
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Tocotrienols, the vitamin e of the 21st century: its potential against cancer and other chronic diseases


Contents lists available at
Biochemical Pharmacology
Tocotrienols, the vitamin E of the 21st century: Its potential against cancer andother chronic diseases
Bharat B. Aggarwal Chitra Sundaram, Seema Prasad, Ramaswamy Kannappan
Cytokine Research Laboratory, Department of Experimental Therapeutics, The University of Texas, M.D. Anderson Cancer Center, 1515 Holcombe Boulevard, Box 143, Houston,TX 77030, USA
Initially discovered in 1938 as a ‘‘fertility factor,'' vitamin E now refers to eight different isoforms that
Received 9 June 2010
belong to two categories, four saturated analogues (a, b, g, and d) called tocopherols and four
Accepted 27 July 2010
unsaturated analogues referred to as tocotrienols. While the tocopherols have been investigatedextensively, little is known about the tocotrienols. Very limited studies suggest that both the molecular
and therapeutic targets of the tocotrienols are distinct from those of the tocopherols. For instance,
suppression of inflammatory transcription factor NF-kB, which is closely linked to tumorigenesis and
inhibition of HMG-CoA reductase, mammalian DNA polymerases and certain protein tyrosine kinases, is
unique to the tocotrienols. This review examines in detail the molecular targets of the tocotrienols and
their roles in cancer, bone resorption, diabetes, and cardiovascular and neurological diseases at both
preclinical and clinical levels. As disappointment with the therapeutic value of the tocopherols grows,
the potential of these novel vitamin E analogues awaits further investigation.
ß 2010 Elsevier Inc. All rights reserved.
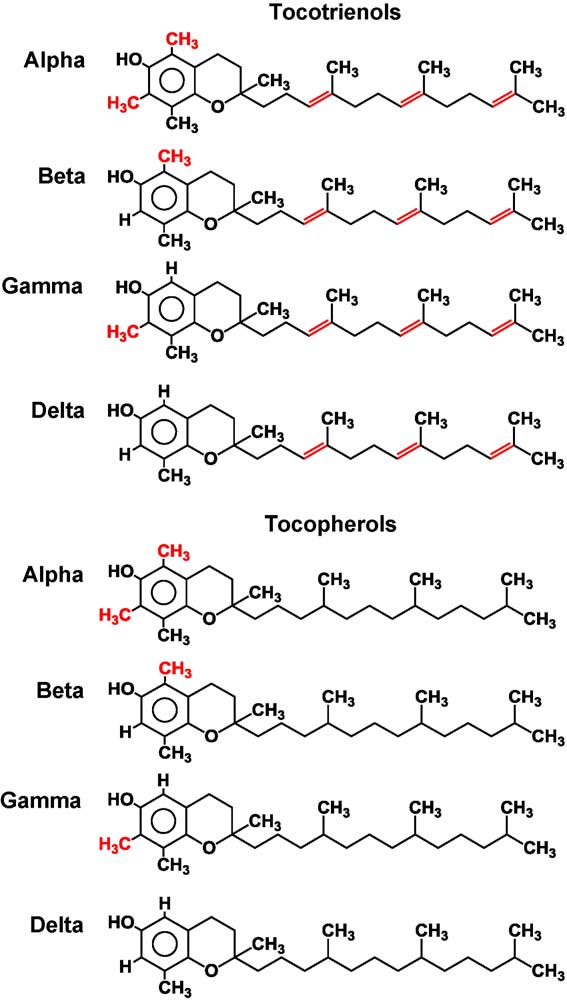
are saturated forms of vitamin E, whereas the tocotrienols areunsaturated and possess an isoprenoid side chain. Some evidence
Preventing beriberi by eating unpolished rice, curing scurvy by
suggests that human tissues can convert tocotrienols to tocopherols
eating citrus fruits, and supporting fertility by eating leafy
. Tocopherols consist of a chromanol ring and a 15-carbon tail.
vegetables—all of these life-sustaining properties of foods are
The presence of three trans double bonds in the tail distinguishes
related to factors that in 1912 came to be called vitamins (vita
tocopherols from tocotrienols. The isomeric forms of tocotrienol are
means life). In 1922, Herbert Evans and Katherine Bishop, two
distinguished by the number and location of methyl groups on the
prominent researchers from Berkeley, first isolated fat-soluble
chromanol rings: a-tocotrienol is 5,7,8-trimethyl; b-tocotrienol is
vitamin E from green leafy vegetables and described it as a fertility
5,8-dimethyl; g-tocotrienol is 7,8-dimethyl and d-tocotrienol is
factor. Vitamin E was named tocopherol in 1924 and synthesized in
8-monomethyl. While leaves and seeds of most plants contain
1938 [for references, see Deficiency of this vitamin is now
tocopherols, tocotrienols are present in only a very small fraction of
known to cause severe degenerative diseases such as ataxia,
plants and b). Although some activities of tocopherols and
Duchenne muscular dystrophy-like muscle degeneration, and
tocotrienols are compared in this review, tocotrienols are the
infertility. Vitamin E is present in most edible oils to various
primary focus.
extents, including those extracted from wheat germ oil, wheat, rice
The name tocotrienol to denote a tocopherol with a true
bran (0.035%), barley (0.012% or 44 mg/g oil), oats (0.03%), coconut
isoprenoid side chain was first suggested by Bunyan et al. , and
(0.019%) and palm (0.044%; 0.78–1.08 mg/g oil) (
the tocotrienols were described in Nature when isolated from the
latex of the rubber plant, Havea brasiliensis, in 1964 The
While alpha-tocopherol was the first vitamin E analogue to be
tocotrienols attracted no real attention until the 1980s and 1990s
recognized, eight chemically distinct analogues are now known,
when their cholesterol-lowering potential and anticancer
consisting of alpha (a), beta (b), gamma (g) and delta (d)-
effects were described Subsequently, rice bran, palm, and
tocopherols (TP) and alpha, beta, gamma and delta-tocotrienols
annatto (90% delta and 10% gamma) oils were described as some of
(T3); all of them are referred to as vitamin E ). The tocopherols
the richest sources of tocotrienols by Tan and his coworkers. Thetocopherols:tocotrienols ratios in rice bran, palm and annatto oilsare 50:50; 25:75 and 0.1:99.9, respectively . Besides tocopher-ols, various isomers of tocotrienols have also been detected in
* Corresponding author. Tel.: +1 713 794 1817.
E-mail address: (B.B. Aggarwal).
human milk .
0006-2952/$ – see front matter ß 2010 Elsevier Inc. All rights reserved.
doi:
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
growth-suppressive effects of this agent. Moreover, inhibition ofmitogen-activated protein kinases (MAPK) such as ERK p38MAPK and JNK is critical to the antiproliferative effects oftocotrienols. The suppression of cyclin D1 expression induced bytocotrienols also plays an important role in the growth-inhibitoryactivities of this vitamin Tocotrienols impedethe survival of various tumor cells by inhibiting expression of cellsurvival proteins such as XIAP, IAP-1, IAP-2, bcl-2, bcl-xl, c-FLIP,TRAF-1, survivin and Bfl-1/A1 . Suppression of the phospho-tidyl-inositol-3-kinase (PI3K)/AKT pathway by tocotrienols couldaccount for its antisurvival activities Downregulation of thetelomerase, c-myc, and raf–ERK signaling pathways has beenlinked to tocotrienol's ability to inhibit cell survival .
Various studies have revealed that tocotrienols can induce
apoptosis in a wide variety of tumor cells. These effects aremediated through activation of both extrinsic and intrinsicpathways by the vitamin. The extrinsic pathways involveinduction of death receptors and activation of caspase-8,which leads to caspase-3 activation The activation of intrinsicpathways by tocotrienols involves mitochondrial depolarizationand is mediated through the upregulation of Bax cleavage of Bid release of cytochrome C , andactivation of caspase-9, which in turn leads to activation ofcaspase-3 . This unsaturated form of vitamin E alsomediates apoptosis through DNA fragmentation andupregulation of p53 in certain cells.
The suppression of angiogenesis by tocotrienols is mediated
through inhibition of VEGF expression and VEGF receptorsignaling Suppression of the matrix metalloproteinase(MMP)-9 gene could also contribute to the angiogenesis-suppres-sive activity . Although TWIST, CXCR4, TNF, FGF, TGF-b,PDGF and IL-8 all have been linked with angiogenesis, whether anyof these pathways is modulated by tocotrienols is poorlyunderstood.
Numerous lines of evidence suggest that tocotrienols exhibit
Fig. 1. Chemical structure of tocotrienols and tocopherols.
potent anti-inflammatory activity. First, activation of the tran-scription factor NF-kB has been closely linked with inflammationSecond, tocotrienols have been shown to suppress the
2. Molecular targets
expression of TNF IL-1 IL-6 , IL-8 induciblenitric oxide synthase and cyclo-oxygenase 2 , all
Like tocopherols, tocotrienols exhibit antioxidant activities, and
of which mediate inflammation. Third, tocotrienols have been
most of its effects can be linked to its antioxidant function.
shown to suppress STAT3 cell-signaling pathway, also involved in
Molecular targets of tocotrienols can be classified as those that are
inflammation Hypoxia-induced factor-1 is another path-
modulated by binding directly and those that are
way that has been linked with inflammation and is modulated by
modulated indirectly. Modulation of various targets by tocotrie-
tocotrienols .
nols may occur at the transcriptional, translational, or post-
Tocotrienols inhibit various protein kinases, including protein
translational levels, or by direct interactions with cellular targets
kinase C p60 Src , IkBa kinase and GSK-3b
For instance, src and 3-hydroxy-3-methyl-glutaryl
Inhibition of HMG-CoA reductase, an enzyme that is rate limiting
coenzyme A (HMG-CoA) reductase are modulated through direct
in the pathway to cholesterol biosynthesis also plays an
binding, whereas inflammatory transcription factors and the genes
essential role in the various activities attributed to this vitamin.
regulated by them and death receptors are modulated indirectly
There are, for instance, reports that the antitumor effects of
and b). Various studies indicate that tocotrienols exhibit
tocotrienols are linked to its ability to inhibit HMG-CoA reductase
antioxidant, antiproliferative, antisurvival, proapoptotic, antian-
Different isomeric forms of tocotrienols vary in their
giogenic, and anti-inflammatory activities.
ability to lower cholesterol, as follows: d > g > a > b . The
The antioxidant activities of this vitamin E (tocotrienols) are
reduction of HMG-CoA occurs through two separate mechanisms,
mediated through induction of antioxidant enzymes such as
first the enhancement of degradation of the reductase protein and
superoxide dismutase , NADPH:quinone oxidoreductase
second the decrease in efficiency of translation of the reductase
and glutathione peroxidase , which quench free radicals
such as superoxide radicals (). The antiproliferative
The modification by tocotrienols of various cell-signaling
activity of tocotrienols are mediated through modulation of
pathways described here has been linked to its effects against
growth factors such as vascular endothelial growth factor (VEGF)
cancer, diabetes, and cardiovascular and neurological diseases.
, basic fibroblast growth factor (bFGF) and transforminggrowth factor-beta (TGF-b) HER2/neu and interleukin-6
3. In vitro studies
(IL-6) Cyclin-dependent kinases (CDK2, CDK4, CDK6) andtheir inhibitors, such as p21, p27 and p53 and down-
Numerous in vitro studies indicate that tocotrienols exhibit
regulation of Rb phosphorylation also mediate the
anticancer, cardioprotective, and neuroprotective effects ).
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
Table 1A list of molecular targets modulated by tocotrienols in various cell types.
Apoptotic regulators
Transcription factors
Adhesion molecules
Modulation of various targets by tocotrienol at transcription, translation, post-translation or by direct interaction are indicated by the superscripts 1, 2, 3 or 4, respectively.
12-LOX, 12-lipoxygenase; Apo A, apolipoprotein A; bFGF, basic fibroblast growth factor; CDK, cyclin-dependent kinases; C/EBPa; CCAAT/enhancer-binding protein-alpha;CHOP, C/EBP homologous protein; COX-2, cyclo-oxygenase-2; Cyt C, cytochrome C; DR5, death receptor 5; EGFR, endothelial growth factor receptor; eNOS, endothelial nitricoxide synthase; ER-a, estrogen receptor alpha; ERK, extracellular signal-regulated kinase; FLIP, FLICE-like inhibitory protein; GGT, gamma-glutamyl transpeptidase; GPx,glutathione peroxidase; GSH, reduced glutathione; GST, glutathione S-transferase; HIF-1a, hypoxia-inducible factor-1alpha; HMGCR, 3-hydroxy-3-methyl-glutarylcoenzyme A reductase; hTERT, human telomerase reverse transcriptase; IAP, inhibitors of apoptosis; ICAM-1, intercellular adhesion molecule-1; Id-1, inhibitor ofdifferentiation; IFN, interferon; IKK, IkB kinase; IL, interleukin; iNOS, inducible nitric oxide synthase; JNK, c-Jun NH(2)-terminal kinase; LDL-R, low-density lipoproteinreceptor; MAO-A, monoamine oxidase A; MAPK, mitogen-activated pathway kinase; MMP, matrix metalloproteinase; NF-kB, nuclear factor-kappa B, NQO1,NAD(P)H:quinone oxidoreductase; PARP, poly (ADP-ribose) polymerase; PDK, phosphoinositide-dependent protein kinase; PDK-1, Pl3K-dependent kinase 1; Pl3K,phosphoinositide 3-kinases; PF-4, platelet factor-4; PGE, prostaglandin; p-GSK3b, phospho-glycogen synthase kinase3 beta; PKC, protein kinase C; PLA(2), phospholipaseA(2); PPAR, peroxisome proliferator-activated receptors; PXR, pregnane X receptor; SOD, super oxide dismutase; SREBP, sterol regulatory element binding proteins; STAT,signal transducer and activator protein; SXR, steroid and xenobiotic receptor; TGF-b RII, tissue growth factor-beta receptor II; TIMP, tissue inhibitor of metalloproteinases;TNF, tumor necrosis factor; TRAF, TNF receptor-associated factor 1; TX-B2, thromboxane B2; VCAM-1, vascular cell adhesion molecule; VEGFR, vascular endothelial growthfactor receptor; XIAP, X-linked inhibitor of apoptosis protein.
3.1. Anticancer effects
enzyme in cholesterol biosynthesis and reduce theexpression of adhesion molecules and monocyte–endothelial cell
Tocotrienols have been shown to suppress proliferation and
induce apoptosis in wide variety of tumor cells including those of thebreast
3.3. Neuroprotective effects
, liver , lung , stomach skin, pancreas , and prostate A number of
Various reports suggest that tocotrienols are neuroprotective,
mechanisms have been proposed by which tocotrienols induce
as indicated by its ability to suppress glutamate-induced activation
apoptosis in these cancer cells, as already described. Some additional
of c-Src kinase Tocotrienols also have activity against
mechanisms involve induction of death receptor-5, as described
Parkinson disease .
recently . Interaction of tocotrienols with estrogen receptors hasbeen implicated in studies of breast cancer cells Various
4. Animal studies with tocotrienols
results indicate that g- and d-tocotrienol exhibit greater anticanceractivity than a- or b-tocotrienol .
4.1. Anticancer effects
3.2. Cardioprotective effects
Tocotrienols exhibit activity in different models of both
prevention and treatment of cancer ). Perhaps the first
Tocotrienols' cardioprotective effects are mediated through
report about the therapeutic potential of tocotrienols for cancer in
their antioxidant mechanisms and their ability to suppress
animal models was by Kato et al., who in 1985 showed that
inflammation, and inhibit HMG-CoA reductase, a rate-limiting
tumor-bearing rats administered with tocotrienols had an
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
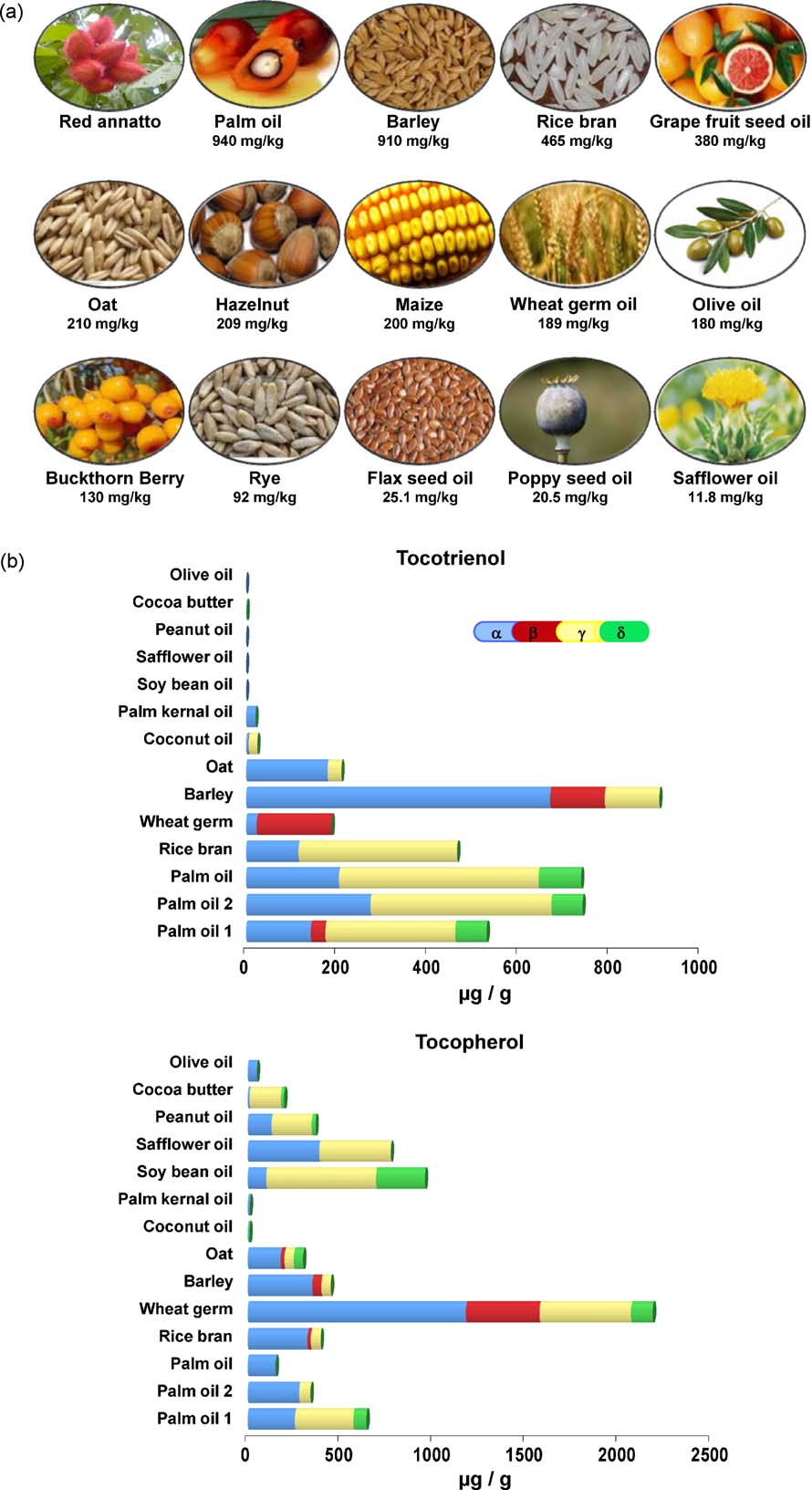
Fig. 2. (a) Natural sources of tocotrienols. For reference see red annatto ; palm oil rice bran oil grape seed oil, maize, wheat germ oil hazel nut ;olive oil ; buckthorn berry ; rye ; oat and barley flax seed oil, poppy seed oil, safflower oil . (b) Content of tocotrienol and tocopherol isomers fromvarious sources. For reference see
extended life span . Komiyama et
al. observed antitumor
a-tocotrienol as an antitumor agent They also showed
activity when tocotrienols were administered intraperitoneally to
that tocotrienols are better antioxidants than tocopherols. The
mice with established murine Meth A fibrosarcoma. They showed
growth of highly metastatic B16 melanoma in female mice was
that tocotrienols were more effective than a-tocopherol, and
inhibited by tocotrienols, and d-tocotrienol was more active than
among the tocotrienols, g-tocotrienol was more effective than
g-tocotrienol in this setting . In mice implanted with
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
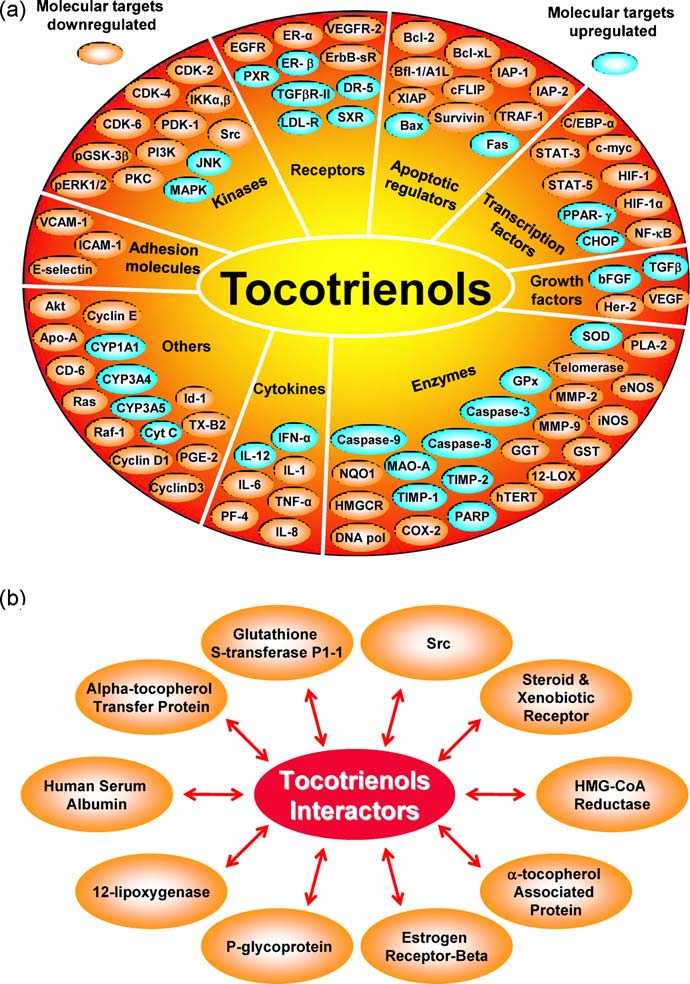
Fig. 3. (a) Molecular targets of tocotrienols. (b) Proteins that directly interact with tocotrienols.
hepatoma, both g-tocotrienol and d-tocotrienol delayed tumor
lymphoblastoid cells. They showed that g- and d-tocotrienol
growth, and when examined for levels of tocotrienols, the tumors
derived from palm oil exhibit strong activity against tumor
contained a specific accumulation of these analogues .
promotion by inhibiting EBV early antigen expression in Raji cells
The antitumor effects of tocotrienols appear to be mediated in
induced by phorbol ester. However, a- and g-tocopherol and
part through their ability to suppress angiogenesis .
dimers of g-tocotrienol or g-tocopherol lack this activity . Iqbal
Suppression of angiogenesis is mediated through reduction in
et al. showed that feeding tocotrienol-rich fraction (TRF; 10 mg/
serum levels of VEGF and inhibition of the PI3K–AKT pathway. The
kg) to DMBA-administered rats suppressed mammary carcino-
inhibition of HMG-CoA reductase and the consequent decrease in
genesis, and this correlated with declines in serum cholesterol,
serum cholesterol level has been linked with the tumor-suppres-
low-density lipoprotein (LDL)-cholesterol, and HMG-CoA reduc-
sive action of tocotrienols . Tocotrienols have also been shown
tase protein Wada et al. examined the effect of 0.05% oral
to enhance the antitumor effects of other agents. In one study, d-
tocotrienols on spontaneous liver carcinogenesis in male mice and
tocotrienol was reported to enhance the growth-suppressive
on glycerol-induced lung tumor promotion in male mice initiated
effects of lovastatin in the B16 melanoma model in mice .
with 4-nitroquinolone 1-oxide . Incidence of liver and lung
g-Tocotrienol preferentially sensitized human prostate cancer in
tumors was almost 80% lower in treated animals than in untreated
nude mice to radiation .
Besides antitumor effects against established tumors, toco-
Tocotrienols have been shown to prevent chemical-induced
trienols have also been shown to be effective in cancer prevention
carcinogenesis of the liver and found to suppress 2-
models. Sundram et al. showed that palm oil, one of the richest
acetylaminofluorene (AAF)-induced hepatocarcinogenesis
dietary sources of tocotrienols, is effective in preventing 7,12-
In another study, Rahmat et
al. examined the effect of
dimethylbenz[a]anthracene (DMBA)-induced mammary carcino-
long-term administration of tocotrienols on hepatocarcinogenesis
genesis in rats, but corn oil and soybean oil, which contain
in rats. Liver carcinogenesis was induced by diethylnitrosamine and
tocopherols but not tocotrienols, lack this activity . Gould et al.
AAF in rats fed a diet containing 30 mg/kg tocotrienols for 9 months.
reported a statistically significant increase in tumor latency in the
Expression of biomarkers of liver carcinogenesis such as glutathione,
DMBA-induced rat mammary tumor model with tocotrienols but
alkaline phosphatase, and gamma-glutamyl transpeptidase was
not with tocopherols Inhibition of tumor promotion by
enhanced by the carcinogens but attenuated by tocotrienols,
various palm-oil tocotrienols was also reported by Goh et al.
decreasing the impact of the carcinogens. A similar study by others
in an in vitro assay utilizing the activation of Epstein–Barr virus
confirmed these findings . All these studies suggest that
(EBV) early antigen expression in EBV-genome-carrying human
tocotrienols have potential to both prevent and treat cancer.
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
Table 2In vitro studies with tocotrienols for effects against cancer, cardiovascular and neurodegenerative diseases.
Anticancer effect
Inhibited estrogen receptor-negative and -positive cell proliferation
Inhibited growth of cells irrespective of estrogen receptor status
Induced cell death by DNA fragmentation
Suppressed preneoplastic mammary epithelial cell proliferation
Induced apoptosis through caspase pathway
Induced apoptosis through mitochondria-mediated death pathway
Induced apoptosis through TGF-beta–Fas–JNK-signaling pathways
Inhibited cell proliferation and induced apoptosis in neoplastic mammary cells
Exhibited synergism with statin in suppressing proliferation of tumor cells
Exhibited synergism with phytochemicals in suppressing proliferation of tumor cells
Exhibited synergism with celecoxib in suppressing proliferation of tumor cells
Exhibited synergism with erlotinib/gefitinib in suppressing tumor cell proliferation
Inhibited proliferation by arresting cell-cycle progression
Inhibited tumor cell growth by suppressing HMGR activity
Induced apoptosis in tumor cells through endoplasmic reticulum stress
Inhibited proliferation through downregulation of Id1 protein
Reduced cell viability and induced apoptosis via the mitochondrial pathway
Inhibited colony formation through death receptor-5 and CHOP upregulation
Inhibited growth and colony formation through DNA fragmentation
Induced apoptosis and inhibited cell proliferation through cell-cycle arrest
Showed synergistic inhibition of cancer cell growth
Reduced cell viability and proliferation through DNA fragmentation
Exerted antiproliferative effect by inducing S phase arrest
Induced Bax and Bid regulated apoptosis
Induced apoptosis on accumulation of cells in G1 phase through mutation of ras genes
Suppressed survival and invasion capacity of the tumor cells
Enhanced cisplatin-induced cytotoxicity in mesothelioma cells
Induced apoptosis through downregulation of the Raf–ERK signaling pathway
Inhibited cell migration and invasion through downregulation of matrix metalloproteinase
Induced apoptosis via mitochondria-dependent apoptosis pathway
Inhibited cell proliferation and potentiated lovastatin-mediated growth suppression
Induced apoptosis by activating procaspases and accumulating sub-G1 cell population
Induced apoptosis and cycle arrest at G1 phase
Inhibited cell growth
Inhibited cellular proliferation and accelerated apoptotic events
Suppressed cell proliferation and invasion through multiple-signaling pathways
Activated caspase-dependent programmed cell death
Inhibited growth of human and mouse tumor cells
Inhibited tumor promotion in human lymphoblastoid cells
Inhibited both proliferation and tube formation and minimized tumor angiogenesis
Inhibited angiogenesis and telomerase activity
Inhibited pol lambda activity and angiogenesis
Cardiovascular diseases
Inhibited surface cell expression and adhesion
Inhibited cholesterol biosynthesis
Inhibited glutamate-induced death of HT4 neuronal cells
Inhibited H2O2-induced neuronal death and oxidative stress-mediated cell death
Attenuated homocysteic acid-induced neurotoxicity
Prevents oxidative stress stimulated cell death of cortical neurons cells
Protected methylmercury-induced neuronal cell death
CHOP, C/EBP homologous protein; ERK, extracellular signal-regulated kinase; HMGR, 3-hydroxy-3-methyl-glutaryl coenzyme A reductase; Id1, inhibitor of differentiation;JNK, c-Jun N-terminal kinase; TGF, transforming growth factor.
4.2. Cardioprotective effects
that the tocotrienols' effects were more pronounced than those ofa-tocopherol TRF from palm oil can reduce total cholesterol
Persistent hypertension is one of the risk factors for strokes,
and LDL-cholesterol levels through downmodulation of hepatic
heart attacks, and heart failure and is a leading cause of chronic
HMG-CoA reductase activity . Whether rice bran oil with its
renal failure. Most of the cardioprotective effects of tocotrienols
high content of g-oryzanol and g-tocotrienol has the same effect
are mediated through their ability to inhibit a rate-limiting
has been investigated in rats . A rice bran oil diet lowered
enzyme in cholesterol biosynthesis and their antioxidant and anti-
plasma triglyceride, LDL-cholesterol and hepatic triglyceride
inflammatory activities. In one study, tocotrienols significantly
concentrations and increased hepatic cholesterol 7-alpha-hydrox-
depressed age-related increases in systolic blood pressure of
ylase, hepatic LDL receptor, and HMG-CoA reductase mRNA in rats.
spontaneously hypertensive rats, and the investigators concluded
The g-oryzanol and g-tocotrienol in rice bran oil can lead to
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
Table 3A list of animal studies with tocotrienols for pharmacokinetics and for effects against cancer, cardiovascular, diabetes, neurodegenerative diseases, bone metabolism andother diseases.
Increased LLU-alpha concentration by oral administration in rats
Selective uptake of T3 into the rat skin
Distribution and bioavailability of a-, g-T3 in rats elevated by sesame
Distribution and metabolism in adipose tissues and skin of rats
NOAEL of toxicity by T3 in rats
Stimulated sodium excretion in vivo in rats
Bioavailability (delivered by ip and im than oral) in rats
Effective distribution of T3 homologues to rat eye tissues
Preferential absorption of a-T3 than g- and d-T3
Postprandial levels of the natural vitamin E-T3 in human circulation
Tissue distribution and accumulation in adipose tissue
Fast intestinal uptake of g-T3
More extensive metabolism of g-T3 than g-TP in rats
Bioavailability of d-T3 is longer in pancreas with no toxicity
Anticancer effects
Inhibited mammary carcinogenesis in female rats
Effective against sarcoma, Ehrlich and IMC carcinoma
Reduced the severity of AAF-induced hepatocarcinogenesis in rats
Attenuated DEN and AAF-induced hepatocarcinogenesis in rats
Reduced AAF-induced increase in enzyme activities in rats
Inhibited tumor promotion
Suppressed the growth of B16 melanoma in mice
Inhibited chemical-induced cancer in rats
Suppressed DMBA-induced mammary tumors and hypercholesterolemia
Inhibited TPA-induced skin carcinogenesis
Delayed the onset, incidence and size of human breast cancer in nude mice
Suppressed liver and lung carcinogenesis in mice
Radio sensitized human prostate tumors in nude mice
Potentiated lovastatin-induced growth suppression
Reduced UVB-induced sunburn and incidence of tumor in hairless mice
Delayed tumor growth in mice hepatoma
Suppressed tumor growth via angiogenesis
Chemosensitizer in hormone refractory prostate cancer
Suppressed neovascularization in tumor cell-implanted mice
Anti-angiogenic effect in BALB/c mice model, reduced VEGF
Cardiovascular disorders
Depressed the age-related increase in blood pressure of SHR
Decreased levels of MDA and preserved continuity of IEL in rabbit aorta
Reduction in atherosclerotic lesion size by d-P25-T3 in mice
Increased nitric oxide activity and reduced the blood pressure in rats
Activated NO–cGMP pathway and reduced ischemia/reperfusion myocardial injury in rats
Reduced myocardial infarct size in rats
Reduced autophagy during MI by elevating Beclin, LC3-II and mTOR signaling in rats
Diabetes mellitus
Prevented increase in AGE in streptozotocin-induced diabetic rats
Protected against oxidative damage in diabetic KKAy mice
Reduced the antioxidant biomarker level in mice
Reduced serum creatinine level, creatinine clearance, U albumin and protein excretion in ODS rats
Modulated streptozotocin-induced inflammation in diabetic rats
Modulated diabetes-induced cognitive impairment
Lowered the blood glucose level and improved dyslipidemia in diabetic rats
Improved insulin sensitivity in mice through activation of PPARs
Inhibited glutamate-induced pp60(c-Src) kinase activation and death of HT4 cells
Expressed T3 sensitive genes in the developing rat fetal brain
Modulated 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration
Prevented cerebral infarction induced by MCA occlusion
Protected against glutamate- and stroke-induced neurodegeneration
Inhibited c-Src activity leading to prevention of glutamate-induced neurodegeneration
Attenuated oxidative–nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy
Prevented intracerebroventricular STZ-induced cognitive impairment and oxidative–nitrosative stress
Ameliorated behavioral and biochemical alterations in the rat model of alcoholic neuropathy
Prevented chronic alcohol-induced cognitive dysfunction by suppression of neuroinflammation
Inhibited glutamate-induced activation of phospholipase A2
Reduced bone resorption to a greater extent than bone formation in thyrotoxic rats
Helped in normal bone calcification in female
Reduced body fat mass and increased bone calcium content in adrenalectomized rats
Reversed nicotine-induced bone loss in rats
Reversed free radical-induced bone loss in rats
Exhibited antioxidant activity and prevented imbalance in bone metabolism due to free radicals in rats
Acted as an anabolic agent for bone in normal male rats
Improved normal bone structure
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
Table 3 (Continued )
Inhibited cholesterol esterase activity in rats
Reduced lipid peroxidation and enhanced superoxide dismutase activity in SHR
Inhibited HMGCR, increased HDL, lowered LDL and TC in pigs
Improved the lipid profile, lowered TG and increased HDL-c in rats
Enhanced cholesterol catabolism by increasing LDL-R and HMG-CoA level in rat liver
Increased fecal excretion of neutral sterol and bile acids in rats
Suppressed Akt phosphorylation in 3T3-L1 preadipocytes in rats
Enhanced proliferation and function of spleen and MLN lymphocytes
Prevented aspirin-induced gastric lesion
Blocked stress-induced changes in the gastric acidity and gastrin level in rats
Maintained renal morphology against iron induced renal dysfunction
AAF, 2-acetylaminofluorene; AGE, advanced glycosylation end-products; d-P25-T3, didesmethyl tocotrienol; DEN, diethyl nitrosamine; DMBA, 7,12-dimethylbenz[a]an-thracene; HDL, high-density lipoprotein; HDL-c, HDL cholesterol; HMG-CoA, 3-hydroxy-3-methyl-glutaryl coenzyme A reductase; HMGCR, HMG-CoA reductase; IEL, internalelastic lamina; LDL, low-density lipoprotein; LDL-R, LDL receptor; LLU-alpha, 2,7,8-trimethyl-2-(beta-carboxyethyl)-6-hydroxy chroman; LP, lipid profile; MCA, middlecerebral artery; MDA, malondialdehyde; MI, myocardial infarct; MLN, mesenteric lymph node; m-TOR, mammalian target of rapamycin; NF-kB, nuclear factor-kappa B;NOAEL, No-observed-adverse-effect level; NO–cGMP, nitric oxide–cyclic GMP; ODS, osteogenic disorder shionogi; PPAR, peroxisome proliferator-activated receptors; SHR,spontaneously hypertensive rats; STZ, streptozotocin; TC, total cholesterol; TG, triglyceride; TPA, 12-O-Tetradecanoyl-phorbol-13-acetate; VEGF, vascular endothelialgrowth factor.
increased neutral sterol and bile acid excretion in feces via
superoxide dismutase activity . The investigators concluded
upregulation of cholesterol synthesis and catabolism. Chou et al.
that antioxidant supplementation with g-tocotrienol may prevent
observed that rice bran oil improved lipid abnormalities, reduced
development of increased blood pressure, reduce lipid peroxides in
the atherogenic index and suppressed the hyperinsulinemic
plasma and blood vessels and enhance total antioxidant status,
response in rats with streptozotocin/nicotinamide-induced type
including superoxide dismutase activity.
2 diabetes mellitus
Myocardial ischemic injury results from severe impairment of
In atherosclerosis, build-up of fatty materials such as choles-
coronary blood supply and produces a spectrum of clinical
terol leads to artery wall thickening. Nafeeza et al. investigated the
syndromes. Although all of the tocotrienol isomers have cardio-
effect of TRF on the microscopic development of atherosclerosis
protective properties against myocardial ischemic injury, g-
and lipid peroxidation in the aortas of rabbits. After 10 weeks of
tocotrienol was the most protective. The differential interaction
treatment with TRF, cholesterol-fed rabbits had lower aortic
of MAPK with caveolin 1/3 in conjunction with proteasome
contents of malondialdehyde, less intimal thickening and greater
stabilization plays a unique role in tocotrienols-mediated cardi-
preservation of the internal elastic lamina than untreated rabbits
oprotection, possibly by altering the availability of prosurvival and
. Because TRF lowered lipid peroxidation, which in turn
antisurvival proteins .
reduces intimal thickening and preserves the internal elastic
In a study of the cardioprotective properties of g-tocotrienol in
lamina, they concluded that the antioxidant activities of TRF could
combination with resveratrol, the two agents acted synergistically,
reduce experimental atherosclerosis.
providing a greater degree of cardioprotection than either alone
TRF and isomers of tocotrienols can improve postischemic
. The basis of this effect is their ability to induce autophagy
ventricular function and reduce myocardial infarct size. They exert
accompanied by activation of Beclin and LC3-II as well as mTOR
this cardioprotective effect through downmodulation of c-Src and
signaling while simultaneously generating a greater amount of
upregulation of phosphorylation of Akt, thus generating a survival
survival signal through activation of the Akt–Bcl-2 survival pathway.
signal A 6-week treatment of diet supplemented with eitherd-P(21)-T3, d-P(25)-T3, g-T3, or TRF showed significant effects on
4.3. Effects against diabetes mellitus
lipid metabolism in swine expressing hereditary hypercholester-olemia Levels of serum total cholesterol, LDL-cholesterol,
In diabetes the blood glucose level is persistently high because
apolipoprotein B, platelet factor 4, thromboxane B(2), glucose,
of insufficient insulin production or insulin resistance. TRF
triglycerides, and glucagon were reduced in all of the treatment
prevented increases in serum levels of advanced glycosylation
groups relative to the control. The hepatic HMG-CoA reductase
end-products (AGE) in normal rats and decreased blood glucose
activity was lower, and cholesterol and fatty acid levels in various
and glycated hemoglobin levels in diabetic rats . In a similar
tissues were lower in all of the treatment groups.
study, TRF treatment not only reduced serum glucose and glycated
Activation of the nitric oxide–cGMP pathway is associated with
hemoglobin concentrations, it also reduced plasma total choles-
myocardial protection against ischemia; in ischemia, the function
terol, LDL-cholesterol and triglyceride levels and increased levels
of this pathway is disturbed. Esterhuyse et al. investigated the
of high-density lipoprotein (HDL)-cholesterol, as compared to the
effects of red palm oil on the myocardial nitric oxide–cGMP
untreated group Tocotrienols exert these effects through
signaling pathway . Treatment with red palm oil increased
increasing superoxide dismutase activity and levels of vitamin C in
aortic output and increased levels of cGMP and polyunsaturated
plasma and decreasing levels of plasma and aorta malondialde-
fatty acid in rat hearts. Their findings suggest that dietary red palm
hyde and 4-hydroxynonenal and oxidative DNA damage. Thus TRF
oil protects via the nitric oxide–cGMP pathway and/or changes in
lowers blood glucose level and oxidative stress markers, improves
polyunsaturated fatty acid composition during ischemia/reperfu-
dyslipidemia, and maintains vessel wall integrity. A combination
sion. As red palm oil contains both tocopherols and tocotrienols, it
of insulin and tocotrienol treatment attenuated the diabetic
is unclear which of these constituents exerted the cardioprotective
condition and reversed neuropathic pain through modulation of
effect. Newaz et al. determined the effects of g-tocotrienol on lipid
oxidative–nitrosative stress and release of inflammatory cytokines
peroxidation and total antioxidant status of spontaneously
and caspase-3 in diabetic rats . In another study,
hypertensive rats. Their study showed that a 3-month antioxidant
suppression of the NF-kB signaling pathway by tocotrienols
trial with g-tocotrienol reduced blood and plasma concentrations
prevented diabetes-associated cognitive deficits. Rats with strep-
of lipid peroxides and improved total antioxidant status and
tozotocin-induced diabetes were treated with oral tocotrienols
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
(25 mg/kg, 50 mg/kg or 100 mg/kg body weight) for 10 weeks,
expression profiling. HO-3, LINE-1, and ApoB are some of the vitamin
which significantly prevented behavioral, biochemical and molec-
E-sensitive genes affected by vitamin E treatment
ular changes associated with diabetes, in part through suppression
A cerebral infarction is an ischemic kind of stroke caused by a
of activation of the NF-kB signaling pathway .
disturbance in the blood vessels supplying blood to the brain. a-
Oxidative stress is considered to be a key factor in the
Tocopherol, a-tocotrienol and g-tocopherol significantly de-
development of diabetes and its complications. Kanaya et
creased the size of cerebral infarcts in the mice middle cerebral
examined the antioxidative effects of a crude lipophilic rice bran
artery occlusion model, while g-tocotrienol, d-tocopherol and d-
extract, Ricetrienol, which contains a-tocopherol, tocotrienols, and
tocotrienol showed no effect . Tiwari et al. demonstrated the
phytosterol, in obese diabetic KKAy mice While Ricetrienol
effectiveness of tocotrienols in attenuation of alcoholic neuropathy
did not affect hyperglycemia, body weight, or hyperlipidemia, it
. Treatment with a-tocopherol and tocotrienols (mixture of
did significantly suppress elevation of plasma malondialdehyde
a-, b-, g-tocotrienol) for 10 weeks significantly improved
and significantly increase glutathione peroxidase (GPx) mRNA
nociceptive threshold, paw-withdrawal threshold and superoxide
expression at the 0.1% concentration; the authors suggested that
dismutase levels and decreased tumor necrosis factor alpha (TNF-
Ricetrienol exerts a protective effect against oxidative damage in
a) and IL-1b levels in male Wistar rats. In another study, they
diabetes mellitus. Yoshida et al. evaluated the antioxidant
investigated the effect of a-tocopherol and a-tocotrienol against
properties of natural and synthetic dietary antioxidants by using
the biomarker, total hydroxyoctadecadienoic acid (tHODE) .
pairment and oxidative–nitrosative stress in rats. Both isoforms
Remarkable increases in tHODE and total 8-iso-prostaglandin F
effectively attenuated the reductions in glutathione and catalase
(2alpha) (t8-iso-PGF (2alpha)) levels were observed in the plasma,
and reduced the malondialdehyde, nitrite and cholinesterase
erythrocytes, liver and brain of mice that were fed an a-
activity in the brains of these rats, but the effect was more potent
tocopherol-stripped (E-free) diet, whereas levels of these markers
with tocotrienols .
were reduced in mice treated with the E-free diet supplementedwith a lipophilic antioxidant (0.04% by wt) containing a-
4.5. Effects on bone metabolism
tocopherol, a-tocotrienol, and g-tocopherol.
al. investigated the mechanism through which
Tobacco smoking has been identified as a risk factor in the
tocotrienols reduce blood glucose levels in patients and in
development of osteoporosis, vitamin E supplements reverse
preclinical animal models . They proposed that tocotrienols
nicotine-induced bone loss and stimulate bone formation
function as peroxisome proliferator-activated receptor (PPAR)
Another group has shown that tocotrienols can reverse nicotine-
modulators. PPARs are ligand-regulated transcription factors that
induced bone loss in rats (Bone histomorphometric
play essential roles in energy metabolism. Synthetic PPARa and
parameters of adult male rats treated with TRF or g-tocotrienol but
PPARg ligands have been used recently in the treatment of
not with g-tocopherol (60 mg/kg) following nicotine treatment
hyperlipidemia and diabetes. Both a- and g-tocotrienol activated
showed significantly higher trabecular thickness and less eroded
PPARa, while d-tocotrienol alone activated PPARa, PPARg, and
surface than the control group. Tocotrienols are slightly superior to
PPARd in reporter-based assays. Tocotrienols enhanced the
tocopherols in attenuating the effects of tobacco; g-tocotrienol
interaction between the purified ligand-binding domain of PPARa
especially may have therapeutic potential to repair bone damage
and the receptor-interacting motif of coactivator PPARg coacti-
caused by chronic smoking. This vitamin is an anabolic agent for
vator-1alpha. They also found that TRF improved whole-body
bone in normal male rats .
glucose utilization and insulin sensitivity of diabetic Db/Db mice
Other studies have shown that tocotrienols can reverse
by selectively regulating PPAR target genes . All of these
glucocorticoid-induced or free radical-induced bone loss in
results indicate that tocotrienols have antidiabetic potential.
adrenalectomized rats and improve normal bonestructure possibly through its antioxidant activity
4.4. Neuroprotective effects
in bone Maniam et al. investigated the effects of vitamin Eon lipid peroxidation and antioxidant enzyme levels in rat bones
Numerous reports indicate that tocotrienols exhibit neuropro-
. They found that palm-oil tocotrienols at the dose of 100 mg/
tective effects under a wide variety of conditions
kg body weight significantly reduced the level of thiobarbituric
Chopra and her group noted neuroprotection by tocotrienols in
acid-reactive substance in the femur while significantly increasing
an experimental model of diabetic neuropathy in the rat
glutathione peroxidase activity compared to the control group;
model of alcoholic neuropathy in chronic alcohol-induced
these effects were not observed in rats treated with g-tocopherol.
cognitive dysfunction in rats , in intracerebroventricular
Tocotrienols also showed a protective effect against free radical
streptozotocin-induced cognitive impairment and oxidative–nitro-
damage in the rat femur bones. Long-term glucocorticoid
sative stress in rats , in diabetic nephropathy and in
treatment is associated with severe side effects, such as obesity
diabetes-associated cognitive deficits , all through suppression
and osteoporosis. Ima-Nirwana et al. showed that treatment with
of proinflammatory pathways. Sen and his group have examined
g-tocotrienol (60 mg/kg body weight/day) reduced body fat mass
extensively the prevention of glutamate-induced neurodegenera-
and increased fourth lumbar vertebra bone calcium content in rats,
tion by tocotrienols . They found that
while a-tocopherol was ineffective Therefore, palm-oil-
modulation of c-Src, 12-lipoxygenase and PLA2 is involved in the
derived g-tocotrienol has the potential to be utilized as a
neuroprotective effects of tocotrienols. Khanna et al. showed that a
prophylactic agent in prevention of the skeletal side effects of
subattomole quantity of a-tocotrienol, but not g-tocopherol,
long-term glucocorticoid and tobacco use.
protected neurons from glutamate challenge. Rats given a a-tocotrienol supplement showed more protection against stroke-
4.6. Immunomodulatory effects
induced injury through downregulation of c-Src activation and 12-lipoxygenase phosphorylation at the stroke site . Roy et al.
al. demonstrated the immunoregulatory effects of
reported that dietary tocotrienols are bioavailable to both mother
dietary a-tocopherol and mixture of tocotrienols on humoral- and
and fetal brains and that the enrichment is greater in fetal brain
cell-mediated immunity . Their results showed that toco-
tissue. They also identified a specific set of vitamin E-sensitive genes
pherols or tocotrienols feeding enhanced expression of interfer-
in the developing rat fetal brain using GeneChip microarray
on-gamma, IgA, and IgG, but not IgE, and decreased the proportion
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
of CD4+ T cells. Interestingly, tocotrienols decreased the expres-
ability of a-tocotrienol was approximately 28%, while the
sion of TNF-a. These investigators concluded that oral adminis-
bioavailability of g- and d-tocotrienol were around 9%
tration of tocopherols and tocotrienols affects the proliferation
Phase I cytochrome p450 3A4 enzyme and P-glycoprotein at the
and function of spleen and mesenteric lymph node lymphocytes.
gastrointestinal epithelium are implicated in the oral absorption oftocotrienols. Preferential absorption of a-tocotrienol over g-
4.7. Gastroprotective effects
tocotrienol and d-tocotrienol in rats is in agreement with otherreports in pigs and lymphatic cannulated rats . These
al. compared the impacts of tocopherols and
differences may be linked to the number of methyl groups in the
tocotrienols on gastric acidity, gastric tissue content of parameters
chromanol ring, as a-tocotrienol has three, g-tocotrienol has two,
such as malondialdehyde and prostaglandin E2, and serum levels
and d-tocotrienol has one methyl group, and thus they have
of gastrin and glucagon-like peptide-1 in rats exposed to restraint
stress. They found that tocotrienol-treated animals, both stressed
In another study, following a single oral administration of d-
and non-stressed, had comparable gastric acidity and gastrin levels
tocotrienol (100 mg/kg), the peak plasma concentration was
. Both tocopherols and tocotrienols had gastroprotective
57 � 5 mmol/l, the time required to reach peak plasma concentration
effects against damage by free radicals generated in stress
was 2 h, and the plasma half-life was 3.5 h. The tocotrienols were
conditions, but only tocotrienols had the ability to block stress-
cleared from plasma and liver within 24 h, but clearance from the
induced changes in gastric acidity and gastrin level. Another group
pancreas was delayed . d-Tocotrienol was 10-fold more
showed that tocotrienols can prevent aspirin-induced gastric
concentrated in the pancreas than in the tumor and no toxicity
lesions through their ability to limit lipid peroxidation .
was shown by d-tocotrienol (100 mg/kg) in mice. Intestinal epithelialcells absorb g-tocotrienol faster than a-tocopherol. Tocotrienol
5. Pharmacokinetics of tocotrienol
isomers accumulated rapidly in Caco2 cells treated with micelles ofvitamin E isomers consisting of bile salts, lysophospholipids, free fatty
Numerous studies on the pharmacokinetics, organ and tissue
acid, and 2-monoacylglycerols and was greater than the accumula-
distribution and toxicity of tocopherols and tocotrienols have been
tions of corresponding tocopherol isomers This finding shows
carried out . Yap et al. determined the pharmacokinetics
that the difference in saturation of the side chains of tocopherols and
and bioavailability of a-, g-, and d-tocotrienol given via oral,
tocotrienols, rather than the difference in their rings, was responsible
intravenous, intramuscular and intraperitoneal routes in rats. They
for the rapid epithelial transport into the Caco2 cell membranes. a-
found that oral absorption of all forms of tocotrienols was
Tocopherol, a-tocotrienol and g-tocotrienol can all be retained
incomplete and that absorption of tocotrienols given via the
abundantly by the skin of rats and mice, but only a-tocopherol is
intramuscular or intraperitoneal routes was negligible; they
retained by the liver, kidney, and plasma of these animals
concluded that these routes of administration should be avoided
Dietary sesame seeds can elevate absorption and concentrations of a-
. They also found that a-tocotrienol had greater bioavailabil-
and g-tocotrienol in skin and adipose tissue Kawakami et al.
ity than g-tocotrienol and d-tocotrienol. The absolute bioavail-
investigated the distribution of tocotrienols in rats and reported that
Table 4Effects of tocotrienols in human subjects.
Biological effect
Plasma transport and tissue concentrations of T3 in humans
a- and g-T3 are metabolized to carboxyethyl-hydroxychroman derivatives and excreted in human urine
Pharmacokinetics and bioavailability of a-, g- and d-T3 varies under different food status
Lipolysis and droplet size affects T3 absorption from self-emulsifying formulations
Postprandial metabolic fate of T3-rich vitamin E differs significantly from that of a-TP
Daily supplementation of TRF did not induce immunomodulatory changes in healthy human volunteers
Neoplastic disorders
T3 concentration of adipose tissue of human breast with cancer
T3 levels in adipose tissue of benign are higher than that in malignant breast lumps in patients in Malaysia
Higher prediagnostic serum levels is associated with lower risk of developing prostate cancer
Cardiovascular and metabolic disorders
T3 lowers serum cholesterol in hypercholesterolemic humans
Palmvitee lowered both serum total cholesterol and LDL-cholesterol in humans
T3 induced decrease in cholesterol in hypercholesterolemic subjects
T3 attenuates collagen-induced platelet aggregation in patients with hyperlipidemia and carotid stenosis
T3 modulate cardiovascular diseases risk parameters of hypercholesterolemic humans
T3 had no effect on serum lipids, lipoproteins, or platelet function in men with mildly elevated serum lipid
a-Tocotrienyl acetate supplement decreased LDL oxidation in hypercholesterolemic humans
T3 exhibit synergistic effects with lovastatin on lipid parameters in hypercholesterolemic humans
T3 mixture does not improve cardiovascular disease risk factors in men and women with hypercholesterolemia
TRF (100 mg/day) suppressed serum cholesterol by in hypercholesterolemic humans
T3 is beneficial in prevention and treatment of type 2 diabetic patients with hyperlipidemia
T3 elevated plasma T3 levels but had no effect on lipid profile in healthy humans
T3 but not TP reduced fasting serum lipid levels in patients with mild hypercholesterolemia
T3 with citrus flavonoids decreased serum cholesterol levels in hypercholesterolemic subjects
T3 (self-emulsifying preparation) improved arterial compliance in 36 healthy male
Anti-aging effect
T3 (160 mg � 8 months) reduced DNA damage in older healthy adults (64)
T3 improves long-term clinical outlook and survival in patients with neurodegenerative familial dysautonomia
Topical a-T3 supplementation inhibited lipid peroxidation after benzoyl peroxide treatment of human skin
T3, tocotrienols; TP, tocopherols; TRF, tocotrienol-rich fraction; LDL, low-density lipoprotein.
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
Fig. 4. Physiological functions of tocotrienols.
g-tocotrienol was significantly distributed to the adipose tissue and
When the tocotrienol analogues were given at the same dose,
that the adipose tissue concentration increased from 1.1 nmol/g to
plasma levels of a-tocotrienol were twice those of g-tocotrienol
10.2 nmol/g according to rice bran tocotrienols intake .
and 10 times higher than those of d-tocotrienol. Another study
Nakamura et al. examined the 13-week oral toxicity of a
showed that a- and g-tocotrienol are metabolized to carbox-
tocotrienol preparation in rats and found that the no-observed-
yethyl-hydroxychroman derivatives and excreted in human urine
adverse-effect level of tocotrienols was 0.019% in the diet (i.e.,
. When human subjects (n = 6) consumed 125 mg of
120 mg/kg body weight/day for male and 130 mg/kg body -
tocotrienyl acetate daily for the first week, 500 mg daily for the
weight for female rats). A decrease in total cholesterol was
second week, 125 mg daily for the third week and 500 mg daily for
observed in males in line with the hypocholestrolemic activity of
the fourth week, only 1–2% of a-tocotrienol and 4–6% of g-
this vitamin .
tocotrienol metabolites was recovered in the urine. To overcomethe limited oral bioavailability of tocotrienols, self-emulsifying
6. Clinical studies with tocotrienols
formulations have been tested in healthy human volunteers withfavorable results .
Numerous clinical studies have been performed to examine
bioavailability and various therapeutic effects of tocotrienols in
6.2. Effects on cardiovascular system
About 50% of persons consuming the typical Western diet will
6.1. Pharmacodynamics and pharmacokinetics
die of coronary heart disease or stroke. Hypercholesterolemiaand inflammation of the coronary artery are the major risk
In a double-blind placebo-controlled study, the bioavailability
factors for development of coronary heart disease. While dietary
of purified a-, g- or d-tocotrienol (250 mg/day for 8 weeks) in
fat has been associated with coronary heart disease, a diet of
hypercholesterolemic humans was examined. At the end of the
predominantly plant foods, such as rice, oats and barley (all of
study period, plasma levels of a-tocotrienol, g-tocotrienol and d-
which contain tocotrienols), can retard this disease. While some
tocotrienol were 0.8 mM, 0.54 mM and 0.09 mM, respectively
studies indicate that tocotrienols have cardioprotective proper-
The preferential absorption of a-tocotrienol in humans
ties in humans others have failed to show the
noted here is in agreement with that noted in rats Hayes et
al. reported that tocotrienols were transported by chylomicrons
In the first study ever performed on the effects of tocotrienols in
and disappeared from the plasma during chylomicron clearance
human subjects, 22 healthy volunteers took one capsule daily
Another study investigated the pharmacokinetics and
containing a palm-oil-vitamin E concentrate (palmvitee) that
bioavailability of a single oral dose (300 mg) of a-tocotrienol, g-
comprised approximately 18 mg tocopherols, 42 mg tocotrienols
tocotrienol and d-tocotrienol in healthy volunteers (N = 8) under
and 240 mg palm olein for 30 days The investigators
fed and fasting conditions. Oral bioavailability of all tocotrienol
observed decreases in total cholesterol ranging from 5% to 35.9%
analogues was markedly increased when taken with food, with
and in LDL-cholesterol from 0.9% to 37% . These cholesterol-
peak plasma concentrations (1.52–5.87 mM) occurring between 3
lowering effects were attributed to tocotrienols, as tocopherols has
and 5 h after ingestion. The biological half-lives of a-tocotrienol, g-
been shown in human subjects to lack these effects Qureshi
tocotrienol and d-tocotrienol were 2.3 h, 4.4 h, and 4.3 h,
al. performed a double-blind, crossover 8-week study
respectively. The half-life of a-tocopherol is about 20 h; thus
comparing the effect of 200 mg palmvitee/day with that of
the half-lives of the tocotrienols are 4.5- to 8.7-fold shorter .
300 mg corn oil (which lacks tocotrienols) on the serum lipid
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
levels of 25 hypercholesterolemic human subjects. The serum
risk for cardiovascular disease, the presence of even a borderline-
cholesterol levels of seven subjects decreased by 31% during the 4-
high-risk LDL-cholesterol level signals the need for aggressive
week period of treatment that included tocotrienols, and this effect
LDL-lowering therapy. Thus Baliarsingh et al. investigated the
persisted even 2 weeks after the capsules were discontinued .
therapeutic impacts of tocotrienols on serum and lipoprotein lipid
Later, in another clinical trial, Qureshi et al. administered TRF from
levels in patients with type 2 diabetes in a randomized, double-
rice bran oil in a 12-week double-blind study in 21 hypercholes-
blind, placebo-controlled design involving 19 subjects with type 2
terolemic subjects. A 12% decrease in total cholesterol and 16%
diabetes and hyperlipidemia. After 60 days of TRF treatment,
reduction in LDL-cholesterol were noted in subjects given TRF
subjects showed average declines of 23%, 30%, and 42% in serum
during the 4-week period in which the dose was 200 mg but not in
total lipids, total cholesterol, and LDL-cholesterol, respectively.
the placebo group given 1.2 g corn oil. Furthermore, a 17% decrease
Tocotrienols mediated a reduction of LDL-cholesterol level from an
in lipoprotein-a was noted in the treated group, which is
average of 179 mg/ml to 104 mg/ml. No hypoglycemic effect was
remarkable as most cholesterol-lowering drugs do not affect
observed in these patients because their glucose and glycated
hemoglobin levels at baseline were close to normal values
The antioxidant benefit of tocotrienols has been reported in a
These findings suggest that daily intake of dietary TRF by
group of patients with cerebrovascular diseases. Both tocopherols
individuals with type 2 diabetes will be useful in the prevention
and 240 mg mixed tocotrienols were used in this trial In
and treatment of hyperlipidemia and atherogenesis.
another clinical trial, low doses of TRF for 25 weeks were found toexhibit synergistic effects with lovastatin on various lipid
parameters in hypercholesterolemic humans Further studiesrevealed that the effect of TRF on serum cholesterol levels was dose
Familial dysautonomia, a genetic neurodegenerative disorder
dependent when administered at 25 mg/day, 50 mg/day, 100 mg/
affecting primarily individuals of Ashkenazi Jewish descent, is
day, or 200 mg/day. The maximum decrease (25%) was seen at the
caused by mutations in the IKBKAP gene, which encodes the
100 mg/day dose .
IkappaB kinase complex-associated protein (IKAP). The more
In contrast to these studies, Mensink et
common or major mutation causes aberrant splicing, resulting in a
randomized, double-blind, placebo-controlled trial in 20 mildly
truncated form of IKAP. Tissues from individuals homozygous for
hypercholesterolemic men who received 140 mg tocotrienols and
the major mutation contain both mutant and wild-type IKAP
20 mg a-tocopherol for 4 weeks, that this regimen had no effect on
transcripts. The apparent leaky nature of this mutation prompted a
serum lipid levels . Whether the negative results were due to
search for agents capable of elevating the level of expression of the
the presence of the tocopherols are not clear, but a-tocopherol has
wild-type IKAP transcript. It has been shown that tocotrienols can
been shown to neutralize the HMG-CoA reductase-inhibitory
increase the transcription of IKAP mRNA in familial dysautonomia-
activity of tocotrienols These results do agree, however, with
derived cells, with corresponding increases in the correctly spliced
those of Wahlqvist et al. In another double-blind, placebo-
transcript and normal protein. Because ingestion of tocotrienols
controlled study, the serum cholesterol-lowering efficacy of
elevates IKAP and MAO-A in familial dysautonomia patients, Rubin
purified a-, g- or d-tocotrienol (250 mg/day) for 8 weeks in
et al. examined their impact on the frequency of hypertensive
hypercholesterolemic humans was examined. Although at the end
crises and cardiac function in individuals with this disorder. After
of the study period, plasma levels of a-tocotrienol, g-tocotrienol
3–4 months of tocotrienol ingestion, approximately 80% of patients
and d-tocotrienol were 0.98 mM, 0.54 mM and 0.09 mM, respec-
reported a significant (�50%) decrease in the number of crises. In a
tively, no change in serum or LDL-cholesterol levels were observed
smaller group of patients, a postexercise increase in heart rate and
. Alpha-tocotrienol did decrease the oxidizing potential of
a decrease in the QT interval were observed . On the basis of
LDL. Mustad et al. also showed a lack of effect of supplementation
these findings, the authors hypothesized that tocotrienol therapy
of tocotrienols (200 mg/day) on hypercholesterolemia .
improves the long-term clinical outlook and survival of individuals
Another study examined the effects of three doses of
with familial dysautonomia.
tocotrienol-rich vitamin E (TRE) on plasma tocotrienol isomer
The free radical theory of aging suggests that free radicals are
concentrations, arterial compliance, plasma total antioxidant
the leading cause of deteriorating physiologic function during
status, aortic systolic blood pressure, and serum total cholesterol
senescence. Free radicals attack cellular structures or molecules
and LDL-cholesterol levels in healthy men. This randomized,
such as DNA, resulting in various modifications to the DNA.
blinded endpoint, placebo-controlled clinical trial with a parallel
Accumulation of unrepaired DNA contributes to a variety of
design involved 36 male subjects who took either an oral placebo
disorders associated with the aging process. Chin and his
or TRE at doses of 80 mg, 160 mg, or 320 mg daily for 2 months.
coworkers performed a randomized, double-blinded, placebo-
Baseline tocotrienol isomer concentrations were low and in some
controlled study to evaluate the effect of Tri E tocotrienol on DNA
subjects, not detectable. At the end of the study period, all TRE-
damage. Sixty-four subjects aged 37–78 years completed the
treated groups showed significant increases in a-, d- and g-
study. A daily dose of 160 mg of Tri E tocotrienol was given for 6
tocotrienol concentrations from baseline relative to the placebo
months. Blood samples were analyzed for DNA damage using the
group. There was a linear dose and blood level relationship for all
comet assay, frequency of sister chromatid exchange, and
the isomers. There was no significant difference between groups,
chromosome 4 aberrations. Results showed that this treatment
however, in pulse wave velocity, arterial compliance, plasma total
significantly reduced DNA damage as measured by comet assay
antioxidant status, aortic systolic blood pressure, or serum levels of
after 3 months and that DNA damage remained low at 6 months.
total cholesterol or LDL-cholesterol from baseline to end of
The frequency of sister chromatid exchange was also reduced
treatment. Groups receiving 160 mg or 320 mg of TRE showed
after 6 months of supplementation, most markedly in the subjects
significant reductions in their aortic systolic blood pressure, and
older than 50 years, while urinary levels of 8-hydroxy-20-
the group receiving 320 mg showed a significant 9.2% improve-
deoxyguanosine (8-OHdG) were significantly reduced. A strong
ment in total antioxidant status .
positive correlation was observed between sister chromatid
The progression of atherosclerosis is more rapid in individuals
exchange with age, whereas weak positive correlations were
with type 2 diabetes than in the general population, and 80% of
observed in DNA damage and 8-OHdG, which were reduced with
those with type 2 diabetes will die of an atherosclerotic event.
supplementation . However, no translocation or stable
Since in these patients hyperglycemia per se confers increased
insertion was observed in chromosome 4. Thus Tri E tocotrienol
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
Table 5Tocotrienols are more potent than tocopherols.
T3 is more potent than TP in reducing gamma-glutamyl transpeptidase and glutathione S-transferase
T3 is more potent than TP in inducing apoptosis of tumor cells
T3s are more potent than TP in inhibiting growth and inducing apoptosis of mouse mammary epithelial cells
T3s preferentially accumulate than TP in mouse mammary epithelial cells
T3s are more effective than TP in preventing glutamate-induced neuronal cell death
T3s, but not TP, inhibited both the proliferation and tube formation of bovine aortic endothelial cells
T3s are more readily transferred and incorporated into the membranes than TP
T3 had greater peroxy radical scavenging activity than TP in liposomal membrane
T3, not TP inhibited human endothelial cell proliferation and suppressed tumor-induced angiogenesis
T3, not TP reduced VEGF-stimulated tube formation in HUVEC
T3 protects astrocytes better than TP from H2O2-induced-cell loss and apoptosis
T3 is more effective than TP in protecting against glutamate-induced cell death in HT4 neuron cell
T3s are more potent than TP in protecting cerebellar granule cells against methyl mercury toxicity
Accumulation and secretion rate of T3 isomers in Caco2 cells is faster than TP isomers; oral administration caused faster appearance
and disappearance of T3 than TP
T3 is more effective than TP in suppressing LPS-induced IL-6, PGE2 production from macrophages
T3 were more effective in inhibiting the growth of sarcoma 180, Ehrlich carcinoma, and IMC carcinoma than TP
T3 showed significant increase in DMBA-induced tumor latency than TP
T3 showed 40–60 times higher antioxidant activity against induced lipid peroxidation and 6.5 times better protection of
cytochrome P-450 against oxidative damage than TP
Reduction of linoleic acid desaturation was more clear with T3 than with TP
No T3 in plasma but platelet concentration of d-T3 doubled; TP was found in LDL and HDL in human; T3 deposited in adipose
tissue while TP was detected in all tissue except adipose in hamster
Lymphatic transport and recovery of T3 was twice higher than that of TP in thoracis duct-cannulated rats
T3 feeding (0.2% in diet) gave higher CD4+/CD8+ ratio than TP in mesenteric lymph node lymphocytes
T3 exerted stronger antioxidant activity than TP in vivo
T3 (60 mg/kg body weight/day) was more effective than TP in reducing body fat mass and preventing steroid-induced osteoporosis
Concentration of T3 increased markedly in eye tissue than TP
T3 but not a-TP reduced the serum levels of IL-1 and IL-6 in rats
T3 (60 mg/kg body weight) was better than TP in protecting bone resorption caused by free-radicals
T3 has the ability to block the stress-induced changes in the gastric acidity and gastrin level than TP
T3 are detected in postprandial (fasted) human plasma earlier than TP but at significantly lower level than TP
T3 is a better antioxidant than TP in a deep fat frying system
Total cholesterol and LDL-C levels declined in T3 group but not in those on TP
T3 is superior than TP in suppressing nicotine-induced loss of calcium from bone
T3 but not TP reduced the levels of lipid peroxidation and increased GPO activity in the femur of rats
T3, but not TP can maintain the noradrenalin level and prevent gastric lesions in rats exposed to stress
T3 is more extensively than TP metabolized to sulfated CEHC form
T3 was superior than TP, in reversing nicotine-induced bone loss in rats
T3 has better effects than TP on static and dynamic bone histomorphometric parameters
T3 is better than TP as an anabolic agent for bone in normal male rats
CEHC, 2-(beta-carboxyethyl)-6hydroxychromon; DMBA, 7,12-dimethylbenz(a)anthracene; GPO, glutathione peroxidase; HDL, high-density lipoprotein; HUVEC-humanumbilical vein endothelial cells; IL, interleukins; LDL, low-density lipoprotein; LPS, lipopolysaccharide; PGE2, prostaglandin-2; T3, tocotrienols; TP, tocopherols; VEGF-vascular endothelial growth factor.
supplementation may be beneficial by reducing free radical
cells and in inducing apoptosis Almost millimolar doses of
damage as indicated by reductions in DNA damage, sister
tocopherols were required for antiproliferative effects The
chromatid exchange frequency and urinary 8-OHdG level. Topical
authors showed that these differences could be linked to
application of a-tocotrienol has been shown to prevent benzyl
preferential accumulation of tocotrienols as compared to toco-
peroxide-induced lipid peroxidation of human skin
pherols. These differences may also be due to a-tocopheroltransfer protein, which binds to a-tocopherol with higher affinity
7. Tocotrienols vs. tocopherols
than to tocotrienols . When their respective effects onproliferation of bovine endothelial cells, a marker of angiogenesis,
Tocotrienols differ from tocopherols in that the former contain
were measured, only tocotrienols (not tocopherols) inhibited this
three double bonds in their isoprenoid side chain while the latter
proliferation . Another study showed that oral administration
do not; this may account for the differences in their efficacy and
of tocotrienols but not tocopherols blocked tumor-induced
potency in vitro and in vivo ) . While over
angiogenesis. These investigators showed that tocotrienols down-
30,000 papers have been published on tocopherols, fewer than 600
regulated VEGF receptor expression in HUVEC cells and blocked
exist on tocotrienols, most published within the last 5 years.
VEGF signaling .
Tocopherols are present mainly in corn, wheat and soybeans,
Suarna et al. reported that when rats or humans were treated
whereas tocotrienols occur mainly in barley, oats, palm, and rice
with tocotrienols and tocopherols, tocotrienols provided oxidative
bran. Although tocopherols and tocotrienols are structurally very
protection but tocopherols did not a-Tocopherol has been
similar and both are metabolized through similar mechanisms
reported to attenuate the inhibitory effects of tocotrienols on
involving initial v-hydroxylation followed by five cycles of b-
HMG-CoA activity . Tocotrienols have been shown to be
oxidation tocotrienols have been found to exhibit superior
converted to tocopherols in vivo High concentrations of g-
antioxidant activity . McIntyre et
tocotrienol but not a-tocopherol were cytotoxic to astrocytes. This
tocotrienols were several-fold more effective than tocopherols in
difference was attributed to the greater prooxidant activity of
inhibiting the proliferation of mouse mammary tumor epithelial
tocotrienols at high concentrations. At low concentrations,
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
Table 6Comparative effects of various tocotrienol isomers.
g-T3 is 30� more active than a-T3 or d-T3 in inhibiting cholesterol biosynthesis in HepG2 cells
g-T3 is more active than a-T3, and d-T3 in oxidation of lipids and protein in brain mitochondria
a-T3 exhibits faster lymphatic transport and higher absorption in rats than gT3 and d-T3
g-T3 is more active than a-T3, and d-T3 in oxidation of lipids and protein in liver microsomes
g-T3 and d-T3 but not a-T3 inhibit growth of both ER+ and ER� breast cancer cells
d-T3 are more potent than other T3s in promoting apoptosis of breast cancer cells
a-T3 was more active than g-T3 or d-T3 in preventing LDL oxidation in hypercholesterolemic humans
g-T3 is more active than a-T3 or d-T3 in lowering total cholesterol in high fat diet fed hamsters
g-T3 was more active than a-T3 or d-T3 in inducing PXR-mediated gene expression
a-T3 but not g-T3 or d-T3 can prevent cerebral infarction in mice
a-T3 is more potent than other T3 as antioxidant and as a prooxidant
a-T3, but not g-T3 or d-T3 exhibit neuroprotective action in rat striatal neuron cells
d-T3 is more active than b > g > a-T3 in inhibiting proliferation and tube formation of bovine aortic endothelial cells
d-T3 is more potent than other isomers in inhibiting VEGF-stimulated tube formation by HUVEC
d-T3 is more active than a-T3, b-T3 or g-T3 in suppression of adhesion of monocytes to endothelial cells via VCAM-1
d-T3 is more active than other T3 in suppression of tumors in vitro and in vivo
g-T3 is better than a-T3 as an anti-oxidant
g-T3 was more cardioprotective than a-T3 or d-T3 but d-T3 was most active in stabilizing proteasomes
d-T3 is more active than a-T3 or d-T3 in inhibiting DNA polyl and angiogenesis
d-T3 is more active than g-T3 and a-T3 as an antioxidant in rat liver microsomal membranes and cells
d-T3 is more active than d-T3 in stimulating ubiquitination and degradation of HMG-CoA reductase
d-T3 was more active than other isomers in induces cell death in AR� and AR+ prostate cancer cell lines
g-T3 is more potent than a-T3 or d-T3 in inhibiting proliferation and inducing apoptosis of HeLa cells
g-T3 is better than d-T3 in promoting bone formation in male rats
AR, androgen receptor; ER, estrogen receptor; LDL, low-density lipoprotein; HUVEC, human umbilical vein endothelial cells; PXR, pregnane X receptor; T3, tocotrienols;VCAM-1, vascular cell adhesion molecule; VEGF, vascular endothelial growth factor.
however, tocotrienols were found to be antioxidant and to protect
While various studies have indicated that a-tocotrienol is highly
cells from hydrogen peroxide-induced killing This is
neuroprotective , d- and g-tocotrienol have been shown to
consistent with studies showing that tocotrienols are more
exhibit the greatest anticancer effects. In vitro studies suggest that
effective than tocopherols in protecting against glutamate-induced
there may be as much as a 30-fold difference in the ability of a, g,
cell death in HT4 neuron cell culture . Whether these
and d isomers of tocotrienol to inhibit cholesterol biosynthesis
differences were due to the differential rates of uptake of
The antioxidant capacity of these three isomers is a-
tocotrienols and tocopherols by the neuronal cells is controversial
tocotrienol > g-tocotrienol > d-tocotrienol The antioxidant
. Tocotrienols were many times as potent as tocopherols in
activity of a-tocotrienol is similar to that of a-tocopherol
protecting cerebellar granule cells against methyl mercury
McIntyre et al. showed that various tocotrienols differ in their
toxicity, an effect that was linked to the difference in the
potency in inhibiting the proliferation of mouse mammary tumor
antioxidant potency of the two forms of the vitamin . In
epithelial cells and in inducing apoptosis They identified the
vitro studies showed that tocotrienols have greater anti-inflam-
relative potencies of these three isomers as d-tocotrienol > g-
matory activity than tocopherols as measured by lipopolysaccha-
tocotrienol > a-tocotrienol. These observations agree with that of
ride-induced production of IL-6 and prostaglandin E2 .
Inkouchi et al., who showed that the relative potencies for the
Mishima et al. showed that a-tocotrienol and g-tocotrienol were
suppression of proliferation of bovine and human endothelial cells
more effective than a-tocopherol in preventing cerebral infarction
and tube formation were d-tocotrienol > b-tocotrienol > g-toco-
trienol = a-tocotrienol All these reports point to
Any number of mechanisms could account for the difference in
differences in the mechanisms of action of the tocotrienol isomers.
potency of tocotrienols and tocopherols. First, because of structuraldifferences, tocotrienols may be more uniformly distributed in the
lipid bilayer. Second, the chromanol ring of tocotrienols mayinteract more efficiently with the lipid bilayer than that of
While a lot is known about tocopherols, very little is known
tocopherols. Third, tocotrienols may have a higher recycling
about tocotrienols. There is some evidence, however, that
efficiency Fourth, cellular uptake of tocotrienols is 70 times
tocotrienols may be superior in its biological properties, and that
higher than that of tocopherols All of these factors may
its anti-inflammatory and antioxidant activities could prevent
contribute to tocotrienol's greater efficacy. It has also been shown
cancer, diabetes, and cardiovascular and neurodegenerative
that tocotrienol isomers are accumulated and secreted at greater
). Tocotrienols were discovered a half-century
rates in Caco2 cells than tocopherol isomers. When administered
ago, but most of their biology has been revealed only in the last
orally to mice, tocotrienols appeared faster in the plasma but at
decade. More clinical and preclinical studies are needed to fully
lower levels than tocopherols
realize their potential. Disappointment with tocopherols, as
Alpha-tocotrienol mediates some of its effect by inhibiting
indicated by two recent very large randomized controlled clinical
HMG-CoA reductase activity , while a-tocopherol induces
trials for prevention of prostate cancer , is growing as it fails
HMG-CoA reductase activity .
to meet expectations. These trials showed that there was astatistically nonsignificant increased risks of prostate cancer in
8. Tocotrienol isoforms
vitamin E (a-tocopherol acetate) group. Similar negative resultswere previously reported with vitamin E when its effect was
The isoforms of tocotrienols, which differ in their number of
examined on cancer cardiovascular events . Are tocotrienols,
methyl groups, also differ in their biological activities (
the vitamin E that we should be investigating in the 21st century?
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
pheryl succinate on breast cancer cell lines irrespective of HER-2/neu ex-pression. Life Sci 2010;86:668–75.
[27] Wu SJ, Ng LT. Tocotrienols inhibited growth and induced apoptosis in human
The authors thank Ms Kathyrn Hale for carefully reviewing this
HeLa cells through the cell cycle signaling pathway. Integr Cancer Ther
manuscript and providing valuable comments. Dr. Aggarwal is the
[28] Agarwal MK, Agarwal ML, Athar M, Gupta S. Tocotrienol-rich fraction of palm
Ransom Horne, Jr., Professor of Cancer Research. This work was
oil activates p53, modulates Bax/Bcl2 ratio and induces apoptosis indepen-
supported by grant from the Malaysian Palm Oil Board, a core grant
dent of cell cycle association. Cell Cycle 2004;3:205–11.
from the National Institutes of Health (CA-16 672), a program
[29] Samant GV, Wali VB, Sylvester PW. Anti-proliferative effects of gamma-
project grant from National Institutes of Health (NIH CA-124787-
tocotrienol on mammary tumour cells are associated with suppression ofcell cycle progression. Cell Prolif 2010;43:77–83.
01A2), and grant from Center for Targeted Therapy of M.D.
[30] Elangovan S, Hsieh TC, Wu JM. Growth inhibition of human MDA-mB-231
Anderson Cancer Center.
breast cancer cells by delta-tocotrienol is associated with loss of cyclin D1/CDK4 expression and accompanying changes in the state of phosphorylationof the retinoblastoma tumor suppressor gene product. Anticancer Res
[31] Wali VB, Bachawal SV, Sylvester PW. Combined treatment of gamma-toco-
[1] Sen CK, Khanna S, Rink C, Roy S. Tocotrienols: the emerging face of natural
trienol with statins induce mammary tumor cell cycle arrest in G1. Exp Biol
vitamin E. Vitam Horm 2007;76:203–61.
Med (Maywood) 2009;234:639–50.
[2] Qureshi AA, Sami SA, Salser WA, Khan FA. Synergistic effect of tocotrienol-rich
[32] Sun W, Wang Q, Chen B, Liu J, Liu H, Xu W. Gamma-tocotrienol-induced
fraction (TRF(25)) of rice bran and lovastatin on lipid parameters in hyper-
apoptosis in human gastric cancer SGC-7901 cells is associated with a
cholesterolemic humans. J Nutr Biochem 2001;12:318–29.
suppression in mitogen-activated protein kinase signalling. Br J Nutr
[3] Qureshi AA, Sami SA, Salser WA, Khan FA. Dose-dependent suppression of
serum cholesterol by tocotrienol-rich fraction (TRF25) of rice bran in hyper-
[33] Park SK, Sanders BG, Kline K. Tocotrienols induce apoptosis in breast cancer
cholesterolemic humans. Atherosclerosis 2002;161:199–207.
cell lines via an endoplasmic reticulum stress-dependent increase in extrinsic
[4] Bunyan J, Mc HD, Green J, Marcinkiewicz S. Biological potencies of epsilon-
death receptor signaling. Breast Cancer Res Treat 2010.
and zeta-1-tocopherol and 5-methyltocol. Br J Nutr 1961;15:253–7.
[34] Ahn KS, Sethi G, Krishnan K, Aggarwal BB. Gamma-tocotrienol inhibits
[5] Dunphy PJ, Whittle KJ, Pennock JF, Morton RA. Identification and estimation
nuclear factor-kappaB signaling pathway through inhibition of receptor-
of tocotrienols in Hevea latex. Nature 1965;201:521–2.
interacting protein and TAK1 leading to suppression of antiapoptotic gene
[6] Whittle KJ, Dunphy PJ, Pennock JF. The isolation and properties of delta-
products and potentiation of apoptosis. J Biol Chem 2007;282:809–20.
tocotrienol from Hevea latex. Biochem J 1966;100:138–45.
[35] Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell
[7] Qureshi AA, Burger WC, Peterson DM, Elson CE. The structure of an inhibitor of
cycle progression and cell proliferation by down-regulation of cyclins. FASEB
cholesterol biosynthesis isolated from barley. J Biol Chem 1986;261:10544–50.
J 2002;16:1952–4.
[8] Kato A, Yamaoka M, Tanaka A, Komiyama Ka, Umezawa I. Physiological effect
[36] Samant GV, Sylvester PW. gamma-Tocotrienol inhibits ErbB3-dependent
of tocotrienol. J Jpn Oil Chem Soc (Yukugaku) 1985;34:375–6.
PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell
[9] Sundram K, Khor HT, Ong AS, Pathmanathan R. Effect of dietary palm oils on
mammary carcinogenesis in female rats induced by 7,12-dimethylbenz(a)an-
[37] Eitsuka T, Nakagawa K, Miyazawa T. Down-regulation of telomerase activity
thracene. Cancer Res 1989;49:1447–51.
in DLD-1 human colorectal adenocarcinoma cells by tocotrienol. Biochem
[10] Tan B. Tocotrienols: The New Vitamin E. Spacedocnet,
Biophys Res Commun 2006;348:170–5.
[38] Shah S, Sylvester PW. Tocotrienol-induced caspase-8 activation is unrelated
[11] Kobayashi H, Kanno C, Yamauchi K, Tsugo T. Identification of alpha-, beta-,
to death receptor apoptotic signaling in neoplastic mammary epithelial cells.
gamma-, and delta-tocopherols and their contents in human milk. Biochim
Exp Biol Med (Maywood) 2004;229:745–55.
Biophys Acta 1975;380:282–90.
[39] Rickmann M, Vaquero EC, Malagelada JR, Molero X. Tocotrienols induce
[12] van Haaften RI, Haenen GR, Evelo CT, Bast A. Tocotrienols inhibit human
apoptosis and autophagy in rat pancreatic stellate cells through the mito-
glutathione S-transferase P1-1. IUBMB Life 2002;54:81–4.
chondrial death pathway. Gastroenterology 2007;132:2518–32.
[13] Comitato R, Nesaretnam K, Leoni G, Ambra R, Canali R, Bolli A, et al. A novel
[40] Sakai M, Okabe M, Tachibana H, Yamada K. Apoptosis induction by
mechanism of natural vitamin E tocotrienol activity: involvement of ERbeta
gamma-tocotrienol in human hepatoma Hep3B cells. J Nutr Biochem
signal transduction. Am J Physiol Endocrinol Metab 2009;297:E427–37.
[14] Upadhyay J, Misra K. Towards the interaction mechanism of tocopherols and
[41] Xu WL, Liu JR, Liu HK, Qi GY, Sun XR, Sun WG, et al. Inhibition of proliferation
tocotrienols (vitamin E) with selected metabolizing enzymes. Bioinformation
and induction of apoptosis by gamma-tocotrienol in human colon carcinoma
HT-29 cells. Nutrition 2009;25:555–66.
[15] Zhou C, Tabb MM, Sadatrafiei A, Grun F, Blumberg B. Tocotrienols activate the
[42] Shah SJ, Sylvester PW. Tocotrienol-induced cytotoxicity is unrelated to
steroid and xenobiotic receptor, SXR, and selectively regulate expression of
mitochondrial stress apoptotic signaling in neoplastic mammary epithelial
its target genes. Drug Metab Dispos 2004;32:1075–82.
cells. Biochem Cell Biol 2005;83:86–95.
[16] Khanna S, Roy S, Park HA, Sen CK. Regulation of c-Src activity in glutamate-
[43] Takahashi K, Loo G. Disruption of mitochondria during tocotrienol-induced
induced neurodegeneration. J Biol Chem 2007;282:23482–90.
apoptosis in MDA-MB-231 human breast cancer cells. Biochem Pharmacol
[17] Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, et al. Molecular basis
of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of
[44] Sun W, Xu W, Liu H, Liu J, Wang Q, Zhou J, et al. gamma-Tocotrienol induces
glutamate-induced neurodegeneration. J Biol Chem 2003;278:43508–15.
mitochondria-mediated apoptosis in human gastric adenocarcinoma SGC-
[18] Lee SP, Mar GY, Ng LT. Effects of tocotrienol-rich fraction on exercise endur-
7901 cells. J Nutr Biochem 2009;20:276–84.
ance capacity and oxidative stress in forced swimming rats. Eur J Appl Physiol
[45] Sylvester PW, Shah S. Intracellular mechanisms mediating tocotrienol-in-
duced apoptosis in neoplastic mammary epithelial cells. Asia Pac J Clin Nutr
[19] Newaz MA, Nawal NN. Effect of gamma-tocotrienol on blood pressure, lipid
peroxidation and total antioxidant status in spontaneously hypertensive rats
[46] Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle
(SHR). Clin Exp Hypertens 1999;21:1297–313.
arrest and apoptosis selectively in human prostate cancer cells. Biochem
[20] Hsieh TC, Wu JM. Suppression of cell proliferation and gene expression by
Biophys Res Commun 2006;346:447–53.
combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in
[47] Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Tomita S, Shirakawa H, et al.
estrogen receptor-positive MCF-7 breast cancer cells. Int J Oncol 2008;
Tocotrienol inhibits secretion of angiogenic factors from human colorectal
adenocarcinoma cells by suppressing hypoxia-inducible factor-1alpha. J Nutr
[21] Adam A, Marzuki A, Ngah WZ, Top GM. Nitrofurantoin-induced hepatic and
pulmonary biochemical changes in mice fed different vitamin E doses.
[48] Miyazawa T, Shibata A, Nakagawa K, Tsuzuki T. Anti-angiogenic function of
Pharmacol Toxicol 1996;79:334–9.
tocotrienol. Asia Pac J Clin Nutr 2008;17(Suppl 1):253–6.
[22] Renuka Devi R, Arumughan C. Antiradical efficacy of phytochemical extracts
[49] Nakagawa K, Eitsuka T, Inokuchi H, Miyazawa T. DNA chip analysis of
from defatted rice bran. Food Chem Toxicol 2007;45:2014–21.
comprehensive food function: inhibition of angiogenesis and telomerase
[23] Weng-Yew W, Selvaduray KR, Ming CH, Nesaretnam K. Suppression of tumor
activity with unsaturated vitamin E, tocotrienol. Biofactors 2004;21:5–10.
growth by palm tocotrienols via the attenuation of angiogenesis. Nutr Cancer
[50] Shibata A, Nakagawa K, Sookwong P, Tsuduki T, Oikawa S, Miyazawa T. delta-
Tocotrienol suppresses VEGF induced angiogenesis whereas alpha-tocoph-
[24] Rashid SA, Halim AS, Muhammad NA. The effect of vitamin E on basic
erol does not. J Agric Food Chem 2009;57:8696–704.
fibroblast growth factor level in human fibroblast cell culture. Med J Malay
[51] Liu HK, Wang Q, Li Y, Sun WG, Liu JR, Yang YM, et al. Inhibitory effects of
gamma-tocotrienol on invasion and metastasis of human gastric adenocar-
[25] Shun MC, Yu W, Gapor A, Parsons R, Atkinson J, Sanders BG, et al. Pro-
cinoma SGC-7901 cells. J Nutr Biochem 2010;21:206–13.
apoptotic mechanisms of action of a novel vitamin E analog (alpha-TEA) and a
[52] Shirode AB, Sylvester PW. Synergistic anticancer effects of combined gamma-
naturally occurring form of vitamin E (delta-tocotrienol) in MDA-MB-435
tocotrienol and celecoxib treatment are associated with suppression in Akt
human breast cancer cells. Nutr Cancer 2004;48:95–105.
and NFkappaB signaling. Biomed Pharmacother 2010;64:327–32.
[26] Pierpaoli E, Viola V, Pilolli F, Piroddi M, Galli F, Provinciali M. Gamma- and
[53] Kuhad A, Chopra K. Attenuation of diabetic nephropathy by tocotrienol:
delta-tocotrienols exert a more potent anticancer effect than alpha-toco-
involvement of NFkB signaling pathway. Life Sci 2009;84:296–301.
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
[54] Norazlina M, Lee PL, Lukman HI, Nazrun AS, Ima-Nirwana S. Effects of vitamin
[81] Sakai M, Okabe M, Yamasaki M, Tachibana H, Yamada K. Induction of
E supplementation on bone metabolism in nicotine-treated rats. Singapore
apoptosis by tocotrienol in rat hepatoma dRLh-84 cells. Anticancer Res
Med J 2007;48:195–9.
[55] Ahmad NS, Khalid BA, Luke DA, Ima Nirwana S. Tocotrienol offers better
[82] Wada S, Satomi Y, Murakoshi M, Noguchi N, Yoshikawa T, Nishino H. Tumor
protection than tocopherol from free radical-induced damage of rat bone.
suppressive effects of tocotrienol in vivo and in vitro. Cancer Lett 2005;229:
Clin Exp Pharmacol Physiol 2005;32:761–70.
[56] Wu SJ, Liu PL, Ng LT. Tocotrienol-rich fraction of palm oil exhibits anti-
[83] Kashiwagi K, Harada K, Yano Y, Kumadaki I, Hagiwara K, Takebayashi J, et al. A
inflammatory property by suppressing the expression of inflammatory med-
redox-silent analogue of tocotrienol inhibits hypoxic adaptation of lung
iators in human monocytic cells. Mol Nutr Food Res 2008;52:921–9.
cancer cells. Biochem Biophys Res Commun 2008;365:875–81.
[57] Jiang Q, Yin X, Lill MA, Danielson ML, Freiser H, Huang J. Long-chain carbox-
[84] Nakashima K, Virgona N, Miyazawa M, Watanabe T, Yano T. The tocotrienol-
ychromanols, metabolites of vitamin E, are potent inhibitors of cyclooxy-
rich fraction from rice bran enhances cisplatin-induced cytotoxicity in hu-
genases. Proc Natl Acad Sci USA 2008;105:20464–9.
man mesothelioma H28 cells. Phytother Res 2010.
[58] Kashiwagi K, Virgona N, Harada K, Kido W, Yano Y, Ando A, et al. A redox-
[85] Yano Y, Satoh H, Fukumoto K, Kumadaki I, Ichikawa T, Yamada K, et al.
silent analogue of tocotrienol acts as a potential cytotoxic agent against
Induction of cytotoxicity in human lung adenocarcinoma cells by 6-O-car-
human mesothelioma cells. Life Sci 2009;84:650–6.
boxypropyl-alpha-tocotrienol, a redox-silent derivative of alpha-tocotrienol.
[59] Bachawal SV, Wali VB, Sylvester PW. Combined gamma-tocotrienol and
Int J Cancer 2005;115:839–46.
erlotinib/gefitinib treatment suppresses Stat and Akt signaling in murine
[86] Chang PN, Yap WN, Lee DT, Ling MT, Wong YC, Yap YL. Evidence of gamma-
mammary tumor cells. Anticancer Res 2010;30:429–37.
tocotrienol as an apoptosis-inducing, invasion-suppressing, and chemother-
[60] Ozer NK, Boscoboinik D, Azzi A. New roles of low density lipoproteins and
apy drug-sensitizing agent in human melanoma cells. Nutr Cancer 2009;61:
vitamin E in the pathogenesis of atherosclerosis. Biochem Mol Biol Int
[87] McAnally JA, Gupta J, Sodhani S, Bravo L, Mo H. Tocotrienols potentiate
[61] Sen CK, Khanna S, Roy S, Packer L. Molecular basis of vitamin E action,
lovastatin-mediated growth suppression in vitro and in vivo. Exp Biol Med
tocotrienol potently inhibits glutamate-induced pp60(c-Src) kinase activa-
tion and death of HT4 neuronal cells. J Biol Chem 2000;275:13049–55.
[88] Hussein D, Mo H. Delta-tocotrienol-mediated suppression of the proliferation
[62] Sylvester PW, Shah SJ, Samant GV. Intracellular signaling mechanisms medi-
of human PANC-1, MIA PaCa-2, and BxPC-3 pancreatic carcinoma cells.
ating the antiproliferative and apoptotic effects of gamma-tocotrienol in
neoplastic mammary epithelial cells. J Plant Physiol 2005;162:803–10.
[89] Constantinou C, Hyatt JA, Vraka PS, Papas A, Papas KA, Neophytou C, et al.
[63] Qureshi AA, Pearce BC, Nor RM, Gapor A, Peterson DM, Elson CE. Dietary
Induction of caspase-independent programmed cell death by vitamin E
alpha-tocopherol attenuates the impact of gamma-tocotrienol on hepatic 3-
natural homologs and synthetic derivatives. Nutr Cancer 2009;61:864–74.
hydroxy-3-methylglutaryl coenzyme A reductase activity in chickens. J Nutr
[90] Conte C, Floridi A, Aisa C, Piroddi M, Galli F. Gamma-tocotrienol metabolism
and antiproliferative effect in prostate cancer cells. Ann N Y Acad Sci
[64] Elson CE, Qureshi AA. Coupling the cholesterol- and tumor-suppressive
actions of palm oil to the impact of its minor constituents on 3-hydroxy-
[91] Yap WN, Chang PN, Han HY, Lee DT, Ling MT, Wong YC, et al. Gamma-
3-methylglutaryl coenzyme A reductase activity. Prostaglandins Leukot
tocotrienol suppresses prostate cancer cell proliferation and invasion
Essent Fatty Acids 1995;52:205–7.
through multiple-signalling pathways. Br J Cancer 2008;99:1832–41.
[65] Iqbal J, Minhajuddin M, Beg ZH. Suppression of 7,12-dimethylbenz[alpha]an-
[92] Comitato R, Leoni G, Canali R, Ambra R, Nesaretnam K, Virgili F. Tocotrienols
thracene-induced carcinogenesis and hypercholesterolaemia in rats by toco-
activity in MCF-7 breast cancer cells: involvement of ERbeta signal transduc-
trienol-rich fraction isolated from rice bran oil. Eur J Cancer Prev
tion. Mol Nutr Food Res 2010;64:327–32.
[93] Kamat JP, Sarma HD, Devasagayam TP, Nesaretnam K, Basiron Y. Tocotrienols
[66] Parker RA, Pearce BC, Clark RW, Gordon DA, Wright JJ. Tocotrienols regulate
from palm oil as effective inhibitors of protein oxidation and lipid peroxida-
cholesterol production in mammalian cells by post-transcriptional suppres-
tion in rat liver microsomes. Mol Cell Biochem 1997;170:131–7.
sion of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J Biol Chem
[94] Yu W, Simmons-Menchaca M, Gapor A, Sanders BG, Kline K. Induction of
apoptosis in human breast cancer cells by tocopherols and tocotrienols. Nutr
[67] Pearce BC, Parker RA, Deason ME, Qureshi AA, Wright JJ. Hypocholester-
olemic activity of synthetic and natural tocotrienols. J Med Chem 1992;35:
[95] Chao JT, Gapor A, Theriault A. Inhibitory effect of delta-tocotrienol, a HMG
CoA reductase inhibitor, on monocyte–endothelial cell adhesion. J Nutr Sci
[68] Ali H, Shirode AB, Sylvester PW, Nazzal S. Preparation, characterization, and
Vitaminol (Tokyo) 2002;48:332–7.
anticancer effects of simvastatin-tocotrienol lipid nanoparticles. Int J Pharm
[96] Pearce BC, Parker RA, Deason ME, Dischino DD, Gillespie E, Qureshi AA, et al.
Inhibitors of cholesterol biosynthesis. 2. Hypocholesterolemic and antioxi-
[69] Bachawal SV, Wali VB, Sylvester PW. Enhanced antiproliferative and apopto-
dant activities of benzopyran and tetrahydronaphthalene analogues of the
tic response to combined treatment of gamma-tocotrienol with erlotinib or
tocotrienols. J Med Chem 1994;37:526–41.
gefitinib in mammary tumor cells. BMC Cancer 2010;10:84.
[97] Theriault A, Chao JT, Gapor A. Tocotrienol is the most effective vitamin E for
[70] McIntyre BS, Briski KP, Gapor A, Sylvester PW. Antiproliferative and apoptotic
reducing endothelial expression of adhesion molecules and adhesion to
effects of tocopherols and tocotrienols on preneoplastic and neoplastic
monocytes. Atherosclerosis 2002;160:21–30.
mouse mammary epithelial cells. Proc Soc Exp Biol Med 2000;224:292–301.
[98] Khanna S, Roy S, Parinandi NL, Maurer M, Sen CK. Characterization of the
[71] Wali VB, Bachawal SV, Sylvester PW. Suppression in mevalonate synthesis
potent neuroprotective properties of the natural vitamin E alpha-tocotrienol.
mediates antitumor effects of combined statin and gamma-tocotrienol treat-
J Neurochem 2006;98:1474–86.
ment. Lipids 2009;44:925–34.
[99] Liu X, Yamada N, Osawa T. Assessing the neuroprotective effect of antiox-
[72] Wali VB, Bachawal SV, Sylvester PW. Endoplasmic reticulum stress mediates
idative food factors by application of lipid-derived dopamine modification
gamma-tocotrienol-induced apoptosis in mammary tumor cells. Apoptosis
adducts. Methods Mol Biol 2009;580:143–52.
[100] Komiyama K, Iizuka K, Yamaoka M, Watanabe H, Tsuchiya N, Umezawa I.
[73] Guthrie N, Gapor A, Chambers AF, Carroll KK. Inhibition of proliferation of
Studies on the biological activity of tocotrienols. Chem Pharm Bull (Tokyo)
estrogen receptor-negative MDA-MB-435 and -positive MCF-7 human breast
cancer cells by palm oil tocotrienols and tamoxifen, alone and in combina-
[101] He L, Mo H, Hadisusilo S, Qureshi AA, Elson CE. Isoprenoids suppress the
tion. J Nutr 1997;127:S544–8.
growth of murine B16 melanomas in vitro and in vivo. J Nutr 1997;127:
[74] Nesaretnam K, Guthrie N, Chambers AF, Carroll KK. Effect of tocotrienols on
the growth of a human breast cancer cell line in culture. Lipids 1995;30:
[102] Hiura Y, Tachibana H, Arakawa R, Aoyama N, Okabe M, Sakai M, et al. Specific
accumulation of gamma- and delta-tocotrienols in tumor and their antitu-
[75] Nesaretnam K, Stephen R, Dils R, Darbre P. Tocotrienols inhibit the growth of
mor effect in vivo. J Nutr Biochem 2009;20:607–13.
human breast cancer cells irrespective of estrogen receptor status. Lipids
[103] Nakagawa K, Shibata A, Yamashita S, Tsuzuki T, Kariya J, Oikawa S, et al. In
vivo angiogenesis is suppressed by unsaturated vitamin E, tocotrienol. J Nutr
[76] Shah S, Gapor A, Sylvester PW. Role of caspase-8 activation in mediating
vitamin E-induced apoptosis in murine mammary cancer cells. Nutr Cancer
[104] Kumar KS, Raghavan M, Hieber K, Ege C, Mog S, Parra N, et al. Preferential
radiation sensitization of prostate cancer in nude mice by nutraceutical
[77] Sylvester PW, Nachnani A, Shah S, Briski KP. Role of GTP-binding proteins in
antioxidant gamma-tocotrienol. Life Sci 2006;78:2099–104.
reversing the antiproliferative effects of tocotrienols in preneoplastic mam-
[105] Gould MN, Haag JD, Kennan WS, Tanner MA, Elson CE. A comparison of
mary epithelial cells. Asia Pac J Clin Nutr 2002;11(Suppl. 7):S452–9.
tocopherol and tocotrienol for the chemoprevention of chemically induced
[78] Sylvester PW, Shah SJ. Mechanisms mediating the antiproliferative and apo-
rat mammary tumors. Am J Clin Nutr 1991;53:1068S–70S.
ptotic effects of vitamin E in mammary cancer cells. Front Biosci 2005;10:
[106] Goh SH, Hew NF, Norhanom AW, Yadav M. Inhibition of tumour promotion
by various palm-oil tocotrienols. Int J Cancer 1994;57:529–31.
[79] Yang Z, Xiao H, Jin H, Koo PT, Tsang DJ, Yang CS. Synergistic actions of
[107] Iqbal J, Minhajuddin M, Beg ZH. Suppression of diethylnitrosamine and 2-
atorvastatin with gamma-tocotrienol and celecoxib against human colon
acetylaminofluorene-induced hepatocarcinogenesis in rats by tocotrienol-
cancer HT29 and HCT116 cells. Int J Cancer 2010;126:852–63.
rich fraction isolated from rice bran oil. Eur J Cancer Prev 2004;13:515–20.
[80] Har CH, Keong CK. Effects of tocotrienols on cell viability and apoptosis in
[108] Ngah WZ, Jarien Z, San MM, Marzuki A, Top GM, Shamaan NA, et al. Effect of
normal murine liver cells (BNL CL.2) and liver cancer cells (BNL 1ME A.7R.1),
tocotrienols on hepatocarcinogenesis induced by 2-acetylaminofluorene in
in vitro. Asia Pac J Clin Nutr 2005;14:374–80.
rats. Am J Clin Nutr 1991;53:1076S–81S.
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
[109] Rahmat A, Ngah WZ, Shamaan NA, Gapor A, Abdul Kadir K. Long-term
histomorphometry parameters in normal male rats. J Bone Miner Metab
administration of tocotrienols and tumor-marker enzyme activities during
hepatocarcinogenesis in rats. Nutrition 1993;9:229–32.
[136] Shuid AN, Mehat Z, Mohamed N, Muhammad N, Soelaiman IN. Vitamin E
[110] Shamaan NA, Wan Ngah WZ, Ibrahim R, Jarien Z, Top AG, Abdul Kadir K. Effect
exhibits bone anabolic actions in normal male rats. J Bone Miner Metab
of tocotrienol on the activities of cytosolic glutathione-dependent enzymes
in rats treated with 2-acetylaminofluorene. Biochem Pharmacol 1993;45:
[137] Maniam S, Mohamed N, Shuid AN, Soelaiman IN. Palm tocotrienol exerted
better antioxidant activities in bone than alpha-tocopherol. Basic Clin Phar-
[111] Koba K, Abe K, Ikeda I, Sugano M. Effects of alpha-tocopherol and tocotrienols
macol Toxicol 2008;103:55–60.
on blood pressure and linoleic acid metabolism in the spontaneously hyper-
[138] Norazlina M, Ima-Nirwana S, Abul Gapor MT, Abdul Kadir Khalid B. Toco-
tensive rat (SHR). Biosci Biotechnol Biochem 1992;56:1420–3.
trienols are needed for normal bone calcification in growing female rats. Asia
[112] Chen CW, Cheng HH. A rice bran oil diet increases LDL-receptor and HMG-
Pac J Clin Nutr 2002;11:194–9.
CoA reductase mRNA expressions and insulin sensitivity in rats with strep-
[139] Ima-Nirwana S, Suhaniza S. Effects of tocopherols and tocotrienols on body
tozotocin/nicotinamide-induced type 2 diabetes. J Nutr 2006;136:1472–6.
composition and bone calcium content in adrenalectomized rats replaced
[113] Chou TW, Ma CY, Cheng HH, Chen YY, Lai MH. A rice bran oil diet improves
with dexamethasone. J Med Food 2004;7:45–51.
lipid abnormalities and suppress hyperinsulinemic responses in rats with
[140] Gu JY, Wakizono Y, Sunada Y, Hung P, Nonaka M, Sugano M, et al. Dietary
streptozotocin/nicotinamide-induced type 2 diabetes. J Clin Biochem Nutr
effect of tocopherols and tocotrienols on the immune function of spleen and
mesenteric lymph node lymphocytes in Brown Norway rats. Biosci Biotech-
[114] Nafeeza MI, Norzana AG, Jalaluddin HL, Gapor MT. The effects of a tocotrie-
nol Biochem 1999;63:1697–702.
nol-rich fraction on experimentally induced atherosclerosis in the aorta of
[141] Azlina MF, Nafeeza MI, Khalid BA. A comparison between tocopherol and
rabbits. Malays J Pathol 2001;23:17–25.
tocotrienol effects on gastric parameters in rats exposed to stress. Asia Pac J
[115] Das S, Lekli I, Das M, Szabo G, Varadi J, Juhasz B, et al. Cardioprotection with
Clin Nutr 2005;14:358–65.
palm oil tocotrienols: comparision of different isomers. Am J Physiol Heart
[142] Nafeeza MI, Fauzee AM, Kamsiah J, Gapor MT. Comparative effects of a
Circ Physiol 2008;294:H970–8.
tocotrienol-rich fraction and tocopherol in aspirin-induced gastric lesions
[116] Qureshi AA, Peterson DM, Hasler-Rapacz JO, Rapacz J. Novel tocotrienols of
in rats. Asia Pac J Clin Nutr 2002;11:309–13.
rice bran suppress cholesterogenesis in hereditary hypercholesterolemic
[143] Freiser H, Jiang Q. Gamma-tocotrienol and gamma-tocopherol are primarily
swine. J Nutr 2001;131:223–30.
[117] Esterhuyse AJ, Toit ED, Rooyen JV. Dietary red palm oil supplementation
methylchroman and sulfated long-chain carboxychromanols in rats. J Nutr
protects against the consequences of global ischemia in the isolated perfused
rat heart. Asia Pac J Clin Nutr 2005;14:340–7.
[144] Hattori A, Fukushima T, Yoshimura H, Abe K, Imai K. Production of LLU-alpha
[118] Das M, Das S, Wang P, Powell SR, Das DK. Caveolin and proteasome in
following an oral administration of gamma-tocotrienol or gamma-tocoph-
erol to rats. Biol Pharm Bull 2000;23:1395–7.
[145] Husain K, Francois RA, Hutchinson SZ, Neuger AM, Lush R, Coppola D, et al.
[119] Lekli I, Ray D, Mukherjee S, Gurusamy N, Ahsan MK, Juhasz B, et al. Co-
Vitamin E delta-tocotrienol levels in tumor and pancreatic tissue of mice
ordinated autophagy with resveratrol and gamma-tocotrienol confers syn-
after oral administration. Pharmacology 2009;83:157–63.
ergetic cardioprotection. J Cell Mol Med 2009.
[146] Ikeda I, Imasato Y, Sasaki E, Sugano M. Lymphatic transport of alpha-,
[120] Wan Nazaimoon WM, Khalid BA. Tocotrienols-rich diet decreases advanced
gamma- and delta-tocotrienols and alpha-tocopherol in rats. Int J Vitam
glycosylation end-products in non-diabetic rats and improves glycemic
Nutr Res 1996;66:217–21.
control in streptozotocin-induced diabetic rats. Malays J Pathol 2002;24:
[147] Ikeda S, Niwa T, Yamashita K. Selective uptake of dietary tocotrienols into rat
skin. J Nutr Sci Vitaminol (Tokyo) 2000;46:141–3.
[121] Budin SB, Othman F, Louis SR, Bakar MA, Das S, Mohamed J. The effects of
[148] Ikeda S, Toyoshima K, Yamashita K. Dietary sesame seeds elevate alpha- and
palm oil tocotrienol-rich fraction supplementation on biochemical param-
gamma-tocotrienol concentrations in skin and adipose tissue of rats fed the
eters, oxidative stress and the vascular wall of streptozotocin-induced dia-
tocotrienol-rich fraction extracted from palm oil. J Nutr 2001;131:2892–7.
betic rats. Clinics (Sao Paulo) 2009;64:235–44.
[149] Kawakami Y, Tsuzuki T, Nakagawa K, Miyazawa T. Distribution of tocotrie-
[122] Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-kappabeta
nols in rats fed a rice bran tocotrienol concentrate. Biosci Biotechnol Biochem
signaling pathway by tocotrienol can prevent diabetes associated cognitive
deficits. Pharmacol Biochem Behav 2009;92:251–9.
[150] Khosla P, Patel V, Whinter JM, Khanna S, Rakhkovskaya M, Roy S, et al.
[123] Kuhad A, Chopra K. Tocotrienol attenuates oxidative–nitrosative stress and
Postprandial levels of the natural vitamin E tocotrienol in human circulation.
inflammatory cascade in experimental model of diabetic neuropathy. Neuro-
Antioxid Redox Signal 2006;8:1059–68.
[151] Saito Y, Yoshida Y, Nishio K, Hayakawa M, Niki E. Characterization of cellular
[124] Kanaya Y, Doi T, Sasaki H, Fujita A, Matsuno S, Okamoto K, et al. Rice bran
uptake and distribution of vitamin E. Ann N Y Acad Sci 2004;1031:368–75.
extract prevents the elevation of plasma peroxylipid in KKAy diabetic mice.
[152] Tanito M, Itoh N, Yoshida Y, Hayakawa M, Ohira A, Niki E. Distribution of
Diabetes Res Clin Pract 2004;66(Suppl. 1):S157–60.
tocopherols and tocotrienols to rat ocular tissues after topical ophthalmic
[125] Yoshida Y, Hayakawa M, Habuchi Y, Itoh N, Niki E. Evaluation of lipophilic
administration. Lipids 2004;39:469–74.
antioxidant efficacy in vivo by the biomarkers hydroxyoctadecadienoic acid
[153] Tasaki M, Umemura T, Inoue T, Okamura T, Kuroiwa Y, Ishii Y, et al.
and isoprostane. Lipids 2007;42:463–72.
Induction of characteristic hepatocyte proliferative lesion with dietary
[126] Fang F, Kang Z, Wong C. Vitamin E tocotrienols improve insulin sensitivity
exposure of Wistar Hannover rats to tocotrienol for 1 year. Toxicology
through activating peroxisome proliferator-activated receptors. Mol Nutr
Food Res 2010;54:345–52.
[154] Tsuzuki W, Yunoki R, Yoshimura H. Intestinal epithelial cells absorb gamma-
[127] Tiwari V, Kuhad A, Bishnoi M, Chopra K. Chronic treatment with tocotrienol,
tocotrienol faster than alpha-tocopherol. Lipids 2007;42:163–70.
an isoform of vitamin E, prevents intracerebroventricular streptozotocin-
[155] Yap SP, Yuen KH, Lim AB. Influence of route of administration on the
induced cognitive impairment and oxidative–nitrosative stress in rats. Phar-
absorption and disposition of alpha-, gamma- and delta-tocotrienols in rats.
macol Biochem Behav 2009;93:183–9.
J Pharm Pharmacol 2003;55:53–8.
[128] Tiwari V, Kuhad A, Chopra K. Tocotrienol ameliorates behavioral and bio-
[156] Qureshi AA, Qureshi N, Hasler-Rapacz JO, Weber FE, Chaudhary V, Crenshaw
chemical alterations in the rat model of alcoholic neuropathy. Pain
TD, et al. Dietary tocotrienols reduce concentrations of plasma cholesterol,
apolipoprotein B, thromboxane B2, and platelet factor 4 in pigs with inher-
[129] Tiwari V, Kuhad A, Chopra K. Suppression of neuro-inflammatory signaling
ited hyperlipidemias. Am J Clin Nutr 1991;53:1042S–6.
cascade by tocotrienol can prevent chronic alcohol-induced cognitive dys-
[157] Nakamura H, Furukawa F, Nishikawa A, Miyauchi M, Son HY, Imazawa T, et al.
function in rats. Behav Brain Res 2009;203:296–303.
Oral toxicity of a tocotrienol preparation in rats. Food Chem Toxicol
[130] Khanna S, Parinandi NL, Kotha SR, Roy S, Rink C, Bibus D, et al. Nanomolar
vitamin E alpha-tocotrienol inhibits glutamate-induced activation of phos-
[158] O'Byrne D, Grundy S, Packer L, Devaraj S, Baldenius K, Hoppe PP, et al. Studies
pholipase A2 and causes neuroprotection. J Neurochem 2010;112:1249–60.
of LDL oxidation following alpha-, gamma-, or delta-tocotrienyl acetate
[131] Khanna S, Roy S, Slivka A, Craft TK, Chaki S, Rink C, et al. Neuroprotective
supplementation of hypercholesterolemic humans. Free Radic Biol Med
properties of the natural vitamin E alpha-tocotrienol. Stroke 2005;36:
[159] Hayes KC, Pronczuk A, Liang JS. Differences in the plasma transport and tissue
[132] Roy S, Lado BH, Khanna S, Sen CK. Vitamin E sensitive genes in the developing
concentrations of tocopherols and tocotrienols: observations in humans and
rat fetal brain: a high-density oligonucleotide microarray analysis. FEBS Lett
hamsters. Proc Soc Exp Biol Med 1993;202:353–9.
[160] Yap SP, Yuen KH, Wong JW. Pharmacokinetics and bioavailability of alpha-,
[133] Mishima K, Tanaka T, Pu F, Egashira N, Iwasaki K, Hidaka R, et al. Vitamin E
gamma- and delta-tocotrienols under different food status. J Pharm Phar-
isoforms alpha-tocotrienol and gamma-tocopherol prevent cerebral infarc-
tion in mice. Neurosci Lett 2003;337:56–60.
[161] Lodge JK, Ridlington J, Leonard S, Vaule H, Traber MG. Alpha- and gamma-
[134] Hermizi H, Faizah O, Ima-Nirwana S, Ahmad Nazrun S, Norazlina M. Benefi-
tocotrienols are metabolized to carboxyethyl-hydroxychroman derivatives
cial effects of tocotrienol and tocopherol on bone histomorphometric param-
and excreted in human urine. Lipids 2001;36:43–8.
eters in Sprague–Dawley male rats after nicotine cessation. Calcif Tissue Int
[162] Yap SP, Yuen KH. Influence of lipolysis and droplet size on tocotrienol
absorption from self-emulsifying formulations. Int J Pharm 2004;281:67–78.
[135] Mehat MZ, Shuid AN, Mohamed N, Muhammad N, Soelaiman IN. Beneficial
[163] Ajuluchukwu JN, Okubadejo NU, Mabayoje M, Ojini FI, Okwudiafor RN,
effects of vitamin E isomer supplementation on static and dynamic bone
Mbakwem AC, et al. Comparative study of the effect of tocotrienols and -
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
tocopherol on fasting serum lipid profiles in patients with mild hypercho-
system, and in rat and human lipoproteins. Biochim Biophys Acta
lesterolaemia: a preliminary report. Niger Postgrad Med J 2007;14:30–3.
[164] Baliarsingh S, Beg ZH, Ahmad J. The therapeutic impacts of tocotrienols in
[191] Mazlan M, Sue Mian T, Mat Top G, Zurinah Wan Ngah W. Comparative
type 2 diabetic patients with hyperlipidemia. Atherosclerosis 2005;182:
effects of alpha-tocopherol and gamma-tocotrienol against hydrogen per-
oxide induced apoptosis on primary-cultured astrocytes. J Neurol Sci
[165] Qureshi AA, Bradlow BA, Brace L, Manganello J, Peterson DM, Pearce BC, et al.
Response of hypercholesterolemic subjects to administration of tocotrienols.
[192] Shichiri M, Takanezawa Y, Uchida K, Tamai H, Arai H. Protection of cerebellar
granule cells by tocopherols and tocotrienols against methylmercury toxici-
[166] Qureshi AA, Bradlow BA, Salser WA, Brace LD. Novel tocotrienols of rice bran
ty. Brain Res 2007;1182:106–15.
modulate cardiovascular disease risk parameters of hypercholesterolemic
[193] Yam ML, Abdul Hafid SR, Cheng HM, Nesaretnam K. Tocotrienols suppress
humans. Nutr Biochem 1997;8:290–8.
proinflammatory markers and cyclooxygenase-2 expression in RAW264.7
[167] Qureshi AA, Qureshi N, Wright JJ, Shen Z, Kramer G, Gapor A, et al. Lowering of
macrophages. Lipids 2009;44:787–97.
serum cholesterol in hypercholesterolemic humans by tocotrienols (palm-
[194] Serbinova EA, Packer L. Antioxidant properties of alpha-tocopherol and
vitee). Am J Clin Nutr 1991;53:1021S–6.
alpha-tocotrienol. Methods Enzymol 1994;234:354–66.
[168] Rasool AH, Rahman AR, Yuen KH, Wong AR. Arterial compliance and vitamin
[195] Miyazawa T, Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M.
E blood levels with a self emulsifying preparation of tocotrienol rich vitamin
Anti-angiogenic potential of tocotrienol in vitro. Biochemistry (Mosc)
E. Arch Pharm Res 2008;31:1212–7.
[169] Roza JM, Xian-Liu Z, Guthrie N. Effect of citrus flavonoids and tocotrienols on
[196] Lippman SM, Klein EA, Goodman PJ, Lucia MS, Thompson IM, Ford LG, et al.
serum cholesterol levels in hypercholesterolemic subjects. Altern Ther
Effect of selenium and vitamin E on risk of prostate cancer and other cancers:
Health Med 2007;13:44–8.
the Selenium and Vitamin E Cancer Prevention Trial (SELECT). J Am Med
[170] Tan DT, Khor HT, Low WH, Ali A, Gapor A. Effect of a palm-oil-vitamin E
concentrate on the serum and lipoprotein lipids in humans. Am J Clin Nutr
[197] Lonn E, Bosch J, Yusuf S, Sheridan P, Pogue J, Arnold JM, et al. Effects of long-
term vitamin E supplementation on cardiovascular events and cancer: a
[171] Tomeo AC, Geller M, Watkins TR, Gapor A, Bierenbaum ML. Antioxidant
randomized controlled trial. J Am Med Assoc 2005;293:1338–47.
effects of tocotrienols in patients with hyperlipidemia and carotid stenosis.
[198] Schroeder MT, Becker EM, Skibsted LH. Molecular mechanism of antioxidant
synergism of tocotrienols and carotenoids in palm oil. J Agric Food Chem
[172] Mensink RP, van Houwelingen AC, Kromhout D, Hornstra G. A vitamin E
concentrate rich in tocotrienols had no effect on serum lipids, lipoproteins, or
[199] Sookwong P, Nakagawa K, Yamaguchi Y, Miyazawa T, Kato S, Kimura F.
platelet function in men with mildly elevated serum lipid concentrations. Am
Tocotrienol distribution in foods: estimation of daily tocotrienol intake of
J Clin Nutr 1999;69:213–9.
Japanese population. J Agric Food Chem 2010;58:3350–5.
[173] Mustad VA, Smith CA, Ruey PP, Edens NK, DeMichele SJ. Supplementation
[200] Hassanein MM, Abedel-Razek AG. Chromatographic quantitation of some
with 3 compositionally different tocotrienol supplements does not improve
bioactive minor components in oils of wheat germ and grape seeds produced
cardiovascular disease risk factors in men and women with hypercholester-
as by-products. J Oleo Sci 2009;58:227–33.
olemia. Am J Clin Nutr 2002;76:1237–43.
[201] Amaral JS, Casal S, Alves MR, Seabra RM, Oliveira BP. Tocopherol and
[174] Rasool AH, Yuen KH, Yusoff K, Wong AR, Rahman AR. Dose dependent
tocotrienol content of hazelnut cultivars grown in Portugal. J Agric Food
elevation of plasma tocotrienol levels and its effect on arterial compliance,
plasma total antioxidant status, and lipid profile in healthy humans supple-
[202] Cunha SC, Amaral JS, Fernandes JO, Oliveira MB. Quantification of tocopherols
mented with tocotrienol rich vitamin E. J Nutr Sci Vitaminol (Tokyo)
and tocotrienols in Portuguese olive oils using HPLC with three different
detection systems. J Agric Food Chem 2006;54:3351–6.
[175] Stampfer MJ, Willet W, Castelli WP, Taylor JO, Fine J, Hennekens CH. Effect of
[203] Kallio H, Yang B, Peippo P, Tahvonen R, Pan R. Triacylglycerols, glyceropho-
vitamin E on lipids. Am J Clin Pathol 1983;79:714–6.
spholipids, tocopherols, and tocotrienols in berries and seeds of two subspe-
[176] Wahlqvist ML, Krivokuca-Bogetic Z, Lo CS, Hage B, Smith R, Lukito W.
cies (ssp. sinensis and mongolica) of Sea Buckthorn (Hippophae rhamnoides). J
Differential serum responses of tocopherols and tocotrienols during vitamin
Agric Food Chem 2002;50:3004–9.
supplementation in hypercholesterolemic individuals without change in
[204] Milagros Delgado-Zamarreno M, Bustamante-Rangel M, Sierra-Manzano S,
coronary risk factors. Nutr Res 1992;12(Suppl.).
Verdugo-Jara M, Carabias-Martinez R. Simultaneous extraction of tocotrie-
[177] Anderson SL, Rubin BY. Tocotrienols reverse IKAP and monoamine oxidase
nols and tocopherols from cereals using pressurized liquid extraction prior to
deficiencies in familial dysautonomia. Biochem Biophys Res Commun
LC determination. J Sep Sci 2009;32:1430–6.
[205] Panfili G, Fratianni A, Irano M. Normal phase high-performance liquid
[178] Rubin BY, Anderson SL, Kapas L. Can the therapeutic efficacy of tocotrienols in
chromatography method for the determination of tocopherols and tocotrie-
neurodegenerative familial dysautonomia patients be measured clinically?
nols in cereals. J Agric Food Chem 2003;51:3940–4.
Antioxid Redox Signal 2008;10:837–41.
[206] Bozan B, Temelli F. Chemical composition and oxidative stability of flax,
[179] Chin SF, Hamid NA, Latiff AA, Zakaria Z, Mazlan M, Yusof YA, et al. Reduction
safflower and poppy seed and seed oils. Bioresour Technol 2008;99:6354–9.
of DNA damage in older healthy adults by Tri E tocotrienol supplementation.
[207] Hafid SR, Radhakrishnan AK, Nesaretnam K. Tocotrienols are good adjuvants
for developing cancer vaccines. BMC Cancer 2010;10:5.
[180] Weber SU, Thiele JJ, Han N, Luu C, Valacchi G, Weber S, et al. Topical alpha-
[208] Naito Y, Shimozawa M, Kuroda M, Nakabe N, Manabe H, Katada K, et al.
tocotrienol supplementation inhibits lipid peroxidation but fails to mitigate
Tocotrienols reduce 25-hydroxycholesterol-induced monocyte–endothelial
increased transepidermal water loss after benzoyl peroxide treatment of
cell interaction by inhibiting the surface expression of adhesion molecules.
human skin. Free Radic Biol Med 2003;34:170–6.
[181] Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramem-
[209] Noguchi N, Hanyu R, Nonaka A, Okimoto Y, Kodama T. Inhibition of THP-1 cell
brane mobility in the antioxidant properties of alpha-tocopherol and alpha-
adhesion to endothelial cells by alpha-tocopherol and alpha-tocotrienol is
tocotrienol. Free Radic Biol Med 1991;10:263–75.
dependent on intracellular concentration of the antioxidants. Free Radic Biol
[182] Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, et al.
Structural and dynamic membrane properties of alpha-tocopherol and al-
[210] Mizushina Y, Nakagawa K, Shibata A, Awata Y, Kuriyama I, Shimazaki N, et al.
pha-tocotrienol: implication to the molecular mechanism of their antioxi-
Inhibitory effect of tocotrienol on eukaryotic DNA polymerase lambda and
dant potency. Biochemistry 1993;32:10692–9.
angiogenesis. Biochem Biophys Res Commun 2006;339:949–55.
[183] Yoshida Y, Niki E, Noguchi N. Comparative study on the action of tocopherols
[211] Makpol S, Shamaan NA, Jarien Z, Top AG, Khalid BA, Wan Ngah WZ. Different
and tocotrienols as antioxidant: chemical and physical effects. Chem Phys
starting times of alpha-tocopherol and gamma-tocotrienol supplementation
and tumor marker enzyme activities in the rat chemically induced with
[184] Birringer M, Pfluger P, Kluth D, Landes N, Brigelius-Flohe R. Identities and
cancer. Gen Pharmacol 1997;28:589–92.
differences in the metabolism of tocotrienols and tocopherols in HepG2 cells.
[212] Ong FB, Wan Ngah WZ, Shamaan NA, Md Top AG, Marzuki A, Khalid AK.
J Nutr 2002;132:3113–8.
Glutathione S-transferase and gamma-glutamyl transpeptidase activities in
[185] Kamal-Eldin A, Appelqvist LA. The chemistry and antioxidant properties of
cultured rat hepatocytes treated with tocotrienol and tocopherol. Comp
tocopherols and tocotrienols. Lipids 1996;31:671–701.
Biochem Physiol C 1993;106:237–40.
[186] Sigounas G, Anagnostou A, Steiner M. DL-Alpha-tocopherol induces apoptosis
[213] Ong FB, Wan Ngah WZ, Top AG, Khalid BA, Shamaan NA. Vitamin E glutathi-
in erythroleukemia, prostate, and breast cancer cells. Nutr Cancer 1997;28:
one S-transferase and gamma-glutamyl transpeptidase activities in cultured
hepatocytes of rats treated with carcinogens. Int J Biochem 1994;26:
[187] Hosomi A, Arita M, Sato Y, Kiyose C, Ueda T, Igarashi O, et al. Affinity for alpha-
tocopherol transfer protein as a determinant of the biological activities of
[214] Shibata A, Nakagawa K, Sookwong P, Tsuzuki T, Oikawa S, Miyazawa T. Tumor
vitamin E analogs. FEBS Lett 1997;409:105–8.
anti-angiogenic effect and mechanism of action of delta-tocotrienol. Bio-
[188] Inokuchi H, Hirokane H, Tsuzuki T, Nakagawa K, Igarashi M, Miyazawa T.
chem Pharmacol 2008;76:330–9.
[215] Landes N, Pfluger P, Kluth D, Birringer M, Ruhl R, Bol GF, et al. Vitamin E
activates gene expression via the pregnane X receptor. Biochem Pharmacol
[189] Miyazawa T, Tsuzuki T, Nakagawa K, Igarashi M. Antiangiogenic potency of
vitamin E. Ann N Y Acad Sci 2004;1031:401–4.
[216] Uto-Kondo H, Ohmori R, Kiyose C, Kishimoto Y, Saito H, Igarashi O, et al.
[190] Suarna C, Hood RL, Dean RT, Stocker R. Comparative antioxidant activity of
Tocotrienol suppresses adipocyte differentiation and Akt phosphorylation in
tocotrienols and other natural lipid-soluble antioxidants in a homogeneous
3T3-L1 preadipocytes. J Nutr 2009;139:51–7.
B.B. Aggarwal et al. / Biochemical Pharmacology 80 (2010) 1613–1631
[217] Bi S, Liu JR, Li Y, Wang Q, Liu H, Yan YG, et al. gamma-Tocotrienol modulates
[234] Nakano M, Onodera A, Saito E, Tanabe M, Yajima K, Takahashi J, et al. Effect of
the paracrine secretion of VEGF induced by cobalt (II) chloride via ERK
astaxanthin in combination with alpha-tocopherol or ascorbic acid against
signaling pathway in gastric adenocarcinoma SGC-7901 cell line. Toxicology
oxidative damage in diabetic ODS rats. J Nutr Sci Vitaminol (Tokyo)
[218] Li F, Tan W, Kang Z, Wong CW. Tocotrienol enriched palm oil prevents
[235] Ima-Nirwana S, Kiftiah A, Sariza T, Gapor Abd, Khalid MTBAK. Palm vitamin E
atherosclerosis through modulating the activities of peroxisome prolifera-
improves bone metabolism and survival rate in thyrotoxic rats. Gen Phar-
tors-activated receptors. Atherosclerosis 2010;211:278–82.
[219] Wang Q, Theriault A, Gapor A, Adeli K. Effects of tocotrienol on the intracel-
[236] Rukmini C, Raghuram TC. Nutritional and biochemical aspects of the
lular translocation and degradation of apolipoprotein B: possible involve-
hypolipidemic action of rice bran oil: a review. J Am Coll Nutr 1991;10:
ment of a proteasome independent pathway. Biochem Biophys Res Commun
[237] Khor HT, Ng TT. Effects of administration of alpha-tocopherol and tocotrie-
[220] Theriault A, Wang Q, Gapor A, Adeli K. Effects of gamma-tocotrienol on ApoB
nols on serum lipids and liver HMG CoA reductase activity. Int J Food Sci Nutr
synthesis, degradation, and secretion in HepG2 cells. Arterioscler Thromb
Vasc Biol 1999;19:704–12.
[238] Poveda E, Ayala P, Milena R, Ordonez E, Baracaldo C, Delgado W, et al. Effects
[221] Galli F, Stabile AM, Betti M, Conte C, Pistilli A, Rende M, et al. The effect of
of vegetal oil supplementation on the lipid profile of Wistar rats. Biomedica
alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman
metabolites on prostate cancer cell proliferation. Arch Biochem Biophys
[239] Gupta A, Chopra K. Effect of tocotrienols on iron-induced renal dysfunction
and oxidative stress in rats. Drug Chem Toxicol 2009;32:319–25.
[222] Brigelius-Flohe R. Induction of drug metabolizing enzymes by vitamin E. J
[240] Fairus S, Nor RM, Cheng HM, Sundram K. Postprandial metabolic fate of
Plant Physiol 2005;162:797–802.
tocotrienol-rich vitamin E differs significantly from that of alpha-tocopherol.
[223] Clarke MW, Burnett JR, Croft KD. Vitamin E in human health and disease. Crit
Am J Clin Nutr 2006;84:835–42.
Rev Clin Lab Sci 2008;45:417–50.
[241] Radhakrishnan AK, Lee AL, Wong PF, Kaur J, Aung H, Nesaretnam K. Daily
[224] Yap WN, Zaiden N, Tan YL, Ngoh CP, Zhang XW, Wong YC, et al. Id1,
supplementation of tocotrienol-rich fraction or alpha-tocopherol did not
inhibitor of differentiation, is a key protein mediating anti-tumor
induce immunomodulatory changes in healthy human volunteers. Br J Nutr
responses of gamma-tocotrienol in breast cancer cells. Cancer Lett
[242] Rautalahti M, Albanes D, Hyvonen L, Piironen V, Heinonen M. Effect of
[225] McIntyre BS, Briski KP, Tirmenstein MA, Fariss MW, Gapor A, Sylvester
sampling site on retinol, carotenoid, tocopherol, and tocotrienol concentra-
PW. Antiproliferative and apoptotic effects of tocopherols and tocotrie-
tion of adipose tissue of human breast with cancer. Ann Nutr Metab
nols on normal mouse mammary epithelial cells. Lipids 2000;35:
[243] Nesaretnam K, Gomez PA, Selvaduray KR, Razak GA. Tocotrienol levels in
[226] Wali VB, Sylvester PW. Synergistic antiproliferative effects of gamma-
adipose tissue of benign and malignant breast lumps in patients in Malaysia.
tocotrienol and statin treatment on mammary tumor cells. Lipids
Asia Pac J Clin Nutr 2007;16:498–504.
[244] Weinstein SJ, Wright ME, Lawson KA, Snyder K, Mannisto S, Taylor PR, et al.
[227] Saito H, Kiyose C, Yoshimura H, Ueda T, Kondo K, Igarashi O. Gamma-
Serum and dietary vitamin E in relation to prostate cancer risk. Cancer
tocotrienol, a vitamin E homolog, is a natriuretic hormone precursor. J Lipid
Epidemiol Biomarkers Prev 2007;16:1253–9.
[245] Nur Azlina MF, Nafeeza MI. Tocotrienol and alpha-tocopherol reduce corti-
[228] Kausar H, Bhasin G, Zargar MA, Athar M. Palm oil alleviates 12-O-tetrade-
costerone and noradrenalin levels in rats exposed to restraint stress. Phar-
canoyl-phorbol-13-acetate-induced tumor promotion response in murine
skin. Cancer Lett 2003;192:151–60.
[246] Kamat JP, Devasagayam TP. Tocotrienols from palm oil as potent inhibitors of
[229] Nesaretnam K, Ambra R, Selvaduray KR, Radhakrishnan A, Reimann K, Razak
lipid peroxidation and protein oxidation in rat brain mitochondria. Neurosci
G, et al. Tocotrienol-rich fraction from palm oil affects gene expression in
tumors resulting from MCF-7 cell inoculation in athymic mice. Lipids
[247] Raederstorff D, Elste V, Aebischer C, Weber P. Effect of either gamma-
tocotrienol or a tocotrienol mixture on the plasma lipid profile in hamsters.
[230] Yamada Y, Obayashi M, Ishikawa T, Kiso Y, Ono Y, Yamashita K. Dietary
Ann Nutr Metab 2002;46:17–23.
tocotrienol reduces UVB-induced skin damage and sesamin enhances toco-
[248] Osakada F, Hashino A, Kume T, Katsuki H, Kaneko S, Akaike A. Alpha-
trienol effects in hairless mice. J Nutr Sci Vitaminol (Tokyo) 2008;54:117–23.
tocotrienol provides the most potent neuroprotection among vitamin E
[231] Yap WN, Zaiden N, Luk SY, Lee DT, Ling MT, Wong YC, et al. In vivo evidence of
analogs on cultured striatal neurons. Neuropharmacology 2004;47:
gamma-tocotrienol as a chemosensitizer in the treatment of hormone-re-
fractory prostate cancer. Pharmacology 2010;85:248–58.
[249] Palozza P, Verdecchia S, Avanzi L, Vertuani S, Serini S, Iannone A, et al.
[232] Qureshi AA, Salser WA, Parmar R, Emeson EE. Novel tocotrienols of rice bran
Comparative antioxidant activity of tocotrienols and the novel chromanyl-
inhibit atherosclerotic lesions in C57BL/6 ApoE-deficient mice. J Nutr
polyisoprenyl molecule FeAox-6 in isolated membranes and intact cells. Mol
Cell Biochem 2006;287:21–32.
[233] Newaz MA, Yousefipour Z, Nawal N, Adeeb N. Nitric oxide synthase activity in
[250] Song BL, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation
blood vessels of spontaneously hypertensive rats: antioxidant protection by
of 3-hydroxy-3-methylglutaryl coenzyme a reductase stimulated by delta-
gamma-tocotrienol. J Physiol Pharmacol 2003;54:319–27.
and gamma-tocotrienols. J Biol Chem 2006;281:25054–61.
Source: http://tocomax.com.br/site/artigos/5.pdf
Checklist for Full-Time Missionary Recommendation MISSIONARY DEPARTMENT50 E NORTH TEMPLE ST RM 345 WSALT LAKE CITY UT 84150-5400 To the Bishop or Branch President Ensure that family members and others contributing to the Church's missionary funds are aware that contributions belong to the Church for use Review the Church Handbook of Instructions, Book 1: Stake Presidencies
Fractional Flow Reserve–Guided PCI versus Medical Therapy in Stable Coronary Disease Bernard De Bruyne, M.D., Ph.D., Nico H.J. Pijls, M.D., Ph.D., Bindu Kalesan, M.P.H., Emanuele Barbato, M.D., Ph.D., Pim A.L. Tonino, M.D., Ph.D., Zsolt Piroth, M.D., Nikola Jagic, M.D., Sven Mobius-Winckler, M.D., Gilles Rioufol, M.D., Ph.D., Nils Witt, M.D., Ph.D., Petr Kala, M.D., Philip MacCarthy, M.D., Thomas Engström, M.D.,










