
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Doi:10.1016/j.jsbmb.2005.08.019

Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
The xenoestrogen bisphenol A in the Hershberger assay: androgen
receptor regulation and morphometrical reactions indicate no major effects
Tsuyuki Nishino , Thilo Wedel , Oliver Schmitt , Katja B¨uhlmeyer , Martin Sch¨onfelder ,
Christian Hirtreiter , Thorsten Schulz , W. K¨uhnel , H. Michna
Institute of Public Health Research, Technical University of Munich, Connollystraße 32, 80809 Munich, Germany
Institute of Anatomy, University of Luebeck, Germany
Institute of Anatomy, University of Rostock, Germany
Institute of Organic Chemistry, University of Regensburg, Germany
Received 24 January 2005; accepted 31 August 2005
We evaluated androgen-like effects of bisphenol A (BPA) using orchiectomized Wistar rats. Animals were treated p.o. either with vehicle
or with 3, 50, 200, 500 mg/kg bw/day BPA (n = 13) for 7 days. One group was treated s.c. with 1 mg/kg bw/day testosterone propionate (TP).
Flutamide (FL) (3 mg/kg bw/day, p.o.) was used to antagonize androgen effects of the suprapharmacological dose (500 mg/kg bw/day) of BPA.
Androgen-like effects of BPA on prostates and seminal vesicles were assessed by the Hershberger assay, densitometric analysis of androgen
receptor (AR) immunoreactivity, cell proliferation-index and a morphometric analysis. Absolute weights of prostates and seminal vesicles
were not increased by BPA, whereas the relative weights were increased at higher doses of BPA, most likely due to a decrease in body weight.
Staining intensity for AR immunoreactivity was increased at low but not at higher doses of BPA in comparison to the orchiectomized rats.
BPA at all doses tested did not cause an increase of the cell proliferation-index. Epithelial height and glandular luminal area were increased
by low doses of BPA, whereas higher doses caused a decrease of these parameters. The data provide evidence that BPA does not exert major
androgenic effects.
2005 Elsevier Ltd. All rights reserved.
Keywords: Bisphenol A; Prostate; Seminal vesicle; Immunohistochemistry; Morphometry; Densitometry; Androgen receptor regulation; PCNA; MIB-5;
Proliferation markers
Recent in vitro studies demonstrate, in fact, that xeno-
biotics can bind with estrogen receptors and activate them,
BPA is a chemical monomer used primarily to make
resulting in gene expression BPA slightly induced
epoxy-resins, polycarbonate (PC) plastic products and the
MCF-7 cell proliferation at a level of 0.1 M and maxi-
flame retardant tetrabromobisphenol A A number of
mum proliferation at 10 M Also in in vivo studies,
so-called xenobiotics, including pesticides (p,p�-DDT), plas-
BPA showed estrogenic activity. Plasma free testosterone lev-
ticizers (BPA) and a variety of other industrial chemicals
els were dramatically decreased following 8 weeks of BPA
(polychlorinated biphenyls) contain a phenolic ring that mim-
treatment It was claimed by vom Saal that, the expo-
ics the A-ring of estradiol and have been reported to have
sure of pregnant mice to extremely low concentrations of
hormonal or antihormonal activity Although the level
certain xenobiotics, for instance, results in offspring with
of exposure to these xenobiotics may be, if any, very low, they
lower sperm production, increased prostate size or alters
may exert their potential toxicity or endocrine disturbance in
maternal behaviour, postnatal growth rate and reproduc-
human beings and wildlife.
tive function in female mice work groups, in
contrast, found an uterotrophic response (increase in uter-
∗ Corresponding author. Tel.: +49
89 289 24571; fax: +49 89 289 24572.
ine wet weight) at doses up to 100 mg/kg BPA for 3 days
E-mail address: [email protected] (T. Nishino).
0960-0760/$ – see front matter 2005 Elsevier Ltd. All rights reserved.

T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
Also Gupta an enhancement of the anogen-
stitution (OX) and to a vehicle treated intact control group
ital distance and the prostate size of fetuses, when pregnant
(Intact). Propylene glycol was purchased from Merck, Darm-
CD-1 mice were treated with BPA in the microgram range
stadt, Germany. TP and FL were kindly provided by Schering
per kg bw/day. BPA induces inappropriate androgen receptor
AG, Berlin, Germany. After the treatment animals were sacri-
activation and mitogenesis in prostatic adenocarcinoma cells
ficed by decapitation and seminal vesicles and prostates were
(LNCaP). Takao reported a significant decrease in plasma
harvested surgically, weighed and immediately fixed in 4%
free testosterone levels at 50 g BPA/ml in drinking water
neutral buffered paraformaldehyde for 24 h.
(14 mg/kg/day). No significant effect (although a trend in the
same direction) was observed neither after 4 weeks of expo-
sure nor after 4 and 8 weeks exposure to 5 g of BPA/ml
drinking water (0.14 mg/kg/day)
After fixation the specimens (prostate, seminal vesicle)
In addition, Kim et al. did not detect any androgenic or
were dehydrated in ascending series of alcohol, embed-
anti-androgenic activities of BPA in Hershberger assay
ded in paraffin and cut in sections of 5 m thickness (10
the BPA doses used were 10–1000 mg/kg/day.
sections per specimen). For the immunohistochemical visu-
The present study was carried out to clarify the andro-
alization of androgen receptors and proliferation markers
genic potential of BPA in a broad dose range from "ultralow",
the following primary antibodies were applied using the
"pharmacological" to "suprapharmacological" in rats using
standard procedure protocols provided by the manufac-
the standard Hershberger assay with additionally androgen-
turer: anti-androgen-receptor (1:100, sc-815, rabbit poly-
clonal, Santa Cruz Biotechnology, CA, USA), anti-androgen-
Additional parameters such as morphometric and qualita-
receptor (1:100, 554224, mouse monoclonal, BD PharMin-
tive data are therefore required to determine the androgenicity
gen, Germany), anti-PCNA (1:200, PC-10, mouse mono-
of a given substance, in particular if the expected effects are
clonal, Novocastra, New Castle, United Kingdom) and anti-
of lower degree.
MIB-5 (1:100, M 7248 mouse monoclonal, DakoCytoma-
tion, Denmark).
2. Materials and methods
2.4. Densitometry and morphometry
2.1. Animals and housing
Intensity of immunohistochemical staining was deter-
mined densitometrically, while epithelial height and luminal
Wistar rats (male HdrBrHan from Harlan Winkelmann,
area of the glandular ducts were measured morphometri-
Borchen, Germany), weighing about 150 g (age of 2 weeks)
cally (KS 100, KS RUN, Zeiss-Vision, Jena, Germany).
were separated into different groups by randomized proce-
Microscopy was performed with an Axiophot light micro-
dure. They received tap water and ssniff R 10, laboratory stan-
scope (Zeiss, Jena, Germany) equipped with a high resolution
dard rat diet (in pellet form) ad libitum (ssniff Spezialdi¨aten
scanner camera (Axiocam, Zeiss, Germany).
GmbH, Soest, Germany). Groups of 2–4 animals were kept
All images had a uniform size of 1300 × 1030 pixel. Since
in Makrolon cages type IV with ssniff bedding (3/4 Faser) at
the images were generated by using a 20× objective and 1.0
22 ± 3 ◦C, a relative humidity of 30–70% and artificial 24 h
optovar the final resolution of the edge lengths of one pixel
light. After acclimatization animals were orchiectomized
in the resulting image is 0.32 m. This resolution was large
under Ketanest/Rompun—anesthesia (Ketanest 10 mg/kg bw
enough for deciding which profile of glandular ductus in the
from Parke-Davis, Berlin, Germany and Rompun 2 mg/kg bw
field of vision was suitable for densitometric measurements.
from Bayer AG, Leverkusen, Germany).
Gray values were transformed pixel by pixel into optical den-
2.2. Treatment of animals
Five measurements were performed within each section.
Five sections were examined per animal resulting in 25 mea-
Seven days after orchiectomy animals were partitioned
surements for each animal. The mean values were calculated
into eight groups (n = 13 in each group). They were treated
and compared between the different groups.
p.o. with 3, 50, 200 and 500 mg BPA/kg/day dissolved
For morphometric measurements the software package
in propylene glycol for 7 days. BPA was purchased from
KS 100 3.0 (Zeiss-Vision, Jena, Germany) was used. The
Fa. Bayer (PtNr. 97.001/Prod.Nr. 04111095, CasNr. 80-05,
epithelial height was determined by using a 40× objective
Leverkusen, Germany). Another group of orchiectomized
and 1.0 optovar. For the determination of the luminal area
animals was treated s.c. with testosterone propionate (TP)
a 10× objective and 1.0 optovar was used. The quantitative
1 mg/kg bw in arachis oil.
assessment of proliferating cells was performed using a 40×
combination with 500 mg
objective and 1.0 optovar. One thousand cells per section
sible androgen effects of UNCORRECTED PROOF
Flutamide (FL) 3 mg/kg bw p.o. in
BPA was used to antagonize pos-
the "suprapharmacological" dose
were counted excluding those which due to the section did
of BPA. These groups were compared to vehicle (propylene
not show a nucleus to avoid overestimation of the total cell
glycol) treated, orchiectomized rats without any other sub-
number. A two-sided t-test at a significance level of p < 0.05

T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
Fig. 1. Comparison of absolute wet weights of the whole prostate in (mg)
Fig. 2. Comparison of body weights in (g) between the Intact group, orchiec-
between the Intact group, OX group and TP group in relation to the BPA-
tomized group (OX) and TP group in relation to the BPA-treated groups (3,
treated groups (3, 50, 200, 500 and 500 + FL). Asterisks indicate statistically
50, 200, 500 and 500 + FL). Asterisks indicate statistically significant dif-
significant differences (p < 0.05), which refer to the castrated control group.
ferences (p < 0.05), which refer to the castrated control group.
was applied for statistical comparison. Data were depicted as
500 + FL) for seminal vesicles, respectively. In the prostate
mean ± standard deviation (S.D.).
tissue the intensity of staining was significantly higher in both
the Intact and TP group (t-test, p < 0.05, n = 13) compared
to the OX group. BPA at lower doses (3 and 50 mg/kg bw)
3. Results
increased AR immunoreactivity and staining intensity of
prostate tissue, but reduced them at higher doses (200 and
3.1. Hershberger assay
500 mg/kg bw). In seminal vesicles, the intensity of AR stain-
ing was reduced by orchiectomy in comparison with the Intact
In contrast to TP, BPA induced no effect on absolute
control. The treatment of orchiectomized animals with BPA
weights of prostate (and seminal vesicle (not shown).
showed no dose-dependent effects (not shown).
BPA at high doses of 200 and 500 mg/kg bw caused a decrease
Using the anti-androgen-receptor monoclonal antibody
in body weights and a significant increase in relative
(1:100, 554224, mouse monoclonal, Novocastra, New cas-
weights of prostate and seminal vesicle (not shown). A simul-
tle, United Kingdom) the staining intensity of AR in the
taneous administration of FL had no further effect in BPA
prostates revealed the following optical density values:
treated animals. Animals treated with 200, 500 and 500 + FL
104 ± 20 (Intact), 56 ± 17 (OX), 77 ± 22 (TP), 99 ± 7 (BPA
BPA showed severe signs of gastro-intestinal toxicity.
3), 103 ± 23 (BPA 50), 78 ± 16 (BPA 200), 49 ± 23 (BPA
500) and 36 ± 6 (BPA 500 + FL). Statistical analysis con-
3.2. Densitometric analysis
firmed that the intensity of staining was significantly higher
in both the intact and TP group (t-test, p < 0.05, n = 13)
The staining intensity after incubation with the poly-
compared to the OX group (Thus, the
clonal antibody (1:100, sc-815, rabbit polyclonal, Santa Cruz
data obtained for both antibodies used provide evidence that
Biotechnology, CA, USA) directed against AR was 75 ± 21
orchiectomy results in a reduced staining intensity of AR,
(Intact), 43 ± 12 (OX), 61
whereas substitution with TP enhances the immunoreactive
57 ± 19 (BPA 50), 48 ± 18
signal of AR. The intensity of staining was significantly
33 ± 10 (BPA 500*FL) for
UNCORRECTED PROOF
± 24 (TP), 65 ± 27 (BPA 3),
(BPA 200), 39 ± 9 (BPA 500) andthe prostates and 42 ± 17 (Intact),
increased in prostate after treatment with lower doses of
11 ± 10 (OX), 19 ± 11 (TP), 8 ± 5 (BPA 3), 11 ± 15 (BPA
BPA (3 and 50 mg/kg bw). At 500 mg/kg bw staining inten-
50), 7 ± 3 (BPA 200), 3 ± 1 (BPA 500) and 15 ± 17 (BPA
sity was similar to the castrated control, but the combina-

T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
to the Intact and TP group (Whereas
orchiectomy caused a considerable decrease of cell prolif-
eration, administration of TP could reverse this effect and
induced a cell proliferation index similar to the Intact group.
The assessment of both proliferation markers revealed that
BPA showed at all doses tested no stimulation of proliferat-
ing activity in prostate.
3.4. Morphometry
The mean epithelial height of prostate glands measured
17 ± 2 m in both the Intact and TP group, whereas the mean
epithelial height was significantly (t-test, p < 0.05, n = 13)
decreased to 11 ± 1 m in the OX group. BPA treated groups
displayed following data: 14 ± 2 m (BPA 3), 14 ± 2 m
(BPA 50), 10 ± 1 m (BPA 200), 9 ± 1 m (BPA 500) and
8 ± 1 m (BPA 500 + FL). Similar significant (t-test, p < 0.05,
n = 13) data were obtained for the epithelial height of sem-
inal vesicle glands: 18 ± 3 m (Intact), 9 ± 2 m (OX) and
15 ± 2 m (TP), 11 ± 1 m (BPA 3), 11 ± 1 m (BPA 50),
8 ± 1 m (BPA 200), 8 ± 1 m (BPA 500) and 8 ± 1 m
(BPA 500 + FL), respectively
Fig. 3. Densitometric values of the Intact group, orchiectomized group
The mean luminal area of prostate glands was
(OX), TP group and the BPA-treated groups (3, 50, 200, 500 and 500 + FL)
131 000 ± 40 000 m2 in the Intact group, 7000 ± 5000 m2
after immunohistochemical staining (monoclonal antibody) of the androgen
in the OX group and 87 000 ± 30 000 m2 in the TP
receptor in the prostate. Asterisks indicate statistically significant differences
group. BPA treated groups showed following data:
(p < 0.05), which refer to the castrated control group.
23 000 ± 16 000 m2 (BPA 3), 25 000 ± 12 000 m2 (BPA
50), 15 000 ± 10 000 m2 (BPA 200), 4000 ± 500 m2 (BPA
tion of BPA (500 mg/kg bw) with FL significantly reduced
500) and 4000 ± 3000 m2 (BPA 500 + FL). The luminal
the staining intensity Absolute organ
area in the OX group was significantly (t-test, p < 0.05, n = 13)
weights (prostate, seminal vesicle) at 200 and 500 mg/kg/day
reduced compared to the Intact and TP group. In seminal vesi-
(40/100 fold the NOAEL (no-observed-adverse-effect-level))
cles the mean luminal area measured 113 000 ± 80 000 m2
were not significantly altered (The NOAEL is
(Intact), 7000 ± 5000 m2 (OX) and 151 000 ± 86 000 m2
the greatest concentration or amount of a substance e.g.
(TP), 35 000 ± 43 000 m2 (BPA 3), 27 000 ± 20 000 m2
BPA, found by experiment or observation, which causes
(BPA 50), 8000 ± 3000 m2 (BPA 200), 4000 ± 1000 m2
no detectable adverse alteration of morphology, functional
(BPA 500) and 3000 ± 2000 m2 (BPA 500 + FL), respec-
capacity, growth, development, or life span of the target
tively. Similarly as observed for the prostates the luminal
organism under defined conditions of exposure.
area in the OX group was significantly (t-test, p < 0.05, n = 13)
reduced compared to the Intact and TP group (
3.3. Cell proliferation
These morphologic observations clearly reveal that
orchiectomy causes a substantial reduction of both epithe-
The assessment of cell proliferation markers yielded the
lial height and luminal area of the prostate gland and seminal
following data in rat prostates: The percentage of immunore-
vesicles. If TP is substituted both parameters return to values
active epithelial cells for MIB-5 was 85 ± 9% in the Intact
similar to those found in the Intact group. Lower doses of
group, 9 ± 1% in the OX group and 90 ± 2% in the TP
BPA caused an increase in epithelial height and luminal area
group. BPA treated groups displayed: 10 ± 1% (BPA 3),
of prostate and seminal vesicle, while high doses reduced the
8 ± 1% (BPA 50), 2% (BPA 200), 1% (BPA 500) and 1%
epithelial height of prostate significantly in comparison to the
(BPA 500 + FL). The percentage of immunoreactive epithe-
lial cells for MIB-5 in the OX group was significantly (t-test,
p < 0.05, n = 13) reduced compared to the intact and TP group
Similar results were obtained for the rela-
tive amount of cells immunoreactive for PCNA: 90 ± 9% in
the Intact group, 10 ± 2%
4.1. Methodologic approaches
TP group, 2% (BPA 3), 4%
(BPA 500) and 6 ± 1%
UNCORRECTED PROOF
in the OX group and 88 ± 9% in the
(BPA 50), 2% (BPA 200), 5 ± 1%
(BPA 500 + FL). The percentage of
4.1.1. Hershberger assay
epithelial cells immunoreactive for PCNA in the OX group
Although the Hershberger assay is a valid quantitative
was significantly (t-test, p < 0.05, n = 13) reduced compared
method for evaluating androgenic or anti-androgenic proper-

T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
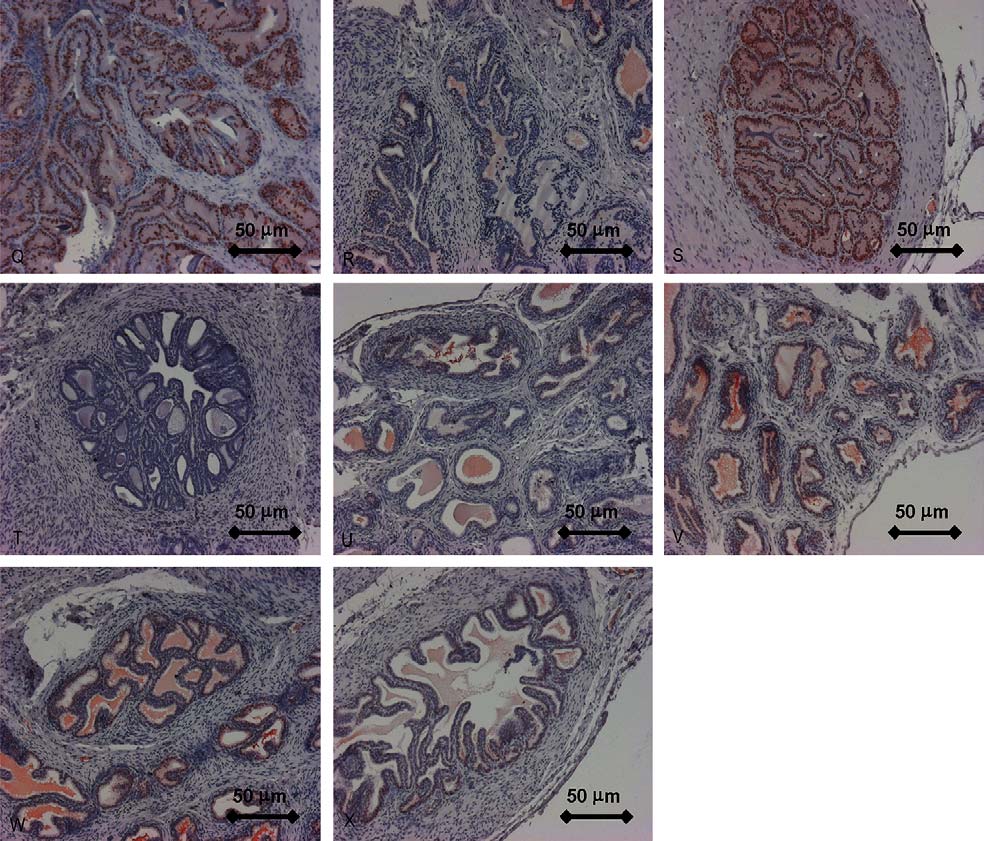
Fig. 4. All panels show
UNCORRECTED PROOF
photographs of the prostate. Zones are described according to McNeal Immunohistochemical staining of androgen receptor
(monoclonal antibody) showing the transition zone of the Intact group (A), OX group (B) and TP group (C) in relation to the BPA-treated groups (3, 50, 200,500 and 500 + FL). Original magnification 20×. I–P: Immunohistochemical staining of MIB-5 showing the peripheral zone of the Intact group (I), OX group(J) and TP group (K) in relation to the BPA-treated groups (3, 50, 200, 500 and 500 + FL). Original magnification 10×. Q–X: Immunohistochemical staining ofPCNA showing the transition zone of the intact group (Q), OX group (R) and TP group (S) in relation to the BPA-treated groups (3, 50, 200, 500 and 500 + FL).
Original magnification 10×.

T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
Fig. 4. (Continued ).
ties of substances by measuring the organ weight of seminal
advantages of a computer-assisted densitometry are a faster
vesicles and prostates, the findings obtained by this assay
scoring procedure of sections from large series and a higher
provide only limited information on the specificity of the
reliability. However, the disadvantage of a semiquantitative
observed effects when only the reactions of the organ weights
approach is the possibility that relevant signals can easily be
are judged: For example, the growth of seminal vesicles can
missed, so that comparative studies should be based on rather
be stimulated not only by androgens but also by estrogenic
substances, well known as a paradoxical effect of estrogens
4.3. Influence of BPA
Morphologic and functional analysis of cellular param-
eters in male accessory organs may allow a more subtle
In this study lower doses of BPA (3 and 50 mg/kg bw/day)
and reliable assessment of the (anti-) androgenicity of sub-
were found to cause an enhancement in staining intensity of
stances; in previous studies we analyzed the regulation of
AR in rat prostate. Morphometric data showed that lower
tenascin expression Since the amount of nuclear AR
doses of BPA cause an increase in epithelial height and lumi-
present in the rat prostate has been demonstrated to be influ-
nal area of prostate and seminal vesicle, while high doses sig-
enced by androgens densitometric analysis of AR-
nificantly reduce the epithelial height of prostate. These find-
immunoreactive cells in prostates and seminal vesicles was
ings are similar to those observed in testosterone propionate
performed by using immunohistochemical methods
substituted castrated rats. Gupta androgen-like
effects of BPA in pregnant CD-1 mice at 0.05 mg/kg/day by
4.2. Densitometric analysis
observing an enhancement of the anogenital distance and the
size of the prostate in fetuses. The androgen receptor (AR)
It has been previously described that the concentrations
binding affinity in prostate of fetuses was also increased sig-
of biochemically active substances can be estimated from
nificantly. In contrast, Kim et al. did not found any androgenic
the optical density of the
or anti-androgenic activities of BPA in Hershberger assay at
a result, we found a mark
10–1000 mg/kg/day
AR-positive cells after
UNCORRECTED PROOF
immunoreactive signal sed decrease in staining intensity of
orchiectomy in comparison to the con-
BPA at all doses tested exerted no significant effects on
trol group. This effect of orchiectomy was mostly reversed
absolute weights of prostate and seminal vesicle. High doses
by an administration of a pharmacological dose of TP. The
of BPA (200 and 500 mg/kg bw/day) caused a significant

T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
Fig. 5. Quantitative comparison of (a) MIB-5-immunoreactive and (b)PCNA-immunoreactive epithelial prostatic cells between the Intact group,
Fig. 6. Comparison of epithelial height of (a) prostate and (b) seminal vesicle
OX group and TP group in relation to the BPA-treated groups (3, 50, 200,
between the Intact group, OX group and TP group in relation to the BPA-
500 and 500 + FL). Asterisks indicate statistically significant differences
treated groups (3, 50, 200, 500 and 500 + FL). Asterisks indicate statistically
(p < 0.05), which refer to the
significant differences (p < 0.05), which refer to the castrated control group.
than to an androgenic effect of this substance. An oral pre-
These effects of BPA may
UNCORRECTED PROOF
castrated control group.
increase in relative weights of prostates and seminal vesicles.
be due rather to a toxicity-related
dictable no effect concentration (PNECoral) of 33 mg/kg food
significant decrease in body weights and well known gen-
has been derived for the secondary poisoning assessment
eral side effects, e.g. loss of appetite and diarrhea
from a NOAEL of 50 mg/kg bw (based on a reduction in litter
T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
cell proliferation in epithelial prostatic cells, but effects on
androgen receptor immunoreactivity and epithelial height of
prostate and seminal vesicles, glandular area of prostates and
seminal vesicles was observed. Overall, in standard devel-
opmental studies in rodents, there is no convincing evidence
that BPA is a developmental toxicant
The estrogenic activity of BPA has been mostly observed
in higher doses up to 100 mg/kg bw/day mech-
anism concerning the androgen-like effect of the low-doses
of BPA on the epithelial cells of the prostate is not clear at
the present time. It may be difficult to correlate this BPA-
effect with the known estrogenic property of this compound
BPA had almost no effect on the seminal vesi-
cle. Estrogens have been known to stimulate the development
of the fibrous tissue and muscular walls of both prostate and
seminal vesicle stimulating the epithelium and
secretory activity Moreover, it seems very improbable,
that the increase of the immunoreactive AR in the prostatic
epithelial cells (not in the seminal vesicle) is up-regulated
by estrogens. It is also unlikely that BPA, via an activation of
adrenal androgen synthesis (the production of corticosteroid-
binding globulin CBG and an activation of adrenal functions,
leads to an increased steroidogenesis including androgen pro-
duction), stimulates the prostate selectively, although estro-
gens have been considered to be one of the controllers of
adrenal androgen secretion Further studies on the low-
dose effects of BPA using an antiandrogen may be necessary
to elucidate the selective effects on the prostate in the rat.
Based on the present data, the densitometric analysis of
AR-immunoreactivity and the assessment of both cell mor-
phology and cell proliferation proved to be independent and
sensitive parameters for the evaluation of androgen effects
on prostates and seminal vesicles. The combined application
of these parameters may provide an additional tool to test
the broad spectrum of endocrine active substances such as
endocrine disruptors, which are actually discussed on their
potential risk to the environment and humans.
The authors wish to thank the director of the Institute of
Anatomy (Univ. Prof. Dr. med. J. Westermann) for his gen-
erous support. We gratefully acknowledge Mrs. H. Strauch-
mann, Mrs. K. Budler, Mrs. L. Gutjahr, and Mrs. U. M¨uller-
Horn for their excellent technical assistance.
Fig. 7. Comparison of luminal glandular area of (a) prostate (m2) and (b)seminal vesicle (m2) between the Intact group, OX group and TP groupin relation to the BPA-treated groups (3, 50, 200, 500 and 500 + FL). Aster-
isks indicate statistically significant differences (p < 0.05), which refer to thecastrated control group.
[1] T. Zincke, Mittheilungen aus dem chemischen Laboratorium der Uni-
versitat Marburg, Justus Liebigs Annalen Chemie 343 (1905) 75–99.
[2] S. Krimski, Hormonal Chaos, The Johns Hopkins University Press,
Baltimore-London, 2000, 1–54.
UNCORRECTED PROOF
three-generation multi-dose level feeding study
represents the concentration below
[3] J. Ashby, J. Odum, D. Paton, P.A. Lefevre, N. Beresford, J.P.
which an unacceptable risk should not occur. At 3 and 50 mg
Sumpter, Re-evaluation of the first synthetic estrogen, 1-keto-1,2,3,4-
BPA/kg bw/day no effect on body weight, organ weight,
tetrahydrophenantrene, and bisphenol A, using both the ovariec-
T. Nishino et al. / Journal of Steroid Biochemistry & Molecular Biology xxx (2005) xxx–xxx
tomized rat model used in 1933 and additional assays, Toxicol. Lett.
[18] S.E. De Jongh, Paradoxe Wirkungen von Follikelhormon (Menfor-
115 (2000) 231–238.
mon) bei m¨annlichen Tieren; ihre Beeinflussbarkeit durch m¨annliches
[4] F. Paris, P. Balaguer, B. Terouanne, N. Servant, C. Lacoste,
Hormon, Arch. Int. Pharmacodyn. 50 (1935) 348–355.
J.P. Cravedi, J.C. Nicolas, C. Sultan, Phenylphenols, biphenols,
[19] S.E. De Jongh, De beinvloeding van het mannelijk genitaal apparaat
bisphenol-A and 4-tert-octylphenol exhibit alpha and beta estrogen
door hormonen, Hormoon VII (1937) 42–51.
activities and antiandrogen activity in reporter cell lines, Mol. Cell
[20] J. Freud, Conditions of hypertrophy of the seminal vesicles in rats,
Endocrinol. 193 (2002) 43–49.
Biochem. J. 27 (1933) 1438–1445.
[5] S.C. Nagel, F.S. vom Saal, K.A. Thayer, M.G. Dhar, M. Boech-
[21] G. Vollmer, H. Michna, K. Ebert, R. Knuppen, Androgen ablation
ler, W.V. Welshons, Relative binding affinity-serum modified access
induces tenascin expression in the rat prostate, Prostate 25 (1994)
(RBA-SMA) assay predicts the relative in vivo bioactivity of the
xenoestrogens bisphenol A and octylphenol, Environ. Health Per-
[22] R.J. Moore, J.M. Gazak, J.D. Wilson, Regulation of cytoplasmic
spect. 105 (1997) 10–16.
dihydrotestosterone binding in dog prostate by 17-estradiol, J. Clin.
[6] H.S. Kim, S.Y. Han, S.D. Yoo, B.M. Lee, K.L. Park, Potential
Invest. 63 (1979) 351–357.
estrogenic effects of bisphenol-A estimated by in vitro and in vivo
[23] J.P. Blondeau, E.E. Baulieu, P. Robel, Androgen-dependent reg-
combination assays, J. Toxicol. Sci. 26 (2001) 111–118.
ulation of androgen nuclear receptor in the rat ventral prostate,
[7] T. Takao, W. Nanamiya, I. Nagano, K. Asaba, K. Kawabata, K.
Endocrinology 110 (1982) 1926–1932.
Hashimoto, Exposure with the environmental estrogen bisphenol A
[24] C. Huggins, C.V. Hodges, Studies on prostatic cancer. I. The effect
disrupts the male reproductive tract in young mice, Life Sci. 65
of castration, of estrogen and of androgen injection on serum phos-
(1999) 2351–2357.
phatases in metastatic carcinoma of the prostate, J. Urol. 167 (2002)
[8] W.V. Welshons, S.C. Nagel, K.A. Thayer, B.M. Judy, F.S. vom Saal,
Low-dose bioactivity of xenoestrogens in animals: fetal exposure to
[25] C. Rolf, E. Nieschlag, Potential adverse effects of long-term
low doses of methoxychlor and other xenoestrogens increases adult
testosterone therapy, Baillieres Clin. Endocrinol. Metab. 12 (1998)
prostate size in mice, Toxicol. Ind. Health 15 (1999) 12–25.
[9] F.S. vom Saal, P.S. Cooke, D.L. Buchanan, P. Palanza, K.A. Thayer,
[26] L.B. Nabors, E. Songu-Mize, R.R. Mize, Quantitative immunocy-
S.C. Nagel, S. Parmigiani, W.V. Welshons, A physiologically based
tochemistry using an image analyzer. II. Concentration standards
approach to the study of bisphenol A and other estrogenic chemi-
for transmitter immunocytochemistry, J. Neurosci. Methods 26 (1)
cals on the size of reproductive organs, daily sperm production and
(1988) 25–34.
behaviour, Toxicol. Ind. Health 14 (1998) 239–260.
[27] R.R. Mize, R.N. Holdefer, L.B. Nabors, Quantitative immunocyto-
[10] P.L. Palanza, K.L. Howdeshell, S. Parmigiani, F.S. vom Saal, Expo-
chemistry using an image analyzer. I. Hardware evaluation, image
sure to a low dose of bisphenol A during fetal life or in adulthood
processing and data analysis, J. Neurosci. Methods 26 (1) (1988)
alters maternal behavior in mice, Environ. Health Perspect. 110
(2002) 415–422.
[28] W.I. De Boer, P.S. Hiemstra, J.K. Sont, E. De Heer, K.F. Rabe,
[11] K.L. Howdeshell, A.K. Hotchkiss, K.A. Thayer, J.G. Vandenbergh,
J.H.J.M. Van Krieken, P.J. Sterk, Image analysis and quantification
F.S. vom Saal, Environmental toxins: exposure to bisphenol A
in lung tissue, Clin. Exp. Allergy 31 (2001) 504–508.
advances puberty, Nature 401 (1999) 763–764.
[29] R.E. Morissey, J.D. George, C.J. Price, R.W. Tyl, M.C. Marr, C.A.
[12] P. Diel, T. Schulz, K. Smolnikar, E. Strunck, G. Vollmer, H.
Kimmel, The developmental toxicity of bisphenol A in rats and mice,
Michna, Ability of xeno- and phytoestrogens to modulate expres-
Fundam. Appl. Toxicol. 8 (1987) 571–582.
sion of estrogen-sensitive genes in rat uterus: estrogenicity profiles
[30] K. Yamasaki, M. Sawaki, S. Noda, N. Imatanaka, M. Takatsuki,
and uterotrophic activity, J. Steroid Biochem. Mol. Biol. 73 (2000)
Subacute oral toxicity study of ethynylestradiol and bisphenol A,
based on the draft protocol for the "Enhanced OECD Test Guideline
[13] C.M. Markey, C.L. Michaelson, E.C. Veson, C. Sonnenschein, A.M.
no. 407", Arch. Toxicol. 76 (2002) 65–74.
Soto, The mouse uterotrophic assay: a reevaluation of its validity in
[31] R.W. Tyl, C.B. Myers, M.C. Marr, B.F. Thomas, A.R. Keimowitz,
assessing the estrogenicity of bisphenol A, Environ. Health Perspect.
D.R. Brine, M.M. Veselica, P.A. Fail, T.Y. Chang, J.C. Seely, R.L.
109 (2001) 55–60.
Joiner, J.H. Butala, S.S. Dimond, S.Z. Cagen, R.N. Shiotsuka, G.D.
[14] H. Tinwell, R. Joiner, I. Pate, A. Soames, J. Foster, J. Ashby,
Stropp, J.M. Waechter, Three-generation reproductive toxicity study
Uterotrophic activity of bisphenol A in the immature mouse, Regul.
of dietary bisphenol A in CD Sprague-Dawley rats, Toxicol. Sci. 68
Toxicol. Pharmacol. 32 (2000) 118–126.
(2002) 121–146.
[15] C. Gupta, Reproductive malformation of the male offspring following
[32] European Chemicals Bureau: EU-Risk-Assessment Summary Vol. 37
maternal exposure to estrogenic chemicals (44516), Proc. Soc. Exp.
(2003) on 4,4'-isopropylidenephenol (bisphenol-A), CAS#: 80-05-7,
Biol. Med. 224 (2000) 61–68.
EINECS#: 201-245-8.
[16] H. Kim, S. Han, T. Kim, S. Kwack, R. Lee, I. Kim, J. Seok, B. Lee,
[33] K. David, J. Freud, S.E. de Jongh, Conditions of hypertrophy of
S. Yoo, K. Park, No androgenic/anti-androgenic effects of bisphenol-
seminal vesicle in rats II. The effect of derivatives of oestrone (Men-
A in Hershberger assay using immature castrated rats, Toxicol. Lett.
formon), Biochem. J. 28 (1934) 1360–1367.
5 (135) (2002) 111, 1–2.
[34] W.D. Odell, L.N. Parker, Control of adrenal androgen production,
[17] M. Oberholzer, M. Oestreicher, H. Christen, M. Bruehlmann, Meth-
Endocr. Res. 10 (1984) 617–630.
ods in quantitative image analysis, Histochem. Cell Biol. 105 (1996)
[35] J.E. McNeal, Normal histology of the prostate, Am. J. Surg. Pathol.
12 (1988) 619–633, Publication I.03.149.
UNCORRECTED PROOF
Source: http://neuroviisas.med.uni-rostock.de/publications/Nishino-2005.pdf
Marketing Strategy tiasnimbas business school nyenrode business universiteit C H A P T E R 2 You don't win silver.You lose gold.Nike1 DEFINING THE BUSINESS To assess where and how companies compete in the present day, the marketer must analyse the internal and external environments of the company. The most important of these analyses revolve around the customer; the customer value proposition; the business model; and the industry and macro environments in which the company competes. Following Abell (1980), we agree that defining the business is the true start-ing point of strategic market planning.2 In Abell's perspective, a business is defined in terms of three different dimensions: the customer groups a business unit serves; the functions its offering fulfills for these customer groups; and the technologies that are deployed to realise these functions. Abell argues:
To discuss any of the information contained in this guide, or further assistance, please call the Myeloma Support Line on 1800 MYELOMA (1800 693 566) A Myeloma Support Nurse will answer your call in confidence Publication date: August 2014 toll free: 1300 632 100 myeloma support line: 1800 693 566 Myeloma Australia PO Box 5017, Burnley, Vic 3121












