
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Drug prices to plummet benefitspro
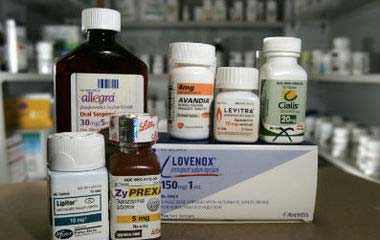
Trending: Consumer Driven Health Care Compliance Legal
Follow us:
Drug prices to plummet
The cost of prescription medicines used by
July 25, 2011
By Linda A. Johnson
mil ions of people every day is about toplummet.
Tags
Core/Group, Consumer Driven
Health Care, Employer-paid
The next 14 months wil bring generic versions
of seven of the world's 20 best-selling drugs,
including the top two: cholesterol fighter Lipitorand blood thinner Plavix.
The magnitude of this wave of expiring drugspatents is unprecedented. Between now and
(AP Photo/Matt Rourke)
2016, blockbusters with about $255 bil ion inglobal annual sales wil go off patent, notesEvaluatePharma Ltd., a London research firm.
Generic competition wil decimate sales of the brand-name drugs and slash the cost to patients and companies that provide healthbenefits.
Top drugs getting generic competition by September 2012 are taken by mil ions every day: Lipitor alone is taken by about 4.3mil ion Americans and Plavix by 1.4 mil ion. Generic versions of big-selling drugs for blood pressure, asthma, diabetes,depression, high triglycerides, HIV and bipolar disorder also are coming by then.
The flood of generics wil continue for the next decade or so, as about 120 brand-name prescription drugs lose market exclusivity,according to prescription benefits manager Medco Health Solutions Inc.
"My estimation is at least 15 percent of the population is currently using one of the drugs whose patents wil expire in 2011 or2012," says Joel Owerbach, chief pharmacy officer for Excel us Blue Cross Blue Shield, which serves most of upstate New York.
Those patients, along with businesses and taxpayers who help pay for prescription drugs through corporate and governmentprescription plans, col ectively wil save a fortune. That's because generic drugs typical y cost 20 percent to 80 percent less thanthe brand names.
Doctors hope the lower prices wil significantly reduce the number of people jeopardizing their health because they can't affordmedicines they need.
Dr. Nieca Goldberg, director of The Women's Heart Program at NYU Langone Medical Center in Manhattan, worries aboutpatients who are skipping checkups and halving pil s to pare costs.
"You can pretty much tel by the numbers when I check the patient's blood pressure or cholesterol levels," that they've not takentheir medications as often as prescribed, she says.
Even people with private insurance or Medicare aren't fil ing all their prescriptions, studies show, particularly for cancer drugswith copays of hundreds of dollars or more.
The new generics wil slice copayments of those with insurance. For the uninsured, who have been paying ful price, the savingswil be much bigger.
Daly Powers, 25, an uninsured student who works two part-time jobs at low wages, says he often can't afford the $220 a monthfor his depression and attention deficit disorder pil s. He couldn't buy either drug in June and says he's struggling with hisSpanish class and his emotions. He looks forward to his antidepressant, Lexapro, going generic early next year.
"It'd make all the difference in the world," says Powers, of Bryan, Texas.
July 25, 2011
By Linda A. Johnson
Generic medicines are chemically equivalent to the original brand-name drugs and work just as wel for
nearly al patients.
Core/Group,Consumer DrivenHealth Care,
When a drug loses patent protection, often only one generic version is on sale for the first six months, so
the price fal s a little bit initial y. Then, several other generic makers typical y jump in, driving prices down
dramatical y.
Last year, the average generic prescription cost $72, versus $198 for the average brand-name drug,according to consulting firm Wolters Kluwer Pharma Solutions. Those figures average all prescriptions, fromshort-term to 90-day ones.
Average copayments last year were $6 for generics, compared with $24 for brand-name drugs given preferred status by aninsurer and $35 for nonpreferred brands, according to IMS Health.
Among the drugs that recently went off patent, Protonix, for severe heartburn, now costs just $16 a month for the generic, versusabout $170 for the brand name. And of the top sellers that soon wil have competition, Lipitor retails for about $150 a month, Plavixcosts almost $200 a month and blood pressure drug Diovan costs about $125 a month. For those with drug coverage, their out-of-pocket costs for each of those drugs could drop below $10 a month.
Jo Kelly, a retired social worker in Conklin, Mich., and her husband, Ray, a retired railroad mechanic, each take Lipitor and twoother brand-name medicines, plus some generic drugs. Both are 67, and they land in the Medicare prescription "doughnut hole,"which means they must pay their drugs' ful cost by late summer or early fal each year. That pushes their monthly cost for Lipitorto about $95 each, and their combined monthly prescription cost to nearly $1,100.
Generic Lipitor should hit pharmacies Nov. 30 and cost them around $10 each a month.
"It would be a tremendous help for us financial y," she says. "It would allow us to start going out to eat again."
For people with no prescription coverage, the coming savings on some drugs could be much bigger. Many discount retailers andgrocery chains sell the most popular generics for $5 a month or less to draw in shoppers.
The impact of the coming wave of generics wil be widespread — and swift.
Insurers use systems that make sure patients are switched to a generic the first day it's available. Many
July 25, 2011
By Linda A. Johnson
health plans require newly diagnosed patients to start on generic medicines. And unless the doctor writes"brand only" on a prescription, if there's a generic available, that's almost always what the pharmacist
Consumer DrivenHealth Care,
"A blockbuster drug that goes off patent wil lose 90 percent of its revenue within 24 months. I've seen it
happen in 12 months," says Ben Weintraub, a research director at Wolters Kluwer Pharma Solutions.
The looming revenue drop is changing the economics of the pharmaceutical industry.
In the 1990s, big pharmaceutical companies were wildly successful at creating pil s that mil ions of peopletake every day for long-term conditions, from heart disease and diabetes to osteoporosis and chronic pain. The drugs areenormously profitable compared with drugs that are prescribed for short-term ailments.
The patents on those blockbusters, which were filed years before the drugs went on sale, last for 20 years at most, and manyexpire soon.
In recent years, many drug companies have struggled to develop new blockbuster drugs, despite multibil ion-dollar researchbudgets and more partnerships with scientists at universities and biotech companies. The dearth of successes, partly becausethe "easy" treatments have already been found, has turned the short-term prognosis for "big pharma" anemic.
"The profit dollars that companies used to reinvest in innovation are no longer going to be coming," warns Terry Hisey, lifesciences leader at consultant Deloitte LLP's pharmaceutical consulting business. He says that raises "long-term concerns aboutthe industry's ability to bring new medicines to market."
But pharmaceutical companies can save bil ions when they stop promoting drugs that have new generic rivals, and U.S. drug and
biotech companies are stil spending more than $65 bil ion a year on R&D.
Drug companies have received U.S. approval for 20 drugs this year and expect approval for other important ones the next fewyears. Eventually, those wil help fil the revenue hole.
For now, brand-name drugmakers are scrambling to adjust for the bil ions in revenue that wil soon be lost. Typical y, they raiseprices 20 percent or more in the final years before generics hit to maximize revenue. Some also contract with genericdrugmakers for "authorized generics," which give the brand-name company a portion of the generic sales.
Brand-name companies also are trimming research budgets, partnering with other companies to share drug development costsand shifting more manufacturing and patient testing to low-cost countries.
Pharmaceutical companies have cut about 10 percent of U.S. jobs in four years, from a peak of about 297,000 to about 268,000,according to Labor Department data. Nearly two-thirds of the cuts came in the last 1 1/2 years, partly because of big mergersthat were driven by the need to bulk up drugs in development and boost profits in the short term by cutting costs.
Drug companies also are trying to grow sales by putting more sales reps in emerging markets, such as China and India, and bydiversifying into businesses that get little or no generic competition. Those include vaccines, diagnostic tests, veterinarymedicines and consumer health products.
As the proportion of prescriptions fil ed with generic drugs jumped to 78 percent in 2010, from 57 percent in 2004, annualincreases in prescription drug spending slowed, to just 4 percent in 2010. According to the Generic Pharmaceutical Association,generics saved the U.S. health care system more than $824 bil ion from 2000 through 2009, and now save about $1 bil ion everythree days.
The savings are only going to get greater as our overweight population ages. People who take their medicines regularly oftenavoid costly complications and hospitalizations, says AARP's policy chief, John Rother, which produces even bigger savings thanthe cheaper drugs.
In addition, many patients taking a particular brand-name drug wil defect when a slightly older rival in the same class goesgeneric.
Global sales of Lipitor peaked at $12.9 bil ion in 2006, the year Zocor, an older drug in the statin class that reduces badcholesterol, went generic. Lipitor sales then declined slowly but steadily to about $10.7 bil ion last year. That stil wil make Lipitorthe biggest drug to go generic.
For patients, it's a godsend.
Douglas Torok, 59, of Erie, Pa., now spends nearly $290 every three months for insulin for his Type 2 diabetes, plus four dailypil s — Lipitor, Plavix and two generics — for his blood pressure and cholesterol problems. The $40,000-a-year foundrysupervisor fears not being able to cover the out-of-pocket costs when he retires and doesn't have a generous prescription plan.
In the meantime, once Lipitor and Plavix get generic competition his copayments wil plunge.
"I wil pay $16 for 90 days," says Torok, who hopes to travel more. "It's a big deal for me on my income."
Brand-name drugs going off patent through 2015: http://www.medcohealth.com/art/corporate/anticipatedfirsttime_generics.pdf
Brand-name and generic drug price comparisons: https://www.flrx.com/calculator/generic/advanced_calculator.html
Copyright 2011 Associated Press. All rights reserved. This material may not b e pub lished, b roadcast, rewritten, or redistrib uted.
House approves debt bil ; Senate
Enrol ment lags in
Americans can benefit from a dose of
retirement savings
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
Estimated Dates of Possible First Time Generic/ Rx-to-OTC Market Entry
2009 US Retail Sales:
Brand Name
Generic name
(in millions)
latanoprost $520
Aromasin®
Concerta®
extended-release
triamcinolone nasal
Nasacort AQ®
(previously approved, not
Levaquin®
levofloxacin $1,633
Uroxatral®
alfuzosin extended-
Anzemet®
Zyprexa® and Zyprexa®
Malarone®
atovaquone/proguanil $60
minocycline extended-
Solodyn®
(previously approved,
briefly launched)
Lipitor®
atorvastatin $6,054
Tazorac®
Combivir®
lamivudine/zidovudine $280
Clarinex® & Clarinex-D®
desloratadine and
(planning OTC prior to
generic availability)
Lexapro2
escitalopram $2,557
Seroquel®
quetiapine $3,483
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
2009 US Retail Sales:
Brand Name
Generic name
(in millions)
Gabitril®
rosiglitazone $437
Avandamet®
Avandaryl®
Avalide®
hydrochlorothiazide
Provigil®
Plavix®3
(approved 1/06 and
briefly launched 8/06)
Viramune®
Lescol® and Lescol® XL
ethinyl estradiol/
Femcon® Fe
fenofibrate $1,350
Singulair®
pioglitazone $2,783
levalbuterol inhalation
Xopenex® (not HFA)4
Revatio®
Diovan® and Diovan®
HCT5
Diovan HCT: $1,376
hydrochlorothiazide
ziprasidone $976
Lidoderm®
lidocaine topical patch
Atacand® and Atacand
HCT® (16/12.5 and
Atacand HCT: $73
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
2009 US Retail Sales:
Brand Name
Generic name
(in millions)
32/12.5 strengths)
hydrochlorothiazide
Maxalt® and
Maxalt-MLT®
Maxalt-MLT: $229
Actoplus Met®
extended-release
Opana® ER
(previously approved, not
omega-3-acid ethyl
Valcyte®
valganciclovir $188
zolmitriptan $166
Allegra-D® 24 hr
Fosamax Plus D™
Vivelle-DOT®
estradiol extended-
Rilutek®
(generic approved, not
(generic approved, not
Niaspan®
Advicor®
lovastatin/niacin $80
AcipHex®
(tablets approved but not
Cymbalta®
duloxetine $2,621
Rapamune®
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
2009 US Retail Sales:
Brand Name
Generic name
(in millions)
Micardis® and
Micardis® HCT
Micardis HCT: $198
hydrochlorothiazide
ethinyl estradiol/
Loestrin® 24 Fe7
mesalamine delayed-
Renagel®
Viracept®
esomeprazole $5,551
Celebrex®
celecoxib $1,581
Lunesta®
eszopiclone $805
risedronate $762
capecitabine $294
Teveten® and
Teveten® HCT
hydrochlorothiazide
moxifloxacin $475
Vigamox®
ophthalmic solution
Copaxone®
glatiramer injection
Cipro® HC
hydrocortisone otic
Namenda®
(generic approved
4/14/10, not launched)
Lumigan®
ophthalmic solution
Sustiva®
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
2009 US Retail Sales:
Brand Name
Generic name
(in millions)
Renvela®
Oxytrol®
oxybutynin extended-
Abilify®
aripiprazole $3,583
Avodart®
dutasteride $461
Welchol®
colesevelam $271
Travatan® and
travoprost ophthalmic
Travatan Z®
aspirin/dipyridamole
extended-release
Gleevec®
Androgel®
testosterone gel
Patanol®
olopatadine solution
Combivent®
albuterol/ipratropium
enfuvirtide injection
Coreg CR®
carvedilol extended-
release capsules
ethinyl estradiol/
Ortho Evra®
transdermal system
dexmethylphenidate
Focalin XR®
extended-release
Crestor®
rosuvastatin calcium
Benicar® & Benicar®
Benicar HCT: $583
hydrochlorothiazide
ezetimibe $1,111
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
2009 US Retail Sales:
Brand Name
Generic name
(in millions)
Sandostatin®
octreotide injection
Spectracef®
cefditoren pivoxil
Vytorin®
ezetimibe/simvastatin $1,233
Cancidas®
caspofungin injection
Velcade®
bortezomib injection
Nasonex®
mometasone nasal
ethinyl estradiol/
Nuvaring®
etonogestrel vaginal
Sensipar®
Tarceva®
rivastigmine extended-
Exelon® Patch
Hepsera®
adefovir dipivoxil
Vesicare®
solifenacin $407
Reyataz®
pregabalin $1,566
ibandronate $588
sildenafil $1,001
Revlimid®
lenalidomide $99
Detrol® LA
tolterodine $756
mesalamine delayed-
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
2009 US Retail Sales:
Brand Name
Generic name
(in millions)
exenatide injection
Chantix®
varenicline $493
Crixivan®
Truvada®
tenofovir/emtricitabine $1,039
tiotropium powder for
Spiriva®
Vyvanse®
lisdexamfetamine $776
Thalomid®
thalidomide $172
Trilipix®
Janumet®
metformin/sitagliptin $431
Januvia®
sitagliptin $1,260
Dollar amount reflects combined sales of all strengths and formulations of the product, unless otherwise indicated; source IMS Health 1 Several companies have announced the settlement of patent litigation relating to Desloratadine Tablets, 5 mg (Clarinex). Pursuant to these settlements, the generic manufacturers will have the right to market Desloratadine Tablets, 5 mg, in the U.S. on July 1, 2012, or earlier in certain circumstances. Clarinex and Clarinex D will likely be switched to OTC availability prior to generic availability. 2 Lexapro's '712 patent was originally slated to expire in December 2009, which included an additional six months of pediatric exclusivity. The U.S. Patent & Trademark Office granted the patent an 828-day extension in March 2006. Therefore, the estimated date for generic availability changed to March 2012. A recent patent litigation decision has also sided with Forest, upholding the validity of a key patent on Lexapro and keeping generics off the market until patent expiration in 2012. 3 Generic Plavix was approved 1/20/06 with paragraph IV certification giving Apotex 180-day exclusivity. Apotex launched their generic "at risk" on August 8, 2006. A preliminary injunction was granted on August 31, ordering Apotex to halt its sales of generic Plavix. The judge did not require Apotex to recall clopidogrel that had already
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Estimated dates are subject to change due to patent litigation, additional patents, exclusivities…
been distributed. Bristol-Myers Squibb/Sanofi's patent infringement trial against Apotex
began January 22, 2007.
4 Mylan launched their first-time generic for Xopenex inhalation solution 0.25% with 180
day exclusivity in September 2009. In April 2008, Breath Limited received FDA
approval for their generic Xopenex inhalation solution 0.021%, .0042%, and 0.0103%.
Sepracor settled a patent dispute with them which will allow Breath to market generic
versions of the drug beginning August 2012 with a 180-day exclusive license. In March
2009, Sepracor entered into a Settlement and License Agreement with Teva and Barr.
The agreement permits Barr and Teva to launch generic versions of Xopenex inhalation
solution dosages under terms of a non-exclusive license commencing on February 17,
2013.
5 Diovan received pediatric exclusivity, extending the drug's patent protection in the US
until September 2012.
6 Patent litigation between Teva and Merck is ongoing. The U.S. Court of Appeals for the
Federal Circuit court is reviewing an appeal by Merck. If the Appeals court agrees that
Merck's patent is unenforceable, Teva may launch their generic at any time.
7 Warner Chilcott sued Watson for patent infringement in 2006. A settlement was
reached in January 2009. Warner Chilcott sued Lupin for patent infringement in
September 2009.
8 In October 2007, Teva received FDA approval of its ANDA for Actonel 5 mg, 30 mg,
and 35 mg tablets but did not launch due to ongoing patent litigation. In February 2008,
the U.S. District Court for the District of Delaware found Procter & Gamble's U.S. Patent
No. 5,538,122 covering Actonel to be valid. Teva intends to appeal this decision.
9 Boehringer Ingelheim sued Teva/Barr for patent infringement in July 2007. The
companies announced a settlement agreement in August 2008.
Note: Pulmicort dry powder inhalation and Advair have been removed from the table.
These both contain inhaled corticosteroids, and the FDA has not determined a standard
for bioequivalence for inhaled corticosteroids in multi-dose inhalers (MDIs) or dry
powder inhalers (DPIs). Therefore, generic availability may be significantly delayed. At
this time, there is no estimated date of generic approval.
Information current as of January 2011. Estimated dates are subject to change due to patent
litigation, additional patents, exclusivities…
Medco is a registered trademark of Medco Health Solutions, Inc.
2011 Medco Health Solutions, Inc. All rights reserved.
Source: http://www.1stopbenefits.com/apps/Prescription%20Generic%20Entries%20in%20Market.pdf
Policies of the University of North Texas Health Science Center 14.315 Physician Delegation to Pharmacists under a Drug Therapy Management Protocol Policy Statement. UNTHSC shall require pharmacists and physicians to sign a delegated drug therapy management protocol before pharmacists may provide drug therapy management to patients in a UNT Health practice site.
Calidad de viday esquizofrenia Primera edición, octubre 2008Título: Calidad de vida y Esquizofrenia: . Estudio realizado con la población perteneciente a las Asociaciones de Salud Mental de la Comunidad Autónoma de Madrid© Edita AMAFERuiz Perelló, 7- 28028 MadridTeléf. 91 3612768 - Fax 91 3613001Correo electrónico : [email protected] - www.amafe.org© Para la presente edición:Obra Social Caja MadridPlaza de Celenque, 228013 Madrid© Autores: Mª Teresa Ruiz Jiménez, Juan Pedro Núñez Partido, Rafael Jódar Anchía,Rufino Meana Peón.Fotografías: M.ª Jesús Martínez de MiguelTodos los derechos reservados. Esta publicación no puede ser reproducida, registradao transmitida, parcial o totalmente, en ninguna forma ni por medio alguno sin permiso del Editor.Diseño, preimpresión e impresión:Mk Direct, Lagasca, 135 - 28006 MadridDepósito Legal:





