
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa

Contents lists available at
Neuroscience Letters
Functional and effective connectivity of anterior insula in anorexia nervosa and
Kyung Ran Kim , Jeonghun Ku , Jung-Hyun Lee , Hyeongrae Lee , Young-Chul Jung
a Department of Psychiatry, Yonsei University College of Medicine,South Korea
b Institute of Behavioral Science in Medicine, Yonsei University College of Medicine,South Korea
c Department of Biomedical Engineering, Keimyung University,South Korea
d Mind & Mind Eating Disorder Clinic,South Korea
e MEG Center, Department of Neurosurgery, Seoul National University College of Medicine,South Korea
� Functional and effective connectivity of the insula in women with eating disorders was analyzed.
� Anorexia nervosa demostrated synchrony between the insula and the right inferior frontal cortex.
� Bulimia nervosa demonstrated synchrony between the insula and the medial orbitofrontal cortex.
The anterior insula has been proposed to play a crucial role in eating disorders. How-
Received 23 April 2012
ever, it is still poorly understood how the anterior insula is involved in anorexia nervosa
Received in revised form 25 May 2012
(AN) and bulimia nervosa (BN), which are characterized by opposite motivational responses to
Accepted 31 May 2012
food. We applied a cue-reactivity paradigm using blood oxygen level-dependent functional magnetic
resonance imaging in women with AN (N = 18) and BN (N = 20) and age-matched healthy controls (N = 20).
We defined the left anterior insula as a region-of-interest and performed seed-based functional connec-
tivity and effective connectivity MRI analysis. In response to food images compared to non-food images,
both the AN group and BN group demonstrated increased activity in the left anterior insula. In the AN
Functional connectivity
group, the left anterior insula demonstrated significant interactions with the right insula and right infe-
Effective connectivity
rior frontal gyrus. In the BN group, the left anterior insula demonstrated significant interactions with the
medial orbitofrontal cortex. The distinct patterns of functional and effective connectivity of the anterior
insula may contribute to the different clinical features of AN and BN.
2012 Elsevier Ireland Ltd. All rights reserved.
The anterior insula forms the primary gustatory cortex
and processes the physical properties of food and their reward
Anorexia nervosa (AN) and bulimia nervosa (BN) are charac-
properties The anterior insula is also implicated in a wide
terized by pathological eating behaviors that generally result in
range of conditions and behaviors, including integrating interocep-
severe food restriction and body weight loss in AN, and recurrent
tive information, which is the sense of the physiological condition
binge-eating episodes without weight loss in BN and BN
of the body imaging studies consistently
are recognized as separate eating disorders with categorical cri-
reported aberrant activities in the anterior insula and proposed
teria. However, it has not yet been determined whether AN and
that the anterior insula might account for the aversive interocep-
BN share a primary disturbance of appetitive pathways and what
tive experiences in subjects with AN contrast, subjects with
neural correlates underlie their different clinical presentations.
BN are known to lack of appetite control and experience intense
food craving, which likely results in binge-eating episodes
The anterior insula activity has also reported to be altered in sub-
jects with BN, however the anterior insula has gained less attention
∗ Corresponding author at: Department of Psychiatry, Yonsei University College
compared to the reward circuitry
of Medicine, 696-6 Tanbul-dong Gwangju-si, Geonggi-do 464-100, South Korea.
In this study, we used a cue-reactivity paradigm by present-
Tel.: +82 31 760 9405; fax: +82 31 761 7582.
E-mail address: (Y.-C. Jung).
ing food images and measured the subjective responses and neural
1 Both these authors contributed equally to this paper.
responses in women with AN and BN. We hypothesized the anterior
0304-3940/$ – see front matter
2012 Elsevier Ireland Ltd. All rights reserved.
K.R. Kim et al. / Neuroscience Letters 521 (2012) 152–157
insula as the region of interest (ROI) and conducted a seed-based
using the Analysis of Functional Neuroimage program (AFNI)
functional connectivity and effective connectivity analysis in order
The first 7 time points in all the time series data were discarded
to compare the functional aspects of the anterior insula circuitry in
to allow for T1 equilibrium effects. The rest of the data were
these two eating disorders. Functional connectivity is operationally
performed slice timing correction, motion correction of all slices
defined as the temporal correlations between spatially remote neu-
within a volume, and mean-based intensity normalization to
rophysiological events which reveals the strength of relationship
convert the data from arbitrary intensity units to units of percent
between regions whereas effective connectivity is closer to
signal modulation. Further processing included spatial smoothing
the intuitive notion of a connection and represents the contrib-
(Gaussian filter with 8 mm full-width at half-maximum [FWHM]).
utory influence one region exerts over another expected
Spatial normalization was performed to transform Talairach
this pluralistic approach would provide a better appreciation of the
space using Montreal Neurological Institute (MNI) N27 tem-
role of anterior insula in AN and BN.
plate provided in AFNI (bilinear interpolation, spatial resolution:
2 mm × 2 mm × 2 mm).
2. Methods
The response to each stimulus category (food vs. non-food)
compared with the fixation baseline was calculated using decon-
volution regions that showed a response to any stimulus
type were included in the analysis each subject, the regres-
Participants included 58 right-handed women (aged 20–35): 18
sion model provided a brain activity map to each stimulus type
had a current DSM-IV diagnosis of AN (6 restricted type, 12 binge
for the whole brain. Contrast "Food versus Non-food" was calcu-
eating/purging type), 20 had a current DSM-IV diagnosis of BN, and
lated for each subject. One-sample t-tests and two-sample t-test
20 were healthy control age-matched women (HC) who were in
were performed for comparisons and the threshold was p < 0.001
normal weight range (There was no significant difference
(uncorrected) for multiple comparisons and the cluster threshold
between the three groups in age (F = 2.463, p = 0.095) or years of
was k > 50.
education (F = 2.937, p = 0.061). Screening and diagnosis were made
using the Structured Clinical Interview for DSM-IV (SCID) and were
confirmed by a psychiatrist. Any subject who had a current or his-
2.4. Region of interest
tory of a psychiatric comorbid Axis I diagnosis according to SCID
were excluded. This study was carried out under the guidelines
We defined a cluster within the anterior insula as a seed to inves-
for the use of human participants established by the Institutional
tigate its functional and effective connectivity. In order to compare
Review Board. Following a complete description of the scope of
the functional connectivity of the two groups with an identical
the study, written informed consent was obtained from all of the
seed, we combined AN and BN as one group (N = 38) and identi-
fied a common cluster in the anterior insula (x = −35, y = 7, z = −4;
2.2. Stimuli and procedure
The participants were instructed to abstain from eating for 6 h
2.5. Functional connectivity analysis
preceding the MR scanning session, which began between 600 pm
and 700 pm. The participants passively viewed food images and
The assessment of cortical networks was performed using
non-food images, which had been matched for complexity and
a seed-based correlation approach. The preprocessed fMRI data
color composition. The food images were selected among high-
were temporally band-pass filtered (0.01–0.08 Hz) to reduce low
calorie food items in order to amplify the subjective and neural
frequency fluctuation of the signal in the BOLD for functional
responses The images were presented through an MR com-
connectivity analysis The reference time series during the
patible goggle system, while the participants were instructed to
task were extracted by averaging time series from voxels in the
imagine tasting the food items or using the non-food items. Each
subject-specific ROIs within the left anterior insula. The functional
category had 40 pictures, which were presented alternatively in
connectivity maps of the left anterior insular were obtained by a
a block design. Each block consisted of 8 pictures, which were
correlation analysis being conducted with the reference time series
each presented for 2800 ms with a 200 ms inter-stimulus interval.
and time series from the whole brain using a voxel-wise approach,
There was a fixation baseline (24 s) between each block. To con-
which correlation coefficients were converted to Z-values rep-
firm their attention, participants pressed a button every time the
resenting functional connectivity strength with the insular seed
picture changed. We measured the hunger state and food craving
using Fisher's Z transformation (two-tailed threshold of uncor-
before and after the MR scanning session using a 11-point Likert
rected p < 0.001)
scale ("not hungry" to "very hungry") and the State Food Craving
Questionnaire (FCCQ-S)
2.6. Effective connectivity analysis
2.3. MRI acquisition
We also performed the structural vector autoregressive (SVAR)
Functional images were acquired on a 1.5 T MRI scan-
which allows us to study a realistic model of network relations by
ner (General Electric, Milwaukee, WI) by using a gradient
considering both contemporaneous and lagged interactions
echo-planar imaging sequence (TR = 2.5 s; TE = 14.3 ms; flip
addition to the left anterior insula seed, three additional ROIs were
angle = 90; field of view = 240 mm; 64 × 64 × 30 matrix with
selected from the functional connectivity analysis: right anterior
3.75 mm × 3.75 mm × 5 mm spatial resolution; 30 axial slices;
insula, right inferior frontal gyrus and medial orbitofrontal cor-
thickness = 5 mm).
tex (With the four ROIs, we extracted a
cal dataset was obtained for each subject by using a fast
time series for each ROI from the preprocessed fMRI data and put
spoiled gradient echo sequence (TR = 8.5 s, TE = 1.8 ms, flip
them into the SVAR analysis process. After obtaining the network
angle = 12, field of view = 240 mm, 256 × 256 × 256 matrix with
coefficients among the three regions, we conducted a within-group
0.94 mm × 0.94 mm × 1.5 mm spatial resolution, 116 axial slices,
analysis and set the threshold, p < 0.05 uncorrected, to get the sig-
and slice thickness = 1.5 mm). The fMRI data were preprocessed
K.R. Kim et al. / Neuroscience Letters 521 (2012) 152–157
Demographic and clinical variables of subjects.
Duration of illness (y)
Frequency of binge (per month)
Frequency of vomiting (per month)
AN > BN > HC
AN: anorexia nervosa, BN: bulimia nervosa, HC: healthy control, BMI: body mass index, BDI: Beck Depression Inventory, BAI: Beck Anxiety Inventory, EDI: eating disorder
2.7. Statistical analysis
3.2. Within-group comparisons of brain activations
Analyses of variance (ANOVAs) for repeated measures were
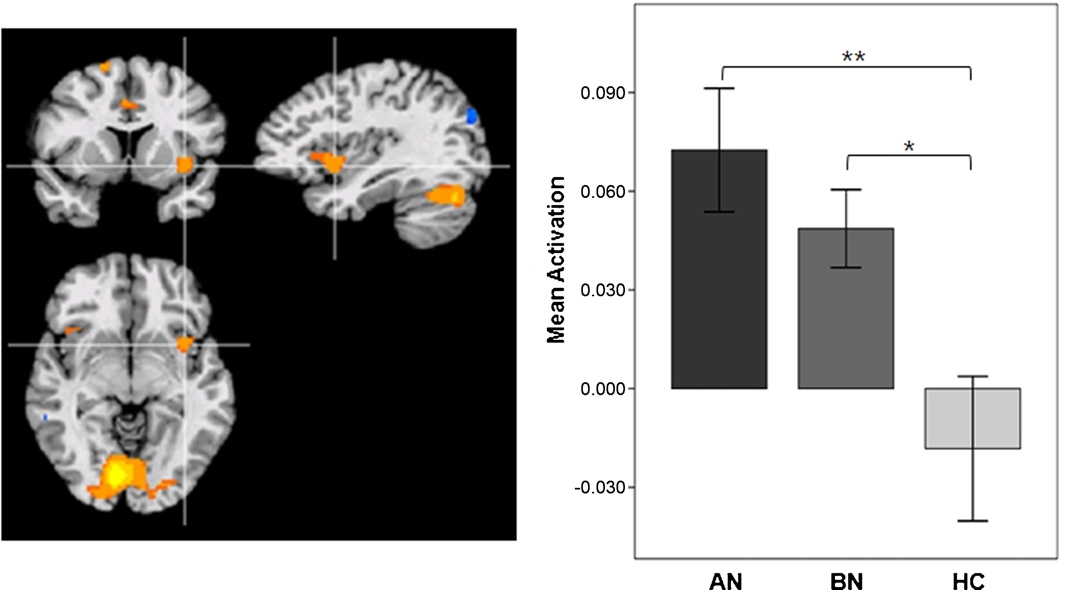
In response to food images, increased activity was observed in
used to compare hunger rating and food craving, which were
the left anterior insula in both the AN group and BN group. In addi-
rated before and after the scanning session. Between-group
tion, food > non-food contrast demonstrated increased activity in
comparison of the ROI activity to food images were con-
the inferior frontal gyrus (IFG), superior frontal gyrus, anterior cin-
ducted by computing analyses of covariance (ANCOVA) with
gulate cortex (ACC), visual cortex and cerebellum in the AN group.
body mass index (BMI), Beck Depression and Anxiety Inven-
By contrast, increased activity was demonstrated only in the visual
tory (BDI, BAI) scores as covariates to exclude confounding
cortex and cerebellum in the BN group ANCOVA analy-
effects of these variables. Between-group comparison of func-
sis indicated that the left anterior insula activity was significantly
tional connectivity were conducted by ANOVA. Statistical analyses
increased in the eating disorder groups, when compared to the
were conducted by using SPSS (Chicago, IL) with two-tailed
HC group (F = 3.141, p = 0.015). However, there was no significant
p < 0.05.
difference between the two eating disorder groups (
3.3. Between-group comparisons of brain activations
3. Results
In comparison to the HC group, the AN group demonstrated
greater activity to food images in the right IFG, superior frontal
3.1. Subjective hunger and food craving
gyrus, ACC, and cerebellum, whereas the BN group demonstrated
increased activity in the right middle frontal gyrus, right insula and
ANOVA revealed a significant difference between groups in sub-
cerebellum. Comparing between the AN group and the BN group,
jective hunger (F2, 55 = 5.162, p = 0.009), but the effect of session
the AN group demonstrated increased activity in the ACC, whereas
(before vs. after) was not significant (F1, 55 = 3.088, p = 0.084). Post
the BN group demonstrated increased activity in the middle tem-
hoc analysis showed that the rating for subjective hunger was sig-
nificantly higher in BN group (p = 0.19) compared to that of the AN
group. By contrast, there was no significant difference between
3.4. Functional and effective connectivity analysis
groups in food craving (F2, 55 = 0.878, p = 0.421) and the effect of
session was not significant on the FCQ-S scores (F1, 55 = 0.134,
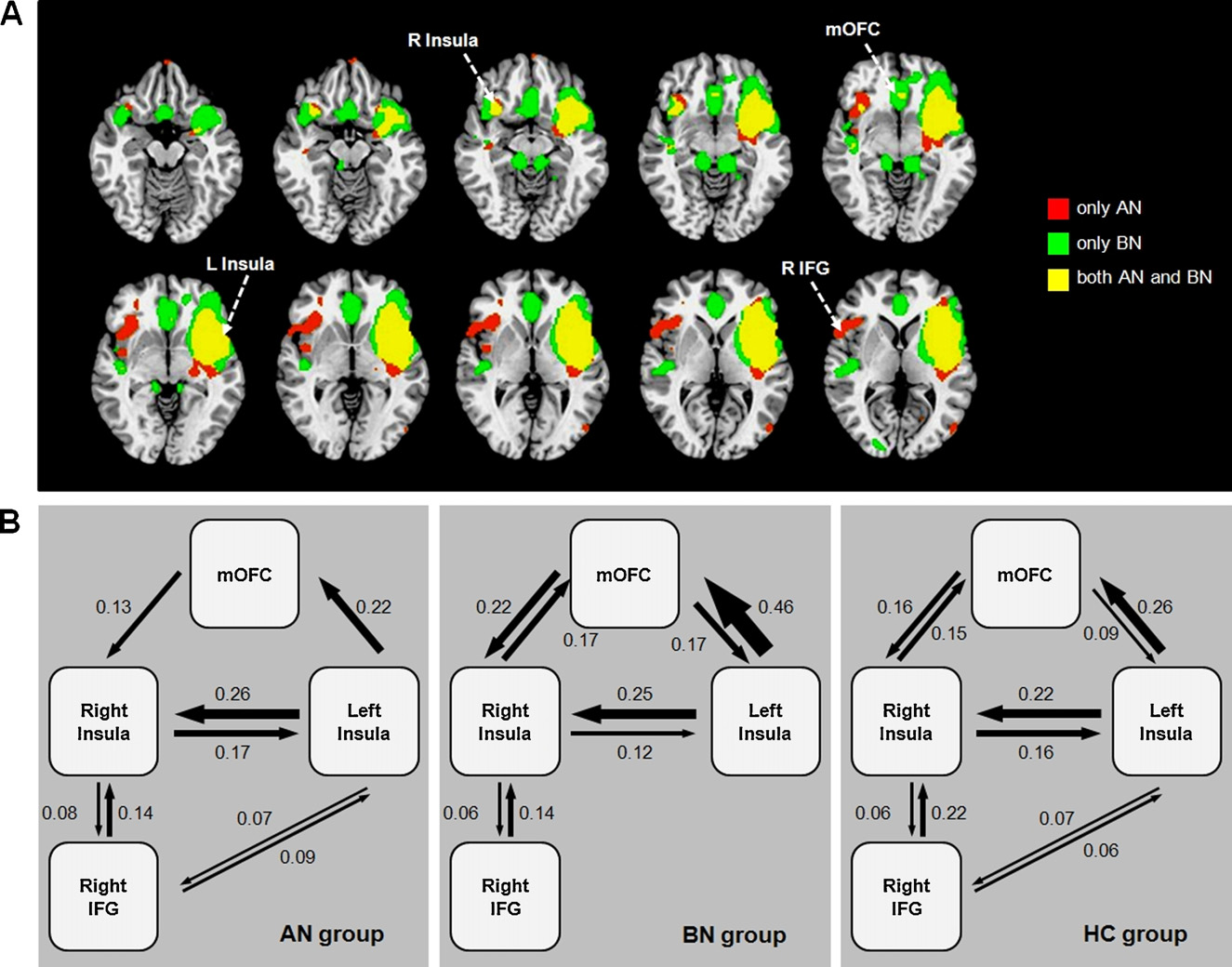
The AN group demonstrated significant correlations between
the left anterior insula and the right IFG/insula cluster. In contrast,
Fig. 1. Region of Interest (left anterior insula; x = −35, y = 7, z = −4) and mean activity to food images relative to non-food images. **p < 0.01, *p < 0.05.
K.R. Kim et al. / Neuroscience Letters 521 (2012) 152–157
Within-group and between-group comparisons of brain activations to food images.
Inferior frontal gyrus
Superior frontal gyrus
Anterior cingulate cortex
Middle frontal gyrus
Inferior frontal gyrus
Superior frontal gyrus
Anterior cingulate cortex
Inferior parietal lobule
Middle frontal gyrus
Postcentral gyrus
Inferior parietal lobule
Anterior cingulate cortex
Middle temporal gyrus
AN: anorexia nervosa, BN: bulimia nervosa, HC: healthy control.
a Regions significant at uncorrected p < 0.001, k > 50 at whole brain.
b Regions significant at FDR-corrected p < 0.05, k > 10 at whole brain.
the BN group showed prominent functional connectivity between
adjustments in cognitive control whereas the right IFG is
the left anterior insula and the mOFC
known to be a key locus of inhibitory motor control The
The functional connectivity between the left anterior insula and
right IFG is involved not only in stopping response when instructed
the mOFC was significantly stronger in the BN group compared to
by an external signal, but also when an individual prepares to
the AN group and HC group (F2, 55 = 6.690, p = 0.003).
stop an upcoming response tendency the task, there
The AN group and BN group demonstrated bidirectional influ-
were significant correlations between the left anterior insula and
ences between the left and right insula. However, the causal
the right IFG-insula activities in AN group. This is consistent with
outflow from the right IFG to the left insula was significant only
structural connectivity, as the insula is known to have reciprocal
in the AN group (
projections with the IFG A number of cases have previously
reported anorexia nervosa symptoms in patients with right pre-
frontal lesions The right IFG/insula activities has been
proposed to represent top-down cognitive effort to trigger an
We investigated the functional aspects of the anterior insula
avoidance tendency to food might be related to the
in subjects with AN and BN. The AN group and BN group both
discrepancy between the ratings of subjective hunger and food
demonstrated altered left anterior insula activity as a common
craving, which probably indicates the aversive or ambivalent moti-
neural response to food-related stimuli, but demonstrated distinct
vational response of the AN group in our study. This was supported
patterns of functional and effective connectivity that should poten-
by the effective connectivity results which showed that the interac-
tially underlie their different motivational responses to food.
tions between the right IFG and the left insula was significant only
Both the AN and BN group demonstrated increased activity in
in the AN group, but not in the BN group.
the left anterior insula in response to food cue, which likely repre-
There FCQ scores show that food craving was not induced
sents their increased salience attribution to the food related cues.
significantly in the subjects, which may explain why no activi-
The anterior insula forms the primary gustatory cortex
ties were observed in the reward circuit that were highlighted
is interconnected with the amygdala, ventral ACC and the OFC,
in previous studies Activities of the mOFC have been
which regions are consistently reported to be involved in visual
reported to correlate positively with subjective pleasantness
processing of food stimuli not only in patients with eating disorders
toward food-related cues well as alcohol- and nicotine-
also in unaffected healthy controls
related cue These findings support the hypothesis that
The AN group demonstrated increased activities in the dor-
the mOFC would play similar roles in both substance crav-
sal ACC and the right IFG. The dorsal ACC has been repeatedly
ing and food craving Previous studies has pointed out
described to signal conflict monitoring and trigger compensatory
that increased activations in the reward circuit to food-related
K.R. Kim et al. / Neuroscience Letters 521 (2012) 152–157
Fig. 2. (A) Functional connectivity map of the left anterior insula seed in the AN group and the BN group. (B) Effective connectivity between the left anterior insula, right
anterior insula, medial orbitofrontal cortex (mOFC) and right inferior frontal gyrus (IFG) in the AN group, BN group and HC group.
cues is not limited to BN but also observed in AN
Our findings showed the functional connectivity between the
left anterior insula and the mOFC was stronger in the BN
Our data extend the understanding of the role of the ante-
group compared to the AN group. It seems plausible to specu-
rior insula in eating disorders through revealing its functional and
late that the functional interaction between these two regions
effective connectivity. In the AN group, the anterior insula showed
might vary during the course of illness and potentially con-
significant correlations with the right insula/IFG, which might play
tribute to the degree of food craving in patients with eating
a leading role in the negative interoceptive evaluation and avoid-
ance toward food-related cues. In the BN group, the anterior insula
There are some limitations to this study. The number of subjects
demonstrated stronger functional connectivity with the mOFC,
was not large enough to compare between the subtypes of anorexia
which likely represents the enhanced reward sensitivity toward
nervosa (restrict type = 6; binge-eating/purging = 12). The two sub-
food stimuli. The distinct pattern of the functional and effective
types have different clinical features and it could be argued that
connectivity of the anterior insula may underlie the different clin-
AN binge-eating/purge type and BN have similar responses to food.
ical features of AN and BN.
Another limitation is the brain patterns should have been influ-
enced by confounding factors associated with peripheral changes
Conflict of interest
that accompany AN and BN. In fact, the BMI has been reported to
modulate food-related responses in the insula. Another thing to
The authors declare no conflict of interest.
consider is that our subjects were treatment-seeking patients tak-
ing selective serotonin reuptake inhibitors (SSRIs). The effects of
SSRI on fMRI were reported not to be significant comparing eat-
ing disorder patients who were taking SSRI and those who were
not we could not confirm this in our study because
The authors would like to thank Edith V. Sullivan (Stanford
majority of the patients (N = 36) were taking SSRI medication.
University) for her suggestions for improving the manuscript, and
The medication effect should have compounded and attenuated
Kang-Joon Yoon (St. Peter's Hospital, Korea) for providing tech-
the food craving scores as well as the neural responses in our
nical support. This study was supported by a grant from Yonsei
University College of Medicine (6-2009-0117).
K.R. Kim et al. / Neuroscience Letters 521 (2012) 152–157
Appendix A. Supplementary data
[17] Z. Ellison, J. Foong, R. Howard, E. Bullmore, S. Williams, J. Treasure, Func-
tional anatomy of calorie fear in anorexia nervosa, Lancet 352 (1998)
Supplementary data associated with this article can be
[18] K. Friston, C.D. Frith, R.S. Frackowiak, Time-dependent changes in effec-
found, in the online version, at
tive connectivity measured with PET, Human Brain Mapping 1 (1993)
[19] K.J. Friston, C.D. Frith, P.F. Liddle, R.S. Frackowiak, Functional connectivity: the
principal-component analysis of large (PET) data sets, Journal of Cerebral Blood
Flow and Metabolism 13 (1993) 5–14.
[20] D. Fuhrer, S. Zysset, M. Stumvoll, Brain activity in hunger and satiety: an
[1] A.R. Aron, From reactive to proactive and selective control: developing a richer
exploratory visually stimulated FMRI study, Obesity (Silver Spring) 16 (2008)
model for stopping inappropriate responses, Biological Psychiatry 69 (2009)
[21] G.H. Glover, Deconvolution of impulse response in event-related BOLD fMRI,
[2] S. Asahi, Y. Okamoto, G. Okada, S. Yamawaki, N. Yokota, Negative correlation
Neuroimage 9 (1999) 416–429.
between right prefrontal activity during response inhibition and impulsive-
[22] E. Houy, B. Debono, P. Dechelotte, F. Thibaut, Anorexia nervosa associated with
ness: a fMRI study, European Archives of Psychiatry and Clinical Neuroscience
right frontal brain lesion, International Journal of Eating Disorders 40 (2007)
254 (2004) 245–251.
[3] J.R. Augustine, Circuitry and functional aspects of the insular lobe in primates
[23] W.H. Kaye, J.L. Fudge, M. Paulus, New insights into symptoms and neurocircuit
including humans, Brain Research. Brain Research Reviews 22 (1996) 229–244.
function of anorexia nervosa, Nature Reviews Neuroscience 10 (2009) 573–584.
[4] M.S. Beauchamp, K.E. Lee, J.V. Haxby, A. Martin, FMRI responses to video and
[24] J.G. Kerns, J.D. Cohen, A.W. MacDonald 3rd, R.Y. Cho, V.A. Stenger, C.S. Carter,
point-light displays of moving humans and manipulable objects, Journal of
Anterior cingulate conflict monitoring and adjustments in control, Science 303
Cognitive Neuroscience 15 (2003) 991–1001.
(2004) 1023–1026.
[5] B. Biswal, F.Z. Yetkin, V.M. Haughton, J.S. Hyde, Functional connectivity in the
[25] P. Monteleone, E. Castaldo, M. Maj, Neuroendocrine dysregulation of food
motor cortex of resting human brain using echo-planar MRI, Magnetic Reso-
intake in eating disorders, Regulatory Peptides 149 (2008) 39–50.
nance in Medicine 34 (1995) 537–541.
[26] S. Moreno, C.S. Warren, S. Rodriguez, M.C. Fernandez, A. Cepeda-Benito, Food
[6] C. Bohon, E. Stice, Reward abnormalities among women with full and sub-
cravings discriminate between anorexia and bulimia nervosa. Implications for
threshold bulimia nervosa: a functional magnetic resonance imaging study,
‘success' versus ‘failure' in dietary restriction, Appetite 52 (2009) 588–594.
International Journal of Eating Disorders 44 (2011) 585–595.
[27] H. Myrick, R.F. Anton, X. Li, S. Henderson, D. Drobes, K. Voronin, M.S. George,
[7] S.J. Brooks, O.G. O‘Daly, R. Uher, H.C. Friederich, V. Giampietro, M. Brammer, S.C.
Differential brain activity in alcoholics and social drinkers to alcohol cues:
Williams, H.B. Schioth, J. Treasure, I.C. Campbell, Differential neural responses
relationship to craving, Neuropsychopharmacology 29 (2004) 393–402.
to food images in women with bulimia versus anorexia nervosa, PLoS One 6
[28] H. Ogawa, Gustatory cortex of primates: anatomy and physiology, Neuro-
(2011) e22259.
science Research 20 (1994) 1–13.
[9] A. Cepeda-Benito, D.H. Gleaves, T.L. Williams, S.A. Erath, The development and
[29] E.T. Rolls, C. McCabe, Enhanced affective brain representations of choco-
validation of the state and trait food-craving questionnaires, Behavior Therapy
late in cravers vs. non-cravers, European Journal of Neuroscience 26 (2007)
31 (2000) 151–173.
[10] G. Chen, D.R. Glen, Z.S. Saad, J. Paul Hamilton, M.E. Thomason, I.H. Gotlib, R.W.
[30] S. Santel, L. Baving, K. Krauel, T.F. Munte, M. Rotte, Hunger and satiety in
Cox, Vector autoregression, structural equation modeling, and their synthesis
anorexia nervosa: fMRI during cognitive processing of food pictures, Brain
in neuroimaging data analysis, Computers in Biology and Medicine 41 (2011)
Research 1114 (2006) 138–148.
[31] A. Schienle, A. Schafer, A. Hermann, D. Vaitl, Binge-eating disorder: reward sen-
[11] F.A. Cowdrey, R.J. Park, C.J. Harmer, C. McCabe, Increased neural processing of
sitivity and brain activation to images of food, Biological Psychiatry 65 (2009)
rewarding and aversive food stimuli in recovered anorexia nervosa, Biological
Psychiatry 70 (2011) 736–743.
[32] D.M. Small, R.J. Zatorre, A. Dagher, A.C. Evans, M. Jones-Gotman, Changes in
[12] R.W. Cox, AFNI: software for analysis and visualization of functional mag-
brain activity related to eating chocolate: from pleasure to aversion, Brain 124
netic resonance neuroimages, Computers and Biomedical Research 29 (1996)
(2001) 1720–1733.
[33] M. Trummer, S. Eustacchio, F. Unger, M. Tillich, G. Flaschka, Right hemispheric
[13] A.D. Craig, How do you feel – now? The anterior insula and human awareness,
frontal lesions as a cause for anorexia nervosa report of three cases, Acta Neu-
Nature Reviews Neuroscience 10 (2009) 59–70.
rochirurgica 144 (2002) 797–801, discussion 801.
[14] C.S. Culbertson, J. Bramen, M.S. Cohen, E.D. London, R.E. Olmstead, J.J. Gan, M.R.
[34] R. Uher, T. Murphy, M.J. Brammer, T. Dalgleish, M.L. Phillips, V.W. Ng, C.M.
Costello, S. Shulenberger, M.A. Mandelkern, A.L. Brody, Effect of bupropion
Andrew, S.C. Williams, I.C. Campbell, J. Treasure, Medial prefrontal cortex activ-
treatment on brain activation induced by cigarette-related cues in smokers,
ity associated with symptom provocation in eating disorders, American Journal
Archives of General Psychiatry 68 (2011) 505–515.
of Psychiatry 161 (2004) 1238–1246.
[15] I.E. de Araujo, E.T. Rolls, M.L. Kringelbach, F. McGlone, N. Phillips, Taste-
[35] S. Yaxley, E.T. Rolls, Z.J. Sienkiewicz, Gustatory responses of single neurons
olfactory convergence, and the representation of the pleasantness of flavour,
in the insula of the macaque monkey, Journal of Neurophysiology 63 (1990)
European Journal of Neuroscience 18 (2003) 2059–2068.
[16] J.R. Duann, J.S. Ide, X. Luo, C.S. Li, Functional connectivity delineates distinct
[36] Y. Zhou, M. Liang, L. Tian, K. Wang, Y. Hao, H. Liu, Z. Liu, T. Jiang, Functional dis-
roles of the inferior frontal cortex and presupplementary motor area in stop
integration in paranoid schizophrenia using resting-state fMRI, Schizophrenia
signal inhibition, Journal of Neuroscience 29 (2009) 10171.
Research 97 (2007) 194–205.
Source: http://yonseil.co.kr/doc/Neuroscience_letters.pdf
Cutaneous Fungal Infections oDermatophytosis - "ringworm" disease of the nails, hair, and/or stratum corneum of the skin caused by fungi called dermatophytes. oDermatomycosis - more general name for any skin disease caused by a fungus. • Etiological agents are called dermatophytes - "skin plants". Three important anamorphic genera, (i.e., Microsporum, Trichophyton, and Epidermophyton), are involved in ringworm.
COMPANY NOTE Target Estimate Change UK Healthcare Pharmaceuticals September 12, 2013 AstraZeneca PLC (AZN LN) Price target 2,850.00p Respiratory Market 2025: Too Little, Too Late Key Takeaway AstraZeneca does not look well equipped to deal with the evolving respiratorymarket, which will see pressure from new entrants, treatment modalities and








