
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Journal course 3: etiology, mechanisms, and anesthesia implications of autoimmune myasthenia gravis
AANA Journal Course
Update for nurse anesthetists
*6 CE Credits
Etiology, mechanisms, and anesthesia implications
of autoimmune myasthenia gravis
MAJ Thomas E. Ceremuga, CRNA, MSN, AN, USA
Xiang-Lan Yao, MD, PhD
Joseph T. McCabe, PhD
Myasthenia gravis (MG) is the prototypical neurological
nicotinic acetylcholine receptor at the neuromuscular junction.
autoimmune disease. It is characterized by muscle weakness
Anesthesia management of the patient with MG is challenging
that progressively worsens on repetition but improves with
and requires specific management; however, safe and success-
rest. Muscle weakness and fatigability arise from defective or
ful outcomes are achievable. This course emphasizes the
decreased acetylcholine receptors at the neuromuscular junc-
autoimmune neuromuscular defect in MG, current treatments
tions, where nerve signals from spinal motor neurons that
for this syndrome, contraindications of certain anesthetic
drugs in this condition, and anesthetic management of a
innervate muscles cannot effectively induce muscle contrac-
patient with MG in the operating room environment.
tion. Several mechanisms of pathogenesis lead to the MG syn-
drome. The most prevalent cause of MG is an autoimmune dis-
Key words: Acetylcholine receptor, autoimmune disease, myas-
order in which the patient produces antibodies that attack the
thenia gravis, receptor, neuromuscular junction.
At the conclusion of this course, the reader should be
Myasthenia gravis (MG) is the prototypical neurologi-
cal autoimmune disease. Willis first described the mal-
1. Discuss the pathologic processes related to the
ady in 1672, but it was not until 1895 that Jolly used
neuromuscular junction in autoimmune myas-
the name, myasthenia gravis.1 Jolly described a condi-
thenia gravis.
tion of 2 boys who exhibited muscle weakness that pro-
2. Identify the cellular autoimmune events occur-
gressively worsened on repetition but improved with
ring in myasthenia gravis.
rest.1 Muscle weakness and fatigability arise from
3. Describe the various modalities used in the treat-
defective or decreased acetylcholine receptors (AChRs)
ment of myasthenia gravis.
at the neuromuscular junctions (NMJs), where nerve
4. Identify the pharmacologic agents that reduce
signals from spinal motor neurons that innervate mus-
neuromuscular transmission in patients with
cles cannot effectively induce muscle contraction.
myasthenia gravis and should be avoided in the
Various mechanisms of pathogenesis lead to the MG
syndrome. Congenital myasthenias are caused by a
5. Discuss the prudent delivery of anesthesia and the
variety of genetic defects (eg, ion channels or subunits
anesthetic plan for patients with myasthenia
of AChR mutations) of the presynaptic or postsynaptic
machinery of the NMJ.2
Lambert-Eaton myasthenic syn-
* The American Association of Nurse Anesthetists is accredited as a provider of continuing education in nursing by the American Nurses Credentialing
Center Commission on Accreditation. The
AANA Journal course will consist of 6 successive articles, each with objectives for the reader and sources for
additional reading. At the conclusion of the 6-part series, a final examination will be printed in the
AANA Journal. Successful completion will yield the
participant 6 CE credits (6 contact hours), code number: 24623, expiration date: July 31, 2003.
AANA Journal/August 2002/Vol. 70, No. 4
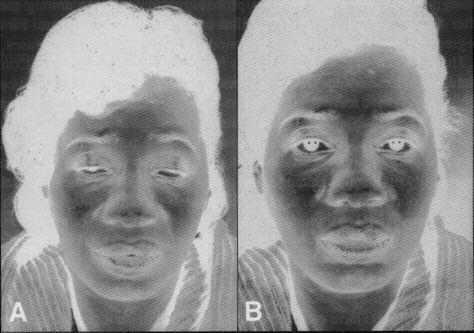
Figure 1.
drome frequently is associated with neoplasms and
involves a dysregulation of presynaptic acetylcholine(ACh) release. It seems that the presynaptic defectinvolves an alteration in calcium channels and, conse-quently, decreased release of acetylcholine into thesynapse.3,4 The most common cause of MG is anautoimmune disorder in which the patient producesantibodies that attack the nicotinic acetylcholine recep-tor at the NMJ.5 This article emphasizes the autoim-mune neuromuscular defect in MG, current therapy forthis syndrome, contraindications of certain anestheticdrugs in this condition, and anesthetic management ofa patient with MG in the operating room environment.
History and review of the literature
• Autoimmune neuromuscular MG. The hallmark
(A) Patient with myasthenia gravis with ptosis
features of autoimmune MG are fatigue, increasing
(B) Patient after receiving 10 mg of edrophonium
weakness with repetitive motion, and a higher inci-
Reproduced by permission of Mosby, Inc.)
dence in women.6 Characteristics and symptoms of MGreflect the dysfunctional AChR at the NMJ, including
Nerve terminal endings are located presynaptically in
generalized weakness in 85% of patients and limited
the primary synaptic clefts at the synapse. The muscle
weakness of ocular muscles in 15% of patients with
surface area, postsynaptically, is enlarged by invagina-
ocular MG.7 While ocular MG is less prevalent, ocular
tions of the plasma membrane into secondary synaptic
muscle problems, such as ptosis or diplopia, usually are
folds. The AChRs are located primarily at the distal
the initial complaint, with subsequent progression to
extents of the folds, in closer apposition to the nerve
the generalized disease.8 Patients with generalized MG
terminals.6 There is continual AChR turnover, in which
complain of dysphagia, dysphonia, proximal limb mus-
old receptors are internalized and degraded, and new
cle weakness, and even exacerbation to dyspnea or ven-
receptors are synthesized and inserted into the synaptic
tilatory failure (myasthenic crisis).9 In 85% to 95% of
folds.6 Skeletal muscle sodium channels are located in
patients with MG, a thymic abnormality, such as thy-
the depths of the folds, and AChE, the enzyme that
moma or thymic hyperplasia, may be responsible for
hydrolyzes ACh, is located at the basal lamina of the
secretion of AChR antibodies.10
secondary synaptic fold.6
Diagnostic testing for MG includes pharmacologic,
The generation of a muscle action potential and,
electrophysiologic, and laboratory testing. Edropho-
ultimately, muscle contraction begins with the depolar-
nium, an anticholinesterase (anti-AChE), is adminis-
ization of the presynaptic nerve terminal. This leads to
tered to inhibit the enzyme that degrades ACh; there-
calcium influx via channels and calcium-dependent
fore, more ACh remains at the synapse. The patient
fusion of synaptic vesicles with presynaptic nerve ter-
with MG (Figure 1) usually demonstrates a temporary
minal membrane in the nerve boutons. Fusion allows
reversal of muscle weakness with edrophonium.11
the release of ACh into the synapse, where the neuro-
Nerve conduction tests, such as repetitive nerve stimu-
transmitter then can diffuse across the synaptic cleft
lation, also are performed to verify the MG diagnosis.
and bind to AChRs. If a large enough quantity of ACh
Motor response is monitored after the nerve is stimu-
is released, a muscle endplate potential is reached,
lated repetitively at the rate of 2 Hz, and the patient
resulting in postsynaptic depolarization and muscle
with MG usually exhibits a gradual decrease in ampli-
contraction. ACh is removed by AChE hydrolysis and
tude.12 In addition, serum AChR antibodies are assayed
to confirm the diagnosis; results for 90% to 95% of
Adult human AChR (Figure 3) is part of a super-
patients with MG are positive.10 The positive results
family of neurotransmitter-gated ion channels, and its
from these testing modalities point to a defect in the
pentameric (5-subunit) structure includes 2 α and 1
NMJ in the patient with MG.
each of the β, δ, and ε subunits. Fetal AChR is similar,
• The neuromuscular junction. Proper functioning of
except a γ subunit is substituted for the ε subunit.13,14
the NMJ is required for impulse propagation and mus-
The fetal AChR is retained in adult thymic myoid
cle contraction. The NMJ is a complex structure, com-
cells15 and adult ocular muscle fibers.16 The adult
posed of the motor nerve terminal, postsynaptic mus-
AChR subunits (see Figure 3) are believed to be
cle surface, and specialized basal lamina (Figure 2).
arranged around a central ion channel in the following
AANA Journal/August 2002/Vol. 70, No. 4
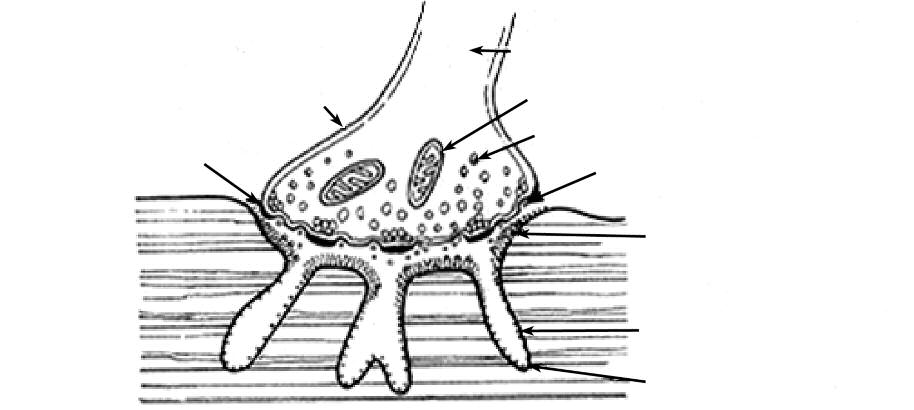
Figure 2. Normal human neuromuscular junction
Reproduced by permission of Mosby, Inc.)
Figure 3. Acetylcholine receptor (AChR) subunits: fetal (left) and adult (right)
Subunits: α1, β1, γ, δ, ε
at subunit interface
Reproduced by permission of John Wiley & Sons, Inc.)
order: α1εα1δβ1.17 The ACh binding sites are formed
4) 2 adjacent AChRs.21 These properties and character-
at the union of the α1 and ε and the α1 and δ sub-
istics of the main immunogenic region facilitate the
units.18 Both binding sites must be occupied by an ago-
pathogenic mechanisms involved in the autoimmune
nist (ACh) for the ion channel to open.19 The binding
response to AChRs.18
of ACh to both sites results in a conformational change
The evidence that links AChR antibodies as the
in the AChR and channel opening.14 Conversely, if an
causative factor in MG includes the following: (1) Of
antagonist (eg, vecuronium) binds one site, channel
patients with MG symptoms, 85% have these antibod-
opening is prevented.18
ies.10 (2) Immunoglobulin G (IgG) has been found at
• Autoimmune pathology. A region located on the
the neuromuscular endplate.22 (3) Plasmapheresis to
extracellular tip of the α1 subunits has been described
reduce circulating antibodies provides temporary
as the main immunogenic region. Since the main
symptomatic relief.23 (4) Healthy animals injected with
immunogenic regions are located at the outer (extra-
antibodies against AChR produce MG signs.24 There
cellular) portion of the AChR, they are easily recog-
also is the possibility that the antibodies bind to or near
nized by antibodies.20 A single antibody is unable to
the ligand-binding site.7 Antibodies also can cross-link
cross-link 2 α1 subunits but easily cross-links (Figure
AChRs resulting in internalization, increased degrada-
AANA Journal/August 2002/Vol. 70, No. 4
Figure 4. Cross-linked acetylcholine receptors (AChRs) by antibody to main immunogenic region (MIR)
AChRs cross-linked
by antibody to the MIR
Reproduced by permission of John Wiley & Sons, Inc.)
tion rate, and a decrease in AChR density at the end-
patients with MG.12 There seem to be different trigger
plate. This increase in AChR degradation correlates
mechanisms of autoimmunity for the various forms of
well with clinical manifestations of MG.25
MG. The patient with rheumatoid arthritis may trigger
Moreover, complement-mediated destruction of the
an MG syndrome by taking penicillamine, which is
NMJ occurs as a result of AChR antibodies. Antibodies
believed to react covalently with AChRs, producing
bind to the C9 component of complement (part of the
new antigenic sites.31 This MG condition is reversible
immune system involved in cell destruction), trigger-
with the termination of penicillamine. A paraneoplastic
ing an inflammatory cell response, endplate membrane
immune response may account for the 12% of patients
degradation, and destruction of junctional folds that
with MG who have a thymoma, for they have different
harbor AChR-abundant membranes (Figure 5). This
HLA marker frequencies than do other patients with
inevitably would reduce the membrane surface avail-
MG. This indicates a probable difference in immune
able for AChR insertion.26 Therefore, the autoimmune
system genetic background.32 They have not only high
response in MG affects many components of the
levels of AChR antibodies, but also antibodies to sev-
machinery at the postsynaptic membrane, resulting in
eral muscle proteins in the interior of the cells.33 In
altered depolarization of muscle tissue.
most MG cases, the immunogen is likely the native
Morphological studies of the NMJ in MG demon-
muscle AChR or a closely related protein. The fetal type
strate the following postsynaptic anomalies (Figure 6):
(γ subunit) has been implicated as the immunogen, as
decreased quantity of AChR,27 widened and decreased
selective reaction with fetal AChRs has been reported
machinery of the postsynaptic fold, and increased gap
with antibodies from patients with MG.34
between presynaptic and postsynaptic membranes.28
Molecular mimicry by microbes also has been sug-
Therefore, the primary pathologic mechanism of MG is
gested to be responsible for the autoimmune response
a reduction in AChRs and, thus, a reduction in the end-
in MG, in which bacterial or viral proteins initiate the
plate potential that is not strong enough to reach
immune response, then reaction with the AChR leads
threshold potential, depolarization of muscle mem-
to epitope (antigenic determinant) spreading. In addi-
brane, and resultant muscle contraction.12 If this trans-
tion, both bacteria and viruses can express superanti-
mission failure occurs at many junctions, the strength
gens that nonspecifically activate many T and B cells.18
of the muscle contraction is reduced and the patient
Although the B cells synthesize the autoantibodies to
becomes weak. Normally, only 25% to 30% of AChRs
AChR, there is evidence for a T-cell role in autoimmu-
are necessary for neuromuscular transmission. The
nity,35 as T cells from patients with MG seem to
remaining 70% to 75% of receptors represent a "safety
respond to AChR stimulation and aid in the production
margin."29 In MG, there is a decrease in the number of
of AChR antibodies.36 Helper T cells found in patients
functional AChR and a decrease in the safety margin.
with MG can increase the synthesis of anti-AChR anti-
• Cellular autoimmunity mechanisms. The precipi-
bodies. In MG, T-helper lymphocytes are required to
tating events that cause MG are not completely under-
cooperate with B lymphocytes in promoting autoanti-
stood, but evidence implicates the thymus, as altered
body synthesis.37 The autoantibodies in MG are poly-
thymic function has a 90% prevalence in this disease.30
clonal and heterogeneous and recognized different epi-
For example, thymoma or hyperplasia of the thymus
topes on the AChR.38,39
occurs in high frequency in patients with MG, T and Bcells have an active role in antibody formation, myoid
State of the art
cells of the thymus gland have the same type of surface
• Treatment of MG. Treatment modalities usually
as AChR, and thymectomies usually are beneficial for
reflect the rate of progression, severity, and weakness
AANA Journal/August 2002/Vol. 70, No. 4
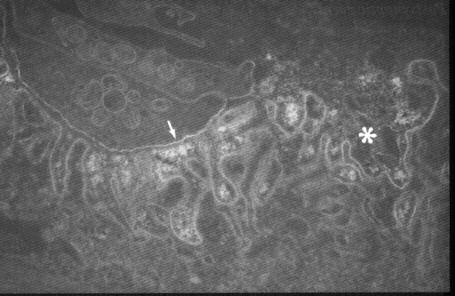
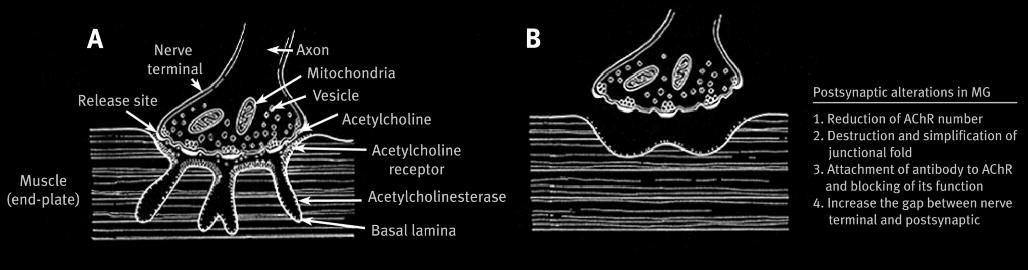
Figure 5. (A) Normal neuromuscular junction: arrow is synaptic cleft, asterisk is the secondary cleft. (B) Lytic C9
complement component of myasthenic neuromuscular junction showing synaptic degeneration; asterisk is the
junctional folds without nerve terminal and arrrow is presynaptic staining.
Reproduced by permission of Mosby, Inc.)
Figure 6. (A) Normal neuromuscular junction; (B) neuromuscular junction in myasthenia gravis (MG)
AChR = acetylcholine receptor
Reproduced by permission of Mosby, Inc.)
distribution of the patient. Age, sex, and the presence
that autoreactive T cells are activated in the thymus.42
of concomitant diseases also influence long-term ther-
Studies indicate that thymectomies decrease T-cell reac-
apy decisions. In general, the treatment for MG consists
tivity against disease-specific antigens and provide
of 5 modalities: anticholinesterase drugs, immunosup-
symptomatic relief .43 In addition, if the source of the
pressants, thymectomy, plasmapheresis, and intra-
immunogen is thymic myoid cells, their elimination
venous immunoglobulins (IgG).40
may decrease the immune response44 as a possible
Anticholinesterases usually are the initial therapy
reservoir of AChR antibody-producing B cells may be
and provide symptomatic improvement in muscular
removed with this operation.45 Plasmapheresis usually
strength, as they inhibit the enzyme that degrades ACh.
is used as a short-term treatment in patients with
This allows ACh to remain longer at the NMJ, increas-
extreme weakness. This treatment is believed to
ing the probability for ACh binding to AChRs.
remove circulating AChR antibodies and immune com-
Immunosuppressant drugs (eg, corticosteroids, aza-
plexes, often resulting in rapid improvement, which
thioprine, and cyclosporine) are administered to
lasts 6 to 8 weeks.40
decrease the immune response and modulate mecha-
In addition to plasmapheresis, intravenous immu-
nisms in cellular immunity.40 Since thymic abnormali-
noglobulins (IgG) have been used in the extremely
ties (hyperplastic changes and neoplasias) are prevalent
weakened patient with acute MG. The mechanism by
and evidence suggests they are intricately involved in
which IgG improves MG symptoms is unclear but is
many forms of MG, surgical thymectomies are also per-
speculated to involve the interaction between autoanti-
formed.41 As mentioned previously, it is hypothesized
bodies and anti-idiotypic (nonspecific) antibodies in
AANA Journal/August 2002/Vol. 70, No. 4
IgG preparations.46 Future treatment modalities are
kanamycin, gentamicin, neomycin, amikacin),54,55
being considered with a more cell-directed approach,
erythromycin,56 and polymyxin B sulfate9 have all been
such as monoclonal antibodies directed against helper
implicated in this action. It seems that calcium glu-
T cells and administration of immunotoxins that would
conate is effective in reversing this aminoglycoside-
destroy B cells specific for AChRs.7,47
induced muscle weakness, whereas calcium chloride
• Anesthesia pharmacology contraindications. The
partially antagonizes the neuromuscular block pro-
hallmark symptom of muscle weakness, especially after
duced by polymyxin B.55 In addition, 2 cases have been
repetitive stimuli, can lead to dangerous and life-threat-
reported involving ciprofloxacin and increased neuro-
ening situations for the patient with MG. One such
muscular blockade.57,58 There are several cardiovascular
environment or condition is surgery (often an elective
drugs that also have demonstrated worsening of MG
thymectomy) and, more important, the administration
and should be given with caution or avoided. Pro-
of anesthesia during the surgical procedure. MG is a
cainamide seems to potentiate MG,59 β-blockers seems
condition of particular interest to anesthesia as it
to also potentiate MG because of their depressant
involves the NMJ, the site of action of many commonly
effects on the NMJ,60 and antiepileptic drugs, especially
used anesthetic drugs. There are many pharmacologic
phenytoin, can decrease muscle strength.9 These med-
agents used in anesthesia that can lead to devastating
ications should be avoided whenever possible.
consequences and even precipitate a myasthenic crisisin the patient with MG.
Current practice of anesthesia in MG
• Muscle relaxants. The response of the patient with
• Preoperative care. An MG severity classification sys-
MG to muscle relaxants is difficult to predict, and
tem by Osserman and Genkins (Table) has been
administration of these drugs should be monitored
described: I, ocular signs and symptoms only; IIA, gener-
closely with a peripheral nerve stimulator.48 The drugs
alized mild muscle weakness; IIB generalized moderate
that are used to treat MG (anticholinesterases) have an
weakness and/or bulbar dysfunction; III, acute fulminat-
effect on the response to muscle relaxants. For exam-
ing manifestations and/or respiratory dysfunction; and IV,
ple, the anticholinesterase, pyridostigmine, not only
late, severe, generalized MG.61 This grading system can be
inhibits the AChE enzyme, but also decreases plasma
useful as an indication for perioperative complications.53
cholinesterase activity. Plasma cholinesterase is respon-
There is approximately a 10% incidence of other
sible for degrading succinylcholine, a depolarizing neu-
autoimmune diseases that occur concomitantly with
romuscular blocker, and ester-type local anesthetics.48
MG, including hypothyroidism (10% occurrence),
In addition, patients with MG treated with pyridostig-
rheumatoid arthritis, systemic lupus erythematous, and
mine show a marked resistance to succinylcholine,
pernicious anemia. It is important to optimize these
which causes depolarization of muscle endplates, and
conditions before elective surgery for the patient with
this is thought to be the result of the reduced number
MG. This includes optimizing a euthyroid state, evalu-
of AChRs at the NMJ.49 Thus, there may be prolonged
ating cervical spine involvement in the patient with
effects of anesthetic drugs from these medications.
rheumatoid arthritis, and relief or lessening of systemic
Conversely, patients with MG are extremely sensitive to
lupus erythematous manifestations.48,62
nondepolarizing muscle relaxants (eg, curare). Studies
The respiratory status of the patient should be eval-
demonstrate the increased sensitivity to various nonde-
uated with spirometry, as MG affects both the inspira-
polarizing muscle relaxants (competitive antagonists)
tory and expiratory muscles.54 Pulmonary function
such as atracurium50 and vecuronium.51 However, the
use of short- and intermediate-acting muscle relaxants
Myasthenia gravis severity classification system
by Osserman and Genkins61
is acceptable with judicious titration and peripheralnerve monitoring, with the ability to reverse theireffects at the end of surgery.52 In addition, the use of
these shorter-acting muscle relaxants may avoid the
Ocular signs and symptoms
need for reversal with anti-AChE, which can trigger a
Generalized mild muscle weakness
cholinergic crisis. A cholinergic crisis is characterizedby muscle weakness and respiratory insufficiency sim-
Generalized moderate weaknessand/or bulbar dysfunction
ilar to that seen with MG. It is precipitated by an excessof the anti-AChE agent.53
Acute fulminating manifestations
• Miscellaneous drugs. Certain antibiotics have been
and/or respiratory dysfunction
reported to reduce neuromuscular transmission in
Late, severe, generalized myasthenia
patients with MG and should be avoided during the
perioperative period. Aminoglycosides (streptomycin,
AANA Journal/August 2002/Vol. 70, No. 4
tests show low vital capacity, normal total lung capac-
should be used judiciously, as patients with MG have
ity, normal or elevated residual volume, and decreased
very little respiratory reserve. There also is a high like-
maximal inspiratory and expiratory pressures.63 How-
lihood of a need for postoperative ventilatory support;
ever, patients with MG maintain a normal response to
therefore, the patient should be counseled for the pos-
carbon dioxide and an intact ventilatory drive.64 About
sibility of endotracheal tube intubation and ventilatory
15% of patients with MG have thymomas that, if they
support following surgery.48 The following preopera-
become large enough, can cause airway collapse and
tive criteria correlate with the need for postoperative
occlusion at the induction of general anesthesia. These
ventilatory support in the patient undergoing thymec-
patients should undergo chest computed tomography
tomy: (1) disease duration greater than 6 years, (2)
and flow volume spirometry to evaluate the severity of
presence of chronic obstructive pulmonary disease, (3)
this mediastinal mass and the potential for tracheal
pyridostigmine dose greater than 750 mg/per day dur-
ing the 48 hours before surgery, and (4) preoperative
A thorough cardiac assessment should be con-
vital capacity less than 2.9 L.68
ducted, especially for conduction defects, ST and T
The anesthetic plan or management of the myas-
wave changes, and arrhythmias (bradycardia, ventricu-
thenic patient should be individualized according to
lar premature contractions, atrial fibrillation) that are
the severity of the disease and the nature of the surgi-
observed in patients with MG. Significant arrhythmias
cal procedure. Whenever possible, regional or local
should be evaluated and treated by a cardiologist before
anesthesia should be used rather than general anesthe-
surgery.65 A small percentage of patients with MG are
sia. However, the amount of local anesthetic may need
reported to have myocarditis, which may be related to
to be reduced, especially ester local anesthetics in a
MG or to an associated autoimmune disorder. These
patient receiving anticholinesterase drugs, since ester
patients demonstrate impaired left ventricular filling
local anesthetics are degraded by plasma cholin-
that usually is reversed by an anticholinesterase.66
esterases. Furthermore, the level of block for spinal or
When symptoms of impaired cardiac function are dis-
epidural anesthesia must be controlled closely to pre-
covered, referral to cardiology for further evaluation
vent a high thoracic block that could weaken accessory
(eg, echocardiography) and optimization should be
respiratory muscles, resulting in dyspnea or acute res-
piratory failure.53 In addition, a combined technique of
As a result of the weakened musculature of the
general anesthesia and regional anesthesia (epidural
oropharynx, the patient with MG is at high risk for pul-
block) can provide excellent muscle relaxation without
monary aspiration of gastric contents.48 Therefore, it is
the use of neuromuscular blockers. This has been
prudent to prophylactically administer sodium citrate
demonstrated in laparoscopic surgery with immediate
to neutralize gastric acids, a gastrointestinal prokinetic
tracheal extubation postoperatively.69
medication (eg, metoclopramide) to increase gastric
• Intraoperative care. Standard monitoring should
motility, and a histamine (H2) blocker to decrease gas-
be used for every patient with MG undergoing surgery:
tric acid production.48
temperature, electrocardiogram, blood pressure, pulse
Preoperative management goals include optimiza-
oximetry, in-line carbon dioxide, and ventilation rate,
tion of anticholinesterase therapy, weaning of corticos-
and an arterial line should be inserted for obtaining
teroids to the lowest possible dose, and, if needed,
samples for blood gas analysis that can guide the tim-
plasmapheresis to prepare the patient for surgery.53
ing of extubation. In addition, for a large thymoma
Plasmapheresis is recommended for patients with MG
case, central venous access should be obtained, as the
with a vital capacity of less than 2 L7 and leads to tem-
potential for blood loss is increased.53
porary remission in 45% of cases.53 However, caution
Induction of anesthesia with a short-acting intra-
needs to be taken in administering drugs metabolized
venous agent is appropriate for the patient with MG;
by plasma cholinesterases, such as succinylcholine and
however, one should anticipate an exaggerated respira-
mivacurium, as their action may be prolonged.53
tory depressant effect. The intubation of the trachea
• Premedication. There are varying regimens for the
usually requires the use of muscle relaxation in the
administration of anticholinesterase (anti-AChE) drugs
patient without MG; however, this may be accom-
to the patient with MG. One regimen suggests admin-
plished without muscle relaxation by exploiting the
istering one half the usual morning dose for patients
existing weakness and the relaxing effects of volatile
with class I or II MG and the full dose for more severe
gas anesthetics on skeletal muscle.48 As an alternative,
cases.67 Other anesthesiologists withhold anti-AChE
lower doses of muscle relaxants may be used prudently.
drugs on the morning of surgery in order to decrease
The maintenance of anesthetic depth for surgery
the dose of muscle relaxant needed.
often is achieved by the use of nitrous oxide and a
Preoperative sedation with opioids and anxiolytics
volatile anesthetic gas. The muscle-relaxing properties
AANA Journal/August 2002/Vol. 70, No. 4
associated with volatile anesthetic gases usually reduce
control are effective in decreasing postoperative compli-
or even eliminate the dose of muscle relaxants needed,
cations. It also is extremely important to avoid drugs
as neuromuscular transmission is reduced by about
known to increase the muscle weakness of MG.
50%.51 In addition, anesthetic gases dissipate at the endof surgery, which allows for the evaluation of skeletal
muscle strength during the early postoperative period.
Myasthenia gravis most commonly is an acquired
If a muscle relaxant is required, a short- or intermedi-
autoimmune disease that is exemplified by production of
ate-acting nondepolarizing muscle relaxant, such as
AChR antibodies. Decreased AChR numbers at the NMJ
mivacurium or vecuronium, is used with one half to
are manifested as a decreased amplitude of endplate
two thirds the normal dose administered. Careful mon-
potential, which is represented clinically as muscle
itoring with a peripheral nerve stimulator should be
weakness. The AChR antibodies are present in 80% to
conducted. Opioids are used with caution due to their
90% of cases and are produced by B cells in a T
ventilatory depressant effects. Intravenous general
cell–dependent manner, and a pathologic thymus is
anesthesia with propofol also has been used success-
implicated to have an important role in MG genesis and
fully; it provides easy control of depth and quick recov-
progression. IgG and complement components are
ery and avoids consequences at the NMJ.70
deposited on the postsynaptic membrane, and destruc-
• Postoperative care. Postoperatively, the endotra-
tive mechanisms may consist of increased degradation of
cheal tube often is left in place until demonstration of
AChRs, cross-linking of AChRs, and blockage of AChRs.
adequate levels of ventilation are observed. Good indi-
Since the NMJ involves the site of action of many com-
cations of the need for postoperative ventilatory sup-
monly used anesthetic drugs, anesthesia providers must
port are the aforementioned preoperative criteria.
understand the pathophysiology of MG, be cognizant of
Gracey et al describe recent surgery (especially thymec-
the many drug interactions that can be detrimental to the
tomy) as the most common reason for respiratory fail-
myasthenic patient, and administer anesthetics that
ure in MG.71 Generally, MG classes III and IV have a
would most benefit the patient with MG.
high incidence of postoperative respiratory failure. Cri-teria for extubation of the patient with MG are strin-
1. Keesey J. Myasthenia gravis. Arch Neurol. 1998;55:745-746.
gent and consist of an awake patient who can maintain
2. Vincent A, Newland C, Croxen R, Beeson D. Genes at the junction:
a head lift for more than 5 seconds and generate a sus-
candidates for congenital myasthenic syndromes. Trends Neurosci.
tained negative inspiratory force of more than –25 cm
3. Kim YI, Neher E. IgG from patients with Lambert-Eaton syndrome
2O.52 In addition, the patient's respiratory rate should
blocks voltage-dependent calcium channels. Science. 1988;239:405-
be less than 30 per minute and vital capacity more than
10 mL/kg; arterial blood gases should reflect a PaO2 of
4. Lang B, Vincent A, Murray NM, Newsom-Davis J. Lambert-Eaton
more than 90 mm Hg, a PaCO
myasthenic syndrome: immunoglobulin G inhibition of Ca2 flux in
2 of less than 50 mm Hg,
and pH more than 7.30.72 Another reason the patient
tumor cells correlates with disease severity. Ann Neurol. 1989;25:265-271.
with MG may have respiratory difficulty postopera-
5. Lefvert AK, Bergstrom K, Matell G, Osterman PO, Pirskanen R.
tively is bilateral vocal cord abductor weakness (stri-
Determination of acetylcholine receptor antibody in myasthenia
dor), and this should be evaluated.73
gravis: clinical usefulness and pathogenetic implications. J NeurolNeurosurg Psychiatry. 1978;41:394-403.
Postoperative analgesia can be achieved by cautious
6. Boonyapisit K, Kaminski HJ, Ruff RL. Disorders of neuromuscular
administration of oral or parenteral opioid analgesics
junction ion channels. Am J Med. 1999;106:97-113.
or by using regional anesthesia techniques. With opi-
7. Drachman DB. Myasthenia gravis. N Engl J Med. 1994;330:1797-
oid administration, it is imperative to monitor the res-
piratory status of the patient with MG, as ventilatory
8. Grob D, Arsura EL, Brunner NG, Namba T. The course of myasthe-
nia gravis and therapies affecting outcome. Ann N Y Acad Sci. 1987;
reserve is decreased and the patient is more prone to
respiratory depression. As an alternative, epidural nar-
9. Wittbrodt ET. Drugs and myasthenia gravis: an update. Arch Intern
cotics provide excellent postoperative analgesia for the
patient with MG with a much lower incidence of respi-
10. Lindstrom JM, Seybold ME, Lennon VA, Whittingham S, Duane DD.
ratory depression.74
Antibody to acetylcholine receptor in myasthenia gravis: prevalence,clinical correlates, and diagnostic value. Neurology. 1976;26:1054-
Furthermore, the reintroduction of the patient's pre-
operative medications in the early postoperative period
11. Daroff RB. The office Tensilon test for ocular myasthenia gravis. Arch
is very important, especially the anticholinesterases. Pre-
operative medications must be continued as soon as pos-
12. Pourmand R. Myasthenia gravis. Dis Mon. 1997;43:65-109.
sible, especially since the improvement of MG symp-
13. Le Novere N, Changeux JP. Molecular evolution of the nicotinic
acetylcholine receptor: an example of a multigene family in excitable
toms is delayed after a thymectomy.75 Efforts to optimize
cells. J Mol Evol. 1995;40:155-172.
preoperative respiratory function and postoperative pain
14. Kaminski HJ, Ruff RL. Insights into possible skeletal muscle nicotinic
AANA Journal/August 2002/Vol. 70, No. 4
acetylcholine receptor (AChR) changes in some congenital myasthe-
thenia or disease restricted to ocular muscles. Clin Exp Immunol.
nias from physiological studies, point mutations, and subunit substi-
tutions of the AChR. Ann N Y Acad Sci. 1993;681:435-450.
39. Vincent A, Newsom-Davis J. Acetylcholine receptor antibody char-
15. Schluep M, Willcox N, Vincent A, Dhoot GK, Newsom-Davis J.
acteristics in myasthenia gravis, III: patients with low anti-AChR
Acetylcholine receptors in human thymic myoid cells in situ: an
antibody levels. Clin Exp Immunol. 1985;60:631-636.
immunohistological study. Ann Neurol. 1987;22:212-222.
40. Massey JM. Acquired myasthenia gravis. Neurol Clin. 1997;15:577-
16. Horton RM, Manfredi AA, Conti-Tronconi BM. The "embryonic"
gamma subunit of the nicotinic acetylcholine receptor is expressed
41. Castleman B. The pathology of the thymus gland in myasthenia
in adult extraocular muscle. Neurology. 1993;43:983-986.
gravis. Ann N Y Acad Sci. 1966;135:496-505.
17. Kreienkamp HJ, Maeda RK, Sine SM, Taylor P. Intersubunit contacts
42. Wekerle H, Ketelsen UP. Intrathymic pathogenesis and dual genetic
governing assembly of the mammalian nicotinic acetylcholine
control of myasthenia gravis. Lancet. 1977;1:678-680.
receptor. Neuron. 1995;14:635-644.
43. Ahlberg R, Yi Q, Pirskanen R, et al. The effect of thymectomy on
18. Lindstrom JM. Acetylcholine receptors and myasthenia. Muscle
autoreactive T- and B-lymphocytes in myasthenia gravis. J Neuroim-
19. Karlin A, Kao PN, DiPaola M. Molecular pharmacology of the nico-
44. Kao I, Drachman DB. Thymic muscle cells bear acetylcholine recep-
tinic acetylcholine receptor. Trends Pharmacol Sci. 1986;4:304-308.
tors: possible relation to myasthenia gravis. Science. 1977;195:74-75.
20. Beroukhim R, Unwin N. Three-dimensional location of the main
45. Scadding GK, Vincent A, Newsom-Davis J, Henry K. Acetylcholine
immunogenic region of the acetylcholine receptor. Neuron.
receptor antibody synthesis by thymic lymphocytes: correlation
with thymic histology. Neurology. 1981;31:935-943.
21. Conti-Tronconi B, Tzartos S, Lindstrom J. Monoclonal antibodies as
46. Cosi V, Lombardi M, Piccolo G, Erbetta A. Treatment of myasthenia
probes of acetylcholine receptor structure, 2: binding to native
gravis with high-dose intravenous immunoglobulin. Acta Neurol
22. Engel AG, Lambert EH, Howard FM. Immune complexes (IgG and
47. Swain SL, Dialynas DP, Fitch FW, English M. Monoclonal antibody
C3) at the motor endplate in myasthenia gravis: ultrastructural and
to L3T4 blocks the function of T cells specific for class 2 major his-
light microscopic localization and electrophysiologic correlations.
tocompatibility complex antigens. J Immunol. 1984;132:1118-1123.
Mayo Clin Proc. 1977;52:267-280.
48. Stoelting RK, Dierdorf SF. Anesthesia and Co-existing Disease. 3rd ed.
23. Newsom-Davis J, Vincent A. Combined plasma exchange and
New York, NY: Churchill Livingstone; 1993:439-444.
immunosuppression in myasthenia gravis. Lancet. 1979;2:688.
49. Eisenkraft JB, Book WJ, Mann SM, Papatestas AE, Hubbard M.
24. Lennon VA, Lambert EH. Myasthenia gravis induced by monoclonal
Resistance to succinylcholine in myasthenia gravis: a dose-response
antibodies to acetylcholine receptors. Nature. 1980;285:238-240.
25. Schonbeck S, Chrestel S, Hohlfeld R. Myasthenia gravis: prototype
50. Smith CE, Donati F, Bevan DR. Cumulative dose-response curves for
of the antireceptor autoimmune diseases. Int Rev Neurobiol.
atracurium in patients with myasthenia gravis. Can J Anaesth.
26. Engel AG, Fumagalli G. Mechanisms of acetylcholine receptor loss
51. Nilsson E, Meretoja OA. Vecuronium dose-response and mainte-
from the neuromuscular junction. Ciba Found Symp. 1982;90:197-224.
nance requirements in patients with myasthenia gravis. Anesthesiol-
27. Fambrough DM, Drachman DB, Satyamurti S. Neuromuscular junc-
tion in myasthenia gravis: decreased acetylcholine receptors. Sci-
52. Baraka A. Anaesthesia and myasthenia gravis. Can J Anaesth.
28. Engel AG, Tsujihata M, Lindstrom JM, Lennon VA. The motor end
53. Krucylak PE, Naunheim KS. Preoperative preparation and anes-
plate in myasthenia gravis and in experimental autoimmune myas-
thetic management of patients with myasthenia gravis. Semin Thorac
thenia gravis; a quantitative ultrastructural study. Ann N Y Acad Sci.
Cardiovasc Surg. 1999;11:47-53.
54. Book WJ, Abel M, Eisenkraft JB. Anesthesia and neuromuscular dis-
29. Paton WD, Waud DR. The margin of safety of neuromuscular trans-
eases. Anesthesiol Clin North America. 1996;14:515-542.
mission. J Physiol. 1967;191:59-90.
55. Snavely SR, Hodges GR. The neurotoxicity of antibacterial agents.
30. Hohlfeld R, Wekerle H. The role of the thymus in myasthenia gravis.
Ann Intern Med. 1984;101:92-104.
Adv Neuroimmunol. 1994;4:373-386.
56. May EF, Calvert PC. Aggravation of myasthenia gravis by erythro-
31. Penn AS, Low BW, Jaffe IA, Luo L, Jacques JJ. Drug-induced autoim-
mycin. Ann Neurol. 1990;28:577-579.
mune myasthenia gravis. Ann N Y Acad Sci. 1998;841:433-449.
57. Moore B, Safani M, Keesey J. Possible exacerbation of myasthenia
32. Compston D, Vincent A, Newsom-Davis J, Batchelor J. Clinical,
gravis by ciprofloxacin. Lancet. 1988;1:882.
pathological, HLA antigen, and immunological evidence for diseaseheterogeneity in myasthenia gravis. Brain. 1981;103:579-601.
58. Mumford CJ, Ginsberg L. Ciprofloxacin and myasthenia gravis. BMJ.
33. Baggi F, Mantegazza R, Vincent A, Newsom-Davis J. HLA-A2–
restricted T-cell line recognizing an epitope of the human acetylcho-
59. Drachman DA, Skom JH. Procainamide: a hazard in myasthenia
line receptor. Ann N Y Acad Sci. 1993;681:276-279.
gravis. Arch Neurol. 1965;13:316-320.
34. Vincent A, Willcox N, Hill M, Curnow J, MacLennan C, Beeson D.
60. Herishanu Y, Rosenberg P. beta-Blockers and myasthenia gravis [let-
Determinant spreading and immune responses to acetylcholine
ter]. Ann Intern Med. 1975;83:834-835.
receptors in myasthenia gravis. Immunol Rev. 1998;164:157-168.
61. Osserman KE, Genkins G. Studies in myasthenia gravis. Review of a
35. Richman DP, Antel JP, Patrick JW, Arnason BG. Cellular immunity
twenty-year experience in over 1200 patients. Mt Sinai J Med.
to acetylcholine receptor in myasthenia gravis: relationship to his-
tocompatibility type and antigenic site. Neurology. 1979;29:291-296.
62. Christensen PB, Jensen TS, Tsiropoulos I, et al. Associated autoim-
36. Hohlfeld R, Toyka KV, Michels M, Heininger K, Conti-Tronconi B,
mune diseases in myasthenia gravis: a population-based study. Acta
Tzartos SJ. Acetylcholine receptor-specific human T-lymphocyte
Neurol Scand. 1995;91:192-195.
lines. Ann N Y Acad Sci. 1987;505:27-38.
63. Zulueta JJ, Fanburg BL. Respiratory dysfunction in myasthenia
37. Raghel S, Lisak R. Immune regulation and myasthenia gravis. Ann N
gravis. Clin Chest Med. 1994;15:683-691.
Y Acad Sci. 1998;841:211-224.
64. Borel CO, Teitelbaum JS, Hanley DF. Ventilatory drive and carbon
38. Vincent A, Newsom-Davis J. Acetylcholine receptor antibody char-
dioxide response in ventilatory failure due to myasthenia gravis and
acteristics in myasthenia gravis, I: patients with generalized myas-
Guillain-Barré syndrome. Crit Care Med. 1993;21:1717-1726.
AANA Journal/August 2002/Vol. 70, No. 4
65. Gibson TC. The heart in myasthenia gravis. Am Heart J. 1975;90:
74. Kirsch JR, Diringer MN, Borel CO, Hanley DF, Merritt WT, Bulkley
GB. Preoperative lumbar epidural morphine improves postoperative
66. Johannessen KA, Mygland A, Gilhus NE, Aarli J, Vik-Mo H. Left
analgesia and ventilatory function after transsternal thymectomy in
ventricular function in myasthenia gravis. Am J Cardiol. 1992;69:
patients with myasthenia gravis. Crit Care Med. 1991;19:1474-1479.
75. Loach AB, Young AC, Spalding JM, Smith AC. Postoperative man-
67. Girnar DS, Weinreich AI. Anesthesia for transcervical thymectomy
agement after thymectomy. Br Med J. 1975;1:309-312.
in myasthenia gravis. Anesth Analg. 1976;55:13-17.
68. Leventhal SR, Orkin FK, Hirsh RA. Prediction of the need for post-
operative mechanical ventilation in myasthenia gravis. Anesthesiol-
MAJ Thomas E. Ceremuga, CRNA, MSN, AN, USA, is a graduate student
in the Neuroscience Program, Uniformed Services University of the
69. Hubler M, Litz RJ, Albrecht DM. Combination of balanced and
Health Sciences, Bethesda, Md.
regional anaesthesia for minimally invasive surgery in a patient withmyasthenia gravis. Eur J Anaesthesiol. 2000;17:325-328.
Xiang-Lan Yao, MD, PhD, is a research assistant professor, Depart-
ment of Anatomy, Physiology and Genetics, Uniformed Services Univer-
70. O'Flaherty D, Pennant JH, Rao K, Giesecke AH. Total intravenous
sity of the Health Sciences, Bethesda, Md.
anesthesia with propofol for transsternal thymectomy in myastheniagravis. J Clin Anesth. 1992;4:241-244.
Joseph T. McCabe, PhD, is a professor and vice chairman, Depart-
71. Gracey DR, Divertie MB, Howard FM Jr. Mechanical ventilation for
ment of Anatomy, Physiology & Genetics, Uniformed Services University
respiratory failure in myasthenia gravis: two-year experience with
of the Health Sciences, Bethesda, Md.
22 patients. Mayo Clin Proc. 1983;58:597-602.
72. Gorback MS, Moon RE, Massey JM. Extubation after transsternal
thymectomy for myasthenia gravis: a prospective analysis. South
The opinions or assertions contained herein are the private ones of the
Med J. 1991;84:701-706.
authors and are not to be construed as official or reflecting the views of
73. Colp C, Kriplani L, Nussbaum M. Vocal cord paralysis in myasthe-
the US Department of Defense or the Uniformed Services University of
nia gravis following anesthesia. Chest. 1980;77:218-220.
the Health Sciences.
AANA Journal/August 2002/Vol. 70, No. 4
Source: http://web.unair.ac.id/admin/file/f_66373_jcourse3_0802_p301-310.pdf
Reporte sobre el Incremento de Advertencias Internacionales sobre Drogas Psiquiátricas Comité de Ciudadanos en Defensa de los Derechos Humanos LOS GOBIERNOS ADVIERTEN SOBRE LOS PELIGROS El 30 de junio de 2006, La Suprema Corte de Justicia de Alaska reconoció los peligros que provocan las drogas psiquiátricas, declarando que: "Las drogas psiquiátricas ‘afectan la mente, el comportamiento, las
DIFLUCAN® (Fluconazole Tablets) (Fluconazole Injection - for intravenous infusion only) (Fluconazole for Oral Suspension) DESCRIPTION DIFLUCAN® (fluconazole), the first of a new subclass of synthetic triazole antifungal agents, is available as tablets for oral administration, as a powder for oral suspension and as a sterile solution for intravenous use in glass and in Viaflex® Plus plastic containers. Fluconazole is designated chemically as 2,4-difluoro-α,α1-bis(1H-1,2,4-triazol-1-ylmethyl) benzyl alcohol with an empirical formula of C13H12F2N6O and molecular weight 306.3. The








