forming osteoblasts and bone-
density (BMD) is decreased, bone microarchitecture is de-
resorbing osteoclasts , which is ultimately determined
teriorated, and a variety of proteins in bone are also
by the proliferation and differentiation of progenitors ofthese two bone-associated cells.
Osteoclasts are derived from hematopoietic cells within
* Correspondence: 1
the monocyte/macrophage lineage [and regulated by the
College of Pharmacy, Natural Products Research Institute, Seoul National
University, San 56-1 Sillim-dong, Gwanak-gu, Seoul 151-742, KoreaFull list of author information is available at the end of the article
2014 Chung et al.; licensee BioMed Central Ltd. This is an Open Access article distributed under the terms of the CreativeCommons Attribution License which permits unrestricted use, distribution, andreproduction in any medium, provided the original work is properly credited. The Creative Commons Public DomainDedication waiver applies to the data made available in this article,unless otherwise stated.
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
combined action of the receptor activator of the NF-κB
ligand (RANKL) However, osteoblasts induce the
Female ICR mice (18–20 g, 8 weeks old) were purchased
increase in bone mass by inhibiting the ability of osteo-
from Central Laboratory Animal Inc. (Seoul, Korea). Ani-
clasts. Osteoblasts also express RANKL and osteopro-
mals were housed under standard laboratory conditions
tegerin (OPG), a decoy receptor of RANKL, which are
with free access to food and water. The temperature was
essential factors regulating the formation and activation
thermostatically regulated to 22°C ± 2°C, and a 12-hour
of osteoclasts (osteoclastogenesis) Therefore, the
light/dark schedule was maintained. Prior to their use, they
balance of RANKL and OPG mainly contributes to bone
were allowed one week for acclimatization within the work
remodeling because RANKL stimulates osteoclastogen-
area environment. All animal experiments were carried out
esis, while OPG suppresses bone resorption [Yet,
in accordance with the Institutional Animal Care and Use
excessive osteoclastogenesis and defects in osteoblasto-
Committee Guidelines of Ewha Womans University (per-
genesis are associated with bone diseases such as osteo-
mission number: EWHA2010-2-07).
porosis and rheumatoid arthritis [].
At 9 weeks of age, mice were bilaterally OVX, and 8 mice
JSOG-6 (named Bogangyeongol-hwan) consists of a mix-
were Sham-operated (Sham). After 1 week of recovery from
ture of six crude drugs, and each drug has been widely used
surgery, the OVX mice were randomly divided into 5
in traditional medicine to treat various bone disorders such
groups of 8 mice per group (OVX control, 17β-estradiol
as arthritis, degenerative disc disease and osteoporosis. Of
(E2, 20 μg/kg), and JSOG-6 (50, 150, or 450 mg/kg)).
the six components of JSOG-6, Harpagophytum procum-
JSOG-6 was orally administered in distilled water (0.3 mL)
bens var. sublobatum (Engl.) Stapf, radix, called Devil's claw,
for 12 weeks, and the same volume of distilled water was
is an herbal substance commonly used by patients with
used in the Sham- and OVX control groups. After 12 weeks
osteoarthritis (OA). Anti-inflammatory activities of the
of treatment, the animals were sacrificed, and blood sam-
root extracts of H. procumbens var. sublobatum have
ples were collected for serum isolation. The femur bones
also been reported in various inflammation models
were dissected and divested of soft tissue for analysis of tra-
Another component of JSOG-6, Drynaria for-
tunei (Kunze ex Mett.) J. Sm., rhizome also showed ananti-osteogenic effect in a bone-resorption model [
Analysis of serum bone biomarkers
as well as anti-inflammatory properties [Poria cocos
Serum calcium (Ca) and phosphorus (P) were measured
F.A.Wolf, sclerotium and Rehmannia glutinosa
as previously described ]. The serum concentrations
(Gaertn.) DC., radix exhibited anti-inflammatory
of osteocalcin (OCN) and alkaline phosphatase (ALP)
effects. Furthermore, Panax ginseng C.A.Mey., radix
activity were assayed using an ELISA kit (Biomedical
showed an anti-osteoporotic effect in an OVX rat model
Technologies Inc., Stoughton, MA, USA) and QuantiChrome
These reported data suggest that JSOG-6 might
ALP assay kit (DALP-250, BioAssay Systems, CA, USA)
have the potential to alleviate the symptoms of bone
according to the manufacturer's instructions, respectively.
The serum levels of C-terminal telopeptides (CTx), bone
In the present study, we investigated the activities of
resorption biomarkers that indicate osteoclastic activity,
JSOG-6 in vitro and in in vivo bone-remodeling models
were also analyzed using commercial ELISA kits (Serum
and examined its underlying molecular mechanism.
CrossLaps, Nordic Bioscience, Herlev, Denmark). Thetartrate-resistant acid phosphatase (TRAP) concentrationwas determined by a mouse TRAP assay kit (Suomen
Bioanalytikka Oy, Turku, Finland).
Preparation of test samplesA mixture of six crude drugs (Harpagophytum procumbens
Analysis of bone microarchitecture
var. sublobatum (Engl.) Stapf, radix (120 g), Drynaria fortu-
Bone microarchitecture of the femur was scanned using a
nei (Kunze ex Mett.) J. Sm., rhizome (120 g), Equus asinus
micro-computed tomography (μCT system, SkyScan 1076,
L., gelatinized (120 g), Poria cocos F.A.Wolf, sclerotium
Aart-selaar, Belgium) in the region of 0.6–2.1 mm from
(120 g), Rehmannia glutinosa (Gaertn.) DC., radix (120 g),
the growth plate. The X-ray source was set at a voltage of
and Panax ginseng C.A.Mey., radix (120 g)) was boiled in
50 kV and a current of 200 μA and filtered with a 0.5 mm
tap water (10 L) for 6 h, and the extract was freeze-dried to
aluminum filter. The scanning angular rotation was 180°
obtain the JSOG-6 extracts (259 g, 35.6%). The crude drugs
with an angular step of 0.5°. The voxel size was fixed at
were purchased from an herbal market in Seoul, Korea,
8.9 μm. The morphometric index of the bone region was
and authenticated by Dr. S.H. Lee, Jaseng Hospital of
determined from the microtomographic data using a 3D
Korean Medicine in Seoul, Korea. Voucher specimens of
image (SkyScan). The following measures characterizing
the plants used in this study were deposited in the herbar-
the three-dimensional structure of a trabecular bone were
ium at Jaseng Hospital of Oriental Medicine.
determined: the ratio of bone components to volume of
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
interest (BV/TV,%), trabecular thickness (Tb.Th, mm), tra-
(2 × 104 cells/mL) were incubated with or without vari-
becular separation (Tb.Sp, mm), trabecular bone number
ous concentrations of JSOG-6. After 4 days, the cells
(Tb.N, mm-1), and structure model index (SMI).
were washed with PBS and fixed with 70% ethanol for
BV/TV indicates the ratio which a trabecular bone is
5 min and then extracted into lysis solution (10 mM Tris,
occupied with a given volume of interest, usually mea-
0.1% Triton X-100 buffer (pH 7.5). Enzyme activity was
sured as a% value. Tb.Th and Tb.Sp define the shape of
determined using p-nitrophenylphosphate (p-NPP) as a
a trabecular bone, whereas Tb.N implies the number of
substrate. The color change reflecting the conversion of p-
traversals made per unit length on a random linear path
NPP to p-nitrophenol was measured at 405 nm. The pro-
across a trabecular bone through a given volume of inter-
tein concentration of cell lysates was measured using the
est. SMI quantifies the relative prevalence of rod-, plate-,
Bradford assay.
or sphere-shapes in a trabecular bone structure.
Mineralization assay
α-Modified minimal essential medium (α-MEM), fetal bo-
The calcium deposition of MC3T3-E1 cells was deter-
vine serum (FBS), sodium pyruvate, L-glutamine, antibiotic-
mined by a previously reported method MC3T3-
antimycotic solution, and trypsin-EDTA were purchased
E1 cells (2 × 104 cells/mL) were incubated with or
from Invitrogen Co. (Grand Island, NY, USA). RANKL
without various concentrations of JSOG-6 for 14 days.
and macrophage-colony stimulating factor (M-CSF) were
The cells were washed twice with PBS and fixed with
purchased from R&D systems (Minneapolis, MN, USA). As-
70% ethanol for 30 min. The fixed MC3T3-E1 cells
were stained with 2% Alizarin Red S solution (pH 4.0)
2,5-diphenyltetrazolium bromide (MTT), and other chemicals
for 5 min. The plate was washed several times by dis-
were obtained from Sigma (St. Louis, MO, USA) unless
tilled water, and then the cells were observed under a
Cell cultureMouse calvaria MC3T3-E1 cells, obtained from American
Real-time reverse transcriptase-polymerase chain reaction
Type Culture Collection (ATCC, Rockville, MD, USA),
(Real-time RT-PCR)
were cultured in α-MEM supplemented with 10% heat-
Lipopolysaccharide (LPS), a cell component of Gram-
inactivated FBS, 100 units/mL penicillin, 100 μg/mL
negative bacteria, is an important mediator of pathological
streptomycin, and 0.25 μg/mL amphotericin B. Cells
bone destruction associated with inflammation. MC3T3-
were incubated at 37°C and 5% CO2 in a humidified
E1 cells were stimulated with 1 μg/mL LPS in the presence
or absence of JSOG-6 for 48 h. Total cellular RNA wasextracted with TRI reagent (Sigma, St. Louis, MO, USA)
Evaluation of growth inhibitory potential
according to the manufacturer's recommended procedure.
MC3T3-E1 cells (1 × 104 cells/mL in 96-well plates)
Total RNA (1 μg) was reverse-transcribed using oligo-(dT)15
were treated with various concentrations of JSOG-6
primers and avian myeloblastosis virus (AMV) reverse tran-
and incubated at 37°C in a humidified atmosphere with
scriptase (Promega, Madison, WI, USA).
5% CO2. After JSOG-6 treatment for 72 h, MTT solu-
Real-time RT-PCR was conducted with a MiniOpti-
tion (5 mg/mL in PBS) was added to the medium (final
con system (Bio-Rad, Hercules, CA, USA) using 5 μL
concentration 500 μg/mL) and further incubated for
of the reverse-transcription product, iQ™ SYBR® Green
4 h. The medium was discarded and 200 μL of (100% or
Supermix (Bio-Rad, Hercules, CA, USA) and primers
10%) DMSO was added to each well to dissolve the for-
for a total volume of 20 μL. Standard thermal cycler
mazan. The absorbance was measured at 570 nm. The
conditions were employed (95°C for 20 s, 40 cycles of
effect of JSOG-6 on cell growth was calculated as a per-
95°C for 20 s, 56°C for 20 s, and 72°C for 30 s, followed
centage relative to solvent-treated control incubations,
by 95°C for 1 min, and 55°C for 1 min). The threshold
and the IC50 values were calculated using non-linear
cycle (CT), the fractional cycle number at which the
regression analysis (percent cell proliferation versus
amount of amplified target gene reaches a fixed thresh-
old, was determined by MJ Opticon Monitor software.
The mean threshold cycle (CT) value for each transcript
Alkaline phosphatase (ALP) activity
was normalized by dividing it by the mean CT value for
The ALP activity was determined according to a previ-
the β-actin transcript for that sample. Normalized tran-
ously described method MC3T3-E1 cells were in-
script levels were expressed relative to those obtained
cubated in osteogenic medium containing 400 μM
from the control. Real-time RT-PCR primer sequences
ascorbic acid and 5 mM β-glycerophosphate. The cells
are listed in Table
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
Table 1 Sequences of target gene-specific primers used in real-time PCR
Preparation of total cell lysates
(pH 6.8), 4% SDS, 10% glycerol, 0.006% bromophenol
MC3T3-E1 cells (5 × 105 cells/mL in 60 mm dish)
blue, 50 mM sodium fluoride, 5 mM sodium orthova-
were incubated with or without various concentra-
nadate, and 2% β-mercaptoethanol). Cell lysates were
tions of JSOG-6 for 48 h. To obtain total cell lysates,
boiled for an additional 20 min and stored at −20°C.
the cells were washed with ice-cold PBS and lysed in
The protein content of cell lysates was determined by
boiling 2X sample loading buffer (250 mM Tris–HCl
the BCA method.
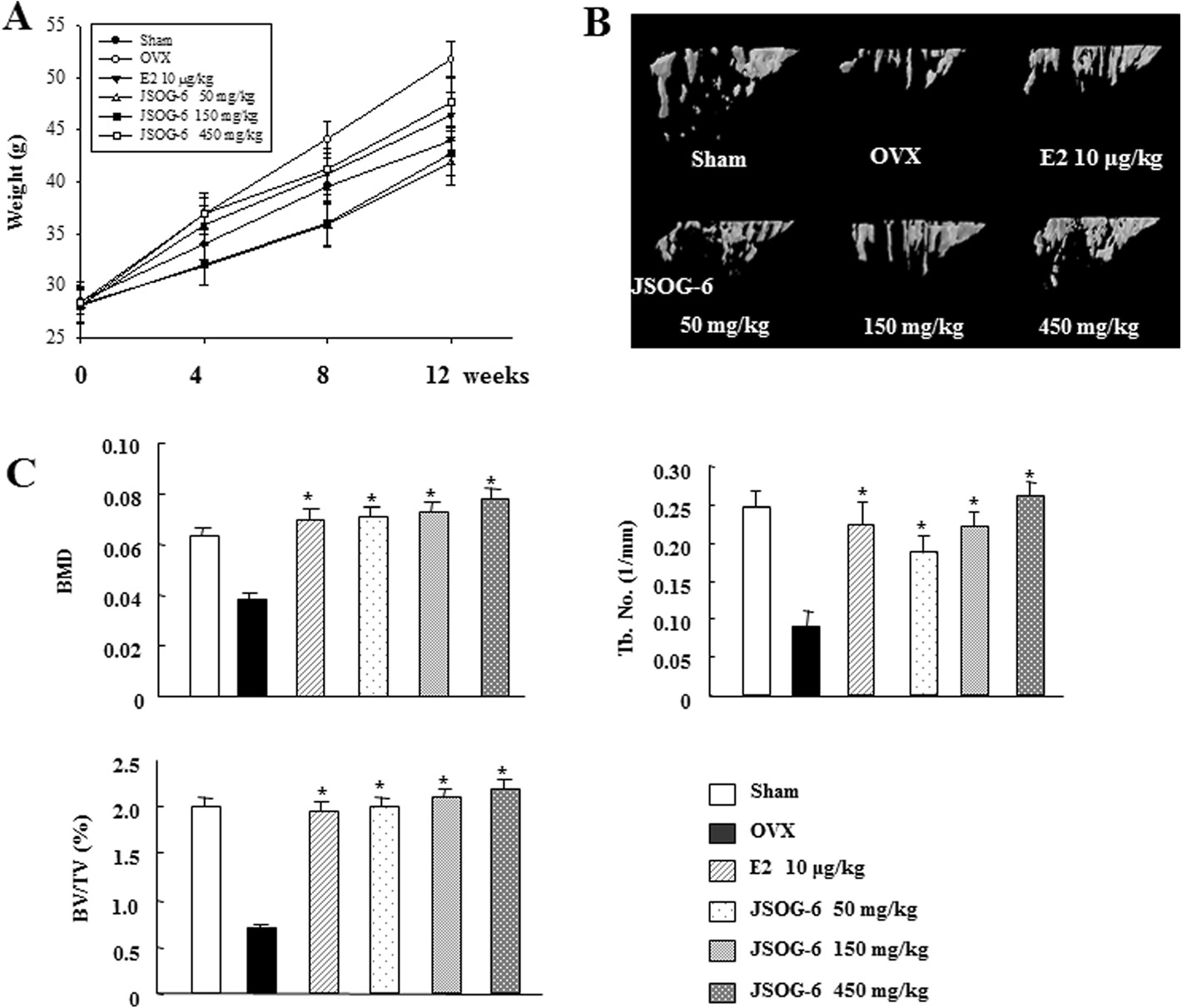
Figure 1 Effect of JSOG-6 on bone loss in OVX mice. (A) Change in body weight 12 weeks after ovarietomy. (B) Effect of JSOG-6 on bone 3DmicroCT image of the distal femur in OVX mice. (C) Effect of JSOG-6 on the bone morphometric parameters BMD, BV/TV (%), and Tb.No (1/mm)as analyzed with micro-CT SkyScan CTAN software. Data represent the mean ± S.D. (n = 8). *P < 0.01 indicates statistically significant differencesfrom the OVX mice group.
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
Table 2 Effect of JSOG-6 on the serum parameters in OVX mice
Calcium concentration
Potassium concentration
Osteocalcin level
The serum levels of calcium, potassium, ALP, CTx, OCN, and TARP were analyzed as described in the . Data represent the mean ± S.D. (n = 8). *P < 0.01indicates statistically significant differences from the OVX mice group.
Figure 2 Effect of JSOG-6 on ALP activity in MC3T3-E1 cells. (A) Cell viability was measured by the MTT method as described in the(B) MC3T3-E1 cells (2 × 104 cells/mL) were incubated with JSOG-6 in the presence of ascorbic acid and β-glycerophosphate for 4 days.
The ALP activity was corrected for the amount of protein. Data represent the mean ± S.D. (n = 3). *P < 0.05, **P < 0.01 indicates statisticallysignificant differences from the control group. N.C., negative control; P.C., positive control (ascorbic acid + β-glycerophosphate).
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
Western blot analysis
M-CSF. The medium was replaced with 50% volume
Equal amounts of cell lysates (40 50 μg) were subjected
with and without JSOG-6 every 2 days, and the culture
to 8% and 10% SDS-PAGE and electrotransferred onto
was terminated after 5 days.
polyvinylidene difluoride (PVDF) membranes (Millipore,MA, USA). Membranes were blocked in PBST (PBS with0.1% Tween-20) containing 5% non-fat dry milk for 1 h at
Tartrate-resistant acid phosphatase (TRAP) staining
room temperature. After washing 3 times with PBST,
Osteoclast differentiation was assessed by TRAP (Sigma-
membranes were incubated with primary antibodies
Aldrich, St. Louis, MO, USA) activity. 5 days after stimulat-
against OPG, RANKL, c-Fos, and TRAF6 (Santa Cruz
ing the cells with M-CSF and RANKL (100 and 30 ng/mL,
Biotechnology, Santa Cruz, CA, USA), p-ERK, ERK (Cell
respectively), the cells were washed with PBS and fixed with
Signaling, Danvers, MA, USA), and β-actin (Sigma) for
4% paraformaldehyde for 5 min. The cells were rinsed in
3 h at room temperature or overnight at 4°C. Membranes
de-ionized water and incubated in tartrate-staining solution
were washed 3 times with PBST and incubated with the
in the dark for 1 h at 37°C. The cells were rinsed in de-
corresponding secondary antibodies (Santa Cruz) for
ionized water and allowed to air dry. TRAP-positive multi-
90 min at room temperature. The blots were washed 3
nucleated cells containing 3 or more nuclei were counted
times with PBST and visualized using an enhanced
as osteoclasts.
chemiluminescence (ECL) Western blotting detectionsystem (Lab Frontier, Suwon, Korea).
Murine bone marrow-derived osteoclasts
All experiments were repeated at least 3 times. Data
BMCs were isolated from 4-week-old mice as previously
are presented as the mean ± SD for the indicated num-
described [The cells were plated into 96-well plates
ber of independently performed experiments. The statis-
in 30 ng/mL of M-CSF for 24 h. Next, the cells were
tical significance within a parameter was evaluated by
treated with the indicated concentrations of JSOG-6 in
one-way analysis of variation (ANOVA) coupled with
the presence of 100 ng/mL of RANKL and 30 ng/mL of
Dunnett's t-test.
Figure 3 Effect of JSOG-6 on the mineralization of MC3T3-E1 cells. (A) MC3T3-E1 cells (2 × 104 cells/mL) were incubated with JSOG-6 inthe presence of ascorbic acid and β-glycerophosphate for 14 days. Mineralized nodule formation was assessed by Alizarin red S staining.
Data represent the mean ± S.D. (n = 3). *P < 0.01 indicates statistically significant differences from the control group. (B) Representativemicroscopic observation of JSOG-6 on the formation of calcification nodules with staining Alizarin red S. N.C., negative control; P.C., positivecontrol (ascorbic acid + β-glycerophosphate).
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
demonstrated by treatment with E2, a positive control,
Effect of JSOG-6 on bone loss in an OVX-induced
in the same experimental condition. In addition, the
microstructural index parameters were observed in the
The anti-osteoporotic activity of JSOG-6 was primarily per-
OVX-induced bone loss model (Figure C). The BMD
formed in an in vivo experiment employing an OVX-
of the OVX group was markedly reduced by 38.7% in
induced bone loss mouse model. Body weight change was
comparison with the Sham group. The BMD of the tra-
monitored during the administration of JSOG-6. As shown
becular bone of the femur in mice treated with JSOG-6
in Figure , the increase in body weight in the OVX
(50 mg/kg) was shown to be 83.0% higher than that in
group was significantly higher than that in the Sham-
the OVX group and was not significantly different from
operation group (P < 0.01). However, mice given an oral ad-
that in the Sham control group (P < 0.01). E2 also in-
ministration of JSOG-6 for 12 weeks after OVX showed a
creased the BMD by 80.9% in the OVX-induced bone
relatively slow increase in body weight compared to the
loss mouse model. The BV/TV was also markedly re-
OVX group. The estradiol (E2, 10 μg/kg)-treated group
duced in the OVX group (64.7%) compared with the Sham
showed a similar result. The destruction of the trabecular
group, but the treatment of OVX mice with JSOG-6 or E2
bone of the femur was also observed by 3D-μCT. As illus-
resulted in a significant increase in the BV/TV in the
trated in Figure OVX caused a significant deterioration
OVX-induced bone loss model (P < 0.01). The Tb.No in
of trabecular bone architecture compared with the Sham-
the OVX group was 63.8% lower than that of the Sham
control group (P < 0.01). However, treatment with JSOG-6
group, but JSOG-6 treatment (50, 150, and 450 mg/kg)
retarded or recovered the destruction of the trabecular
significantly recovered the Tb.No value to 113.3, 145.6,
bone of the femur in a dose-dependent manner in the
and 192.2%, respectively, in a dose-dependent manner
OVX-induced bone loss model. The protective effect on
than the OVX group. The E2-treated group also showed a
trabecular bone microarchitecture was also clearly
150% recovery of Tb.No compared to the OVX group.
1 10 25 100
1 10 25 100
1 10 25 100
JSOG-6 ( g/ml)
JSOG-6 ( g/ml)
JSOG-6 ( g/ml)
1 10 25 100
1 10 25 100
1 10 25 100
JSOG-6 ( g/ml)
JSOG-6 ( g/ml)
JSOG-6 ( g/ml)
Figure 4 Effect of JSOG-6 on osteoblastic gene expression. MC3T3-E1 cells (2 × 104 cells/mL) were treated with the indicated concentrationsof JSOG-6 for 48 h, and the mRNA levels of osteoblastic genes were examined using real-time PCR. The results are presented as a relative expressionlevel compared to unstimulated cells and were normalized to β-actin. Data represent the mean ± S.D. (n = 3). *P < 0.05, **P < 0.01 indicates statisticallysignificant differences from the control group.
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
Effect of JSOG-6 on serum biochemical markers in the
RANKL play important roles in the maintenance of bone
mass and the regulation of bone remodeling. The OPG/
The effects of JSOG-6 on the bone metabolic bio-
RANKL ratio is the major index of osteoclastogenic stimu-
markers were also determined with the serum of blood
lation. JSOG-6 treatment induced the expression of OPG
samples collected in OVX mice. The serum levels of cal-
mRNA in a concentration-dependent manner, whereas
cium and potassium were not significantly different in
JSOG-6 significantly suppressed the expression of RANKL
the Sham, JSOG-6 treatment, and OVX groups (P <
mRNA (*P < 0.05, **P < 0.01). In addition, JSOG-6 signifi-
0.01) (Table The serum levels of the bone formation
cantly increased the OPG/RANKL ratio in MC3T3-E1 cells
markers ALP and OCN were significantly increased in
(*P < 0.01) (Figure ). These findings suggest that JSOG-6
the OVX group compared to the Sham group. However,
might modulate the process of osteoclastogenesis via its ef-
JSOG-6 treatment decreased the elevated serum levels of
fect on the OPG/RANKL gene expressions.
ALP and OCN in the OVX mice (P < 0.01). In addition, theserum level of TRAP, which is responsible for enhanced os-teoclastogenesis and activation of mature osteoclasts for
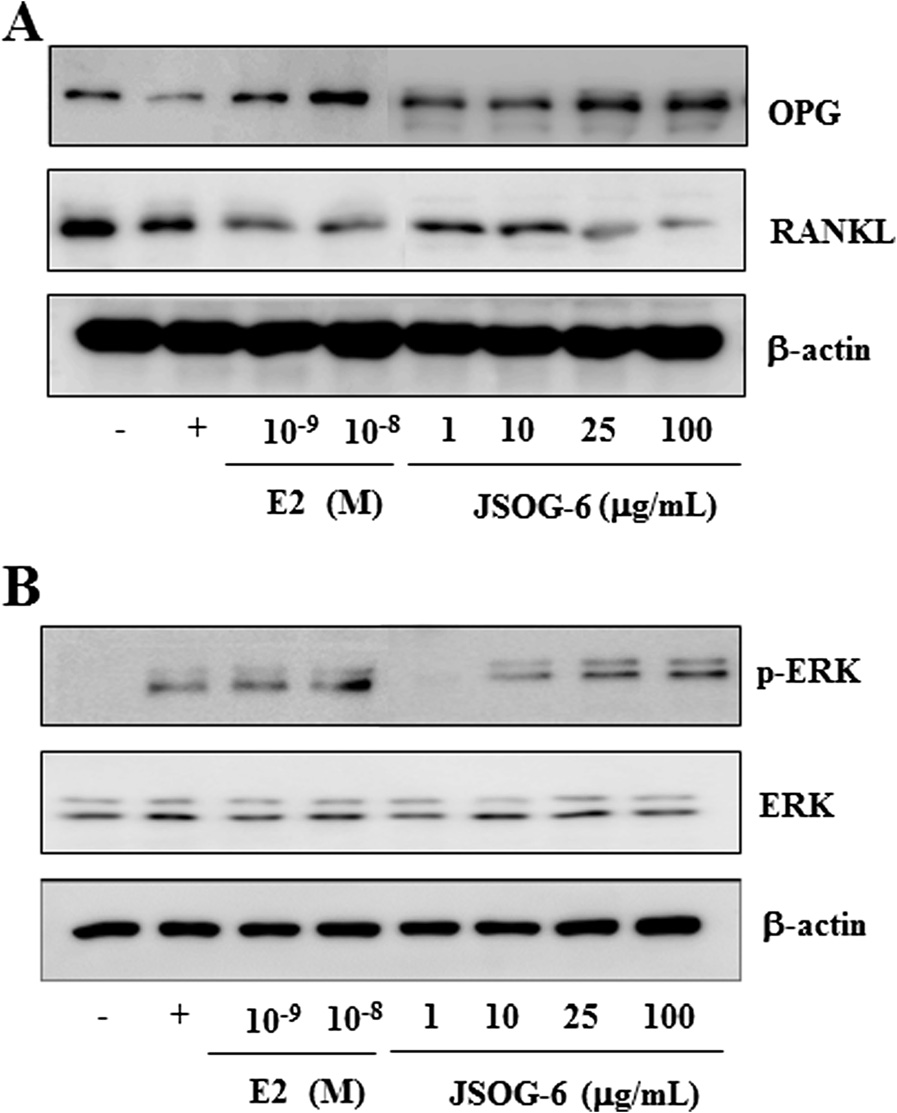
Effect of JSOG-6 on the protein levels of OPG, RANKL, and
bone resorption, was increased in the OVX group, but
ERK in MC3T3-E1 cells
JSOG-6 treatment significantly reduced TRAP activity in a
The effect of JSOG-6 on the protein expressions of OPG,
dose-dependent manner (P < 0.01). The increase in an add-
RANKL, and ERK in MC3T3-E1 cells was evaluated to con-
itional bone resorption marker, CTx, in the OVX group
firm the activity of JSOG-6 on osteogenic differentiation. As
was also inhibited by JSOG-6 treatment (P < 0.01) (Table
shown in Figure , JSOG-6 increased the protein expres-sion of OPG in a concentration-dependent manner, but
Effect of JSOG-6 on the ALP activity of MC3T3-E1 cells
the protein expression of RANKL was suppressed. In
The ALP staining activity, a marker of osteoblastic differ-
addition, JSOG-6 treatment induced ERK phosphorylation
entiation , was evaluated in osteoblastic MC3T3-El
cells. There was no effect on MC3T3-El cell viability withJSOG-6 treatment up to 100 μg/mL as determined by theMTT assay (>95% cell viability) (Figure A). Therefore,the cells were treated with up to 100 μg/mL JSOG-6 tounderstand the biological effects of JSOG-6 without caus-ing cytotoxicity. The MC3T3-El cells were differentiatedin the presence of ascorbic acid and β-glycerophosphate inthe cell culture medium. After 4 days of differentiation,JSOG-6 was found to enhance the density and ALP activ-ity in a concentration-dependent manner (Figure ).
Effect of JSOG-6 on the mineralization of MC3T3-E1 cellsExtracellular matrix mineralization is one of the markers ofosteoblastic maturation. Alizarins red S staining was usedto evaluate extracellular matrix calcium deposition. As illus-trated in Figure when the cells were simultaneouslytreated with JSOG-6 for 14 days, the calcification nodules,represented in red, in MC3T3-E1 cells were increased in aconcentration-dependent manner. These results indicatethat the effect of JSOG-6 on the ALP activity and calciumdeposition stimulated osteoblast differentiation.
Effect of JSOG-6 on osteoblastic gene expressionThe effects of JSOG-6 on osteoblast differentiation was fur-ther elucidated using the analysis of expression of osteo-
Figure 5 Effect of JSOG-6 on the protein levels of OPG, RANKL,
genic differentiation mediators mRNA by real-time RT-
and ERK in MC3T3-E1 cells. (A, B) MC3T3-E1 cells (2× 104 cells/mL)
PCR. After 2 days of differentiation, JSOG-6 (100 μg/mL)
were incubated for 48 h and then treated with JSOG-6 for 48 h. After
caused a significant (P < 0.01) increase in the expressions of
incubation, total cell extracts were obtained and subjected to Western
ALP and OPN mRNA, whereas the expression of OCN
blot analysis as described in the Data were representative ofthree separate experiments. β-Actin was used as an internal standard.
mRNA was decreased by JSOG-6 treatment. OPG and
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
Figure 6 Effect of JSOG-6 on RANKL-induced osteoclast differentiation. (A) Cell viability was measured by the MTT method as described inthe (B) Bone marrow cells (1 × 104 cells/mL) were incubated with JSOG-6 in the presence of M-CSF (30 ng/mL) and RANKL (100 ng/mL)for 5 days. Osteoclastogenesis was confirmed by TRAP staining. Data represent the mean ± S.D. (n = 3). **P < 0.01 indicates statistically significantdifferences from the control group. (C) The expression of TRAP was determined by Western blot analysis as described in the Data arerepresentative of three separated experiments. β-Actin was used as an internal standard.
Effect of JSOG-6 on osteoclast differentiation
JSOG-6 is an herbal preparation derived from six trad-
TRAP staining was performed to evaluate the effect of
itional medicines that have been clinically used to treat
JSOG-6 on RANKL-induced osteoclast differentiation. As
inflammation-associated diseases in oriental Korean medi-
depicted in Figure , no cytotoxic effect on BMCs was ob-
cine. However, the exact pharmacological effect and its
served at a test concentration up to 100 μg/mL JSOG-6 as
underlying mechanisms of action remain to be elucidated.
determined by the MTT assay (>85% cell survival rate).
Therefore, the present study was performed to investigate
BMCs were allowed to differentiate into osteoclasts in the
the anti-osteoporotic activity of JSOG-6 in an OVX mouse
presence of RANKL and M-CSF for 5 days. JSOG-6
model and to further elucidate its underlying mechanisms
inhibited the formation of TRAP-positive cells during
of actions both in vitro and in vivo.
RANKL-induced osteoclast differentiation in a concentration-
The OVX animal model has been widely used to study
dependent manner (Figure Western blot analysis showed
postmenopausal osteoporosis mimicked by estrogen in-
that JSOG-6 also suppressed the protein expression of
sufficiency Deterioration of trabecular 3D micro-
TRAF6 in RANKL-stimulated cells (Figure ).
architecture is apparent in the OVX mouse model We found that JSOG-6 prevented the deterioration of
microstructural parameters in the distal femur in mice.
Osteoporosis is an age-dependent skeletal metabolic dis-
Oral administration of JSOG-6 restored bone loss back
ease in which patients suffer from lower bone density
in OVX mice (Figure These results indicated that
and lower bone mass compared to healthy individuals
JSOG-6 was effective not only in preserving bone mass
The disease is also associated with a homeostatic
but also in rescuing the deterioration of bone microarch-
imbalance between bone modeling and bone resorption.
itecture associated with OVX mice.
Although the main cause of the disease is not clear, en-
Biochemical markers of bone turnover have been deter-
docrinologic, nutritional, and genetic factors are thought
mined previously, allowing the evaluation of the status of
to be highly involved in osteoporosis.
bone remodeling . Analyses of the serum levels of ALP
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
and OCN, typical biomarkers of osteoblastic activity, and
M-CSF and RANKL are important cytokines that
CTx and TRAP, biomarkers of bone resorption, showed
cause osteoclast precursors to differentiate into activated
that the serum concentrations of ALP, OCN, CTx and
osteoclasts . The present study showed that JSOG-6
TRAP in OVX mice were significantly higher than those
suppressed RANKL-induced differentiation of osteoclasts
in the Sham group. However, the levels of ALP and
by down-regulating the protein expression of TRAF6 in
TRAP in the JSOG-6-treated group were equivalent to
BMCs (Figure These in vitro findings were well corre-
the levels in the Sham group (Table These data sug-
lated with the in vivo anti-osteoporotic effects of JSOG-6,
gest that JSOG-6 most likely prevents bone loss through
and thus JSOG-6 might be applicable to the clinic for im-
decreased bone turnover.
proving age-dependent bone destruction disease.
In addition, the mechanisms underlying the cellular
effects of JSOG-6 were investigated in osteoblasts and oste-
oclasts. Osteoblasts, bone-forming cells, synthesize and
The present study provides evidence that JSOG-6 has a po-
regulate the deposition and mineralization of the extracellu-
tential anti-osteoporotic activity both in vitro and in vivo.
lar matrix of bone [In this study, JSOG-6 treatment
The underlying mechanisms of action of JSOG-6 are corre-
was found to increase the ALP activity in a concentration-
lated with the induction of ALP activation in osteoblasts,
dependent manner in MC3T3-E1 cells (Figure ). JSOG-6
the increase in calcified bone matrix, and the reduction of
also led to an increase of calcium deposition in MC3T3- E1
osteoclast formation. These data provide a pharmaco-
logical basis for the use of JSOG-6 as a potential thera-
To further explore the mechanism responsible for the ef-
peutic strategy for protecting against osteoporotic bone
fect of JSOG-6 on the regulation of osteoblasts, the markers
loss in clinic.
of bone formation in MC3T3-E1 cells were detected byreal-time RT-PCR. LPS leads to the intracellular induction
Competing interestsThe authors declare that they have no competing interest.
of p38, JNK phosphorylation, and NFκB in macrophagesand monocytes, and promotes the differentiation and sur-
Authors' contributions
vival of osteoclasts through the production of several fac-
Conceived and designed the experiments: HJC, LC, and SKL. Performed theexperiments HJC and LC. Analyzed the data: HJC, LC, and SKL. Contributed
tors such as PGE2, interleukin 1, RANKL, and TNF
reagents, materials and analysis tools: HJC, LC, JSS, JL, IHH, and SKL. Wrote
The levels of the bone formation biomarkers ALP, OPN,
the paper: HJC, HJP, and SKL. Editing the paper: HJC, HJP, and SKL. All
and OPG/RANKL mRNAs were up-regulated by JSOG-6
authors read and approved the final manuscript.
treatment (Figure
RANKL drives osteoclastogenesis by providing an
This work was supported by a grant from the JASENG Hospital of Korean
important signal to osteoclast progenitors through the
membrane-anchored receptor RANK in osteoclasts. Oste-
oblasts also synthesize and secrete OPG, a decoy receptor
1College of Pharmacy, Natural Products Research Institute, Seoul National
of RANKL, which blocks the interaction between RANKL
University, San 56-1 Sillim-dong, Gwanak-gu, Seoul 151-742, Korea. 2JasengSpine and Joint Research Institute, Jaseng Medical Foundation, Jaseng
and RANK. Therefore, the expression of OPG/RANKL
Hospital of Korean Medicine, Seoul 135-896, Korea.
plays an essential role in modulating bone remodeling[OPG was also able to block the interaction of
Received: 13 December 2013 Accepted: 29 May 2014Published: 6 June 2014
RANKL with osteoclast cells, thus suppressing osteoclas-togenesis . The data showed that JSOG-6 up-regulated
the protein expression of OPG and down-regulated that of
Alexander JM, Bab I, Fish S, Mül er R, Uchiyama T, Gronowicz G, Nahounou M,Zhao Q, White DW, Chorev M, Gazit D, Rosenblatt M: Human parathyroid
RANKL (Figure ). Therefore, the suppression of the
hormone 1–34 reverses bone loss in ovariectomized mice. J Bone Miner Res
protein expressions of OPG and RANKL by JSOG-6 might
be a plausible partial mechanism of action of its modula-
Raisz LG: Pathogenesis of osteoporosis: concepts, conflicts, andprospects. J Clin Invest 2005, 115:3318–3325.
tion of bone remodeling.
Takayanagi H: Inflammatory bone destruction and osteoimmunology.
Various intracellular signaling pathways are involved in
J Periodontal Res 2005, 40:287–293.
the regulation of osteoblast differentiation. ERK is consid-
Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG:Mechanotransduction in osteoblast regulation and bone disease.
ered to be an essential function in the proliferation and
Trends Mol Med 2009, 15:208–216.
differentiation of osteoblasts ]. In this study, JSOG-6
Harada S, Rodan GA: Control of osteoblast function and regulation of
treatment induced the activation of ERK (Figure These
bone mass. Nature 2003, 15:349–355.
Rahman MM, Bhattacharya A, Fernandes G: Conjugated linoleic acid
findings also suggest that JSOG-6 might have potential in
inhibits osteoclast differentiation of RAW264.7 cells by modulating
improving bone formation.
RANKL signaling. J Lipid Res 2006, 47:1739–1748.
Bone formation is regulated by crosstalk between bone-
Rodan GA, Martin TJ: Therapeutic approaches to bone diseases.
Science 2000, 289:1508–1514.
forming osteoblasts and bone-resorbing osteoclasts. Unbal-
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ: Modulation
anced osteoclastogenesis causes bone loss in osteoporosis
of osteoclast differentiation and function by the new members of the
Chung et al. BMC Complementary and Alternative Medicine 2014, 14:184
tumor necrosis factor receptor and ligand families. Endocr Rev 1999,
the long-term dosing of ovariectomized rats. J Pharmacol Exp Ther 1996,
Teitelbaum SL: Bone resorption by osteoclasts. Science 2000, 289:1504–1508.
Cano A, Dapía S, Noguera I, Pineda B, Hermenegildo C, del Val R, Caeiro JR,
Boyle WJ, Simonet WS, Lacey DL: Osteoclast differentiation and activation.
García-Pérez MA: Comparative effects of 17beta-estradiol, raloxifene and
Nature 2003, 423:337–342.
genistein on bone 3D microarchitecture and volumetric bone mineral
Martin TJ: Historically significant events in the discovery of RANK/RANKL/
density in the ovariectomized mice. Osteoporos Int 2008, 19:793–800.
OPG. World J Orthop 2013, 4:186–197.
Bahlous A, Kalai E, Hadj Salah M, Bouzid K, Zerelli L: Biochemical markers of
Hadjidakis DJ, Androulakis II: Bone remodeling. Ann N Y Acad Sci 2006,
bone remodeling: recent data of their applications in managing
postmenopausal osteoporosis. Tunis Med 2006, 84:751–757.
Inaba K, Murata K, Naruto S, Matsuda H: Inhibitory effects of devil's claw
Owen TA, Aronow MS, Barone LM, Bettencourt B, Stein GS, Lian JB:
(secondary root of Harpagophytum procumbens) extract and
Pleiotropic effects of vitamin D on osteoblast gene expression are
harpagoside on cytokine production in mouse macrophages. J Nat Med
related to the proliferative and differentiated state of the bone cell
2012, 64:219–222.
phenotype: dependency upon basal levels of gene expression, duration
Sanders M, Grundmann O: The use of glucosamine, devil's claw
of exposure, and bone matrix competency in normal rat osteoblast
(Harpagophytum procumbens), and acupuncture as complementary and
cultures. Endocrinology 1991, 128:1496–1504.
alternative treatments for osteoarthritis. Altern Med Rev 2011, 16:228–238.
Stein GS, Lian JB: Molecular mechanisms mediating proliferation/
Fiebich BL, Muñoz E, Rose T, Weiss G, McGregor GP: Molecular targets of
differentiation interrelationships during progressive development of the
the antiinflammatory Harpagophytum procumbens (devil's claw):
osteoblast phenotype. Endocr Rev 1993, 14:424–442.
inhibition of TNFα and COX-2 gene expression by preventing activation
Suda K, Woo J-T, Takami M, Sexton PM, Nagai K: Lipopolysaccharide
of AP-1. Phytother Res 2012, 26:806–811.
supports survival and fusion of preosteoclasts independent of TNF-α,
Chen LL, Lei LH, Ding PH, Tang Q, Wu YM: Osteogenic effect of Drynariae
IL-1, and RANKL. J Cell Physiol 2002, 190:101–108.
rhizoma extracts and Naringin on MC3T3-E1 cells and an induced rat
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, Sato Y,
alveolar bone resorption model. Arch Oral Biol 2011, 56:1655–1662.
Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E,
Anuja GI, Latha PG, Suja SR, Shyamal S, Shine VJ, Sini S, Pradeep S, Shikha P:
Morinaga T, Higashio K: Identity of osteoclastogenesis inhibitory factor
Rajasekharan S. Anti-inflammatory and analgesic properties of Drynaria
(OCIF) and osteoprotegerin (OPG): amechanism by which OPG/OCIF
quercifolia (L.) J. Smith. J Ethnopharmacol 2010, 11:456–460.
inhibits osteoclastogenesis in vitro. Endocrinology 1998, 139:1329–1337.
Nukaya H, Yamashiro H, Fukazawa H, Ishida H, Tsuji K: Isolation of inhibitors
Teitelbaum SL: Osteoclasts, integrins, and osteoporosis. J Bone Miner
of TPA-induced mouse ear edema from Hoelen, Poria cocos.
Metab 2000, 18:344–349.
Chem Pharm Bull 1996, 44:847–849.
Bord S, Ireland DC, Beavan SR, Compston JE: The effects of estrogen on
Han Y, Jung HW, Lee JY, Kim JS, Kang SS, Kim YS, Park YK: 2,5-
osteoprotegerin, RANKL, and estrogen receptor expression in human
dihydroxyacetophenone isolated from Rehmanniae Radix Preparata
osteoblasts. Bone 2003, 32:136–141.
inhibits inflammatory responses in lipopolysaccharide-stimulated
Hu Y, Chan E, Wang SX, Li B: Activation of p38 mitogen-activated protein
RAW264.7 macrophages. J Med Food 2012, 15:505–510.
kinase is equired for osteoblast differentiation. Endocrinology 2003,
Kim HR, Cui Y, Hong SJ, Shin SJ, Kim DS, Kim NM, So SH, Lee SK, Kim EC,
Chae SW, Chae HJ: Effect of ginseng mixture on osteoporosis in
Jadlowiec J, Koch H, Zhang X, Campbell PG, Seyedain M, Sfeir C:
ovariectomized rats. Immunopharmacol Immunotoxicol 2008, 30:333–345.
Phosphophoryn regulates the gene expression and differentiation ofNIH3T3, MC3T3-E1, and human mesenchymal stem cells via the integrin/
Zhang Y, Lai WP, Leung PC, Wu CF, Wong MS: Short- to mid-term effects
MAPK signaling pathway. J Biol Chem 2004, 17:53323–53330.
of ovariectomy on bone turnover, bone mass and bone strength in rats.
Ross FP, Teitelbaum SL: Alphavbeta3 and macrophage colony-stimulating
Biol Pharm Bull 2007, 30:898–903.
factor: partners in osteoclast biology. Immunol Rev 2005, 208:88–105.
Chen WF, Wong MS: Genistein modulates the effects of parathyroid
Takayanagi H, Sato K, Takaoka A, Taniguchi T: Interplay between interferon
hormone in human osteoblastic SaOS-2 cells. Br J Nutr 2006, 95:1039–1047.
and other cytokine systems in bone metabolism. Immunol Rev 2005,
Bhargavan B, Gautam AK, Singh D, Kumar A, Chaurasia S, Tyagi AM, Yadav DK,
Mishra JS, Singh AB, Sanyal S, Goel A, Maurya R, Chattopadhyay N:
Khosla S: Minireview: the OPG/RANKL/RANK system. Endocrinology 2001,
Methoxylated isoflavones, cajanin and isoformononetin, have non-
estrogenic bone forming effect via differential mitogen activated proteinkinase (MAPK) signaling. J Cell Biochem 2009, 1:388–399.
Takahashi N, Yamada H, Yosiki S, Roodman GD, Mundy GR, Jones SJ,
doi:10.1186/1472-6882-14-184Cite this article as: Chung et al.: Effects of JSOG-6 on protection
Boyde A, Suda T: Osteoclast-like cell formation and its regulation by
against bone loss in ovariectomized mice through regulation of osteoblast
osteotropic hormones in mouse bone marrow cultures.
differentiation and osteoclast formation. BMC Complementary and Alternative
Endocrinology 1998, 122:1373–1382.
Medicine 2014 14:184.
Katagiri T, Yamaguchi A, Ikeda T, Yoshiki S, Wozney JM, Rosen V, Wang EA,Tanaka H, Omura S, Suda T: The non-osteogenic mouse pluripotent cellline, C3H10T1/2, is induced to differentiate into osteoblastic cells by re-combinant human bone morphogenetic protein-2. Biochem Biophys ResCommun 1990, 172:295–299.
Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, Kahn AJ,Suda T, Yoshiki S: Recombinant human bone morphogenetic protein-2stimulates osteoblastic maturation and inhibits myogenic differentiation
Submit your next manuscript to BioMed Central
in vitro. J Cell Biol 1991, 113:681–687.
and take full advantage of:
Hohenhaus MH, McGarry KA, Col NF: Hormone therapy for the preventionof bone loss in menopausal women with osteopenia: is it a viable
• Convenient online submission
option? Drugs 2007, 67:2311–2321.
Levine JP: Effective strategies to identify postmenopausal women at risk
• Thorough peer review
for osteoporosis. Geriatrics 2007, 62:22–30.
• No space constraints or color figure charges
Frolik CA, Bryant HU, Black EC, Magee DE, Chandrasekhar S: Time-dependent changes in biochemical bone markers and serum cholesterol
• Immediate publication on acceptance
in ovariectomized rats: effects of raloxifene HCl, tamoxifen, estrogen,
• Inclusion in PubMed, CAS, Scopus and Google Scholar
and alendronate. Bone 1996, 18:621–627.
• Research which is freely available for redistribution
Sato M, Bryant HU, Iversen P, Helterbrand J, Smietana F, Bemis K, Higgs R,Turner CH, Owan I, Takano Y, Burr DB: Advantages of raloxifene overalendronate or estrogen on nonreproductive and reproductive tissues in
Submit your manuscript at www.biomedcentral.com/submit
Source: http://www.jaseng.ru/wp-content/uploads/2016/02/Effects-of-JSOG-6-on-protection-against-bone-loss-in-ovariectomized-mice-through-regulation-of-osteoblast-differentiation-and-osteoclast-formation.pdf
Curriculum Vitae JACK MERRIT GWALTNEY, JR. December 24, 1930, Norfolk, Virginia B.A. University of Virginia 1948-1952 M.D. University of Virginia 1952-1956 Summary of Career: University Hospitals of Cleveland, Cleveland, Ohio Residency, Internal Medicine University Hospitals of Cleveland, Cleveland, Ohio Chief Resident, Internal University of Virginia Hospital
Royal Victoria Eye & Ear Hospital Research Foundation Adelaide Road, Dublin 2 Tel: (01) 6343630 Fax: (01) 6614670Email: [email protected] www.researchfoundation.ie MISSION STATEMENT & GOVERNANCE STRUCTURE 5 SERVICES 6 GENETICS RESEARCH 8 RETINAL RESEARCH 10 PATHOLOGY RESEARCH 11 OCULAR IMUNOLOGY, INFLAMMATION AND CORNEAL RESEARCH 12






