
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Time-course gait analysis of hemiparkinsonian rats following 6-hydroxydopamine lesion


Contents lists available at
Behavioural Brain Research
Time-course gait analysis of hemiparkinsonian rats following6-hydroxydopamine lesion
Tsung-Hsun Hsieh , Jia-Jin J. Chen , Li-Hsien Chen , Pei-Tzu Chiang , Hsiao-Yu Lee
a Institute of Biomedical Engineering, National Cheng Kung University, Tainan, Taiwanb Institute of Basic Medical Sciences, National Cheng Kung University College of Medicine, Tainan, Taiwanc Department of Digital Media Design and Management, Far East University, No.49, Zhonghua Rd., Xinshi Dist., Tainan City, 74448, Taiwan
Gait disturbances similar to those of human Parkinson's disease (PD) can be observed in animals after
Received 20 August 2010
administration of neurotoxin 6-hydroxydopamine (6-OHDA) to induce unilateral nigrostriatal dopamine
Received in revised form 10 March 2011
depletion. However, the relationship between gait disturbances and dopamine depletion following 6-
Accepted 14 March 2011
OHDA infusion has not been determined. The present study investigated the longitudinal changes ofspatiotemporal gait patterns using a walkway system to acquire footprints and lateral limb images over
a 6-week period following unilateral 6-OHDA injection into the medial forebrain bundle of rats. Our
Parkinson's disease
results indicated that hemiparkinsonian rats exhibited changes in gait patterns, as compared to normal
controls, and pre-lesion levels, including a significantly decreased walking speed and step/stride length
Gait patternDopaminergic neurons
as well as an increased base of support and foot angle. The relative percentage of the gait cycle was alsoaltered, showing an increase in the stance to swing ratio, which was more evident in the affected hindlimb.
Time-course observations showed that these gait disturbances occurred as early as 4 days post-lesionand gradually increased up to 42 days post-injury. The extents of gait disturbances were compared withconventional apomorphine-induced turning behavior and akinesia bar tests, which were also apparentat 4 days post-lesion but remained relatively unchanged after 28 days. Our time-course gait analysis ofa unilateral 6-OHDA rodent model provides insight into the compensatory changes of motor functionsduring the 6-week development of a nigrostriatal lesion, which might be useful for future objectiveassessment of novel treatments for human PD subjects.
Crown Copyright 2011 Published by Elsevier B.V. All rights reserved.
1,2,3,6-tetrahydropyridine (MPTP) or by infusion of the neurotoxin6-hydroxydopamine (6-OHDA) in rats. Whereas MPTP injection
Gait disturbances are commonly observed in subjects with
causes acute and bilateral lesions in the nigrostriatal dopaminer-
Parkinson's disease (PD) resulting from a degeneration of dopamin-
gic system, unilateral injection of 6-OHDA into the rat SN, medial
ergic (DA) neurons in the substantia nigra (SN) the
forebrain bundle (MFB) or striatum (Str) has commonly been used
hallmark changes of gait following PD include temporal asymme-
to induce the changes of motor dysfunction observed in the hemi-
try, which manifests as an inability to maintain internal gait rhythm
parkinsonian rat model
walking speed, increased cadence and increased dou-
Several animal behavior tests have been devised to assess the
ble stance time n addition, PD subjects exhibit abnormal spatial
functional deficits and to quantify the behaviors that are simi-
indices of gait patterns, which typically include short steps
lar to human PD symptoms, including a rotation test for severity
freezing gait decreased stride length gait abnor-
of dopamine depletion bar test for akinesia a
malities become more pronounced in the advanced stages of PD,
stepping test for rigidity gait impairment is the car-
inducing further disability or limitation of mobility. To understand
dinal sign of PD in humans, gait analysis is used to quantify the
the development of PD and to further explore effective thera-
multifaceted and complex motor functions in PD animal models
peutic strategies for improved management of gait disturbances,
studies investigating PD gait disorders in rats often
it is important to have relevant PD animal models, which can
used footprints to monitor abnormalities in the spatial parameters
be obtained by systemic administration of 1-methyl-4-phenyl-
of gait. For example, the rodent hind paws were inked, and therodent was then allowed to walk on paper strips. Based on foot-print assessment, rats with a unilateral PD lesion were found to
∗ Corresponding author. Tel.: +886 6 597 9566x7652.
display a shuffling gait, motor asymmetries and short stride lengths
E-mail addresses: , (H.-Y. Lee).
that resemble the key features of the human PD gait
0166-4328/$ – see front matter. Crown Copyright 2011 Published by Elsevier B.V. All rights reserved.
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
ever, temporal data regarding the gait cycle in PD rats is insufficient
mirror reflected the image of the rat's paws for convenient observation with a dig-
due to the limitations of the inked footprint assessment system.
ital camera (EX-F1, Casio, Japan). For image capture, the camera was set to record
The recent development of computer-assisted automatic gait anal-
simultaneously a direct lateral view and a reflected underview of the walking track.
For lateral kinematical data acquisition, the rats were shaved and marked with red
ysis, such as CatWalk, provides objective quantification of static
on the skin of the lateral side of the bilateral hindlimbs before each test session. The
and dynamic gait parameters from footprint analysis and has been
marked landmarks included the lateral malleolus and the fifth metatarsal head as
applied to bilateral 6-OHDA lesion rats Other simple video-
identified by palpation while moving the joints. Use of colored landmarks provided
based gait analysis systems have implemented a reflective mirror
an easy way to determine the stance and swing phases of the gait cycle from heelcontact to toe off.
in a confined, transparent walking track for simultaneous recording
Before the experiment, the rats were acclimated to the walkway by allowing
of the plantar and sagittal views of the rat's hindlimbs; this allows
them to walk freely on the track for 20 min before formal recording. The walking
assessment of spatiotemporal and kinematics data in varied, freely
task was repeated in both directions, thus permitting the recording of the move-
ment of each hindlimb. The walking task was repeated until five or six satisfactory
Although gait analysis in rats has been employed in vari-
walks of at least 4 steps without pause were obtained. Only the hindlimb steppingpatterns were analyzed in our present video-based gait analysis system. The digital
ous neuroscience studies the literature is scant regarding
images obtained from each trial were processed with a threshold setting to detect
the time-course changes of motor behaviors or locomotion func-
the boundary of the soles, and critical points for derivation of paw indices were
tions during the development of the 6-OHDA hemiparkinsonian
determined using Matlab software (MatWorks, version 7.6., R2008a). After identifi-
rat model. Understanding the relationships between development
cation of sequential footprints, four spatial parameters, including step length, stridelength, base of support (BOS) and foot angle, and three temporal gait parameters, i.e.,
of motor disturbances and the degrees of DA cell loss might
walking speed, stance/swing phase time and stance/swing ratio, were determined.
provide some insight into the quantitative assessment of novel
Each gait parameter was averaged for at least 20 footsteps.
therapeutic strategies for PD. Thus, the aims of the present studywere to provide a detailed analysis of the time-course changes in
gait spatiotemporal parameters and to observe the corresponding
Impairment of the initiation of movement or akinesia has been commonly char-
acterized by bar tests for immobility, stepping or cylinder tests bar
dopamine loss in the rat's brain for 6 weeks following unilateral
test was adopted in this study to observe the akinesia phenomenon of PD rats. During
6-OHDA injection.
the bar test, each rat was placed gently on a table. Each forepaw was placed alter-nately on a horizontal acrylic bar (0.7 cm diameter), which was suspended 9 cm
2. Materials and methods
above the table surface. The forepaw nearest the camera was recorded. The totaltime (in s) spent by each paw on the bar, i.e., the amount of time from the placing of
the forepaw on the bar to the first complete removal of the paw from the bar, wasrecorded
Animal studies were conducted on 41 adult male Wistar rats with a body weight
range of 350–450 g and age range of 8–12 weeks at experimental onset. The ani-
2.3.3. Analysis of apomorphine-induced spontaneous rotation
mals were separated into two groups for evaluating motor behaviors and DA cell
A conventional behavioral assessment using apomorphine-induced rotation
loss. Sixteen rats (eight normal control and eight lesioned rats) were assigned for
was performed to quantify the unilateral nigrostriatal lesion-induced motor asym-
time-course assessment of motor behaviors for six weeks. The other 25 rats were
metry after ipsilateral 6-OHDA injection rotational tests were
separated into five subgroups, which were sacrificed at pre-lesion and at 1, 7, 21 and
performed with apomorphine (0.5 mg/kg in 0.1% ascorbic acid, i.p.; Sigma) injec-
42 days for evaluating the degree of DA neuron loss following the 6-OHDA lesion. All
tion of PD rats. The rats were placed individually in a 30-cm-diameter round bowl
rats were obtained from the Laboratory Animal Center, National Cheng Kung Uni-
and assessed over a 60-min period stickers of two different colors were
versity, Taiwan. The rats were housed at 25 ◦C with a 12/12 h light/dark cycle and
pasted on the rat's back for easy identification of torso direction from the vec-
continuous water and food. All experiments followed the Guide for the Care and Use
tor change derived from the centers of the color circles. For precise calculation of
of Laboratory Animals.
the number of rotations after apomorphine injection, the rotational behavior wasrecorded using a digital video camera, which was analyzed at 10-min intervals using
2.2. Chronic hemiparkinsonian rat model
an image analysis program written in Matlab. The net number of rotations was cal-culated as the difference between the number of contralateral rotations and the
For the 6-OHDA lesion, the rat was anaesthetized with intraperitoneal
number of ipsilateral rotations with respect to the 6-OHDA injection side.
400 mg/kg chloral hydrate and placed into a stereotactic apparatus (Stoelting, IL,USA) to prevent head movement using a 45◦ non-puncture ear bar with the nose
position at 3.3 mm below the interaural line. A 2-cm incision was made, and the areawas carefully cleared to expose the line of bregma. To cause destruction of the nigros-
For evaluating DA neuron loss, tyrosine hydroxylase (TH) staining at five time
triatal pathway, which results in near total depletion of dopamine in the ipsilateral
points post-lesion was performed. The animals were deeply anaesthetized with
Str and the SN g/l of 6-OHDA (dissolved in 0.02% ascorbic saline, Sigma
an overdose of pentobarbital and perfused transcardially with 0.9% saline and 4%
Chemical Co., USA) was injected intra-cranially into the MFB (anterior–posterior:
paraformaldehyde (PFA) in 0.1 M phosphate buffer solution (PBS). Brains were
−4.3 mm from the bregma; lateral: 1.6 mm with respect to the midline and ven-
removed and post-fixed for 3 days in the same fixative and dehydrated in 30%
tral 8.2 mm from skull surface;) according to the stereotaxic brain atlas of Paxinos
sucrose in 0.02 M PBS until the brain sank. The brains were cut into 30-m sec-
and Watson was done on left side of the brain using a 26-gauge 10-l
tions containing the Str and the SN on a cryostat (Thermo Shandon Ltd., UK). Every
Hamilton microsyringe mounted vertically on the stereotactic frame. The syringe
fourth section was selected from the region spanning from −5.20 mm to −5.80 mm
was lowered through the burr hole, and the toxin was infused at a rate of 0.5 l/min
in the SN and from +1.70 mm to +2.30 mm in the Str with respect to the bregma
with a syringe pump, giving a total volume of 4 l. The needle was left in the brain
free-floating sections were quenched for 10 min in 0.3% H2O2/PBS and
for at least 5 min to prevent back filling along the injection tract
rinsed in a 1:200 dilution of concentrated IHC Wash Solution with distilled water
Rats with successful lesions were typically slower in their general activity and
for 5 min. Rinsing was repeated 3 times. All sections were soaked in nonspecific
had a tendency to turn toward the ipsilateral lesion side, but had a tendency to turn
antibody binding solution that was blocked by Ready-To-Use IHC Blocking Solu-
toward the contralateral side after apomorphine injection effectiveness of
tion for 15 min. The sections were subsequently incubated with a 1:1000 dilution
the MFB lesion was verified by an apomorphine-induced rotational test at 2 weeks
of rabbit primary anti-TH (cat #AB125, Millipore) with Ready-To-Use IHC Antibody
following the lesion f apomorphine-induced contralateral rotation behavior
Diluent for 15–18 h at room temperature and then incubated in IgG anti-Rabbit
did not occur, the rat was excluded from further statistical analysis.
IHC Antibody (Bethyl). Immunostaining was visualized by peroxidase reaction withstabilized, metal-enhanced diaminobenzidine for approximately 5–10 min with
2.3. Behavioral tests
enhanced visualization by hematoxylin and, finally, bluing solution for 1–2 min. Sec-tions were mounted on chromalum-coated slides, dehydrated in ascending alcohol
Three motor behavior tests (gait, bar and drug-induced rotation) were per-
concentrations, cleared in xylene and coverslipped in DPX. The TH-positive neurons
formed in same sequence on same day. For each test, there were at least 2 h of
in the SN from both hemispheres were counted manually in each section loss
resting time between each test.
rate of TH-positive cells in the lesion hemisphere was calculated and normalized asthe percentage of TH-positive neurons with respect to the unlesioned side.
2.3.1. Spatiotemporal analysis of gait patterns
A walking track equipped with a video-based system was modified from pre-
2.5. Experimental design and statistical analysis
vious studies for acquiring more spatiotemporal parameters of gait in this studywalking track apparatus consisted of a plexiglass chamber 80 (l) × 6
For motor behavioral testing, eight PD-lesioned and another eight normal
(w) × 12 (h) cm with a mirror tilted at 45◦ underneath the walking track. The tilted
animals were pre-tested (gait, bar and rotation tests) at least two days before
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
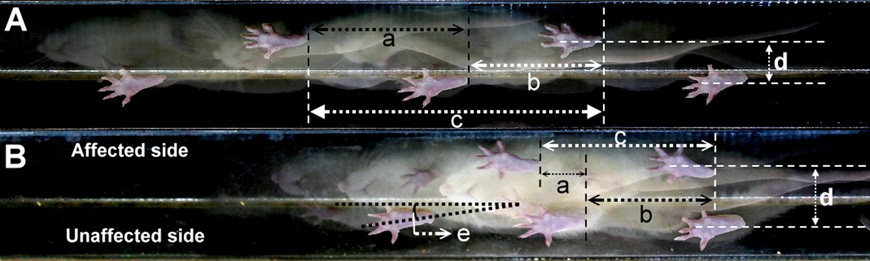
Fig. 1. Characteristics of stepping footprint during locomotion in (A) a pre-surgery and (B) a 42-day post-unilateral PD lesioned rat. Note the shorter step lengths of the
affected side (a) and the unaffected side (b) in the lesioned rat relative to the pre-surgery rat. The stride lengths (c) are also shorter, in contrast to the wider BOS (d) and larger
foot angle (e) relative to the pre-surgery level.
injection to establish baseline data. After induction of a unilateral 6-OHDA lesion,
rection post hoc test when the main effect of time was significant. Also, a paired
motor behavior test sessions were performed on the first and fourth day post-
t-test was performed to investigate the differences between the ipsilateral (unaf-
lesion, then at weekly intervals up to 6 weeks (i.e., 1, 7, 14, 21, 28, 35 and 42
fected) and contralateral (affected) sides over the time-course. For bar and rotation
days post-lesion) under the same environmental conditions. For immunohisto-
tests, a one-factor analysis (time) repeated-measures ANOVA was used to compare
chemistry analysis, five out of 25 lesioned rats were sacrificed at each of five
pre- and post-values at each time point followed by a Bonferroni correction post
specific time points (i.e., pre-lesion and 1, 7, 21 and 42 days post-lesion) for
hoc test in PD rats. For immunohistochemistry analysis, a one-way ANOVA was also
TH staining.
performed to compare between groups followed by a Tukey's post hoc test. Data
For statistical analysis of gait measurements, a two-way repeated measure anal-
were analyzed using SPSS version 17.0 (SPSS Inc., USA) with the significance level
ysis of variance (ANOVA) was used to test both group (PD versus normal) and time
set at p < 0.05 for each assessment. All data were presented as the average ± standard
factors. Multiple within-subject comparisons were taken with the Bonferroni cor-
error of the mean (SEM).
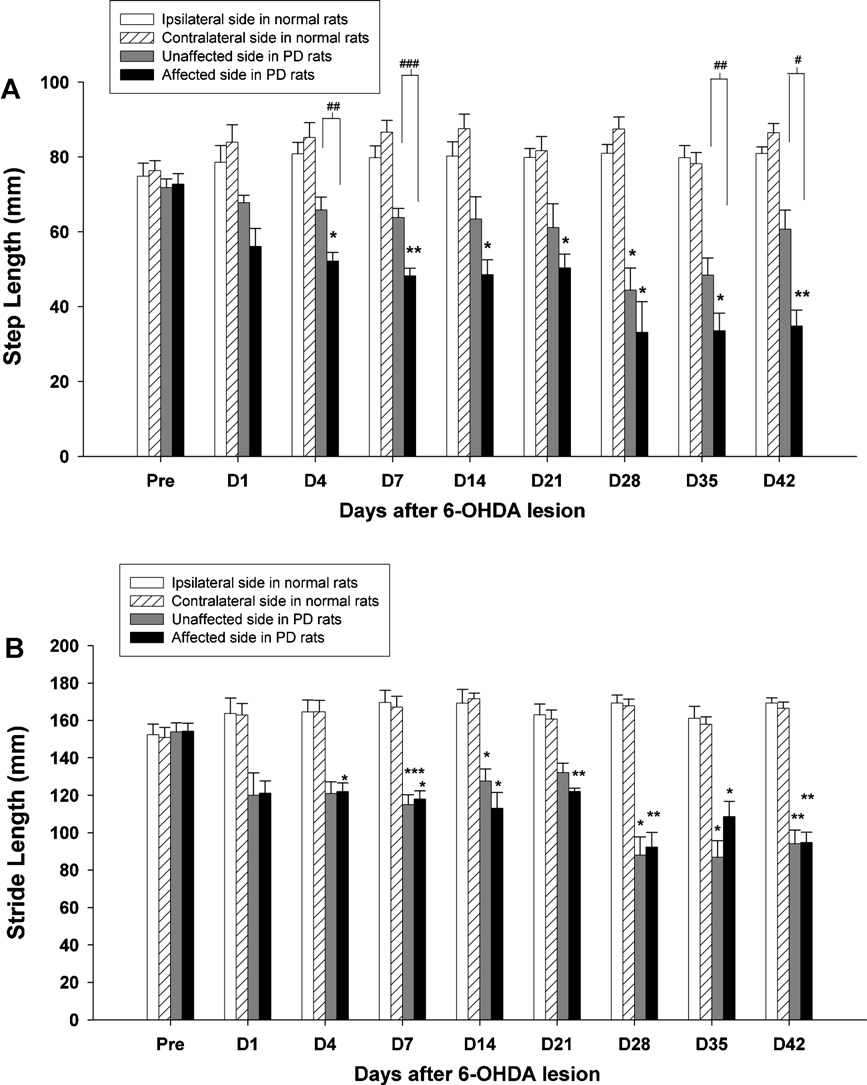
Fig. 2. Time-course changes in the step length (A) and stride length (B) of the ipsilateral (left) and contralateral (right) hindlimbs were observed over 42 days in hemi-
parkinsonian rats. Asterisks represent significant differences as compared to the baseline data before surgery by using a paired t-test with a Bonferroni correction (*p < 0.05,
**p < 0.01, ***p < 0.001). Significant changes between two hindlimbs are represented with a square bracket (paired t-tests, #p < 0.05, ##p < 0.01, ###p < 0.001). Note that the
step length dropped significantly in the affected side (right) after 4 days post-lesion, and the stride length of the lesioned hemisphere (right) was particularly evident from
the earliest (day 4) until the last (day 42) time points. Data represent the mean (±SEM) length (mm) of the hindlimb during gait.
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
3. Results
and contralateral limb (F8,56 = 4.89; p < 0.001) groups (F1,7 = 175.80;p < 0.001 in ipsilateral limb and F1,7 = 224.93; p < 0.001 in contralat-
3.1. Spatiotemporal footprint analysis
eral limb) and a significant time × group interaction (F8,56 = 5.41;p < 0.001 in ipsilateral limb and F8,56 = 7.09; p < 0.001 in contralat-
Eight normal rats and eight PD rats completed gait analysis
within 42 days post-lesion. a representative series of
In the normal control group, no significant differences were
footprint images captured from a pre-lesion rat (and a rat
found in all gait parameters across the test time when compared to
at 42 days post-lesion (The post-lesion footprints clearly
pre-surgery baseline data (all p > 0.05). In PD rats, a post hoc analy-
showed shorter steps, especially on the affected side, whereas the
sis with a Bonferroni correction in the time effect showed that the
pre-lesion animal exhibited a relatively consistent stride length.
step and stride lengths in the affected side gradually decreased and
Furthermore, the ventral view of the footprints showed that post-
reached a significant difference at 4 days post-lesion (p < 0.05) fol-
lesion rats walked with a wider BOS than the pre-lesion rats. The
lowed by a progressive increase of severity up to the sixth week of
bilateral stride length in the post-lesion animals was markedly
observation (p < 0.01). Furthermore, the difference in step length of
shorter than that of the pre-lesion animals, as exemplified in
the affected side revealed a decreasing time-course trend, with the
Compared to pre-lesion measurements, post-lesion data
unaffected side showing a slower trend than the affected side.
showed significant differences in the spatial gait parameters of the
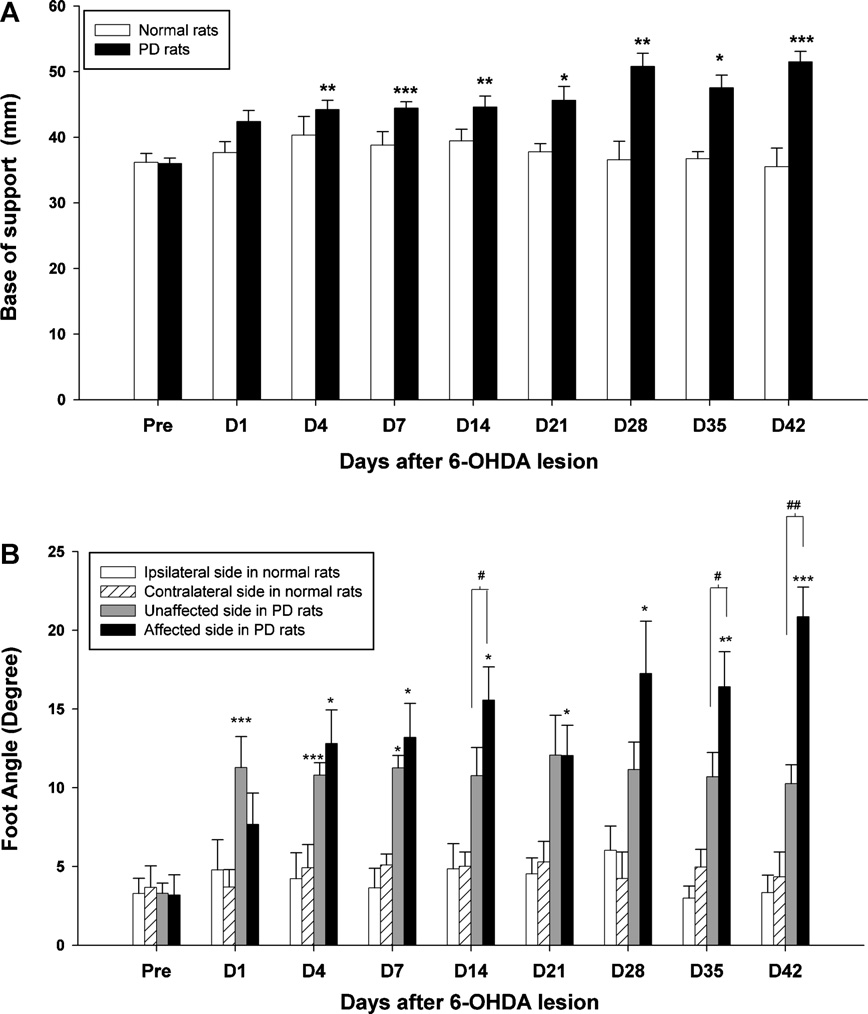
Regarding the BOS, there was a significant time × group interac-
affected (contralateral) side of the lesioned rats.
tion (F8,56 = 3.53, p = 0.00). The main effect for time was significant
the time-course changes of bilateral step length and stride length. A
(F8,56 = 3.45, p = 0.003). A significant group difference was also
two-factor ANOVA on the step length over the 42 days showed a sig-
observed (F1,7 = 59.48, p < 0.001). shows that post-lesion rats
nificant time × group interaction in the ipsilateral limb (F8,56 = 3.30,
showed a significantly larger BOS at 7 days post-lesion compared to
p = 0.004) and the contralateral limb (F8,56 = 8.24, p < 0.001) as well
pre-lesion levels (p < 0.001), with a gradual but persistent increase
as significant effects of time (F8,56 = 2.44, p = 0.02 in ipsilateral limb;
up to the end of the measurement period (p < 0.001). shows
F8,56 = 3.93, p = 0.001 in contralateral limb) and group (F1,7 = 159.97,
the time-course change of the foot angle. Much more external rota-
p < 0.001 in ipsilateral limb; F1,7 = 452.6, p < 0.001 in contralateral
tion was exhibited in both the ipsilateral and contralateral sides
limb). For stride length, a two-factor ANOVA showed signifi-
one day after the lesion in comparison with the pre-lesion state.
cant effects of time in the ipsilateral limb (F8,56 = 5.77; p < 0.001)
Interestingly, the foot angle in the unaffected side reached a sig-
Fig. 3. Time-course changes of the BOS (A) and foot angle (B) of the hindlimbs after 6-OHDA injection through 42 days. Asterisks represent significant differences in the
foot angle between measured values and pre-surgery data (*p < 0.05, ***p < 0.001) by using Bonferroni correction post hoc tests. The bracket indicates significant differences
between the hindlimbs of the two sides using a paired t-test (#p < 0.05, ##p < 0.01).
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
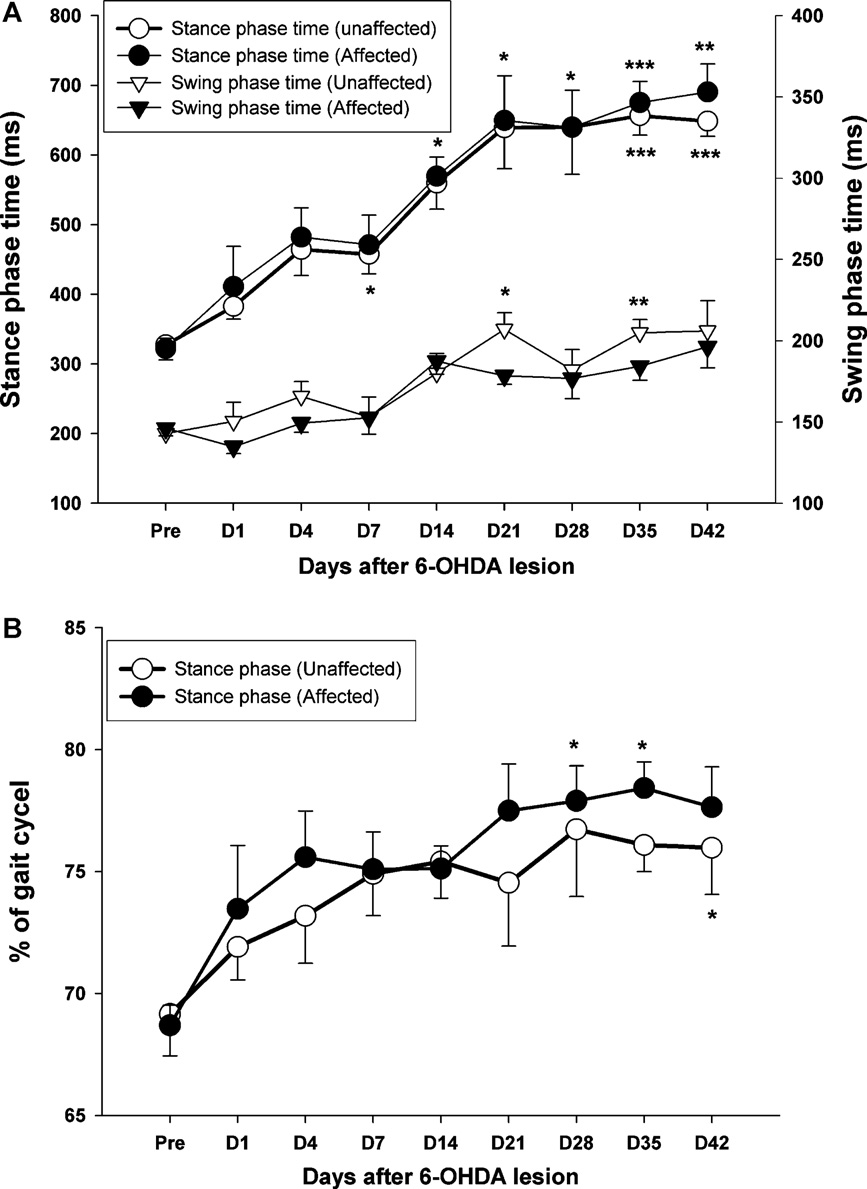
Fig. 4. (A) The time-course changes in the duration of stance and swing phase within 42 days of observation. Note the gradually increased trend in both phases, which was
maintained at relatively high levels until 21 days after PD lesion. (B) The percentage of stance phase in the full gait cycle also shows a gradual increase either in the affected
side (black circle) or the unaffected side (white circle) following 6-OHDA injection. *p < 0.05, **p < 0.01, ***p < 0.001 as compared to pre-operative values with a Bonferroni
correction.
nificant difference at the second time point (4 days post-lesion)
(F8,56 = 6.51, p < 0.001), as reflected in the main effect for test days. A
and remained substantially stable throughout the remaining time
significant group effect was also observed on the changes of walk-
points. In contrast, the foot angle of the affected side exhibited a
ing speed (F1,7 = 224.07, p < 0.001). The time × group interaction
continuous increase over the full observation period.
was found to be significantly different (F8,56 = 320.69, p < 0.001).
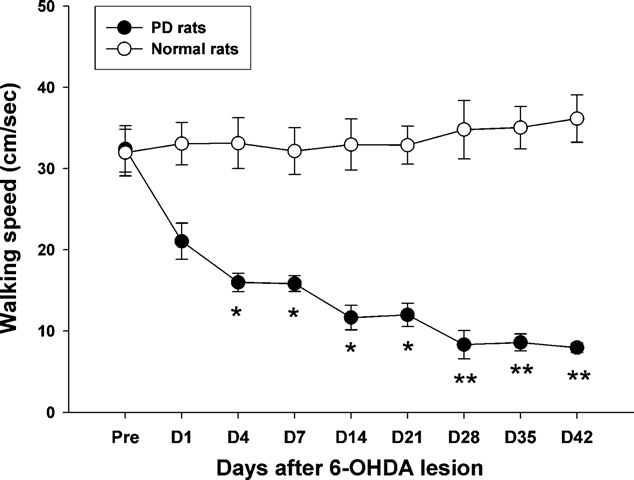
With regard to temporal gait parameters after unilateral
For post hoc comparisons, the time-course changes
dopaminergic lesion, the data in show that the precise dura-
of walking speed. A significant difference clearly appears at day 4
tion of swing and stance phase can be clearly observed by high
post-lesion (p = 0.011). Interestingly, walking speed showed a con-
speed video recording. A significant increase in the stance phase
tinuously decreasing trend over the time-course of observation,
time could be seen at 14 days post-lesion in the affected hindlimb,
although the most dramatic change in walking speed occurred by
but no apparent change was seen in the swing phase time until 21
the first post-lesion test. From day 28 to 42, the decrease in walking
days post-lesion (p < 0.05). Furthermore, shows that there
speed seemed to reach a plateau. The average walking speed was
was a significant difference in the percentage of stance phase as
30.8 ± 2.3 cm/s at the pre-lesion state, which was quite different
compared to the pre-lesion level in the affected side by 28 days
than the final speed (days 28–42) of approximately 8.0 ± 0.7 cm/s
post-lesion (p < 0.05). The time-course measurements also indi-
(p = 0.004).
cated progressive increase in the percentage of the stance phasein the affected hindlimb, from 69% at pre-surgery to 78% at 42 days
post-lesion, as shown in
The walking speed of the post-lesion animals was also severely
shows the time-course changes of post-lesion paw
decreased. A significant difference was observed across time
immobility according to the bar test for both limbs. One-way
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
Fig. 7. Time-measurement changes in the rotational response to apomorphine. Bars
Fig. 5. Time-course changes of walking speed in normal and PD lesion rats while
represent the mean (±SEM) number of net contralateral rotations performed by the
rats performed the walkway locomotion test during the 42 days. Note the gradual
animals as the total number of full body rotations in 60 min. **p < 0.01, ***p < 0.001
decrease in the walking speed, which started to reach significance at day 4 after 6-
as the significant difference compared with the pre-lesion stage with the Bonferroni
OHDA injection. Levels of significance: *p < 0.05; **p < 0.01 as compared to pre-values
correction post hoc test.
with the Bonferroni correction. Data are expressed as the means ± SEM.
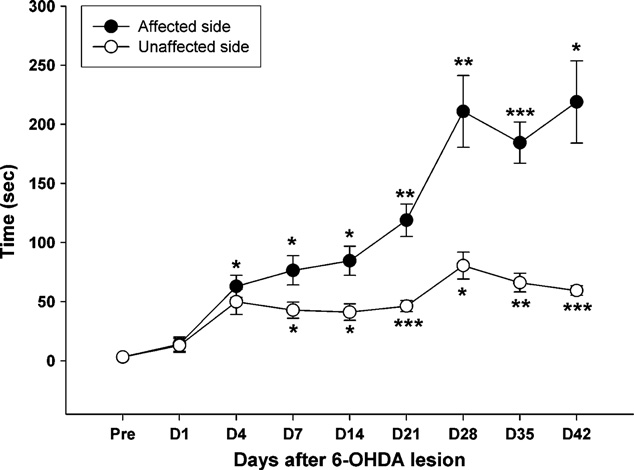
repeated measures of ANOVA revealed a significant effect of time
sistent asymmetry in turning behavior difference (p = 0.001). The
in the ipsilateral limb (F
time-course measurement showed a gradual increase in the rota-
8,56 = 18.27, p < 0.001) and contralateral
tion number until 21 days post-lesion, after which a plateau was
8,56 = 10.65, p < 0.001). Following 6-OHDA lesion, both limbs
exhibited similar trends of increasing immobility. Compared with
reached and the values remained basically unchanged.
the pre-lesion level, the bar test scores showed a statistically signif-icant increase (p = 0.012) at approximately 4 days after the PD lesion
3.4. Histological tests
in the affected forelimb, but the unaffected side did not reach statis-tical significance until 7 days post-lesion (p = 0.026). The behavior
The results of the TH-immunohistochemistry in the Str and the
asymmetry in the forelimbs became evident at day 4 post-lesion
SN at pre-lesion and at 1, 7, 21 and 42 days post-lesion are shown
and remained statistically significant from day 4 until the end of
in At the Str and SN regions, infusion of 6-OHDA induced
observation (paired t-tests, t = 3.07, p < 0.02).
a progressive loss of TH-immunoreactive cells. A mild lesion wasalready apparent at 1 day post-injection in the ipsilateral Str and
3.3. Apomorphine-induced rotation behavior
SN. A decrease in TH-immunoreactive density on the side of theinfusion was markedly apparent at 1 week post-lesion, a trend
depicts the time-course changes in rotation behavior
which progressed throughout the course of observation. Six weeks
for the test animals from pre-lesion until the end of the test
after unilateral 6-OHDA infusion, tyrosine hydroxylase immunore-
period. Post-lesion animals revealed significant time-dependent
activity in the Str and SN was less detectable on the side of the
contralateral rotation to the side of infusion following apomorphine
infusion. The quantification of DA neuron loss in the SN at each time
point is presented in n our longitudinal analysis, the aver-
8,56 = 21.65, p < 0.001). At the first day after unilat-
eral 6-OHDA administration, the rats displayed occasional rotation,
age TH cell loss in the SN region was 11.30 ± 1.10%, 67.13 ± 5.28%,
but the behavior did not reach a level of significance (p = 0.416).
88.66 ± 4.68% and 95.43 ± 2.97% at 1, 7, 21 and 42 days post-
However, at 4 days post-lesion, the injured animals revealed con-
lesion, respectively. All lesioned groups had significant differencesin the time factor (one-way ANOVA: F4,20 = 58.29, p < 0.001). TheTukey's post hoc analysis indicated that the survival rates of theTH-immunoreactive neurons reached a significant level after 7 dayspost-lesion (p < 0.001).
The present study investigated time-course changes of motor
behaviors, including gait spatiotemporal patterns, conventionalbar and rotational behavioral and TH-immunohistochemistry pat-terns of DA neuron loss for 6 weeks following unilateral 6-OHDAlesion in rats. As compared to the pre-lesion state, animals withthe development of unilateral dopamine depletion exhibited grad-ual reduction in step/stride length and walking speed but anincrease in BOS and foot angle. The gait cycle of stance and swingphases were also affected. Additional behavioral tests, includingapomorphine-induced rotation and the akinesia bar test furtheridentified asymmetric motor behavior during the 6 weeks of grad-ual DA neuron loss following 6-OHDA injury.
Fig. 6. Time-course changes of akinesia observed from the bar test scores of 6-
OHDA lesioned rats. *p < 0.05, **p < 0.01, ***p < 0.001, significantly different from the
The time-course observation of gait patterns and behavior
pre-lesion time point with the Bonferroni correction post hoc test.
tests helped confirm behavioral compensation and quantify the
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
Fig. 8. Representative microphotographs demonstrating TH-immunoreactive fibers in the SN (A) and Str (B) from animals sacrificed at pre-surgery as well as at 1, 7, 21 and
42 days post-6-OHDA lesion. At pre-lesion, neurons in the bilateral hemisphere (darkly stained TH-positive cells), are present throughout the SN. Note that obvious reduction
of TH-immunoreactive neurons in the lesioned hemisphere (left side) can be observed after 7 days of 6-OHDA lesion.
relative dopaminergic cell depletion at the measurement time
variation after day 4, whereas the affected side showed a progres-
points, which provided a clearer picture of the development of
sively increasing trend until day 28, followed by a plateau for the
induced PD from pre-lesion to full symptom manifestation. After
remainder of the test (Similarly, the rotational response
the unilateral injection of 6-OHDA, gradual impairment in gait
(progressively increased up to day 21 and reached a rela-
performance reached a plateau at around 28 days post-lesion
tively stable plateau. According to our time-course measurement
(Similar observations could be found in the bar test
of TH-immunoreactive neurons in the SN, the DA loss increased
and induced rotational behavior test. Across the 6 weeks of bar
slowly from 89% at 21 days to 95% at 42 days post-lesion
tests, the immobility duration of the unaffected side showed little
indicating that the depletion of DA neurons also reached a plateauat about the same time points of 21 or 28 days. Thus, rats withinfusion of 6-OHDA in the MFB displayed a gradual development ofdifficulties in locomotion, which was well correlated to progressiveloss of nigrostriatal DA neurons.
According to our detailed spatial gait analysis, our seven-day
post-lesion data revealed significant modification of step/stridelength and foot angle. At the same time point, the majority of DAdepletion (about 67%) was observed from our TH staining. Inter-estingly, these results concurred with those of previous studiesdemonstrating that approximately 75% of DA cell loss in rats 68% of DA cell loss in PD patients pronounced locomo-tion deficits. Also, the asymmetric gait patterns became evident at4 days post-lesion. The asymmetry in locomotion can be attributedto the significant reduction in step length, stride length and footangle in the affected hindlimb of hemiparkinsonian rats. However,a smaller step length, stride length and foot angle than those of nor-mal rats were also found in the unaffected hindlimb. Although theSN of the intact hemisphere did not experience dopamine deple-tion, the level of gait performance of the unaffected hindlimb also
Fig. 9. Time-dependent histogram representing the percentage of neuron survival
showed mild impairment, which has also been reported in a previ-
rates determined by quantification of TH-immunoreactive neurons of the SN in the
ous study using electromyographic (EMG) recordings
lesioned hemisphere as compared to the SN of the intact hemisphere. Values are
work also indicated that the intact hindlimb plays an important
expressed as the mean ± SEM. Note that TH-immunoreactive neuron loss becomesapparent after 7 days post-lesion as compared to the pre-lesion value (***p < 0.001,
compensatory role during locomotion, carrying more weight to
Bonferroni correction post hoc test).
support the unaffected side during propulsion a
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
significant increase in the BOS and foot angle of both hindlimbs was
of gait initiation, akinesia and locomotion. Deep brain stimulation
found in the PD rats. The progressive decrease in walking speed can
of the PPN has been proposed as a new therapeutic means for gait
explain a compensatory increase in the BOS and foot angle, which
restoration in animal models or PD patients the
seems to be necessary to increase balance and stability during loco-
loss of neurons and the changes of neuronal activity in the PPN
following 6-OHDA lesion in rodent studies still reveal certain dis-
With regard to gait temporal information, our results showed
crepancies the mechanisms of impaired
that dopamine deficiency induced significant changes in stance and
gait development from animal models may provide a basis for
swing duration of the gait cycle after 7 days post-lesion. In addition
implementing new therapeutic approaches for functional recovery
to the elongation of swing and stance time as compared to pre-
of PD subjects, such as repetitive transcranial magnetic stimula-
lesion measurements, the gait cycle showed a gradual increase in
tion (rTMS), a non-invasive brain stimulation technique, which
the percentage of stance phase (The extension of both swing
may modulate brain activity and improve motor performance in
and stance duration indicated both the slowness of hindlimb move-
ment and a strongly reduced walking speed slow steppingpattern in the affected hindlimb might reflect akinesia or hypoki-
nesia induced by dopamine depletion, which was supported by theforelimb akinesia observed during the bar test. The extended dura-
The present study characterized the time-course development
tion of hindlimb swing and stance during locomotion indicated that
of rodent gait impairment and the extent of cell loss in the SN
the PD rats had difficulty in initiating steps with the affected limb
from the immediate post-lesion state to the stable plateau state
due to the 6-OHDA injury observation of an ele-
following 6-OHDA injection. The unilateral 6-OHDA rat model
vated percentage of stance phase and a decreased percentage of
study has generated interesting findings regarding the pre-lesion to
swing phase has been demonstrated previously in both human
full-symptom time-course development of gait impairment, rota-
and animal studies can be explained by a delayed onset
tional response, bar test behavior and TH-immunohistochemistry.
of the swing phase due to akinesia or muscle rigidity.
The time-course changes in gait impairment started as soon as
Compared to dynamic gait analysis, the bar test is useful for eval-
day four post-lesion and progressively increased to a peak level
uating the motor asymmetry and akinesia of the forelimbs under
around four weeks post-lesion; they then persisted at a plateau
static conditions increase in the immobilized duration
state over the remaining 6 weeks of the test period. The rotational
observed in our study confirms the well-known phenomenon that
response to apomorphine and the bar test for akinesia also pro-
an established nigrostriatal lesion causes akinesia/bradykinesia in
vided similar developmental curves. The video-based methodology
the bar test with the values of pre-surgery and
and the experimental data provide new insight into the progres-
unaffected forelimb, we observed that the unilateral 6-OHDA lesion
sive changes affecting rodent gait pattern during the development
caused akinesia in bilateral limbs but was especially severe in the
of nigrostriatal lesions and suggest that the hemiparkinsonian rat
contralateral side. Regarding animal behavior, the rats tended to
model can be used as an animal model for human Parkinson's dis-
avoid the use of the affected forepaws after unilateral DA lesions.
ease. Future researchers may use the presented methodology and
Clearly, the forelimbs play an important role in supporting the body
data for enhanced understanding of the general mechanisms of PD
weight during walking in four-legged animals avoiding
and in the development of novel treatment protocols for functional
the use of a forelimb would also contribute to the abnormal gait
recovery from PD.
patterns, which could be confirmed from our time-course obser-vation of bar test data showing gradual aggravation similar to ourgait data.
Researchers often start to perform therapeutic examination or
manipulation after confirmation of the PD rodent model from rota-
The authors would like to thank the National Science Council and
tional behavior tested at 2–3 weeks post-lesion
the National Health Research Institutes of Taiwan for financially
data showed that the rotational response presented at day 4 and
supporting this work under contract numbers NSC 96-2628-E-269-
progressively increased to day 21 post-lesion. These results agree
001-MY3 and NHRI-EX98-9535EI. We also would like thank the
with previous findings that the rotational response to apomorphine
anonymous reviewers for their constructive comments.
was present at about 3 days post-lesion that sig-nificant dopaminergic cell loss occurred at early time points. In
Appendix A. Supplementary data
addition, previous work reported that the minimal dopaminergiccell loss required to elicit a rotational response was about 40–50%
Supplementary data associated with this article can be found, in
for SN was very close to our TH-immunoreactive
the online version, at .
cell counts in the SN, interpolated from a DA neuron loss of 11%at day 1 and 67% at day 7 post-lesion Our results alsoshowed that apomorphine-induced rotational changes correlated
well with the progressive losses in dopamine neurons over time-
[1] Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update.
course observations of six weeks.
Trends Neurosci 2007;30:194–202.
Although the relationship and mechanisms between dopamine
[2] Giladi N, Treves TA, Simon ES, Shabtai H, Orlov Y, Kandinov B, et al. Freez-
depletion and motor behavior are not fully understood, it is
ing of gait in patients with advanced Parkinson's disease. J Neural Transm
believed that the onset of gait pattern changes and reach-
[3] Chee R, Murphy A, Danoudis M, Georgiou-Karistianis N, Iansek R. Gait freezing
ing a plateau at 28 days after lesion is highly related to
in Parkinson's disease and the stride length sequence effect interaction. Brain
dopamine depletion studies have also suggested that
dopamine-depleted rats change their somatosensory and/or pro-
[4] Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Fieuws S, Broens-Kaucsik
E. Abnormalities of the spatiotemporal characteristics of gait at the onset of
prioceptive input undergo asymmetric reticulospinal
freezing in Parkinson's disease. Mov Disord 2001;16:1066–75.
tract activation may in turn result in an abnormal
[5] Blin O, Ferrandez AM, Serratrice G. Quantitative analysis of gait in Parkinson
gait pattern. Furthermore, in addition to gait disturbances caused
patients: increased variability of stride length. J Neurol Sci 1990;98:91–7.
[6] Truong L, Allbutt H, Kassiou M, Henderson JM. Developing a preclinical model
by the loss of dopamine in the SN, the involvement of the peduncu-
of Parkinson's disease: a study of behaviour in rats with graded 6-OHDA lesions.
lopontine nucleus (PPN) may play an important role in the control
Behav Brain Res 2006;169:1–9.
T.-H. Hsieh et al. / Behavioural Brain Research 222 (2011) 1–9
[7] Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats:
[27] Olsson M, Nikkhah G, Bentlage C, Bjorklund A. Forelimb akinesia in the rat
an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol
Parkinson model: differential effects of dopamine agonists and nigral trans-
plants as assessed by a new stepping test. J Neurosci 1995;15:3863–75.
[8] Mabrouk OS, Marti M, Salvadori S, Morari M. The novel delta opioid recep-
[28] Miklyaeva EI, Martens DJ, Whishaw IQ. Impairments and compensatory adjust-
tor agonist UFP-512 dually modulates motor activity in hemiparkinsonian
ments in spontaneous movement after unilateral dopamine depletion in rats.
rats via control of the nigro-thalamic pathway. Neuroscience 2009;164:
Brain Res 1995;681:23–40.
[29] Hausdorff JM, Cudkowicz ME, Firtion R, Wei JY, Goldberger AL. Gait variability
[9] Fantin M, Auberson YP, Morari M. Differential effect of NR2A and NR2B subunit
and basal ganglia disorders: stride-to-stride variations of gait cycle timing in
selective NMDA receptor antagonists on striato-pallidal neurons: relationship
Parkinson's disease and Huntington's disease. Mov Disord 1998;13:428–37.
to motor response in the 6-hydroxydopamine model of parkinsonism. J Neu-
[30] Wang Y, Bontempi B, Hong SM, Mehta K, Weinstein PR, Abrams GM, et al. A
comprehensive analysis of gait impairment after experimental stroke and the
[10] Lindner MD, Plone MA, Francis JM, Blaney TJ, Salamone JD, Emerich DF.
therapeutic effect of environmental enrichment in rats. J Cereb Blood Flow
Rats with partial striatal dopamine depletions exhibit robust and long-lasting
behavioral deficits in a simple fixed-ratio bar-pressing task. Behav Brain Res
[31] Marin C, Aguilar E, Mengod G, Cortes R, Obeso JA. Effects of early vs. late
initiation of levodopa treatment in hemiparkinsonian rats. Eur J Neurosci
[11] Lindner MD, Plone MA, Francis JM, Emerich DF. Validation of a rodent model of
Parkinson's disease: evidence of a therapeutic window for oral Sinemet. Brain
[32] Hefti F, Melamed E, Sahakian BJ, Wurtman RJ. Circling behavior in rats with
Res Bull 1996;39:367–72.
partial, unilateral nigro-striatal lesions: effect of amphetamine, apomorphine,
[12] Metz GA, Tse A, Ballermann M, Smith LK, Fouad K. The unilateral 6-OHDA rat
and DOPA. Pharmacol Biochem Behav 1980;12:185–8.
model of Parkinson's disease revisited: an electromyographic and behavioural
[33] Hudson JL, van Horne CG, Stromberg I, Brock S, Clayton J, Masserano J, et al.
analysis. Eur J Neurosci 2005;22:735–44.
Correlation of apomorphine- and amphetamine-induced turning with nigros-
[13] Amende I, Kale A, McCue S, Glazier S, Morgan JP, Hampton TG. Gait dynamics
triatal dopamine content in unilateral 6-hydroxydopamine lesioned rats. Brain
in mouse models of Parkinson's disease and Huntington's disease. J Neuroeng
Rehabil 2005;2:20.
[34] Steiner H, Kitai ST. Unilateral striatal dopamine depletion: time-dependent
[14] Chang JY, Shi LH, Luo F, Woodward DJ. Neural responses in multiple
effects on cortical function and behavioural correlates. Eur J Neurosci
basal ganglia regions following unilateral dopamine depletion in behav-
ing rats performing a treadmill locomotion task. Exp Brain Res 2006;172:
[35] Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and
assessment of forelimb sensorimotor outcome in unilateral rat models of
[15] Klein A, Wessolleck J, Papazoglou A, Metz GA, Nikkhah G. Walking pattern anal-
stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharma-
ysis after unilateral 6-OHDA lesion and transplantation of foetal dopaminergic
progenitor cells in rats. Behav Brain Res 2009;199:317–25.
[36] Schneider JS, Peacock V. Differential effects of GDNF treatment on rotational
[16] Vlamings R, Visser-Vandewalle V, Koopmans G, Joosten EA, Kozan R, Kaplan S,
asymmetry, skilled forelimb use deficits and sensory neglect in unilateral 6-
et al. High frequency stimulation of the subthalamic nucleus improves speed of
OHDA-lesioned rats. Restor Neurol Neurosci 1998;13:205–12.
locomotion but impairs forelimb movement in Parkinsonian rats. Neuroscience
[37] Prentice SD, Drew T. Contributions of the reticulospinal system to the postu-
ral adjustments occurring during voluntary gait modifications. J Neurophysiol
[17] Chaniary KD, Baron MS, Rice AC, Wetzel PA, Ramakrishnan V, Shapiro SM.
Quantification of gait in dystonic Gunn rats. J Neurosci Methods 2009;180:
[38] Ferraye MU, Debu B, Fraix V, Goetz L, Ardouin C, Yelnik J, et al. Effects of pedun-
culopontine nucleus area stimulation on gait disorders in Parkinson's disease.
[18] Yu P, Matloub HS, Sanger JR, Narini P. Gait analysis in rats with peripheral nerve
injury. Muscle Nerve 2001;24:231–9.
[39] Rauch F, Schwabe K, Krauss JK. Effect of deep brain stimulation in the peduncu-
[19] Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Amster-
lopontine nucleus on motor function in the rat 6-hydroxydopamine Parkinson
dam: Elsevier Academic Press; 2005.
model. Behav Brain Res 2010;210:46–53.
[20] Mabrouk OS, Volta M, Marti M, Morari M. Stimulation of delta opioid recep-
[40] Nandi D, Liu X, Winter JL, Aziz TZ, Stein JF. Deep brain stimulation of the
tors located in substantia nigra reticulata but not globus pallidus or striatum
pedunculopontine region in the normal non-human primate. J Clin Neurosci
restores motor activity in 6-hydroxydopamine lesioned rats: new insights
into the role of delta receptors in parkinsonism. J Neurochem 2008;107:
[41] Stefani A, Lozano AM, Peppe A, Stanzione P, Galati S, Tropepi D, et al. Bilat-
eral deep brain stimulation of the pedunculopontine and subthalamic nuclei in
[21] Fischer DA, Ferger B, Kuschinsky K. Discrimination of morphine- and
severe Parkinson's disease. Brain 2007;130:1596–607.
haloperidol-induced muscular rigidity and akinesia/catalepsy in simple tests
[42] Breit S, Bouali-Benazzouz R, Benabid AL, Benazzouz A. Unilateral lesion of the
in rats. Behav Brain Res 2002;134:317–21.
nigrostriatal pathway induces an increase of neuronal activity of the peduncu-
[22] Paille V, Henry V, Lescaudron L, Brachet P, Damier P. Rat model of Parkin-
lopontine nucleus, which is reversed by the lesion of the subthalamic nucleus
son's disease with bilateral motor abnormalities, reversible with levodopa, and
in the rat. Eur J Neurosci 2001;14:1833–42.
dyskinesias. Mov Disord 2007;22:533–9.
[43] Florio T, Scarnati E, Confalone G, Minchella D, Galati S, Stanzione P, et al. High-
[23] Blandini F, Levandis G, Bazzini E, Nappi G, Armentero MT. Time-
frequency stimulation of the subthalamic nucleus modulates the activity of
course of nigrostriatal damage, basal ganglia metabolic changes and
pedunculopontine neurons through direct activation of excitatory fibres as
behavioural alterations following intrastriatal injection of 6-hydroxydopamine
well as through indirect activation of inhibitory pallidal fibres in the rat. Eur J
in the rat: new clues from an old model. Eur J Neurosci 2007;25:
[44] Elahi B, Chen R. Effect of transcranial magnetic stimulation on Parkinson
[24] Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG, Sung YH, et al. Treadmill exercise
motor function–systematic review of controlled clinical trials. Mov Disord
suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-
induced Parkinson's rats. Neurosci Lett 2007;423:12–7.
[45] Fang JH, Chen JJJ, Hwang IH, Huang YZ. Review: repetitive transcranial mag-
[25] Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional
netic stimulation over the human primary motor cortex for modulating motor
selectivity. Brain 1991;114(Pt 5):2283–301.
control and motor learning. J Med Biol Eng 2010;30:193–201.
[26] Muir GD, Whishaw IQ. Ground reaction forces in locomoting hemi-
[46] Chang YJ, Hsieh TH, Huang YM, Hsu MJ, Wong AM. A lack of modulation of motor
parkinsonian rats: a definitive test for impairments and compensations. Exp
evoked potential in sensory-impaired individuals with spinal cord injuries. J
Brain Res 1999;126:307–14.
Med Biol Eng 2011;31:37–43.
Source: http://icl.cs.utk.edu/news_pub/submissions/1-s2.0-S0166432811002221-main.pdf
Praktikumsdokumentation Informationen zur Vorbereitung auf ein Praktikum an der Diani Maendeleo Academy Kontakt und Informationen E-Mail: [email protected]. Web: http://girlshope.de/ Praktikumsdokumentation Überblick über das Praktikum . 2 Praktikumsdauer und Bewerbungsfristen . 2 Praktikumsbetreuung und Aufgaben . 2
UNIVERSIDAD TÉNICA DEL NORTE FACULTAD CIENCIAS DE LA SALUD ESCUELA DE ENFERMERÍA Tesis de Grado previo a la obtención del título de LICENCIADA FACTORES QUE INCIDEN EN LOS TRASTORNOS AFECTIVOS DE LOS Y LAS ADOLESCENTES QUE INGRESAN AL SERVICIO DE MEDICINA INTERNA DEL HOSPITAL SAN VICENTE DE PAÚL DE IBARRA POR INTENTO AUTOLÍTICO EN EL PERIODO MAYO 2006 Narciza Santos Vila.












