
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Trainorlab.ucdavis.edu


Author's personal copy
Available online at www.sciencedirect.com
Hormones and Behavior 53 (2008) 192 – 199
Rapid effects of estradiol on male aggression depend on photoperiod in
reproductively non-responsive mice
Brian C. Trainor a,b,⁎, M. Sima Finy b, Randy J. Nelson b
a Department of Psychology, University of California, Davis, CA 95616, USA
b Departments of Psychology and Neuroscience, Institute for Behavioral Medicine Research, Ohio State University, Columbus, OH 43210, USA
Received 27 July 2007; revised 12 September 2007; accepted 21 September 2007
Available online 29 September 2007
In three genuses and four species of rodents, housing in winter-like short days (8L:16D) increases male aggressive behavior. In all of these
species, males undergo short-day induced regression of the reproductive system. Some studies, however, suggest that the effect of photoperiod onaggression may be independent of reproductive responses. We examined the effects of photoperiod on aggressive behavior in California mice(Peromyscus californicus), which do not display reproductive responsiveness to short days. As expected, short days had no effect on plasmatestosterone. Estrogen receptor alpha and estrogen receptor beta immunostaining did not differ in the lateral septum, medial preoptic area, bednucleus of the stria terminalis, or medial amygdala. However, males housed in short days were significantly more aggressive than males housed inlong days. Similar to previous work in beach mice (Peromyscus polionotus), estradiol rapidly increased aggression when male California micewere housed in short days but not when housed in long days. These data suggest that the effects of photoperiod on aggression and estrogensignaling are independent of reproductive responses. The rapid action of estradiol on aggression in short-day mice also suggests that nongenomicmechanisms mediate the effects of estrogens in short days.
2007 Elsevier Inc. All rights reserved.
Keywords: Aggressive behavior; Peromyscus californicus; California mouse; c-fos; Nongenomic effects; Estrogen receptor alpha; Estrogen receptor beta
conditions (Haemisch et al., 1994; Marashi et al., 2003). Thesestudies document that the environmental context has important
The physiological bases of aggressive behavior are typically
effects on the function of physiological pathways that regulate
examined under constant environmental conditions. However,
the environment can have important effects on aggression and
Several laboratories have reported a consistent effect of
can even alter the effects of specific physiological systems. For
photoperiod (day length) on male aggression in rodents. In
example, adverse behavioral effects associated with the short
Syrian hamsters (Mesocricetus auratus) (Garrett and Campbell,
form of the monoamine oxidase A (MAOA) gene are typically
1980; Jasnow et al., 2002; Caldwell and Albers, 2004), Siberian
observed only in individuals who have been exposed to adverse
hamsters (Phodopus sungorus) (Jasnow et al., 2000; Demas et
environmental conditions (Caspi et al., 2003; Kim-Cohen et al.,
al., 2004; Wen et al., 2004), beach mice (Peromyscus polionotus)
2006; Frazzetto et al., 2007). In rodents, males are typically
(Trainor et al., 2007a), and deer mice (Peromyscus maniculatus)
more aggressive when confronted in a familiar environment
(Trainor et al., 2007b), males are more aggressive in a resident–
(e.g., a resident–intruder test) as compared to a neutral setting
intruder test when tested in short days (8L:16D) as opposed to
(Bester-Meredith et al., 1999), and males from some mouse
long days (16L:8D). This effect has been considered paradox-
strains are more aggressive when housed in environmentally
ical, because in each of these species housing in short days
enriched housing compared to the standard laboratory housing
causes regression of testes and a corresponding decrease intestosterone (Jasnow et al., 2000, 2002; Trainor et al., 2006c). InSiberian hamsters there is evidence that the effect of photoperiod
⁎ Corresponding author. Department of Psychology, 1 Shields Ave.,
on aggression is independent of gonadal responses (Demas et al.,
University of California, Davis, CA 95616, USA.
E-mail address: [email protected] (B.C. Trainor).
2004). Photoperiod has no significant effect on reproductive
0018-506X/$ - see front matter 2007 Elsevier Inc. All rights reserved.
doi:10.1016/j.yhbeh.2007.09.016
Author's personal copy
B.C. Trainor et al. / Hormones and Behavior 53 (2008) 192–199
physiology or aggression in CD-1 mice (Trainor et al., 2006a),
estrogens regulate aggressive behavior. Estrogen receptor alpha
but this stock of mice has been bred in captivity since at least the
and beta are expressed in the LS, medial preoptic area (MPOA),
1920s. Recent studies in P. polionotus suggest that changes in
BNST, ventromedial hypothalamus (VMH), and MEA. These
estrogen signaling are involved in mediating the effect of photo-
brain areas are part of a circuit that regulates social behavior
period on aggression in rodents.
(Newman, 1999; Goodson, 2005), including aggression
Estrogens can affect aggression in male vertebrates because
(Nelson and Trainor, 2007). It was previously reported that, as
aromatase present in the brain can convert androgens to estro-
in P. polionotus, fadrozole increased aggression in P. californicus
gens. The effects of estrogens on male aggression are variable,
housed in long days (Trainor et al., 2004). In the current study, we
increasing aggression in some species and decreasing aggression
tested whether estradiol acts rapidly to increase aggression in
in others (Trainor et al., 2006b). This could reflect differences in
short-day P. californicus.
the expression of estrogen receptor (ER) subtypes. Selectivedeletion of ERα is associated with reduced male aggression in
domestic mice (Ogawa et al., 1997; Scordalakes and Rissman,2003). The deletion of ERβ is generally associated with
increased aggression (Ogawa et al., 1999; Nomura et al.,2006), although this effect appears to be context-dependent
P. californicus were obtained from Dr. Catherine Marler (University of
Wisconsin, Madison, WI, USA). California mice form monogamous mating
(Nomura et al., 2002). Deletion of both receptors is associated
pairs in the field (Ribble and Salvioni, 1990) and males do not respond to short
with increased male aggression (Ogawa et al., 2000). In male
days by reducing testes mass (Nelson et al., 1995). Males were individually
P. polionotus, and P. maniculatus, ERα expression in the lateral
housed and provided with filtered tap water and Teklad 8640 food (Harlan,
septum (LS) and bed nucleus of the stria terminalis (BNST) is
Madison, WI) ad libitum. Field studies show that male P. californicus defend
increased in short days whereas ERβ expression in the BNST
exclusive territories (Ribble and Salvioni, 1990), which indicates that to acertain extent, single housing approximates the social organization of young
and medial amygdala (MEA) is increased in long days (Trainor
unpaired males in this species. All experimental procedures were approved
et al., 2007b). This led to the attractive hypothesis that changes
by the Ohio State University Institutional Animal Care and Use Committee
in ER expression mediate the effect of photoperiod on
and animals were maintained in accordance with the recommendations of the
National Institutes of Health Guide for the Care and Use of Laboratory Animals.
The estrogen receptor hypothesis was tested with hormone
manipulation experiments (Trainor et al., 2007a). In male
Effect of photoperiod on behavior and physiology
P. polionotus, treatment with the aromatase inhibitor fadrozoleincreased aggression in long days but decreased aggression in
Males (residents) were randomly assigned to be housed in long (n = 9,
LD 16:8) or short (n = 7, LD 8:16) days for 8 weeks. One week before resident–
short days. The estrogen receptor hypothesis was not supported
intruder aggression tests, retroorbital blood samples were collected under light
because the effects of fadrozole were reversed by co-treatment
isoflurane anesthesia. Plasma samples were frozen at −80 °C. Resident–intruder
with either ERα or ERβ selective agonists, regardless of
tests were conducted 1 h after lights out (1500 EDT) under dim red light. A
photoperiod. These data suggested that differential expression of
group-housed, sexually inexperienced male intruder (weight matched to within
estrogen receptors could not explain the effect of photoperiod on
4 g) was introduced into each resident's cage for 7 min. Observations werevideotaped and scored offline by an observer blind to treatment assignments.
aggression. Instead, it appears that photoperiod changes the
One hour after aggression tests residents were anesthetized with isoflurane and
molecular activity of estrogen receptors. Results from a micro-
rapidly decapitated. This time course was chosen because previous studies have
array experiment showed that estrogen-dependent gene expres-
reported that maximal c-fos expression occurs between 1 and 2 h after
sion in the BNST was decreased in short days compared to long
stimulation (Kovacs and Sawchenko, 1996; Hoffman and Lyo, 2002). Brains
days (Trainor et al., 2007a). Furthermore, estradiol injections
were quickly removed and fixed in 5% acrolein overnight at 4 °C. Brains werethen transferred to 30% sucrose for 24 h and frozen for immunocytochemistry.
increased aggression within 15 min in male P. polionotus housed
Plasma was collected from trunk blood samples to measure post-encounter
in short days, but had no effect on males housed in long days.
testosterone and testes were dissected and weighed. Total testosterone was
These data suggested that estrogens increase aggression in short-
measured with a 125I testosterone RIA kit (DSL-4100; Diagnostic Systems
day mice by activating nongenomic mechanisms, as it is
Laboratories, Webster, TX). The intra-assay coefficient of variation was 2.8%.
generally thought that 15 min is insufficient time for changes
Hormone concentrations were not normally distributed and were log trans-formed for statistical analyses.
in estrogen-dependent changes in gene expression to occur
Immunocytochemistry for ERα and ERβ was conducted using the methods
(Vasudevan and Pfaff, 2006). Together, these results suggest that
of Trainor et al. (2007b). Briefly, six brains from each group were sectioned at
the effect of photoperiod on aggression is independent of
40 μm on a cryostat and free-floating sections were then incubated in 1% sodium
changes in estrogen receptor expression and is mediated instead
borohydride in 0.1 M phosphate-buffered saline (PBS) for 10 min. Sections were
by changes in receptor activity (genomic or nongenomic).
then rinsed in 20% normal goat serum and 0.3% hydrogen peroxide in PBS for20 min. Alternate sections were incubated in either primary ERα antibody
In the present study we examined the effect of photoperiod
(C1355, Upstate Biotechnology, concentration 1:20 K), primary ERβ (D7N,
on aggression in California mice (P. californicus), a species in
Invitrogen, Carlsbad, CA, concentration 1:400), or primary c-fos (rabbit anti c-
which males do not regress testes when housed in short days
fos, Chemicon 1:10 K) in 1% normal goat serum in 0.5% PBS + triton X (PBS
(Nelson et al., 1995). We hypothesized that if the effect of
TX) for 40 h at 4 °C. Sections were rinsed in PBS and incubated for 2 h with
photoperiod is indeed independent of gonadal responses to short
biotinylated goat–anti-rabbit antibody (1:500, Vector Laboratories, Burlingame,CA) in PBS + TX. The sections were rinsed in PBS and then incubated for 30 min
days, then male California mice would increase aggressive
in avidin–biotin complex (ABC Elite kit, Vector Laboratories). After rinses in
behavior in short days. We also examined whether photoperiod
PBS, the sections were developed in hydrogen peroxide and diaminobenzidine
influences the effects of estrogen receptor expression and how
for 2 min. Sections were mounted on gel-coated slides, dehydrated and
Author's personal copy
B.C. Trainor et al. / Hormones and Behavior 53 (2008) 192–199
coverslipped. A Nikon E800 microscope was used to capture representative
generally occur on a time scale of hours or days, whereas nongenomic effects of
photomicrographs of each of the following brain areas using a mouse brain atlas
estrogens can occur within minutes or seconds (Nabekura et al., 1986). Previous
(Paxinos and Franklin, 2002): ventral lateral septum (bregma 0.26), BNST
studies have used the delivery of water soluble cE2 to study the nongenomic
(bregma 0.02), MPOA (bregma 0.02), VMH (bregma −1.70), paraventricular
effects of estrogens on reproductive behavior (Cross and Roselli, 1999; Cornil
nucleus (PVN, bregma −1.22), and posterodorsal MEA (bregma −1.82). In these
et al., 2006). Fifteen minutes after injections males were tested in resident–
areas the number of ER and c-fos immunoreactive (-ir) cells was counted using
intruder aggression tests as described above.
Image J (NIMH, Bethesda, MD) by an observer unaware of treatmentassignments. We counted the number of cells in a box in the MPOA (l × w,
400 × 250 μm), LS (330 × 480 μm), BNST (500 × 350 μm), PVN (330 × 480 μm),and MEA (450 × 450 μm) similar to our previous study quantifying estrogenreceptor expression in P. polionotus and P. maniculatus (Trainor et al., 2007b). In
Effects of photoperiod on physiology, receptor expression, and
the VMH, the number of ERα and c-fos positive cells in a circle with a radius of
180 μm was counted. In the anterior hypothalamus (bregma −1.22) we countedc-fos positive cells (box size, 520 × 520 μm) but not ER positive cells because
Photoperiod did not affect testes mass (Fig. 1A; t14 = 1.15,
there are no ERα or ERβ cells in this region of the brain in this species. The
p = 0.27), baseline testosterone concentrations (Fig. 1B, t
aggressive behavior data and cell counts from the males assigned to the long-day
group in this study were also analyzed (as virgin males) in the accompanying
p = 0.42), or the number of ERα-ir cells in the LS (Figs. 2A and B,
paper examining the effect of parental experience on aggression and estrogen
t10 = 0.28, p = 0.80), BNST (t10 = 1.26, p = 0.26), MPOA (Figs. 2C
receptor expression (Trainor et al., 2008-this issue).
and D, t10 = 0.17, p = 0.90), MEA (t10 = 1.1, p = 0.28), PVN(t10 = 0.28, p = 0.80), or VMH (t10 = 1.40, p = 0.2). There was
Effects of acute estradiol injections in long days and short days
also no effect of photoperiod on the number of ERβimmunostained cells in the BNST (Figs. 2E and F, t10 = 0.96,
Male California mice were randomly assigned to be housed in long or short
p = 0.36), MPOA (t
days for 8 weeks. All males were then bilaterally castrated under isoflurane
10 = 0.74, p = 0.48), PVN (t10 = 1.11, p = 0.26),
anesthesia. Each male was implanted with a Silastic implant (1.47 mm i.d.;
or MEA (Figs. 2G and H, t10 = 0.54, p = 0.60).
1.96 mm o.d.) packed with 1 mm of testosterone. All males were also implanted
Despite the absence of differences in estrogen receptor
with an osmotic minipump (model 2002, Alzet, Palo Alto, CA) containing
expression or testosterone concentrations, males housed in
fadrozole (0.25 mg/kg/day), a potent aromatase inhibitor. After surgery all
short days were significantly more aggressive than males
animals were treated with an s.c. injection of buprenorphine (0.38 mg/kg). After
housed in long days. Males housed in short days showed
10 days, all males were tested in a resident–intruder aggression test. Fifteenminutes before testing, each male was injected with either saline or 100 μg/kg
shorter attack latencies (Fig. 1C, t14 = 2.90, p = 0.01) and more
cyclodextrin conjugated estradiol (cE
offensive attacks (Fig. 1D, t
2). The genomic effects of estrogens
14 = 2.23, p = 0.04) in aggression
Fig. 1. Effect of photoperiod on physiology and behavior. There was no effect of photoperiod on testes mass (A) or baseline plasma testosterone (B). In resident–intruder aggression tests, males housed in short days showed significantly shorter mean attack latency (C) and increased bites (D) compared to males housed in longdays. ⁎p b 0.05. For all panels; long days n = 9, short days n = 7.
Author's personal copy
B.C. Trainor et al. / Hormones and Behavior 53 (2008) 192–199
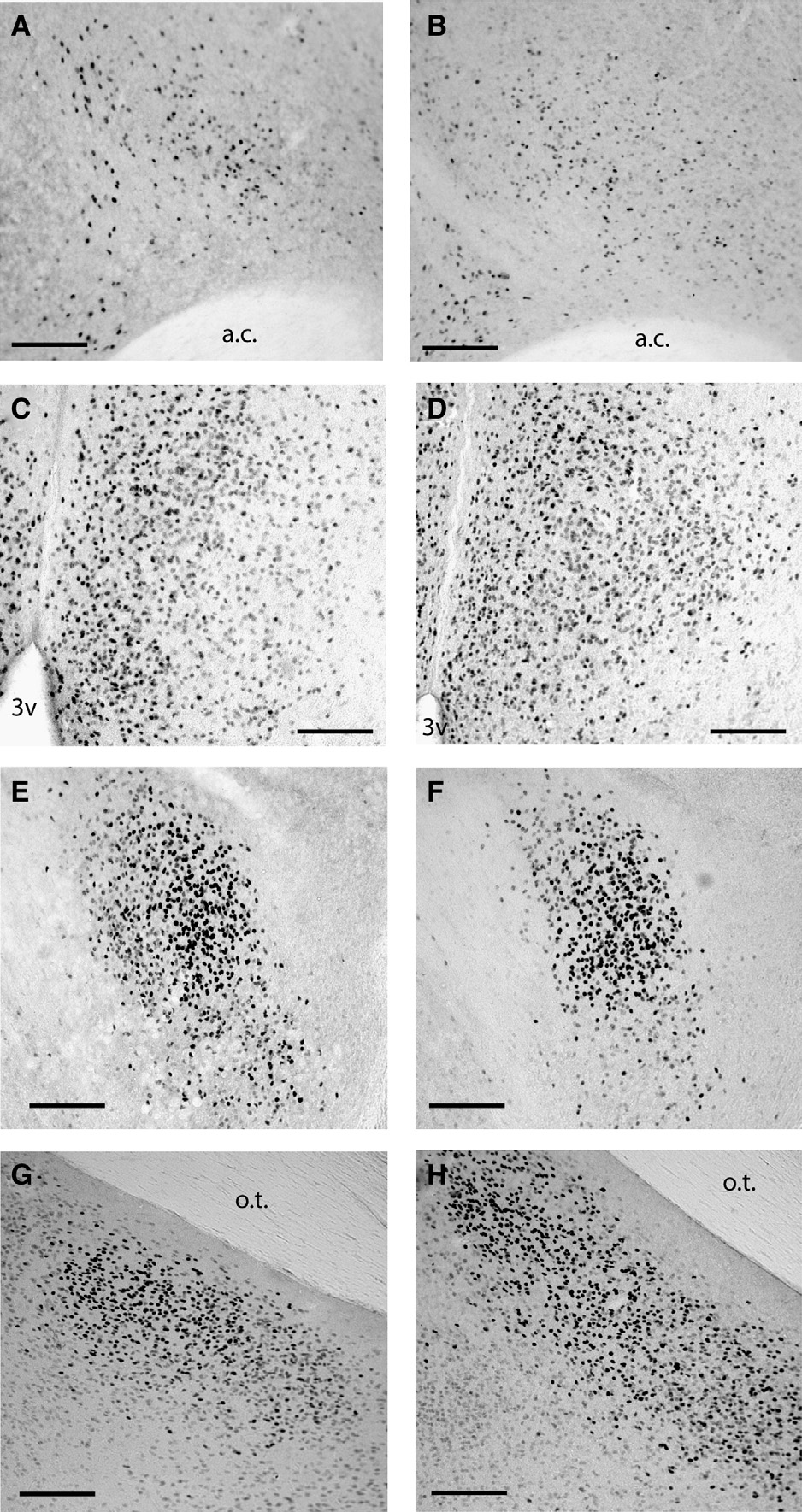
Fig. 2. Representative photomicrographs of estrogen receptor alpha immunoreactivity in long days (A, C) and short days (B, D) in the lateral septum (A, B) and medialpreoptic area (C, D). Representative photomicrographs of estrogen receptor beta immunoreactivity in long days (E, G) and short days (F, H) in the bed nucleus of thestria terminalis (E, F) and medial amygdala (G, H). Abbreviations: anterior commissure (ac), third ventricle (3v). Scale bars = 100 μm.
Author's personal copy
B.C. Trainor et al. / Hormones and Behavior 53 (2008) 192–199
tests than males housed in long days. In general the frequency
were no significant photoperiod by injection interactions (all
of boxing in long-day (mean ± SE, 6.8 ± 2.4) and short-day
p's N 0.16), planned comparisons showed that the effects of
(5.4 ± 2.2) mice was low, and unaffected by photoperiod (t14 =
estradiol injections were stronger when mice were housed in
0.69, p = 0.41).
short days. Estradiol injections caused a significant increase in
One hour after aggression tests, males housed in short days
bites in short (Fig. 3A, p b 0.01), but not long days (p N 0.05).
(0.38 ± 0.1 ng/ml) had significantly higher testosterone concen-
Similarly, estradiol injections caused a significant decrease in
trations compared to long-day males (0.12 ± 0.2 ng/ml, t14 =
attack latency in short days (Fig. 3B, p b 0.05) but not long days
2.26, p = 0.04). In the MPOA there were more c-fos positive
(p N 0.05). There were no significant differences for boxing
cells in short-day males compared to long-day males (Supple-
behavior (Fig. 3C).
mentary Table 1, t10 = 2.80, p = 0.02). There were no significantdifferences in c-fos positive cells in any other brain region.
Rapid effects of estradiol on aggression
The biology of P. californicus allowed us to test hypotheses on
the relationship between photoperiod and aggression from a
We detected significant main effects of estradiol injections
unique perspective. The effects of photoperiod on aggression in
on biting (F1,32 = 4.94, p b 0.05) and attack latency (F1,32 = 4.37,
P. californicus occur in the absence of gonadal regression, a result
p b 0.05), but not boxing (F1,32 = 0.1, p N 0.05). Although there
that supports the hypothesis that the effect of photoperiod onaggression is independent of changes in gonadal hormones(Demas et al., 2004; Wen et al., 2004). We also observed thatphotoperiod affected aggression in the absence of changes in ERαand ERβ expression. This result supports the hypothesisdeveloped in P. polionotus that the effects of photoperiod onaggression are independent of changes in ER expression (Trainoret al., 2007a). Finally, we observed that in P. californicus estradiolinjections act rapidly to increase aggression in mice housed inshort days but not long days. These data suggest that estrogensincrease aggression by activating nongenomic pathways in short,but not long days. These data indicate that the environment hasimportant effects on how estrogens regulate aggression.
Previously, the effect of photoperiod on aggression had only
been observed in species that undergo gonadal regression andreduced testosterone when housed in short days (Matochiket al., 1986; Jasnow et al., 2000, 2002; Caldwell and Albers,2004; Trainor et al., 2007b). Our results demonstrate that theeffect of photoperiod on aggression can be dissociated fromreproductive responses, because P. californicus were more ag-gressive in short days in the absence of reproductive changes.
A previous study reported that exogenous testosterone admin-istered to short-day Siberian hamsters decreased aggressivebehavior in a resident–intruder test (Jasnow et al., 2000), but asubsequent report showed that short-day non-responders (whichdo not decrease testes size or testosterone production in shortdays) are just as aggressive as short-day males with regressedtestes (Wen et al., 2004). Our results suggest that the effect ofphotoperiod on aggression occurs independently of changes ingonadal testosterone. Studies in hamsters suggest that the pri-mary source of aromatizable androgen in Peromyscus may beproduced in the brain, most likely from adrenal steroids such asdehydroepiandrosterone (DHEA) (Demas et al., 2004). DHEAcan be converted to the aromatizable androgen androsteindioneby 3 β-hydroxysteroid dehydrogenase.
In closely related P. polionotus and P. maniculatus, increased
aggression in short days is associated with increased expression
Fig. 3. Rapid effects of estradiol on aggression in a resident–intruder test.
of ERα and decreased expression of ERβ (Trainor et al.,
Estradiol injections (100 μg/kg) administered 15 min before aggression tests
2007b). In contrast, we observed in the present study that
caused a significant increase in bites (A) and a decrease in attack latency (B) in
increased aggression in short days occurs in the absence of
short-day males but not long-day males. There was no effect of photoperiod orestradiol injections on boxing behavior (C). ⁎⁎p b 0.01, ⁎p b 0.05.
differences in ERα and ERβ expression in the hypothalamus
Author's personal copy
B.C. Trainor et al. / Hormones and Behavior 53 (2008) 192–199
and limbic system. It has been hypothesized that the effect of
These findings suggest that corticosterone might inhibit aggres-
photoperiod on aggression is independent of changes in ERα or
sion in rats via genomic processes and increase aggression via
ERβ in the brain (Trainor et al., 2007a), and the current ex-
nongenomic processes, similar to how estrogens appear to
perimental results are consistent with this hypothesis. Although
regulate aggression in Peromyscus. An intriguing possibility is
we have not tested this directly, we suspect that the effect of
that nongenomic estrogen receptor and glucocorticoid receptor
photoperiod on ERα and ERβ expression in P. polionotus is
activity may tap into similar second messenger systems to
related to changes in testosterone and resulting negative feed-
facilitate aggressive behavior. Presently, it is unclear which
back (Clancy and Michael, 1994). We observed no effect of
pathways mediate the rapid actions of estrogens or glucocorti-
estradiol injections on aggression in P. californicus housed in
coids on aggressive behavior.
long days whereas studies of wild-type Mus musculus (housed
We have demonstrated in two species of Peromyscus that
in a 12-h light cycle) report that a similar dose of estradiol
estrogens act rapidly to increase aggression in short days and
increases aggression in males (Nomura et al., 2006). These
that this effect is weaker or absent in long days. A previous
contrasting results could be attributed to several factors. First, in
study reported that estrogens inhibited aggression in P.
the Nomura study estradiol was administered over a 3-week
californicus housed in long days (14 L) (Trainor et al., 2004),
period via implants whereas we conducted our tests 15 min after
indicating that a photoperiod-mediated reversal of the effects of
a subcutaneous injection. A 3-week time period is sufficient to
estrogens on aggression in Peromyscus is not limited to a single
induce genomic changes mediated by estrogen receptors, and
species. It remains unspecified how differences in day length
most researchers agree that 15 min is not enough time for
can exert such a profound effect on hormone action. One
genomic effects to occur. In P. californicus, treatment with
intriguing possibility is suggested by in vitro studies. In cell
fadrozole for 10 days is associated with increased aggression.
culture, melatonin interacts with the DNA binding domain of
This suggests that estrogens may indeed affect aggression in
ERα and inhibits its transcriptional activity (Rato et al., 1999;
P. californicus by affecting gene expression, but in the opposite
Kiefer et al., 2002, 2005). Mice housed in short days have
direction observed in M. musculus. This raises a second pos-
increased melatonin concentrations in the brain for prolonged
sibility. Species differences in estrogen receptor expression
periods of time compared to mice housed in long days, and
may contribute to differences in how estrogens affect aggres-
recent data suggest that classical estrogen receptors can have
sion. For example, ERα positive cells are present in the PVN of
nongenomic effects (Abraham et al., 2004). One possible
P. californicus but not M. musculus, whereas ERα positive cells
explanation for our results is that melatonin may inhibit the
are present in the AHA of M. musculus but not P. californicus
transcriptional activity of classical estrogen receptors without
(Merchenthaler et al., 2004). The AHA is known to have im-
interfering with nongenomic activity. This would explain why
portant effects on aggressive behavior in rodents (Ferris et al.,
nongenomic action of estradiol is more prevalent in short days.
1997), so the absence of ERα in this area may influence how
Another possibility is that melatonin could affect the secretion of
estrogens affect aggression in Peromyscus. The availability
adrenal hormones that could provide the substrate for aromatiz-
of selective ER agonists should facilitate examination of
able androgen in the brain. Studies in M. musculus (Paterson and
the effects of the two ER subtypes on behavior in different
Vickers, 1981) and P. sungorous (Demas et al., 2004) have
species that exhibit different distributions of estrogen receptor
demonstrated that the facilitating effect of melatonin on male
aggression can be blocked via adrenalectomy. It is also possible
Estradiol acts within 15 min to increase aggression in
(and perhaps likely) that melatonin affects aggressive behavior
P. californicus housed in short days, suggesting that estrogens
by working at multiple levels of hormone function
act nongenomically to increase aggression. This result is con-
sistent with a previous study in P. polionotus which reported
The effects of photoperiod on aggressive behavior were first
that estradiol injections increased bites in short- but not long-
described in species that exhibit reproductive suppression in
day mice (Trainor et al., 2007a). It is thought that such rapid
short days (Garrett and Campbell, 1980; Jasnow et al., 2000).
behavioral effects of estradiol must be mediated by nongenomic
Our observations in P. californicus, a species that does not
processes (Nilsson et al., 2001; Vasudevan and Pfaff, 2006).
exhibit reproductive inhibition in short days, suggest that these
Previous studies showed that estradiol acts rapidly to increase
findings may be applicable to a wider array of species,
male mating behavior in rats (Cross and Roselli, 1999) and quail
including humans. There is some evidence that components
(Cornil et al., 2006), but these effects did not differ from those
of aggression in humans exhibit seasonal rhythms. Patients
observed in response to systemic estradiol treatment. Non-
diagnosed with seasonal affective disorder tend to be more
genomic effects of glucocorticoids on behavior have been
likely to exhibit anger attacks compared to patients diagnosed
reported in several species. In rough skinned newts (Taricha
with non-seasonal depression (Winkler et al., 2006). Anger and
granulosa), corticosterone rapidly inhibits male mating behav-
hostility scores, as measured using Emotion Rating scales
ior (Moore and Miller, 1984), presumably by binding to
(Beck et al., 1961), have also reported seasonal variation, with
glucocorticoid receptors positioned at the membrane (Orchinik
higher scores being recorded in winter (Harmatz et al., 2000).
et al., 1991). Corticosterone also acts rapidly to increase ag-
Accumulating evidence indicates that the effect of photoperiod
gression in rats (Mikics et al., 2004) and mice (Poole and Brain,
on aggression is decoupled from reproductive responses, sug-
1974). In contrast, over longer time frames corticosterone
gesting that hamsters and Peromyscus may be effective models
appears to inhibit rat aggression (Haller et al., 2001, 2004).
for studying seasonal changes in aspects of human behavior.
Author's personal copy
B.C. Trainor et al. / Hormones and Behavior 53 (2008) 192–199
Jasnow, A.M., Huhman, K.L., Bartness, T.J., Demas, G.E., 2000. Short-day
increases in aggression are inversely related to circulating testosteroneconcentrations in male Siberian hamsters (Phodopus sungorus). Horm.
We thank P. Gallagher for the technical assistance. This work
Behav. 38, 102–110.
supported by NIH MH076313 to B.C.T., SBS Undergraduate
Jasnow, A.M., Huhman, K.L., Bartness, T.J., Demas, G.E., 2002. Short days and
Research Award to M.S.F., and NIH MH57535 to R.J.N.
exogenous melatonin increase aggression of male Syrian hamsters(Mesocricetus auratus). Horm. Behav. 42, 13–20.
Appendix A. Supplementary data
Kiefer, T., Ram, P.T., Yuan, L., Hill, S.M., 2002. Melatonin inhibits estrogen
receptor transactivation and cAMP levels in breast cancer cells. BreastCancer Res. Treat. 71, 37–45.
Supplementary data associated with this article can be found,
Kiefer, T.L., Yuan, L., Dong, C., Burow, M.E., Hill, S.M., 2005. Differential
in the online version, at doi:10.1016/j.yhbeh.2007.09.016.
regulation of estrogen receptor alpha, glucocorticoid receptor and retinoicacid receptor alpha transcriptional activity by melatonin is mediated viadifferent G proteins. J. Pineal Res. 38, 231–239.
Kim-Cohen, J., Caspi, A., Taylor, A., Williams, B., Newcombe, R., Craig, I.W.,
Moffitt, T.E., 2006. MAOA, maltreatment, and gene-environment interac-
Abraham, I.M., Todman, M.G., Korach, K.S., Herbison, A.E., 2004. Critical
tion predicting children's mental health: new evidence and a meta-analysis.
in vivo roles for classical estrogen receptors in rapid estrogen actions on
Mol. Psychiatry 11, 903–913.
intracellular signaling in mouse brain. Endocrinology 145, 3055–3061.
Kovacs, K.J., Sawchenko, P.E., 1996. Sequence of stress-induced alterations in
Beck, A.T., Ward, C.H., Mendelson, M., Mock, J., Erbaugh, J., 1961. An
indicies of synaptic and transcriptional activation in parvocellular
inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571.
neurosecretory neurons. J. Neurosci. 16, 262–273.
Bester-Meredith, J.K., Young, L.J., Marler, C.A., 1999. Species differences in
Marashi, V., Barnekow, A., Ossendorf, E., Sachser, N., 2003. Effects of different
paternal behavior and aggression in Peromyscus and their associations with
forms of environmental enrichment on behavioral, endocrinological, and
vasopressin immunoreactivity and receptors. Horm. Behav. 36, 25–38.
immunological parameters in male mice. Horm. Behav. 43, 281–292.
Caldwell, H.K., Albers, H.E., 2004. Effects of photoperiod on vasopressin-
Matochik, J.A., Miernicki, M., Powers, J.B., Bergondy, M.L., 1986. Short
induced aggression in Syrian hamsters. Horm. Behav. 46, 444–449.
photoperiods increase ultrasonic vocalization rates among male Syrian
Caspi, A., McClay, J., Moffitt, T.E., Mill, J., Martin, J., Craig, I.W., Taylor, A.,
hamsters. Physiol. Behav. 38, 453–458.
Poulton, R., 2003. Role of genotype in the cycle of violence in maltreated
Merchenthaler, I., Lane, M., Numan, S., Dellovade, T., 2004. Distribution of
children. Science 297, 851–854.
estrogen receptor alpha and beta in the mouse central nervous system: in
Clancy, A.N., Michael, R.P., 1994. Effects of testosterone and aromatase
vivo autoradiographic and immunocytochemical analyses. J. Comp. Neurol.
inhibition on estrogen receptor-like immunoreactivity in male rat brain.
473, 270–291.
Neuroendocrinology 59, 552–560.
Mikics, E., Kruk, M.R., Haller, J., 2004. Genomic and non-genomic effects of
Cornil, C.A., Dalla, C., Papadopoulou-Daifoti, Z., Ballien, M., Balthazart, J.,
glucocorticoids on aggressive behavior in male rats. Psychoneuroendocri-
2006. Estradiol rapidly activates male sexual behavior and affects brain
nology 29, 618–635.
monoamine levels in the quail brain. Behav. Brain Res. 166, 110–123.
Moore, F.L., Miller, L.J., 1984. Stress-induced inhibition of sexual behavior:
Cross, E., Roselli, C.E., 1999. 17beta-Estradiol rapidly facilitates chemoinves-
corticosterone inhibits courtship behaviors of a male amphibian (Taricha
tigation and mounting in castrated male rats. Am. J. Physiol. 276,
granulosa). Horm. Behav. 18, 400–410.
Nabekura, J., Oomura, Y., Minami, T., Minzuno, Y., Fukuda, A., 1986. Mech-
Demas, G.E., Polacek, K.M., Durazzo, A., Jasnow, A.M., 2004. Adrenal
anism of the rapid effects of 17β-estradiol on medial amygdala neurons.
hormones mediate melatonin-induced increases in aggression in male
Science 233, 226–228.
Siberian hamsters (Phodopus sungorus). Horm. Behav. 46, 582–591.
Nelson, R.J., Trainor, B.C., 2007. Neural mechanisms of aggression. Nat. Rev.,
Ferris, C.F., Melloni, R.H., Koppel, G., Perry, K.W., Fuller, R.W., Delville, Y.,
Neurosci. 8, 536–546.
1997. Vasopressin/Serotonin interactions in the anterior hypothalamus
Nelson, R.J., Gubernick, D.J., Blom, J.M., 1995. Influence of photoperiod,
control aggressive behavior in golden hamsters. J. Neurosci. 17, 4331–4340.
green food, and water availability on reproduction in male California mice
Frazzetto, G., Di Lorenzo, G., Carola, V., Proietti, L., Sokolowska, E.,
(Peromyscus californicus). Physiol. Behav. 57, 1175–1180.
Siracusano, A., Gross, C., Troisi, A., 2007. Early trauma and increased risk
Newman, S., 1999. The medial extended amygdala in male reproductive
for physical aggression during adulthood: the moderating role of MAOA
behavior. A node in the mammalian social behavior network. Ann. N.Y.
genotype. PLoS One 2, e486.
Acad. Sci 877, 242–257.
Garrett, J.W., Campbell, C.S., 1980. Changes in social behavior of the male
Nilsson, S., Makela, S., Treuter, E., Tujague, M., Thomsen, J.S., Andersson, G.,
golden hamster accompanying photoperiodic changes in reproduction.
Enmark, E., Pettersson, K., Warner, M., Gustaffson, J.-A., 2001. Mechan-
Horm. Behav. 14, 303–318.
isms of estrogen action. Physiol. Rev. 81, 1535–1565.
Goodson, J.L., 2005. The vertebrate social behavior network: evolutionary
Nomura, M., Durbak, I., Chan, J., Gustafsson, J.A., Smithies, O., Korach, K.S.,
themes and variations. Horm. Behav. 48, 11–22.
Pfaff, D.W., Ogawa, S., 2002. Genotype/Age interactions on aggressive
Haemisch, A., Voss, T., Gartner, K., 1994. Effects of environmental enrichment
behavior in gonadally intact estrogen receptor beta knockout (βERKO) male
on aggressive behavior, dominance hierarchies, and endocrine states in male
mice. Horm. Behav. 41, 288–296.
DBA/2J mice. Physiol. Behav. 56, 1041–1048.
Nomura, M., Andersson, S., Korach, K., Gustafsson, J., Pfaff, D., Ogawa, S.,
Haller, J., van de Schraaf, J., Kruk, M.R., 2001. Deviant forms of aggression in
2006. Estrogen receptor-beta gene disruption potentiates estrogen-inducible
glucocorticoid hyporeactive rats: a model for ‘pathological' aggression?
aggression but not sexual behaviour in male mice. Eur. J. Neurosci. 23,
J. Neuroendocrinol. 13, 102–107.
Haller, J., Halasz, J., Mikics, E., Kruk, M.R., 2004. Chronic glucocorticoid
Ogawa, S., Lubahn, D.B., Korach, K.S., Pfaff, D.W., 1997. Behavioral effects
deficiency-induced abnormal aggression, autonomic hypoarousal, and social
of estrogen receptor gene disruption in male mice. Proc. Natl. Acad. Sci.
deficit in rats. J. Neuroendocrinol. 16, 550–557.
U. S. A. 94, 1476–1481.
Harmatz, M.G., Well, A.D., Overtree, C.E., Kawamura, K.Y., Rosal, M.,
Ogawa, S., Chan, J., Chester, A.E., Gustafsson, J., Korach, K.S., Pfaff, D.W.,
Ockene, I.S., 2000. Seasonal variation of depression and other moods: a
1999. Survival of reproductive behaviors in estrogen receptor beta gene-
longitudinal approach. J. Biol. Rhythms 15, 344–350.
deficient (βERKO) male and female mice. Proc. Natl. Acad. Sci. U. S. A.
Hoffman, G.E., Lyo, D., 2002. Anatomical markers of activity in
96, 12887–12892.
neuroendocrine systems: are we all "fos-ed out"? J. Neuroendocrinol. 14,
Ogawa, S., Chester, A.E., Hewitt, S.C., Walker, V.R., Gustafsson, J., Smithies,
O., Korach, K.S., Pfaff, D.W., 2000. Abolition of male sexual behaviors in
Author's personal copy
B.C. Trainor et al. / Hormones and Behavior 53 (2008) 192–199
mice lacking estrogen receptors α and β (αβERKO). Proc. Natl. Acad. Sci.
differences in aggression. Horm. Behav. 50, 338–345.
U. S. A. 97, 14737–14741.
Trainor, B.C., Kyomen, H.H., Marler, C.A., 2006b. Estrogenic encounters: how
Orchinik, M., Murray, T.F., Moore, F.L., 1991. A corticosteroid receptor in
interactions between aromatase and the environment modulate aggression.
neuronal membranes. Science 252, 1848–1851.
Front. Neuroendocrinol. 27, 170–179.
Paterson, A.T., Vickers, C., 1981. Melatonin and the adrenal cortex: relationship
Trainor, B.C., Martin, L.B.I., Greiwe, K.M., Kuhlman, J.R., Nelson, R.J., 2006c.
to territorial aggression in mice. Physiol. Behav. 27, 983–987.
Social and photoperiod effects on reproduction in five species of Peromys-
Paxinos, G., Franklin, K.B.J., 2002. The Mouse Brain in Stereotaxic
cus. Gen. Comp. Endocrinol. 148, 252–259.
Coordinates. Academic Press, New York.
Trainor, B.C., Lin, S., Finy, M.S., Rowland, M.R., Nelson, R.J., 2007a.
Poole, A.E., Brain, P., 1974. Effects of adrenalectomy and treatments with
Photoperiod reverses the effects of estrogens on male aggression via genomic
ACTH and glucocorticoids on isolation-induced aggressive behavior in male
and non-genomic pathways. Proc. Natl. Acad. Sci. U. S. A. 104, 9840–9845.
albino mice. Prog. Brain Res. 41, 465–472.
Trainor, B.C., Rowland, M.R., Nelson, R.J., 2007b. Photoperiod affects
Rato, A.G., Pedrero, J.G., Martinez, M.A., Del Rio, B., Lazo, P.S., Ramos, S.,
estrogen receptor alpha, estrogen receptor beta, and aggressive behavior.
1999. Melatonin blocks the activation of estrogen receptor for DNA binding.
Eur. J. Neurosci. 26, 207–218.
FASEB J. 13, 857–868.
Trainor, B.C., Finy, M.S., Nelson, R.J. 2008-this issue. Paternal aggression in a
Ribble, D.O., Salvioni, M., 1990. Social organization and nest cooccupancy in
biparental mouse: parallels with maternal aggression. Horm. Behav.
Peromyscus californicus, a monogamous rodent. Behav. Ecol. Sociobiol. 26,
Vasudevan, N., Pfaff, D.W., 2006. Membrane initiated actions of estrogens in
Scordalakes, E.M., Rissman, E.F., 2003. Aggression in male mice lacking
neuroendocrinology: emerging principles. Endocr. Rev. 28, 1–19.
functional estrogen receptor α. Behav. Neurosci. 117, 38–45.
Wen, J., Hotchkiss, A., Demas, G., Nelson, R., 2004. Photoperiod affects
Trainor, B.C., Bird, I.M., Marler, C.A., 2004. Opposing hormonal mechanisms
neuronal nitric oxide synthase and aggressive behaviour in male Siberian
of aggression revealed through short-lived testosterone manipulations and
hamsters (Phodopus sungorus). J. Neuroendocrinol. 16, 916–921.
multiple winning experiences. Horm. Behav. 45, 115–121.
Winkler, D., Pjrek, E., Konstantinidis, A., Praschak-Rieder, N., Willeit, M.,
Trainor, B.C., Greiwe, K.M., Nelson, R.J., 2006a. Individual differences in
Stastny, J., Kasper, S., 2006. Anger attacks in season affective disorder. Int.
estrogen receptor α in select brain nuclei are associated with individual
J. Neuropsychopharmacol. 9, 215–219.
Source: http://trainorlab.ucdavis.edu/uploads/5/2/3/2/52321699/californicus_photoperiod.pdf
24-hour protection from frequent heartburn 24h heartburn protection for less than £1 a day: Frequent heartburn sufferers may need multiple daily doses to achieve long term relief, which can be costly. With over 8 million adults in the UK experiencing heartburn How quickly will Nexium Heartburn medicines: a daily cost comparison
mal laut, mal Luise Heute gibt es nicht nur ordentlich etwas auf die Ohren, sondern auch auf die Augen. Wenn etwa Johannes Deutsch Töne und Klänge sichtbar macht, entsteht ein Kaleidoskop an Farben und Formen. Für Ruhe sorgen danach Helfer gegen Über- lautes von außen und schallschluckende Leisetreter aus Glas für Innen. Im folgenden Earobic-Bereich lassen wir den Werkstoff







