
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Doi:10.1016/j.pnpbp.2007.01.023



Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848 – 857
Effects of haloperidol and its pyridinium metabolite on plasma membrane
permeability and fluidity in the rat brain
Tetsuhito Murata a,⁎, Nobuyuki Maruoka a,b, Naoto Omata a, Yasuhiro Takashima a,
Kazuo Igarashi c, Fumiyo Kasuya d, Yasuhisa Fujibayashi b, Yuji Wada a
a Department of Neuropsychiatry, University of Fukui, Fukui 910-1193, Japan
b Biomedical Imaging Research Center, University of Fukui, Fukui 910-1193, Japan
c Department of Chemistry, Virginia Polytechnic Institute and State University, Blacksburg, VA 24061-0212, USA
d Faculty of Pharmaceutical Sciences, Kobe-gakuin University, Nishi-ku, Kobe 651-21, Japan
Received 26 August 2006; received in revised form 10 January 2007; accepted 23 January 2007
Available online 3 February 2007
The use of antipsychotic drugs is limited by their tendency to produce extrapyramidal movement disorders such as tardive dyskinesia and
parkinsonism. In previous reports it was speculated that extrapyramidal side effects associated with the butyrophenone neuroleptic agenthaloperidol (HP) could be caused in part by the neurotoxic effect of its pyridinium metabolite (HPP+). Although both HPP+ and HP have beenshown to induce neurotoxic effects such as loss of cell membrane integrity, no information exists about the difference in the neurotoxic potency,especially in the potency to induce plasma membrane damage, between these two agents. In the present study, we compared the potency of theinteraction of HPP+ and HP with the plasma membrane integrity in the rat brain. Membrane permeabilization (assessed as [18F]2-fluoro-2-deoxy-
D-glucose-6-phosphate release from brain slices) and fluidization (assessed as the reduction in the plasma membrane anisotropy of 1,6-diphenyl1,3,5-hexatriene) were induced by HPP+ loading (at ≥ 100 μM and ≥ 10 μM, respectively), while comparable changes were induced only at ahigher concentration of HP (= 1 mM). These results suggest that HPP+ has a higher potency to induce plasma membrane damage than HP, andthese actions of HPP+ may partly underlie the pathogenesis of HP-induced extrapyramidal side effects.
2007 Elsevier Inc. All rights reserved.
Keywords: Haloperidol pyridinium; Membrane fluidity; Membrane permeability; Neurotoxicity; Parkinsonism; Tardive dyskinesia
(TD) () and parkinsonism (
Antipsychotic drugs have been widely used to treat
TD is characterized
psychiatric disorders, including schizophrenia, but the use of
by involuntary movements of facial, buccal, and masticatory
these drugs is limited by their tendency to produce a range of
muscles. Parkinsonism is characterized by motor deficits such
extrapyramidal movement disorders such as tardive dyskinesia
as bradykinesia, rigidity, resting tremor and impairment ofpostural reflexes. The underlying pathophysiological mechan-isms of the development of these movement disorders as side
Abbreviations: 2DG, 2-deoxy-D-glucose; DHAA, dehydroabietic acid;
effects of antipsychotic therapy still remain to be elucidated.
DMSO, dimethyl sulfoxide; DPH, 1,6-diphenyl-1,3,5-hexatriene; [18F]FDG,
The butyrophenone neuroleptic agent haloperidol {4-(4-
nol, HP} was introduced into therapy over 40 years ago, and is
still one of the most widely used antipsychotic drugs
dine; PSL, photostimulated luminescence; TD, tardive dyskinesia; TLC, thin-
HP shares some structural
Corresponding author. Tel.: +81 776 61 8363; fax: +81 776 61 8136.
E-mail address: (T. Murata).
0278-5846/$ - see front matter 2007 Elsevier Inc. All rights reserved.
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
MPTP is a contaminant in a synthetic heroin substitute and has
To develop a sensitive assay for detecting cell membrane
been identified as the toxic agent responsible for an irreversible
permeability changes, several laboratories developed a proce-
neurodegenerative condition very similar to Parkinson's disease
dure which utilizes the leakage of radio-labeled 2-deoxy-D-
glucose-6-phosphate (2DG-6-phosphate) from cells
generally accepted that the toxic effects of MPTP are mediated
by 1-methyl-4-phenylpyridine (MPP+), the pyridinium metab-
Advantages of this method include: (1) It uses a biochemically
olite of MPTP HP is extensively
well-characterized probe, 2DG. (2) 2DG is rapidly taken up into
metabolized in the liver, with only about 1% of the administered
cells via a glucose transporter (GLUT) and phosphorylated to a
dose excreted unchanged in the urine
metabolically inert compound, 2DG-6-phosphate. (3) The
The major biotransformation pathways of HP in humans
method is highly sensitive because of the high level of
have been extensively characterized (
accumulated intracellular radioactivity and the comparatively
HP undergoes dehydration to the tetrahydropyridine derivative,
small size of 2DG.
"Membrane fluidity" is the mobility of the membrane
tetrahydropyridine (HPTP), a compound with structural features
components such as lipids and proteins, and is assessed by
similar to those of MPTP. Both HP and HPTP undergo oxidation
measuring the Brownian motion of fluorescent probes incorpo-
to the pyridinium metabolite, 4-(4-chlorophenyl)-1-[4-(4-fluor-
rated into the membrane bilayer ). 1,6-
ophenyl)-4-oxobutyl]-pyridinium (HPP+). HPP+ shares some
diphenyl-1,3,5-hexatriene (DPH) is the most frequently
structural similarity and toxic actions with MPP+, which
employed fluorescent probe which distributes throughout the
suggests that HPP+ might induce extrapyramidal side effects
hydrophobic core of the membrane bilayer (
(). HPP+ has been shown to be present in
), and is widely used to quantify membrane
significant quantities in urine
plasma and post-mortem
When DPH is immobilized in a rigid matrix and
brain samples ) in schizophrenic patients
excited by polarized light, it emits fluorescence that is polarized
treated with HP. In patients undergoing treatment with HP, a
parallel to the exciting light. To the extent that DPH moves
significantly linear relationship was found between the HP dose
during the lifetime of the excited state, the polarization of its
and the serum concentration of HPP+, as well as between the
fluorescent emission is reduced. Thus, a decrease in fluores-
serum concentrations of HP and HPP+ (It has
cence polarization reflects increased mobility of the probe in the
also been shown that in psychiatric patients who were treated
membrane, i.e., an increase in the membrane fluidity. "Aniso-
with HP, the severity of TD and parkinsonism was associated
tropy" is a term often used in the fluorescence polarization field,
with an increased blood level of HPP+
and is also inversely related to fluidity.
These reports led us to speculate that
In the present study, to investigate the effects of HPP+ and
extrapyramidal side effects associated with HP could be caused
HP on plasma membrane permeability, [18F]2-fluoro-2-deoxy-
in part by neurotoxic effects of HPP+.
D-glucose ([18F]FDG) uptake in fresh rat brain slices was
Both HPP+ and HP have been reported to induce neuronal
serially and two-dimensionally measured using a dynamic
positron autoradiography technique (
The results were compared with the leakage of
cytoplasmic lactate dehydrogenase (LDH) from the slices into
Although several laboratories have previously
the medium, which is generally accepted as an indicator of
reported differences in the neurotoxic properties between HPP+
plasma membrane damage and cell injury
). Also, to investigate the effects of these agents on plasma
membrane fluidity, plasma membrane anisotropy in the rat brain
there is not yet a consensus of opinion.
was measured spectrofluorometrically using DPH as a fluores-
Generally, it is well known that antipsychotic drugs act as
cent probe. Additional studies were conducted to measure
antagonists against various neurotransmitter receptors on the
TBARS levels after the addition of HPP+ and HP to evaluate
plasma membrane. However, the targets of these drugs are so
whether oxidative stress is involved in the mechanisms of
diverse that none of the specific interactions of the drugs is
membrane alteration induced by these agents.
likely to be the sole mechanism of action. Also, since thesedrugs have low molecular weight and high lipophilicity, it
seems unlikely that they would remain exclusively at the cellsurface and react only with specific receptors. Rather, it is
2.1. Dynamic positron autoradiography technique
natural to assume that they would become widely distributedthroughout the cell and interact directly with the plasma
All animal procedures were approved by the Animal Care
membrane. Indeed, both HPP+ () and HP
and Use Committee of University of Fukui and conducted in
have been shown to induce loss of cell
accordance with the Guide for the Care and Use of Laboratory
membrane integrity in vitro. However, no information exists
Animals as adopted and promulgated by the National Institutes
about the difference in the potency to induce plasma membrane
of Health. Male Wistar rats (250–300 g) were decapitated and
damage between these two agents.
their brains were removed. Sagittal brain slices (300 μm in
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
tivity of [18F]FDG was 1–2 Ci/mmol at the end of the synthesis,and the total concentration (labeled plus unlabeled) used in theexperiment was 0.45–1.16 μg/ml (2.5–6.4 μM). After 1 h ofpre-incubation, the slices were incubated in Krebs–Ringersolution containing [18F]FDG diluted to 150 kBq/ml. The sliceswere then incubated with various concentrations of HP orHPP+, and the effects of these agents on [18F]FDG uptake wereevaluated. We additionally examined whether the [18F]FDGuptake during the loading of either HPP+ or HP was influencedby addition of a GLUT inhibitor cytochalasin B (300 μM)
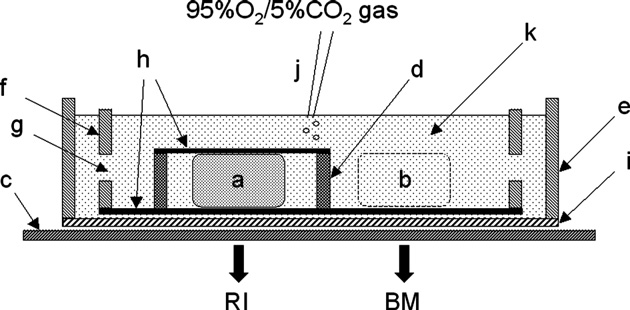
Fig. 1. Schematic view of the apparatus for the incubation of brain slices and the
(Sigma Chemical Co., St. Louis, MO, USA), in order to
detection of the radioactivity signal on a radioluminography plate. a, brain slice
evaluate whether the [18F]FDG uptake was mediated via
(300 μm thick); b, 300-μm-thick bathing solution layer; c, radioluminography
GLUTs. HP and cytochalasin B were dissolved in dimethyl
plate; d, stainless steel ring; e, outer chamber; f, inner chamber; g, hole on the side
sulfoxide (DMSO). HPP+ was dissolved in methanol. The final
wall of inner chamber; h, nylon net (80 μm thick); i, polyvinylidene chloride film(10 μm thick); j, polytetrafluoroethylene catheter for bubbling; k, Krebs–Ringer
concentration of the vehicle (DMSO or methanol) in the
solution containing [18F]FDG. RI and BM are defined as the radioactivity signal
incubation medium was 0–1%, depending on the solubility of
[photostimulated luminescence (PSL)/mm2] on the radioluminography plate
the test compounds. DMSO and methanol at these concentra-
detected beneath the brain region of interest and the bathing medium solution,
tions had no effect on [18F]FDG uptake.
respectively. Images were obtained in a dark environment at 36 °C.
The exposed radioluminography plates (BAS-MP 2040S,
Fuji Photo Film Co., Tokyo, Japan) were scanned using a BAS-
thickness) were prepared with a microslicer (DTK-2000,
1500 (Fuji Photo Film Co.). The pixel size was 100 μm. The
Dosaka EM, Kyoto, Japan), and incubated as previously
regions of the brain slices were identified by referring to a brain
described (). The system
map of the rat ). The image data
is shown schematically in The outer chamber was filled
obtained were quantitatively analyzed as follows. The radioac-
with Krebs–Ringer solution and the inner chamber was
tivity of 18F decreases with the same time course (half-
immersed in it. The bottom of the inner chamber was made of
life = 109.7 min) in both the brain slices and the surrounding
a nylon net, and the bottom of the outer chamber was a 10-μm-
bathing medium, and it is not necessary to compensate for the
thick polyvinylidene chloride film that was penetrable to the
radioactive decay when the radioactivity pixel value of a region
beta and gamma rays of 18F. The prepared slices were placed in
of interest is divided by that of the bathing medium. Thus, the
the inner chamber and covered with a 300-μm-thick stainless
relative increment of [18F]FDG uptake in the region of interest
steel ring whose upper side was covered by a nylon net. The
can be expressed in decay-corrected form by the following ratio:
incubation volume was 80 ml. During the incubation, theKrebs–Ringer solution was bubbled with a mixture of 95% O2
Relative Uptake Ratio ¼ ðRI−BMÞ=BM
and 5% CO2. 18F was produced by 18O (p,n) 18F nuclearreactions, and [18F]FDG was produced by the method of
where RI is defined as the radioactivity signal [photostimulated
using an automated [18F]FDG synthesis
luminescence (PSL)/mm2] on the radioluminography plate
system (NKK Co. Ltd., Tokyo, Japan). The specific radioac-
detected beneath the region of interest, and BM as the average
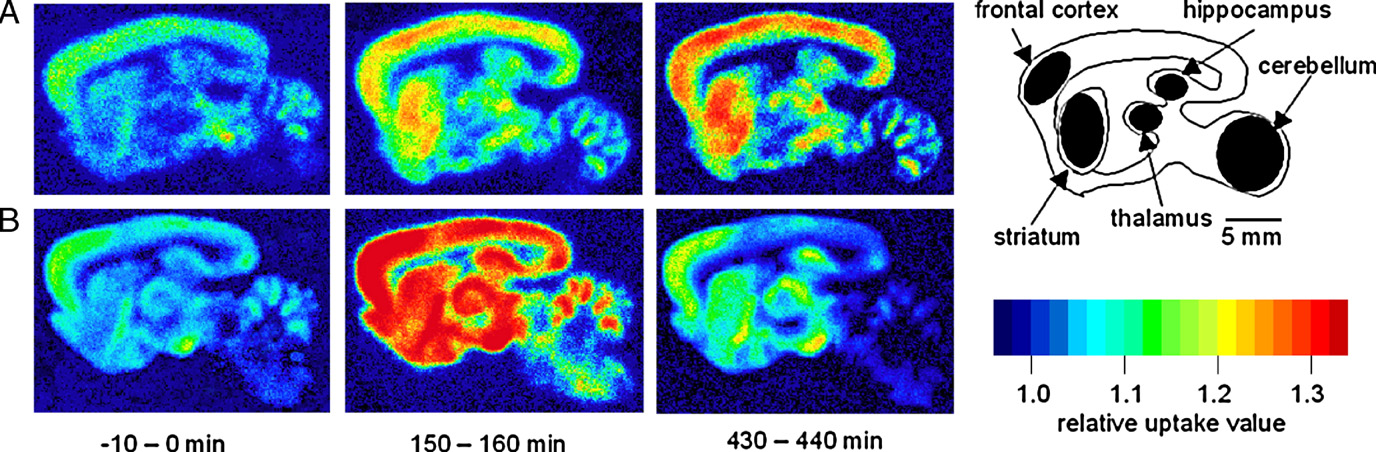
Fig. 2. Time-resolved pseudocolor images of [18F]FDG uptake in sagittally sectioned rat brain slices. Time zero is when HPP+ was added to the incubation medium.
Two typical slices under the control condition with its diagram (A) and before and after the loading of 100 μM HPP+ (B) for three representative time periods (−10–0 min, 150–160 min, and 430–440 min) are shown. The filled regions in the diagram represent the five brain regions examined in the present study (frontal cortex,caudate putamen, thalamus, hippocampus, and cerebellum). For decay correction, the color-coding was based on the relative uptake ratio (see text for furtherexplanation).
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
membranes were diluted in 50 mM Tris–HCl buffer, pH 7.4,and mixed with the fluorescent probe DPH (0.8 mg protein/ml,3.3 μM DPH). The concentration of DPH used was based onprevious reports and the DPH stock solution (1 mM)was prepared in tetrahydrofuran. The mixture was incubated at25 °C for 10 min, and then the reaction was stopped by theaddition of a large volume of the above buffer, and the mixturewas centrifuged at 15,000 ×g and 4 °C for 20 min. The resultantpellet was washed twice with the buffer and resuspended in the
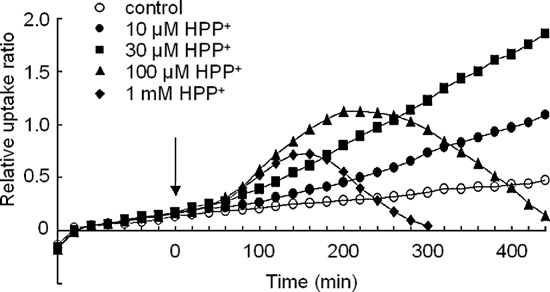
Fig. 3. Effect of treatment with various concentrations of HPP+ on the time–
same buffer. Various concentrations of HP or HPP+ were added
course of [18F]FDG uptake in the striatum. Ordinate: relative uptake ratio of18F-radioactivity (see text for further explanation). Abscissa: time in minutes.
to DPH-labeled membrane solutions (0.07 mg protein/ml) and
The point at which the drug was applied (= time zero) is indicated by the
incubated at 36 °C for 10 min. The 10-min incubation period
arrow. Values are the means obtained for six slices (SD is omitted).
was based on a pilot experiment in which the maximum effectof HP or HPP+ on the membrane anisotropy described below
radioactivity signal (PSL/mm2) on the radioluminography plate
was reached after 10 min of incubation, which is in accord with
detected beneath the bathing medium solution surrounding each
the findings for alcohols () and other organic
brain slice.
solvents The finalconcentration of the vehicle (DMSO or methanol) in the
2.2. [18F]FDG metabolite analysis
reaction mixture was 0–1%, depending on the solubility of thetest compound. DMSO or methanol alone at these concentra-
In order to measure the release of the [18F]FDG metabolite
tions had no effect on membrane fluidity. Fluorescence
from the brain slices induced by the administration of HP or
measurements were carried out at 36 °C with a Beacon 2000
HPP+, metabolite analysis was performed by thin-layer
fluorescence polarization system (Invitrogen Corporation, San
chromatography (TLC) on Whatman LK6DF silica gel plates
Diego, CA, USA). The excitation and emission wavelengths
(Clifton, NJ, USA) with a solvent system of acetonitrile/water
were 330 and 420 nm, respectively. The steady-state fluores-
(95:5). The metabolites in the incubation medium were sampled
cence polarization (P) was expressed using the formula
after the administration of HP or HPP+ and separated by TLC.
The TLC plates were exposed to a radioluminography plate.
P ¼ ðIt−I8Þ=ðIt þ I8Þ
2.3. Assessment of plasma membrane damage and cell injury
where I‖ and I⊥ are the emission intensities parallel and
Leakage of LDH from the slices into the incubation medium
perpendicular, respectively, to the plane of the excitation light.
was measured using an LDH Cytotoxicity Detection Kit (Takara
The fluorescence anisotropy (A) was calculated from the
Bio Inc., Shiga, Japan) according to the manufacturer's
fluorescence polarization value using the formula (
instructions. Results were expressed as percentage of control,
which was considered as 100%.
A ¼ 2P=ð3−PÞ
2.4. Fluorescence anisotropy measurement
The amount of protein was determined with a Bio-Rad
In this study, the P2 fraction of was
protein assay kit (Hercules, CA, USA) using bovine serum
prepared as a crude synaptosomal membrane fraction. Although
albumin as the standard.
the P2 fraction is enriched in synaptosomes but also containsrelatively large amounts of myelin fragments and free mitochon-dria, it can be obtained in 70–80 min and the long preparationtimes of the original method () can beavoided. Briefly, the rat brain tissue was homogenized in 10 vol of0.32 M sucrose using an Ultrasonic Disrupter (UR-20P, TomySeiko Co. Ltd., Tokyo, Japan). Each homogenate was centrifugedat 1000 ×g and 4 °C for 10 min. The supernatant was removed andcentrifuged at 12,000 ×g and 4 °C for 20 min. The resultant pelletwas suspended in 20 vol of 50 mM Tris–HCl buffer, pH 7.4, andwashed twice by centrifugation at 12,000 ×g and 4 °C for 20 min.
The final pellet was resuspended in the same buffer and used for
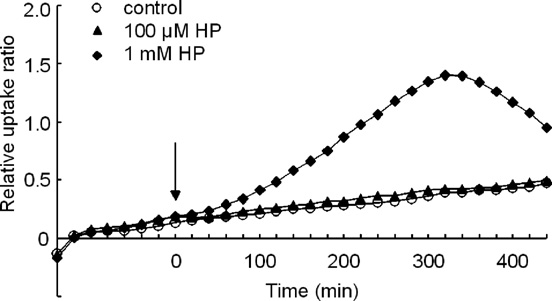
Fig. 4. Effect of treatment with various concentrations of HP on the time–course of [18F]FDG uptake in the striatum. Ordinate: relative uptake ratio of
the measurement of membrane anisotropy.
18F-radioactivity (see text for further explanation). Abscissa: time in minutes.
Membrane anisotropy was measured by the method of
The point at which the drug was applied (= time zero) is indicated by the
arrow. Values are the means obtained for six slices (SD is omitted).
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
2.5. Lipid peroxidation assay
Brain slices were treated with either 1 mM HPP+ or 1 mM HP
for 60 min. The slices were then homogenized and aliquots wereused to determine the levels of thiobarbituric acid-reactivesubstances (TBARS) using Lipid Peroxidation Test Wako(Wako Pure Chemical Industries Ltd, Osaka, Japan) accordingto the manufacturer's instructions. In this assay, lipid peroxidationis quantified by measuring malondialdehyde (MDA), an endproduct of oxidative lipid degradation. MDA forms a 1:2 adductwith thiobarbituric acid (TBA) and produces a TBA pigmentwhich can be measured by fluorometry or spectrophotometry
Fig. 6. Effect of treatment with 1 mM HPP+ and 1 mM HP on LDH leakage from
(The interaction of 50 μM Fe2+ and 3 mM
brain slices into the bathing medium 480 min after the administration of the
hydrogen peroxide was used as a positive control. The quantities
drugs. Samples treated with 1% Triton X-100 were defined as positive controls.
of TBARS were expressed as nanomoles per milligram of protein,
Results are expressed as percentage of control, which was considered as 100%,
and presented as a percentage of control values.
and represent the means ± SD obtained in six slices. The Mann–Whitney U-testwas used to evaluate the significance of differences. ⁎P b 0.05 compared withcontrol values.
HPP+ was synthesized as previously described
before and after the loading of 100 μM HPP+ (B) for three
). The purity of the HPP+ was 97–98%. HP,
representative time periods (−10–0 min, 150–160 min, and
DPH and cytochalasin B were purchased from Sigma Chemical
430–440 min). Time zero is defined as the time when HPP+ or
Co. (St. Louis, MO, USA). The LDH Cytotoxicity Detection
HP was introduced into the bathing medium containing brain
Kit was purchased from Takara Bio Inc. (Shiga, Japan). The
slices. Rat brain slices from five representative brain regions
Bio-Rad protein assay kit was obtained from Bio-Rad
(frontal cortex, striatum, thalamus, hippocampus, and cerebel-
Laboratories Inc. (Hercules, CA, USA). The Lipid Peroxidation
lum) were analyzed.
Test Wako was obtained from Wako Pure Chemical Industries
To depict dynamic changes of [18F]FDG uptake, the relative
Ltd. (Osaka, Japan). All other chemicals were from Nacalai
uptake ratio at 10-min intervals in the striatum before and after
Tesque Inc. (Kyoto, Japan).
loading various concentrations (10, 30, 100 μM and 1 mM) ofHPP+ or HP was plotted against time [the results for the control
2.7. Statistical analysis
condition and for the HPP+ loading (10, 30, 100 μM and 1 mM)at 20-min intervals are shown in The slope of the graph
The presented values are shown as the means ± SD. The
indicates the rate of [18F]FDG uptake. The slope of the graph
Mann–Whitney U-test was used to evaluate the significance of
for 10–30 μM HPP+ was definitely increased during the entire
differences. P b 0.05 was considered statistically significant.
time course. However, when slices were loaded with buffercontaining ≥100 μM HPP+, the slope of the graph initially
increased, then gradually decreased and finally became negative(this finding may reflect the outflow of [18F]FDG metabolites
shows time-resolved images of [18F]FDG uptake of
from the brain slices). Similar results were obtained in each of
two typical brain slices under the control condition (A) and
the brain regions examined (data not shown).
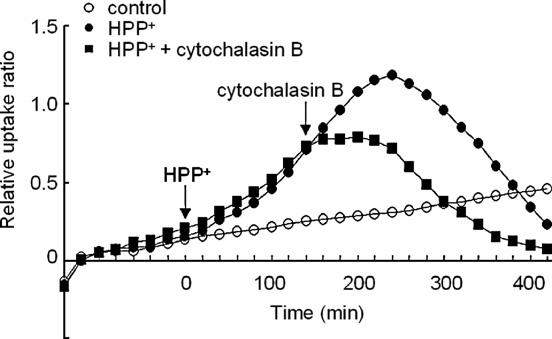
Fig. 5. Effect of treatment with 100 μM HPP+ on the time–course of [18F]FDGuptake in the striatum. HPP+ was administered alone or co-administered with
Fig. 7. Effects of treatment with various concentrations of HPP+ or HP on the
a GLUT inhibitor cytochalasin B (300 μM). Ordinate: relative uptake ratio of
DPH fluorescence anisotropy as an index of membrane fluidity. Data represent
18F-radioactivity (see text for further explanation). Abscissa: time in minutes.
the means ± SD obtained for six samples. The Mann–Whitney U-test was used
The point at which each agent was applied is indicated by an arrow. Values are
to evaluate the significance of differences. ⁎P b 0.05 compared with control
the means obtained for six slices (SD is omitted).
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
In contrast, the slope of the graph for 10–100 μM HP was
Treatment with either 1 mM HPP+ or 1 mM HP did not
similar to that of the control. Significant changes in the slope of
significantly alter TBARS levels in comparison to those in the
the graph (i.e., initial increase and subsequent gradual decrease
in the slope of the graph) were induced by HP loading only atthe concentration of 1 mM [the results for the control condition
and for the HP loading (100 μM and 1 mM) at 20-min intervalsare shown in Similar results were obtained in each of the
Previous studies have shown differences in the neurotoxic
regions examined (data not shown).
properties between HPP+ and HP. Sagittal mouse brain slices
The enhancement of the [18F]FDG uptake induced by both
incubated in vitro with HP (10 nM) showed time- and
HPP+ (≥10 μM) and HP (1 mM) is inhibited by addition of
concentration-dependent inhibition of complex I
300 μM cytochalasin B in each of the brain regions examined
). However, similar concentrations of HPP+ failed to
(the results for the 100 μM HPP+ loading, and cytochalasin B
inhibit complex I activity, and comparable inhibition was
loading at 140 min after the administration of HPP+ in the
obtained only at a 10,000-fold higher concentration of HPP+
striatum are shown in ). Therefore, it is suggested that the
(100 μM) (). On the other hand, because
enhancement of the [18F]FDG uptake was mediated via GLUTs.
the neuronal accumulation of HP metabolites via monoamine
The TLC data suggested that the major [18F]FDG metabolite
transporters could facilitate subsequent toxic events in the
released from the slices as a result of the administration of HPP+
(≥100 μM) or HP (1 mM) was [18F]FDG-6-phosphate, not
laboratories examined the interactions of HPP+ and HP with the
[18F]FDG (data not shown). Because [18F]FDG-6-phosphate
monoamine transporters and demonstrated that HPP+ was more
cannot be transported via GLUTs, the efflux of [18F]FDG-6-
active for inhibiting monoamine uptake into cells
phosphate was probably not mediated by GLUTs; rather, it is
likely that the increased plasma membrane permeability of the
). Thus, no consensus has
cells allowed intracellular [18F]FDG-6-phosphate to leak from
yet been reached as to the difference in the potency of inducing
neurotoxicity between HPP+ and HP.
The LDH assay revealed that in the case of 1 mM HPP+ and
The present study is the first to investigate the difference in
1 mM HP exposure, which began to induce the release of [18F]
the potency of inducing plasma membrane damage between
FDG-6-phosphate around 200–300 min after the administration
HPP+ and HP by using [18F]FDG-6-phosphate leakage as an
of the drug ), no significant increase in the release of
index of membrane permeabilization and DPH fluorescence
LDH was observed even at 480 min after the administration of
anisotropy as an index of membrane fluidization. In this study,
the drug (). These results indicate that the leakage of LDH
increases in membrane permeability and fluidity were induced
is clearly slower than that of [18F]FDG-6-phosphate.
by HPP+ loading (≥100 μM and ≥10 μM, respectively)
At concentrations ≥ 10 μM, HPP+ induced a dose-dependent
). Similar concentrations of HP failed to affect
decrease in anisotropy (i.e., an increase in membrane fluidity),
membrane permeability or fluidity, and comparable changes
while 10–100 μM HP induced no significant changes in
were obtained only at a higher concentration of HP (1 mM)
anisotropy; a significant decrease in anisotropy was obtained
These results suggest that HPP+ has a higher
by HP loading only at the concentration of 1 mM [the results for
potency for inducing plasma membrane damage than HP.
the control condition, for the HPP+ loading (10 and 100 μM) and
We found that when the release of [18F]FDG-6-phosphate
for the HP loading (100 μM and 1 mM) are shown in ].
was already induced at 480 min after the administration of bothHPP+ and HP, no significant increase in the release of LDH wasobserved, which suggests that the leakage of LDH is clearlyslower than that of [18F]FDG-6-phosphate Apossible explanation for this is that HPP+ and HP could initiallyinduce small membrane holes that became enlarged with time.
The holes were not initially large enough to allow the leakage oflarger molecules such as LDH, but a small increase in hole sizewith time eventually enabled the leakage of LDH. The lagbetween the leakage of [18F]FDG-6-phosphate and LDH couldreflect the time needed for the hole to become large enough forLDH leakage to be possible.
Amphiphilic molecules have both hydrophobic and hydro-
philic moieties and accumulate in the membrane bilayer and can
Fig. 8. Effect of treatment with 1 mM HPP+ and 1 mM HP on the formation of
consequently affect the membrane fluidity. For example, the
thiobarbituric acid-reactive substances (TBARS) in rat brain slices. Data
amphiphilic peptide gramicidin-S penetrates and accumulates in
represent the means ± SD obtained in six slices. Samples treated with 50 μM
the membrane bilayer. The penetration of gramicidin-S creates a
FeSO4 and 3 mM H2O2 were examined as positive controls. The quantities of
space in the interior of the membrane, resulting in an increase in
TBARS were expressed as percentage of the control values. The Mann–
the movement of the acyl chains of phospholipid molecules and
Whitney U-test was used to evaluate the significance of differences. ⁎P b 0.05compared with control value.
thus in the membrane fluidization ). The
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
accumulation of amphiphilic molecules destabilizes the mem-
evaluate whether the [18F]FDG uptake was mediated via GLUTs.
brane structure, resulting in the release of membrane components
The enhancement of the [18F]FDG uptake induced by both HPP+
and a concomitant enhancement of permeability
(≥10 μM) and HP (≥1 mM) was prevented by addition of a
). Antipsychotic drugs such as HP and chlorpromazine
GLUT inhibitor cytochalasin B (). Therefore it is suggested
are known to have an amphiphilic nature
that the enhancement of the [18F]FDG uptake was mediated via
). Although HPP+ possesses a fixed
GLUTs, rather than the GLUT-independent [18F]FDG influx
positive charge, which renders the molecule hydrophilic, it can
(passive diffusion) due to membrane damage. Nevertheless, we
still be considered to be lipophilic because of its complex alkyl
could not exclude the possibility that the membrane permeability
aromatic structure (). Therefore, it seems
is underestimated in dynamic positron autoradiography since the
reasonable to categorize HPP+ as an amphiphilic agent. In the
summation of radioactivities derived from [18F]FDG taken up
present study, increased lipophilicity of HP and HPP+ did not
into the tissue and [18F]FDG-6-phosphate that leaked out from the
necessarily correlate with potency to interact with the membrane,
tissue was measured in this method.
since HPP+ [partition coefficient (log P) = 1.23], which is less
It has been reported that haloperidol induces the generation
lipophilic than HP (log P = 3.85) was
of reactive oxygen species in rat neurons (
more potent as a membrane destabilizer than HP. Accordingly, it is
Treatment with haloperidol led to increased lipid peroxidation
suggested that the ability to interact with the membrane is not due
and concomitant reductions in brain levels of glutathione in rats
to the mere increase of lipophilicity of HP and HPP+, but mainly
to the amphiphilic nature of HP and HPP+. Our results are also
and humans ). Free radicals have been
consistent with a previous report which showed that when
reported to increase plasma membrane permeability (
erythrocytes were transformed into stomatocytes by the addition
Several laboratories have
of cationic amphiphilic agents, membrane fluidization was always
reported that oxidative stress and lipid peroxidation increase
observed, thus indicating a close correlation between stomatocyte
membrane fluidity
formation and an increase in membrane fluidity ).
These reports prompted us to speculate that HPP+ and HP may
Our data showed that HPP+ (≥ 10 μM) and HP (1 mM)
induce oxidative stress and lipid peroxidation, and consequently
enhanced the [18F]FDG uptake in the initial phase after the drug
increase membrane permeabilization and fluidization. Based on
administration. A possible explanation for the mechanism by
this hypothesis, TBARS levels after the addition of HPP+ and
which HPP+ or HP induced enhancement of the [18F]FDG
HP were measured. Contrary to our hypothesis, treatment with
uptake is the drug-induced inhibition of mitochondrial activity.
either 1 mM HPP+ or 1 mM HP did not alter the TBARS levels
It has been shown that both HPP+ (
significantly (suggesting that HPP+ and HP did not
induce lipid peroxidation. Therefore, it is unlikely that oxidative
inhibit complex I in the mitochondrial
stress is involved in the mechanisms of membrane alteration
respiratory chain. It has also been demonstrated that HPP+ is a
induced by HPP+ and HP.
stronger inhibitor of mitochondrial respiration than MPP+
HPP+ is mainly produced by the microsomal-catalyzed
() which is well known to be a
oxidation of HP with cytochrome P450, which is distributed
mitochondrial complex I inhibitor (). In
principally in the liver (
the present study, the enhancement of glycolytic activity may
have served to compensate for the decrease in aerobic
). Enzyme activity responsible for the conversion of
metabolism when oxidative phosphorylation was inhibited by
HP to HPP+ was not found in rat brain mitochondria (
HPP+ or HP at the mitochondrial respiratory chain level.
Therefore, it seems unlikely that HPP+ was gen-
Dehydroabietic acid (DHAA), which is known to be an
erated during the incubation of the brain slices. This, however,
amphiphilic compound, was reported to cause a dose-dependent
may not be the case, since HP and HPTP are biotransformed by
reduction in the cellular ATP content and a concomitant
mouse brain preparations to HPP+ ).
enhancement of the glycolytic activity of rainbow trout
Consequently, it is also possible that HPP+ is formed from
hepatocytes, suggesting that ATP depletion is likely due to
HP in the brain slices, which may have partly affected the
increased consumption of cellular ATP caused by amphiphilic
membrane alteration induced by HP in the present study.
action of DHAA in the cell membrane ().
The therapeutic use of antipsychotic drugs is limited by their
Therefore, in the present study, through their amphiphilic
tendency to produce a range of extrapyramidal movement
properties, HP and HPP+ could induce ATP depletion and
disorders such as TD
enhancement of glycolytic activity. It has been suggested that
and parkinsonism
these mechanisms cause further ATP depletion, and depletion of
ATP to a critically low level increases membrane permeability
Although the pathophysiological basis of TD remains unclear,
TD has been attributed to the supersensitivity of dopamine
It could be argued that GLUT-independent [18F]FDG influx
(passive diffusion) would be induced if a membrane structure is
suggested that antipsychotic drugs fluidize
damaged. Therefore, we additionally examined whether the
membranes, thus promoting fusion between the neurovesicle
enhancement of the [18F]FDG uptake induced by either HPP+ or
membrane and the presynaptic membrane. This membrane-
HP was prevented by addition of a GLUT inhibitor in order to
fluidizing action would thus lead to an increased spontaneous
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
secretion of dopamine, and this may underlie TD. Although
drug-induced parkinsonism is usually attributed to the anti-dopaminergic properties of antipsychotic drugs, a wide variety
For the first time, we investigated the effect of HP and its
of processes have been suggested to be involved in the
pyridinium metabolite HPP+ on plasma membrane perme-
pathogenesis of Parkinson's disease and parkinsonism. Recent-
ability in relation to the effect of these agents on plasma
ly, Volles et al. proposed that membrane permeabilization by
membrane fluidity in the central nervous system. Membrane
protein α-synuclein is a pathogenetic mechanism involved in
permeabilization and fluidization were induced by HPP+ loading
Parkinson's disease; that is, inappropriate membrane permea-
(≥ 100 μM and ≥ 10 μM, respectively). Similar concentrations of
bilization by α-synuclein could cause the degeneration and
HP failed to change membrane permeability and fluidity in this
death of neurons in several ways: unregulated calcium flux into
model; indeed, comparable changes were obtained only at a
the cytosol, depolarization of the mitochondrial membrane, or
higher concentration of HP (1 mM). These results suggested that
leakage of dopamine into the cytoplasm (
HPP+ had a higher potency to induce plasma membrane dam-
These reports together with our
age than HP, which may partly underlie the pathogenesis of
findings prompt us to speculate that the induction of membrane
HP-induced extrapyramidal side effects such as TD and
permeabilization and fluidization by HPP+ may play a role, at
least partially, in the pathogenesis of HP-induced extrapyrami-dal side effects such as parkinsonism and TD. Further studies
will be needed to clarify the relationship between membraneactions of HPP+ and the pathogenesis of HP-induced extrapy-
This work was in part supported by 21st Century COE
ramidal side effects.
program "Biomedical Imaging Technology Integration Program"
One of the limitations of our findings is that the concentra-
from the Japan Society for the Promotion of Science (JSPS).
tions of HPP+ required to produce membrane perturbation inour system are higher than the concentrations of this agent
observed in human plasma, although the concentrations we used
Andreoli SP, Baehner RL, Bergstein JM. In vitro detection of endothelial
are in the same range as those used in previous studies which
cell damage using 2-deoxy-D-3H-glucose: comparison with chromium 51,
investigated HPP+-induced neurotoxicity in vitro (
3H-leucine, 3H-adenine, and lactate dehydrogenase. J Lab Clin Med 1985;106:
Ashwell JD, Schwartz RH, Mitchell JB, Russo A. Effect of gamma radiation on
). The plasma concentrations of HPP+ in patients
resting B lymphocytes. I. Oxygen-dependent damage to the plasmamembrane results in increased permeability and cell enlargement. J Immunol
with schizophrenia taking HP range between 0.2 and 10 nM
(However, HPP+ has been reported to
Avdulov NA, Chochina SV, Igbavboa U, O'Hare EO, Schroeder F, Cleary JP,
accumulate at higher concentrations in rat brain tissue than in
et al. Amyloid β-peptides increase annular and bulk fluidity and induce
lipid peroxidation in brain synaptic plasma membranes. J Neurochem
The concentration of HPP+ in the rat brain has been determined
Avent KM, Riker RR, Fraser GL, Van der Schyf CJ, Usuki E, Pond SM.
to be 0.047−0.27 μg/ml compared to 0.002−0.013 μg/ml in
Metabolism of haloperidol to pyridinium species in patients receiving high
plasma after receiving a total of 10 mg/kg HP by intraperitoneal
doses intravenously: is HPTP an intermediate? Life Sci 1997;61:2383–90.
injection twice a day over a period of 3 days (
Balijepalli S, Boyd MR, Ravindranath V. Inhibition of mitochondrial complex I
Therefore, HPP+ may reach neurotoxic
by haloperidol: the role of thiol oxidation. Neuropharmacology 1999;38:
levels in the brain. In acute toxicity studies in vitro, micromolar
Bloomquist J, King E, Wright A, Mytilineou C, Kimura K, Castagnoli K, et al.
concentrations of HPP+ are toxic to neurons (
1-Methyl-4-phenylpyridinium-like neurotoxicity of a pyridinium metabolite
derived from haloperidol: cell culture and neurotransmitter uptake studies.
J Pharmacol Exp Ther 1994;270:822–30.
Because of the high doses of HPP+ used in acute in vitro
Bryan-Lluka LJ, Siebert GA, Pond SM. Potencies of haloperidol metabolites as
studies and the very low concentrations of HPP+ found in
inhibitors of the human noradrenaline, dopamine and serotonin transportersin transfected COS-7 cells. Naunyn Schmiedeberg's Arch Pharmacol
patients, it is important to consider the possibility that HPP+
which is present at low concentrations chronically in vivo may
Burkhardt C, Kelly JP, Lim YH, Filley CM, Parker Jr WD. Neuroleptic
produce the comparative neurotoxicity seen after higher doses
medications inhibit complex I of the electron transport chain. Ann Neurol
in acute studies in vitro; that is, low concentrations of HPP+ in
the brain in vivo could be toxic when the exposure is over
Eyles DW, McLennan HR, Jones A, McGrath JJ, Stedman TJ, Pond SM.
Quantitative analysis of two pyridinium metabolites of haloperidol in
months or years, rather than hours or days. It is known that
patients with schizophrenia. Clin Pharmacol Ther 1994;56:512–20.
MPTP chronically administered to primates at low doses
Eyles DW, McGrath JJ, Pond SM. Formation of pyridinium species of haloperidol
eventually produces neurotoxicity comparative to that seen
in human liver and brain. Psychopharmacology 1996;125:214–9.
after higher doses in acute studies Based
Eyles DW, Avent KM, Stedman TJ, Pond SM. Two pyridinium metabolites of
on these considerations, it appears reasonable to speculate that
haloperidol are present in the brain of patients at post-mortem. Life Sci1997;60:529–34.
chronic exposure to HP could lead to the gradual accumulation
Fang J, Yu PH. Effect of haloperidol and its metabolites on dopamine and
of HPP+ in individuals and result in brain levels of HPP+ that
noradrenaline uptake in rat brain slices. Psychopharmacology (Berl)
might induce loss of membrane integrity.
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
Fang J, Zuo D, Yu PH. Comparison of cytotoxicity of a quaternary pyridinium
Koh JY, Choi DW. Quantitative determination of glutamate mediated cortical
metabolite of haloperidol (HP+) with neurotoxin N-methyl-4-phenylpyr-
neuronal injury in cell culture by lactate dehydrogenase efflux assay.
idinium (MPP+) towards cultured dopaminergic neuroblastoma cells.
J Neurosci Methods 1987;20:83–90.
Psychopharmacology (Berl) 1995;121:373–8.
Kristensen SR, Horder M. Release of enzymes from quiescent fibroblasts during
Fang J, Baker GB, Silverstone PH, Coutts RT. Involvement of CYP3A4 and
ATP depletion. Enzyme 1988;39:205–12.
CYP2D6 in the metabolism of haloperidol. Cell Mol Neurobiol 1997;17:
Kudo S, Ishizaki T. Pharmacokinetics of haloperidol: an update. Clin Pharmacokinet
Fang J, McKay G, Song J, Remillrd A, Li X, Midha K. In vitro characterization of
Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans
the metabolism of haloperidol using recombinant cytochrome p450 enzymes
due to a product of meperidine-analog synthesis. Science 1983;219:979–80.
and human liver microsomes. Drug Metab Dispos 2001;29:1638–43.
Malik JK, Schwarz LR, Wiebel FJ. Assessment of membrane damage in
Forsman A, Ohman R. Studies on serum protein binding of haloperidol. Curr
continuous cultures of mammalian cells. Chem Biol Interact 1983;45:29–42.
Ther Res Clin Exp 1977;21:245–55.
Manor D, Sadeh M. Muscle fibre necrosis induced by intramuscular injection of
Galili-Mosberg R, Gil-Ad I, Weizman A, Weizman A, Melamed E, Offen D.
drugs. Br J Exp Pathol 1989;70:457–62.
Haloperidol-induced neurotoxicity — possible implications for tardive
Markey SP, Schmuff NR. The pharmacology of the parkinsonian syndrome
dyskinesia. J Neural Transm 2000;107:479–90.
producing neurotoxin MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyri-
Gerlach J, Casey DE. Tardive dyskinesia. Acta Psychiatr Scand 1988;77: 369–78.
dine) and structurally related compounds. Med Res Rev 1986;6:389–429.
Goldstein DB. The effects of drugs on membrane fluidity. Annu Rev Pharmacol
Marsden CD, Jenner P. The pathophysiology of extrapyramidal side-effects of
neuroleptic drugs. Psychol Med 1980;10:55–72.
Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-
Mitchell IJ, Cooper AC, Griffiths MR, Cooper AJ. Acute administration of
microscopic study of cell fragments derived by homogenization and
haloperidol induces apoptosis of neurones in the striatum and substantia
centrifugation. J Anat 1962;96:79–88.
nigra in the rat. Neuroscience 2002;109:89–99.
Grohmann R, Koch R, Schmidt LG. Extrapyramidal symptoms in neuroleptic
Murata T, Omata N, Fujibayashi Y, Waki A, Sadato N, Yoshida S, et al. Dynamic
recipients. Agents Actions Suppl 1990;29:71–82.
changes in glucose metabolism of living rat brain slices induced by hypoxia
Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-
and neurotoxic chemical-loading revealed by positron autoradiography.
carrier-added 2-[18F]-fluoro-2-deoxy-D-glucose using aminopolyether sup-
J Neural Transm 1999;106:1075–87.
ported nucleophilic substitution. J Nucl Med 1986;27:235–8.
Noji S, Takahashi T, Kon H. A spin-label study of the correlation between
Huang J, Tanii H, Ohyashiki T, Hashimoto K. Structure–toxicity relationship of
stomatocyte formation and membrane fluidization of erythrocytes. Biochem
monoketones: in vitro effects on beta-adrenergic receptor binding and Na+/
K+-ATPase activity in mouse synaptosomes. Neurotoxicol Teratol 1993;15:
Ohyashiki T, Sakata N, Matsui K. A decrease of lipid fluidity of the porcine
intestinal brush-border membranes by treatment with malondialdehyde.
Igarashi K, Castagnoli Jr N. Determination of the pyridinium metabolite derived
J Biochem (Tokyo) 1992;111:419–23.
from haloperidol in brain tissue, plasma and urine by high-performance liquid
Omata N, Murata T, Fujibayashi Y, Waki A, Sadato N, Yoshimoto M, et al.
chromatography with fluorescence detection. J Chromatogr 1992;579:
Hypoxic but not ischemic neurotoxicity of free radicals revealed by dynamic
changes in glucose metabolism of fresh rat brain slices on positron
Igarashi K, Kasuya F, Fukui M, Usuki E, Castagnoli Jr N. Studies on the
autoradiography. J Cereb Blood Flow Metab 2000;20:350–8.
metabolism of haloperidol (HP): the role of CYP3A in the production of the
Pai BN, Janakiramaiah N, Gangadhar BN, Ravindranath V. Depletion of
neurotoxic pyridinium metabolite HPP+ found in rat brain following ip
glutathione and enhanced lipid peroxidation in the CSF of acute psychotics
administration of HP. Life Sci 1995;57:2439–46.
following haloperidol administration. Biol Psychiatry 1994;36:489–91.
Igarashi K, Matsubara K, Kasuya F, Fukui M, Idzu T, Castagnoli Jr N. Effect of a
Pappu AS, Hauser G. Alterations of phospholipid metabolism in rat cerebral cortex
pyridinium metabolite derived from haloperidol on the activities of striatal
mince induced by cationic amphiphilic drugs. J Neurochem 1981;37:1006–14.
tyrosine hydroxylase in freely moving rats. Neurosci Lett 1996;214:183–6.
Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. San
Iwahashi K, Anemo K, Nakamura K, Fukunishi I, Igarashi K. Analysis of the
Diego: Academic Press; 1998.
metabolism of haloperidol and its neurotoxic pyridinium metabolite in patients
Post A, Holsboer F, Behl C. Induction of NF-κB activity during haloperidol-
with drug-induced parkinsonism. Neuropsychobiology 2001;44:126–8.
induced oxidative toxicity in clonal hippocampal cells: suppression of NF-
Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic
κB and neuroprotection by antioxidants. J Neurosci 1998;18:8236–46.
indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol
Rajput AH, Rozdilsky B, Hornykiewicz O, Shannak K, Lee T, Seeman P.
Reversible drug-induced parkinsonism. Clinicopathologic study of two
Jeste DV, Caligiuri MP. Tardive dyskinesia. Schizophr Bull 1993;19:303–15.
cases. Arch Neurol 1982;39:644–6.
Johannessen JN. A model of chronic neurotoxicity: long-term retention of the
Rissanen E, Krumschnabel G, Nikinmaa M. Dehydroabietic acid, a major
neurotoxin 1-methyl-4-phenylpyridinium (MPP+) within catecholaminergic
component of wood industry effluents, interferes with cellular energetics in
neurons. Neurotoxicology 1991;12:285–302.
rainbow trout hepatocytes. Aquat Toxicol 2003;62:45–53.
Kalgutkar AS, Zhou S, Fahmi OA, Taylor TJ. Influence of lipophilicity on the
Rollema H, Skolnik M, D'Engelbronner J, Igarashi K, Usuki E, Castagnoli Jr N.
interactions of N-alkyl-4-phenyl-1,2,3,6-tetrahydropyridines and their
MPP+-like neurotoxicity of a pyridinium metabolite derived from haloperidol:
positively charged N-alkyl-4-phenylpyridinium metabolites with cyto-
in vivo microdialysis and in vitro mitochondrial studies. J Pharmacol Exp Ther
chrome P450 2D6. Drug Metab Dispos 2003;31:596–605.
Katsu T, Kobayashi H, Hirota T, Fujita Y, Sato K, Nagai U. Structure–activity
Sagara Y. Induction of reactive oxygen species in neurons by haloperidol.
relationship of gramicidin S analogues on membrane permeability. Biochim
J Neurochem 1998;71:1002–12.
Biophys Acta 1987;899:159–70.
Saporito MS, Heikkila RE, Youngster SK, Nicklas WJ, Geller HM.
Katsu T, Kuroko M, Morikawa T, Sanchika K, Fujita Y, Yamamura H, et al.
Dopaminergic neurotoxicity of 1-methyl-4-phenylpyridinium analogs in
Mechanism of membrane damage induced by the amphipathic peptides
cultured neurons: relationship to the dopamine uptake system and inhibition
gramicidin S and melittin. Biochim Biophys Acta 1989;983:135–41.
of mitochondrial respiration. J Pharmacol Exp Ther 1992;260:1400–9.
Katsu T, Nakao S, Iwanaga S. Mode of action of an antimicrobial peptide,
Sayre LM, Wang F, Hoppel CL. Tetraphenylborate potentiates the respiratory
tachyplesin I, on biomembranes. Biol Pharm Bull 1993;16:178–81.
inhibition by the dopaminergic neurotoxin MPP+ in both electron transport
Kim KC, Burkman AM. Haloperidol causes irreversible damage to rat anterior
particles and intact mitochondria. Biochem Biophys Res Commun
pituitary lactotropes in culture. Res Commun Chem Pathol Pharmacol
Seeman P, Staiman A, Lee T, Chau-Wong M. The membrane actions of
Klawans HL, Goetz CG, Perlik S. Tardive dyskinesia: review and update. Am
tranquilizers in relation to neuroleptic-induced parkinsonism and tardive
J Psychiatry 1980;137:900–8.
dyskinesia. Adv Biochem Psychopharmacol 1974;9:137–48.
T. Murata et al. / Progress in Neuro-Psychopharmacology & Biological Psychiatry 31 (2007) 848–857
Sergent O, Pereira M, Belhomme C, Chevanne M, Huc L, Lagadic-Gossmann
Ulrich S, Neuhof S, Braun V, Danos P, Pester U, Hoy L. Disposition of
D. Role for membrane fluidity in ethanol-induced oxidative stress of primary
haloperidol pyridinium and reduced haloperidol pyridinium in schizophrenic
rat hepatocytes. J Pharmacol Exp Ther 2005;313:104–11.
patients: no relationship with clinical variables during short-term treatment.
Shinitzky M, Inbar M. Microviscosity parameters and protein mobility in
J Clin Psychopharmacol 2000;20:210–9.
biological membranes. Biochim Biophys Acta 1976;433:133–49.
Ulrich S, Sandmann U, Genz A. Serum concentrations of haloperidol
Shinitzky M, Barenholz Y. Fluidity parameters of lipid regions determined by
pyridinium metabolites and the relationship with tardive dyskinesia and
fluorescence polarization. Biochim Biophys Acta 1978;515:367–94.
parkinsonism: a cross-section study in psychiatric patients. Pharmacopsy-
Shivakumar BR, Ravindranath V. Oxidative stress induced by administration of
the neuroleptic drug haloperidol is attenuated by higher doses of haloperidol.
Urano S, Asai Y, Makabe S, Matsuo M, Izumiyama N, Ohtsubo K, et al.
Brain Res 1992;595:256–62.
Oxidative injury of synapse and alteration of antioxidative defense systems
Shivakumar BR, Ravindranath V. Oxidative stress and thiol modification
in rats, and its prevention by vitamin E. Eur J Biochem 1997;245:64–70.
induced by chronic administration of haloperidol. J Pharmacol Exp Ther
Usuki E, Pearce R, Parkinson A, Castagnoli Jr N. Studies on the conversion of
haloperidol and its tetrahydropyridine dehydration product to potentially
Siebert GA, Pond SM, Bryan-Lluka LJ. Further characterisation of the
neurotoxic pyridinium metabolites by human liver microsomes. Chem Res
interaction of haloperidol metabolites with neurotransmitter transporters in
rat neuronal cultures and in transfected COS-7 cells. Naunyn Schmiede-
Usuki E, Bloomquist JR, Freeborn E, Casagnoli K, Van Der Schyf CJ,
berg's Arch Pharmacol 2000;361:255–64.
Castagnoli Jr N. Metabolic studies on haloperidol and its tetrahydropyridinyl
Silvestri S, Seeman MV, Negrete JC, Houle S, Shammi CM, Remington GJ,
dehydration product (HPTP) in C57BL/6 mouse brain preparations.
et al. Increased dopamine D2 receptor binding after long-term treatment with
Neurotox Res 2002;4:51–8.
antipsychotics in humans: a clinical PET study. Psychopharmacology (Berl)
Volles MJ, Lansbury Jr PT. Vesicle permeabilization by protofibrillar
α-synuclein is sensitive to Parkinson's disease-linked mutations and
Subramanyam B, Rollema H, Woolf T, Castagnoli Jr N. Identification of a
occurs by a pore-like mechanism. Biochemistry 2002;41:4595–602.
potentially neurotoxic pyridinium metabolite of haloperidol in rats. Biochem
Volles MJ, Lee SJ, Rochet JC, Shtilerman MD, Ding TT, Kessler JC, et al.
Biophys Res Commun 1990;166:238–44.
Vesicle permeabilization by protofibrillar α-synuclein: implications for the
Subramanyam B, Pond SM, Eyles DW, Whiteford HA, Fouda HG, Castagnoli Jr
pathogenesis and treatment of Parkinson's disease. Biochemistry 2001;40:
N. Identification of potentially neurotoxic pyridinium metabolite in the urine
of schizophrenic patients treated with haloperidol. Biochem Biophys Res
Waddington JL. Spontaneous orofacial movements induced in rodents by very
long-term neuroleptic drug administration: phenomenology, pathophysiol-
Subramanyam B, Woolf T, Castagnoli Jr N. Studies on the in vitro conversion of
ogy and putative relationship to tardive dyskinesia. Psychopharmacology
haloperidol to a potentially neurotoxic pyridinium metabolite. Chem Res
Walum E, Peterson A. Tritiated 2-deoxy-D-glucose as a probe for cell membrane
Tanii H, Huang J, Ohyashiki T, Hashimoto K. Physical–chemical-activity
permeability studies. Anal Biochem 1982;120:8–11.
relationship of organic solvents: effects on Na+/K+-ATPase activity and
Wright AM, Bempong J, Kirby ML, Barlow RL, Bloomquist JR. Effects of
membrane fluidity in mouse synaptosomes. Neurotoxicol Teratol 1994;16:
haloperidol metabolites on neurotransmitter uptake and release: possible role
in neurotoxicity and tardive dyskinesia. Brain Res 1998;788:215–22.
Tanii H, Zhang XP, Ohyashiki T. In vitro influences of alcohols on mouse
Wysowski DK, Baum C. Antipsychotic drug use in the United States, 1976–
synaptosomes, and structure–activity relationships. Arch Toxicol 1995;69:
1985. Arch Gen Psychiatry 1989;46:929–32.
Yokoyama H, Kasai N, Ueda Y, Niwa R, Konaka R, Mori N, et al. In vivo
Tipton KF, Singer TP. Advances in our understanding of the mechanisms of the
analysis of hydrogen peroxide and lipid radicals in the striatum of rats under
neurotoxicity of MPTP and related compounds. J Neurochem 1993;61:
long-term administration of a neuroleptic. Free Radic Biol Med 1998;24:
Ulrich S, Wurthmann C, Brosz M, Meyer FP. The relationship between serum
concentration and therapeutic effect of haloperidol in patients with acuteschizophrenia. Clin Pharmacokinet 1998;34:227–63.
Source: http://fisica.uc.pt/data/20072008/apontamentos/apnt_172_13.pdf
Getränke Havanna vo „Staubfänger"-Phalanx fürs heimatliche Wohnzimmer Havanna von A bis Z Adressen Nummer 169 A an der Ecke der Straßen Virtudes und Amistad. Die Adressen in Havanna sind recht Damit man sich leichter nach dem Weg verwirrend – auf den ersten Blick. Bei erkundigen kann, sind in diesem City- näherer Betrachtung sind sie allerdings
The Expanding Cosmos of Nuclear Receptor Coactivators David M. Lonard1 and Bert W. O'Malley1,*1Department of Molecular and Cellular Biology, Baylor College of Medicine, Houston, TX 77030, USA*Contact: [email protected] DOI 10.1016/j.cell.2006.04.021 About 200 coactivators play a central role in promoting gene expression mediated by nuclear receptors. This diverse group of proteins are key integrators of signals from steroid hormones and have been implicated in cancer and other diseases.












