
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Diltiazem-coating
European Drying Conference - EuroDrying'2011
Palma. Balearic Island, Spain, 26-28 October 2011
Influence of Coating and Formulation Variables on the Dissolution Profile
of Diltiazem HCl Extended Release Pellets
A. Bernardino, A. C. P. Simão, E. T. Katayama, M. B. Bertuzzi, N. S. Maria, M. Nitz1
Mauá Institute of Technology (IMT)
Praça Mauá, 1, São Caetano do Sul, São Paulo, 09580-900, Brazil
Tel.:+55 11 4239 3114, E-mail: [email protected]
Abstract: The development of extended release diltiazem HCl pellets is presented. The release rate was adjusted by introducing a subcoating layer prior to film coating with polyvinyl acetate, and replacing some of the microcrystalline cellulose in the core with lactose. The pellets coated with a mass gain of 2.7% Advantia Prime and 12% Kollicoat SR30D showed a zero order dissolution profile for 9 hours. The amount of drug released was 23% after 3 hours, 65% after 9 hours and 73% after 12 hours, which conforms to the Pharmacopeia acceptance table.
Keywords: film coating, pellets, modified release, extended release
the spraying nozzle (Yang
et al., 1992) and the air
and coating suspension flow rates.
Simply stated, film coating is the process of drying a
There are two main types of modified release:
suspension while it is continuously sprayed over the
sustained and delayed release. The first permits a
surface of particles in a moving or fluidized bed. In
reduction in dosing frequency compared to the
the pharmaceutical industry, the coating of a solid
situation in which the drug is presented in a
dosage form takes place for either aesthetic or
conventional form. The second is the choice when
functional reasons. For multiunit dosage forms, also
the release of the active ingredient comes sometime
known as pellets, film coating is usually performed to
other than promptly after administration. The gastro-
improve efficiency in treatment by modifying the
resistant, also called enteric-release forms belong to
drug release profile. The controlled release
this class. PH-sensitive enteric coatings have been
characteristic is designed so that dosing frequency
used routinely to deliver drug to the small intestine
may be reduced and the risk of side effects limited by
avoiding a higher than necessary drug concentration in the blood (Hamdani
et al., 2002; Salsa
et al.,
This work shows the development of extended
1997). There are a great number of variables
release diltiazem HCl pellets. Variations on coating
involved in this complex process. The challenge of
and core formulation were performed in order to
film coating is to apply the spray droplets uniformly
achieve the desired release rate.
and to dry them at the proper rate.
MATERIAL AND METHODS
There are already many commercial coating suspensions, such as: Aquacoat, Eudragit, Kollicoat
Uncoated pellets
and Surelease. The suspension blend is chosen based
Diltiazem HCl pellets were produced by the
on the desired release profile. Kollicoat type SR 30D,
extrusion-spheronisation process (Santos
et al.,
for instance, is an aqueous dispersion of polyvinyl
Microcrystalline
acetate, suitable for a ph-independent release profile
(Blanver, Brazil), was used as the main filler of the
(Dashevsky
et al., 2004).
pellets. Methocel (low viscosity hypromellose) was
The release profile depends not only on the coating
tested as a binder at 2, 3 and 4%, as seen in Table 1.
formulation itself but also on the process variables
Water was added to the solid mixture of Table 1 in
that affect drying. Examples of process variables that
the ratio 48 g of water per 100 g of solid,
influence the coating efficiency are: the type of fluid
approximately. The mixture was granulated in a
bed contactor, the temperature and humidity of inlet
planetary mixer (Arno, Brazil). The wet mixture was
air, the pressure of the atomizing air, the position of
extruded in a gravity feed lab-scale extruder (Zelus, Brazil). The rotational speed was 40 rpm and the
screen opening 0.8 mm. A 500 g-load of material was
The acceptance values for drug release are shown in
then spheronised on a friction plate (Zelus, Brazil)
for 4.5 min at 900 rpm. The granules were then dried in an oven at 50 ºC for 24 hours. For the coating step,
Table 2. Acceptance Table
350 g of pellets between 0.85 and 1.40 mm were used.
Amount dissolved (%)
Table 1. Pellet composition - dry mass fractions
Not less than 70%
RESULTS AND DISCUSSION
Pellets containing from 2 to 4% Methocel, according
to Table 1, were all suitable for the extrusion-spheronisation process and the size distributions were
The coating suspensions
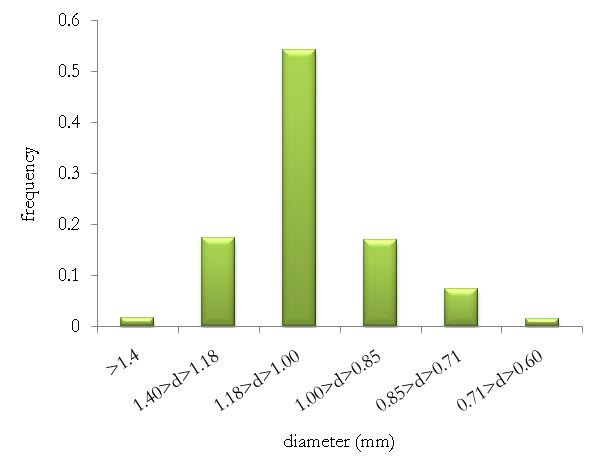
similar. Fig. 1 shows the pellet size distribution for
Two different coating suspensions were used with the
the formulation containing 4% Methocel. It is clear
following commercial powders: Advantia Prime (ISP
that 80% of the pellets are between 0.85 mm and
Technologies) and Kollicoat SR30 D (Basf). The first
1.18 mm. That narrow size distribution is important
is a protective prime coating, which consists of
for industrial production.
HPMC and HPC. The second is an aqueous dispersion of polyvinyl acetate, used to achieve a ph-independent release profile. Advantia Prime is a pre-blended powder, which should simply be added to water and mixed (6.0% w/w of powder). Kollicoat suspension was also prepared by simply mixing with water (1:1).
The coating experiments
The experiments were accomplished in a fluid bed
Fig. 1. Size distribution (pellets with 4% Methocel)
coater column with a Wurster insert (model R-060, Zelus, Brazil). Air suspension coating, known as the
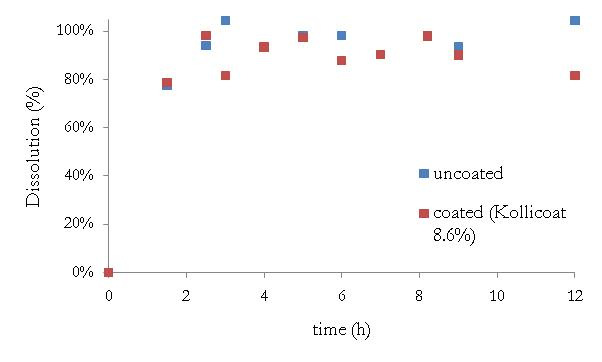
Those pellets were coated with Kollicoat SR30D to a
Wurster process, involves dispersing solid particulate
mass gain of 8.6%. However, when the release
core materials in a supporting air stream and the
profiles of coated and uncoated pellets are compared,
bottom spray coating of the suspended materials. The
one can see no difference (Fig. 2). As diltiazem is
cyclic process is repeated until the desired coating
highly soluble in water, the drug on the surface of
thickness is obtained (Anal, 2008).
pellets dissolves in the water of the coating layer. As a consequence, pores are formed in the polymer layer
A mass of 350 g of pellets was placed in the fluid
and the release rate of the drug is not modified.
bed. Inlet airflow rate was 30 Nm³/h and inlet temperature was adjusted to 60°C. These values were chosen after preliminary testing. The double-fluid atomizing nozzle has an orifice of 0.7 mm. The atomizing air absolute pressure was 1.5 bar. The coating suspensions were kept under agitation during the coating experiments. The feed rates were 3.4 g/min for Advantia and 7.0 g/min for Kollicoat.
The dissolution tests
The coated granules were submitted to dissolution
studies. USP XXXII (2009) test number 1 for
Fig. 2. Release profile of pellets (coated and
extended release diltiazem HCl capsules was
employed. In this analysis 900 mL of water are used as dissolution medium. Apparatus 2 (paddle) was
In order to achieve an extended release profile, a
used at 100 rpm. The drug release in the dissolution
prime coating layer was applied with Advantia
medium was determined by UV absorbance at 237
Prime. The results are shown in Fig. 3. The release
profile was modified significantly, which proves that
spectrophotometer.
the prime layer was really required.


None of the tests conform to the Table 2. The test
The higher solubility of the pellets with lactose
with 10% mass gain of Kollicoat was close to a zero
increased the release rate considerably. That
order kinetic release for the first 6 hours but the
behaviour is shown in Fig. 5. A thicker layer of
amount of drug dissolved after 12 hours was only
Kollicoat was needed to extend drug release. With
55%. The other two tests (4 and 8% Kollicoat layer)
Kollicoat mass gain lower than 10%, approximately
showed a too fast release rate for the first 3 hours and
60% of the drug was released in 3 hours. With a
therefore did not conform to the acceptance table.
Kollicoat mass gain of 12%, a zero order dissolution rate was achieved for 9 hours, which conforms to the limits established in Table 2.
A subcoating layer with Advantia Prime (2.7% mass gain) was effective in reducing the amount of Kollicoat SR30D needed to modify the release of diltiazem HCl. The amount of polymer used to achieve a zero order release kinetic was 12%. The use of 5% lactose in the core made the pellets more
soluble and increased the amount of drug dissolved
Fig. 3. Release profile of pellets with prime and
modified release coatings
ACKNOWLEDGEMENTS
To overcome this obstacle, part of the MCC was replaced with lactose. The idea was to make the
Authors wish to acknowledge FAPESP for the
pellet more soluble and increase the amount of drug
financial support and Mauá Institute of Technology.
released after 12 hours. Lactose accounted for 5% of the total mass, reducing the percentage of MCC to
66%, keeping Methocel at 4% and diltiazem HCl at
Anal, A. K. (2008), Controlled-Release Dosage
Pharmaceutical
Manufacturing Handbook, Wiley-Interscience, New Jersey, 1370p.
Dashevsky, A.; Kolter, K.; Bodmeier, R. (2004), pH-
Independent Release of a Basic Drug from Pellets Coated with the Extended Release Polymer Dispersion Kollicoat SR 30 D and the Enteric Polymer Dispersion Kollicoat MAE 30 DP. Europ. J. Pharm. Biopharm., v.28, n.1, pp. 45-49.
Hamdani, J.; Moës, A. J.; Amighi, K. (2002),
Development and evaluation of prolonged release
Fig. 4. Size distribution (pellets with 5% lactose)
pellets obtained by the melt pelletization process, Int. J. Pharm., v.245, pp.167-177.
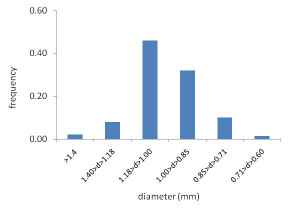
The size distribution was only slightly affected by this change, as shown in Fig. 4, so that more than
Salsa, T; Veiga, F.; Pina, M. E (1997), Oral
80% of the pellets are still between 0.85 mm and
Controlled-Release Dosage Forms. I. Cellulose
Ether polymers in Hydrophilic Matrices. Drug Dev. Ind. Pharm., 23(9), pp.929-938.
Santos et al. (2004), Obtenção de pellets por extrusão
e esferonização farmacêutica – Parte I – Avaliação das variáveis tecnológicas e de formulação, Rev. Bras. Ciências Farm., v.40, n.4, pp.455-470.
Pharmacopeia.
Ghebresellasie, I. (1992), The Effect of Spray
Mode and Chamber Geometry of Fluid-Bed Coating Equipment and Other Parameters on an
Fig. 5. Release profile of pellets with 5% lactose
Aqueous-Based Ethylcellulose Coating. Int. J. Pharm., v.86, n.2-3, pp.247-257.
Source: http://www.uibcongres.org/imgdb/archivo_dpo10992.pdf
British Journal of Anaesthesia 90 (2): 166±72 (2003) DOI: 10.1093/bja/aeg038 Parecoxib sodium has opioid-sparing effects in patients undergoing total knee arthroplasty under spinal anaesthesia² R. C. Hubbard1*, T. M. Naumann2, L. Traylor1 and S. Dhadda1 1Pharmacia, 5200 Old Orchard Road, Skokie, IL 60077, USA. 2Hessing'sche Orthopedic Clinic, Hessingstr, Augsburg, Germany
Kriminelle Auswüchse Unserer heutigen Scheindemokratie Die Fortsetzung des Willkürstaates und des Holocausts mit neuen Mitteln! Euthanasie HEUTE Erlebnisse eines Kreativen Mitbürgers im heimtückisch mordenden neuen deutschen Willkürstaat warum unsere deutschen Behörden ihre









