
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
[9]فريال 644
Eng. &Tech.Journal, Vo 3
l. 3,Part (B), No.2, 2015
Synthesis of Prodrug Polymer as Ring Opening of PVP
Dr. Firyal Mohammed Ali
College of Science, University of AL-Mustansiriya/Baghdad
Email: a
Halah Hamid Humadi
College of Pharmacy, University of AL-Mustansiriya/Baghdad
Email:Toqaquee
Luma Amer Mussa
College of Pharmacy, University of AL-Mustansiriya/Baghdad
Email: baba ahamed
Revised on: 30/7/2014 & Accepted on: 7/8/2014
ABSTRACT In this work a new drug polymer was prepared from reaction of
polyvinylpyrrolidone (PVP) with doxycylin in 10:1 dioxane: dimethelyformad
solvent mixture. The prepared drug polymer was formed with 85% conversion
percentage. The physical properties were studied and intrinsic viscosity was equal to
0.23 dl/g. The drug polymer was characterized by FT-IR and UV spectroscopy. The
swelling % was studied in different non solvents. The elemental analysis and DSC
were analyzed. The controlled release rates for drug polymer were studied in different
pH values at 37oC for 4 days. The softening point of the prepared doxycyclin drug
polymer was 143.4oC to 150.3oC with ΔH=-189.89 J/g.
نﻮﯿﻧﺪﻟوﺮﯾﺎﺑ ﻞﯿﻧﺎﻓ ﻲﻟﻮﺒﻟا ﺔﻘﻠﺣ ﺢﺘ ﺑ
ﻔ ﺔﯾﺮﻤﯿﻟﻮﺑ ﺔﯿﺋاود تﺎﻣﺪﻘﻣ ﺮﯿﻀﺤﺗ
ﺐﯾﺬﻣ ﻲﻓ ﻦﯿﻠﯾﺎﺴﯿﺴﻛوﺪﻟا رﺎﻘﻋ ﻊﻣ نﻮﻨﯾﺪﯿﻟوﺮﯾﺎﺑ ﻞﯿﻨﯾﺎﻓ ﻲﻟﻮﺒﻟا ﻞﻋﺎﻔﺗ ﻦﻣ ﻲﺋاود ﺮﻤﯿﻟ
ﻮﺑ ﺮﻀﺣ ﺚﺤﺒﻟا اﺬھ ﻲﻓ
ﺔﯿﺋﺎﯾﺰﯿﻔﻟا تﺎﻔﺼﻟا ﺖﺳرد
85% ﺔﺒﺴﻨﺑ ﺮﯾﻮﺤﺘﻟا ﺞﺗﺎﻧ نﺎﻜﻓ 10
:1 ﺔﺒﺴﻨﺑ ﺪﯾﺎﻣرﻮﻓ ﻞﯿﺜﻣ ياﺪﻟاو نﺎﺴﻛﻮﯾاﺪﻟا
ﺖﺤﺗ ﺔﻌﺷﻷا ﺔﻄﺳاﻮﺑ ﺞﺗﺎﻨﻟا ﻲﺋاوﺪﻟا ﺮﻤﯿﻟﻮﺒﻟا ﺺﺨﺷو (0.23dl/g) يوﺎﺴﺗ ﻲﺘﻟاو ﺮﻤﯿﻟﻮﺒﻠﻟ ﺔﯾﺮھﻮﺠﻟا ﺔﺟوﺰﻠﻟاو ﺮﺻﺎﻨﻌﻠﻟ ﻖﯿﻗﺪﻟا ﻞﯿﻠﺤﺘﻟا يﺮﺟأو .تﺎﺒﯾﺬﻣﻼﻟا ﻲﻓ ﺔﯾﻮﺌﻤﻟا خﺎﻔﺘﻧﻻا ﺔﺒﺴﻧ ﺖﺴﯿﻗو ،ﺔﯿﺠﺴﻔﻨﺒﻟا قﻮﻓ ﺔﻌﺷﻷاو ءاﺮﻤﺤﻟا لاود ﻲﻓ ﺮﻀﺤﻤﻟا ﺮﻤﯿﻟﻮﺒﻟا ﻦﻣ ﻲﺋاوﺪﻟا رﺮﺤﺘﻟا عﺮﺳ ﺖﺳرد ﻚﻟﺬﻛو
. DSC يراﺮﺤﻟا ﻞﯿﻠﺤﺘﻟا ءاﺮﺟإ ﻢﺗو
ﺮﯿﻐﺘﻟاو 143.4oC -150.3oC يو
ﺎﺴﺗ ﺮﻤﯿﻟﻮ ا
ﺔﻟ ﯿﺳ ﺔﺟرد ﺖﻧﺎﻛو .مﺎ
37 ﻨﻋ ﺔﻔﻠﺘﺨﻣ ﺔﯿﻀﻣﺎﺣ
. /لﻮﺟوﺮﻜﯿﻣ (
9) ﺒﯿﻟﺎﺜﻧﻹا ﻲﻓ
oxycycline is used in prophylaxis against malaria. It should not be used alone for initial treatment of malaria, even when the parasite is doxycycline-
Dsensitive, because the antimalarial effect of doxycycline is delayed. This
delay is related to its mechanism of action, which is to specifically impair the progeny of the apicoplast genes, resulting in their abnormal cell division. The action of polymeric drugs in vivo usually depends on hydrolytic on enzymatic cleavage of drug modify from the polymer [1]. Doxycycline is used in the treatment and prophylaxis of
Eng. &Tech.Journal, Vol.33,Part (B), No.2, 2015 Synthesis of Prodrug Polymer as Ring Opening
of PVP
Bacillus anthraces, it is also effective against Yersinia pestis (the infectious agent of
bubonic plague), and is prescribed for the treatment of Lyme disease [2-5],
ehrlichiosis [6, 7] and Rocky Mountain spotted fever. In fact, because doxycycline is
one of the few medications shown to be effective in treating Rocky Mountain spotted
fever (with the next-best alternative being chloramphenicol), doxycycline is indicated
even for use in children for this illness. Otherwise, doxycycline is not indicated for
use in children less than eight years. Doxycycline, like other antibiotics, will not work
for colds, influenza, or other viral infections. A polymer is a large molecule
composed of many smaller units called monomers that are bonded together. In
addition to eliminating the necessity of removal, the five key advantages [8] that
polymeric drug delivery products can offer are: sustained delivery of drug,
stabilization of the drug, release rate that is less dependent on the drug properties and
steadier release rate with time. In diffusion controlled systems the release rate
typically declines with time.
If an application requires rapid development and commercialization, then the
polymer selection will most likely be made of among those polyesters that have
already received regularly approval. Another factor to be taken into account is the
choice, whether to use homo polymers consisting of single monomeric repeating unit
or copolymers containing multiple monomer species. A review that describes in detail
the relationship between polymer properties and performance in drug delivery
applications have been published [9].
In some cases, the term biodegradation is limited to the description of chemical
processes (the chemical changes that alter either the molecular weight or solubility of
the polymer) [10, 11]
Degradation by erosion normally takes place in devices that are prepared from
soluble polymers. In such instances, the device erodes as water is absorbed into the
system causing the polymer chains to hydrate, swell, disentangle and ultimately
dissolved away from the dosage form. Alternatively, degradation can also result from
chemical changes to the polymer including cleavage of covalent bonds, ionization
and protonation either along the polymer backbone or on pendent side chains [12-14].
The purpose of this research was to synthesize polymer based smart bioactive
doxycycline prodrug polymer and one of the main goals of this work is to investigate
the efficient drug carrier and the effect of pH values on drug release at 37oC .
Chemicals and Apparatus
Doxycycline was provided by the college of Pharmacy and all other chemicals
were purchased from Mereck, and polyvinylpyrrolidinone was obtained from
Fluka.All available chemical reagents were used without further purification. FT-IR
spectra were taken on a Shimadzu spectrophotometer recorder over the range 4000-
400cm-1. Ultraviolet spectra were recorded using Shimadzu UV-Vis recorder.
Differential Scanning Calormeter (DSC) study was carried out on a Shimadzu-60
instrument (Japan) at a heating rate of 10oC min-1, under air (normal), not vacuum.
Temperature range from -140oC up to 600oC. The detector type K for the furnace
temperature.
Experimental
Modification of Polyvinylpyrrolidinon PVP with Doxycycline:
A mixture of (5g, 0.045 mole) of PVP and 10:1 ml of Dioxane: DMF was placed in
a round bottomed flask equipped with a reflux condenser and magnetic stirrer. Then
(1.621g, 0.045 mole) of dissolved Doxycycline was added gradually, refluxed for 1hr.
Eng. &Tech.Journal, Vol.33,Part (B), No.2, 2015 Synthesis of Prodrug Polymer as Ring Opening
of PVP
Then the mixture left for 10 hours. The colorless viscous polymer was precipitated
from 50ml of ethanol; the pure polymer was obtained 85% conversion.
Controlled Release Study:
100 mg of modified drug polymer was placed in a cylinder containing 50ml of
buffer solution with (PH: 7.4 or 1.1) and 50ml dioxane in water bath at 37ᵒC without
stirring. A sample from the release medium was periodically withdrawn and analyzed
by UV. At 300 nm to determine the amount of the released drug. A calibration curve
was constructed with software built in the computerized UV. Spectrophotometer, the
amount 0.1 mg of the released drug was determined directly from the software for 4
days, using the calibration curve in different pH values at 37ᵒC.
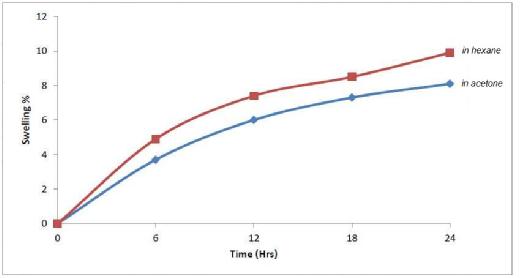
Swelling percentage of prepared polymer was studied and equals to 8% for 26 hours
using a mixed solvent like acetone and 10% hexane. The Swelling percentage was
calculated according to:
∆m= (m1-mo) / mo * 100
When: mo is the weight of the dry drug polymer at time 0.
m1 is the weight of swallowed polymer in non-solvent.
Results and Discussion
Poly (N-vinyl-2-pyrrolidinone) is a white hydroscopic powder, forming hard clear
films. Physical properties are determined on films or powder. The polymer strongly
interacts through dipole-dipole attraction. The ring opening reaction of PVP with OH
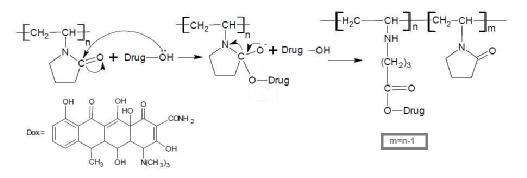
of the drug is illustrated in the mechanism [4] shown in scheme (1):
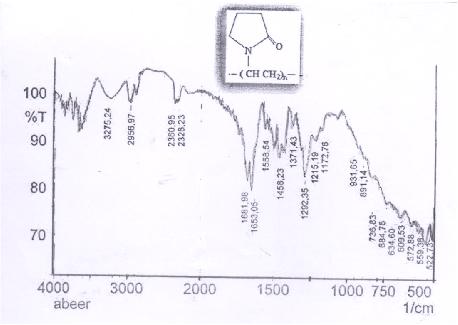
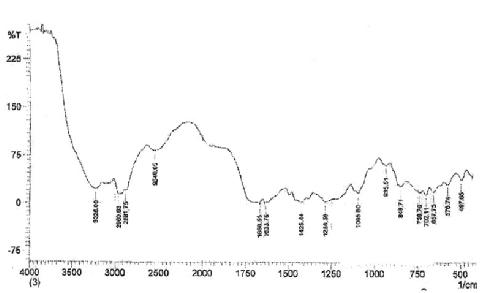
Due to the presence of –OH group, which is strong nucleophile attach, the ring opining of pyrrolidinone produced prodrug polymer. The poly vinyl pyrrolidinon connected with hydroxyl of drug moiety affords both protection and specific transport properties with longer acting release with higher reactivity in suitable site and this type of drug polymer, which hydrolysis in fabrications conditions to delivery of agents, for therapeutic against diseases state, and sustained rate, target delivery of drugs and minimize toxicity and enhanced selectivity. The structural characterization was done by FT-IR spectrum Figure(2) showed peaks at 3275 cm-1 assigned to –NH and the absorption appeared at 1633 cm-1 and 1681 cm-1 assigned to C=O stretching of ester and 3080 cm-1 was attributed to C-H stretching of aromatic ring and peak at 2960 cm-1 assigned to aliphatic C-H stretching; on the other hand, the FT-IR showed peaks at 1580 cm-1 and 1600 cm-1 due to C=C stretching of the aromatic ring of doxycycline, the FT-IR of drug polymer Figure(2), which compared with Figure (1) of FT-IR spectra of PVP [15-17].
Eng. &Tech.Journal, Vol.33,Part (B), No.2, 2015 Synthesis of Prodrug Polymer as Ring Opening
of PVP
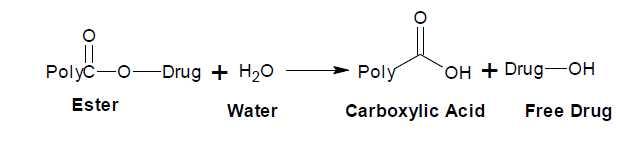
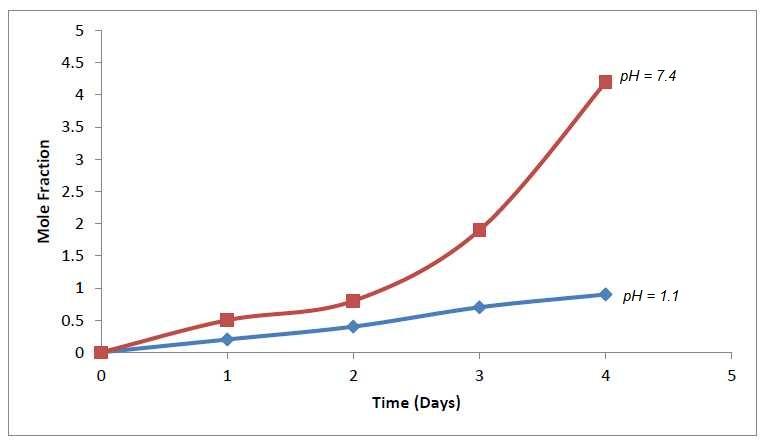
Figure (3) shows the softening point of the drug polymer and it was 143.4-150.3oC, which measured by using differential scanning calorimeter DSC analysis, obtained high thermal stability and ΔH=-189.89J/g. The physical properties of the prepared doxycycline polymer were studied such as intrinsic viscosity, which was measured at 30oC with Ostwald viscometer by using dioxane as solvent ([ η]in= 0.23dl/g). Figure (4) explain the effect of pH values on the rate of controlled release and profiles of mole fraction of doxycycline ratio to total moles present in the sample swelling versus time at pH values 7.4 and 1.1 at 37oC. The only nucleophile acyl substitution reaction that amides are hydrolysis, esters are fairly stable in water, but the ester bond is cleaved on the heating in presence of strong acid or bases. Normally, this cleavage produces a free drug carboxylic polymer. The release of the drug at suitable condition gradually with outside effect, this hydrolysis of ester group, which shown in the following mechanism [14].
The hydrolysis rate of this ester bond acts higher hydrolysis in basic medium, than
acidic medium, due to the more neuclophilic attack of –OH than H2O molecule. In
acid, however, the hydroxyl is protonated giving free drug unit [18-22].
Figure (5) demonstration the Swelling percentage of prepared drug polymer in 10%
hexane and acetone, this equals to 8% for 26 hours.
Conclusion
It was concluded, that the prepared prodrug was analyzed by controlling drug
release, it was found that in a basic medium could hydrolysis higher than acidic
medium, this due to the presence of OH‾ which was a stronger nucleophilic with
respect to H2O or H+, and the rate of release was prolonged about four days, this
indicated the advantage of drug carrier polymer as a doxycycline prodrug delayed
and sustained release of drug over a long time with a corresponding decrease of side
effect.
Figure (1) FT-IR Spectrum of Polyvinylpyrrolidone
Eng. &Tech.Journal, Vol.33,Part (B), No.2, 2015 Synthesis of Prodrug Polymer as Ring Opening
of PVP
Figure (2) FT- IR Spectrum of Polyvinylpyrrolidone with Doxycycline
Figure (3) Thermal Analysis (DSC) Result of Polyvinylpyrrolidone with
Doxycycline
Figure (4) Controlled Release Drug Polymer at 37 ̊
C in Different pH Values
Eng. &Tech.Journal, Vol.33,Part (B), No.2, 2015 Synthesis of Prodrug Polymer as Ring Opening
of PVP
Figure (5) Swelling% of Prepared Drug Polymer
References
[1] Dahl, EL.; Shock, JL.; Shenai, BR.; Gut, J.; DeRisi, JL. and Rosenthal, PJ.
"Tetracyclines Specifically Target the Apicoplast of the Malaria Parasite Plasmodium
Flaciparum", Antimicrob. Agents Chemother., V.50(9) Pp: 3124-3130, 2006.
[2] Nadelman, RB.; Luger, SW.; Frank, E.; Winsniewski, M.; Collins, JJ. and
Wormser, GP. "Comparison of Cefuroxime Axetil and Doxycycline in the Treatment
of Early Lyme Disease", Ann. Intern. Med., V. 117(4) Pp: 273-280, 1992. PMID
1637021.
[3] Luger, SW.; Paparone, P. and Wormser, GP. "Comparison of Cefuroxime Axetil
and Doxycycline in Treatment of Patients with Lyme Desease Associated with
Erythema Migrans", Antimicrob. Agents Chemother., V. 39(3) Pp: 661-667,2003.
PMC 162601. PMID 7793869.
[4] Nadelman, RB.; Nowakowski, J. and Fish, D. "Prophylaxis with Singly-Dose
Doxycycline for the Prevention of Lyme Disease after an Ixodes Scapularis Tick
Bite",N. Engl. J. Med., V. 345(2) Pp: 79-84, 2001.
[5] Karlsson, M.; Hammers-Berggren, S.; Lindquist, L.; Stiernstedt, G. and
Svenungsson, B., "Comparison of intravenous Pencillin G and Oral Doxycycline for
Treatment of Lyme Neuroborreliosis", Neurologe, V. 44(7) pP: 1203-1207, 1994.
[6] Testa B. Prodrugs,bridging pharmacodynamics /pharmacokinetic gaps, Curr Opin
Chem Biol,V.13 pP: 338, 2009.
[7] Karlsson, U.; Bjoersdorff, A.; Massung, RF. and Christensson, B., "Human
Granulocytic Ehrlichiosis-a Clinical Case in Scandinavia", Scand. J. Infect. Dis., V.
33(1) pP: 73-74, 1996.
[8] Leong, K.W. "Biodegradable polymers as drug delivery systems". Tarcha, PJ,
editors. J. Polymers for Controlled Drug Delivery. CRC Press: Boca Raton; Pp: 128,
1991.
[9] Stella VJ, Prodrugs, "some thoughts and current issrrent". J Pharm Sci, V.99 pP:
4755-4765, 2010.
[10] Alzung, B. ''Basic and Clinical Pharmacology' , 2ed. Megrew-hill company, Pp:
754, 2001.
[11] Tulken, .P.M., Eur.J.Clin.Micro.Infect. "Fundamentals of Pharmacology" Pp:
100-106, 1991.
[12] Alam, M.S; Choi, J.H and Lee, DU., "Synthesis of novel Schiff base analogues
of 4-amino-1,5-dimethyl-2-phenylpyrazol-3-one and their evaluation for antioxidant
and anti-inflammatory activity" Bioorg. Med. Chem., V.20 (13), pP: 4103-4108,
2012.
Eng. &Tech.Journal, Vol.33,Part (B), No.2, 2015 Synthesis of Prodrug Polymer as Ring Opening
of PVP
[13] Carlier, M.B.; Scornearux, B.; Zenebergh, A.; Desnottes, J.F. and Tulkens, P.M. J. Antimicrobial Chemo. V. 26. Pp: 27-39, 1990. [14] Firyal, M.A.; Abbas, N.M; and Khudheyer, G.K. "Modification of Poly VinylPyrrolidinone with Amoxilline to Drug Polymer", Fifth Scientific Conference-College of Science-University of Babylon.Vol.5, Pp: 224-229, 2010. [15] Wang, Y.; Zhang, H.; Zhang, G.; Zhou, Q. and Liu,Z. "Studies of the Interaction between Paraquat and Bovine Hemoglobin", Int. J. Biol. Macromol, V. 41(3)Pp: 243-250, 2007. [16] Wang, Y.; Zhang, H.; Zhang, G. and Liu, Z. "Fluorescence Spectroscopic Investigation of the Interaction between Benzidine and Bovine Hemoglobin", J. Mol. Struct., 886C1-3, Pp: 77-84, 2007. [17] Chen, L.and Tianqing, L., "Interaction Behaviors between Chitosan and Hemoglobin", Int. J. Biol. Macromol, V. 42(5) Pp: 441-446, 2008. [18] Francis, A. Carey, "Organic Chemistry", 4th Ed., Pp: 804-805, 2000. [19] Talib, H.M.; Nahzar, M.R. and Ibram U. K. "Chemically cross linked poly (acrylic-co-vinylsulfonic acid hydroxyl for the delivery of Iso-sorbide mono nitrate " J. of the Scientific World. Article ID.V.340737, pP: 2, 2013. [20] Radi, S.; Toubi, Y.; Hamdani, L; Hakkou, A.; Souna, F.; Himri, I. and Bouakka, M.,"Synthesis, Antifungal Activities of some new Bipyrazolic Tripodal derivatives, chem. sci., V.2(4), pP:40-44, 2012. [21] L. Averous, " Polylactic Acid, Synthesis, Properties and Application", CH.21.indd 435, 2011. [22] Yi Li; Yuanyuan Liu; Haowei Wang; Xiaohui Xiong; Ping Wei and Fangshi Li, "Synthesis, Crystal Structure,Vibration Spectral, and DFT Studies of 4-Aminoantipyrine and Its Derivatives, Molecules," V. 18, pP: 877-893, 2013.
Source: http://uotechnology.edu.iq/tec_magaz/2015/volum332015/No.02.B.2015/(9)Text.pdf
Review of India: Country of Origin (COI) Report (Home Office UK Border Agency COI Service) dated May 2012 Prof. Dr. Livia Holden1 15 December 2013 1. Introduction On the 6th November 2013 I was commissioned the reviews of India: Country of Origin (COI) Report (Home Office UK Border Agency COI Service) dated May 2012 and of the Operational Guidance Note India by Chief Inspector of Borders. These
RICERCA BERE MODERATO COME EDUCARE I GIOVANI AD UN USO CONSAPEVOLE DELL'ALCOL A cura di Diego Boerchi, Miriam Magnoni, Italo Piccoli Confederazione Generale dei Consumatori (Confconsumatori) Sede Regionale: Via De Amicis, 17 - 20123 Milano Tel. 02.83241893 Fax 02.58104162 E-mail [email protected] Movimento Difesa del Cittadino Onlus (MDC) Sede regionale: Via Lorenteggio, 145 - 20146 Milano Numero verde 800.090176 Tel. 02.89055396 Fax 02.89055396 E-mail [email protected] Movimento Consumatori (MC) Sede Regionale: Via Cipro, 30 - 25124 Brescia Tel. 030.2427872 Fax 030.2452831 E-mail [email protected] ICS45 Via Bragadino, 2 - 20144 Milano Tel. 02.87382284 Fax 02.92879388 E-mail [email protected] Sito internet ww.ics45.it










