
471-479 pascual.qxd
Acta Neurobiol Exp 2007, 67: 471479
Effects of neonatal maternal deprivation
and postweaning environmental complexity
on dendritic morphology of prefrontal
pyramidal neurons in the rat
Rodrigo Pascual1 and S. Pilar Zamora-León2
1Laboratory of Neuroscience, School of Kinesiology, Faculty of Basic Sciences
and Mathematics, Catholic University of Valparaiso, Avenida Brasil 2950,
Valparaíso, Chile; 2Laboratory of Developmental Neurobiology, Institute of
Basic Science, Catholic University of Maule
Abstract. It has been reported that periodic maternal separation in rats leads to
a variety of endure behavioral, neurochemical and microstructural sequelae
associated with the pathophysiology of anxiety disorders. Since it has been
proposed that these changes might be permanent, we examined whether
environmental complexity aid to recover the structural dendritic impairment
induced by neonatal maternal deprivation in the medial prefrontal cortex of the
rat. In addition, the anxiety-like behavior was assessed in the elevated plus-
maze. Repeated maternal separation between postnatal days 621 (3 hours
daily) significantly reduced the dendritic material in layer II/III pyramidal
neurons and induced anxiety-like behaviors in the elevated plus maze.
Furthermore, environmental stimulation (twice a day, 1 h each) during
12 consecutive days (postnatal days 2335) failed to recover the neuronal and
behavioral disorders induced by neonatal maternal separation. The results
demonstrated that (i) neonatal maternal separation severely altered pyramidal
dendritic outgrowth in close association with anxiety-like behavior assessed in
the elevated plus maze, and (ii) postweaning environmental complexity was
unable to recover neither the prefrontocortical neuronal impairment nor the
novelty-induced anxiety-like behavior triggered by early maternal deprivation.
Correspondence should be
addressed to R. Pascual,
Key words: dendritic impairment, environmental complexity, maternal
separation, medial prefrontal cortex, elevated-plus maze
472 R. Pascual and S.P. Zamora-León
tion may compensate neuronal impairments induced
by neonatal MS. Thus, the objective of the present
Clinical studies have suggested that disruption of the study was to determine (i) whether MS alters dendritic
mother-infant socioemotional bond during the first length and branching of mPFC pyramidal cells, which
year of life constitutes a severe early life stressful are involved in the regulation of complex socio-emo-
experience that may contribute to the pathophysiology tional behaviors, and (ii) if postweaning EC amelio-
of some psychiatric disorders, such as anxiety sub- rates the MS-induced neuronal impairments. In addi-
stance abuse disorders and antisocial behavior (Foley tion, we evaluated the effect of both MS and EC on the
et al. 2004, Lipman et al. 2001, MacMillan et al. 2001). anxiety-like behavior in the elevated plus-maze, the
In addition, several studies performed with laboratory most widely screening assay employed for anxiolytic
animals have clearly demonstrated that repeated mater- and anxiogenic agents in rodents.
nal separation (36 h) during the first 23 weeks of life
have long-term consequences on endocrine, behav-
ioral, and brain development later in life. For example,
maternal separation (MS) in rats showed stress hyper-
Animals and experimental design
reactivity, anxious behavior in the elevated plus-maze
test (EPM), anhedonia, increased ethanol consump-
Adult female Sprague-Dawley rats were mated with
tion, and hyper-reactivity of the hypothalamic-pitu- colony breeder male rats. One week before birth, dams
itary-adrenal (HPA) axis in response to stress (Caldji et were individually housed in standard laboratory
al. 2000, Francis and Meaney 1999, Hofer 1996, Huot Plexiglas cages (40 × 30 × 18 cm), with sawdust as
et al. 2001, Wigger and Neumann 1999). Most of these bedding in an air-conditioned room under standard
behavioral and endocrinal dysfunctions persist laboratory conditions: 12 h light-dark cycle (lights on
throughout adulthood (Daniels et al. 2004). In addtion, at 7:00 AM), 22 ± 2°C, and free access to pellets and
it has been shown that these long-term effects induced water. The date of birth was designated as postnatal
by MS appear to depend upon changes in the structure day 0 (P0). One day after delivery, litters (1014 pups)
and function of the medial prefrontal cortex (mPFC) were cross-fostered and culled to 10 pups each
neurons, which are involved in the regulation of the (5 males and 5 females) and housed together with
stress response (Spencer et al. 2005, Williams et al. a mother in standard rat cages (50 × 30 × 20 cm). Half
2006). In fact, mPFC pyramidal neurons of MS rats of the litters were randomly assigned to the maternal
showed changes in dendritic branching, spine density, separation group (MS,
n=20) and the other half to the
and monoaminergic fiber innervation (Bock et al. mother-reared control group (MR,
n=20). MS pups
2005, Helmeke et al. 2001, Ovtscharoff and Braun were separated every day from their dams for 3 h
2001, Poeggel et al. 2003), indicating that early (1:004:00 PM) from P6 until P21. During that time
adverse socioemotional experiences, such as maternal MS pups were kept individually in small aluminium
deprivation, interfere with the development of neu- foil nests (12 cm diameter, 15 cm height) filled with
ronal and synaptic composition of the mPFC.
clean bedding and placed on a 36°C heating blanket.
On the other hand, several reports in laboratory ani- There was no visual, tactile, olfactory or auditory
mals indicated that environmental complexity (EC) communication between the pups during the depriva-
enhanced the individuals brain and behavioral devel- tion period. After isolation, the pups were placed back
opment. For instance, rats exposed to EC showed an in their home cage. MR pups were left undisturbed,
increase in brain weight, cortical depth (Rosenzweig except for cage cleaning 2 times a weak. At P21, all
and Bennett 1996), dendritic outgrowth (Green et al. animals were weaned and housed in groups of 4 rats
1983), synaptogenesis (Comery et al. 1996) and neuro- per cage under standard laboratory conditions. At P23,
genesis (Olson et al. 2006). Additionally, it has been half of the MS (
n=10) and MR (
n=10) animals were
demonstrated that environmental stimulation during randomly selected (MS-EC and MR-EC groups) and
the postweaning period reverses the effects of MS on transferred to an enriched environment (EC), twice
both HPA axis and behavioral responses to stress a day for 1 h each during 12 consecutive days
(Francis et al. 2002). However, there are no studies (P23P35). The EC consist of a large Plexiglas/alu-
examining whether a similar environmental manipula- minum cage (100 × 100 × 70 cm) with a variety of
Neonatal maternal deprivation 473
Fig. 1. Experimental design. (P1P35) postnatal days; (MR)
maternally-reared animals; (MS) maternally-separated ani-
mals; (MR-EC) maternally-reared animals submitted to
environmental complexity; (MS-EC) maternally-separated
animals submitted to environmental complexity; (EPM) ele-
vated-plus maze.
objects, such as tunnels, shelves, running wheels, lad-
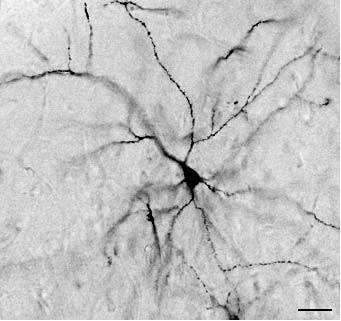
ders, and different kinds of manipulable objects (e.g., Fig. 2. Representative Golgi-impregnated pyramidal neuron
jars, glass balls, wooden objects) that where changed of the rats mPFC. Scale bar is 45 mm.
twice a week in order to avoid habituation. At P36, all
animals were behaviorally evaluated and, at the fol-
Neuronal evaluations
lowing day, sacrificed under deep pentobarbital anes-
thesia (50 mg/kg, i.p.). The experimental design is
In order to study the impact of periodic MS on
shown in Fig. 1.
mPFC pyramidal dendritic development, the brains
All experimental protocols followed guidelines were stained with the Golgi-Cox-Sholl procedure
given at Principles of laboratory animal care (NIH (MR, n=10; MS, n=10; MR-EC, n=10; MS-EC, n=10;
publication No. 86-23, revised 1996) and were Sholl 1953; Fig. 2), technique that stains 15% of ran-
approved by the Institutional Animal Care and Use domly distributed neurons (Pasternak and Woolsey
Committee at Universidad Católica del Maule.
1975). After 60 days of slow impregnation, brains were
dehydrated in ethanol-acetone and ethanol-ether solu-
Elevated plus maze
tions (50%/50%), embedded in celoidin, and hardened
in chloroform vapors. Coronal sections (120 µm thick-
At P36, all animals were evaluated in the elevated ness) were cut using a sledge microtome and mounted
plus maze (EPM). The EPM was made of black with DPX (Fluka).
Plexiglas and consisted of 2 open arms (50 × 15 cm)
In order to qualify for the morphometrical evalua-
and 2 closed arms (50 × 15 × 20 cm). The apparatus tions, pyramidal cells should fulfill the following crite-
was mounted on a fixed base, 50 cm above the floor. ria: (i) have a well-defined pyramidal shape, (ii) show
Each rat was placed in the center of the EPM facing an an adequate staining of the soma and dendrites,
open arm, and was allowed to freely explore the EPM (iii) have no extensive processes overlapping neigh-
for 5 minutes. The number of entries and the time boring neurons, and (iv) be located in a cortical strip
spent in the open arms was recorded. Measures were between 100 and 350 mm under the pial surface
carried out under dim light condition between (2.2 mm and 3.2 mm anterior to bregma; Paxinos and
8:0011:00 PM by 2 observers, who were blind to Watson 1998). The first 1012 cells per brain section
the rearing condition of the pups. The EPM was meeting the above criteria were traced with the aid of
cleaned between each pup with 5% ethanol to remove a camera lucida (Olympus, model BH-DA-LB, 400×
odor cues. This test has been validated in rodents to magnification). Two dendritic variables were quanti-
evaluate anxiety related behaviors (Andreatini and fied: (i) the mean basilar dendritic length/neuron by
Bacellar 2000).
means of an electronic map reader (Pascual et al.
474 R. Pascual and S.P. Zamora-León
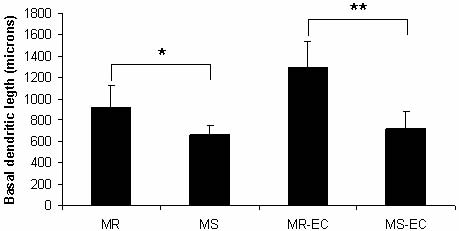
Fig. 4. Effect of maternal separation and environmental
complexity on basal dendritic length. (MR), (MS) maternal-
ly-reared and maternally-separated animals, respectively;
(MR-EC), (MS-EC) maternally-reared and maternally-sepa-
rated animals submitted to environmental complexity,
respectively. *P<0.05, **P<0.01 (means ± SD).
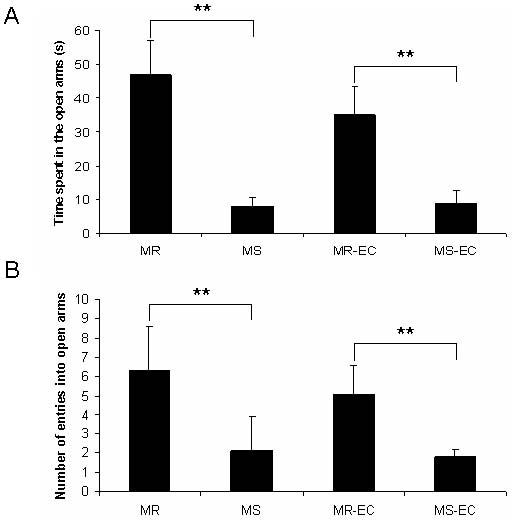
Fig. 3. Effect of maternal separation and environmental
complexity on (A) time spent (s) and (B) number of entries
into the open arms of the elevated-plus maze. (MR), (MS)
maternally-reared and maternally-separated animals, respec-
tively; (MR-EC), (MS-EC) maternally-reared and maternal-
ly-separated animals submitted to environmental complexi-
ty, respectively. **P<0.01 (means ± SD).
Fig. 5. Effect of maternal separation and environmental
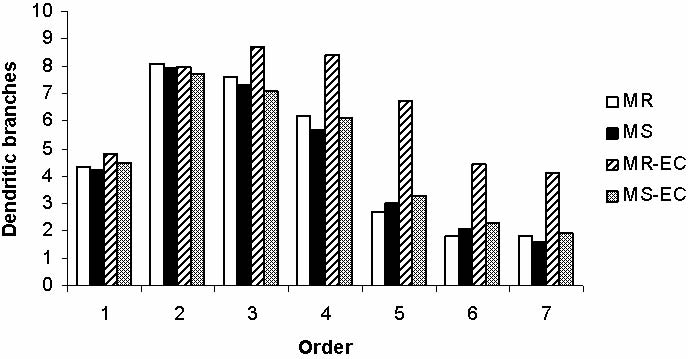
1996), and (ii) the mean basilar dendritic ramifica- complexity on basal dendritic branching. (MR), (MS) mater-
tion/order, determined according to the method of nally-reared and maternally-separated animals, respectively;
Coleman and Riesen (1968). This method states that (MR-EC), (MS-EC) maternally-reared and maternally-sepa-
dendrites coming out directly from the cell body are rated, animals submitted to environmental complexity,
considered as first order, direct branches from first respectively. *P<0.05, **P<0.01.
order dendrites are considered as second order, and so
on. A total of 391 cells were sampled from mPFC (MR: time and did not enter the open arms as many times as
87; MS: 98; MR-EC: 94; MS-EC: 112). All cells were the MR control rats (Fig. 3A and B, respectively;
drawn and analyzed by the same person in a blind man- P<0.01). Unexpectedly, these behavioral alterations
ner to maximize reliability.
appeared to be permanent, since MS environmentally
stimulated animals (MS-EC group) did not differ from
Statistical analysis
MS non-stimulated rats (Fig. 3A and B). On the other
hand, MS rats displayed a decrease in dendritic length
Unpaired two-tailed t-test statistical analyses were compared to the MR control group ( 28%; P<0.05;
performed. Results were expressed as means ± SD and Fig. 4). Again, the EC condition was unable to amelio-
differences were considered significant when P<0.05.
rate the dendritic impairment induced by MS ( 44%;
P<0.01; Fig. 4). Surprisingly, MS did not affect the
number of dendritic branches per neuron (Fig. 5). Only
control rat pyramidal cells submitted to EC condition
Rats exposed to maternal separation (MS group) did (MR-EC group) showed a significant increase in den-
not explore the open arms of the EPM as much as the dritic branching (dendritic orders 47; P<0.05,
control rats (MR group). In addition, MS rats spent less P<0.01; Fig. 5). Those results indicate that post-
Neonatal maternal deprivation 475
vation on mPFC pyramidal cells. Bock and coauthors
(2005) examined the effects of brief and repeated
maternal deprivation periods (P1P3, P5P7, or
P14P16) on dendritic length development in the rats
mPFC. These authors showed, opposite to our results,
that repeated maternal deprivation during the ontoge-
netic stage P5P7 and P14P16 induced a significant
increase in dendritic length of layer II/III pyramidal
neurons. In that report, MS was induced in alternated
and shorter periods (1 h); in our study, the pups were
submitted to MS during continued and prolonged peri-
ods (3 h). This is a critical methodological difference,
since it has been demonstrated that short periods of MS
actually enhance maternal care and leads to an
increased exploratory behavior, less defecation, and
a reduced taste neofobia on handled pups (reviewed in
Sánchez et al. 2001) and a to a more effective hypo-
thalamic-pituitary-adrenal (HPA) axis regulation (Liu
et al. 2000). In addition, short alternating periods of
MS are similar to normal wild-type maternal behavior,
since dams often leave the nest 1530 min to forage
(Jans and Woodside 1990). Therefore, short periods of
MS could enhance dendritic outgrowth (or avoid its
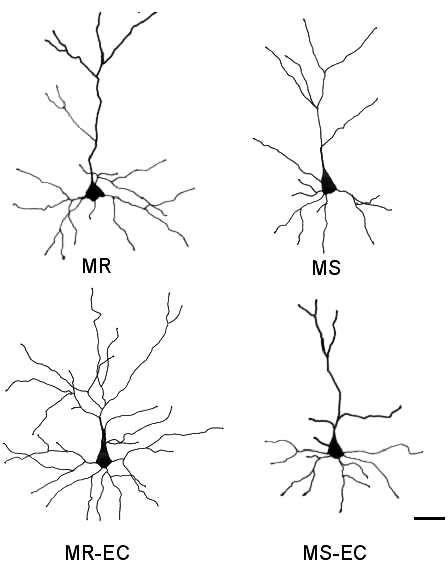
Fig. 6. Representative camera lucida drawings of prefronto-
damage), as reported in the study of Bock and others
cortical layer II/III pyramidal cells. (MR), (MS) maternally-
(2005). On the contrary, longer periods of MS alter
reared and maternally-separated animals, respectively; (MR-
maternal care quality and impair HPA negative feed-
EC), (MS-EC) maternally-reared and maternally-separated
back (Berger et al. 2000, Brake et al. 2004). These
animals submitted to environmental complexity, respective-
findings suggest that postnatal manipulations per-
ly. Scale bar is 50 µm.
formed at different periods can induce opposite effects
on neuronal development.
weaning environmental complexity appears to be
Even though the mechanisms involved in neuronal
unable to offset the neurobehavioral alterations alterations induced by maternal deprivation are not
induced by early maternal separation. Representative well known, we speculate that the endocrine response
camera lucida drawings of mPFC pyramidal cells are induced by stress, and/or changes in neurotrophin
shown in Fig. 6.
expression, could be involved. One of the most well
At weaning, measurements of body weight did not characterized biological features of maternal depriva-
show significant differences between the experimental tion in laboratory animals (McCormick et al. 1998) and
groups (MR: 40.6 ± 4.2; MS: 43.7 ± 3.8; n.s.)
human infants (Buss et al. 2003, Gunnar et al. 2001) is
the hyper-reactivity of the HPA axis in response to
socio-emotional stressful experiences. As a conse-
quence, glucocorticoids (GCs) levels are extremely
We demonstrated that repeated maternal separation elevated (Kalinichev et al. 2002), exerting a broad
during the early preweaning period (P6P21) induced range of deleterious effects on developing GCs recep-
a significant dendritic impairment in mPFC pyramidal tor expressing neurons, i.e., prefrontal (Meaney and
neurons and a significant reduction in the rats explorato- Aitken 1985) or hippocampal pyramidal cells (Meaney
ry behavior in the EPM, alterations that were not attenu- et al. 1988). It has been demonstrated that chronic
ated by postweaning environmental stimulation.
social stress or direct administration of GCs produce
To our knowledge, there is only one report (Bock et dendritic atrophy in prefrontal (Wellman 2001) and
al. 2005) analyzing the impact of early maternal depri- hippocampal (McKittrick et al. 2000) neurons.
476 R. Pascual and S.P. Zamora-León
Alternatively, it has been demonstrated that the adult rats (P90), when the critical dendritic develop-
expression of brain ornithine descarboxylase (ODC), the mental period is currently over (Petit et al. 1988).
first and rate-limiting enzyme in the synthesis of
It is possible that EC, in combination with antide-
endogenous polyamines (Kuhn et al. 1978), is dramati- pressant drugs, may be effective in promoting dendrit-
cally decreased in MR rats. Since ODC is involved in ic outgrowth. This hypothesis is supported by at least
neural growth and differentiation (Shimizu et al. 1965, 2 evidences. First, paroxetine or reboxetine, adminis-
Tabor and Tabor 1984), the reduced ODC activity tered during P21P28, reversed most of the emotional
observed in MS rats could induce dendritic outgrowth abnormalities detected in MS rats (Huot et al. 2001,
abnormalities in specific neuronal groups (Soulet and Ladd et al. 1999). Second, BDNF expression, the
Rivest 2003). On the other hand, since MS significantly major neurotrophin involved in dendritic outgrowth
decreases growth hormone (GH) secretion (Kuhn et al. and plasticity (McAllister 1999), is increased in
1978), and GH can regulate neuronal differentiation response to EC (Spires et al. 2004) and antidepressants
(Turnley 2005), it is possible that the dendritic out- drugs (such as desipramine or phenelzine) (Dias et al.
growth impairment observed in the current study could 2003). Accordingly, it is possible that the administra-
be induced by low GH levels in the brain. Finally, since tion of antidepressants drugs combined with EC could
dendritic outgrowth is regulated by the action of brain- attenuate the dendritic impairment induced by neonatal
derived neurotrophic factor (BDNF; McAllister 1999), MS. Further preclinical studies will be performed in
and MS animals express lower levels of BDNF on both order to prove these hypotheses.
prefrontal and hippocampal neurons (Roceri et al. 2002,
The EPM test is widely used to assess anxiety-like
2004; but see Greisen et al. 2005), we cannot discard behaviors and is based on unconditioned responses to
that the downregulation of BDNF expression could a potentially dangerous environment, i.e., the avoid-
induce neuronal atrophy in MS rats. Further preclinical ance of open and novel spaces. We observed, consis-
studies, at the molecular and cellular levels, are required tent with previous reports, that MS rats entered the
in order to prove these pathophysiological relationships. EPM open arms less times compared to MR controls
Environmental complexity (EC) was not effective (Fig. 3; Daniels et al. 2004, Boccia and Pedersen 2001,
recovering both dendritic impairment and exploratory McIntosh et al. 1999, Romeo et al. 2003). The fact that
behavior in the EPM. Since EC is a condition of inan- the anxiety-like behavior continued even after 30-days
imate and social over-stimulation, it is possible that the of resocialization or EC, suggests that early neonatal
social challenge and the novel environment could act MS produced endured behavioral sequelae. Opposite
as a stressful condition in vulnerable animals, such as to our results, Hellemans and coauthors (2004) showed
MS pups. As a consequence, the therapeutic effects that EC significantly ameliorated the anxiety-like
of the EC on dendritic and behavioral parameters behavior induced by postweaning social isolation. In
described in other studies (Green et al. 1983, that report, social deprivation was imposed later in
Rosenzweig and Bennett 1996) could not be effective development (postweaning period) when the environ-
in previously deprived animals. This suggestion is con- mental hostile experiences may be less harmful. In our
sistent with the fact that EC experienced by control rats study, maternal deprivation was imposed during
(MR) significantly promoted mPFC dendritic develop- preweaning, a more vulnerable period. These opposite
ment (see Figs 4 and 5). However, the dramatic neu- results suggest how important and critical is the time
ronal plasticity observed in mPFC neurons of MR ani- when the deprivation period begins.
mals contrasted with previous data reported by Kolb
and colleagues (2003), study that failed to demonstrate
any significant change in mPFC dendritic outgrowth of
rats submitted to EC. The most critical methodological
The present data demonstrated that neonatal mater-
difference between both studies was the age of the ani- nal deprivation impaired postnatal mPFC neuronal
mals at the time of the EC stimulation. We submitted development and caused long-term anxiety disorders.
the rats to EC during the early postweaning period In addition, postweaning environmental enrichment
(P23P35), when mPFC neurons are highly plastic and was unable to ameliorate any of the neurobehavioral
modifiable (Wedzony et al. 2005); in contrast, Kolb variables studied, suggesting that MS induces perma-
and others (2003) performed the EC manipulation in nent neurobehavioral sequelae.
Neonatal maternal deprivation 477
Dias BG, Banerjee SB, Duman RS, Vaidya BA (2003)
Differential regulation of brain derived neurotrophic fac-
This research was supported by Grant FONDECYT
tor transcripts by antidepressant treatments in the adult rat
brain. Neuropharmacology 45: 553563.
Foley DL, Eaves LJ, Wormley B, Silverg JL, Maes HH,
Kuhn J, Riley B (2004) Childhood adversity,monoaminoe oxidase A, genotype, and risk for conduct
Andreatini R, Bacellar LFS (2000) Animal models: trait or
disorder. Arch Gen Psychiatry 61: 738744.
state measure? The test-retest reliability of the elevated
Francis DD, Meaney MJ (1999) Maternal care and the
plus-maze and behavioral despair. Prog Neuropharmacol
development of stress responses. Curr Opin Neurobiol 9:
Biol Psychiat 24: 549560.
Berger MA, Barros VG, Sarchi MI, Tarazi FI, Antonelli MC
Francis DD, Diorio J, Plotsky PM, Meaney MJ (2002)
(2000) Long-term effects of prenatal stress on dopamine
Environmental enrichment reverses the effects of mater-
and glutamate receptors in adult rat brain. Neurochem
nal separation on stress reactivity. J Neurosci 22:
Res 27: 15251533.
Boccia ML, Pedersen SA (2001) Brief vs. long maternal sep-
Green EJ, Greenough WT, Schlumpf BE (1983) Effects of
arations in infancy: contrasting relationships with adult
complex or isolated environments on cortical dendrites of
maternal behavior and lactation levels of aggression and
middle-aged rats. Brain Res 264: 233240.
anxiety. Psychoneuroendocrinology 26: 657672.
Greisen MH, Altar CA, Bolwing TG, Whitehead R,
Bock J, Gruss M, Becker S, Braun K (2005) Experience-
Wörtwein G (2005) Increased adult hippocampal brain-
induced changes of dendritic spine densities in the pre-
derived neurotrophic factor and normal levels of neuro-
frontal and sensory cortex: correlation with developmen-
genesis in maternal separation rats. J Neurosci Res 79:
tal time windows. Cereb Cortex 15: 802808.
Brake WG, Zhang TY, Diorio J, Meaney MJ, Gratton A
Gunnar MR, Morison SJ, Chisholm K, Schuder M (2001)
(2004) Influence of early postnatal rearing conditions on
Salivary cortisol levels in children adopted from
mesocorticolimbic dopamine and behavioural responses
Romanian orphanages. Dev Psychopathol 13: 611628.
to psychostimulants and stressors in adult rats. Eur J
Hellemans KG, Benge LC, Olmstead MC (2004)
Neurosci 19: 18631874.
Adolescent enrichment partially reverses the social iso-
Buss KA, Schumacher MJR, Dolski I, Kalin NH, Goldsmith
lation syndrome. Dev Brain Res 150: 103115.
H, Davidson RJ (2003) Right frontal brain activity, corti-
Helmeke C, Poeggel G, Braun K (2001) Differential emo-
sol, and withdrawal behavior in 6-month-old infants.
tional experience induces elevated spine densities on
Behav Neurosci 117: 1120.
basal dendrites of pyramidal neurons in the anterior cin-
Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ
gulate cortex of Octodon degus. Neuroscience 104:
(2000) The effects of early rearing environment on the
development of GABAA and central benzodiazepine
Hofer MA (1996) On the nature and consequences of early
receptor levels and novelty-induced fearfulness in the rat.
loss. Psychosom Med 58: 570581.
Neuropsychopharmacology 22: 219229.
Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM
Coleman PD, Riesen AH (1968) Environmental effects on
(2001) Development of adult ethanol preference and
cortical dendritic fields. I. Rearing in the dark. J Anat
anxiety as a consequence of neonatal maternal separation
102: 363374.
in Long-Evans rats and reversal with antidepressant
Comery TA, Stamoudis CX, Irwin SA, Greenough WT (1996)
treatment. Psychopharmacology 158: 366373.
Increased density of multiple-head dendritic spines on
Jans J, Woodside BC (1990) Nest temperature: effect of
medium-sized spiny neurons of the striatum in rats reared in
maternal behavior, pup development, and interaction
a complex environment. Neurobiol Learn Mem 66: 9396.
with handling. Dev Psychobiol 23: 529534.
Daniels WM, Pietersen CY, Carstens ME, Stein DJ (2004)
Kalinichev M, Easterling KW, Plotsky PM, Holtzman SG
Maternal separation in rats leads to anxiety-like behavior
(2002) Long-lasting changes in stress-induced corticos-
and a blunted ACTH response and altered neurotransmit-
terone response and anxiety-like behaviors as a conse-
ter levels in response to a subsequent stressor. Metab
quence of neonatal maternal separation in Long-Evans
Brain Dis 19: 314.
rats. Pharmacol Biochem Behav 73: 131140.
478 R. Pascual and S.P. Zamora-León
Kolb B, Gorny G, Soderpalm AH, Robinson TE (2003)
synaptic composition in the infralimbic cortex of
Environmental complexity has different effects on the
Octodon degus. Neuroscience 104: 3340.
structure of neurons in the prefrontal cortex versus the pari-
Pascual R, Hervias MC, Figueroa HR (1996) Effects of
etal cortex or nucleus accumbens. Synapse 48: 149153.
preweaning environmental stimulation on neuronal and
Kuhn CM, Butler SR, Schanberg SM (1978) Selective depres-
behavioral impairment produced by undernutrition. Biol
sion of serum growth hormone during maternal deprivation
Neonate 70: 165172.
in rat pups. Science 201: 10341036.
Pasternak JF, Woolsey TA (1975) On the selectivity of the
Ladd CO, Huot RL, Thrivikraman KV, Plotsky PM (1999)
Golgi-Cox method. J Comp Neurol 160: 307312.
Reversal of the maternal separation phenotype by reboxe-
Paxinos G, Watson Ch (Eds) (1998) The Rat Brain in
tine. Soc Neurosci Abst 25: 1456.
Stereotaxic Coordinates (2nd ed.) Academic Press, San
Lipman EL, MacMillan HL, Boyle MH (2001) Childhood
abuse and psychiatric disorders among single and married
Petit TD, LeBoutillier JC, Gregorio A, Libstug H (1988) The
mothers. Am J Psychiary 158: 7377.
pattern of dendritic development in the cerebral cortex of
Liu D, Caldji C, Sharma S, Plotsky PM, Meaney MJ (2000)
the rat. Dev Brain Res 41: 209219.
Influence of neonatal rearing conditions on stress-induced
Poeggel G, Helmeke C, Abraham A, Schwabe T, Friedrich P,
adrenocorticotropin responses and norepinepherine release
Braun K (2003) Juvenile emotional experience alters synap-
in the hypothalamic paraventricular nucleus. J Neuro-
tic composition in the rodent cortex, hippocampus, and lat-
endocrinol 12: 512.
eral amygdala. Proc Natl Acad Sci U S A100: 1613716142.
MacMillan HL, Fleming JE, Streiner DL, Lin E, Boyle MH,
Roceri M, Hendriks W, Racagni G, Ellenbroek BA, Riva MA
Jamieson E, Duku EK, Walsh CA, Wong MY, Beardslee
(2002) Early maternal deprivation reduces the expression of
WR (2001) Childhood abuse and lifetime psychopathology
BDNF and NMDA receptor subunits in rat hippocampus.
in a community sample. Am J Psychiatry 158: 18781883.
Mol Psychiatry 7: 609616.
McAllister AK (1999) Neurotrophins and synaptic plasticity.
Roceri M, Cirulli F, Pessina C, Peretto P, Racagni G, Riva MA
Annu Rev Neurosci 22: 295318.
(2004) Postnatal repeated maternal deprivation produces
McCormick CM, Kehoe P, Kovacs S (1998) Corticosterone
age-dependent changes in brain-derived neurotrophic factor
release in response to repeated short episodes of neonatal
expression in selected brain regions. Biol. Psychiatry 55:
isolation: evidence of sensitization. Int J Dev Neurosci 16:
Romeo RD, Mueller A, Sisti HM, Ogawa S, McEwen BS,
McIntosh J, Anisman H, Merali Z (1999) Short- and long-
Brake WG (2003) Anxiety and fear behaviors in adult male
periods of neonatal maternal separation differentially affect
and female C57BL/6 mice are modulated by maternal sep-
anxiety and feeding in adult rats: gender-dependent effects.
aration. Horm Behav 43: 561567.
Dev Brain Res 113: 97106.
Rosenzweig MR, Bennett EL (1996) Psychobiology of plas-
McKittrick CR, Magarinos AM, Blanchard DC, Blanchard
ticity: effects of training and experience on brain and behav-
RJ, McEwen BS, Sakai RR (2000) Chronic social stress
ior. Behav Brain Res 78: 5765.
reduces dendritic arbors in CA3 of hippocampus and
Sánchez MM, Ladd ChO, Plostsky PM (2001) Early adverse
decreases binding to serotonin transporter sites. Synapse
experience as a developmental risk factor for later psy-
chopathology: evidence from rodent and primate models.
Meaney MJ, Aitken DH (1985) (3H)-Dexamethasone binding
Dev Psychopathol 13: 419449.
in rat frontal cortex. Brain Res 328: 176180.
Shimizu H, Kakimoto Y, Sano I (1965) Changes in concentra-
Meaney MJ, Aitken DH, van Berkel Ch, Bhatnagar S,
tion of polyamines in the developing mouse brain. Nature
Sapolsky RM (1988) Effect of neonatal handling on age-
207: 11961197.
related impairments associated with the hippocampus.
Soulet D, Rivest S (2003) Polyamines play a critical role in the
Science 239: 766768.
control of the innate immune response in the mouse central
Olson AK, Eadie BD, Ernst C, Christie BR (2006)
nervous system. J Cell Biol 162: 257268.
Environmental enrichment and voluntary exercise mas-
Spencer SJ, Buller KM, Day TA (2005) Medial prefrontral cor-
sively increase neurogenesis in the adult hippocampus via
tex control of the paraventricular hypothalamic nucleus
dissociable pathways. Hippocampus 16: 250260.
response to psychological stress: possible role of the bed
Ovtscharoff W, Braun K (2001) Maternal separation and
nucleus of the stria terminalis. J Comp Neurol 481:
social isolation modulate the postnatal development of
Neonatal maternal deprivation 479
Spires TL, Grote HE, Varshney NK, Cordery PM, van
Wellman CL (2001) Dendritic reorganization in pyrami-
Dellen A, Blakemore C, Hannan AJ (2004)
dal neurons in medial prefrontal cortex after chronic
Environmental enrichment rescues protein deficits in a
corticosterone administration. J Neurobiol 49:
mouse model of Huntington's disease, indicating a possi-
ble disease mechanism. J Neurosci 24: 22702276.
Wigger A, Neumann ID (1999) Periodic manternal depriva-
Tabor CW, Tabor H (1984) Polyamines. Annu Rev Biochem
tion induces gender-dependent alterations in behavioral
53: 749790.
and neuroendocrine responses to emotional stress in adult
Turnley AM (2005) Growth hormone and SOCS2 regulation
rats. Physiol Behav 66: 293302.
of neuronal differentiation: possible role in mental func-
Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri
tion. Pediatr Endocrinol Rev 2: 366371.
G, Peduto A, Gordon E, Bryant RA (2006) Trauma modu-
Wedzony K, Fijal K, Mackowiak M (2005) Alterations in
lates amygdala and medial prefrontal responses to con-
the dendritic morphology of prefrontal pyramidal neurons
sciously attended fear. Neuroimage 29: 347357.
in adult rats after blockade of NMDA receptors in thepostnatal period. Brain Res 1062: 166170.
Received 19 January 2007, accepted 4 July 2007
Source: http://www.chomiko.ane.pl/pdf/6737.pdf
Bad Axe, Michigan Wednesday, December 28, 2011 The regular meeting of the Huron County Board of Commissioners was held on Wednesday, December 28, 2011, commencing at 10:15 a.m. in the Board of Commissioners room, Third Floor, Huron County Building, Bad Axe, Michigan. PRAYER AND PLEDGE: The meeting was called to order by Chairman Wruble with The Lord's Prayer and Pledge to the Flag of the United States of America. ROLL CALL: Commissioners present: Chairman Ron Wruble, Steve Vaughan, John Nugent, Clark Elftman, John Horny, John Bodis and Dave Peruski. AGENDA: Commissioner Elftman moves Consent Resolution #31 to the New Business agenda. Motion by Vaughan, seconded by Bodis to approve the agenda as amended. Motion carried. APPROVAL OF MINUTES: Motion by Elftman, seconded by Bodis to approve the Committee of the Whole minutes of December 13, 2011 and the minutes of the regular meeting of December 13, 2011 and Committee of the Whole minutes of December 20, 2011. Motion carried. COMMUNICATIONS: Chairman Wruble turns the following communications over to proper committee.
6th Annual North Park University Undergraduate Research Symposium Tuesday, April 17, 2012 North Park University Chicago, Illinois Dr. Rachel Schmale Session 1 John-Tyler Carlson Session 2 Closing Remarks 5:20–5:25 pm Dr. Matthew Schau Following the symposium: Discussion and dinner (served at 5:45 pm) for presenters and advisors in Olssson Lounge, Seminary Building.










