
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Manual_cold_fusion_web_cover
Cold Fusion
Cloning Kit
Cat. #s MC010A-1, MC100A-1, MC101A-1
User Manual
Store the master mixture and positive controls at -20˚C
Store the competent cells at -80˚C.
A limited-use label license covers this
product. By use of this product, you
accept the terms and conditions outlined
in the Licensing and Warranty Statement
contained in this user manual.
Cold Fusion™ Cloning Kit
Cat. # MC010A-1, MC 100A-1, MC 101A-1
Introduction . 2
A. Key Features . 2
B. Applications . 2
C. List of Components . 3
D. Storage . 4
E. Other Reagents Needed . 4
F. Technical Information . 4
II. Protocol . 5
A. Overview . 5
B. Preparation of Linearized Vector . 5
C. Primer Design . 6
D. PCR Requirements . 6
E. Cold Fusion Reaction . 6
F. Transformation . 7
III. Examples . 8
A. Primer Design for Positive Control Reaction . 8
B. Cloning a single DNA Fragment . 8
C. Joining Multiple DNA Fragments . 8
IV. Troubleshooting . 9
V. Technical Support . 11
VI. Licensing and Warranty . 11
888-266-5066 (Toll Free)
650-968-2200 (outside US)
System Biosciences (SBI)
I. Introduction
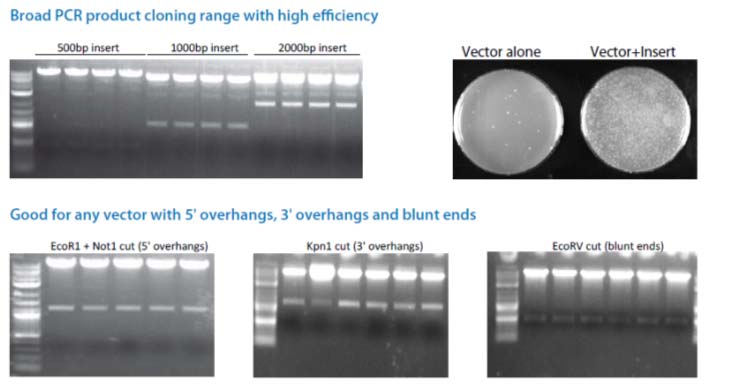
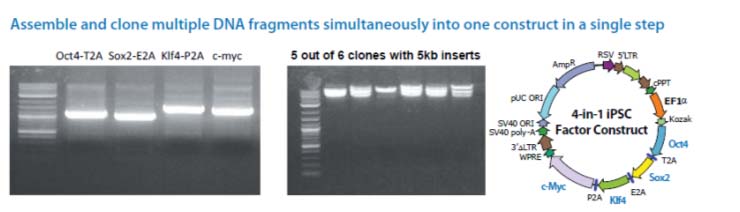
The Cold Fusion technology is a simple, rapid and highly efficient PCR cloning kit. It allows you to directly clone any PCR product(s) to any linearized expression vector, at any site. The PCR fragments can be generated by Taq DNA polymerase or other high fidelity DNA polymerases, with primers that are designed to have 15 bases of homology at the linear ends where the DNA-of-interest will "fuse". The linearized vector can be generated by PCR or restriction enzyme digest (single or double cut). With a one tube simple reaction format, a 5 minute incubation at room temperature, followed by 10 minutes on ice, your PCR product(s) rapidly and accurately fuse into the linearized vector in the desired orientation. The kit is so robust that multiple DNA fragments can be assembled simultaneously and cloned into one construct in a single step. The system is highly efficient, with more than a 95% positive cloning rate.
A. Key Features
Cloning is simple, rapid, accurate and directional Clone any insert, at any site within any vector Restriction enzyme, phosphatase and ligase free system Broad PCR size Joining multiple fragments at once High efficiency with > 95% positive clones
B. Applications
PCR cloning into any vector Gene transfer from one vector to another Add adaptor, linker and tag before or after the insert Gene
High throughput cloning
www.systembio.com
Cold Fusion™ Cloning Kit
Cat. # MC010A-1, MC 100A-1, MC 101A-1
C. List of Components
Cat. No. MC010A-1
Competent cells (1x10 9 cfu /g)
Cat. No. MC100A-1
Competent cells (1x109 cfu /g)
888-266-5066 (Toll Free)
650-968-2200 (outside US)
System Biosciences (SBI)
Cat. No. MC101A-1
Competent cells (1x109 cfu /g)
D. Storage
Store the master mixture and positive controls at -20˚C. Store the competent cells at -80˚C.
E. Other Reagents Needed
Gene-specific
dNTPs, Taq or other high fidelity polymerase, and corresponding buffers for PCR QIAquick PCR Purification Kit (Cat # 28106, Qiagen) QIAquick Gel Extraction kit (Cat # 28704, Qiagen) SOC or LB Broth for transformation of bacteria LB + 100 µg/ ml Ampicillin plates
F. Technical Information
The competent cells provided with the Cold Fusion Cloning Kit has the following genotype:
F' {proAB+ lacIq lacZΔM15 Tn10(TetR) Δ(ccdAB)} mcrA Δ(mrr-hsdRMS-mcrBC) φ80(lacZ)ΔM15 Δ(lacZYA-argF) U169 endA1 recA1 supE44 thi-1 gyrA96 relA1 tonA panD Due to the presence of the Tn10 transposon element, which encodes the tetracycline-resistance gene (TetR), the competent cell will be able to grow in LB-Agar plates containing 10 µg/ml of tetracycline. Cloning of inserts into plasmids containing a TetR marker and transforming the competent cells in the kit will likely lead to the failure of the cloning reaction. In this case, we would recommend another high-quality competent cell that does not have built-in tetracycline resistance (TetR) for best results.
www.systembio.com
Cold Fusion™ Cloning Kit
Cat. # MC010A-1, MC 100A-1, MC 101A-1
II. Protocol
A. Overview
B. Preparation of Linearized Vector
Complete linearization of the vector is critical to achieve a successful Cold Fusion cloning reaction. Incomplete
linearization of the vector will result in high background. The linearized vector can be generated by PCR or restriction
enzyme digest (single or double digest) and should be purified using a gel or PCR purification kit.
Due to the digestion efficiency, different restriction enzymes will generate different levels of background. In general, two enzyme digestion is better than a single enzyme digestion. The further the restriction sites are apart, the better the digestion efficiency. Increasing the enzyme digestion time and the digestion reaction volume will also help reduce the background. For many enzymes, we recommend incubate the digestion reaction between 3 hours and overnight in order to increase linearization and reduce background.
Check the background of your vector by transforming 1g (10-100ng) linearized and purified vector into competent cells. If the background is high, continue digesting the remaining vector for a longer time after the addition of more restriction enzyme(s).
We recommend digesting 2g vector in 50l reaction overnight. Use QIAGEN's QIAquick Spin Gel Extraction kit for gel purification and elute the DNA with 30l dH2O.
888-266-5066 (Toll Free)
650-968-2200 (outside US)
System Biosciences (SBI)
C. Primer Design
Forward Primer Design
The forward primer should contain 18-20 bases complimentary to the 5' end of your gene-of-interest plus 15 bases
corresponding to the vector. Depending on the purpose of your cloning, your primers might also need to contain a
Kozak sequence and/ or ATG start site.
Reverse Primer Design
Reverse primers should be made to the negative strand of DNA. Depending on the vector you are using, the 3' end
of the insert may need to contain a stop codon (TAG, TAA, or TGA). The 3' primer should contain 18-20 bases
complimentary to the 3' end of your gene-of-insert, plus 15 bases corresponding to the vector.
Introducing Restriction Sites
A restriction site can be introduced in the middle of the primer and can be the same as or different than the one used
to linearize the vector. For multiple DNA fragment joining, it is recommended that each PCR product shares 18
base pairs of homology.
D. PCR Requirements
The PCR fragments for the cDNA-of-interest should be generated using
Taq DNA polymerase or other high
fidelity DNA polymerase.
The melting temperature (Tm) should be calculated based on the gene-specific ends of the primer, NOT the
Specific PCR reaction conditions should be optimized for the cDNA-of-interest. After completion of the PCR reaction, gel purify the appropriate band to remove any extra primers or primer
dimmers that will inhibit the Cold Fusion reaction. We recommend the QIAquick PCR Purification Kit (Cat # 28106, Qiagen).
E. Cold Fusion Reaction
Set up the following reaction in a 1.5 ml sterile reaction tube by mixing the following reagents gently and then spin
down briefly to collect the reagents at the bottom of the tube.
Cloning reaction
Linearized destination vector (10-100ng/l) l* PCR insert(s) (20-200ng/l) (for each PCR Product)
www.systembio.com
Cold Fusion™ Cloning Kit
Cat. # MC010A-1, MC 100A-1, MC 101A-1 5x master mix
Positive control reaction
Linearized vector (positive control)
500bp PCR insert (positive control)
Negative Control
Linearized destination vector (10-100ng/l) l* dH2O
* 2:1 or 1:1 molar ratio of insert: vector works well in the Cold Fusion reaction.
For reactions with larger volumes of vector and insert (>8l of vector + insert), double the amount of reaction buffer and enzyme, and add dH20 for a total volume of 20l.
When using Cold Fusion cloning kit for the first time, we strongly recommend that you perform the positive and negative control reactions in parallel with your Cold Fusion cloning reaction. The positive control 500bp PCR insert and linearized vector provided in the kit have already been purified. There is no treatment needed prior to the cloning reaction.
Cold Fusion Reaction Incubation
1. 5 minutes at room temperature 2. 10 minutes one ice
F. Transformation
1. Add
50l Cold Fusion competent cells to the cloning mixture
2. Incubate on ice for 20 minutes 3. Heat shock at 42˚C for 50 seconds 4. Transfer on ice for 2 minutes 5. Add
250l S.O.C medium or LB broth
6. Incubate at 37˚C for an hour 7. Take
100l culture spread on pre-warmed (37˚C) culture plate containing Ampicillin
8. Incubate the plate at 37˚C overnight
888-266-5066 (Toll Free)
650-968-2200 (outside US)

System Biosciences (SBI)
III. Examples
A. Primer Design for Positive Control Reaction
B. Cloning a single DNA Fragment
C. Joining Multiple DNA Fragments
Note: For multiple DNA fragments cloning, depending on the number and the size of each insert, you may obtain
fewer colonies than those with one or two fragments.
www.systembio.com
Cold Fusion™ Cloning Kit
Cat. # MC010A-1, MC 100A-1, MC 101A-1
IV. Troubleshooting
Probable cause
Solution
Check primer sequences to
ensure that they provide 15
bases of homology with the
region flanking the insertion site.
Optimize your PCR amplification reactions so
that you generate pure PCR
Use a different method to purify your PCR product.
It is imperative to obtain as
high a DNA concentration as
concentration in
possible in your Cold Fusion
Both the PCR product and
the linearized vector should
obtained from product or
linearized vector
Do not add more than 10l of
reaction mixture to 50l of
competent cells. Too much
reaction mixture
reaction mixture inhibits the transformation.
Handle the competent cells gently. Do not re-freeze cells after thawing.
Quality of competent cells
may be tested by
poor handling of
transforming a circular
plasmid to determine cells' competency. Competent cells with a transformation efficiency of 1x109 cfu/ μg are recommended.
Wrong antibiotic
Choose plates with the
appropriate concentration of
888-266-5066 (Toll Free)
650-968-2200 (outside US)
System Biosciences (SBI)
Probable cause
Solution
antibiotic in the
the right antibiotic.
It is critical to remove any
uncut vector prior to use in
linearization of
the Cold Fusion reaction. If
necessary, re-digest your vector and gel purify.
2. Large numbers of
If you insert was amplified
from a plasmid, circular DNA
Contamination of
may have carried through
cloning reaction
purification and contaminated
with plasmid with the cloning reaction. We
recommended gel purifying
your PCR product or
linearizing the template DNA before performing PCR.
Plates are too old Make sure that your antibiotic
plates are fresh. Check the
antibiotic resistance of your
Optimize your PCR reaction
to improve the specificity.
Screen more colonies for the
amplified artifacts correct clones.
www.systembio.com
Cold Fusion™ Cloning Kit
Cat. # MC010A-1, MC 100A-1, MC 101A-1
V. Technical
For more information about SBI products and to download manuals in PDF format, please visit our web site:
http://www.systembio.com
For additional information or technical assistance, please call or email us at:
Phone: (650) 968-2200
(888) 266-5066 (Toll Free)
Fax: (650) 968-2277
E-mail:
General
[email protected]
[email protected]
[email protected]
System Biosciences (SBI) 265 North Whisman Rd. Mountain View, CA 94043
VI. Licensing and Warranty
Limited Use License
Use of the Cold Fusion™ Kit (i.e., the "Product") is subject to the following terms and conditions. If the terms and
conditions are not acceptable, return all components of the Product to System Biosciences (SBI) within 7 calendar
days. Purchase and use of any part of the Product constitutes acceptance of the above terms. Purchase of the
product does not grant any rights or license for use other than those explicitly listed in this Licensing and Warranty
Statement. Use of the Product for any use other than described expressly herein may be covered by patents or
subject to rights other than those mentioned. SBI disclaims any and all responsibility for injury or damage which may
be caused by the failure of the buyer or any other person to use the Product in accordance with the terms and
conditions outlined herein. SBI has pending patent applications related to the Product. For information concerning
licenses for commercial use, contact SBI.
Limited Warranty
SBI warrants that the Product meets the specifications described in the accompanying Product Analysis Certificate. If
it is proven to the satisfaction of SBI that the Product fails to meet these specifications, SBI will replace the Product
or provide the purchaser with a refund. This limited warranty shall not extend to anyone other than the original
purchaser of the Product. Notice of nonconforming products must be made to SBI within 30 days of receipt of the
Product.
SBI's liability is expressly limited to replacement of Product or a refund limited to the actual purchase price. SBI's liability does not extend to any damages arising from use or improper use of the Product, or losses associated with the use of additional materials or reagents. This limited warranty is the sole and exclusive warranty. SBI does not provide any other warranties of any kind, expressed or implied, including the merchantability or fitness of the Product for a particular purpose.
888-266-5066 (Toll Free)
650-968-2200 (outside US)
System Biosciences (SBI)
SBI is committed to providing our customers with high-quality products. If you should have any questions or concerns about any SBI products, please contact us at (888) 266-5066.
2013 System Biosciences (SBI), All Rights Reserved.
www.systembio.com
Key Features
List of Components
Cat. No. MC010A-1
Other Reagents Needed
A. Overview
B. Preparation of Linearized Vector
C. Primer Design
Forward Primer Design
Reverse Primer Design
Introducing Restriction Sites
D. PCR Requirements
Cold Fusion Reaction
Cold Fusion Reaction Incubation
Primer Design for Positive Control Reaction
Cloning a single DNA Fragment
Joining Multiple DNA Fragments
Licensing and Warranty
Limited Use License
Source: http://www.bioscience.co.uk/userfiles/pdf/Cold%20Fusion%20Cloning%20Kit%20Manual.pdf
TEAM ORGAN MARCH 27TH, 2009 Sara Moghaddam-Taaheri 1. SPECIFIC AIMS Though nearly 110,000 people are on the organ transplant waiting list, only 77 people receive organ transplants daily. The time an organ can remain outside the body plays a crucial role in the organ transplantation system. The current viability of hearts is limited to a mere four to six hours, due to the limitations of the common cold storage method. This limitation influences several key decisions, such as where a heart can be transported to, or where the surgery can be conducted. Current methods of cold static storage have reached their limits in storage time due to the extent of ischemia-reperfusion (I/R) injury induced during static cold storage. The extent of reperfusion injury is directly proportional to preservation time in cold storage, and research has shown that with static storage methods, heart storage time will not exceed six hours. This essentially means that the demands of the organ transplant list will not be met.
Ausgegeben zu Bonn am 29. Juni 2000 Gesetz zur Änderung von Vorschriften über die Tätigkeit der Steuerberater (7. StBÄndG) . . . . FNA: neu: 610-10/1; 610-10, 610-1-3, 611-10-14, 610-10-6, 610-10, 610-10-6, 610-10-4, 610-10-5, 610-10-9, 610-10-2GESTA: D045 Gesetz über Fernabsatzverträge und andere Fragen des Verbraucherrechts sowie zur Umstel-lung von Vorschriften auf Euro . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .







