ConditionOsteoarthritis of the knee Osteoarthritis of the kneeThis booklet provides information and answers to your questions about this condition. Arthritis Research UK produce and print our booklets entirely from charitable donations. What is osteoarthritis of the knee? Osteoarthritis is the most common form of joint disease, and the knee is one of the most commonly aff ected joints. In this booklet we'll explain how osteoarthritis of the knee develops, what causes it and how it can be treated. We'll also give some hints and tips to help you manage your arthritis and suggest where you can fi nd out more.

Publication.babcock.edu.ng
[Downloaded free from http://www.jcsjournal.org on Tuesday, July 05, 2016, IP: 197.253.33.106]ORIGINAL RESEARCH REPORT Antibiotic susceptibility/resistant gene profiles of
Group B streptococci isolates from pregnant women in
a tertiary institution in Nigeria
Charles J. Elikwu1,2, Oyinlola O. Oduyebo3, Rose I. Anorlu4, Brigitte König5 1Department of Medical Microbiology and Parasitology, Ben Carson School of Medicine, Babcock University, Ilisan‑Remo, Ogun State, 2Department of Medical Microbiology and Parasitology, Lagos University Teaching Hospital, 3Department of Medical Microbiology and Parasitology, College of Medicine, University of Lagos, 4Department of Obstetrics and Gynecology, College of Medicine, University of Lagos, Idi‑Araba, Lagos, Nigeria, 5Institute of Medical Microbiology and Infection Epidemiology, University of Leipzig, Germany ABSTRACT
Background: Penicillin is recommended as the first‑line agent for intrapartum antibiotic
prophylaxis (IAP). Although Group B Streptococcus (GBS) strains are generally susceptible to penicillin with only occasional resistance, they show varying resistance to erythromycin, clindamycin, and tetracycline. Therefore, knowledge of the resistance profile of GBS in the local environment will be useful for administration of appropriate intrapartum antibiotics prophylaxis. Methodology: Rectovaginal swabs collected from pregnant women were cultured
for GBS using conventional media. Kirby–Bauer disc diffusion method was performed according to the Clinical and Laboratory Standards Institute guidelines on GBS isolates to determine the antibiotic susceptibility patterns of the isolates. Inducible clindamycin resistance was detected by using the D‑zone test. Resistance determinants genes were discerned with conventional polymerase chain reaction. Results: Carriage rates of GBS among pregnant women studied
were 19.7%. GBS colonizing the pregnant mothers were uniformly susceptible (100%) to penicillin, vancomycin, and ceftriaxone. Only three (6.5%) of the isolates showed inducible clindamycin resistance. ermA gene was present in all three GBS isolates with inducible macrolide‑lincosamide‑streptogramin resistance. ermB was absent in all the strains tested. mefA/E gene was carried by two of the macrolide‑clindamycin resistance isolates. tetM gene Address for correspondence:
Dr. Charles J. Elikwu, was carried by all isolates with tetracycline resistance phenotypes. Conclusion: In this study,
Department of Medical all GBS isolates were susceptible to penicillin G, the recommended antibiotic for IAP. The Microbiology and Parasitology, presence of resistance to erythromycin and inducible resistance to clindamycin excludes the Ben Carson School of Medicine, use of these agents as alternatives in cases of penicillin allergy. In this case, vancomycin is Babcock University, Ilisan‑Remo, the drug of choice as recommended in the updated Centers for Disease Control guideline.
Ogun State, Nigeria. Key words: Group B streptococci, inducible clindamycin resistance, pregnant women
To reduce the incidence of neonatal disease caused by beta hemolytic GBS, the Centers for Disease Control (CDC) and Maternal intrapartum beta hemolytic Group B Prevention recommends the use of intrapartum antibiotic Streptococcus (GBS) colonization is the primary risk prophylaxis (IAP) in pregnant women who are vagino‑rectal factor for early‑onset disease in infants.[1] In the absence carriers of GBS.[4,5] Penicillin is recommended as the of any intervention, approximately 2% of infants first‑line agent for IAP, while ampicillin is considered as an born to colonized mothers develop early‑onset GBS This is an open access article distributed under the terms of the Creative Commons Attribution‑NonCommercial‑ShareAlike 3.0 License, which allows Access this article online
others to remix, tweak, and build upon the work non‑commercially, as long as the author is credited and the new creations are licensed under the identical terms.
Quick Response Code:
For reprints contact: [email protected]
How to cite this article: Elikwu CJ, Oduyebo OO, Anorlu RI, Konig B.
Antibiotic susceptibility/resistant gene profiles of Group B streptococci isolates from pregnant women in a tertiary institution in Nigeria. J Clin 2016 JOURNAL OF CLINICAL SCIENCES PUBLISHED BY WOLTERS KLUWER - MEDKNOW [Downloaded free from http://www.jcsjournal.org on Tuesday, July 05, 2016, IP: 197.253.33.106]
Elikwu, et al.: Group B streptococci, inducible clindamycin resistance, pregnant women acceptable alternative.[1,6] In penicillin‑allergic women, who clindamycin (as either of these represents a suitable are not at high risk for anaphylaxis, cefazolin is considered alternative in case of penicillin allergy) and tetracycline the agent of choice for intrapartum chemoprophylaxis for epidemiological reasons.
because of its narrow spectrum of activity and ability to achieve high intra‑amniotic concentrations.[1] In pregnant Pregnant women at gestational age <35 weeks or those women at high risk for penicillin anaphylaxis, clindamycin who had had received antibiotics within 2 weeks before is recommended if the organism is susceptible, and presenting at the antenatal clinic were excluded from vancomycin is recommended if there is clindamycin the study. The Health Research and Ethics Committee resistance or if susceptibility is unknown.[1,2,7] of the LUTH, Lagos, Nigeria, approved this study and every participant gave written informed consent before Although GBS strains are generally susceptible to penicillin with only occasional resistance, they show varying resistance rates to erythromycin, clindamycin, Culture of specimens
and tetracycline.[7,8] The most commonly encountered Vagino‑rectal swabs were collected from 300 pregnant macrolide resistance mechanisms among streptococci women and were inoculated into Todd‑Hewitt broth are ribosomal modification by a methylase encoded by supplemented with 15 μg/ml nalidixic acid and an erm gene[9] and drug efflux by a membrane‑bound 10 μg/ml colistin[12] (Biomerieux, Germany). After protein encoded by a mef gene.[10] The presence of the overnight incubation at 37°C, broths were sub‑cultured erm methylase confers resistance to erythromycin and onto Columbia blood agar plates with 5% sheep blood inducible or constitutive resistance to lincosamides and (Oxoid, United Kingdom) while the solid media were streptogramin B (macrolide‑lincosamide‑streptogramin B incubated at 37°C in ambient air for 24–48 h.
phenotype), whereas the presence of the mef pump confers resistance only to 14‑ and 15‑membered macrolides Identification of isolates
(M phenotype). A second efflux mechanism, encoded by All isolates under investigation were presumptively the mreA gene, has been described for GBS.[11] identified as GBS if they were beta hemolytic, Gram‑positive cocci, catalase negative, and showed positive Christie, In view of the aforementioned, a local knowledge of the Atkins and Munch‑Peterson test (CAMP). PCR targeting resistance profile of GBS will be useful for administration of the GBS species‑specific gene, cfb was done for definitive appropriate antibiotics for prophylaxis. This study aimed to identification. Total DNA extraction from overnight evaluate the antibiotic susceptibility profiles of GBS isolates GBS colonies performed using the DN easy blood from a tertiary healthcare institution in Lagos, Southwest and tissue kits (Qiagen, Germany) according to the Nigeria; and characterize antibiotic resistance genes of manufacturer's instructions. PCR was performed on GBS isolates to advise the local community on GBS empiric GBS DNA extracts using GBS‑specific primers ((Sag59) therapy. This will inform the development of rational 5'‑TTCACCAGCTGTATTAGAAGTA‑3' and reverse (Sag190): interventions and antibiotic guidelines in GBS infections.
5'‑GTTCCCTGAACATTATCTTTGAT‑3') defined as previously described.[13] Amplicons were analyzed by electrophoresis in 1.5% agarose gel (Sigma, USA) stained with ethidium bromide using 1 × TAE buffer (buffer solution Study area and population
containing a mixture of Tris base, acetic acid and EDTA) Participants for this study were recruited from the antenatal (40 mM Tris‑acetate, 1 mM ethylenediaminetetraacetic clinic of the Lagos University Teaching Hospital (LUTH), acid (EDTA); pH 8.0) at 70 V for 60 min. The DNA a tertiary care facility located in South West Nigeria. molecular weight marker (100 bp) (Invitrogen, Germany), Molecular studies were performed at the Institute of was run concurrently to serve as reference for defining Medical Microbiology and Infection Epidemiology, the PCR products. Gels were visualized under UV University of Leipzig, Germany.
trans‑illumination (Biometra, Germany) and the results were transmitted to a computer display system (Biometra, Study design
Germany) and stored.
This was a cross‑sectional study conducted at the LUTH, Lagos from December 2010 to October 2011. A pair of Antibiotic susceptibility testing
vagino‑rectal swab were collected from all consenting All GBS isolates were subjected to antibiotic susceptibility mothers at gestation age 35–37 weeks presenting at using the disk diffusion method as recommended by the the antenatal clinic. GBS was detected in the maternal Clinical and Laboratory Standards Institute (CLSI)[8] using specimens by culture and confirmed by polymerase chain the modified Kirby–Bauer method. The antimicrobial reaction (PCR). GBS isolates were tested for antibiotic agents tested includes penicillin G (10U), clindamycin susceptibility using the disk diffusion method. Phenotypical (2 μg), erythromycin (15 μg), vancomycin (30 μg), and molecular antibiotic resistance testing were conducted ciprofloxacin (5 μg), ceftriaxone (30 μg), tetracycline (30 μg) for isolates that showed resistance for erythromycin and and co‑trimoxazole (25 μg) (Oxoid, UK). The diameter of JOURNAL OF CLINICAL SCIENCES, VOLUME 13, NUMBER 3, JULY-SEPTEMBER 2016
[Downloaded free from http://www.jcsjournal.org on Tuesday, July 05, 2016, IP: 197.253.33.106]
Elikwu, et al.: Group B streptococci, inducible clindamycin resistance, pregnant women
the zone of inhibition was measured in millimeters using
resistant to erythromycin were positive for inducible
a meter rule and interpreted according to CLSI guidelines
clindamycin resistance. Erythromycin resistance gene
with reference to tables of interpretative criteria.
ermA was found in the three erythromycin‑clindamycin
Streptococcus agalactiae control strains Ia (2008232728),
inducible resistance and absent in the only one strain
Ib (2008232729), III (2008232582) and V (2008232731)
with intermediate susceptibility [Figure 1]. However,
from CDC were used as control strains. The results were
erythromycin resistance gene ermB was absent in all the
described by frequency number and percentage.
four strains tested. The mefA/E gene was carried by two
of the macrolide‑clindamycin resistance isolates [Figure 2].
Phenotypic and molecular antibiotic resistance testing
The tetM gene was carried by all isolates that showed
were conducted for isolates that showed resistance to
tetracycline resistance phenotypes [Figure 3] while tetO
erythromycin, clindamycin, and tetracycline.
gene was completely absent.
Phenotypic antibiotic resistance testing
Inducible clindamycin resistance phenotype was performed
on all GBS isolates with erythromycin resistance using
GBS is the leading cause of early‑onset neonatal sepsis
the D‑zone disk diffusion method on Mueller‑Hinton
in most countries of the world. Universal screening is
agar supplemented with 5% defibrinated sheep blood
recommended for pregnant women at 35–37 weeks'
(Oxoid, UK) using erythromycin and clindamycin disks as
gestation.[2] Therefore, we conducted antibiotic
recommended by the CLSI.[8] Isolates with blunting of the
susceptibility profiles of GBS isolates with the primary
inhibition zone around the clindamycin disk adjacent to
objective of evaluating the antibiogram among GBS isolates
the erythromycin disk (D‑zone positive) were considered
from Nigerian pregnant women. In this study, we found
to have inducible clindamycin resistance[14,15] and were
presumed to be resistant. Susceptibility to clindamycin with
no blunting defined the M phenotype (efflux mechanism).
Table 1: Antibiotic susceptibility profile of
maternal GBS strains
The CDC S. agalactiae serotype III (2008232582) was used
as a control strain.
Resistance
susceptibility n (%)
Genotypic antibiotic resistance testing
Penicillin G (10U)
Erythromycin (15ug)
Antibiotic resistance genes of isolates resistant to
Clindamycin (2ug)
erythromycin, clindamycin, and tetracycline, was
Vancomycin (30ug)
determined. PCR was performed on GBS resistant isolates
Tetracycline (30ug)
using primers erm A 1/2, erm B 1/2, and mef AE ½
Ceftraixone (30ug)
(BioteZ, Berlin, Germany) for the erm (A), erm (B) and
mef (A) genes respectively. The sequences of the primer
SXT (1.25/23.75ug)
sets and PCR conditions[14,17] were as previously described.
All isolates that expressed phenotypic resistance to
tetracycline were also tested for the presence of the tet (M)
and tet (O) tetracycline resistance determinants[18] using tet
M 1/2 and tet O 1/2 primers respectively (BioteZ, Berlin,
Germany). Amplicons were analyzed by electrophoresis
in 1.5% agarose gel (Sigma, USA) stained with ethidium
bromide using 1 × TAE (40 mM Tris‑acetate, 1 mM EDTA;
pH 8.0) as stated previously.
Fifty‑nine (19.7%) out of 300 pregnant women were
enrolled for the study showed GBS‑vaginal colonization.
All 46 available GBS isolates colonizing the pregnant
mothers were uniformly susceptible (100%) to
penicillin, vancomycin, and ceftriaxone while 44 (95.7%)
and 41 (89.1%) were resistant to tetracycline and
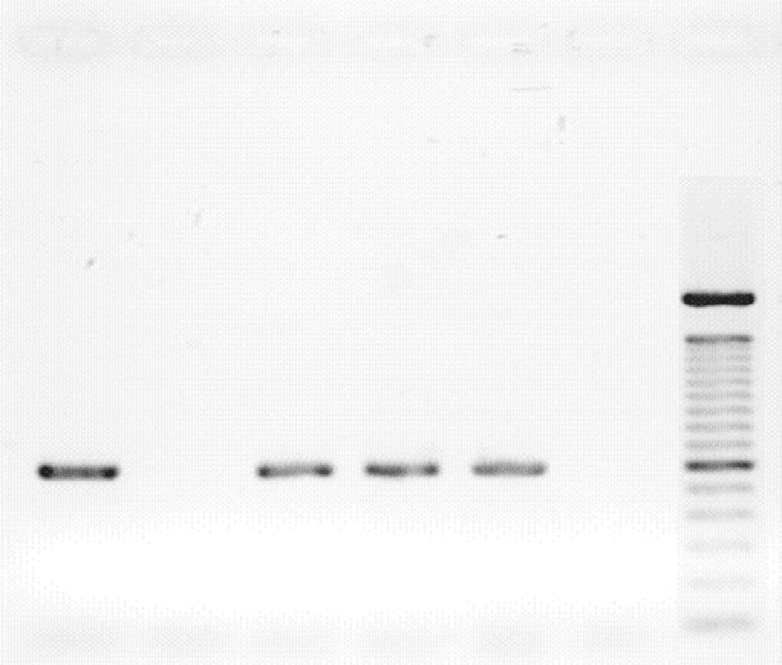
Lanes 1, 3-4: GBS ermA positive isolates
Lane 2: GBS ermA negative isolate
trimethoprim‑sulfamethoxazole, respectively [Table 1].
Lane 5: Positive control
Only three (6.5%) of the isolates showed erythromycin
Lane 6: Negative control
ML: DNA molecular ladder
resistance while another three were resistant to clindamycin
with a lone intermediate susceptibility. All the three isolates
Figure 1: Gel electrophoresis identification of GBS erm (A) resistant gene
JOURNAL OF CLINICAL SCIENCES, VOLUME 13, NUMBER 3, JULY-SEPTEMBER 2016
[Downloaded free from http://www.jcsjournal.org on Tuesday, July 05, 2016, IP: 197.253.33.106]
Elikwu, et al.: Group B streptococci, inducible clindamycin resistance, pregnant women
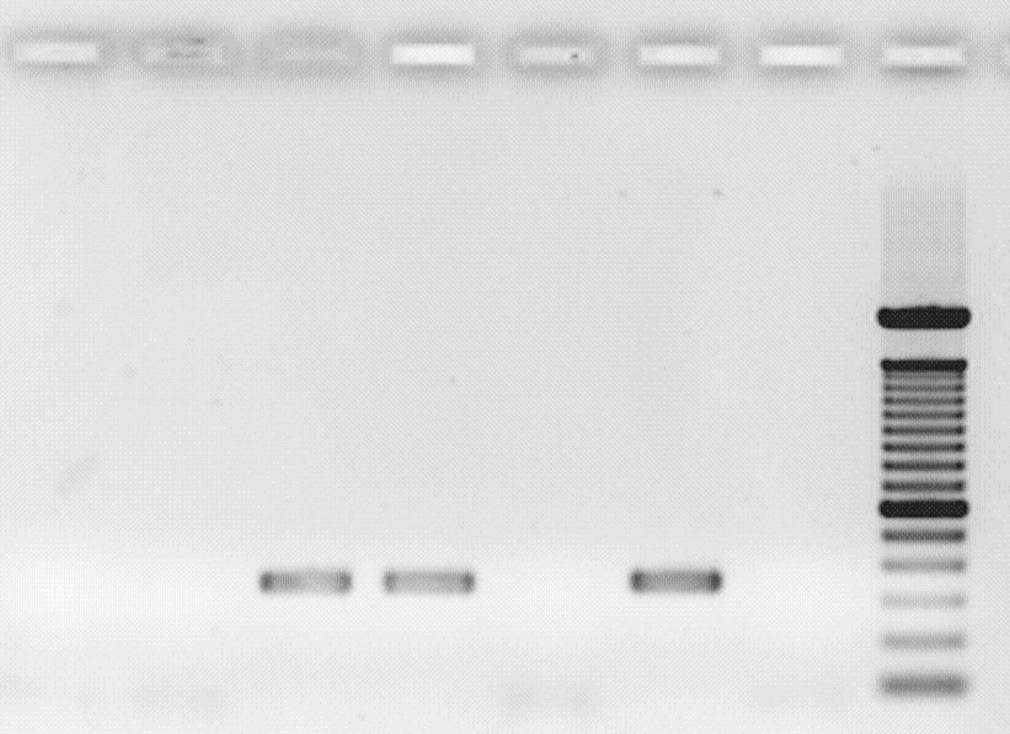
Lanes 1, 3, 4, 6, 8-12: GBS tetM positive isolates
Lanes 2, 5, 7: GBS tetM negative isolates
Lanes 3-4: GBS mef(A/E) positive isolates
Lane 13: Positive control
Lanes 1-2, 5: GBS mef(A/E) negative isolates
Lane 14: Negative control
Lane 6: Positive control
ML: DNA molecular ladder
Lane 7: Negative control
ML: DNA molecular ladder
Figure 3: Gel electrophoresis identification of GBS tetM resistant gene
Figure 2: Gel electrophoresis identification of GBS mef (A/E) resistant gene
Our study provides valuable information about current
that all GBS isolates remain susceptible to penicillin G,
knowledge of antibiogram of GBS isolates among pregnant
the recommended antibiotic for IAP.[2] However, a study
women in Nigeria. It is however limited by the small sample
from Obafemi Awolowo University Teaching Hospital,
size or rather a small size of GBS isolates and the conduct of
Ile‑Ife Nigeria in the same geopolitical zone of our country
the study in a lone institution. Nevertheless, the antibiotics
reported uniform resistance of all GBS isolates to penicillin,
situation is similar across the country as Nigeria currently
ampicillin and clindamycin.[19]
has no policy and guidelines regulating antibiotics in
place; we believe that the results of our study were a true
Though this study recorded a low rate of resistance (6.5%)
reflection of the situation GBS antibiogram among pregnant
to erythromycin and another 6.5% to clindamycin, a
women in Nigeria.
number of literature has showed that the proportions
of GBS isolates with in vitro resistance to clindamycin
Financial support and sponsorship
or erythromycin, have increased over the past two
A short‑term research grant from DAAD (German Academic
decades.[12,20‑22] At this juncture, a limitation of this study
Exchange Program).
is highlighted: A total of 46 GBS isolates analyzed was
low compared to 801 isolates by Milledge et al.[22] whose
Conflicts of interest
study stretched over a 5 years period. Even at that, an
There are no conflicts of interest.
upward antibiotic resistance trend is predicted given the
high rates of antibiotic misuse and abuse in Nigeria.[23]
Resistance to erythromycin is associated frequently but
1. Verani JR, McGee L, Schrag SJ; Division of Bacterial Diseases,
not always with resistance to clindamycin.[15] The
National Center for Immunization and Respiratory Diseases,
CDC guideline on prevention of perinatal GBS disease
Centers for Disease Control and Prevention (CDC). Prevention
recommends that erythromycin is no longer acceptable
of perinatal group B streptococcal disease – Revised guidelines
for empiric prophylaxis because of increasing rates
from CDC, 2010. MMWR Recomm Rep 2010;59:1‑36.
2. Cagno CK, Pettit JM, Weiss BD. Prevention of perinatal
of resistance.[2,20] In such a case clindamycin (900 mg
group B streptococcal disease: Updated CDC guideline. Am
intravenously every 8 h until delivery) is the drug of
Fam Physician 2012;86:59‑65.
choice if the GBS isolate is susceptible to clindamycin and
3. Adair CE, Kowalsky L, Quon H, Ma D, Stoffman J, McGeer A,
erythromycin, and if there is no inducible clindamycin
et al. Risk factors for early‑onset group B streptococcal disease
in neonates: A population‑based case‑control study. CMAJ
resistance. Vancomycin (1 g intravenously every 12 h until
delivery) is recommended if testing shows resistance or
4. Koenig JM, Keenan WJ. Group B Streptococcus and
inducible resistance to clindamycin. If erythromycin and
early‑onset sepsis in the era of maternal prophylaxis. Pediatr
clindamycin susceptibility tests were not performed, or if
Clin North Am 2009;56:689‑708.
5. Jauréguy F, Carton M, Panel P, Foucaud P, Butel MJ,
results are not available at the time of labor, vancomycin
Doucet‑Populaire F. Effects of intrapartum penicillin
should be used in women at high risk of anaphylaxis.[2] The
prophylaxis on intestinal bacterial colonization in infants.
resistance mechanisms of GBS isolates to erythromycin in
J Clin Microbiol 2004;42:5184‑8.
this study was accounted for by the presence of the genes
6. Berner R. Significance, management and prevention of
Streptococcus agalactiae infection during the perinatal
that expresses erythromycin methylases and macrolide
period. Expert Rev Anti Infect Ther 2004;2:427‑37.
efflux in the resistant isolates.
7. Chaudhuri K, Gonzales J, Jesurun CA, Ambat MT,
JOURNAL OF CLINICAL SCIENCES, VOLUME 13, NUMBER 3, JULY-SEPTEMBER 2016

[Downloaded free from http://www.jcsjournal.org on Tuesday, July 05, 2016, IP: 197.253.33.106]
Elikwu, et al.: Group B streptococci, inducible clindamycin resistance, pregnant women
Mandal‑Chaudhuri S. Anaphylactic shock in pregnancy: A
macrolide‑lincosamide‑streptogramin B resistance determinants.
case study and review of the literature. Int J Obstet Anesth
Antimicrob Agents Chemother 1999;43:2823‑30.
16. Clancy J, Petitpas J, Dib‑Hajj F, Yuan W, Cronan M,
8. Clinical and Laboratory Standards Institute. Performance
Kamath AV, et al. Molecular cloning and functional analysis
Standards for Antimicrobial Susceptibility Testing;
of a novel macrolide‑resistance determinant, mefA, from
Twenty‑Second Informational Supplement. Wayne, PA, USA:
Streptococcus pyogenes. Mol Microbiol 1996;22:867‑79.
Clinical and Laboratory Standards Institute; 2013. p. 1‑184.
17. Sutcliffe J, Grebe T, Tait‑Kamradt A, Wondrack L. Detection
9. Weisblum B. Inducible resistance to macrolides, lincosamides
of erythromycin‑resistant determinants by PCR. Antimicrob
and streptogramin type B antibiotics: The resistance
Agents Chemother 1996;40:2562‑6.
phenotype, its biological diversity, and structural elements
18. Trzcinski K, Cooper BS, Hryniewicz W, Dowson CG.
that regulate expression – A review. J Antimicrob Chemother
Expression of resistance to tetracyclines in strains of
1985;16 Suppl A: 63‑90.
methicillin‑resistant Staphylococcus aureus. J Antimicrob
10. Luna VA, Coates P, Eady EA, Cove JH, Nguyen TT,
Roberts MC. A variety of gram‑positive bacteria carry mobile
19. Onipede A, Adefusi O, Adeyemi A, Adejuyigbe E,
mef genes. J Antimicrob Chemother 1999;44:19‑25.
Oyelese A, Ogunniyi T. Group B Streptococcus carriage
11. Clancy J, Dib‑Hajj F, Petitpas JW, Yuan W. Cloning and
during late pregnancy in Ile‑Ife, Nigeria. Afr J Clin Exp
characterization of a novel macrolide efflux gene, mreA, from
Microbiol 2012;13:135‑43. Available from: http://www.ajol.
Streptococcus agalactiae. Antimicrob Agents Chemother
info/index.php/ajcem/article/view/81774. [Last accessed on
2016 Jan 25].
12. Joachim A, Matee MI, Massawe FA, Lyamuya EF. Maternal
20. Lin FY, Whiting A, Adderson E, Takahashi S, Dunn DM,
and neonatal colonisation of group B Streptococcus at
Weiss R, et al. Phylogenetic lineages of invasive and
Muhimbili National Hospital in Dar es Salaam, Tanzania:
colonizing strains of serotype III group B Streptococci from
Prevalence, risk factors and antimicrobial resistance. BMC
neonates: A multicenter prospective study. J Clin Microbiol
Public Health 2009;9:437.
13. Ke D, Ménard C, Picard FJ, Boissinot M, Ouellette M, Roy PH,
21. Edwards MS, Baker CJ. Streptococcus agalactiae. In:
et al. Development of conventional and real‑time PCR assays
Mandell GL, Bennett JE, Dolin R. editors. Principles and
for the rapid detection of group B streptococci. Clin Chem
Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill
Livingstone; 2005. p. 2423‑34.
14. Kataja J, Seppälä H, Skurnik M, Sarkkinen H, Huovinen P.
22. Milledge J, Calis JC, Graham SM, Phiri A, Wilson LK,
Different erythromycin resistance mechanisms in group C
Soko D, et al. Aetiology of neonatal sepsis in Blantyre,
and group G streptococci. Antimicrob Agents Chemother
Malawi: 1996‑2001. Ann Trop Paediatr 2005;25:101‑10.
23. Ekwochi U, Chinawa JM, Obi I, Obu HA, Agwu S. Use and/or
15. Roberts MC, Sutcliffe J, Courvalin P, Jensen LB,
misuse of antibiotics in management of diarrhea among children
Rood J, Seppala H. Nomenclature for macrolide and
in Enugu, Southeast Nigeria. J Trop Pediatr 2013;59:314‑6.
New features on the journal's website
Optimized content for mobile and hand-held devices
HTML pages have been optimized of mobile and other hand-held devices (such as iPad, Kindle, iPod) for faster browsing speed.
Click on [Mobile Full text] from Table of Contents page.
This is simple HTML version for faster download on mobiles (if viewed on desktop, it will be automatically redirected to full HTML version)
E-Pub for hand-held devices
EPUB is an open e-book standard recommended by The International Digital Publishing Forum which is designed for reflowable content i.e. the
text display can be optimized for a particular display device.
Click on [EPub] from Table of Contents page.
There are various e-Pub readers such as for Windows: Digital Editions, OS X: Calibre/Bookworm, iPhone/iPod Touch/iPad: Stanza, and Linux:
Calibre/Bookworm.
E-Book for desktop
One can also see the entire issue as printed here in a ‘flip book' version on desktops.
Links are available from Current Issue as well as Archives pages.
Click on
JOURNAL OF CLINICAL SCIENCES, VOLUME 13, NUMBER 3, JULY-SEPTEMBER 2016
Source: http://publication.babcock.edu.ng/docs/MBBS/elikwuc/1467722257.pdf
Historia natural de una nueva contractura en flexion posterior a un rtr primario-corregido-2
Evolución natural de una nueva contractura en flexión posterior a un reemplazo total de rodilla primario Dr. Andrés Anania Hospital Naval Cirujano Mayor "Dr. Pedro Mal o" Av. Patricias Argentinas 351, C.A.B.A. Objetivo. Investigar la incidencia, la evolución natural y las consecuencias funcionales del desarrol o de una nueva contractura en flexión posterior a un



