
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Pii: s0162-0134(01)00182-9

Journal of Inorganic Biochemistry 84 (2001) 163–170
www.elsevier.nl / locate / jinorgbio
Complexes of Ni(II) and Cu(II) with ofloxacin
Crystal structure of a new Cu(II) ofloxacin complex
Benigno Mac´ıas *, Mar´ıa V. Villa , Inmaculada Rubio , Alfonso
Castineiras , Joaqu´ın
aDepartamento de Qu´ımica
Inorganica, Facultad de Farmacia, Universidad de Salamanca, Campus Unamuno, 37007-Salamanca, Spain
bDepartamento de Qu´ımica
Inorganica, Facultad de Farmacia, Universidad de Santiago de Compostela, Santiago de Compostela, Spain
cDepartamento de Qu´ımica
Inorganica, Facultad de Farmacia, Universidad de Valencia, Valencia, Spain
Received 6 September 2000; received in revised form 25 January 2001; accepted 29 January 2001
Several coordination compounds formed between Ni(II) or Cu(II) with ofloxacin have been synthesised and characterised. According to
elemental chemical analysis and FT-IR spectroscopy data, direct reaction of Ni(II) and Cu(II) salts with ofloxacin leads to formation ofprecipitates for which mass spectrometry demonstrates their polymeric nature. However, crystalline [Cu(oflo) (H O)]?2H O is formed if
the reaction is carried out in the presence of ammonia. This complex crystallises in the triclinic system, space group P-1 with
a59.2887(12), b511.2376(14), c517.874(2) A, a 592.12(3), b 595.39(3), g591.71(3)8 and Z52. The local geometry around theCu(II) ion is a slightly distorted square base pyramid. Electronic spectra, magnetic susceptibility measurements and EPR spectra of thesynthesised complexes indicate a tetragonal environment.
2001 Elsevier Science B.V. All rights reserved.
Keywords: Ofloxacin; Quinolones; Nickel complexes; Copper complexes
metal cations have been reported in the literature, speciallythose dealing with cinoxacin [2–6], although some studies
on coordination compounds between ofloxacin (hereafter
oflo) or ciprofloxacin with metal cations commonly found
zoxacine-6-carboxilic acid), is a nalidixic acid analog with
in several drugs used as antacids have been also reported
broad spectrum antibacterial activity (Scheme 1). It
[7]. These studies have been mainly directed towards
belongs to the fluorquinolones group, which act as specific
identifying the groups directly attached to the metal site,
inhibitors of the bacterial DNA-gyrase, the enzyme respon-
and establishing the structure of the coordination com-
sible for converting double-stranded DNA into a negative
pounds thus formed. In the present work, we report on the
superhelical form [1].
interaction between several Cu(II) and Ni(II) salts with
Studies of interaction between several quinolones with
oflo, analysing the effect of the counteranion in the startingmetal salt on the nature of the compound finally formed, aswell as the role of such anions on the groups relevant inthe coordination to such a cation, determining by X-raydiffraction (XRD) procedures the molecular structure ofone of the complexes isolated.
2.1. Materials and methods
Ofloxacin was provided by Sigma and all reagents used
*Corresponding author. Tel.: 134-923-294-524; fax: 134-923-294-
were of analytical grade.
E-mail address: [email protected] (B. Mac´ıas).
Chemical analyses for carbon, hydrogen, and nitrogen
0162-0134 / 01 / $ – see front matter
2001 Elsevier Science B.V. All rights reserved.
B. Mac´ıas et al. / Journal of Inorganic Biochemistry 84 (2001) 163 –170
were performed on a 2400 elemental analyzer from Perkin-
Crystal data and structure refinement for [Cu(oflo) (H O)]?2H O
Elmer. Nickel and copper were determined on a ICPspectrometer (Perkin-Elmer model 2380 Plasma 2).
Empirical formula
IR spectra were recorded using KBr mulls and a Perkin-
Elmer FT-IR instrument. Electronic spectra were recorded
on a Shimadzu UV-240 double beam with a diffuse
reflectance accessory and a Hewlett-Packard 8452A diode
P-1 (No. 2)
Unit cell dimensions
The room-temperature magnetic moment was measured
by the Faraday method on a AZTEC DSM8 pendulum-
type susceptometer and electron paramagnetic resonance
spectra were recorded at X-band frequencies with a Bruker
The water content in the complexes was determined by
thermal analysis, using a Perkin-Elmer TGA-7 thermobal-
Calculated density (mg / m )
ance and a DTA-7 differential thermal analysis apparatus,
Absorption coefficient (mm
both operating at a heating rate of 58C / min and under
oxygen as the reaction atmosphere.
Crystal size (mm)
u range for data collection (8)
Molecular masses were measured by Servicio de Masas
2 8 5 h 5 12
Autonoma de Madrid, Spain) by the FAB
2 14 5 k 5 11
method with samples held on a nitrobenzyl alcohol (NBA)
2 23 5 l 5 19
matrix and L-SIMS ionization mode, in a VG Autospec
Reflections collected / unique
11012 / 7673 [R
apparatus; the source was maintained at 308C and 35 keV,
Completeness to u 5 28.06 (%)
Max. and min. transmission
ion were used.
Data / restraints / parameters
Goodness-of-fit on F
2.2. Syntheses of the complexes
Final R indices [I . 2s(I )]
R 50.0703, wR 5 0.1433
R indices (all data)
R 5 0.1617, wR 5 0.1698
The complexes have been prepared by direct reaction
Largest diff. peak and hole (e / A )
MS spectra for the compound Cu(oflo)Cl?2.5H O.
B. Mac´ıas et al. / Journal of Inorganic Biochemistry 84 (2001) 163 –170
between oflo with the corresponding metal cations in theform of water-soluble salts, such as chlorides, sulphates ornitrates. Although in preliminary studies 0.1 M NaOH wasadded to the solution in order to improve solubility of oflo,it was observed that addition of the metal cation gave riseto the same effect, the nature of the complexes formedbeing independent on the presence of NaOH. A typicalprocedure was as follows: 0.41 mmol of the metal saltdissolved in 5 ml H O were added to a magnetically
stirred solution containing 0.82 mmol oflo suspended in 25ml H O. As the amount of cation added was increased,
oflo dissolved, the solution attaining an emerald-greencolour in the case of Ni(II); the concentration of the finalsolution (by elimination of the solvent or by drying in adesiccator with concentrated sulphuric acid) gave rise to abright green precipitate with a sponge-like aspect, whichwas filtered and washed with distilled water. For thecopper salts, the solution becomes deep blue and shortlyafter completing the addition of the salt a greenish-blueprecipitates is formed, which is filtered and washed alsowith distilled water. The solids formed have very lowsolubility in water, probably because of their polymericnature (see below). Yields were greater than 90% in bothcases. Experimental data fit well with the calculatedformula if the presence of crystallization water moleculesis assumed, as checked by thermal analysis. Ni(oflo)(SO )
?2.5H O. Calc.: C, 42.2; H,4.7; N, 8.2; Ni, 11.5;
H O loss, 8.9. Found: C, 42.6; H, 4.9; N, 8.2; Ni, 10.9;
H O loss 9.4. Ni(oflo)Cl?2.5H O. Calc.: C, 43.3; H, 4.8; N,
8.4; Ni, 11.8; H O loss, 9.0. Found: C, 43.7; H, 4.9; N,
8.1; Ni, 11.4; H O loss, 9.1. Ni(oflo)(NO )?2.5H O. Calc.:
C, 41.1; H, 4.6; N, 10.7; Ni, 11.2; H O loss, 8.6. Found: C,
41.2; H, 4.8; N, 10.9; Ni, 10.9; H O loss, 8.3.
Fig. 2. FT-IR spectra for the compounds: (a) ofloxacin, (b) Ni(oflo)Cl?
?2.5H O. Calc.: C, 41.8; H, 4.7; N, 8.1;
2.5H O, (c) Ni(oflo)(SO )
?2.5H O, (d) Ni(oflo) (NO )?2.5H O and (e)
Cu, 12.3; H O loss, 8.7. Found: C, 42.0; H, 4.9; N, 8.1;
Ni(oflo) ?3H O.
Cu, 12.2; H O loss, 8.8. Cu(oflo)Cl?2.5H O. Calc.: C,
42.9; H, 4.8; N, 8.3; Cu, 12.6; H O loss, 8.9. Found: C,
Cu(oflo)(NO )?2.5H O. Calc.: N, 40.7; H, 4.6; N, 10.6;
Cu, 12.0; H O loss, 8.5. Found: C, 41.1; H, 4.7; N, 10.8;
Selected bond lengths (A) and angles (8) for [Cu(oflo) (H O)]?2H O
Cu, 12.2; H O loss, 8.5.
Under the experimental conditions used, it was not
possible to isolate the compounds in a crystalline form, but
single crystals were isolated if the synthesis is carried out
in the presence of ammonia as follows: 0.35 mmol of the
metal salt are added to 0.7 mmol oflo previously dissolved
in 10 ml of 1 M NH . A bright green (in the case of
nickel) or deep blue (in the case of copper) solution is
formed, although in the case of the copper solution, it turns
into emerald-green after a few hours. Crystals, which are
separated by filtration, are formed after 2 or 3 days.
Ni(oflo) ?3H O. Calc.: C, 51.8; H, 5.3; N, 10.1; Ni, 7.0,
H O loss, 6.5. Found: C, 51.6; H, 5.4; N, 10.2; Ni, 7.4;
H O loss, 6.8. [Cu(oflo) (OH )]?2H O. Calc.: C, 51.3; H,
5.3; N, 10.0; Cu, 7.6; H O loss, 6.4. Found: C, 51.4; H,
Symmetry transformations used to generate equivalent atoms: [1
5.5; N, 10.2; Cu, 7.9; H O loss, 6.7. The Cu-complex
x 1 1, y, z.
B. Mac´ıas et al. / Journal of Inorganic Biochemistry 84 (2001) 163 –170
crystals prepared could be analysed by XRD in order to
3. Results and discussion
determine their crystalline structure; unfortunately, the Nicomplex crystals did not give rise to adequate diffractions.
3.1. Mass spectrometry
The results obtained support the assumption above about
2.3. X-ray structure determination of [Cu(oflo) (H O)]?
the polymeric nature of the compounds isolated. As shown
in Fig. 1 for the Cu complex prepared from the chloride,
peaks due to m /z values corresponding to different stoich-
A light green block crystal of [Cu(oflo) (H O)]?2H O
iometries (depicted in Fig. 1) are recorded, in addition to
was mounted on a glass fiber and used for data collection.
the most intense signal at m /z 5784.2, corresponding to
Crystal data were collected at 291 K using a Bruker Smart
the molecular ion, [Cu(oflo) ] , and that at m /z 5424.1,
corresponding to [Cu(oflo)] . Similar results were also
MoKa radiation ( l50.71073 A) was used throughout. The
obtained for the Ni complexes, although in this case they
data were processed with SAINT [8] and corrected for
are associated with the NBA matrix. It can be tentatively
absorption using SADABS (transmissions factors: 1.000–
concluded that the original polymer is broken into different
0.644) [9]. The structure was solved by direct methods
fragments, with different sizes, in the ionization chamber.
using the program SHELXS-86 [10] and refined by full-
Both the water molecules and the counteranions seem to be
matrix least-squares techniques against F using SHELXL-97
absent in the detected ionic fragments, so we may conclude
[11]. Positional and anisotropic atomic displacement pa-
they are only weakly bonded to the main fragments.
rameters were refined for all nonhydrogen atoms. Hydro-gen atoms were located from difference syntheses andrefined isotropically. The H atoms of two water molecules,
3.2. IR spectra
O(2) and O(3) in the next tables, were not located. Atomicscattering factors were from the International Tables for
Although the counteranions are not detected by MS,
X-ray Crystallography [12]. Molecular graphics were from
their presence in the solids isolated is definitively con-
PLATON [13]. A summary of the crystal data, experimental
cluded from the FT-IR spectra. So, bands are detected at
details and refinement results are listed in Table 1.
(sulphate) or 1351 cm
Fig. 3. ORTEP diagram for [Cu(oflo) ?H O]?2H O with the atom-labelling scheme.
B. Mac´ıas et al. / Journal of Inorganic Biochemistry 84 (2001) 163 –170
in Fig. 2. The band at 1713 cm
due to the carboxylic
Atomic coordinates ( 310 ) and equivalent isotropic displacement param-
group is not detected in the spectra of any of the
complexes, indicating that this moiety participates in the
bonding to the metal ion [15]. However, the techniquedoes not permit a definitive conclusion about the participa-
tion of the ketonic group in the bonding to the metal; the
corresponding band is recorded at 1622 cm
spectrum of oflo, and a band close to this position in the
spectra of the complexes could be due to the ketonic group
or to the carboxylate group bonded to the metal ion. If this
band is due to the ketonic group, the antisymmetric and
symmetric modes of the carboxylate group would account
for the bands recorded at 1575 and 1570 cm
ly. However, if the ketonic group participates in the
bonding to the metal we would expect a shift of its
stretching band towards lower wavenumbers, thus corre-
sponding to the band recorded at 1575 cm
bands at 1620 ad 1470 cm
would correspond to the
carboxylate group. We should conclude that FT-IR spec-
troscopy, by itself, does not permit a definitive answer to
the way the ligand is bonded to the metal cation. Finally,
the band at 508 cm
could be ascribed to the Ni–O
stretching mode [2,4]. The IR spectra of the Cu(II)
complexes are similar to that Ni(II) complexes.
3.3. Crystal structure of [Cu(oflo) (H O)]?2H O
An ORTEP diagram of the complex [Cu(oflo) (H O)]?
2H O including the atomic numbering scheme is shown in
Fig. 3 and selected bond distances and angles are presented
in Table 2. Atomic coordinates and equivalent isotropic
displacement coefficients are shown in Table 3.
The crystalline structure data definitively demonstrates
the participation of the ketonic group of the oflo molecule
in the bonding to the metal cation, the Cu(II) cation
becoming five coordinated in a square base pyramidal
structure. The copper atom is located 0.205 A above the
average plane defined by oxygens O11, O31, O13 and
O33, which deviate from such average plane 0.039 A
above (O11 and O31) and 0.039 A below (O13 and O33).
The apical position would be occupied by a water mole-
cule and the four corners of the base would be occupied by
four oxygen atoms, two from the carboxylate groups and
the two remaining from the ketonic groups (from two oflo
molecules). The metal atom placed in a crystallographic
inversion centre relates the two bidentate oflo ligands that
bind through one oxygen of the carboxylate group and the
exocyclic carbonyl oxygen. The Cu–O distances are
similar to those reported previously for the corresponding
complex with cinoxacin [5]. The geometry is not totally
regular, although distortions are not too severe. The Cu–O
(carboxylate) distances are slightly lower than the Cu–O
(ketonic) distances. The O–Cu–O angles of a given oflo
molecule are larger than the O–Cu–O angles with oxygen
is defined as one third of the trace of the orthogonalised U
atoms from different oflo molecules, although this effect
could also arise from the intrinsic structure of the oflo
B. Mac´ıas et al. / Journal of Inorganic Biochemistry 84 (2001) 163 –170
molecule. In addition two water molecules (shown in Fig.
suggesting the same chromophore in both states. A broad
4, pertinent crystallographic information given in Tables 2
band is recorded at 650 nm (e 5 12 M
and 3) provide additional crystalline stability through a
shoulder at 740 nm. This band is characteristic of regular
network of hydrogen bond interactions.
or distorted octahedral structures; the main absorption is
associated to transition n ( A → T (F)) and the shoul-
3.4. Electronic spectra
der to the spin-forbidden transition A → E , which is
usually recorded close to the main absorption, specially in
The electronic spectra recorded in solid state and in
complexes where Dq / B is close to 1, where the energies of
aqueous solution of the Ni(II) complexes are similar,
states T (F) and E are very close [16,17]. Other bands
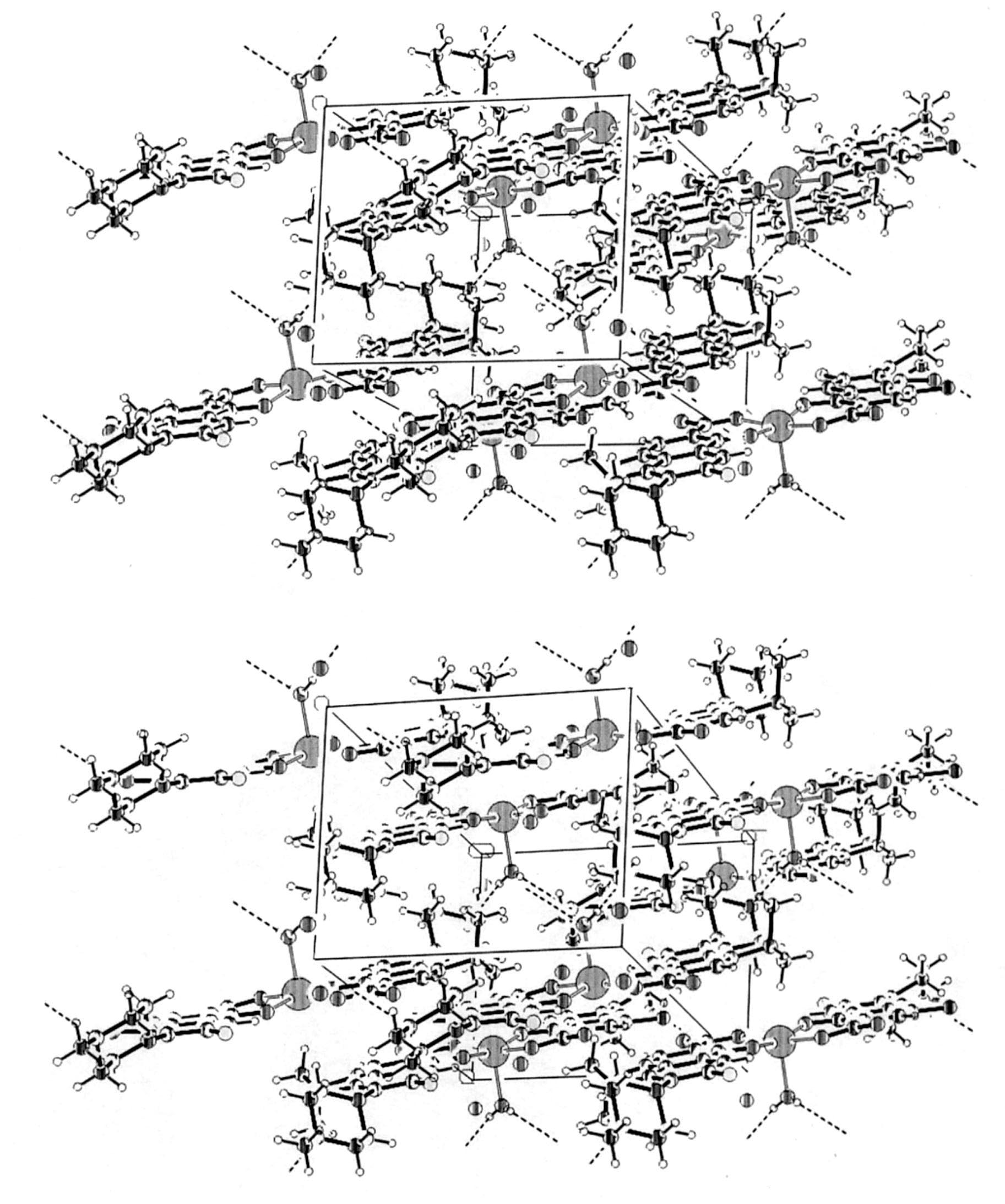
Fig. 4. Stereoscopic view of the unit cell showing the molecular packing and the intermolecular hydrogen bonding (dashed).
B. Mac´ıas et al. / Journal of Inorganic Biochemistry 84 (2001) 163 –170
Fig. 5. Electronic spectra in the solid state for: (top) Cu(oflo)Cl?2.5H O;
(middle) Cu(oflo) (NO )?2.5H O and (bottom) Cu(oflo)(SO )
in the ultraviolet region at 332, 290, and 258 nm (e 5
35 000–65 000 M
) are due to transitions between
energy levels of the ligands.
The solid spectra of the polymeric Cu(II) complexes
(Fig. 5) show a broad asymmetric band with splittings thattentatively correspond to the splitting expected for thelocal C4v symmetry of these cations in the square pyramid
Fig. 6. EPR spectra for the compounds: (a) Cu(oflo)Cl?2.5H O; (b)
?2.5H O and (c) [Cu(oflo) ?H O]?2H O.
3.5. Magnetic susceptibility and EPR spectra
The magnetic moment measured at room temperature
EPR values was obtained by simulation [20]. The EPR
for the Ni complexes is similar in all cases, with m 53.12
spectrum of polycrystalline Cu(oflo)Cl?2.5H O complex
BM, which can be related to an octahedral (regular or
were g 52.11, g 52.17, g 52.41 and A 5150 G, rather
distorted) environment [18]. The value measured is larger
close to those reported for a square base pyramid structure
than the spin-only magnetic moment, 2.83 BM, and such
[21]. The value for the R parameter [R 5 ( g –g ) /( g –g )]
an increase should be due to contributions from the orbital
is 0.25, i.e. lower than 1, thus indicating that the unpaired
electron is located in orbital dx –y . The EPR spectrum of
The value measured for the Cu complexes was m 5
the Cu(oflo)(SO )
?2.5H O is axial slightly rhombic with
1.98 BM, also larger than the spin-only value, 1.73 BM.
g parameters according to the simulation programme, g 5
Such divergence is not uncommon in mononuclear Cu(II)
2.04, g 5 2.07, g 5 2.27 and A 5170 G. Finally, the
complexes due to the mixing-in of some angular moment
values for the crystalline complex [Cu(oflo) (H O)]?2H O
from the closely lying excited states via spin–orbit cou-
were g 5 2.22, g 5 2.00 and A 5 160 G, very close to
those reported in the literature for a square base pyramid
EPR spectroscopy is more sensitive to the chemical
structure, in agreement with the structure concluded for
environment of the metal cation, and thus permits discrimi-
this complex from the XRD data discussed above (Fig. 3).
nation between the properties of the different complexes
Nevertheless, according to all these data, the local geome-
isolated, as shown in Fig. 6 for the Cu complexes. The
try around copper should be close to tetragonal in all cases.
B. Mac´ıas et al. / Journal of Inorganic Biochemistry 84 (2001) 163 –170
[10] G.M. Sheldrick, Acta Cryst. A46 (1990) 467.
[11] G.M. Sheldrick, SHELXL-97. Program for the Refinement of
Crystal Structures, University of Goettingen, Goettingen, 1997.
The authors thank CICYT (grant PM97-0105-C02-02
[12] International Tables for X-ray Crystallography, Vol. C, Kluwer,
and IN96-0252) for financial support. Critical reading of
Dordrecht, 1995.
the manuscript by Professor V. Rives is also acknowledged.
[13] A.L. Spek, PLATON. A Multipurpose Crystallographic Tool, Ut-
recht University, Utrecht, 2000.
[14] K. Nakamoto, in: Infrared and Raman Spectra of Inorganic and
Coordination Compounds, Part B: Applications in Coordination,
Organometallic and Bioinorganic Chemistry, 5th Edition, Wiley,New York, 1997.
[1] V. Aleixandre, G. Herrera, A. Urios, M. Blanco, Antimicrob. Agents
[15] L.J. Bellamy, The Infrared Spectra of Complex Molecules, 3rd
Chemother. 35 (1991) 20.
Edition, Chapman and Hall, London, 1975.
[2] M. Ruiz, R. Ortiz, L.
Perello, Inorg. Chim. Acta 211 (1993) 133.
[16] A.B.P. Lever, Inorganic Electronic Spectroscopy, 2nd Edition,
[3] M. Ruiz, R. Ortiz, L.
Perello, S. Garc´ıa-Granda, M.R.
Dıaz, Inorg.
Elsevier, Amsterdam, 1984.
Chim. Acta 217 (1994) 149.
[17] S.M. Hart, J.C.A. Boeyens, R.D. Hancock, Inorg. Chem. 22 (1983)
[4] M. Ruiz, L.
Perello, R. Ortiz, A.
Castineiras, C.
Canton, J. Inorg. Biochem. 59 (1995) 801.
[18] L. Sacconi, F. Mani, A. Bencini, in: G. Wilkinson (Ed.), Com-
[5] M. Ruiz, R. Ortiz, L.
Perello, J. Latorre, J.
Server-Carrio, J. Inorg.
prehensive Coordination Chemistry, Vol. 5, Late Transitions Ele-
Biochem. 65 (1997) 87.
ments, Pergamon Press, Oxford, 1987, p. 54.
[6] M. Ruiz, L.
Server-Carrio, R. Ortiz, S.
[19] B.N. Figgis, J. Lewis, in: J. Lewis, R.G. Wilkins (Eds.), Modern
M.R. Diaz, E.
Canton, J. Inorg. Biochem. 69 (1998) 231.
Coordination Chemistry: Principles and Methods, Interscience, New
[7] B. Mac´ıas, M. Martinez, A.
Domınguez-Gil, Int. J.
York, 1960, p. 400.
Pharm. 106 (1994) 229.
[20] WINEPR-Simfonia. 1.25, Bruker Analytik, Kalsruhe, 1994–1996.
[8] Bruker, SMART and SAINT. Area Detector Control and Integration
[21] B.J. Hathaway, in: G. Wilkinson (Ed.), Comprehensive Coordination
Software, Bruker Analytical X-ray Instruments Madison, WI, 1997.
Chemistry, Vol. 5, Late Transitions Elements, Pergamon Press,
[9] G.M. Sheldrick, SADABS. Program for Empirical Absorption
Oxford, 1987, p. 656.
Correction of Area Detector Data, University of Goettingen, Goett-ingen, 1997.
Source: http://giqimo.com/wp-content/uploads/murry49.pdf
WASTEWATER TREATMENT SYSTEM WITH SERVICE PRO® CONTROL CENTER MODELS 960 AND TNT ®OWNER'S MANUAL FEATURES AND ADVANTAGES The Singulair system is the finest equipment available Singulair tanks are reinforced precast concrete, manufactured and utilizes the most up-to-date wastewater treatment by the licensed Norweco distributor. Internal walls and baffles
OPEN ACCESS James I. Ausman, MD, PhD For entire Editorial Board visit : University of California, Los Review ArticleMicrovascular decompression for glossopharyngeal neuralgia through a microasterional approach: A case seriesRogelio Revuelta‑Gutiérrez, Andres Humberto Morales‑Martínez, Carolina Mejías‑Soto1, Jaime Jesús Martínez‑Anda, Luis Alberto Ortega‑Porcayo






