
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Doi:10.1016/j.jaci.2006.06.036
Original articles
Salmeterol response is not affected byb2-adrenergic receptor genotype in subjects
with persistent asthma
Eugene R. Bleecker, MD,a Steven W. Yancey, MS,b Leslie A. Baitinger, MS,b
Lisa D. Edwards, PhD,b Michael Klotsman, PhD,b Wayne H. Anderson, PhD,b
and Paul M. Dorinsky, MDb Winston-Salem and Research Triangle Park, NC
Background: Recent studies suggest that there might be an
Conclusion: Response to salmeterol does not vary between
association between albuterol use and worsening asthma in
ADRB2 genotypes after chronic dosing with an inhaled
patients homozygous for arginine (Arg/Arg) at codon 16 of the
b-receptor. However, it is not known whether similar responses
Clinical implications: Analyses from this study indicate that
occur in Arg/Arg patients receiving long-acting b2-agonists.
genetic polymorphisms leading to Arg16Gly sequence changes
Objective: We sought to evaluate the effects of variation in the
within the b2-adrenergic receptor do not affect patients'
b2-adrenergic receptor gene (ADRB2) on clinical response to
responses to recommended asthma therapy with salmeterol
salmeterol administered with fluticasone propionate.
and fluticasone propionate. (J Allergy Clin Immunol
Methods: Subjects (n 5 183) currently receiving short-acting
b2-agonists were randomized to twice-daily therapy withsalmeterol, 50 mg, administered with fluticasone propionate,
Key words: Asthma, salmeterol xinafoate, genotype, polymorphism
100 mg, in a single inhaler or daily therapy with montelukast
(genetics), receptors, b2, adrenergic, fluticasone propionate
for 12 weeks, followed by a 2- to 4-day run-out period.
Results: There was sustained and significant improvement (P <.001) over baseline in all measures of asthma control in subjects
Genetic studies in asthma have led to advances in our
receiving salmeterol, regardless of Arg16Gly genotype.
understanding of the association between gene variants
Morning peak expiratory flow in subjects with the Arg/Arg
and the responses to specific asthma treatments. Recent
genotype showed 89.0 6 16.1 L/min improvement over baseline
studies have revealed the presence of single nucleotide
compared with 93.7 6 12.7 L/min for Gly/Gly subjects and
polymorphisms (SNPs) within ADRB2, the gene coding
92.5 6 11.9 L/min for Arg/Gly subjects. Pairwise changes were
for the b2-adrenergic receptor (b2-AR). Some ADRB2
similar for Arg/Arg compared with Gly/Gly or Arg/Gly
SNPs result in changes within the amino acid sequence
genotypes (estimated differences, 4.7 L/min and 3.5 L/min,
of the b2-AR, leading to alterations of its properties, pos-
respectively). Responses did not appear to be modified by
sibly associated with various asthma-related phenotypes,
haplotype pairs. During the run-out period, all subjects had
including lower pulmonary function and altered broncho-
predictable and similar decreases in measures of asthmacontrol, with no differences between genotypes.
dilator reversibility to short-acting b2-adrenergic Variability in response, attributed to SNPs associated withasthma severity or altered b-agonist pharmacology, mighthave important implications for asthma clinical therapyand could define subgroups of asthmatic patients with
From aWake Forest University School of Medicine, Winston-Salem; and
bGlaxoSmithKline, Research Triangle Park.
The b2-AR is a 413-amino-acid G protein–coupled
Disclosure of potential conflict of interest: E. R. Bleecker has received grant
support from Altana, AstraZeneca, Boehringer-Ingelheim, Centocor,
receptor encoded for by an intronless gene (ADRB2) lo-
Genentech, GlaxoSmithKline, and Novartis; has consultant arrangements
cated on chromosome Variation in the activity
with Aerovance, Altana, AstraZeneca, Centocor, Critical Therapeutics,
of this cell-surface receptor mediates the effects of b2-
Genentech, GlaxoSmithKline, and Novartis; and is on the speakers' bureau
adrenergic agonists on a number of important cellular
for AstraZeneca, GlaxoSmithKline, Genentech, and Merck. M. Klotsman
responses.Screens of ADRB2 have revealed at least 19
and S. W. Yancey are both employed by and own stock in GlaxoSmithKline.
W. H. Anderson, P. M. Dorinsky, L. A. Baitinger, and L. D. Edwards all are
SNPs within the coding and promoter region, some of
employed by GlaxoSmithKline.
which might influence response to b2-agonists.The
Received for publication December 1, 2005; revised June 25, 2006; accepted
most prominent coding SNP is characterized by substitu-
for publication June 27, 2006.
tion of glycine for arginine at codon 16 (Arg16Gly), which
Available online August 29, 2006.
Reprint requests: Eugene R. Bleecker, MD, Pulmonary, Critical Care, Allergy
occurs commonly in the general population (minor allele
and Immunologic Diseases, Center for Human Genomics, Wake Forest
frequency in approximately one sixth of the general
University School of Medicine, Medical Center Blvd, Winston-Salem,
asthma population and about one fourth of the African
NC 27157. E-mail:
American In vitro studies have shown
that the Gly-16 receptor exhibits enhanced downregulation
Ó 2006 American Academy of Allergy, Asthma and Immunologydoi:10.1016/j.jaci.2006.06.036
after b-agonist exposure, but the data remain
J ALLERGY CLIN IMMUNOL
810 Bleecker et al
over a 12-week period and to evaluate clinical asthma
Abbreviations used
stability during withdrawal of therapy.
ADRB2: b2-Adrenergic receptor gene
b2-AR: b2-Adrenergic receptor
BAGS: Beta-agonist Genotype Study
BARGE: Beta Adrenergic Response by Genotype
BUP: b-upstream peptide
FSC: Fluticasone propionate/salmeterol
Data from 2 identical studies, with results published in 2001 and
HWE: Hardy-Weinberg equilibrium
2002, in which DNA was collected were used for these analy-
ICS: Inhaled corticosteroid
ses.The replicate trials were designed to evaluate the efficacy
LABA: Long-acting b
and safety of fluticasone propionate/salmeterol (FSC), 100/50 mg
PEF: Peak expiratory flow
Diskus (GlaxoSmithKline, Research Triangle Park, NC), in
SABA: Short-acting b
adolescent and adult subjects whose asthma was inadequately con-
SLIC: Salmeterol 6 ICSs
trolled with SABAs alone. Subjects were eligible for inclusion if
SNP: Single nucleotide polymorphism
they were 15 years of age or older and had a history of persistent
SOCS: Salmeterol or Corticosteroids
asthma of at least 6 months. All subjects were required to have anFEV1 of between 50% and 80% of predicted value and demonstrate15% or greater reversibility within 30 minutes after 2 puffs (180 mg)of albuterol (Ventolin; GlaxoSmithKline). Spirometry was per-formed according to the American Thoracic Society published
Data analyses of clinical trials suggest that SNPs can
guidelines.Before study enrollment, the aims of these clinical
influence the response to both short-acting b2-agonists
trials and pharmacogenetic analyses were fully explained, and in-
(SABAs) and long-acting b2-agonists
formed consent was obtained from each participant. The study pro-
However, variations in study designs and differences in
tocols were reviewed and approved by the appropriate institutional
sample size have led to inconclusive results.For exam-
review boards.
ple, some studies have reported no change in broncho-dilator response to either SABAsor in
asthmatic subjects with varying b
Eligible subjects entered a 2-week run-in period during which all
2-AR genotypes. In con-
trast, results from the Beta-agonist Genotype Study
participants replaced their oral or inhaled SABAs with albuterol
(BAGS) reported by Israel et showed that B16-Arg/
prescribed as needed for the relief of acute asthma symptoms. PEF,
Arg homozygous subjects had a small decrease in morning
albuterol use, asthma symptoms, and nighttime awakenings wererecorded daily by the subjects on a diary card. After the run-in period,
peak expiratory flow (PEF) when receiving regular albu-
participants meeting randomization criteria (defined as the best FEV
terol, but no change was observed with albuterol used
of between 50% and 80% of predicted value but within 615% of the
on an as-needed basis. Taylor et have extended these
best predose FEV1 obtained at screening and 5 or more days requiring
observations in ADRB2 variations in pulmonary function
albuterol use or a diary card asthma symptom score of
�2 on 3 or
showing that Arg/Arg homozygous subjects receiving
more days by using a 6-point scale [0 5 no symptoms, 5 5 severe
albuterol had a decrease in PEF, as well as an increase in
symptoms] during the previous week) entered the double-blind phase
exacerbations. Interestingly, this effect was not found in
of the study and were randomized to receive one of the following
Arg/Arg homozygous subjects receiving salmeterol.
treatments for a 12-week period: FSC, 100/50 mg Diskus twice daily
More recently, the Beta Adrenergic Response by Geno-
plus placebo montelukast once daily, or oral montelukast, 10 mg once
type (BARGE) study from the Asthma Clinical Research
daily plus placebo Diskus twice daily. The current analyses wererestricted to those participants randomized to therapy with FSC.
Network has shown, using a prospective study design,
Information on the participant's race/ethnicity, medical condition
that Arg/Arg homozygous subjects have reduced peak
and treatment, medical history, and family medical history was
flow and associated responses over time compared with
collected and recorded according to a standardized protocol. Baseline
Gly/Gly homozygous In addition, a recent
data for PEF, albuterol use, asthma symptoms, and nighttime
retrospective analysis of 2 Asthma Clinical Research
awakenings was defined as the mean value over the 7 days before
Network clinical trials has shown decreased pulmo-
randomization. Baseline FEV1 was defined as the randomization visit
nary function with salmeterol in Arg/Arg homozygous
FEV1 measurement. During the study, FEV1 was measured at treat-
ment weeks 1, 4, 8, and 12 and 3 days after treatment.
The clinical implications of these findings could be
significant, considering the recent shift toward the use
of LABAs administered with inhaled corticosteroids
Genotyping was performed in a blinded manner at a central
(ICSs) to achieve asthma control in patients with persistent
laboratory (GlaxoSmithKline) using collected blood samples on all
asthma.This approach is recommended by global
subjects for whom a sample was available. Previously characterized
asthma treatment guidelines in patients who remain symp-
coding SNPs in the b2-AR gene (HUGO nomenclature: ADRB2;sequence accession ID: NM_00002found in the b-upstream pep-
tomatic despite low doses of an In the present
tide (BUP) at nucleotide position 247 from the start codon (BUP-
study we analyzed data from 2 large, identical randomized
Cys/Arg) at amino acid positions 16 (Arg16Gly), 27 (Gln27Glu),
trials in which genetic samples were The
and 164 (Thr164Ile) were genotyped.
aims of these analyses were to evaluate the effects of var-
Departures from Hardy-Weinberg equilibrium (HWE) were
iation in ADRB2 on clinical response to the LABA, salme-
assessed by using a x2 goodness-of-fit test. Assuming that each locus
terol, administered with the ICS, fluticasone propionate,
is in HWE, haplotype probabilities were estimated among white
J ALLERGY CLIN IMMUNOL
Bleecker et al 811
VOLUME 118, NUMBER 4
TABLE I. Localization of SNPs and identification of haplotypes of the b2-AR gene (ADRB2) among 296 white subjects
Corresponding Drysdale
TABLE II. Demographic and baseline characteristics of subjects by Arg16Gly genotype
B16-Arg/Arg (n 5 29)
B16-Arg/Gly (n 5 89)
B16-Gly/Gly (n 5 65)
Mean age (y [SD])
Race/ethnicity (n [%])
FEV1 % reversibility (SD)
FEV1 % predicted (SD)
Nighttime awakenings (SD)
Albuterol use, total puffs (SD)
Rescue-free days (SD)
Asthma symptom scores (SD)
Symptom-free days (SD)
participants by using an expectations/maximization
sex, race/ethnicity, FEV1 percent predicted, reversibility, and the ob-
Genetic analyses were performed by using the HelixTree software
served end-of-study value were conducted on the data collected dur-
package (Golden Helix, Bozeman, Mont).
ing the run-out phase. In the case of FEV1, only one posttreatmentobservation was collected; an analysis of covariance was performed
Statistical analysis
to test for differences in FEV1 among genotypes during the run-out
The primary hypothesis is that Arg/Arg homozygotes do not have
phase. In addition, analyses of the effects of haplotype on clinical
differing clinical responses to salmeterol in the presence of an ICS
outcome were performed.
during treatment or when treatment is discontinued from subjectswith the Arg/Gly or Gly/Gly genotype.
All subjects for whom DNA was obtained were included in the
analyses. The original studies were not powered to detect differences
Baseline distributions
among genotypes; rather, the studies were powered to detect clinicaloutcome differences between FSC and montelukast. However, we
Genetic samples were available for 183 (43%) FSC
performed ANOVAs to test for differences among genotypes (Arg/
recipients from the 2 identical studies. The distribution of
Arg, Arg/Gly, and Gly/Gly) and haplotypes in white subjects at
the BUP-Cys/Arg, Arg16Gly, Gln27Glu, and Thr164Ile
baseline (). Evaluation of the effect of genotype on each clin-
genotypes were all found to be in HWE. ADRB2 haplo-
ical parameter (FEV1, morning PEF, albuterol use, and asthma symp-
type frequencies from unphased genotypes were estimated,
tom scores) during the treatment phase was carried out through the
and haplotype pairs were assigned to white individuals.
use of repeated-measures analyses adjusted for age, sex, race/ethnic-
Haplotype frequencies were not estimated in the other
ity, baseline FEV1 percent predicted, and reversibility. SAS PROCMIXED was used to construct least-squares means, their associated
ethnic groups because of the small sample size.
SEs, and CIs by week, assuming the observations were correlated
There were no significant differences in baseline demo-
within each subject. Similar analyses for daily values of morning
graphic, clinical, and pulmonary function characteristics
PEF, albuterol use, and asthma symptom scores adjusted for age,
across the Arg16Gly genotypes at baseline
J ALLERGY CLIN IMMUNOL
812 Bleecker et al
TABLE III. Changes from baseline in the Arg16Gly genotype at the end of 12 weeks of treatment with FSC
Morning PEF (L/min)
Albuterol use (puffs/d)
Asthma symptom score
Arg16Gly genotype
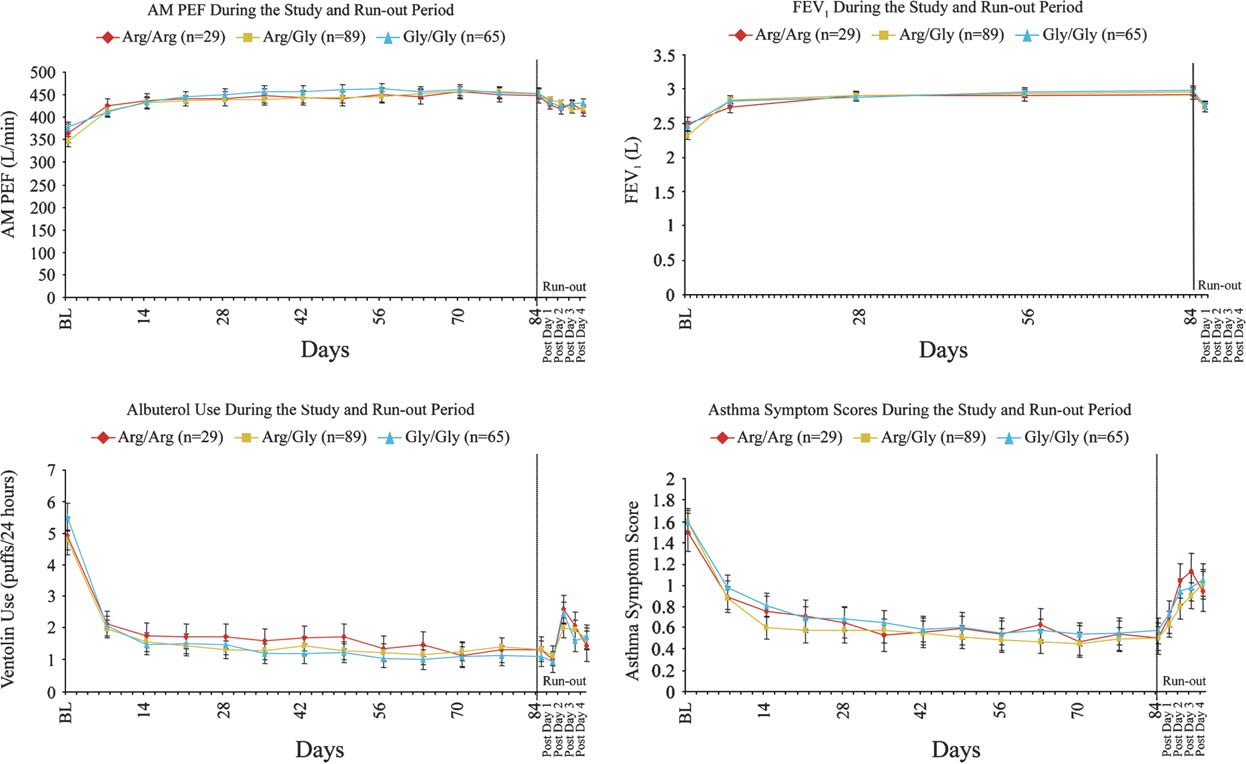
FIG 1. Change from baseline in morning PEF, FEV1, albuterol use, and asthma symptom scores during the12-week treatment period and during the run-out period.
Treatment-phase response to FSC by
not significant on adjustment for multiple comparisons
ADRB2 genotypes and haplotypes
(data not shown).
There was sustained and significant improvement (P <
The clinical responses to FSC were also evaluated with
.001) compared with baseline values in all measures of
respect to other individual ADRB2 SNPs. Pharmacoge-
asthma control in all Arg16Gly genotypes, with no differ-
netic associations between response to FSC and ADRB2
ences observed across genotype subgroups and
BUP-Cys/Arg, Arg16Gly, Gln27Glu, and Thr164Ile gen-
). Specifically, pairwise change from baseline in
otypes and haplotype pairs were evaluated, and neither
morning PEF over the 12 weeks of treatment was similar
measures of pulmonary function, albuterol use, nor asthma
for the Gly/Gly genotype compared with the Arg/Arg
symptom scores were associated with these ADRB2
genotype (estimated difference, 4.7 L/min; P 5 .774)
genotypes With the exception of the rare poly-
and for the Arg/Gly genotype compared with the Arg/Arg
morphism at position 164 and haplotype pairs, no discern-
genotype (estimated difference, 3.5 L/min; P 5 .822;
able associations were observed.
and The treatment responses to FSC for
Because haplotypes might be more informative than
FEV1, albuterol use, and asthma symptoms were also
individual SNPs, associations between haplotype pairs
similar across all genotype subgroups, and no significant
and outcomes were evaluated ). Analysis was
differences across genotype subgroups were observed
restricted to the 4 to 5 most frequently observed haplo-
The results of the montelukast treatment arm
type pairs. No significant associations were identified.
comparison by genotype were similar to those observed
Although it is difficult to definitively rule out a haplotype
in the FSC treatment arm. All observed differences were
effect because of sample size, response to regularly
J ALLERGY CLIN IMMUNOL
Bleecker et al 813
VOLUME 118, NUMBER 4
TABLE IV. Changes from baseline at the end of 12 weeks of treatment with FSC stratified by haplotype
Morning PEF (L/min)
Albuterol use (puffs/d)
Asthma symptom score
*Haplotype pair indicates the haploid genotype pair of each of A, B, and C alleles. For instance, AA indicates an A allele at each of the 2 gene loci on the
chromosome, and AB indicates an A allele at the first locus and a B allele at the second.
P 5 .033 for AB versus CC.
àP 5 .034 for BB versus CC.
TABLE V. Difference between the last 7 days on treatment and the run-out period stratified by genotype forsubjects previously treated with FSC
B16-Arg/Arg (n 5 29)
B16-Arg/Gly (n 5 89)
B16-Gly/Gly (n 5 65)
Morning PEF (L/min)
Total albuterol use
Total asthma symptom score
*No differences between genotypes were noted.
scheduled use of FSC did not appear to be modified
chronic b2-agonist stimulation, morning PEF response
by ADRB2 haplotype pairs. Additional analyses were
for each B16-Arg/Arg subject was examined individually
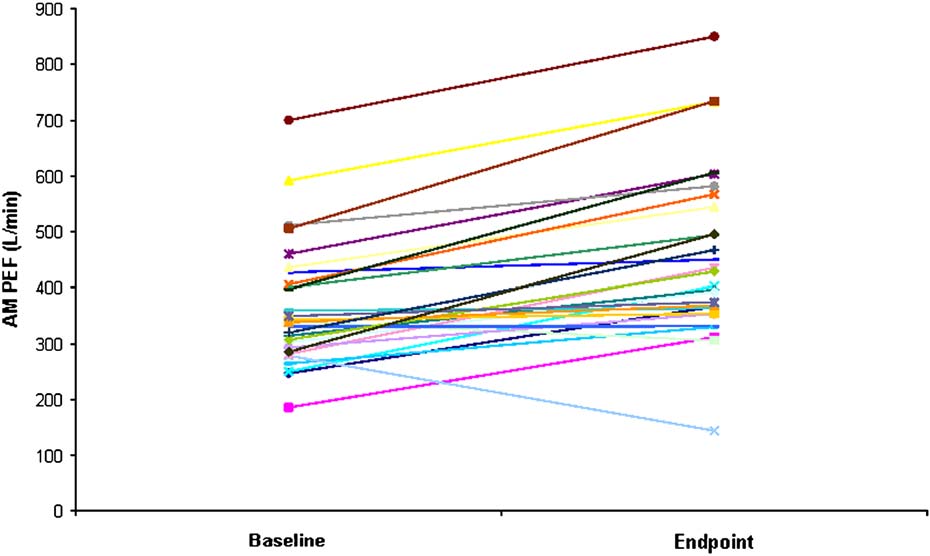
performed by using software for analyzing haplotypes
All but 2 Arg/Arg subjects had improvements over
(haplo.score) for the same phenotypes, and no evidence
baseline at end point in morning PEF, one of whom had
for a significant association was found.
a documented exacerbation on day 71 of the study.
Exacerbations, defined as any event requiring medica-
Furthermore, the responses to treatment with FSC for in-
tion beyond study drug during treatment or the run-out
dividual B16-Gly/Gly and B16-Gly/Arg subjects were
period, were rare. One Arg/Arg subject and 1 Arg/Gly
similar to those of the B16-Arg/Arg subjects (data not
subject experienced an asthma exacerbation during the
treatment phase; no subjects experienced exacerbationsduring the run-out phase.
The number of patients withdrawn was low and
similar across groups. (in this article's OnlineRepository at www.jacionline.org) describes the disposi-
The results of the current study do not indicate a
tion of patients withdrawn during the studies.
differential effect of ADRB2 polymorphisms on responseto FSC therapy in subjects with asthma in this study.
Run-out phase response by genotype
Prior studies suggest that the therapeutic responses to reg-
After 12 weeks of treatment with FSC, treatment was
ularly scheduled therapy with short-acting bronchodila-
discontinued for 2 to 4 days while subjects continued to
tors vary as a result of genetic polymorphisms involving
use albuterol as needed and record daily symptoms and
a variation at the 16th amino acid position of the b2-AR.
PEF on diary cards. Baseline for this run-out period was
A recent report by Wechsler et alanalyzed data from
defined as the average of the last 7 days of the FSC
the Salmeterol or Corticosteroids (SOCS) and Salmeterol
treatment period. During the run-out phase, no differences
6 ICSs (SLIC) trials and concluded that a subset of pa-
were noted in any of the clinical responses to FSC
tients in SLIC with B16-Arg/Arg (n 5 8) do not improve
withdrawal across the genotypes Specifically,
to the same degree as patients with B16-Gly/Gly (n 5 22)
subjects had predictable and similar decreases in morning
receiving salmeterol therapy, either with or without con-
PEF and other measures of asthma control when switched
current ICS therapy. In the SOCS trial, including patients
from FSC to treatment with SABAs alone, regardless of
with persistent asthma treated with salmeterol after ICS
withdrawal, the B16-Arg/Arg patient subset (n 5 12)who received salmeterol alone responded significantly
Specific responses by B16-Arg/Arg genotype
worse than the B16-Gly/Gly subset (n 5 13), as assessed
Because the B16-Arg/Arg genotype is hypothesized
on the basis of changes in morning PEF, compared with
to exhibit enhanced downregulation in the presence of
patients who received placebo. However, in contrast to
J ALLERGY CLIN IMMUNOL
814 Bleecker et al
FIG 2. Change from baseline at end point in morning PEF for individual Arg/Arg subjects (n 5 29).
the study by Wechsler et al, the findings from this study of
In contrast to this study with salmeterol, a retrospective
183 subjects, perhaps the largest genetic analysis to date,
analysis of BAGS results reported small decreases in
show that response to the LABA salmeterol is not
morning PEF over time for B16-Arg/Arg subjects (n 5
affected by the ADRB2 Arg16Gly SNP during chronic
28) who received regularly scheduled albuterol, whereas
dosing in the presence of an ICS. Specifically, there
similar decreases were not observed in either the Arg/Arg
were sustained and quantitatively similar improvements
homozygous subjects who received as-needed albuterol
in lung function, symptoms, and albuterol use during
or in the B16-Gly/Gly subjects (n 5 62) who received
chronic treatment, regardless of Arg16Gly genotype. Al-
regularly scheduled Interestingly, this study
though not powered to definitively evaluate a haplotype
showed a larger decrease in morning PEF after the switch
effect, our haplotype analysis supports the individual
from 4-times-daily albuterol to as-needed albuterol in
SNP results. Drysdale et alshowed the lowest response
B16-Arg/Arg but not B16-Gly/Gly subjects. Results
in their 4/4 haplotype, corresponding to our BB group,
from BARGE appear similar to those from BAGS for
with a total of 14 subjects as opposed to the 25 in our anal-
B16-Arg/Arg subjects receiving regularly scheduled albu-
ysis (). Although we did not measure all 13 pos-
terol, in that there was a differential response between the
sible SNPs, using the SNPs at position 247, 46, 79, and
2 homozygote groups on regular b-agonist therapy, with
491, we were able to evaluate the most frequent haplo-
the Gly/Gly homozygotes showing improvement in PEF
types corresponding to those previously reported.
and other parameters of asthma control.
The results of this study are consistent with the findings
The results of the current study evaluating therapeutic
of Taylor et who examined retrospectively the rela-
responses to salmeterol in patients receiving concomitant
tionship between the frequency of asthma exacerbations
corticosteroids do not support an effect of ADRB2 poly-
and genetic variation at codon 16 of ADRB2 in subjects
morphisms. The contrasting results between BAGS,
receiving placebo, 4-times-daily albuterol, or twice-daily
BARGE, the retrospective analysis of SOCS and SLIC,
salmeterol for 24 weeks. These investigators demon-
and this study can be attributed to various factors, includ-
strated that although subjects treated with 4-times-daily al-
ing sample size, differences in study design, asthma sever-
buterol had a higher number of exacerbations compared
ity, intrinsic activity of the b-agonist evaluated, and
with those receiving placebo, subjects treated with salme-
concomitant use of an ICS.
terol had the lowest number of exacerbations. In addition,
Compounds with lower intrinsic activity are generally
there was no differential response in exacerbations or
believed to result in a lower potential to induce receptor
lung function in subjects receiving salmeterol, regardless
downregulation, a proposed mechanism of reduced
of genotype. Furthermore, a retrospective study by Klots-
response to b-agonists.High intrinsic activity might
man et alshowed that the serial 12-hour FEV1 response
also lead to an opposite response in which response to
to salmeterol after 12 weeks of treatment was similar in
cholinergic tone is enhanced.Salmeterol has the lowest
subjects treated with salmeterol, regardless of concomitant
intrinsic activity of the currently available b-agonist
ICS use or Arg16Gly genotype. The current analysis did
not evaluate salmeterol as monotherapy, which is not rec-
Because ADRB2 is a small gene with significant linkage
ommended in current asthma guidelines and could have
disequilibrium across the 59 promoter and 39 untranslated
contributed to the failure to detect a polymorphic effect
region, functional polymorphisms in partial linkage dis-
of ADRB2 on treatment response.
equilibrium with the Arg16Gly SNP might also be the
J ALLERGY CLIN IMMUNOL
Bleecker et al 815
VOLUME 118, NUMBER 4
cause of the divergent results in the literature because
the present study that there were no associations between
these would not be equally expressed in trials with small
genotypes and the treatment responses to FSC or the
sample sizes.
response to albuterol alone during FSC washout does not
Previous studies have proposed that some patients
eliminate a genetic interaction. In this regard ADRB2
might experience poorer asthma control and adverse side
haplotypes have been described and reported to occur at
effects associated with regular use of the potent SABA
different frequencies based on The haplotype
Because of these results, regular therapy
analysis in this study did not reveal any significant influ-
was compared with as-needed use of SABAs and shown
ence on response, but the sample size, the selection criteria
to have no adverse effects, but regular SABA use provided
of the study population, and the lack of ethnic representa-
no additional therapeutic benefits. Recent pharmacoge-
tion limits these conclusions.
netic data raise questions as to whether there might be a
In summary, this study evaluating associations between
subset of subjects, based on b2-AR genotype, who might
the polymorphic gene encoding for the b-agonist drug
not respond well to chronic SABA therapy. The results of
target and responses to therapy with a long-acting b-
the present study suggest that this is not the case for sal-
agonist showed that response to salmeterol does not vary
meterol when administered with an ICS and that current
by ADRB2 genotypes or haplotypes during chronic dosing
treatment guidelines are appropriate for a broad popu-
in the presence of an ICS. However, larger, prospective,
lation of asthmatic subjects, including those with b2-AR
clinical pharmacogenetic studies with higher power to
evaluate haplotypes across different ethnic/racial groups,
Both clinical trials and observational studies support
as well as genetic epidemiologic studies, are clearly
the therapeutic value of inhaled LABAs in combination
needed to help elucidate this field of great interest.
with ICSs. Specifically, if b-agonist use were associatedwith deleterious outcomes in an at-risk genetic subgroup
We thank Laura Sutton, PharmD, for her assistance in preparing
reported to occur in a small but significant percentage
the manuscript and Deborah Meyers, PhD, for her assistance with
of the asthma population (eg, the B16-Arg/Arg genotype
the haplotype analyses.
is observed in approximately 15% of white subjects),then evaluation of large clinical studies would predictablylead to an increase in adverse outcomes, such as asthma
exacerbations, among b-agonist users compared with
1. Drysdale CM, McGraw DW, Stack CB, Stephens JC, Judson RS, Nan-
nonusers. Long-term clinical trials, including OPTI
dabalan K, et al. Complex promoter and coding region beta2-adrenergicreceptor haplotypes alter receptor expression and predict in vivo respon-
and have highlighted the advantages of
siveness. Proc Natl Acad Sci U S A 2000;97:10483-8.
LABA/ICS therapy in terms of improved asthma control
2. Silverman EK, Kwaitkowski DJ, Sylvia JS, Lazarus R, Drazen JM,
and fewer exacerbations. Similarly, Shrewsbury et
Lange C, et al. Family-based association analysis of b2-adrenergic
in a meta-analysis of data from 3685 subjects, documented
receptor polymorphisms in the childhood asthma management program.
lower frequency of asthma exacerbations and better over-
J Allergy Clin Immunol 2003;112:870-6.
3. Kobilka BK, Dixon RA, Frielle T, Dohlman HG, Bolanowski MA,
all asthma control with a low-dose ICS plus salmeterol
Sigal IS, et al. cDNA for the human b2-adrenergic receptor: a protein
compared with higher doses of an ICS alone. Furthermore,
with multiple membrane-spanning domains and encoded by a gene
a recent pooled analysis of 13 studies including 4020
whose chromosomal location is shared with that of the receptor for
subjects reported fewer asthma exacerbations and hospi-
platelet-derived growth factor. Proc Natl Acad Sci U S A 1987;84:46-50.
talizations for both white and African American subjects
4. Panettieri RA. Airway smooth muscle: an immunomodulatory cell.
receiving an ICS plus LABA compared with an ICS
J Allergy Clin Immunol 2002;110(suppl):S269-74.
These observations, although consistent with
5. Hall IP, Wheatley A, Wielding P, Light SB. Association of the Glu 27
the current study results, do not exclude the possibility
b2-adrenoceptor polymorphism with lower airway reactivity in asthmatic
that individual subjects with asthma might be at greater
subjects. Lancet 1995;345:1213-4.
6. Liggett SB. Update on current concepts of the molecular basis of b
risk with chronic b-agonist therapy. However, examina-
adrenergic receptor signaling. J Allergy Clin Immunol 2002;110(suppl):
tion of results from individual subjects in this study with
the various genotypes (B16-Arg/Arg in particular) did
7. Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of
not indicate that there was a subset of nonresponders. It
the human beta2-adrenergic receptor within the fourth transmembranedomain alters ligand binding and functional properties of the receptor.
could be hypothesized that there might be additional
J Biol Chem 1993;268:23116-21.
gene-gene interaction linkage disequilibrium with other
8. Reihsaus E, Innis M, MacIntyre N, Liggett SB. Mutations in the gene
polymorphisms in ADRB2 that are associated with exacer-
encoding for the b2-adrenergic receptor in normal and asthmatic sub-
bations or as a marker for disease Addition-
jects. Am J Respir Cell Mol Biol 1993;8:334-9.
ally, observational studies reflecting real-world use of
9. Green SA, Turki J, Bejarano P, Hall IP, Liggett SB. Influence of b2-
adrenergic receptor genotypes on signal transduction in human airway
asthma medications support the findings that ICSs plus
smooth muscle. Am J Respir Cell Mol Biol 1995;13:25-33.
LABAs resulted in fewer emergency department visits
10. Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli
and hospitalizations for asthma, less need for supplemen-
VM, et al. The effect of polymorphisms of the b2-adrenergic receptor on
tal albuterol, and overall lower costs compared with an
the response to regular use of albuterol in asthma. Am J Respir Crit CareMed 2000;162:75-80.
ICS alone or an ICS plus leukotriene
11. Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town
Some important limitations should be considered when
GI. Asthma exacerbations during long term beta agonist use: influence
interpreting these data. For example, the observations in
of b2 adrenoceptor polymorphism. Thorax 2000;55:762-7.
J ALLERGY CLIN IMMUNOL
816 Bleecker et al
12. Palmer LJ, Silverman ES, Weiss ST, Drazen JM. Pharmacogenetics of
25. Jewell-Motz EA, Small KM, Theiss CT, Liggett SB. a2A/a2C-adrenergic
asthma. Am J Respir Crit Care Med 2002;165:861-6.
receptor third loop chimera show that agonist interaction with receptor
13. Joos L, Pare PD, Sandford AJ. b2-adrenergic receptor polymorphisms
subtype backbone establishes G protein-coupled receptor kinase phos-
and asthma. Curr Opin Pulm Med 2001;7:69-74.
phorylation. J Biol Chem 2000;275:28989-93.
14. Hancox RJ, Sears MR, Taylor DR. Polymorphism of the b2-adrenoceptor
26. McGraw DW, Almoosa KF, Paul RJ, Kobilka BK, Liggett SB. Antithetic
and the response to long-term b2-agonist therapy in asthma. Eur Respir J
regulation by beta-adrenergic receptors of Gq receptor signaling via
phospholipase C underlies the airway beta-agonist paradox. J Clin Invest
15. Israel E, Chincilli VM, Ford JG, Boushey HA, Cherniak R, Craig TJ,
et al. Use of regularly scheduled albuterol treatment in asthma: geno-
27. Moore RH, Khan A, Dickey BF. Long-acting inhaled b2-agonists in
type-stratified, randomized, placebo-controlled cross-over trial. Lancet
asthma therapy. Chest 1998;113:1095-108.
28. Sears MR, Taylor DR, Print CG, Lake DC, Li Q, Flannery EM, et al.
16. Wechsler ME, Lehman E, Lazarus SC, Lemanske RF, Boushey HA,
Regular inhaled b2-agonist treatment in bronchial asthma. Lancet 1990;
Deykin A, et al. b-adrenergic receptor polymorphism and response to
salmeterol. Am J Respir Crit Care Med 2006;173:519-26.
29. Grainger J, Woodman K, Pearce N, Crane J, Burgess C, Keane A, et al.
17. National Asthma Education and Prevention Program. Guidelines for the
Prescribed fenoterol and death from asthma in New Zealand, 1981-7:
diagnosis and management of asthma. Expert Panel Report II. Bethesda
a further case-control study. Thorax 1991;46:105-11.
(MD): National Institutes of Health, National Heart, Lung, and Blood
30. O'Byrne PM, Barnes PJ, Rodriguez-Roisin R, Runnerstrom E, Sand-
Institute; 1997. NIH publication no. 97-4051.
strom T, Svensson K, et al. Low dose inhaled budesonide and formoterol
18. Global Initiative for Asthma. Global strategy for asthma management
in mild persistent asthma: the OPTIMA randomized trial. Am J Respir
and prevention: NHLBI/WHO Workshop. Bethesda (MD): National
Crit Care Med 2001;164:1392-7.
Institutes of Health, National Heart, Lung, and Blood Institute; 2002.
31. Bateman ED, Boushey HA, Bousquet J, Busse WW, Clark TJH, Pauwels
19. Pearlman DS, White MV, Lieberman AK, Pepsin PJ, Kalberg C, Emmett
RA, et al. Can guideline-defined asthma control be achieved? The Gain-
A, et al. Fluticasone propionate/salmeterol combination compared with
ing Optimal Asthma Control study. Am J Respir Crit Care Med 2004;
montelukast for the treatment of persistent asthma. Ann Allergy Asthma
32. Shrewsbury S, Pyke S, Britton M. Meta-analysis of increased dose of
20. Calhoun W, Nelson HS, Nathan RA, Pepsin PJ, Kalberg C, Emmett
inhaled steroid or addition of salmeterol in symptomatic asthma
A, et al. Comparison of fluticasone propionate-salmeterol combination
(MIASMA). BMJ 2000;320:1368-73.
therapy and montelukast in subjects who are symptomatic on short-
33. Yancey S, Prillaman B, Dorinsky P. Retrospective review of studies
acting B2-agonists alone. Am J Respir Crit Care Med 2001;164:
shows that subjects receiving salmeterol plus and ICS have fewer serious
asthma exacerbations versus subjects on and ICS alone, regardless of
21. American Thoracic Society. Standards for the diagnosis and care of sub-
ethnic origin [abstract]. J Allergy Clin Immunol 2004;113(suppl):S34.
jects with chronic obstructive pulmonary disease (COPD) and asthma.
34. Stempel DA, O'Donnell JC, Meyer JW. Inhaled corticosteroids plus
Am Rev Respir Dis 1987;136:225-44.
salmeterol or montelukast: effects on resource utilization and costs.
22. Liggett SB. b2-adrenergic receptor pharmacogenetics. Am J Respir Crit
J Allergy Clin Immunol 2002;109:433-9.
Care Med 2000;161(suppl):S197-201.
35. Sheth K, Borker R, Emmett A, Rickard K, Dorinsky P. Cost-effective-
23. Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incom-
ness comparison of salmeterol/fluticasone propionate versus montelukast
plete data via the EM algorithm. J R Stat Soc B 1977;39:1-38.
in the treatment of adults with persistent asthma. Pharmacoeconomics
24. Klotsman M, Binnie CG, Dorinsky PM, Yancey SW, Anderson WH.
Pharmacogenetic effect of a b2-adrenergic receptor (ADRB2) polymor-
36. O'Connor RD, O'Donnell JC, Pinto LA, Wiener DJ, Legorreta AP. Two-
phism on b2-agonist responsiveness to salmeterol [abstract]. Am J Respir
year retrospective economic evaluation of three dual-controller therapies
Crit Care Med 2004;169:A582.
used in the treatment of asthma. Chest 2002;121:1028-35.
Source: http://www.okavallc.com/images/bleeker.pdf
Amorphization of Pharmaceuticals by Co- grinding with Neusilin® Amorphization of crystalline drugs can be achieved In a previous report, we discussed solid dispersion through several methods. The most common method methods using Neusilin as an adsorption carrier to is melting and solidifi cation by rapid cooling over liquid improve dissolution and bioavailabilty of poorly water
SUbStance abUSe eDUcation anD policieS Your brain. Use it. Don't abuse it. SUbStance abUSe eDUcation anne arUnDel commUnitY college miSSion . 2 DrUg anD alcohol policY .2-3 Educational Statement .2Guidelines For Discipline .3Procedures For Enforcement .3Legal Sanctions .3






