
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Biology.ucr.edu2
Author's personal copy
Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
Contents lists available at SciVerse ScienceDirect
Pharmacology, Biochemistry and Behavior
Sex differences in cannabinoid receptor-1 (CB1) pharmacology in mice selectivelybred for high voluntary wheel-running behavior
Brooke K. Keeney a, Thomas H. Meek a, Kevin M. Middleton a,b, Loana F. Holness a, Theodore Garland, Jr. a,⁎a University of California, Riverside, Riverside, CA, 92521, USAb California State University San Bernardino, San Bernardino, CA, 92407, USA
The endocannabinoid system (ECS) is involved in regulation of various physiological functions, including
Received 15 June 2011
locomotion, antinociception, emotional states, and motivated behaviors. The ECS has been implicated in
Received in revised form 7 February 2012
regulation of voluntary wheel running in mice via actions at the cannabinoid receptor-1 (CB1). Previously,
Accepted 26 February 2012
we showed that four replicate lines of mice bred for high levels of voluntary wheel running (high-runner
Available online 1 March 2012
or HR lines) sex-specifically (females only) decreased running in response to antagonism of the CB1 receptor,as compared with four unselected Control lines. Here, we administered a CB1 receptor agonist, WIN 55,212-2
(WIN). We predicted that if CB1 activation is involved in the regulation of voluntary wheel running, then HR
mice would show a greater response to CB1 agonism. Following our previous protocols, mice from generation
53 were acclimated to running wheels for 24 days, then received, in random order, either an intra-peritoneal
Experimental evolution
injection of vehicle or a low (0.5 mg/kg), medium (1 mg/kg) or high dosage (3 mg/kg) of WIN. Each mouse
received an injection and then experienced two nights without injections, for a total period of 12 days.
Locomotor activity
Response to WIN was quantified as wheel revolutions, time spent running, and average running speed in
Locomotor performance
the 10–120 min immediately following injection. Injection decreased wheel revolutions in all mice, but
male HR mice decreased their running to a greater degree relative to Controls in response to the high dose
Selective breeding
of WIN over the entire period analyzed, whereas HR females showed a differential response relative to
Sex differencesVoluntary exercise
Controls only in the latter 70–120 min post-injection. These results, in conjunction with our previous
study, show that (a) aspects of endocannabinoid signaling have diverged in four lines of mice bred for highlevels of voluntary exercise and (b) male and female HR mice differ from one another in CB1 signaling as itrelates to wheel running.
2012 Elsevier Inc. All rights reserved.
HR mice of both sexes ran at least 70% more revolutions/day thantheir Control counterparts (Swallow et al., 1998). The divergence
Understanding the control of voluntary behavior is one of the
between HR and Control lines eventually reached a plateau at a differ-
greatest challenges for neurobiology. In particular, knowledge of how
ential of approximately +170% (Kolb et al., 2010; Rhodes et al., 2000;
the brain motivates imperative, yet potentially costly behaviors – such
Swallow et al., 2009). Concomitant with increases in voluntary wheel
as voluntary exercise – is of great relevance to an increasingly inactive
running, HR mice have also undergone a shift toward increased levels
human population (Garland et al., 2011a). The literature reflects an
of spontaneous physical activity (SPA) in cages when wheels are
almost endless array of approaches to study the neurobiology of
absent (Malisch et al., 2009; Rhodes et al., 2001). Likewise, when
exercise; we have chosen to use a long-term selection experiment
running wheels were locked, HR mice spent more time climbing in
that targets high levels of voluntary wheel running in mice (Garland
the locked wheels, apparently trying to run (Koteja et al., 1999).
et al., 2011b; Rhodes et al., 2005; Swallow et al., 2009).
In addition to changes in locomotor behavior (see also Girard et al.,
Within this experiment, four independent, genetically closed lines
2001), the selective breeding regimen has led to changes in capacities
of house mice (Mus domesticus) have been selectively bred since 1993
for aerobic exercise (Kolb et al., 2010; Meek et al., 2009), and in various
(>60 generations) on the basis of their voluntary wheel running on
lower-level morphological and physiological traits that may affect
days 5 and 6 of a 6-day trial (High Runner lines, HR), in parallel
endurance capacity (Garland, 2003). For example, HR mice exhibit
with four unselected Control lines. After 10 generations of selection,
reduced total body mass (Swallow et al., 1999), reduced body fat(Meek et al., 2010; Swallow et al., 2001; Vaanholt et al., 2008), moresymmetrical hind limb bones (Garland and Freeman, 2005), higher
⁎ Corresponding author at: Department of Biology, University of California, Riverside,
circulating corticosterone (Girard and Garland, 2002; Malisch et al.,
Riverside, CA, 92521, USA.
E-mail address: [email protected] (T. Garland,).
2008) and adiponectin levels (Vaanholt et al., 2007), as well as
0091-3057/$ – see front matter 2012 Elsevier Inc. All rights reserved.
doi:10.1016/j.pbb.2012.02.017
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
increased plasticity of some traits in response to wheel access (Gomes
receptor was blocked, female HR mice decreased running to a greater
et al., 2009).
degree than male HR mice or those from Control lines. Although that
Although it is currently not well understood to what degree
study was the first to use pharmacology on both sexes of HR mice to
voluntary exercise can be considered a classical motivated behavior
demonstrate possible differences in neural correlates of wheel running,
(Garland et al., 2011a), several studies in HR mice have documented
we have long known that male and female HR mice have responded
divergences in the neural systems traditionally thought to regulate
differently to selective breeding for high wheel running (Garland et al.,
motivation and reward (see also Bronikowski et al., 2004; Belke and
2011b; Keeney et al., 2008). Specifically, female HR mice have evolved
Garland, 2007). For instance, studies found linetype wheel-running
their higher daily running distances almost entirely by increasing the
differences in response to drugs that affect the D1 receptor system
speed at which they run, whereas males have shown increases in both
(but not in the D2 receptor, serotonergic or opioidergic systems) (Li et
the speed and duration of wheel activity (Garland, 2003; Girard et al.,
al., 2004; Rhodes et al., 2001, 2003, 2005; Rhodes et al., 2001; Rhodes
2001; Keeney et al., 2008; Koteja and Garland, 2001; Rezende et al.,
and Garland, 2003). In addition, Fos immunohistochemistry showed
2009; Rhodes et al., 2000; Swallow et al., 1998, 1999).
HR mice to have a greater proportional increase in activity in some
It is well known that males and females of many species may
brain regions implicated in reward and motivation when wheel access
accomplish a given behavior in different ways, often as a result of
is blocked, consistent with a state of withdrawal (Rhodes et al., 2003).
the influence of either androgens or hormones of the estrous cycle. It
Mathes et al. (2010) hypothesized that many of these differences reflect
is not definitively known to what extent such behaviors as voluntary
an overall dysregulation of dopaminergic signaling.
exercise differ by sex, nor how these putative differences manifest in
Alongside alterations in dopaminergic and reward signaling, we
the brain (see Lightfoot, 2008 for review). The HR and their Control
hypothesized that HR mice differ from Controls in their response to
lines offer a unique system in which to explore the neurobiological
drugs that act upon one of the major receptors (cannabinoid receptor-
underpinnings of an "exerciser" phenotype, as well as how these
1; CB1) of the endocannabinoid system (ECS) (Keeney et al., 2008). The
systems may differ between the sexes. Given the literature, and our
ECS is a complex modulatory system, primarily composed of cannabinoid
previous results for this system, we believe that the CB1 receptor is
receptors, their endogenous ligands (endocannabinoids), and proteins
important in the neural control of voluntary exercise in general, and
involved in the synthesis and modification of endocannabinoids.
specifically to the evolution of the HR phenotype. The role of CB1
Although the role of central cannabinoid signaling as mediated by the
transmission in sex-specific voluntary exercise is not yet clear; nor is
CB1 receptor is not fully understood, cannabinoids likely have a natural
it clear if stimulation of this receptor would have similar results by
role in antinociception, memory, the perception of natural rewards, and
sex or by linetype (HR or Control). To further address these questions,
the regulation of complex locomotor outputs (particularly those paired
we administered a CB1 receptor agonist (WIN 55,212-2) to HR and
with rewarding stimuli) (De Chiara et al., 2010; Iversen, 2003).
Control mice of both sexes and observed their subsequent wheel
Much evidence suggests a relationship between endocannabinoid
running (at the time of peak nightly activity). We predicted that if,
signaling and physical activity (see Fuss and Gass, 2010 for review).
as indicated by our previous study (Keeney et al., 2008), there are
Recent discussion has highlighted the tight involvement of central
sex-specific differences in ECS physiology that underlie high levels of
endocannabinoid activity with the expression of motor behavior (El
voluntary wheel running, then male and female HR mice will differ
Manira and Kyriakatos, 2010), and in particular, voluntary running
from their Control counterparts in their wheel-running response to
(Chaouloff et al., 2011). Specific to rodent systems, it has been
CB1 agonism.
suggested that the ECS may regulate wheel-running behavior(Chaouloff et al., 2011; Dubreucq et al., 2010). To that end, Hill et al.
(2010) have shown that wheel running, a form of voluntary exercise
in rodents (Garland et al., 2011a), increases both CB1 signaling andthe concentration of anandamide within the hippocampal formation
of rats. Likewise, mouse synaptic responses to HU210, a selectivecannabinoid CB1 receptor agonist, were greatly potentiated following
The subjects of study were male and female mice (M. domesticus),
7 or 15 days of wheel access (De Chiara et al., 2010). Similarly, two
originally derived from Hsd:ICR stock (Harlan Sprague Dawley,
weeks of voluntary wheel access was found to sensitize CB1
Indianapolis, Indiana, USA). As discussed in detail elsewhere
receptor-mediated inhibition of striatal GABAergic transmission in
(Swallow et al., 1998), four lines were designated for selection for
mice (Rossi et al., 2009). CB1 knockout mice showed less voluntary
high voluntary wheel running on days 5 and 6 of 6-day period of
wheel running over a period of 6 weeks as compared with their
wheel access (High Runner or HR lines), while four additional lines
wildtype counterparts (Dubreucq et al., 2010); however, they did
were maintained without selective breeding to serve as controls for
not differ statistically in locomotion in an activity cage, exploration
random genetic effects, including drift (Control lines). In brief, the
in an open field, or immobility time in the forced swim test. Similarly,
general selection protocol is as follows. Following birth, mice are
Chaouloff et al. (2011) found that male mice lacking CB1 receptors
weighed, toe-clipped for individual identification, and weaned at
display decreased voluntary running when housed with a running
21 days of age. Mice are then housed four/cage/sex by line. At
wheel for several weeks when compared to wild-type littermates
6–8 weeks of age, mice are housed individually in cages with wheel
(attributable to a decrease in the time spent running). In humans,
(1.12 m circumference) access for a 6-day period. Wheel revolutions
parallels between the psychotropic effects of traditional cannabinoid
are recorded daily in 1-minute intervals by a photocell counter
drugs and the positive feelings associated with sustained, endurance-
attached to the wheel. Revolutions are compiled via customized
type exercise has led some to hypothesize that endocannabinoids may
software (San Diego Instruments, San Diego, California, USA). Following
be involved, at least in part, with a so-called "runner's high" sensation
wheel-testing, breeders are selected for the next generation. In the HR
that may help to motivate exercise behaviors (Dietrich and McDaniel,
lines, a male and a female mouse from each family are selected for
2004). Consistent with this hypothesis, Sparling et al. (2003) showed
having the highest total revolutions during days 5 and 6 of the wheel-
that anandamide, the major endogenous ligand of the ECS, is increased
running trial. In the Control lines, a male and a female mouse from
in the circulation following exercise in trained male college students.
each family are chosen without regard to wheel revolutions. Breeders
In the context of our system, we have previously shown that HR
are then randomly paired within each line, with the exception that
mice have a differential and sex-specific wheel-running response to
sibling pairs are not allowed. Throughout the selection process, and in
intraperitoneal (i.p.) injection of a selective CB1 antagonist (SR141716;
all studies described here, mice are maintained on a standard 12-h
Rimonabant) (Keeney et al., 2008). When transmission at the CB1
light/dark cycle, with ad lib access to water and food.
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
Following our previous protocol (Keeney et al., 2008), mice for the
2.3. Statistical analysis
current study were chosen from among those that underwent theroutine 6-day wheel-running trial. Our sample excluded both the
During the course of experimentation, a total of 4 males and 1
highest and lowest runners from each family. Of the remaining
female were eliminated from the sample because of death before or
mice, one male or female was chosen from each family for a total
during experimentation (3 males), or because they were observed
sample size of 96 (48 males and 48 females), equally representing
to exhibit twirling behavior (running in rapid, small, stereotypic
all 8 independently breeding lines (4 HR, 4 Control). Mice were
circles) in their cages (1 male and 1 female). Individuals with injection
allowed 24 days of acclimation to wheels prior to drug testing.
problems, wheel malfunction or injury were excluded from analysis
While with wheel access, mice were maintained on a 12:12 photoperiod
on a night-by-night basis. Thus, 44 males and 47 females were statis-
with lights on at 03:00 h and lights off at 15:00 h. Placement of mice
tically analyzed (total N = 91).
with wheels was randomized with respect to sex and line, and experi-
Statistical analyses were performed using SAS version 9.1 (SAS
menters were blind to sex, line, and linetype (HR or Control). Animal
Institute, Cary, NC, USA). Analyses were first conducted separately
procedures were in accordance with University guidelines and with
by sex. The primary grouping factors were linetype (HR vs. Control)
the National Institutes of Health Guide for the Care and Use of Laboratory
and dose, with replicate line as a random effect nested within linetype.
Individual was the factor for repeated measures, and we assumedcompound symmetry of the residual covariance matrix in SASProcedure Mixed. In this mixed-model analysis of covariance, the
2.2. Drug protocol
degrees of freedom for testing the effect of linetype, relative to line,are always 1 and 6. For dose and the dose *linetype interaction (tested
WIN 55,212-2 (WIN) was obtained from BIOMOL International, LP
relative to the dose *line [linetype] effect), degrees of freedom are 2
(Enzo Life Sciences International, Inc., Plymouth Meeting, PA), and
and 12. This interaction term is of chief interest because, if statistically
then dissolved in a vehicle solution of DMSO (20% final volume),
significant, it indicates a differential response of the HR and Control
Tween-80 (10% final volume), and physiological saline (70% final
lines to the drug dose. Wheel-freeness (a measure of how long each
volume). Vehicle solutions were added in the order listed, with vigorous
wheel rotates following acceleration to a constant velocity) was
vortexing between steps. This vehicle has been used for the delivery of
recorded four days prior to injections and was included as a covariate
WIN in both rats (French, 1997; Hoffman et al., 2003) and mice
in statistical analyses, as was individual age. After inspection of the
(Kochman et al., 2006). This vehicle solution does not by itself influence
residuals from the statistical models, all wheel-running traits were
open-field locomotor behavior in mice (Gerdeman et al., 2008), nor
transformed by raising to the 0.6 power in order to reduce skewness.
extinguish nightly wheel running in mice from the HR and Control
For analyses of proportional responses, all values were log10-
lines (Keeney et al., 2008).
transformed prior to analyses.
All drug solutions were prepared fresh immediately prior to use.
On the 25th night of wheel-access, mice were divided into three
batches to minimize the length of any disturbance during the activeperiod. Batches were randomized by line and sex. At two hours
3.1. Baseline wheel running and effects of vehicle injection
after lights-off (17:00 h), during typical peak wheel-running activity(Girard et al., 2001; Girard and Garland, 2002; Malisch et al., 2008;
For generation 53, 553 mice representative of all eight lines
Rhodes et al., 2003), a single batch received treatments. The total
underwent the standard 6-day wheel test (N= 272 females, 281
injection period for a batch was roughly two hours (17:00 h–19:00 h).
males). Females from the HR lines (10,004 ±913 rev/day; least-
Each mouse in a batch received one of four treatments (vehicle
squares mean ±standard error) ran 3.01-fold more than Control females
injection; low WIN [0.5 mg/kg]; medium WIN [1 mg/kg]; high WIN
(3323 ± 387 rev/day) on days 5 + 6 (p=0.0005). HR males (7126 ±
[3 mg/kg]) via i.p. injections. Doses were chosen based upon review of
490 rev/day) ran 2.7-fold more than Control males (2636 ±493 rev/
the literature, with specific attention to Patel and Hillard (2006).
day) on days 5+ 6 (p=0.0007).
Injection volumes were adjusted for dose and body-mass of the animal.
Results were similar for the subset of males and females used in
Over the entire experimentation period, injection volumes ranged from
the present experiment (47 males and 47 females from the present
0.104 to 0.242 mL for body masses that ranged from 20.7 g to 48.4 g.
study underwent wheel testing; one male and female did not, but
Following the design of Li et al. (2004) and Keeney et al. (2008),
were included as replacements at a later date due to unexpected
each mouse received one treatment per three-night period until
mortality). Females from the HR lines (8749 ± 843 rev/day) ran 2.92-
every individual had been injected with every treatment (12 nights
fold more than Control females (2995 ± 490 rev/day) on days 5 + 6
total), with 72 h between each injection to avoid carryover effects. Six
(p= 0.0011). For males, HR ran 6599 (±549) as compared with 2680
individuals from batch one were initially tested at a higher maximum
(±392) revolutions/day for Control, yielding a 2.46-fold differential
dose level (10 mg/kg WIN) but did not display any wheel running (or
(p= 0.0011). As expected, Online Supplemental Figure A shows that
activity at all) over the entire testing period. This dose was subsequently
HR mice ran significantly more than C mice in the three days prior to
abandoned, and these individuals received an extra night of injections,
injections. Likewise, females of both linetypes (HR and C) ran more
such that they received all four doses comparable to the rest of the
than males of both linetypes.
sample, making 13 total nights of injections for this subset.
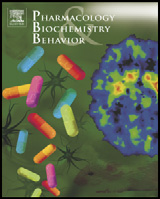
Fig. 1 shows the average wheel-running during the 30 min prior to
Following Keeney et al. (2008), the acute locomotor response to
injections (an hour and forty five minutes past lights off), as well as in
treatment was measured as the total number of wheel revolutions
each 10-minute interval during the first 10–130 min post-injection.
in the period from 10 to 70 min, and also 70 to 120 min post-
Prior to injections, females from the HR lines (273 ± 18 rev/10 min)
injection (not analyzed in our previous study). These time points are
were running 2.95-fold more than Control females (94 ± 18)
consistent with the known time course of WIN in mice (Spina et al.,
(p= 0.022). For males, HR ran 252 (±11 rev, p = 0.0001) as compared
1998), as well as injection effects on HR wheel-running (Keeney et
with 95 (±11 rev, p b 0.0001) revolutions/10 min for Control, yielding a
al., 2008; Li et al., 2004). In addition to wheel revolutions, we also
2.65-fold differential (p b 0.0001). Following vehicle injection, a
analyzed the number of 1-minute intervals with at least one revolution
repeated-measures ANCOVA for the difference between the average
(time spent running), the average running speed (revolutions/minute),
wheel-running during the 30 min prior to injection and the average
and the maximum running speed (revolutions in the single highest
wheel-running during the first 10–70 min following injection (with
1-minute interval) for the same time periods.
covariates of age and wheel-freeness) shows that both female HR
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
Fig. 1. Wheel running revolutions in 10-min bins during intraperitoneal WIN 55,212-2 injections (high WIN (3 mg/kg); medium WIN (1 mg/kg); low WIN (0.5 mg/kg)). (First10-min period after injection is omitted.) Values at −15 min are pooled revolutions in the 30-min period before injections. Values are simple means and standard errors. Pointsare centered on the 5-min mid-point (i.e. the point for the 11–20 min bin is located at 15 min), but have been offset slightly for clarity. WIN 55,212-2 reduced wheel running acutelyin all mice, but for males (lower panel) the reduction was significantly greater for High Runner (HR) lines than for Control lines at the highest dose (Table 1, p for linetype × doseinteraction = 0.0004; Online Supplemental Table B).
(−86±13 rev/10 min, p=0.0007) and Control mice (−36±14,
analyzed in our previous study). For female mice in the first
p = 0.0420) significantly decrease their wheel running, with the
10–70 min post-injection, the reduction in wheel revolutions
decrease being significantly greater for HR females (p=0.0424). The
depended on both dose and linetype. Likewise, there was a significant
trends were similar for males, with HR decreasing by 86 rev/10 min
effect of both dose and linetype on the average and maximum speed,
(±17, p=0.0025) and Controls decreasing by 32 rev/10 min (±17 rev,
but only a statistically significant effect of dose for the amount of time
p=0.0975), and the effect of linetype marginally nonsignificant
spent running. Results were similar 70–120 min post-injection for
(p=0.0674). These decreases may be the result of a natural trend for
females, in that there was a significant effect of both dose and linetype
decreasing wheel running over the course of the night (peak running is
on total revolutions run, as well as the average and maximum running
typically reached around 2 h following lights out, e.g., see Girard and
speed. However, unlike the first 10–70 min, in this period there was a
Garland, 2002; Malisch et al., 2009; Rhodes et al., 2001) and/or an effect
significant effect of both dose and linetype on the time spent running,
of the vehicle injections per se. Without a control group that received no
as well as a dose by linetype interaction for both the total revolutions
injections, we cannot separate these possibilities. For proportional
and average running speed. Thus, for up to 70 min, the wheel running
responses (average running 10–70 min following injection divided by
of females from both linetypes is depressed in a similar fashion for all
average running during the 30 min prior to injections), we observed no
doses. However, inspection of Fig. 1 indicates that during the final
difference between HR and Control lines for either females (p=
70–120 min HR females receiving the 1 mg/kg dose gradually
0.5013) or males (p=0.3212).
resumed running to values near vehicle levels, while wheel runningof Control females given this dose remained depressed.
3.2. Drug response
For males, wheel revolutions, as well as average and maximum
speed, depended on dose and linetype (p b 0.05 for all) for all time
Table 1 shows results of the repeated-measures ANCOVAs for the
periods studied (Fig. 2), with higher doses generally depressing
first 10–70 (corresponding to our previous study: Keeney et al.,
wheel running more for both linetypes. Similar to females, only
2008) and also for 70–120 min post-injection (which was not
dose had a significant effect on the time spent running, both in the
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
Table 1Repeated-measures analyses (SAS procedure mixed) of wheel running (binned in 10-min intervals) during 10–70 and 70–120 min following injections with vehicle, low, mediumor high dose of WIN 55,212-2 in males and females.
Trait and transform used
F for interaction
P for interaction
Females (10–70 min)Revolutions 0.6
Average speed (rpm) 0.6
Maximum speed (rpm) 0.6
Females (70–120 min)Revolutions 0.6
Average speed (rpm) 0.6
Maximum speed (rpm) 0.6
Males (10–70 min)Revolutions 0.6
Average speed (rpm) 0.6
Maximum speed (rpm) 0.6
Males (70–120 min)Revolutions 0.6
Average speed (rpm) 0.6
Maximum speed (rpm) 0.6
Time denotes number of 1-minute intervals with at least one revolution; average speed is revolutions/time; maximum speed is revolutions in the single highest 1-minute interval.
Degrees of freedom are 2 and 12 for dose, 1 and 6 for linetype, and 2 and 12 for the dose *linetype interaction. All p-values are for 2-tailed tests. All analyses also included age and
wheel freeness as covariates (results not shown).
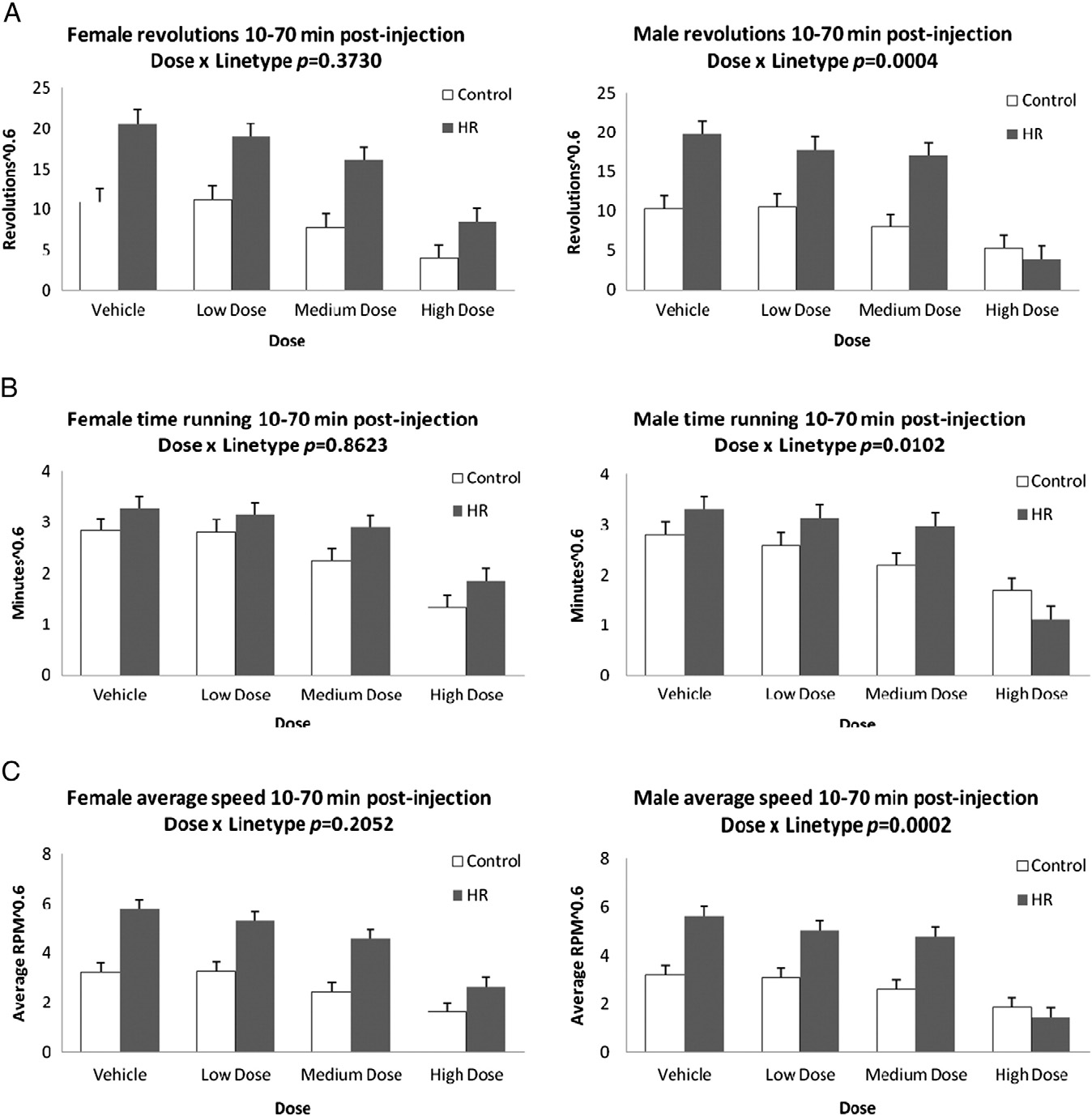
first 10–70 min and in the latter 70–120 min post-injection. Unlike
behavioral endpoints (i.e., similar factorial increase in voluntary
results for females, the reduced wheel running in males was signifi-
wheel running) via at least partially separate mechanisms (Garland
cantly greater for HR lines than for Controls for total revolutions
et al., 2011b).
run, time spent running, as well as average and maximum speed
It is important to note that, as expected from numerous previous
(p b 0.05 for all dose by linetype interactions) in the first 10–70 min
studies (e.g., Garland et al., 2011b; Keeney et al., 2008), HR mice of
post-injection. In addition, males also showed a significant dose by
both sexes differ substantially from Control-line mice in baseline run-
linetype interaction for the total revolutions run and for all measures
ning. In the present study, HR mice ran approximately three-fold
of speed in the 70–120 min post-injection (p b 0.05 for all).
more than Control under baseline conditions (e.g., see Fig. 1), a typicaldifferential. Given this large difference in baseline running, it is possible
that HR and Control mice are operating under considerably differentphysiological regimens during times of peak nightly running, when
Results from the present study show that agonism of the CB1
the present study was conducted. For example, in a given night of
receptor (via i.p. injection of WIN) decreases wheel running in all
wheel running, HR mice could voluntarily approach performances at
groups analyzed. However, male mice from the HR lines decreased
or near their maximal aerobic speed (i.e., almost at their maximal rate
their wheel running to a greater degree in response to WIN, as
of oxygen consumption), unlike the nightly running of typical Control
compared with males from the non-selected Control lines, for both
mice (Girard et al., 2001; Rezende et al., 2005, 2009). Therefore, even
time periods studied (10–70 and 70–120 min post-injection)
nominal increases or decreases in total wheel running during the active
(Table 1, Fig. 2). In contrast, female HR mice had a differential
period could have different physiological consequences for an HR
decrease in wheel running in response to WIN only during the latter
mouse as compared with a Control mouse. These and other differences
time period (70–120 min post-injection). These results complement
between the HR and Control lines (e.g., differences in body fat: Meek et
a prior study, in which we found that female HR mice from generation
al., 2010; Swallow et al., 2001; Vaanholt et al., 2008) may also influence
48 showed altered responsiveness to a selective CB1 receptor antagonist
the pharmacokinetics of WIN. In many cases, exercise is known to alter
(SR141716; Rimonabant) as compared with females from the four non-
the metabolism, absorption, and excretion of drugs (van Baak, 1990),
selected Control lines, while HR males did not differ from their Control
and it is certainly possible that the elevated activity levels of HR mice
counterparts (Keeney et al., 2008). Both the previous and current studies
(or of females relative to males) can affect the dynamics of WIN
show that HR mice differ from Control mice in the magnitude of the
wheel-running response of one sex or the other to the highest dose of
It is of interest that seemingly opposing treatments (Rimonabant
CB1 antagonist or agonist, respectively. In both studies (and sexes),
blocks CB1 receptors, while WIN stimulates them) had negative
this reduction in wheel-running is primarily caused by a decrease in
effects on wheel running in both HR and Control mice. This is consistent
the speed of running, with only HR males during the first 10–70 min of
with other studies of HR mice, which show that to date there has not
WIN injection showing a statistical reduction in the amount of time
been a pharmacological agent that has significantly increased wheel
spent running.
running in HR mice (Rhodes et al., 2001; Rhodes et al., 2005; Rhodes
These results suggest that over the course of selective breeding,
and Garland, 2003; Li et al., 2004). Indeed, the only substance that has
HR mice have evolved to utilize CB1 signaling in a different way
significantly increased wheel running in HR mice has been a high-fat
than Control-line mice during the performance of voluntary wheel
(Western) diet (Meek et al., 2010), which may have had effects on
running (i.e., voluntary exercise: Garland et al., 2011a). Furthermore,
both exercise abilities and motivational aspects of wheel running.
HR mice have done this in a sex-specific manner. Put differently, our
Despite the direction of the effect of pharmacological manipulation of
results show that male and female HR mice have evolved to similar
the CB1 receptor, there is a sex-specific differential in wheel running
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
between HR and Control mice. Similar to Rhodes and Garland (2003),
of selection. In general, the increased daily running distance of HR
we believe that this differential is of prime interest. If the CB1 receptor
mice is accomplished mainly by speed in female HR mice, but by
is important to the performance of HR wheel running, then we might
both speed and duration of running (to a lesser degree than females)
predict that pharmacological manipulations that perturb the specific
in HR males (Garland, 2003; Girard et al., 2001; Keeney et al., 2008;
function of the receptor, regardless of the type of perturbation (stimula-
Koteja and Garland, 2001; Rezende et al., 2009; Rhodes et al., 2000;
tion or blockade), could potentially interfere with such a specific, and
Swallow et al., 1998, 1999). In recent generations HR males (seem-
ingly at a selection limit) can run for as many minutes per day asHR females (Garland et al., 2011b; Rezende et al., 2009). The rodent
4.1. Sex differences
literature supports a generalized trend for higher levels of locomotoractivity in female rodents. This trend seems particularly true for
Several studies have documented robust sex differences in the
wheel running activity (see Lightfoot, 2008 for review). For instance,
evolution of high levels of voluntary wheel running over the course
Eikelboom and Mills (1988) find that female rats run more than
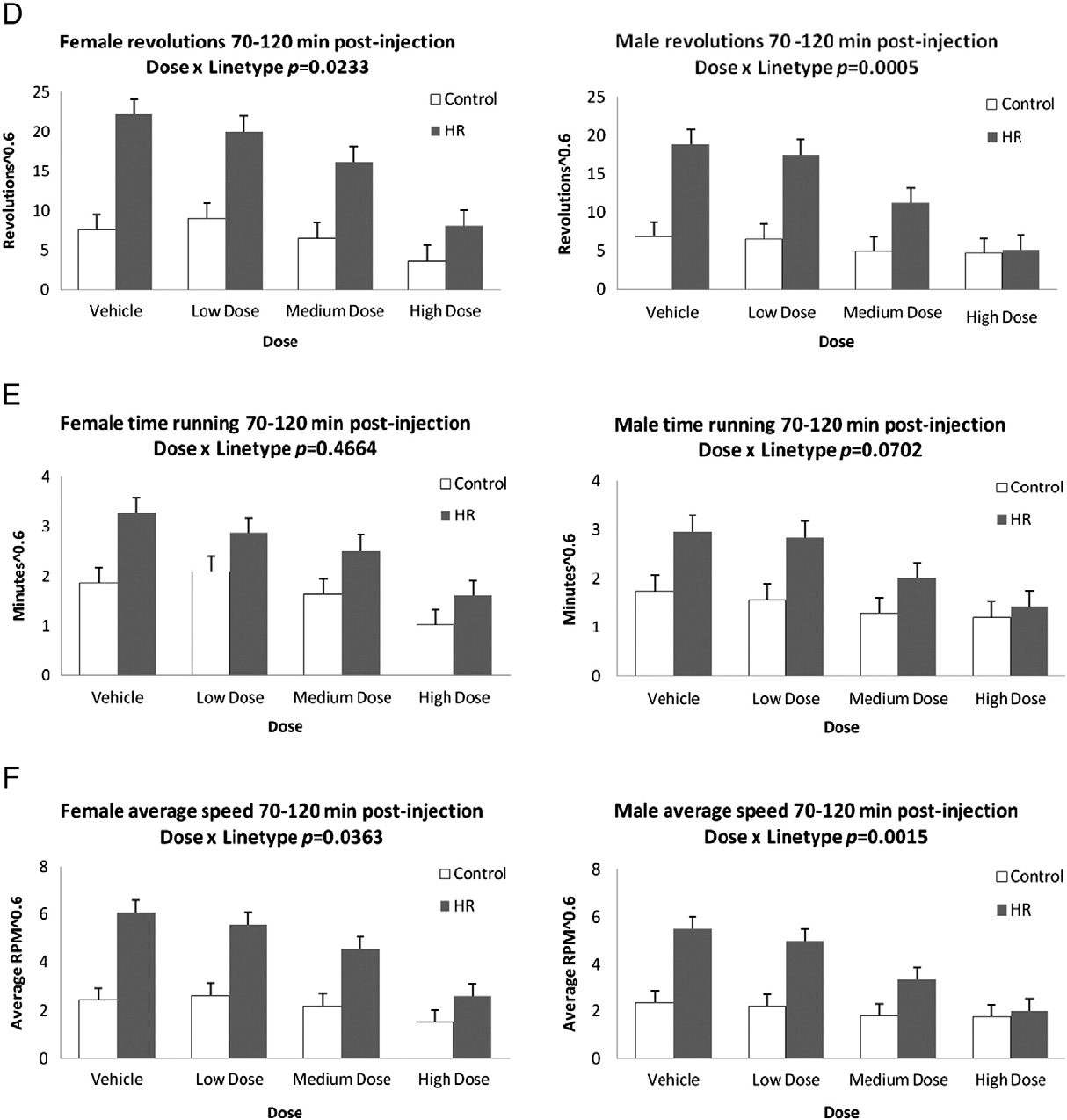
Fig. 2. Least squares means and standard errors from repeated-measures analyses of revolutions, minutes spent running, and revolutions per minute during 10–70 min followinginjection (A,B,C) and 70–120 min following injection (D,E,F) for males and females after injection of the CB1 receptor agonist WIN 55,212-2. Results show a dose by linetype inter-action in the first 10–70 min for total revolutions, time spent running, and average speed of running for males only. During the latter 70–120 min, there is a dose by linetype in-teraction for males and females for total revolutions, as well as average speed of running. Significance levels are presented in Table 1; least squares means and standard errorsare presented in Online Supplemental Table A.
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
Fig. 2 (continued).
males, at a higher speed. Likewise, Konhilas et al. (2004) found that
by pharmacologically blocking CB1 receptors, thus implicating a
female mice of two different strains ran more than male mice of their
modulatory role of estrogen on the ECS. Similarly, although THC and
respective strain, both at higher speeds and for a longer duration.
other cannabinoid agonists are antinociceptive regardless of sex, these
Indeed, Field and Pellis (2008) find significant, overarching differences
effects seem to be stronger in female rats than males (Cohn et al.,
in the ways male and female rats organize their movements across a
1972; for review and other species, see Craft, 2005; Fattore et al., 2008;
wide variety of motor tasks, and suggest that these sex differences in
Tseng and Craft, 2001).
movement are not a byproduct of dimorphisms in body size or shape,
Although it seems evident that female sex hormones can affect
but rather a result of neural differences.
aspects of ECS functioning, there is also evidence that suggests
In the context of our understanding of the voluntary wheel running
male-specific ECS dynamics. For example, Reich et al. (2009) found
of HR mice, it is likely that some of these neural sex differences are
that male rats have higher basal levels of CB1 receptors than females.
associated with the ECS (or factors related to downstream or upstream
Likewise, Miller et al. (2004) showed that CP 55,940, a full agonist at
ECS activity). Several studies suggest that sex-based differences in CB1
both the CB1 and CB2 receptors, increased intake of a highly palatable
signaling are common. For example, Fattore et al. (2007) found that
food reward to a greater degree in male than female rats. In line with
ovarian hormones play a crucial role in the behavioral response of rats
these results, Diaz et al. (2009) found that administration of WIN
to cannabinoids. Similarly, it has been shown in rats that estrogen can
produced a greater degree of hyperphagia in male than female guinea
affect cannabinoid receptor density (Rodriguez de Fonseca et al.,
pigs. It is not yet clear if these dimorphisms represent a clear sex-bias
1994), transcription (González et al., 2000), and signal transduction
in ECS-influenced behaviors (see Fattore and Fratta, 2010 for review).
(Mize and Alper, 2000). Likewise, Hill et al. (2007) reported that the
Given these known sexual dimorphisms in ECS physiology, we
antidepressant effect of estrogen in a rat model of anxiety was prevented
suggest that perhaps the psychotropic effects of ECS activity may
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
play a role in our observations following CB1 receptor agonism and
to have been involved in the development of the HR phenotype.
antagonism in male and female HR mice. As previously mentioned,
Dopamine and endocannabinoids interact (Laviolette and Grace,
it has long been hypothesized that the ECS may contribute to a
2006; Lupica and Riegel, 2005; Maldonado et al., 2006; Pillolla et al.,
pleasurable "runner's high" sensation associated with prolonged
2007), and in some cases, both the ECS and the dopaminergic system
endurance-type exercise (Dietrich and McDaniel, 2004). It has been
have been shown to influence the performance of locomotor behaviors
shown that CB1 signaling can mimic the action of drugs of abuse,
(Beltramo et al., 2000; Giuffrida et al., 1999; Gorriti et al., 2005). Equally
thus producing a rewarding sensation that is capable of conditioning
interesting, but perhaps less expected, is the fact that ECS activity via
behaviors (De Vries and Schoffelmeer, 2005; Maldonado et al., 2006).
both activation and suppression of CB1 transmission results in unique
It is possible that this neurobiological "reward" may motivate, or be
sex differences in running behavior in HR mice. A logical next step
stimulated by, high-intensity (high-speed) running. If it were true that
following both selective CB1 agonism and antagonism would be to
CB1 activity (at least in areas of the brain relevant to the performance
administer mice of both sexes and linetypes an indirect CB1 agonist
of wheel running) is intensity-dependent, then we can predict that
(such as an uptake blocker or FAAH inhibitor). On a more general
relatively high-speed running would be conditioned by the neural
level, it is possible that over the course of selective breeding male and
"pay off" of CB1 activation. Given that HR females tend to run at higher
female HR mice have evolved large-scale differences in how the brain
speeds than males, we would expect females to decrease their wheel
motivates and/or rewards relatively high-speed wheel running.
running to a greater degree when CB1 transmission is blocked, and to
Likewise, although pharmacology can be a useful tool in detecting
a lesser degree (perhaps influenced by the injection itself) when trans-
behavioral correspondences to neural activity, it does not allow us to
mission is stimulated (as this could approach normal CB1 activity during
make quantitative statements about how male and female HR mice
running). This is indeed what we observed (see Table 1), although
might differ with respect to CB1 distribution, regulation or activation
without actual quantification of CB1 dynamics, behavioral observations
in various behavioral contexts. Our two pharmacological tools (receptor
alone are not sufficient evidence of any particular mechanism.
antagonism and agonism) do not fully illuminate the role of the CB1
Of course, it is also possible that the expression or function of the
receptor during HR running. Therefore, additional studies aim to better
CB1 receptor itself is not directly related to promoting voluntary
characterize the involvement of the ECS in voluntary wheel running.
wheel running in HR mice. It has long been understood that receptor
The genetic basis of sex differences in running by HR mice is now
agonist dynamics can affect either a compensatory downregulation or
under study (Hannon et al., 2011; Kelly et al., 2010), and future studies
upregulation of a target receptor protein (Meyer and Quenzer, 2005).
may aim to characterize the extent and nature of how both sexes of HR
Male or female HR mice could have any number of alterations upstream
mice organize and utilize mechanisms of neural reward, with a special
of the CB1 receptors that affect its functionality, including those that
emphasis on an understanding of the dynamics of ECS activity in vivo.
interrupt the synthesis, release or degradation of endocannabinoids. Ifthe ECS is important to network-level neural mechanisms, such as
those that may control overall "motivation" to run, even mutations inindirectly-related genes (e.g. those affecting COX-2, which would in
We thank G. L. Gerdeman for comments on an earlier version of
turn act on 2-arachidonoylglycerol, a common endocannabinoid)
the manuscript and H. Schutz for her help during experimentation.
could have an influence on such a complex phenotype.
Alternately, HR females could be more sensitive to potential negative
Appendix A. Supplementary data
effects of Rimonabant administration (Pacher et al., 2006), while malesare more sensitive to the catalepsy-inducing effects of cannabinoid
Supplementary data to this article can be found online at doi:10.
agonists such as WIN. It is also possible that some or all of these
observed sex differences in the effects of cannabinoids could be due topatterns of drug deposition, as related to differences in body fat. WIN,THC, and other cannabinoids are highly lipophilic, and can be readily
absorbed by fat cells (Nahas et al., 1981). Cortright et al. (1997) found
Belke TW, Garland Jr T. A brief opportunity to run does not function as a reinforcer for
that male rats have a higher percentage of body fat than females,
mice selected for high daily wheel-running rates. J Exp Anal Behav 2007;88:
which led Tseng et al. (2004) to hypothesize that perhaps the behavioral
effects of cannabinoids are less apparent in male rodents due to their
Beltramo M, de Fonseca FR, Navarro M, Calignano A, Gorriti MA, Grammatikopoulos G,
et al. Reversal of dopamine D(2) receptor responses by an anandamide transport
body fat levels. Contrary to this idea, however, male and female HR
inhibitor. J Neurosci 2000;20:3401–7.
mice do not significantly differ in their percentage body fat (Swallow
Bronikowski AM, Rhodes JS, Garland Jr T, Prolla TA, Awad T, Gammie SC. The evolution
et al., 2001), which is very low compared to other common laboratory
of gene expression in the hippocampus in response to selective breeding for in-creased locomotor activity. Evolution 2004;5:2079–86.
strains of mice (Nehrenberg et al., 2009), suggesting that sex differences
Chaouloff F, Dubreucq S, Bellocchio L, Marsicano G. Endocannabinoids and motor be-
in the behavioral response to CB1 manipulation are resultant of more
havior: CB1 receptors also control running activity. Physiology 2011;26(2):76–7.
than just levels of body fat.
Cohn RA, Barratt E, Pirch JH. Differences in behavioral responses of male and female
rats to marijuana. Proc Soc Exp Biol Med 1972;140:1136–9.
Cortright RN, Chandler MP, Lemon PW, DiCarlo SE. Daily exercise reduces fat, protein
and body mass in male but not female rats. Physiol Behav 1997;62:105–11.
Craft RM. Sex differences in behavioral effects of cannabinoids. Life Sci 2005;77:
In summation, our results strongly implicate involvement of the ECS
De Chiara V, Errico F, Musella A, Rossi S, Mataluni G, Sacchetti L, et al. Voluntary exer-
in the performance of wheel running, a type of voluntary exercise in
cise and sucrose consumption enhance cannabinoid CB1 receptor sensitivity in the
rodents (Garland et al., 2011a). When we deconstruct how voluntary
locomotion is accomplished, obviously there is a large contribution of
De Vries TJ, Schoffelmeer ANM. Cannabinoid CB1 receptors control conditioned drug
seeking. Trends Pharmacol Sci 2005;26:420–6.
physical ability; however, of potentially equal importance is an indivi-
Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, et al. Sex differences in the
dual's intrinsic motivation for a potentially taxing and relatively ener-
cannabinoid modulation of appetite, body temperature and neurotransmission at
getically expensive behavior. After more than 50 generations of
POMC synapses. Neuroendocrinology 2009;89:424–40.
Dietrich A, McDaniel WF. Endocannabinoids and exercise. Br J Sports Med 2004;38:
selective breeding for high levels of voluntary wheel running, we have
observed correlated differences in both the ability and seeming "desire"
Dubreucq S, Koehl M, Abrous DN, Marsicano G, Chaouloff F. CB1 receptor deficiency de-
of HR mice to run to on wheels (Garland, 2003; Rhodes et al., 2005;
creases wheel-running activity: consequences on emotional behaviours and hip-pocampal neurogenesis. Exp Neurol 2010;224:106–13.
Belke and Garland, 2007; Swallow et al., 2009; Garland et al., 2011a).
Eikelboom R, Mills R. A microanalysis of wheel running in male and female rats. Physiol
It is interesting that both the dopaminergic system and the ECS seem
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
El Manira A, Kyriakatos A. The role of endocannabinoid signaling in motor control.
Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system
in drug addiction. Trends Neurosci 2006;29:225–32.
Fattore L, Fratta W. How important are sex differences in cannabinoid action? Brit J
Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland Jr T. Circadian pattern of total
and free corticosterone concentrations, corticosteroid-binding globulin, and phys-
Fattore L, Spano MS, Altea S, Angius F, Fadda P, Fratta W. Cannabinoid self-
ical activity in mice selectively bred for high voluntary wheel-running behavior.
administration in rats: sex differences and the influence of ovarian function. Brit
Gen Comp Endocrinol 2008;156:210–7.
J Pharmacol 2007;152:795–804.
Malisch JL, Breuner CW, Kolb EM, Wada H, Hannon RM, Chappell MA, et al. Behavioral
Fattore L, Altea S, Fratta W. Sex differences in drug addiction: a review of animal and
despair and home-cage activity in mice with chronically elevated baseline cortico-
human studies. Womens Health 2008;4:51–65.
sterone concentrations. Behav Genet 2009;39:192–201.
Field EF, Pellis SM. The brain as the engine of sex differences in the organization of
Mathes WF, Nehrenberg DL, Gordon R, Hua K, Garland Jr T, Pomp D. Dopaminergic dys-
movement in rats. Arch Sex Behav 2008;37:30–42.
regulation in mice selectively bred for excessive exercise or obesity. Behav Brain
French ED. Δ9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activa-
tion of cannabinoid CB1 but not opioid receptors. Neurosci Lett 1997;226:159–62.
Meek TH, Lonquich BP, Hannon RM, Garland Jr T. Endurance capacity of mice selective-
Fuss J, Gass P. Endocannabinoids and voluntary activity in mice: runner's high and
ly bred for high voluntary wheel running. J Exp Biol 2009;212:2908–17.
long-term consequences in emotional behaviors. Exp Neurol 2010;224:103–5.
Meek TH, Eisenmann JC, Garland Jr T. Western diet increases wheel running in mice se-
Garland Jr T. Selection experiments: an under-utilized tool in biomechanics and organ-
lectively bred for high voluntary wheel running. Int J Obes 2010;34:960–9.
ismal biology. In: Bels VL, Gasc J-P, Casinos A, editors. Vertebrate biomechanics and
Meyer JS, Quenzer LF. Schizophrenia. In: Meyer JS, Quenzer LF, editors. Psychopharma-
evolution. Oxford, UK: BIOS Scientific Publishers; 2003. p. 23–56.
cology: drugs, the brain and behavior. Sunderland: Sinaver Associates Inc.; 2005.
Garland Jr T, Freeman PA. Selective breeding for high endurance running increases hin-
p. 441–67.
dlimb symmetry. Evolution 2005;59:1851–4.
Miller CC, Murray TF, Freeman KG, Edwards GL. Cannabinoid agonist, CP 55,940, facil-
Garland Jr T, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, et al. The biological
itates intake of palatable foods when injected into the hindbrain. Physiol Behav
control of voluntary exercise, spontaneous physical activity and daily energy ex-
penditure in relation to obesity: human and rodent perspectives. J Exp Biol
Mize AL, Alper RH. Acute and long-term effects of 17-beta estradiol on G(i/o) coupled
neurotransmitter receptor function in the female rat brain as assessed by agonist-
Garland Jr T, Kelly SA, Malisch JL, Kolb EM, Hannon RM, Keeney BK, et al. How to run
stimulated [35S] GTP gamma S binding. Brain Res 2000;859:326–33.
far: multiple solutions and sex-specific responses to selective breeding for high
Nahas G, Leger C, Tocque B, Hoellinger H. The kinetics of cannabinoid distribution and
voluntary activity levels. Proc R Soc B 2011b;278:574–81.
storage with special reference to the brain and testes. J Clin Pharmacol 1981;21:
Gerdeman GL, Schechter JB, French ED. Context-specific reversal of cocaine sensitiza-
tion by the CB(1) cannabinoid receptor antagonist rimonabant. Neuropsychophar-
Nehrenberg DL, Hua K, Estrada-Smith D, Garland Jr T, Pomp D. Voluntary exercise and
its effects on body composition depend on genetic selection history. Obesity
Girard I, Garland Jr T. Plasma corticosterone response to acute and chronic voluntary
exercise in female house mice. J Appl Physiol 2002;92:1553–61.
Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of
Girard I, McAleer MW, Rhodes JS, Garland Jr T. Selection for high voluntary wheel run-
pharmacotherapy. Pharmacol Rev 2006;58:389–462.
ning increases intermittency in house mice (Mus domesticus). J Exp Biol 2001;204:
Patel S, Hillard CJ. Pharmacological evaluation of cannabinoid receptor ligands in a
mouse model of anxiety: further evidence for an anxiolytic role for endogenous
Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopa-
cannabinoid signaling. J Pharmacol Exp Ther 2006;318:304–11.
mine activation of endogenous cannabinoid signaling in dorsal striatum. Nat Neu-
Pillolla G, Melis M, Perra S, Muntoni AL, Gessa GL, Pistis M. Medial forebrain bundle
stimulation evokes endocannabinoid-mediated modulation of ventral tegmental
Gomes FR, Rezende EL, Malisch JL, Lee SK, Rivas DA, Kelly SA, et al. Glycogen storage
area dopamine neuron firing in vivo. Psychopharmacology 2007;191:843–53.
and muscle glucose transporters (GLUT-4) of mice selectively bred for high volun-
Reich CG, Taylor ME, McCarthy MM. Differential effects of chronic unpredictable stress on
tary wheel running. J Exp Biol 2009;212:238–48.
hippocampal CB1 receptors in male and female rats. Behav Brain Res 2009;203:264–9.
González S, Bisogno T, Wenger T, Manzanares J, Milone A, Berrendero F, et al. Sex ste-
Rezende EL, Chappell MA, Gomes FR, Malisch JL, Garland Jr T. Maximal metabolic rates
roid influence on cannabinoid CB(1) receptor mRNA and endocannabinoid levels
during voluntary exercise, forced exercise, and cold exposure in house mice selec-
in the anterior pituitary gland. Biochem Biophys Res Commun 2000;270:260–6.
tively bred for high wheel-running. J Exp Biol 2005;208:2447–58.
Gorriti MA, Ferrer B, del Arco I, Bermúdez-Silva FJ, de Diego Y, Fernandez-Espejo E,
Rezende EL, Gomes FR, Chappell MA, Garland Jr T. Running behavior and its energy cost
et al. Acute delta-9-tetrahydrocannabinol exposure facilitates quinpirole-induced
in mice selectively bred for high voluntary locomotor activity. Physiol Biochem
hyperlocomotion. Pharmacol Biochem Behav 2005;81:71–7.
Hannon RM, Meek TH, Acosta W, Maciel RC, Schutz H, Garland Jr T. Sex-specific heter-
Rhodes JS, Garland Jr T. Differential sensitivity to acute administration of Ritalin, apor-
osis in line crosses of mice selectively bred for high locomotor activity. Behav
morphine, SCH 23390, but not raclopride in mice selectively bred for hyperactive
wheel-running behavior. Psychopharmacology 2003;167:242–50.
Hill MN, Karacabeyli ES, Gorzalka BB. Estrogen recruits the endocannabinoid system to
Rhodes JS, Koteja P, Swallow JG, Carter PA, Garland Jr T. Body temperatures of house
modulate emotionality. Psychoneuroendocrinology 2007;32:350–7.
mice artificially selected for high voluntary wheel-running behavior: repeatability
Hill MN, Titterness AK, Morrish AC, Carrier EJ, Lee TT, Gil-Mohapel J, et al. Endogenous
and effect of genetic selection. J Therm Biol 2000;25:391–400.
cannabinoid signaling is required for voluntary exercise-induced enhancement of
Rhodes JS, Hosack GR, Girard I, Kelley AE, Mitchell GS, Garland Jr T. Differential sensi-
progenitor cell proliferation in the hippocampus. Hippocampus 2010;20:513–23.
tivity to acute administration of cocaine, GBR 12909, and fluoxetine in mice selec-
Hoffman AF, Riegel AC, Lupica CR. Functional localization of cannabinoid receptors and
tively bred for hyperactive wheel-running behavior. Psychopharmacology
endogenous cannabinoid production in distinct neuron populations of the hippo-
campus. Eur J Neurosci 2003;8:524–34.
Rhodes JS, Garland Jr T, Gammie SC. Patterns of brain activity associated with variation
Iversen L. Cannabis and the brain. Brain 2003;126:1252–70.
in voluntary wheel-running behavior. Behav Neurosci 2003;117:1243–56.
Keeney BK, Raichlen DA, Meek TH, Wijeratne RS, Middleton KM, Gerdeman GL, et al.
Rhodes JS, Gammie SC, Garland Jr T. Neurobiology of mice selected for high voluntary
Differential response to a selective cannabinoid receptor antagonist (SR141716:
wheel-running activity. Integr Comp Biol 2005;45:438–55.
rimonabant) in female mice from lines selectively bred for high voluntary wheel-
Rodriguez de Fonseca F, Cebeira M, Ramos JA, Martin M, Fernandez-Ruiz JJ. Cannabi-
running behavior. Behav Pharmacol 2008;19:812–20.
noid receptors in rat brain areas: sexual differences, fluctuations during the estrous
Kelly SA, Nehrenberg DL, Peirce JL, Hua K, Steffy BM, Wiltshire T, et al. Genetic architec-
cycle and changes after gonadectomy and sex steroid replacement. Life Sci
ture of voluntary exercise in an advanced intercross line of mice. Physiol Genomics
Rossi S, De Chiara V, Musella A, Mataluni G, Sacchetti L, Bernardi G, et al. Adaptations
Kochman LJ, dos Santos AA, Fornal CA, Jacobs BL. Despite strong behavioral disruption,
of striatal endocannabinoid system during stress. Mol Neurobiol 2009;39:
Delta 9-tetrahydrocannabinol does not affect cell proliferation in the adult mouse
dentate gyrus. Brain Res 2006;1113:86–93.
Sparling PB, Giuffrida A, Piomelli D, Rosskopf L, Dietrich A. Exercise activates the endo-
Kolb EM, Kelly SA, Middleton KM, Sermsakdi LS, Chappell MA, Garland Jr T. Erythropoietin
cannabinoid system. Neuroreport 2003;14:2209–11.
VO2, max but not voluntary wheel running in mice. J Exp Biol 2010;213:510–9.
Spina E, Trovati A, Parolaro D, Giagnoni G. A role of nitric oxide in WIN 55,212-2 toler-
Konhilas JP, Maass AH, Luckey SW, Ikeda K, Stauffer BL, Olson EN, et al. Sex modifies exer-
ance in mice. Eur J Pharmacol 1998;343:157–63.
cise and cardiac adaptation in the mouse. Am J Physiol Heart Circ 2004;287:H2768–76.
Swallow JG, Carter PA, Garland Jr T. Artificial selection for increased wheel-running be-
Koteja P, Garland Jr T. Forum: response to R. Eikelboom. Anim Behav 2001;61:F25–6.
havior in house mice. Behav Genet 1998;28:227–37.
Koteja P, Garland Jr T, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially
Swallow JG, Koteja P, Carter PA, Garland Jr T. Artificial selection for increased wheel-
selected for high levels of voluntary wheel running. Anim Behav 1999;58:1307–18.
running activity in house mice results in decreased body mass at maturity. J Exp
Laviolette SR, Grace AA. The roles of cannabinoid and dopamine receptor systems in
neural emotional learning circuits: implications for schizophrenia and addiction.
Swallow JG, Koteja P, Carter PA, Garland Jr T. Food consumption and body composition
Cell Mol Life Sci 2006;63:1597–613.
in mice selected for high wheel-running activity. J Comp Physiol B 2001;171:
Li G, Rhodes JS, Girard I, Gammie SC, Garland Jr T. Opioid-mediated pain sensitivity in
mice bred for high voluntary wheel running. Physiol Behav 2004;83:515–24.
Swallow JG, Hayes JP, Koteja P, Garland Jr T. Selection experiments and experimental
Lightfoot JT. Sex hormones' regulation of rodent physical activity: a review. Int J Biol Sci
evolution of performance and physiology. In: Garland Jr T, Rose MR, editors. Exper-
imental evolution: concepts, methods, and applications of selection experiments.
Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a po-
California: University of California Press; 2009. p. 301–51.
tential substrate for cannabinoid receptor antagonist treatment of addiction. Neu-
Tseng AH, Craft RM. Sex differences in antinociceptive and motoric effects of cannabi-
noids. Eur J Pharmacol 2001;430:41–7.
Author's personal copy
B.K. Keeney et al. / Pharmacology, Biochemistry and Behavior 101 (2012) 528–537
Tseng AH, Harding JW, Craft RM. Pharmacokinetic factors in sex differences in D9-
Vaanholt LM, Jonas I, Doornbos M, Schubert KA, Nyakas C, Garland Jr T, et al. Metabolic
tetrahydrocannibinol-induced behavioral effects in rats. Behav Brain Res 2004;154:
and behavioral responses to high-fat feeding in mice selectively bred for high
wheel-running activity. Int J Obes 2008;32:1566–75.
Vaanholt LM, Meerlo P, Garland Jr T, Visser GH, van Dijk G. Plasma adiponectin is in-
van Baak MA. Influence of exercise on the pharmacokinetics of drugs. Clin Pharmacoki-
creased in mice selectively bred for high wheel-running activity, but not by
wheel running per se. Horm Metab Res 2007;39:377–83.
Online Supplemental Table A. Least squares means (all traits transformed by raising to
the 0.6 power) and standard errors from repeated-measures analyses (Table 1) of wheel
running 10-70 and 70-120 minutes following injections, for females and males.
Females 10-70 mins
Females 70-120 mins
Revolutions 0.6
Average Speed 0.6
Maximum Speed 0.6
Males 10-70 mins
Males 70-120 mins
Revolutions 0.6
Average Speed 0.6
Maximum Speed 0.6
All values are means per 10-minute intervals. Time denotes number of 1-minute intervals with at least one revolution; Average Speed is revolutions/time; Maximum Speed is revolutions in the single highest 1-minute interval.
Online Supplemental Table B. Proportional response (dose/vehicle) to WIN 55,212-2 in
the first 10-70 minutes post-injection in females (log10 transformed) and males (log10
transformed). Values are least squares means from SAS Procedure Mixed. Covariates
are wheel freeness and age at time of injection (results not shown).
Females 10-70
Control Standard High
Standard
mins after
Linetype
injection
Revolutions
Average Speed
Maximum Speed
Males 10-70
mins after
injection
Revolutions
Average Speed
Maximum Speed
Online Supplemental Table C. Proportional response (dose/vehicle) to WIN 55,212-2 in
the latter 70-120 minutes post-injection in females (log10 transformed) and males (log10
transformed). Values are least squares means from SAS Procedure Mixed. Covariates
are wheel freeness and age at time of injection (results not shown).
Females 70-120
Standard
Standard
mins after
Linetype
injection
Revolutions
-0.031 0.235 -0.690 0.222 4.110 0.089
0.400 0.248 -0.111 0.239 2.200 0.188
0.446 0.202 0.222 0.191 0.640 0.453
-0.102 0.101 -0.360 0.095 3.460 0.112
0.073 0.097 -0.098 0.093 1.600 0.253
0.166 0.088 0.004 0.084 1.770 0.231
Average Speed
-0.016 0.148 -0.486 0.142 5.210 0.063
0.167 0.136 -0.144 0.131 2.690 0.152
0.207 0.111 0.079 0.105 0.700 0.434
Maximum Speed
-0.010 0.174 -0.484 0.167 3.850 0.097
0.219 0.156 -0.116 0.150 2.370 0.175
0.250 0.129 0.101 0.123 0.700 0.436
Males 70-120
mins after
injection
Revolutions
0.214 0.195 -0.408 0.205 4.770 0.072
0.338 0.226 0.068 0.240 0.660 0.447
0.535 0.243 0.486 0.253 0.020 0.894
-0.002 0.082 -0.241 0.086 4.000 0.093
0.055 0.091 -0.067 0.097 0.840 0.396
0.142 0.096 0.114 0.100 0.040 0.847
Average Speed
0.084 0.115 -0.358 0.120 7.000 0.038
0.115 0.140 -0.100 0.147 1.110 0.333
0.219 0.113 0.197 0.120 0.020 0.897
Maximum Speed
0.118 0.133 -0.356 0.140 5.930 0.051
0.191 0.166 -0.077 0.175 1.220 0.312
0.275 0.133 0.241 0.140 0.030 0.870
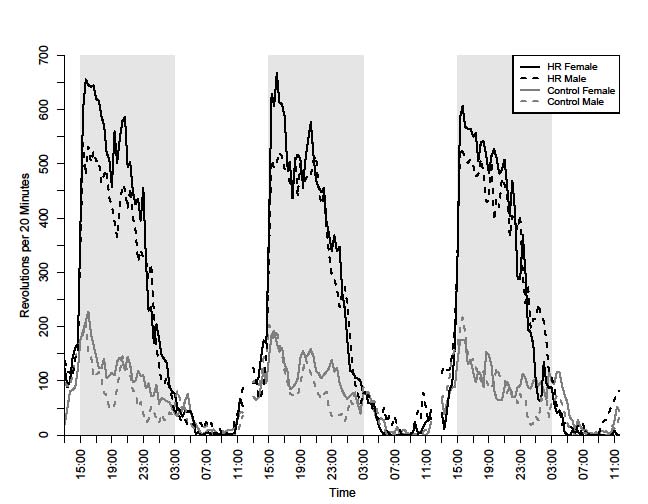
Online Supplemental Figure A. Daily pattern of wheel running (revolutions in 20-min
bins) for mice from selectively bred High Runner (HR) and Control lines during 3 days
before the start of WIN 55,212-2 injections (22 Oct- 24 Oct 2008). Note that females run
more than males in both linetypes. Grey bars indicate lights off.
Source: https://biology.ucr.edu/people/faculty/Garland/Keeney_et_al_2012_sex_differences_in%20cannabinoid_receptor_pharmacology.pdf
Advances in Astronomy and Space Physics, 4, 20-24 (2014) Abundances in the atmosphere of the metal-rich planet-host star HD 77338 I. O. Kushniruk1∗, Ya. V. Pavlenko2,3, J. S. Jenkins4, H. R. A. Jones3 1Taras Shevchenko National University of Kyiv, Glushkova ave., 2, 03127 Kyiv, Ukraine 2Main Astronomical Observatory of the NAS of Ukraine, Akademika Zabolotnoho str., 27, 03680 Kyiv, Ukraine
Stock Report 1-October-2016 Ticker: UCB BBUCB SA S&P Capital IQ Recommendation 12-Mo. Target Price EUR 68.83 (as of 30-September-2016) S&P Capital IQ Equity Analyst Jit Hoong Chan GICS Sector Health Care Summary UCB combines traditional synthetic chemistry and biotechnology approaches in targeting central nervous system (CNS), notably epilepsy, and immune & inflammatory









