
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Jemi.microbiology.ubc.ca
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
Deletion of the Escherichia coli K30 Group I Capsule
Biosynthesis Genes wza, wzb and wzc Confers Capsule-
Independent Resistance to Macrolide Antibiotics
Sandra Botros, Devon Mitchell, Clara Van Ommen
Department of Microbiology and Immunology, University of British Columbia
The Escherichia coli capsule functions to protect bacterial cells from desiccation and environmental stresses. The E.
coli group I capsule is polymerized and transported to the surface of the cells through the action of the wza, wzb
and wzc gene products. It is thought that the presence of a capsule may confer a level of intrinsic antibiotic
resistance. Previous work exploring the role of capsule in antibiotic resistance showed inconsistent results between
different studies, and that the role of capsule in antibiotic resistance may be dependent on antibiotic class. In this
study we sought to examine the role of the E. coli K30 group I capsule in antibiotic resistance across ten different
antibiotic classes. We examined the E. coli K30 strain CWG655Δ[wza-wzb-wzcK30] that has a chromosomal deletion
of three key capsule biosynthesis genes (wza, wzb and wzc) and its isogenic parental strain E69. We quantified the
capsule production of both strains and compared the susceptibility of the strains to ten different antibiotics. In
doing so, we identified macrolide antibiotics as a class of interest and further examined the susceptibility of the
strains to additional macrolides and a ketolide. We observed that CWG655Δ[wza-wzb-wzcK30] exhibited diminished
production of capsular polysaccharides compared to E69 at 21°C, but that both strains produced comparably low
amounts of capsule at 37°C. Contrary to past work on other antibiotic classes, we observed that CWG655Δ[wza-
wzb-wzcK30] was more resistant to macrolide antibiotics, but not ketolides, when compared to E69 at both 21°C and
37°C. From this study, we conclude that a deletion of the capsule biosynthesis genes wza, wzb and wzc confers
resistance to the macrolide family of antibiotics in a mechanism independent of capsule production.
Capsular polysaccharides (CPS) are synthesized,
kanamycin resistance (7) and Song
et. al. reported that
transported and anchored to the surface of the cell by many
capsule could interact with tetracycline, providing
bacterial species, forming a hydrated layer around the cell
resistance via an unknown mechanism (8). Conversely,
that protects it from desiccation and environmental stress
Parmar
et. al found that the capsule did not confer
(1). The
Escherichia coli K30 group I capsule is assembled
resistance to kanamycin or tetracycline, while Drayson
et.
via the Wyz-dependent biosynthesis system, and
al concluded that antibiotic resistance following exposure
polymerized and transported via the action of the Wza,
to sub-inhibitory antibiotic concentrations was conferred in
Wzb and Wzc proteins (3). Wza is found in the outer
a capsule-independent fashion (9, 10). It has been
membrane and polymerizes to form a channel through
suggested by several groups that capsule involvement in
which the CPS is translocated (2). Wzc is an integral
antibiotic resistance is antibiotic class specific, which may
membrane protein of the inner membrane, and participates
explain, in part, the varied and contradictory results seen in
in the polymerization of CPS through its tyrosine
previous work (6-10).
autokinase activity. Wzb is found in the cytoplasm and is
Each of the many classes of antibiotics has a unique size,
the cognate phosphatase of Wzc. (2). Whitfield
et. al.
structure and bacterial target (11). The macrolide family is
developed an
E. coli K30 group I mutant strain,
characterized by the presence of a large 14, 15, or 16-
CWG655Δ[
wza-wzb-wzcK30], that has a chromosomal
membered lactone ring and attached sugar groups (12).
deletion of the
wza,
wzb and
wzc genes resulting in a
Different macrolides vary in ring size and in the chemical
mutant that exhibits decreased surface assembly of group I
groups attached to the ring or sugar moieties (12).
CPS when compared to the isogenic parental strain E69
Macrolides of interest in this study include erythromycin, a
common representative macrolide, as well as its
Previous work suggests that the barrier function of the
capsule may confer a level of antibiotic resistance by
Additionally, a new sub-group of macrolides called
inhibiting access of the antibiotics to the cell (4,5). These
ketolides has been recently developed that includes the
studies have demonstrated that exposure of
E. coli strains
antibiotic telithromycin (12).
to sub-inhibitory concentrations of antibiotics results in an
Macrolides act by binding to the 50s subunit of the
increase in CPS production and a corresponding increase
bacterial ribosome at the 23s rRNA and inhibit protein
in antibiotic resistance (4). However, there has been
synthesis by inducing dissociation of peptidyl-tRNA (13).
conflicting evidence surrounding the direct role of capsule
Four main mechanisms of macrolide resistance have been
in mediating antibiotic resistance. For example, Ganal
et.
previously observed. Firstly, the outer membrane of many
al. reported resistance to kanamycin and streptomycin in a
Gram-negative bacteria can confer resistance (14). For
capsule-dependent fashion (6). In addition, Al Zharani
et.
example, mutations that impair the barrier function of the
al. found that the
E. coli capsule was necessary for
outer membrane were found to increase susceptibility to
Page
1 of
8
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
azithromycin, clarithromycin and roxithromycin (15).
al. (16), with slight modifications. A colony of each cell type was
Secondly, modification of the antibiotic target through
inoculated in 5 ml of either LB or MH media and grown
methylation of the 23s rRNA can confer resistance (14).
overnight at either 21oC or 37oC. We conducted experiments
Thirdly, resistance can be conferred through an efflux
using both LB and MH media because past work by Parmar
et. al. identified differences in the production of capsular polysaccharide
pump (14). Lastly, macrolides can be inactivated by
between strains grown in LB and MH media (9). The following
enzymatic activity in the cell, including that of esterases
day, optical density readings at 660nm for each culture were
and phosphotransferases (14).
measured using a Spectronic 20+ spectrophotometer, and 1 ml of
Given the conflicting evidence surrounding the role of
the same culture was transferred into a sterile microcentrifuge
capsule polysaccharides in antibiotic resistance, we
tube. Next, the 1ml samples were centrifuged using an Eppendorf
examined the role of capsule production on antibiotic
5415D microcentrifuge for 2.5 minutes at 16,100 x g. The
resistance to a range of antibiotics. We examined the
E.
supernatants were discarded and the pellets were washed 3 times
coli K30 group I mutant strain CWG655Δ[
wza-wzb-
with 1ml of 50 mM NaCl. Next, the pellets were re-suspended in 1ml of 50 mM EDTA. The samples were then incubated at 37oC
wzcK30] in addition to its wild type (WT) parental strain
on a shaker for 30min. After incubation, the samples were
E69 (3). We quantified the capsule production of both
pelleted at 16,100 x g and the supernatant containing capsular
strains and compared the susceptibility of the two strains to
polysaccharides was transferred into a sterile microcentrifuge
ten different classes of antibiotics. From this, we identified
tube. The subsequent capsule quantification was performed with
macrolides as a class of interest and further examined the
the phenol-sulphuric acid assay (16). A 1.0 mg/ml carbohydrate
susceptibility of the strains to additional macrolides and a
stock solution containing 0.05% w/v sucrose and 0.05% w/v
ketolide. By examining different macrolide antibiotics as
fructose was used to prepare the standard curve. For capsule
well as a ketolide, we were able to determine if patterns of
quantification, 400 uL of supernatant was combined with 400 uL
antibiotic susceptibility or resistance were specific to an
of 5% phenol and 2 mL of 93% sulphuric acid in a glass test tube. Colour was allowed to develop for 10 min and the absorbance
individual antibiotic or if they applied to the larger
was measured at 490nm on a Spectronic 20+ spectrophotometer.
antibiotic class.
Each experiment was done in replicates of three.
We observed that CWG655Δ[
wza-wzb-wzcK30] produced
Capsule Staining. A colony of each cell type was streaked onto
diminished capsule compared to the WT and showed
either LB or MH solid media and grown overnight at either 21oC
increased resistance to macrolide antibiotics. Overall, our
or 37oC. Colonies were taken from the plates using a sterilized
results suggest that, for macrolide antibiotics, the
E. coli
loop and suspended in 250uL of sterile saline. Capsule staining
K30 group I capsule does not play a role in antibiotic
was performed using a modified version of the Maneval's capsule
resistance, and that CWG655Δ[
wza-wzb-wzc
staining method described by Hughes and Smith (17). First, the
cell suspension in sterile saline was mixed with 250µL of Congo
resistant to macrolides via a mechanism independent of
Red (1% aqueous solution, Sigma Chemical Company C-6767),
capsule but related to the absence of the
wza, wzb and
wzc
spread onto a glass microscope slide using a sterilized loop, and
air-dried for 5-10 minutes. Next, 150µL of Maneval's solution
was then pipetted onto the dried smears (0.047% w/v acid
MATERIALS AND METHODS
fuchsin, JT Baker Chemicals, A355-3; 2.8% w/v ferric chloride,
Bacterial Strains, Preparation of Media and Growth
Fisher Scientific I-89; 4.8% v/v aqueous glacial acetic acid,
Conditions. E. coli K30 strains E69 (serotype: O9a:K30:H12)
Acros, 42322-0025; 3.6% v/v aqueous phenol solution, Invitrogen
and CWG655 [
wza
IS509-037) and allowed to sit for approximately 2 minutes. The
22 min::
aadA Δ(
wza-wzb-wzc) K30::
aphA3 Kmr
Spr] were obtained from the laboratory of Dr. Chris Whitfield
counterstain was washed off with dH2O and the slides were air-
(Department of Molecular and Cellular Biology, University of
dried before being viewed using a light microscope at 1000x
magnification with oil immersion
K30] has a polar
aadA insertion
in the
wza locus corresponding to 22 minutes on the
E. coli K12
Disc Diffusion Assay. Disc diffusion assays were performed
lineage map that eliminates expression of this copy of the
wza-
using a modified version of the Kirby-Bauer method (18). Strains
wzb-wzc locus (3). The second locus of
wza-wzb-wzc was
were grown overnight in liquid culture of LB or MH media at
inactivated using PCR amplification and cloning into the suicide
21°C or 37°C. The optical density of the cultures was measured at
vector pWQ173, which was used to excise parts of
wza and
wzc
660nm using a Spectronic 20+ spectrophotometer and the cultures
as well as all of
wzb (3). In this paper, strain CWG655 is referred
were then diluted with sterile broth to 1 optical density unit. LB
to as either CWG655 Δ[
wza-wzb-wzc]
or MH plates were spread plated with 100µL of the diluted liquid
K30 or as "mutant strain"
while E69 is denoted as "wild type" (WT). All experiments were
cultures. Antibiotic discs (7mm diameter) prepared with either
performed at either 21°C or 37°C. Liquid cultures were incubated
sulfamethoxazole,
on a shaker contained in either a 37°C walk-in incubator or at
polymyxin, vancomycin, erythromycin, tetracycline, gentamycin,
room temperature (approximately 21°C). Plates were incubated in
or norfloxacin (AB-biodisk) obtained from the Department of
either a 37°C walk-in incubator or at room temperature
Microbiology and Immunology at UBC were placed onto the
(approximately 21°C). Bacterial cells were grown in either Luria
plates using sterilized forceps. For the roxithromycin,
Bertani (LB) broth (1.0% w/v tryptone, 0.5% w/v yeast extract,
clarithromycin, and telithromycin disc diffusions, stock solutions
0.5% w/v NaCl, pH 7) or Mueller Hinton (MH) broth (0.2% w/v
of 10mg/mL roxithromycin, clarithromycin and telithromycin
beef extract, 1.75% w/v acid digest of casein, 0.15% starch, pH
were obtained from the lab of Dr. Charles Thompson
7.3, not cation-adjusted) for capsule isolation as well as capsule
(Department of Microbiology and Immunology, UBC) and 10µL
staining. For other capsule staining experiments, as well as for the
of each solution was pipetted onto blank discs. Each experiment
disc-diffusion assay, bacterial cells were grown on plates made
was done is replicates of three, with three or four discs per plate.
from either LB (1.5% agar) or MH (1.7% agar) media.
The plates were incubated for 18 hours at either 21°C or 37°C
Capsule Extraction and Quantification. Capsule extraction
depending on the initial incubation temperature of the liquid
and quantification was performed as outlined by Brimacombe
et
culture, and the diameters of the zones of inhibition were
Page
2 of
8
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
measured in millimetres. An increase in the diameter of the zone of inhibition indicates increased susceptibility and a decrease in the size of the zone of inhibition indicates increased resistance.
Statistical Analysis. Statistical analysis was performed for the
disc diffusion assay as well as for the phenol-sulphuric acid assay. Statistical significance was determined using an unpaired, two tailed t-test (p<0.5). For the phenol-sulphuric acid assay, comparisons were made between CWG655Δ[
wza-wzb-wzcK30] and the WT strain, at both 21oC and 37oC for LB and MH media. Comparisons were also made between 21°C and 37°C for the WT and CWG655Δ[
wza-wzb-wzcK30]. For the disc diffusion assay, comparisons were made between CWG655Δ[
wza-wzb-wzcK30] and the WT strain, at both 21oC and 37oC for LB and MH media.
Deletion of the wza-wzb-wzc genes decreases capsule
production at 21°C but not at 37°C compared to the
WT strain. To confirm decreased capsule biosynthesis
FIG 1 Differences in capsular polysaccharide produced by the
WT strain and CWG655Δ[
ability of CWG655Δ[
wza-wzb-wzcK30] using the phenol-
wza-wzb-wzcK30] compared to the
sulphuric acid capsule quantification method. Strains were
WT strain, we quantified capsular polysaccharide
cultured overnight in 21°C or 37°C shaking incubators in LB liquid
production of both strains at 21°C and 37°C using the
media, and capsule polysaccharide was extracted and quantified using
phenol-sulphuric acid assay. Given that past groups have
the phenol-sulphuric acid assay. * indicates p<0.05, n.s. indicates not
observed decreased capsule production at 37°C, compared
to 21°C, we decided to conduct our analysis at both
culture, this experiment confirmed that differences in
temperatures (9). We expected that CWG655Δ[
wza-wzb-
capsule production between the strains were also observed
wzcK30] would produce less capsular polysaccharide
on solid media. Based on the phenol-sulphuric acid assay
compared to the WT and that both strains would produce
results (Fig. 1), we expected that the WT cells would have
more capsular polysaccharide at 21°C, compared to 37°C.
a larger visible capsule than the CWG655Δ[
wza-wzb-
At 21°C, the WT strain exhibited 14-times greater
production of capsular polysaccharide compared to
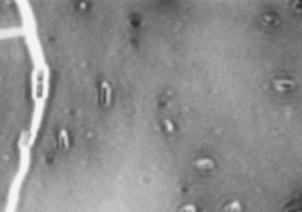
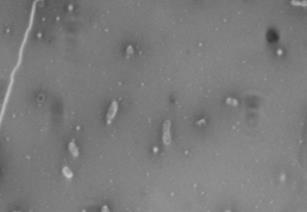
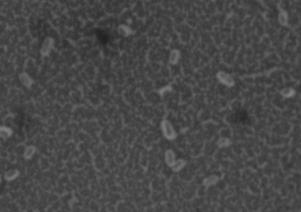
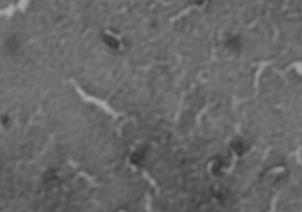
K30] cells at 21°C, but not 37°C. Resulting images of
stained WT cells (Fig. 2A) and CWG655Δ[
wza-wzb-
wza-wzb-wzcK30] when grown in LB broth
(Fig. 1). At 37°C, we found no significant difference in
K30] cells (Fig. 2B) grown at 21°C showed increased
capsule size visible around the WT cells, and not the
capsular polysaccharide production for the WT compared to CWG655Δ[
K30] cells. Both WT (Fig. 2C)
K30] (Fig. 1). Additionally, we
cells and CWG655Δ[
wza-wzb-wzc
observed 12-times greater production of capsular
comparable capsule size at 37°C (Fig. 2D). However, we
polysaccharide for the WT strain at 21°C compared to
observed only minor differences in capsule size between
37°C (Fig. 1). We did not observe a significant difference in capsular polysaccharide production for CWG655Δ[
WT cells grown at 21°C and 37°C (Fig. 2A, 2C). These
results are unexpected given that we observed that the WT
wzb-wzcK30] between 21°C and 37°C (Fig.1). We
produced more capsular polysaccharides at 21°C,
replicated these experiments using both strains grown in
compared to 37°C (Fig. 1). We suspect that our inability to
MH broth and observed a similar trend in which the WT
detect large differences in capsule size is due to disparate
microscope image quality. Despite our inability to detect
wza-wzb-wzcK30] (Supplemental Fig. 1). When
large difference in capsule size between WT cells grown at
grown in MH media we observed a less pronounced
21°C and 37°C, from these results we conclude that, when
difference in polysaccharide production between the two
grown on solid media, CWG655Δ[
wza-wzb-wzc
strains at 21°C, indicating that LB would be a more
decreased capsule size compared to the WT at 21°C, and
suitable media for further study regarding the effects of the
wza-wzb-wzc gene deletion on antibiotic resistance. From
K30] and the WT show similar
capsule sizes at 37°C.
these results, we conclude that the WT strain produces more capsular polysaccharide than CWG655Δ[
exhibits
increased
resistance to erythromycin compared to the WT strain.
wzcK30] at 21°C, but not 37°C.
Due to the increase in capsule production observed for the
WT cells exhibit increased capsule thickness
compared to CWG655Δ[
WT strain compared to CWG655Δ[
wza-wzb-wzc
K30] on solid LB
21°C, we hypothesized that an increase in capsular
agar. To further confirm that CWG655Δ[
wza-wzb-wzcK30]
polysaccharides might influence antibiotic resistance in a
was deficient in capsule compared to the WT, we performed capsule staining using Maneval's staining
class-dependent manner. In addition, differences in antibiotic susceptibility between the WT strain and
procedure, and visualized capsule size using light
microscopy. Given that the antibiotic disc diffusion
K30] were predicted to be more
prominent at 21°C when compared to 37°C, due to the lack
experiments were to be performed on solid media but the
of differential capsule production between the strains
phenol-sulphuric acid assay used cells grown in liquid
Page
3 of
8
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
We observed no significant differences in capsule
production at 37°C between CWG655Δ[wza-wzb-wzcK30]
and the WT strain (Fig. 1). However, we observed
CWG655Δ[wza-wzb-wzcK30] and the WT strain at 37°C (Fig. 3). These results suggest that the differential susceptibility of the strains to erythromycin may not be
due to the physical presence of capsule. From this we
conclude that although the deletion of the wza-wzb-wzc
capsule biosynthesis genes confers increased resistance to
erythromycin, this effect may not be due to decreased capsule production.
Resistance
erythromycin extends to the macrolides clarithromycin
and
ketolide
FIG 2 Differences in capsule thickness of WT and
telithromycin. We observed differences in antibiotic
susceptibility between the WT strain and CWG655Δ[wza-
K30] cells grown on solid LB agar media at
21°C and 37°C. (A) E69 WT cells grown on LB agar at 21°C; (B)
wzb-wzcK30] that varied with antibiotic class (Supplemental
CWG655Δ[wza-wzb-wzcK30] cells grown on LB agar at 21°C. (C) E69
Fig. 2). Additionally, we observed that CWG655Δ[wza-
WT cells grown on LB agar at 37°C. (D) CWG655Δ[wza-wzb-wzcK30]
wzb-wzcK30] exhibited increased resistance to erythromycin
cells grown on LB agar at 37°C. Strains were grown overnight on LB agar plates at 21°C, and cell capsules were stained using Maneval's
compared to the WT (Fig. 3). In order to determine if the
staining protocol and visualized at 1000x magnification. Grey regions
resistance conferred by the wza-wzb-wzc gene deletion was
indicate cell bodies, and white regions indicate capsule.
specific to erythromycin or if it also applied to other
antibiotics in the macrolide class, we conducted further
observed at 37°C (Fig. 1). To test our hypothesis we
conducted a screen of ten antibiotics, each of a different
roxithromycin. Additionally, we used telithromycin, which
antibiotic class, using an antibiotic disc diffusion assay on
is a member of the macrolide sub-group the ketolides. We
LB and MH agar media for both strains at 21°C and 37°C.
examined the susceptibilities of the WT strain and
We observed that CWG655Δ[wza-wzb-wzcK30] exhibited
CWG655Δ[wza-wzb-wzcK30] at both 21°C and 37°C. At
decreased resistance to some antibiotics, such as
21°C, disc diffusion results showed a 3-fold increase in
nitrofurantoin, yet no consistent trends in resistance
susceptibility to roxithromycin for the WT strain compared
changes to many other antibiotics, when compared to the
to CWG655Δ[wza-wzb-wzcK30]. A similar trend of
WT (Supplemental Fig. 2). However, we also observed
increased susceptibility of the WT strain was seen for
that CWG655Δ[wza-wzb-wzcK30] exhibited increased
clarithromycin, but these results were not significant at
resistance to some antibiotics when compared to the WT,
21°C. (Fig 4). When grown at 37°C, we observed at 10-
such as erythromycin and tetracycline (Supplemental Fig.
fold increase in susceptibility to roxithromycin for the WT
2). These results suggest that a deletion of the wza-wzb-
compared to CWG655Δ[wza-wzb-wzcK30] (Fig. 4).
wzc genes can increase, decrease, or have no effect on
Similarly, we observed that the WT was susceptible to
resistance to antibiotics, depending on the antibiotic tested.
clarithromycin with a clear zone of inhibition around the
Based on the observed results, we identified erythromycin
antibiotic disc, while CWG655Δ[wza-wzb-wzcK30] was
as an antibiotic of interest for further study. At 37°C, the
resistant with growth up to the edge of the antibiotic disc.
WT strain had some degree of susceptibility to
At both temperatures, we observed that the WT and
erythromycin, as indicated by the presence of a zone of
CWG655Δ[wza-wzb-wzcK30] were comparably resistant to
the ketolide, telithromycin (Fig. 4). We observed a similar
CWG655Δ[wza-wzb-wzcK30] showed no susceptibility,
trend in results when disc diffusion assays were replicated
growing consistently up to the edge of the disc (Fig. 3A).
on MH media (Supplemental Fig. 4). From these results
The zones of inhibition surrounding the erythromycin discs
we conclude that the wza-wzb-wzc gene deletion confers
appeared as a gradient, not a distinct line (Fig. 3A).
increased resistance to the macrolides clarithromycin and
CWG655Δ[wza-wzb-wzcK30] showed a significant increase
roxithromycin, similar to the pattern seen in erythromycin,
in resistance to the erythromycin compared to the WT
but this does not extend to the ketolide telithromycin. This
strain (Fig. 3B). We observed a similar pattern at 21°C, but
effect is independent of physical capsule presence.
at this temperature results did not reach significance (Fig.
3B). Additionally, we obtained similar results with a disc
DISCUSSION
diffusion assay performed on MH agar, where
In this study, comparison of capsule production
between the WT strain and CWG655Δ[wza-wzb-
increased erythromycin resistance at 37°C and a similar
but less pronounced result at 21°C compared to the WT
revealed that CWG655Δ[wza-wzb-wzcK30]
exhibited 14-times less CPS production than the WT
strain (Supplemental Fig. 3).
strain at 21°C . This is consistent with the expected
Page 4 of 8
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
results, due to the chromosomal deletion of the capsule biosynthesis genes wza, wzb and wzc, as described by Whitfield et al. (3). Our results are consistent with the findings of Parmar et al., who examined a strain deficient in only the Wza channel-forming protein necessary for capsule assembly, and demonstrated decreased CPS production by that mutant strain (9).
We observed a greater difference in capsule
production between the WT strain and CWG655Δ[wza-
wzb-wzcK30] at 21°C than at 37°C, and an overall
increase in capsule production at 21°C for the WT (Fig.
1). Although the group I E. coli capsule was not previously thought to be thermoregulated (2), studies by
Parmar et. al., Drayson et. al, and Stout et. al., and have reported increased capsule production at 21°C compared to 37°C (9, 10, 19). This observation is of interest because some previous studies that have failed to find differences in antibiotic resistance based on the presence or absence of capsule carried out experiments at only 37°C (20). For example, Naimi et. al. examined the role of capsule in streptomycin susceptibility with organisms grown at 37°C and observed no difference in susceptibility between a wza mutant and its isogenic WT strain (20). A possible explanation for their result is
that the strains were producing comparable levels of
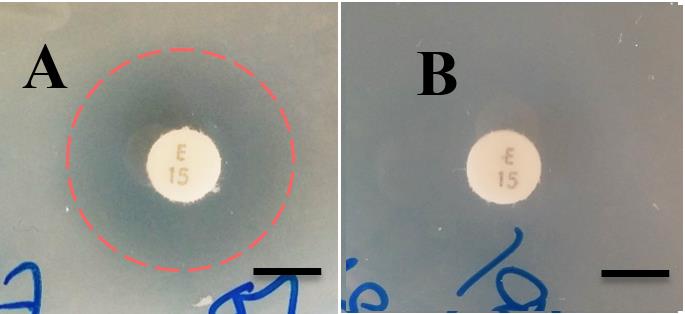
FIG 3 Susceptibility of CWG655Δ[wza-wzb-wzcK30] and the WT
strain to erythromycin via disc diffusion assay. (A, B)
polysaccharides at 37°C. However, other studies have
Representative disc diffusion results showing that the WT is
examined the same strains at both 21°C and 37°C and
susceptible to erythromycin, as seen by a zone of clearance around the
also failed to find differences in antibiotic resistance to
erythromycin disc indicated by a red dashed circle. CWG655Δ[wza-
streptomycin between WT and capsule deficient
wzb-wzcK30] is shown to be resistant to erythromycin, as seen by the
mutants (9, 10). The previous findings on the topic of
lack of inhibition around the erythromycin disc. Scale bars = 7mm; (C) Differences in susceptibility of the WT and CWG655Δ[wza-wzb-
capsule-dependent
wzcK30] to erythromycin at 21°C and 37°C. Disc diffusion assays were
contradictory, however, as there have been other groups
carried out using antibiotic discs on LB agar plates. An increase in the
that have suggested a link between capsule production
diameter of the zone of inhibition indicates an increase in
and resistance to certain antibiotics, such as kanamycin,
susceptibility. * indicates p<0.05, n.s. indicates non-significant. Dashed line indicates diameter of antibiotic disc.
tetracycline, and streptomycin (4, 7, 8). Therefore, we
suggest that capsule may influence antibiotic resistance
compared to the WT strain (Supplementary Fig. 2). Our
for specific classes of antibiotics.
results indicate that our understanding of the role of
In this study, we used a screen of ten different
capsule in antibiotic resistance should be modified to
antibiotics to compare the susceptibilities of the WT
suggest that an increase in capsule production can either
strain and CWG655Δ[wza-wzb-wzcK30] using the disc
increase, decrease or have no effect on antibiotic
diffusion assay for antibiotic resistance. Due to the
resistance depending upon the antibiotic class being
limited number of replicates we performed, the majority
of antibiotics tested showed no significant difference in
Due to the increase in resistance observed by
susceptibility between the two strains (Supplementary
CWG655Δ[wza-wzb-wzcK30] to erythromycin, we
Fig. 2). Indeed, at 21°C we noticed no significant
decided to further investigate macrolides as our
differences between the antibiotic susceptibilities of
antibiotic family of focus. In this study, we
either strain for any of the antibiotics tested.
demonstrated that CWG655Δ[wza-wzb-wzcK30] showed
Nonetheless, the general trend supports the observation
increased resistance to the macrolide antibiotics
in the literature that the presence of an intact capsule
erythromycin, clarithromycin, and roxithromycin, but
can increase resistance to a variety of antibiotics (4, 7,
not the ketolide antibiotic telithromycin, when
8), or have no effect (9, 10). However, we also
compared to the WT strain (Fig. 3, 4). We observed
identified another possibility: the absence of wza, wzb,
comparable results at 21°C and 37°C. However,
and wzc in our mutant may increase antibiotic
differences in susceptibility to erythromycin and
clairithromycin between the WT and CWG655Δ[wza-
CWG655Δ[wza-wzb-wzcK30] exhibited an increase in
wzb-wzcK30] only reached significance at 37°C (Fig. 3,
resistance to both tetracycline and erythromycin
4). We also observed that there were no significant
Page 5 of 8
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
increased resistance observed in CWG655Δ[wza-wzb-wzcK30] in the context of OM permeability. Whitfield et. al. observed that the CWG655Δ[wza-wzb-wzcK30] strain shows depleted surface assembly of CPS (3). These results were replicated in this study (Fig.1, 2). Whitfield et. al. also observed that CWG655Δ[wza-wzb-wzcK30] produces capsular oligosaccharides with a low degree of polymerization that are attached to the lipid A moiety of LPS and form an alternate glycoform of LPS called K-LPS (3). We speculate that the increased resistance to macrolides exhibited by CWG655Δ[wza-wzb-wzcK30] to macrolides may be related to the formation of K-LPS, and therefore an altered OM structure and permeability (C. Whitfield, personal correspondence).
This notion is supported by previous studies that have
FIG 4 Differences in susceptibility of the WT strain and
CWG655Δ[
found that modifications in OM permeability alter
wza-wzb-wzcK30] to the macrolides clarithromycin,
roxithromycin, and the ketolide telithromycin at 21°C and 37°C.
macrolide susceptibility. Vaara found that mutations
Disc diffusion assays were carried out on LB agar plates. An increase
that affected OM structure and increased permeability,
in the diameter of the zone of inhibition indicates an increase in
such as mutations in lipid A synthesis in E. coli,
susceptibility. * indicates p<0.05, ** indicates p<0.005, n.s. indicates
decreased the MICs of erythromycin, roxithromycin,
non-significant. Dashed line indicates diameter of antibiotic disc.
clarithromycin and azithromycin (23). Similarly, Buyuk
differences in CPS production at 37°C between the WT
et. al. found that certain strains of Pseudomonas
aeruginosa are more susceptible to macrolides due to
together these results suggest that the presence or
increased membrane permeability (45). Finally, Farmer
absence of capsule does not play a role in antibiotic
et. al observed that the MIC of azithromycin was
resistance to macrolides for these strains, but that the
increased 8 times with the addition of a magnesium
absence of the wza, wzb and wzc genes may play a role
supplementation that decreased membrane permeability
in the increase in resistance of CWG655Δ[wza-wzb-
(25). These previous findings lend support to our
proposed model wherein the wza-wzb-wzc deletion
These results are consistent with past
observations made by Drayson et. al which suggested
confers macrolide resistance through an alteration of the
that antibiotic resistance can be conferred in a capsule-
OM structure that causes changes in OM permeability.
independent fashion (10). Given that our data suggest
The last observation of significance in this study is
that the absence of wza, wzb and wzc increases
that both the WT strain and CWG655Δ[wza-wzb-
antibiotic resistance in a capsule-independent manner, a
wzcK30] exhibited comparable levels of resistance to
discussion of the potential mechanisms of resistance
telithromycin, with but differing levels of susceptibility
to macrolides that were close derivatives of
A variety of mechanisms for resistance to macrolides
erythromycin (Fig. 3, 4). Different macrolide antibiotics
have been observed (21). Given that capsule is
vary in chemical components that are attached to the
associated with the outer membrane (OM) and wza, wzb
lactone ring or sugar moieties (11). Clarithromycin is
and wzc, are involved in capsule assembly we suggest
derived from erythromycin by substituting a methoxy
that the mechanism of most relevance to this study is
group for the C-6 hydroxyl group of erythromycin (26),
the role of the OM as a permeability barrier. Typically,
while roxithromycin has an N-oxime side chain
macrolide antibiotics are used to treat Gram-positive
attached to the lactone ring (27). Telithromycin is a
infections because the OM of Gram-negative bacteria
member of a macrolide derivative family called
can confer a level of resistance that makes clinical use
ketolides, which have a further modified structure from
of macrolides, particularly erythromycin, challenging
typical macrolides in that a keto functional group is
for those types of infections (22). This is thought to be
substituted for the sugar moiety at C-3 on the lactone
due to the hydrophobic nature of the macrolides, which
ring (26). A methoxy group replaces the hydroxyl group
can prevent them from passing the charged lipid A
at C-6 and C-11-12 is cyclized to make a carbamate
component of LPS present in the OM (22).
group with an imidazo-pyridyl group attachment (26).
Because our mutant strain lacks three genes and their
We suggest that the difference we see in susceptibility
corresponding protein products, we cannot identify a
may be due to structural differences between
single gene product that, when absent, confers the
telithromycin and the macrolides. Most literature in this
area suggests that ketolides have increased activity
developed a model as a potential explanation for the
macrolides; however, limited data is available regarding
Page 6 of 8
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
mechanisms of resistance to ketolides (26). Recent
work could also focus on elucidating the mechanism of
work has reported that few bacterial strains exhibit
resistance to telithromycin used by both E69 and
telithromycin resistance (26). In the literature, it appears
CWG655Δ[wza-wzb-wzcK30]. Given that the incidence of
that those strains that are resistant to ketolides exhibit
telithromycin is rare, future studies could attempt to
their resistance through mechanisms that are common
explain the observed resistance. For example, the strains
to those for macrolide resistance. Walsh et al. reported
could be examined in an attempt to determine if they
two mechanisms of telithromycin resistance: target
harbour genes that have been identified in the literature as
modification by methylation of the 23s rRNA by the
being involved in telithromycin resistance (28).
We observed that the WT strain produced 12-times
erm(B) methylase gene or efflux mediated by the
greater levels of capsular polysaccharides at 21°C
mef(B) gene (28). With our limited data we cannot
compared to 37°C. Past publications have reported similar
develop any conclusive hypotheses regarding the source
results (9). Given that group I capsule production is not
known to be thermoregulated (2), future work could
examine the potential mechanisms behind these results by
mechanisms include exclusion due to its size or charge,
examining the expression of genes involved in capsule
or the presence of any of the resistance mediating genes
assembly at 21°C and 37°C.
described above.
Finally, future experiments could be conducted to further
In this study our aim was to examine the
examine the other observed results in our antibiotic screen.
CWG655Δ[wza-wzb-wzcK30] mutant strain and its WT
That is, higher replicate screens could, and should, be
parental strain E69, with particular focus on the role of
conducted in order to determine the significance of
the K30 group I capsule in antibiotic resistance to
observed trends, and to further confirm the results
multiple antibiotic families. We observed that
observed herein. Moreover, certain antibiotics could be
further studied in order to aid in the explanation of the
K30] produced less capsular
polysaccharide than the WT at 21°C, but not 37°C. We
results. For example, tetracycline resistance was increased
also observed that CWG655Δ[wza-wzb-wzc
in the absence of capsule in this study and further work
increased resistance to macrolides, but not the ketolide
could focus on elucidating this mechanism. Additionally,
subgroup, when compared to the WT at 21°C and 37°C.
for norfloxacin at 21°C the WT strain showed increased resistance compared to CWG655Δ[
Taken together, our data suggest that a deletion of the
wza-wzb-wzcK30], but
this result was reversed at 37°C. Future work could focus
wza, wzb and wzc genes confers resistance to macrolide,
on attempting to replicate this result and examine the
but not ketolide, antibiotics via a capsule-independent
mechanism behind it. Indeed, these are but two of several
mechanism. Overall, we conclude that the absence of
examples of antibiotics that could be further studied in
the capsule biosynthesis genes wza, wzb and wzc
relation to the role of capsule in antibiotic resistance.
confers increased resistance to macrolide antibiotics.
FUTURE DIRECTIONS
We would like to thank the Department of Microbiology and
Although we have indicated that the absence the wza, wzb,
Immunology at the University of British Columbia for their
and wzc genes is important for macrolide resistance, and
funding and support. We would like the thank Dr. David Oliver,
that this phenomenon is capsule-independent, the
Jia Wang and Céline Michaels, for their guidance, instruction,
mechanism of resistance remains unknown. The clearest
and support, as well as the staff of the media room for providing
and most pressing direction to be taken from this study is
us with equipment and supplies. In addition, we would like to
an examination of the effect of single gene mutations in
thank the Department of Pharmacology at UBC for gifting the
wza, wzb or wzc on resistance to macrolide family
Department of Microbiology with many of the antibiotic disks used in our experiments. Further, we would like to thank Dr.
antibiotics. Since this study was conducted using a mutant
Chris Whitfield at the University of Guelph for providing the E.
with a deletion of all three of these capsule assembly
coli strains and for his valuable insight into our work. Finally we
genes, a causal relationship cannot be established between
would like Dr. Charles Thompson at the University of British
the absence of any one gene (or a combination) and the
Columbia for providing us with the macrolide antibiotics used in
observed increase in resistance to macrolides. Further
our experiments.
study is warranted to determine whether or not the
observed effect can be traced to a single gene product, or
REFERENCES
whether the effect is the result of a combination of
1. Reid, AN, Szymanski, CM. 2010. Biosynthesis and assembly of
deletions. Additionally, the effect of other outer membrane
capsular polysaccharides-Chapter 20, p. 351-373. In Moran, A
genes could be studied in order to determine whether this
(ed), Microbial Glycobiology: Structure, Relevance and Application. Elsevier Inc, London, UK.
phenomenon is unique to the capsule biosynthesis genes
2. Whitfield, C. 2006. Biosynthesis and assembly of capsular
wza, wzb and wzc or whether deletions in other membrane
polysaccharides in Escherichia coli. Annu. Rev. Biochem. 75:39-
channels, kinases, etc. are sufficient to induce the
observed, macrolide-resistant phenotype.
3. Reid, AN, Whitfield, C. 2005. Functional analysis of conserved
We observed that the WT strain and CWG655Δ[wza-
gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition
wzb-wzcK30] exhibited telithromycin resistance. Future
Page 7 of 8
Journal of Experimental Microbiology and Immunology (JEMI)
Copyright April 2015, M&I UBC
between Wza and Wzc for colanic acid biosynthesis. J. Bacteriol.
17. Hughes RB, Smith, A. 2011. Capsule stain protocols. ML
Microbe Library, ASM. Accessed on 9th March 2015 at
4. Lu, E, Trinh, T, Tsang, T, Yeung, J. 2008. Effect of growth in
sublethal levels of kanamycin and streptomycin on capsular
18. Hudzicki, J. 2009. Kirby-Bauer disk diffusion susceptibility test
Escherichia coli B23. J. Exp. Microbiol. Immunol. 12:21-26.
protocol. ML Microbe Library, ASM. Accessed on 8th February
5. Slack, MP, Nichols, WW. 1982. Antibiotic penetration through
bacterial capsules and exopolysaccharides. J. Antimicrob.
Chemother. 10:368-372.
6. Ganal, S, Guadin, C, Roensch, K, Tran, M. 2007. Effects of
19. Stout, V, Gottesman, S. 1990. RcsB and RcsC: a two-
streptomycin and kanamycin on the production of capsular
component regulator of capsule synthesis in Escherichia coli. J.
polysaccharides in Escherichia coli B23 cells. J. Exp. Microbiol.
Bacteriol. 172:659-669.
Immunol. 11:54-99.
20. Naimi, I, Nazer, M, Ong, L, Thong, E. 2009. The role of wza in
7. Al Zahrani, F, Huang, M, Lam, B, Vafaei, R. 2013. Capsule
extracellular capsular polysaccharide levels during exposure to
formation is necessary for kanamycin tolerance in Escherichia
sublethal doses of streptomycin. J. Exp. Microbiol. Immunol.
coli K-12. J. Exp. Microbiol. Immunol. 17:24-28.
Vol. 13:36-40.
8. Song, C, Sun, XF, Xing, SF, Xia, PF, Shi, YJ, Wang, SG.
21. Leclercq, R. 2002. Mechanisms of resistance to macrolides and
2013. Characterization of the interactions between tetracycline
lincosamides: nature of the resistance elements and their clinical
antibiotics and microbial extracellular polymeric substances with
implications. Clin. Infect. Dis. 34:482-492.
spectroscopic approaches. Environ. Sci. Pollut. Res. Int. 21:
22. Poole, K. 2002. Outer membranes and efflux: the path to
multidrug resistance in Gram-negative bacteria. Curr. Pharm.
9. Parmar, S, Rajwani, A, Sekhon, S, Suri, K. 2014. The
Biotechnol. 3:77-98.
Escherichia coli K12 capsule does not confer resistance to either
23. Vaara, M. 1993. Outer membrane permeability barrier to
tetracycline or streptomycin. J. Exp. Microbiol. Immunol.18:76-
azithromycin, clarithromycin, and roxithromycin in gram-
negative enteric bacteria. Antimicrob. Agents Chemother.
10. Drayson R, Leggat T, Wood M. 2011. Increased antibiotic
37:354-356.
resistance post-exposure to sub-inhibitory concentrations is
24. Buyck, J, Tulkens, PM, Van Bambeke, F. 2011. Increased
independent of capsular polysaccharide in Escherichia coli. J.
susceptibility of Pseudomonas aeruginosa to macrolides in
Exp. Microbiol. Immunol. 15:36-42.
biologically-relevant media by modulation of outer membrane
11. Walsh, C. 2003. Antibiotics: actions, origins, resistance.
permeability and of efflux pump expression, p. 17-20. In
American Society for Microbiology (ASM).
Anonymous 51st Interscience conference on antimicrobial agents
12. Omura, S. 2002. Macrolide antibiotics: chemistry, biology, and
and chemotherapy, Chicago.
practice. Academic Press.
25. Farmer, S, Li, ZS, Hancock, RE. 1992. Influence of outer
13. Tenson, T, Lovmar, M, Ehrenberg, M. 2003. The mechanism
membrane mutations on susceptibility of Escherichia coli to the
of action of macrolides, lincosamides and streptogramin B
dibasic macrolide azithromycin. J. Antimicrob. Chemother.
reveals the nascent peptide exit path in the ribosome. J. Mol.
29:27-33.
Biol. 330:1005-1014.
26. Zuckerman, JM. 2004. Macrolides and ketolides: azithromycin,
14. Leclercq, R. 2002. Mechanisms of resistance to macrolides and
clarithromycin, telithromycin. Infect. Dis. Clin. North Am.
lincosamides: nature of the resistance elements and their clinical
18:621-649.
implications. Clin. Infect. Dis. 34:482-492.
27. Takashima, H. 2003. Structural consideration of macrolide
15. Vaara, M. 1993. Outer membrane permeability barrier to
antibiotics in relation to the ribosomal interaction and drug
azithromycin, clarithromycin, and roxithromycin in gram-
design. Curr. Top. Med. Chem. 3:991-999.
negative enteric bacteria. Antimicrob. Agents Chemother.
28. Walsh, F, Willcock, J, Amyes, S. 2003. High-level
37:354-356.
telithromycin resistance in laboratory-generated mutants of
16. Brimacombe CA, Stevens A, Jun D, Mercer R, Lang AS,
Streptococcus pneumoniae. J. Antimicrob. Chemother. 52:345-
Beatty JT. 2013. Quorum-sensing regulation of a capsular
polysaccharide receptor for the Rhodobacter capsulatus gene
transfer agent (RcGTA). Mol. Microbiol. 87:802-817
Page 8 of 8
Source: http://jemi.microbiology.ubc.ca/sites/default/files/Botros%20et%20al..pdf
Contributions of low molecule number and enetics chromosomal positioning to stochastic gene expression Attila Becskei1, Benjamin B Kaufmann1,2 & Alexander van Oudenaarden1 The presence of low-copy-number regulators and switch-like signal propagation in regulatory networks are expected to increasenoise in cellular processes. We developed a noise amplifier that detects fluctuations in the level of low-abundance mRNAs in
PROCEEDINGS, Kenya Geothermal Conference 2011 Kenyatta International Conference Center, Nairobi, November 21-22, 2011 HEALTH SPA TOURISM: A POTENTIAL USE OF SAGOLE THERMAL SPRING IN LIMPOPO PROVINCE, SOUTH AFRICA Tshibalo, Azwindini Ernest University of South Africa Preller Street, Muckleneuk Ridge, Pretoria ABSTRACT The Sagole Spa thermal spring is located in Limpopo Province, South Africa, and has a water temperature of about 45°C. The spa flourished in the 1980s as a site for recreation and tourism, but its condition declined for various reasons after 1994, which saw the advent of the new democratic government. However, the water temperature and flow rate have remained the same since the 1980s.The research study sought to identify the most beneficial potential development projects for the thermal spring. The following research methods were used to identify the potential projects: literature review, focus group interviews, site visits and observation, and water sample collection and analysis. Health spa tourism was identified as a potentially viable development project for the spa. Some minerals and trace elements with curative power were identified in the thermal water. The environmental, social and economic impacts and the feasibility of establishing the health spa tourism project were assessed. Development costs and potential benefits were also analyzed. It is concluded that health spa tourism can benefit Sagole, a rural area in Limpopo, South Africa.









