
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Ast 248, lecture 10
AST 248, Lecture 10
Department of Physics & Astronomy
449 ESS Bldg.
Stony Brook University
The Search for Intelligent Life in the Universe
Abundances of the Elements
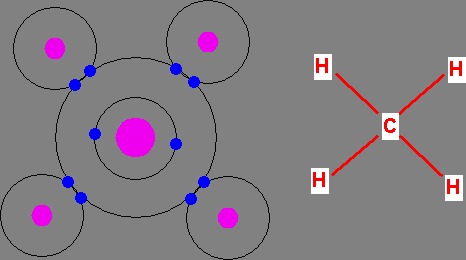
Composition of Biological Compounds
I Organisms are made from long chains of monomers called polymers.
I A monomer is a molecule made from individual atoms.
I An atom is one of the approximately 100 stable elements, or which 4
are ultra-important and 2 more are moderately important.
I The number of bonds are the maximum number of covalent bonds
elements can form. A covalent bond is formed when electrons areshared among atoms.
I Even though some elements can share
more electrons, e.g., P, C has themost flexibility in forming moleculesof different shapes and sizes.
I Most molecules that are carbon-based
are called organic molecules and canbe very complex. Elemental carbon,cabonates, CO2 and cyanides areconsidered inorganic.

Major Types of Organic Biological Molecules
I Lipids (fats and oils) – although polar, are poorly soluble in water, so they
are not found as individual molecules but weakly bonded aggregatesor macromolecules. They store energy and are useful in membranes.
I Carbohydrates – polar, soluble, molecules with many OH hydroxyl groups
attached. Sugars are ring-like carbohydrates, polysaccharides arelinear or branched networks of carbohydrates. Carbohydrates storeenergy and provide structural support.
I Proteins – most complex macromolecules found, linear trains of amino
acids. Like polysaccharides, they polymerize by releasing water.
Provide structure and act as catalysts (called enzymes).
I Nucleic Acids – Largest macromolecules found, are collections of
individual nucleotides linked into linear polymers. Each nucleotideconsists of one sugar, one nitrogeneous base and one or morephosphate groups. Examples: DNA and RNA.
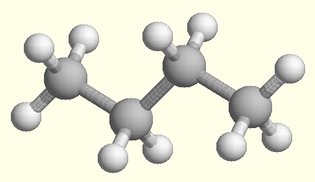
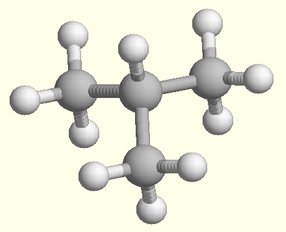
Isomers and Chirality
I When larger groups of atoms are assembled, there may be multiple
ways to bond the same number of atoms. Each of the different waysis called an isomer.
I Some isomers show handedness: these are called stereoisomers and
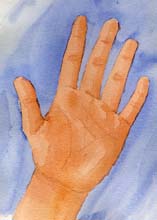
are either left- or right-handed. They are mirror images of eachother. In general, life utilizes only one handedness (e.g., left-handedamino acids are used to make proteins).
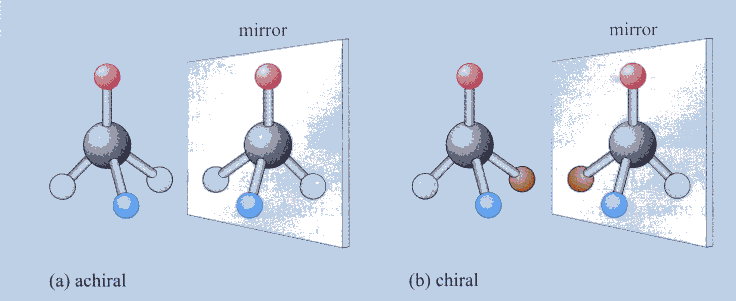
Carbon Isomers and Chirality
I Chirality is the quality that makes handedness unique.
I If less than 4 different things are linked to the central carbon, the
molecule is achiral.
I If 4 different things are linked to the central carbon, the molecule is
I Humans are 9:1 right-handed, but terrestrial biochemistry contains only
left-handed amino acids.
I Meteorites contain amino acids in nearly equal proportions of left- and
right-handed varieties. The ratio is left:right = 1.08:1, and no oneknow why. One possible explanation has to do with circularlypolarized UV light in the stellar association where the Sun formed,as is observed in the Orion nebula.
I Left-and right-handed molecules can have very different properties.
Drugs especially can be affected. Some examples are:
I Orange and lemon peels get aromas from limonene, but it is left-handed in
oranges and right-handed in lemons, which obviously smell different.
I The molecule carvone makes the smells of mint (left) and caraway (right).
I The drug ibuprofen, if left-handed, is 4 times as strong as the other kind.
I The sedative Darvon is an isomer of the cough medicine ingredient Novrad.
I The drug thalidomide is used to control morning sickness, but its mirror
image causes birth defects.
I Ethambutol: one handedness is used to treat tuberculosis, the other causes
I Naproxen: one handedness is used to treat arthritis, but the other causes
liver poisoning.
I Molecular activity can also be affected by handedness. Penicillin kills
only bacteria in cell walls but not the cells themselves because ofhandedness.
Building Blocks and Selectivity
I The largest organic molecules are polymers, or long chains of
monomers, which often containing repeating units or patterns.
I One could view monomers as lego blocks: an infinite variety are
possible, but only a select few are actually used.
I Monomers tend to be simple, because otherwise it might be too
difficult or take too long to form them, either deliberately or bychance.
I Examples: there are 35 isomers of C9H20 and 60 trillion isomers of
I Example of selectivity: About 20 different amino acids are found in
living material although virtually billions are possible to be formed inthe laboratory.
I Another example: Proteins are made of chains of about 100 amino
acids. About 20 different amino acids are used. About 100,000different proteins found in living material although it is possible toform 119!/(100!19!) = 5 · 1021 combinations.
I Selectivity is extended by utilizing only a single handedness.
Kinds of polymers and their monomers:
I proteins – amino acids
I carbohydrates – sugars
I nucleic acids – bases, sugars, phosphates
I lipids – fatty acids
Major Domains of Life
Based on rRNA data, proposed by C. Woese
Differences Between Archaea and Bacteria
I Bacteria and Archaea both have 70S ribosomes, but have different
ribosomal RNA (rRNA) shapes.
I Archaea have 3 RNA polymerases like eukaryotes and their
ribosomes work more like eukaryotes.
I Bacteria have only 1 RNA polymerase.
I Bacteria and Archaea have different types of cell walls.
I Bacteria have cell walls containing peptiodoglycan.
I Archaea have cell walls lacking peptiodoglycan.
I Bacteria and Archae have different cell membranes.
I Bacteria have cell membranes with ester bonds.
I Archae have cell membranes with ether bonds which enclose lipids
with hydrocarbons rather than fatty acids. Monolayers and histonesenhance DNA stability.
Prokaryotes Cells without nuclei
Eukaryotes Cells with nuclei
Major Differences:
I Prokaryotes form bacteria domains
eubacteria and archaea.
I Prokaryotes form single-celled organ-
isms, but can cluster into colonies.
I Euacteria are enclosed by cell walls made of cross-linked peptidoglycan
chains (amino acids + sugar) which maintains size and shape of cell.
I Metabolism in prokaryotes is complex and more diverse.
I Prokaryotic genome is smaller, and its DNA is not attached to histone
I Most prokaryotic DNA is present in a single circular chromosome, and
replication begins at a single point and proceeds around the circle inboth directions.
I Prokaryotes obtain new genes by conjugation (transferral),
transformation (absorbed from environment) and transduction(transferral by viruses or phages.
I Ribosomes of eubacteria differ in molecular detail from those of
eukaryotes and archaea prokaryotes.
I Chemical processses that provide energy
and nutrients to cells. Without cell'spresence, these reactions would occur tooslowly to be useful. Cell's primarypurpose is to speed up these reactions.
I Metabolism requires both sources of
raw materials and energy to breakdown old molecules andmanufacture new ones.
I Cells produce a large variety
of products from a verylimited set of startingmaterials, utilizing adiverse array of enzymes.
I Regardless of where energy
comes from, cells utilizethe same molecule (ATP,adenosine triphosphate)to store and releaseenergy. ATP iscompletely recyclable.
Alberts, Bray, Johnson, Lewis, Raff, Roberts, Walter, Garland Publishing: Taylor Francis Group
Metabolic Sources
Heterotroph Organism which gets its carbon by eating (animals, many
microscopic organisms)
Autotroph Organism which gets its carbon from CO2 in the
atmosphere or dissolved in water (most plants)
I Sunlight (photosynthesis) photo-
I Organic (food) chemo-
I Inorganic (chemicals) chemo-
inorganic chemicals (e.g., Fe, S, NH3)
organic compounds
organic compounds
organic compounds
plants, photosynthetic bacteria
extremophile archaea and bacteria
some bacteria and archaea
animals, many microbes
Metabolism and the Implications for Life
Cell structures are a probable basis for extraterrestrial life.
I General nature of metabolic classes implies they apply to
I Any type of complex metabolism requires existence of
some kind of structure that allows carbon and energy tocome together and manufacture or break down molecules.
Water plays key roles and might be universally required.
I Water is a polar solvent, opposite sides have opposite
I Organic chemicals are readily available for reactions by
being dissolved in water if they are polar (hydrophilic asopposed to hydrophobic).
I Water provides the medium
for transporting chemicals toand within cells and trans-
porting waste products away.
I Water is essential in cellular
metabolic reactions, such asthe ATP-ADP cycle.
Source: http://www.astro.sunysb.edu/lattimer/AST248/lec_10.pdf
Il cour da las alps! Ideen, Produkte und Geschichten aus Tschlin – Strada – Martina Ideas, prodots ed istorgias da Tschlin – Strada – Martina Bun TschlinCH-7559 TschlinTel. +41 (0)81 866 33 03 Tschlin, Strada, Martina – Herz der Alpen! Tschlin, Strada, Martina – Il cour da las alps Das Dorf im Dreiländereck wo pfiffige Ideen nur so sprudelnIm untersten Unterengadin im Dreiländereck von Schweiz, Österreich und Italien liegt Tschlin wie auf einem Adlerhorst auf einer wunderschönen Sonnenterrasse hoch über dem Inn. Die zwei Fraktionen Strada und Martina liegen 400m weiter unten, sanft eingebettet ins Tal, direkt am Inn. In Tschlin gedeiht neben guten Einfällen auch die weit herum bekannte, biologische Biera Engiadinaisa. Andere hochwertige Produkte und Dienst-leistungen aus der Gemeinde, zusammengefasst unter dem Label Bun Tschlin, reichen vom hausgemachten Likör, edlem Schafmilchjogurt und Zie-genkäse bis zum Fleisch von schottischen Hochlandrindern.Abseits der grossen Touristenströme und doch in unmittelbarer Nähe des Skizentren Scuol, Samnaun-Ischgl, Nauders-Tirol und Schöneben im Vinschgau ist die Umgebung von Tschlin ein Paradies für Schneesportler. Auch im Sommer kommt der Wanderer von Tschlin aus auf Touren in allen Schwierigkeitsgraden auf seine Kosten. Für das Wohl der Gäste sorgen charmante kleine Pensionen und kreative Restaurants und es gibt einige Sehenswürdigkeiten zu entdecken. Tschlin bietet alles, was das Herz des ruhesuchenden Reisenden begehrt.
Reversal of Reserpine-Induced Orofacial Dyskinesia And Catalepsy by Sida Cordifolia Navneet Khurana, Pushpendra Kumar Jain, Yogesh Pounikar, Shailendra Patil & Asmita Gajbhiye Department of Pharmaceutical Sciences, Dr. Hari Singh Gour Central University, Sagar, Madhya Pradesh, India E-mail : [email protected] Abstract - Reserpine-induced catalepsy is an animal model used to mimic the behavioural symptoms of Parkinson's disease (PD) in experimental animals. The present study was designed to investigate the effect of aqueous and hydro-ethanolic extracts of Sida cordifolia (AESC and EESC respectively), in reserpine-induced orofacial dyskinesia and catalepsy along with lipid peroxidation evaluated by the levels of thiobarbituric acid like reactive substances (TBARS) in rat forebrain. Sida cordifolia is a well know Ayurvedic plant which has been administered anciently for nervous disorders such as hemiplegia, facial paralysis and PD. It also possesses significant in vitro and ex vivo antioxidant activity. Repeated administration of reserpine (1 mg/kg; s.c.) on alternate days (day 1, 3 and 5) for a period of 5 days significantly increased the vacuous chewing movements (VCM), tongue protrusions (TP), orofacial bursts (OB) and catalepsy along with increased forebrain TBARS levels in rats which was dose-dependently reversed by AESC (50, 100 and 250 mg/kg; p.o.) treatment. No significant effect on these behavioural parameters was observed following varying dose (50, 100 and 250 mg/kg; p.o.) treatment of EESC in reserpine treated rats. These findings suggest the involvement of antioxidant activity along with other underlying mechanisms for the ameliorative effect of AESC in reserpine-induced orofacial dyskinesia and catalepsy. It predicts the scope of AESC in the possible treatment of neuroleptic-induced orofacial dyskinesia and PD.












