
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
(microsoft word - 303 307 301 340 305 350 301.doc)
Acne vulgaris, a chronic inflammatory disorder in adolescents consists of the
pilosebaceous fol icles, characterized by comedones, papules, pustules, cysts, nodules and oftenscars, chiefly on face, neck and upper trunk (Lal a et al., 2001). It may cause disfiguration andpermanent scar ing and it may also have an adverse ef ect on psychological development,resulting in profound emotional scar ing, which may lead to social phobias, withdrawal fromsociety, and clinical depression. The four main pathogenic factors in the development of acne areincreased sebum production, disorders of the microflora, cornification of the pilosebaceous duct,and inflammation (Ozkan et al., 2000).
Normal skin commensals including Propionibacterium acnes,
Propionibacterium granulosum, Staphylococcus epidermidis and Malassezia furfur, proliferaterapidly during puberty and are often involved in the development of acnes (Hamnerius, 1996).
The predominant organism in the fol icular flora is the anaerobic organism, Propionibacterium
P. acnes is an immobile, gram positive, lipophilic anaerobe that colonizes in the
fol icular duct. Evidence demonstrates the role of P. acnes in the production of acne. Although arelationship exists between increased levels of P. acnes and acne development in teenage years, acor elation with acne severity has not been demonstrated. Local inflammation caused by thisorganism results from antibody production (i.e., IgG, IgM). The combination of complementactivation and secretion of chemotactic factors lead to an array of immunological responses (e.g.,mast cel degranulation, neutrophil chemotaxis) and the release of lipases, proteases,hyaluronidases, reactive oxygen species (ROS), and lysosomal enzymes. This process damagesthe epithelial layer of the fol icle and causes its contents to be spil ed onto the skin surface,resulting in inflammatory acne. Further, lipase from P. acnes breaks down glyceride to free fat yacids (FFAs) and glycerol. FFAs directly compromise the integrity of the fol icular environment
resulting in the release of IL-1-∝, which possesses proinflammatory properties as mentionedpreviously.
The general therapy in the treatment of acne vulgaris includes oral and topical
therapy employing comedolytics (benzoyl peroxide, tretinoin, azaleic acid) and antibiotics(tetracycline, erythromycin, etc.) for both oral and topical use (Song et al., 2004). Focusing ontopical therapy, benzoyl peroxide is considered a potent antimicrobial agent against bacteria andyeast as wel as a mild keratolytic. Its mechanism of action may involve the release of freeoxygen radicals that harm bacterial proteins. Tretinoin is a retinoic acid (vitamin A acid) thatincreases the turnover rate and decreases the aggregation of fol icular cel s. Topical retinoidsef ectively target the formation of microcomedones, thereby sustaining remission and preventingthe formation of new lesions. Azelaic acid possesses antibacterial activity against P. acnes byinhibiting thiorodoxin reductase, thus preventing the synthesis of bacterial DNA. It also targetsmicrocomedone formation by affecting the turnover rate of fol icular epithelial cel s. Azelaic acidis suggested for mild to moderate inflammatory acne due to its ef ects on chemotaxis suppressionand at enuation of inflammatory mediator production. Additional y, it reduces a number of P.
acnes. Clindamycin and erythromycin preparations reduce comedonal and inflammatory acne.
These agents are often used as monotherapy for mild papular and pustular acne. They may alsoserve as good alternatives to oral antibiotics by minimizing systemic ef ects.
These drugs have several side effects such as skin ir itation, dry skin, peeling,
burning, erythrema sunlight sensitivity, abnormal skin pigmentation, edema, blistering, scabs(Brand et al., 2003). In addition, antibiotics resistance has been increasing in prevalence withinthe dermatologic set ing (Swanson, 2003).
Plants used in folk medicine have been accepted as one of the main sources of
drug discovery and development. In Thailand, there is a rich treasury of ethnobotanicalknowledge and over past decades several research has been carried out on this subject. During ourstudies, we have noticed the fol owing herbal remedies being used in the treatment of skindiseases and related inflammatory. Thus, Thai medicinal plants have been extensively studied asthe alternative treatments for acne vulgaris.
In the present study, 18 medicinal plants, including (Centella asiatica, Zingiber
officinalis, Ocimum americanum, Ocimum sanctum, Boesenbergia pandurata, Piper betle, Senna
alata, Alpinia galanga, Punica granatum, Morus alba, Azadirachta indica, Cinnamomum verum,
Plumbago zeylanica, Dioscorea membranacea, Syzygium aromaticum, Andrographis paniculata,
Cymbopogon citratus, Rhinacanthus nasutus, which have been traditional y used as antimicrobialand anti-inflammatory agents (9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539; STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO, 2537; Biswas et al., 2002; Farnsworth andBunyapraphatsara, 1992; Navar o et al., 1996; Wannissorn, et al. 2005) were investigated forantibacterial activities against P. acnes.
1.2 Literature review
1.2.1 Acne vulgaris
1.2.1.1 Definition
Acne vulgaris is a self-limited disease, seen primarily in the adolescent age
range, involving the sebaceous fol icles. There are usual y a variety of lesions (Figure 1.1)consisting of comedos, papules, pustules, nodules, cysts, and, as sequelae to active lesions, pit edor hypertrophic scar (Fitzpatrick et al., 1979).
Figure 1.1 Lesions of acne vulgaris (www.selebean.com)
This disease is suf iciently common that has often been termed physiology. It is
occasional y present at birth. However, it is not until puberty begins that it becomes a commonproblem. The disease may be an early manifestation of the puberal spectrum, although in the veryyoung patient the predominant lesions are comedos, and inflammatory lesions are rare. In girls, itmay precede menarche by more than a year. The greatest number of cases is seen during themiddle-to-late teen-age period, then the incidence of the disease decreases. However, particularlyin women, acne may persist through the third decade or even later. Acne appears to af ect malesmore severely during adolescence, whereas similar prevalence is observed in females during thesecond decade of life. Dark skinned populations may be at higher risk for developing abnormalskin color changes and scar ing as a result of acne. Because acne af ects visible parts of the body(e.g., face, neck, upper trunk), this disorder may negatively impact self-esteem (Fitzpatrick et al.,1979).
Classification and symptoms
Acne may be classified as comedonal, papular, pustular, cystic, and nodular.
Comedonal acne is noninflammatory and divided into two types: whiteheads and blackheads.
Whiteheads (closed comedo) present as fresh or white colored, raised bumps whereas blackheads(open comedo) present as open pores containing dark colored skin roughage consisting ofmelanin, sebum, and fol icular cel s. Papules appear as red, solid, elevated lesions often less than5 mm in diameter. Pustules are circumscribed skin elevations containing purulent material. Cystsand nodules are solid, elevated lesions involving deeper dermal and subcutaneous tissue. Cystsare less than 5 mm in diameter whereas nodules exceed 5 mm.
According to the combined acne severity classification, acne is stratified
into three severity levels (Table 1.1): mild, moderate, and severe based on lesion type andquantity of lesions (Song et al., 2004).
Mild acne displays less than 30 lesions comprised of comedos or inflammatory
papules and pustules. Moderate acne displays 30 to 125 comedos, papules, or pustules. Severeacne exhibits more than 125 comedos, papules, pustules, cysts, or nodules. Severe acne is moreprone to scar ing after the condition abates.
Table 1.1 The combined acne severity classificationAcne Severity
Comedos or inflammatory papules and pustules
Comedos, papules, or pustules
Comedos, papules, pustules, cysts, or nodules
Table 1.2 il ustrates a useful approach to grade the severity of facial. The
grading system al ows for the classification of acne by first separating a body site of involvement(e.g., forehead, nose, chest), then identifying the primary lesion type (i.e., comedo, papule,pustule, cyst, nodule) in that area, and lastly counting the number of lesions and grading themaccordingly (Song et al., 2004). The chest and back are evaluated separately from the face
because of the potential variance on overal severity and treatment outcomes. This system isscaled to grades 0, I, I , II , and IV.
Table 1.2 Dif erent parameters of the grading system
Presence of severe
inflammatory acne
Symptoms of acne may present dif erently according to acne types. Patients with
comedos are asymptomatic. Papules and pustules may cause itching at the site of involvement.
Cysts and nodules are often painful.
Pathogenesis of acne vulgaris
The pathogenesis of acne involves multiple physiological factors. These include
fol icular hyperproliferation, increased sebum production due to higher androgen levels, andcolonization of P. acnes. Novel concepts have emerged to help bet er understand its pathogenesis,these include variations in target cel sensitivity, biological markers, neuroendocrine, genetic, andenvironmental factors (Song et al., 2004).
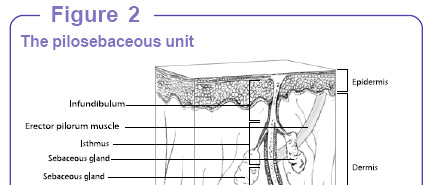
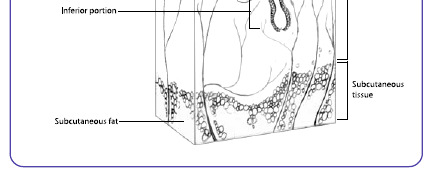
Figure 1.2 The pilosebaceous unit (Song et al., 2004)
Acne develops in the fol icular pilosebaceous unit (Figure 1.2). These units are
largest on the face, neck, and upper trunk where acne is most distributed. The hair fol icle wherethe pilosebaceous unit is embedded consists of three main parts including infundibulum, isthmus,and bulb. The infundibulum starts from the epidermal surface down to the sebaceous unit. Theisthmus is where the arrector pili muscle and sebaceous gland are found and it lies between theinfundibulum and bulb. The sebaceous gland produces sebum that empties into the fol icle.
Typical y, the fol icular bulb is situated in the subcutaneous tissue. The cel s that make up theepidermis and the cel s that line the infundibulum are the same.
The first step in the formation of an acne lesion is the production of a
microcomedone. Clinical y, a microcomedone is unobservable to the naked eye. An importantpathophysiological process in comedogenesis is fol icular hyperproliferation. Keratinocytes in thecomedo proliferate at a higher rate compared to those in a normal fol icle and the cohesiveness ofthese keratinocytes is enhanced (Figuer 1.3). These abnormal y differentiated keratinocytesaggregate in the fol icle along with sebum, lipids, and bacteria, occluding the fol icle andultimately forming an open or closed comedo.
Lipids, androgens, and local cytokines are believed to contribute to acne
production. Introduction of specific lipids into the sebaceous fol icle may be associated withhyperproliferation. High levels of free fat y acids (i.e., squalene, squalene oxide) demonstrated anaugmentation in epithelial keratinocyte adhesion (Leyden, 1995). Likewise, a decrease in certainfat y acids (i.e., linoleic acid) led to increased basal keratinocyte proliferation and unusualkeratinocyte dif erentiation. Various cytokines may also play a contributory role in acneproduction. In vivo cultures demonstrate interleukin-1-∝ (IL-1-∝) may inducehyperkeratinization via its proinflammatory properties and stimulation of abnormal desquamation(Ingham et al., 1992). This cytokine also results in chemoat raction of polymorphonuclearleukocytes (PMNLs) and possibly the production of pustules. Suggestively, other cytokines arethought to be involved and include tumor necrosis factor-∝, interferon-γ, epidermal growthfactor, and transforming growth factor-∝.
Figure 1.3 Comparison between normal skin and skin with acne (Song et al., 2004)
Androgens contribute to microcomedone formation through increased sebum
production (Song et al., 2004). Sebum is composed of glycerides, wax esters, and cholesterol.
Androgens are considered to be the major sebotropica hormones. A marked rise in androgenlevels ( adrenarchea), occurring as early as 7 to 8 years of age to puberty, stimulates the growthand dif erentiation of sebocytes in the sebaceous glands and the production of sebum. Androgens
bind via a coupling mechanism to androgen receptors on sebocytes, leading to gene transcriptionand initiating the differentiation of sebocytes.
Evidence demonstrates the role of P. acnes in the production of acne (Song et
al., 2004). Although a relationship exists between increased levels of P. acnes and acnedevelopment in teenage years, a cor elation with acne severity has not been demonstrated. Localinflammation caused by this organism results from antibody production (i.e., IgG, IgM). Thecombination of complement activation and secretion of chemotactic factors lead to an ar ay ofimmunological responses (e.g., mast cel degranulation, neutrophil chemotaxis) and the release oflipases, proteases, hyaluronidases, reactive oxygen species (ROS), and lysosomal enzymes. Thisprocess damages the epithelial layer of the fol icle and causes its contents to be spil ed onto theskin surface, resulting in inflammatory acne. Further, lipase from P. acnes breaks down glycerideto free fat y acids (FFAs) and glycerol. FFAs directly compromise the integrity of the fol icularenvironment resulting in the release of IL-1-α, which possesses proinflammatory properties asmentioned previously. New evidence suggests glycerol also contributes to the negative ef ects of
Recent concepts on the pathogenesis of acne suggest sebaceous glands on certain
areas of the body demonstrate a greater response to androgens.
Neuroendocrine factors may also play a role in the pathogenesis of acne (Song et
al., 2004). The skin contains a mesh of peripheral sensory nerves comprised of various fibersincluding C fibers that may play a role in skin inflammation that ultimately leads to acne. Aneuropeptide known as substance P may also lead to acne by facilitating hyperkeratinization,enlarging sebaceous glands, and increasing sebum production.
The skin encounters various physical stresses compromising its function as a
bar ier. Corticotropinreleasing hormone (CRH) is an endocrine hormone released during periodsof stress suggesting that CRH and its receptors (i.e., CRH-R1, CRH-R2) may serve as amechanism in acne development. Genetic factors may predispose certain individuals todeveloping acne. A retrospective study involving 1,557 sets of twins demonstrated a significantassociation with the development of acne (p<0.0001). In contrast, individual factors such as lipidprofile and blood glucose levels did not cor elate with the development of acne. Additional y,aber ant genetic coding may interfere with normal androgen receptor production and/or function,
thus preventing sebum production and acne development. Environmental factors such as smokingand sun exposure may possibly influence acne production. Ultraviolet A and B rays may inducesebum production and decrease IL-1-α and interleukin-10 (IL-10). This finding contradicts theaforementioned theory that IL-1-α induces acne thru its proinflammatory properties. While thesefindings are not firmly conclusive, it appears that acne at least in part involves a dysfunctionalimmune system represented by a struggle to balance IL-1-α levels in the body (Lee et al., 2003).
Treatment (Fitzpatrick et al., 1979; Song et al., 2004)
Various treatment options exist according to drug class, mechanism of action,
dosage form, route of administration, and cost. Selection of the most appropriate therapy shouldbe based on a comprehensive evaluation of the patient and the pharmacological profile of theagents. The available treatment options for acne are as fol ows.
A. Physical therapy: There are many treatments including acne surgery, laser
and phototherapy, cryotherapy, and using intralesional corticosteriods.
B. Systemic therapy: The two major systemic modalities used in acne are
antibiotics and estrogenic hormones. Both represent major steps forward in the therapy of thisdisease. This therapy uses for moderate to severe cases.
C. Local therapy: The general therapy including topical agents tretinoin,
adapalene, tazarotene, azelaic acid, salicylic acid, benzoyl peroxide, and antibacterials(clindamycin, erythromycin).
Tretinoin is a retinoic acid (vitamin A acid) that increases the turnover rate and
decreases the aggregation of fol icular cel s. These topical retinoids effectively target theformation of microcomedones, thereby sustaining remission and preventing the formation of newlesions. Furthermore, these agents mimic antibiotic action by altering immune response anddecreasing inflammatory lesions. Tretinoin may significantly reduce both noninflammatory andinflammatory lesions by 81% and 71%, respectively (p<0.05) (Song et al., 2004). Becausetretinoin and retinoid analogs work wel against microcomedones and inflammatory lesions,cur ent practice standards are now suggesting topical retinoids as first-line agents in acnetreatment. Maintenance therapy with retinoids is now recommended in mild to moderate acne to
prevent the relapse of microcomedone formation often observed when initial treatment isterminated.
Common adverse ef ects include sunlight sensitivity, abnormal skin
pigmentation, irritation, erythema, edema, blistering, and scabs. Initial application of tretinoinmay cause flaring of the skin that resolves on its own after 2 to 3 months. Al ergic contactdermatitis may also occur, but the incidence is rare. To minimize the incidence of skin ir itation, alower concentration, frequency, and amount of medication (i.e., a pea-sized amount) should beinitiated and then gradual y increased. Patients should also be advised to apply tretinoin on a dryskin surface using dry fingers 30 minutes after washing.
Adapalene is a third generation topical retinoid analog that selectively binds to
retinoic acid receptors (RARs) in the epidermis. This controls hyperproliferation of fol icularepithelial cel s and halts the production of microcomedones. Adapalene shares similar propertiesto that of tretinoin and works wel against mild to moderate comedonal and inflammatory acne.
Adapalene is available as a 0.1% topical gel, cream, and solution. A meta-analysis of five clinicalstudies compared 900 subjects who received adapalene 0.1% or tretinoin 0.025% gel. This meta-analysis concluded that adapalene is similar or superior in ef icacy compared to tretinoin.
However, the conclusion of this study is limited because it only compared the lowest strength oftretinoin.
Common adverse ef ects associated with adapalene use include erythema,
scaling, dryness, pruritis, and burning sensation of the skin. An initial flare (i.e., pruritis, burningsensation, acne) may also occur in some patients. This agent should be applied at bedtime on adry, washed surface.
Tazarotene is a third generation synthetic topical retinoid prodrug original y
approved for the treatment of psoriasis. Its active form, tazarotenic acid, binds specifical y toRARs as does adapalene. Tazarotene is available as a topical cream or gel in 0.05% and 0.1%strengths. While only the 0.1% strength is indicated for the treatment of acne, the lowertazarotene strength (0.05%) may also be ef ective. Thus, both strengths may in fact be beneficialin acne therapy. Tazarotene is an ef ective alternative to tretinoin and adapalene. Studies suggesttazaroteneds superior ef icacy to tretinoin and equal efficacy to adapalene. When compared toadapalene, tazarotene may be useful in patients with a history of noncompliance as wel as
possibly of er a lower cost benefit based on every other day tazarotene therapy versus once dailyadapalene therapy.
Common adverse ef ects such as desquamation, burning or stinging sensations,
dry skin, erythema, and pruritis are found. Patients should apply a thin layer of this drug oncedaily in the evening after washing the face. The skin surface should be dry when applying thisdrug. Tazarotene is approved only for mild to moderate facial acne.
Azelaic acid possesses antibacterial activity against P. acnes by inhibiting
thiorodoxin reductase, thus preventing the synthesis of bacterial DNA. This drug also targetsmicrocomedone formation by affecting the turnover rate of fol icular epithelial cel s. Azelaic acidalso has activity against inflammatory acne. Azelaic acid is indicated for mild to moderateinflammatory acne. Azelaic acid is available as a topical 15% gel and 20% cream. This drug iswel tolerated because it does not cause the same degree of ir itation to the skin as do the otheragents.
Azelaic acid may also cause al ergic contact dermatitis. This medication should
be applied thinly to a dry, washed surface and massaged whol y to the af ected area twice daily.
Salicylic Acid/Sulfur/Resorcinol: These topical agents provide keratolytic and to
a lesser degree, antibacterial properties that aid in the treatment of acne. The combination of theseagents with benzoyl peroxide (BPO) is believed to have synergistic activity against acne. For thispurpose, a lotion containing BPO and sulfur is available as a prescription. Similarly, combinedsalicylic acid (SA) and BPO is more effective than either agent alone in the reduction of totalacne lesions.
Common adverse ef ects include brown scaling with resorcinol, unpleasant odor
with sulfur, and skin irritation and stinging with SA.
Benzoyl Peroxide is considered a potent antimicrobial agent against bacteria and
yeast as wel as a mild keratolytic. Its mechanism of action may involve the release of freeoxygen radicals that harm bacterial proteins. BPO has been shown to lower P. acnes count bet erthan topical antibiotics (e.g., clindamycin). The onset of clinical improvement improved fromseveral days to a few weeks and P. acnes count improved by 90% to 98% (Gol nick et al, 2003).
In a study using BPO 6% gel and clindamycin phosphate 1% lotion applied twice daily for 2weeks, BPO lowered P. acnes count significantly greater and quicker compared to clindamycin (p
<0.01) (Gans and Kligman, 2002). Unlike topical clindamycin and erythromycin, bacterialresistance does not develop to BPO. Although the superiority of topical antibiotics (e.g.,erythromycin, clindamycin) over BPO has not been demonstrated, the combination of the two hasdemonstrated bet er tolerance and efficacy in reducing P. acnes levels compared to topicalantibiotics alone (Gol nick et al, 2003). An important caveat to using these combination agents isthe degree of clinical ef ects do not consistently relate to changes in P. acnes count. However,combination therapy is not associated with an increased risk of resistant bacteria compared to theantibiotic alone. BPO is recommended for mild to moderately severe acne and is intended onlyfor topical use. Because the Food and Drug Administration (FDA) does not classify BPOgeneral y recognized as safe (GRAS), caution should be taken to prevent overuse of this product.
BPO is available in concentrations ranging from 1% to 10% and vehicles including creams, gels,lotions, washes, and cleansers. Gels are more potent, stable, and provide a predictable drugrelease compared to creams and lotions. Cleansers enable administration during showering, thusexpanding the coverage area and minimizing noncompliance. Some of the combination productsneed to be reconstituted with sterile water or alcohol, while some are premixed.
Common adverse ef ects associated with BPO use include erythema, peeling,
and dryness, particularly in alcohol-based preparations. Up to 3% of patients may experiencecontact dermatitis. Initiating BPO at a low dose and titrating the dose higher may help minimizethis occur ence. BPO should be applied twice daily to dry, washed skin, but patients should beforewarned that BPO could bleach clothes. Further, it should be applied to the entire face, inaddition to the af ected area(s). This helps prevent the development of future comedos.
Antibacterials (clindamycin, erythromycin): Topical antibacterial agents are
indicated for inflammatory acne due to its ef ects on chemotaxis suppression and at enuation ofinflammatory mediator production. Additional y, they reduce the number of P. acnes.
Clindamycin and erythromycin preparations reduce comedonal and inflammatory acne. Theseagents are often used as monotherapy for mild papular and pustular acne. They may also serve asgood alternatives to oral antibiotics by minimizing systemic ef ects. Topical antibiotics incombination with topical retinoids are more ef ective than antibiotics alone in treating acnelesions. As mentioned earlier, topical retinoids facilitate the action of antibiotics. Normal y,
topical antibiotics should not be continued more than 6 to 8 weeks if no improvement is observed,unless improvement occurs earlier at which time they should be discontinued.
Common adverse ef ects associated with topical antimicrobials are mild and
include erythema, pruritis, peeling, dryness, and burning sensation. Topical clindamycin has beenrarely reported to cause Pseudomembraneous colitis (P. colitis) compared to oral dosage forms.
Antibiotic resistance is also a problem with topical antibiotic use, but this has not shown to causesignificant acne inflammation as a consequence of growing resistant P. acnes numbers.
1.2.2 Medicinal plants used as antibacterial agents
Eighteen Thai medicinal plants were selected for investigation of antibacterial
activity against P. acnes. Selection of plants was based on their traditional y uses asantimicrobial, anti-inflammatory agents in primary healthcare and/or previously reports onantimicrobial and/or anti-inflammatory activities. The data of 18 Thai medicinal plants were asfol ows.
1.2.2.1 Centella asiatica
Scientific name: Centella asiatica Linn.
Synonym: Hydrocotyle asiatica
Common name: Asiatic pennyworth, Gotu kola, Bua bok, Pa-na-e-khaa-doh
(Karen-Mae Hong Son), Phak waen (Peninsulin), Phak nok (Northern)
Family name: Umbel iferae
Botanical description: The plant is a perennial herb (Figure 1.4). The stems are
long, creeping and rooting at the nodes. Leaves are simple, 2-10 fascicled at the node, orbicularreinform, 1-7 cm long, 1.5-9 cm broad, entire, crenate or lobulate. Petioles are 4-10 cm long.
Flowers are 3-4-flowered umbel. There are 2-5 umbels arising in axil ary. Peduncles are 0.5-5 cmlong, erected at first then curved; pedicels are almost none. Each flower has 2-3 bracts; 5 sepals; 5petals which are 1-1.5 cm long, purple, 5 stamens alternate with petals. Fruits are flat ened, 2-3mm long, 3-4 mm broad (Farnsworth and Bunyapraphatsara, 1992).

Figure 1.4 Centella asiatica Linn.
Part used: Fresh leaves
Traditional uses: For health promotion, wound healing, to enhance wound
repair, stomach ulcers, treatment of skin diseases, wounds, burns (Farnsworth andBunyapraphatsara, 1992)
Chemical constituents: Sugars, glycoside, tannin, potassium sulfate,
magnesium carbonate, asiaticoside, asiatic acid, madecassic acid (Shukla et al., 1999)
Biological activity: It has been reported that asiaticoside isolated from C.
asiatica exhibited wound healing. Topical applications of 0.2% solution of asiaticoside produced56% increase in hydroxyproline, 57% increase in tensile strength, increased col agen content andbet er epithelisation. Solution of asiaticoside (0.4 %) over punch wounds increasedhydroxyproline content, tensile strength, col agen content and epithelisation thereby facilitatingthe healing in vivo (Shukla et al., 1999). In addition cream and injection preparation ofPennyworth are used to treat wound healing or wound after operation (STJ9I9U <TST99;U FGALPVO9DT ;C:P@ULMCNO, 2537).
1.2.2.2 Zingiber officinale
Scientific name: Zingiber officinale Roscoe
Synonym: Amomum angustifolium Salisb., Amomum zingiber L.
Common name: Ginger, Khing, Khing klaeng, Khing daeng (Chanthaburi),
Khing phueak (Chianmai), Sa-e (Karen-Mae Hong Son)
Family name: Zingiberaceae

Botanical Description: It is a herb, having horizontal, white or pale yel ow,
fleshy and aromatic rhizome; and stem leafy (Figure 1.5). Leaves are lanceoate, 12-20 by 1.5-2cm; tapering gradual y to the apex; nar owing to base and clasping the stem by their long sheaths.
Inflorescencs are borne separately on a bladeless leaf-sheath; consisting of flowers zygomorphic,with bracts and bracteoles subtending the flowers, bracts closely appressed against each other;calyx shortly 3 lobed; corol a tubular, divided into 3 subequal lobes; fertile stamen one only; veryrarely flowers. Fruit is a dehiscent capsule (Farnsworth and Bunyapraphatsara, 1992).
Figure 1.5 Zingiber officinale Roscoe
Part used: Rhizome
Traditional uses: Enhancing appetite, for longevity, flatulence, stomach
discomfort, carminative, anti-emetic, prevent peptic ulcer, anti-bacterial and anti-inflammatoryproperties (Farnsworth and Bunyapraphatsara, 1992)
Chemical constituents: Amino acid, zingiberene, gingerol, camphor, calcium,
zingiberol, citral, zingirol, zingiberine, bisabolene, ∝-curcumene, linalool, cineole, gingerol andgingerone (Wang and Ng, 2005)
Biological activity: It has been reported that ginger rhizomes exerted antifungal
activity toward various fungi including Botrytis cinerea, Fusarium oxysporum, Mycosphaerella
arachidicola, and Physalospora piricola (Wang and Ng, 2005).
It has been reported that the extract of ginger significantly inhibited gr A
hemolytic Streptococci, Staphylococcus aureus, Streptococcus faecalis with the ef ects beingmore pronounced against the first 2 organisms (Farnsworth and Bunyapraphatsara, 1992).
1.2.2.3 Ocimum americanum
Scientific name: Ocimum americanum Linn.
Synonym: Ocimum basilicum Linn. Var. citratum
Common name: Hoary basil, Hairy basil, American basil, Lemon basil, Spice
basil, Lime basil, Perennial basil, Thai basil, Thai lemon basil, Wild basil, Mang lak,Komkokhaao (Northern)
Family name: Labiatae
Botanical description: The plant is an erect herb, 30-50 cm high, much
branched; consisting of stem and branches striate, more or less pubescent, with strong odor(Figure 1.6). Leaves are simple, opposite, 2.5-5 by 1-2.5 cm; having blade lanceolate to el iptic;apex and base acute; margin entire or nar owly ovate tooth; both surfaces glabrous and glandulardot ed; petiole slender, 1-2.5 cm long. Inflorescences are in terminal raceme-like, simple orbranched, 7-15 cm long; consisting of bract 2-3(-5) mm long, tip acute, hairy; pedicels very shortor sessile; flowers white or purple; calyx campanulate, 2-2.5 mm long (to 4-4.5 mm in fruit), 2-lipped, the upper lip large with decur ent margin, the lower with 4 nar ow pointed teeth, hairyinside, outside scat ered with white hair; corol a campanulate, 2-lipped, the upper truncate,subequal y 4 lobed, the low entire; stamens 4, in 2 pairs, exserted; style 2 lobes. Fruit is composedof 4 dry 1-seeded nutlets. Nutlet is el ipsoid, 1.2 mm long, black, dot ed (Farnsworth andBunyapraphatsara, 1992).

Figure 1.6 Ocimum americanum Linn.
Part used: Leaves
Traditional uses: Leaves are useful in skin infections like eczema, carminative,
anti-inflammation, skin diseases, antifungal (Wannissorn, et al. 2005).
Chemical constituents: Borneol, L-β-cadinene, 1-8 cineol, β-caryohyl ene,
eugenol, limonene, linalool, methyl chavicol, myrcene(STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO, 2537)
Biological activity: It has been reported that an alcohol extract of Hairy basil
showed bactericidal ef ects on both gram positive and gram negative bacteria (Farnsworth andBunyapraphatsara, 1992).
In addition, it had antimicrobial activity against zoonotic enteropathogens including
Salmonella spp., Escherichai coli O157, Campylobacter jejunii and Clostridium perferingens(Wannissorn et al., 2005).
1.2.2.4 Ocimum sanctum
Scientific name: Ocimum sanctum Linn.
Synonym: Ocimum tenuiflorum Linn.

Common name: Basil, Sacred basil, Holy basil,Tulsi, Kaphrao, Komko, Komko
dong (Chiang Mai), Ka phrao khaao, Ka phrao daeng (Central), Ho-kwo-suu, Ho-tuu-pluu(Karen-Mae Hong Son), Im-khim-lam (Shan-Mae Hong Son)
Family name: Labiatae
Botanical description: Ocimum sanctum is an erect herb, 30-60 cm high,
having much branches and soft hairs al over (Figure 1.7). Leaves are simple, opposite; el iptic orel iptic-oblong; 2-4.5 by 1-2.5 cm; consisting of apex and base acute or obtuse; margin remotelyser ate; short hairs along the vein; petiole 1-3 cm long. Flowers are smal , borne on the axis, interminal raceme-like or panicle, 8-14 cm long. Each flower consists of bract ovate, tip acute, 2-3mm long, margin hairy; pedicels 3-4.5 mm long, pubescent; calyx 2.5 mm long (to 3-3.5 mm infruit), 2-lipped, upper lip suborbicular, reflexed, the lower longer, 4 teethed; corol a 2-lipped, 3.5-4 mm long; stamens 4, in 2 pairs, filament slender, exserted, the upper pair with a smalappendage at base; style 2-lobed. Fruit contains 4 dry 1-seeded nutlets. Nutlets are el ipsoid, 1.2mm long, glabrous (Farnsworth and Bunyapraphatsara, 1992).
Figure 1.7 Ocimum sanctum Linn.
Part used: Leaves
Traditional uses: For the improvement of blood circulation, treatment of skin
diseases, urticaria, chronic cough, carminative, antiflatulence (Farnsworth and Bunyapraphatsara.
1992).
Chemical constituents: Camphor, cineol, eugenol, limonene, pinene, sabinene,
terpineol, ocimol, linalool
Biological activity: Fixed oil of O. sanctum was found to possess anti-
inflammatory activity against car ageenan and different other mediator-induced paw edema inrats. It also inhibited arachidonic acid and leukotriene-induced paw edema. The anti-inflammatory activity of O. sanctum supported the dual inhibition of arachidonate metabolism asindicated by its activity in inflammation models that are insensitive to selective cyclooxygenaseinhibitors (Singh et al., 1996). Water and hot water extracts of O. sanctum (0.5 ml/disc) showedno antibacterial activity against Bacillus subtilis both H-17 (rect+) and M-45 (rec -) (Farnsworthand Bunyapraphatsara, 1992).
1.2.2.5 Boesenbergia pandurata
Scientific name: Boesenbergia pandurata Roxb.
Synonym: Gastrochilus panduratus Ridl., Boesenbergia rotunda Linn.,
Kaempferia pandurata Roxb.
Common name: Finger root, Kra chaai (general), Ka aen, Ra aen, Chee-puu,
See-phuu (Shan-Maehongson), Waan phra aa thit (Bangkok)
Family name: Zingiberaceae
Botanical description: The plants are rhizomeatous herbs; having roots
cylindrical, fascicled, 6-10 cm long; tip acute, out side light brown, inside yel ow, scented (Figure1.8). Leafy shoot is very short, consisting of 3-4-leaved; petioles 12-25 cm long, tincted red;blade el iptic or oblong, 10-30 cm long, 5-10 cm wide; apex acute; base cuneate or obtuse; marginentire. Inflorescences are terminal, subsessile, enclosed by the leaf sheaths; bearing 2-rankedbracts each subtending a single flower. Bracts are linear-lanceolate up to 5 cm long. Bracteolesare as long as the bracts but nar ower. The uppermost flower opens first. Calyx is about 2 cm,bifid. Corol a is pink, tube exceeding the bracts; lobes about 1.5 cm, oblong. Label um is bag-shaped about 2.5 cm long, 2 cm wide. Lateral staminodes are slightly shorter than corol a lobesand mot led purple. Fruits are el ipsoid (Farnsworth and Bunyapraphatsara, 1992).

Figure 1.8 Boesenbergia pandurata Roxb.
Part used: Rhizomes
Traditional uses: Treatment of stomatopathy, for health promotion, for
dysentery, ringworm, chloasma, abscesses, antiflatulence and carminative (STJ9I9U <TST99;UFGA LPVO9DT ;C:P@ULMCNO, 2537; Farnsworth and Bunyapraphatsara, 1992)
Chemical constituents: 1,5-Cineol, dl-pinostrobin, camphor, flavonoid,
chromene (STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO, 2537), boesenbergin A, boesenberginB, panduratin A, cardomin, cardomonin, pinostrobin, pinocembrin, alpinetin, isopanduratin and 5-hydroxy-7-methoxyflavanone (Hwang et al., 2004)
Biological activity: It has been reported that B. pandurata had antibacterial
activity against Bacillus subtilis (STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO, 2537).
Isopanduratin A isolated from B. pandurata had antibacterial activity against
Streptococcus mutans. The minimum inhibitory concentration (MIC) of isopanduratin A was 4mg/l. The bactericidal test showed that isopanduratin A completely inactivated S. mutans at 20mg/l in 1 min. Significant inhibitory activity of isopanduratin A was also observed against S.
sobrinus, S. sanguinis and S. salivarius with MIC of 4 mg/l. Thus isopanduratin A could beemployed as a potential antibacterial agent for preventing dental caries (Hwang et al., 2004).
1.2.2.6 Piper betle
Scientific name: Piper betle Linn.

Synonym: Chavica betle Miq., Chavica auriculata Miq.
Common name: Betal vine, Phlu, See-keh (Malay-Narathiwat)
Family name: Piperaceae
Botanical description: The plant is stout creeper, climbing by adventitious roots
at the nodes, quite glabrous (Figure 1.9). Leaves are simple, alternate, broadly ovate or rounded,5-18 by 2-10 cm, having apex acute or acuminate, unequal y rounded at the base or broadly heart-shaped, coriaceous, having prominent vein beneath. Flowers are very minute, in cylindrical maleor female spikes, pendulous, male spikes are 2-12 cm long, having pedulous, 1.5-3 cm long,female spikes are long-peduncled, without calyx and corol a, having one smal bract with eachflower; ovary with one cel and one ovule. Fruit is a berry, smal , round, pulpy; containing oneglobose seed (Farnsworth and Bunyapraphatsara, 1992).
Figure 1.9 Piper betle Linn.
Part used: Leaves
Traditional uses: Al ergy, inflammation, beating by insect, kil insect
(insecticide), help driving gas out, help curing urticaria and treatment of skin diseases(STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO, 2537)
Chemical constituents: Chavicol, chavibitol, cineol, eugenol, carvacrol,
caryophyl ene, β-sitosterol (Ramji et al., 2002)
Biological activity: It has been reported that al ylpyrocatechol (APC) from P.
betle leaves showed promising activity against obligate oral anaerobes responsible for halitosis.
The biological studies with APC indicated that the potential to reduce methylmercaptan andhydrogen sulfide was mainly due to the anti-microbial activity as established using dynamic invitro models (Ramji et al., 2002).
The ether leaf extract of the leaves P. betle had good antimicrobial activity
against Trichophyton mentagrophytes, T. rubrum and Epidermophyton floccosum, Staphylococcus
aureus and p-hemolytic Streptococcus Group A (G:ggT<:G@U =>OC:h9iCiNM FGA j9HSMNh k>DT<NhT,2528).
1.2.2.7 Senna alata
Scientific name: Senna alata Linn.
Synonym: Cassia bracteata, Cassia herpetica, Herpetica alata, Cassia alata
Common name: Ringworm bush, Candle bush, Acapulo, Calalabra bush,
Chumhet thet (Central, Peninsular), Kheekhaak, Lapmuen luang, Maak Kaling thet (Northern),Chumhet yai (Central), Ta-see-pho (Karen-Mae Hong Son)
Family name: Leguminosae
Botanical description: The plant is an erect shrub, about 1-2-(5) m high (Figure
1.10). Leaves are paripinnate, 30-60 cm long; consisting of 8-20 pairs of leaflets, each leaflet isoblong or el iptic oblong, rounded at both ends, 5-15 by 3-4 cm, glabrous; the petiolules arerobust, 2-3 cm long. Flowers are densely in axil ary racemes, about 20-50 cm long, 3-4 cm broad.
The bracts are caduceus, 2-3 by 1-2 cm. The pedicels are very short about 2-4 m long. There are5, unequal, oblong, 10-20 by 6-7mm, green sepals. The petals are bright yel ow, ovate-orbicularto spathulate, short-clawed, 2 by 1-1.5 cm. There are 9-10 stamens; 2 large, 4 smal and 3-4stamens are reduced. The anthers are opening by apical pores. There is only one pistil andglabrous ovary. Fruit is a thick, flat ened, wing, glabrous pod, 10-15 by 1.5-2 cm; the wings are 5mm broad. Seeds are about 50, flat ened, more or less quadrangular, 7-10 by 5-8 mm, black(Farnsworth and Bunyapraphatsara, 1992).
Figure 1.10 Senna alata Linn.
Part used: Fresh or dry leaves
Traditional uses: It can be used to cure eczema, treatment of skin diseases,
ringworm, Tinea versicolor and laxative (STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO, 2537).
Chemical constituents: Aloe-emodin, chrysophanol, emodin, sennoside, rhein,
kaempferol (9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539)
Biological activity: It has been reported that ethanolic extract of S. alata leaves
exhibited high activity against various species of dermatophytic fungi but low activity againstnon-dermatophytic fungi. However, bacterial and yeast species showed resistance against in vitrotreatment with the extract. The minimum inhibitory concentration (MIC) values of the extractagainst Trichophyton mentagorphytes vat. interdigitale, Trichophyton mentagrophytes var.
mentagorophyte, Trichophyton rubrum and Microsporum gypseum were 125 mg/ml, whereasagainst Microsporum canis was 62.5 mg/ml (Ibrahim and Osman, 1995).
Crude ethanol and water extracts of the bark had antimicrobial activity against
fungi, Candida albicans. In addition, crude extract of the leaves had antimicrobial activity againstbacteria, Staphylococcus aureus (Somchit et al., 2003).
The volatile oil from the leaves showed antibacterial activity. A 95% alcohol
extract of the leaves exhibited bactericidal activity against Bacillus subtilis, Serratia marcescens,
Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae. A 50% alcohol extract of
the aerial part of S. alata possessed the bactericidal activity against B. subtilis, S. aureus,
Salmonella typhosa and E. coli (25.0 µg/ml) and also against Agrobacterium tumefaciens(Farnsworth and Bunyapraphatsara, 1992).
1.2.2.8 Alpinia galanga
Scientific name: Alpinia galanga Linn.
Synonym: Languas galanga Linn.
Common name: Greater Galangal, Katuk karohinee (Central), Khaa, Khaa
luang (Northern), She-ae-khoei, Sa-e-choei (Karen-Mae Hong Son)
Family name: Zingiberaceae
Botanical description: The plant is a perennial herb with underground stem, 2-
2.5 m high, having aerial stem leafy rhizome with conspicuously nodes and internodes, slightlyaromatic (Figure 1.11). Leaves are simple, rising from the under ground stem; 20-50 by 7-11 cm;glabrous; consisting of blade lanceolate or el iptic-oblong; apex and base acute; margin entire;petiole 5-7 mm long, slightly hairy, and leaf-sheath. Inflorescence is terminal raceme, 15-30 cmlong, having peduncle glabrous, rachis minutely hairy, bearing many smal flowers; pedicel 8-9mm long; bract ovate, 2.5 cm long; calyx greenish white, about 8 mm long, tubular, with 3-toothed, hairy; corol a short tubular, 2.5-3 cm long, apex divided into 3 lobes, the upper lobebroader, the lower lobes oblong, spreading when anthesis; lip distinctly clawed, 1.5-2 cm long;stamen 1, curved, 2.5 cm long, filament flat ened, anther 6-7 mm long; ovary suborbicular, 4 mmlong, 3 locule, 1-2 ovule in each locule. Fruit is globose or el ipsoid, 1 cm in diameter, orange-red, black when ful y matured; containing 2-3 seeds (Farnsworth and Bunyapraphatsara, 1992).
Figure 1.11 Alpinia galanga Linn.
Part used: Rhizomes
Traditional uses: The old tuber is used to prick and apply on the area of eczema
or mix with alcohol for applying on the urticaria area, treating indigestion, nausea and flatulence,Tinea versicolor, Tinea capitis, abscesses, ring worm, stomach upset (Farnsworth andBunyapraphatsara, 1992).
Chemical constituents: Methyl cinnamate, cineol, eugenol, α-pinene, 1′-
acetoxychavicol acetate, terpinen-4-ol, acetoxychavicol acetate (Oonmet a-aree et al., 2005), 1,8-cineole, kaempferol, quercetin (Farnsworth and Bunyapraphatsara, 1992)
Biological activity: It has been reported that A. galanga extract had inhibitory
ef ect against Staphylococcus aureus 209Ps. The minimum inhibitory concentration (MIC) of thegalangal extract was 0.325 mg/ml and the minimum bactericidal concentration (MBC) at 1.3mg/ml. The major compound of the extract was D,L-1′-acetoxychavicol acetate, which wasidentified by GC-MS and NMR (Oonmet a-aree et al., 2005).
The ethanolic and chloroform extracts of A. galanga rhizome showed antifungal
activity against Microsporum gypseum, Trichophyton rubrum (Archararit et al., 1984). Somereports showed that A. galanga was safety, no ef ect of acute and chronic toxicity (Mokkhasmit et
al., 1971) and no effect of mutatogenic activity (Rompelberg et al., 1995).
1.2.2.9 Punica granatum
Scientific name: Punica granatum Linn.
Synonym: Punica nana Linn.
Common name: Pomegranate, Tubtim, Siae lin (Chinese), Phi laa (Nong khai),
Philaa khaao (Nan), Ma koh (Northern), Maak-chang (Shan-Mae Hong Son).
Family name: Punicaceae
Botanical description: The plant is an erect shrub up to 3 m high, much
branched from the base, having branchlets slender, often ending in a spine (Figure 1.12). Leavesare simple; oblong-lanceolate, 1-9 by 0.5-2.5 cm; consisting of obtuse or emarginated apex; base
acute, shiny, glabrous. Flowers are showy, orange red, about 3 cm in diam, 1-5 borned at branchtips, the others solitary in highest leaf-axils, sessile or subsessile; consisting of calyx 2-3 cm long,tubular, lobes erect or recurved, thick, coriaceous; petals the same numbers as the calyx lobes,rounded or very obtuse, from edge of hypanthium, caduceus; stamen numerous within upper halfof hypanthium, filament free; inferior ovary, ovules numerous, style1, stigma capitate. Fruit isglobose berry, crowded by persistent calyx-lobes, having pericarp leathery fil ed with numerousseeds, which are sur ounded by pink and red, transparent, juicy, acid, pleasant-tasting pulp(Farnsworth and Bunyapraphatsara, 1992).
Figure 1.12 Punica granatum Linn.
Part used: Fruit rind
Traditional uses: Anthelmintic, astringent, for wound heeling, pus-foamimg,
antidysentery, cough, antidiar heal (Navarro et al., 1996)
Chemical constituents: Gal otannic acid, tannin (Navar o et al., 1996),
pel etierine pulp (Farnsworth and Bunyapraphatsara, 1992).
Biological activity: It has been reported that methanolic extract of
P. granatum possessed strong in vitro antibacterial activities against Escherichia coli and
Staphylococcus aureus (Melendez and Capriles, 2005). In addition, it has been reported that P.
granatum possessed strong in vitro antimicrobial activity against S. aureus, E. coli, Pseudomonas
aeruginosa and Candida albicans with MIC values of 0.62, 10.0, 10.0 and 10.0 mg/mlrespectively (Navarro et al., 1996). The peel extract of pomegranate had astringent activitybecause of tannin and gal otannin acid. Thus it could treat diarrhea.
1.2.2.10 Morus alba
Scientific name: Morus alba Linn.
Common name: Mulbery, Mhon
Family name: Moraceae
Botanical description: Mulber y is a deciduous tree, 30-50 feet tal with almost
equal spread, dense with a rounded top. The leaves are, simple, green and polymorphic (manyshapes). Flowers are inconspicuous greenish-white in the early spring. The fruits go from white topink to dark red or purple, are smal fleshy drupes and very tasty (Figure 1.13).
Figure 1.13 Morus alba Linn.
Part used: Leaves, bark, roots
Traditional uses: Reduce blood cholesterol, reduce blood sugar, anti
hypertension. It is helpful in cough, dyspepsia, facial dropsy, oedema and injury(www.motherherbs.com).
Chemical constituents: Flavonoid (mulber ofuran G, morusin and camphor)
(9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539; Sohn et al., 2004) gamma aminobutyric acid, phytosterol, sitosterol, fiber, deoxynojirimycin, calcium, vitamin A, vitamin B1,vitamin B2, vitamin C, sodium, potassium
Biological activity: It has been reported that some prenylated flavonoids from
M. alba had antimicrobial activities against Escherichia coli, Salmonella typhimurium,
Staphylococcus epidermidis and S. aureus. Mulber ofuran G and albanol B showed strongantibacterial activity with 5-30 µg/ml of MICs (Sohn et al., 2004). M. alba leaf methanolicextract and its fractions (chloroform, butanol, and aqueous fractions) were found to inhibit NOproduction in LPS-activated RAW264.7 macrophages without an appreciable cytotoxic ef ect atconcentration from 4 to 100 µg/ml. In addition, M. alba leaf extract and its fractions significantlydecreased the production of TNF-α (Choi and Hwang, 2005).
1.2.2.11 Azadirachta indica
Scientific name: Azadirachta indica A. Juss.
Synnonym: Antelaea azadirachta (L.) Adelb., Azedarach fraxinifolia Moench,
Melia azadirachta L., M. fraxinifolia Adelb., M. indica (A. Juss.) Brandis, M. pinnata
Common name: Neem, Neem-tree, Indian lilac, White cedar (Eng.), Margosa
tree (Port.), Azad-darakht-ihindi (Persian), Tamaka, Bowtamaka, Tama, Sadao india, Sadao thai(Thai).
Family name: Meliaceae
Botanical description: Medium sized tree, up to 15 m tal , rarely 25 m, with
short, straight bole and long spreading branches, forming a dense, large, oval or rounded crown.
Evergreen or, under extreme heat and drought, deciduous. Old bark turning dark grey, thick andfur owed. Leaves imparipinately compound with 7-17 pairs of leaflets, which are ovate orlanceolate, falcate with uneven base and dentate margins, 6-8 cm long, 1-3 cm wide.
Inflorescence a 10-30 cm long panicle with many, smal white to cream coloured flowers (Figure1.14). Neem is sometimes confused with the chinaberry, Melia azedarach L., but they are easilydistinguished by the leaves. Azadirachta spp. have simple pinnate leaves, while those of Meliaspp. are 2-to 3-pinnate (Schmidt and Joker, 2000).
Figure 1.14 Azadirachta indica (www.homedd.com)
Part used: Leaves
Traditional uses: Dental diseases, skin diseases, leprosy, skin ulcers, iching and
burning sensation (Biswas et al., 2002)
Chemical constituents: Isoprenoids (diterpenoids and triterpenoids),
protomeliacins, limonoids, azadirone, gedunin, nimbin, salanin, nimboline and azadirachtin(Biswas et al., 2002, Poddar and Mahato, 1988)
Biological activity: It has been reported that A. indica leaf extract had
antibacterial activity against Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae,
Neisseria gonorrhea, Proteus vulgaris, Pseudomonas aeruginosa, Corynebacterium diptheriae,
Neisseria spp., Salmonella spp. (Talwar et al., 1997). In addition, the leaf extract of A. indica hadanthelmintic activity and antidysentery. The lotion from leaf extract of A. indica was used fortreatment of acute or chronic symptoms of skin diseases, 3-4 and 2 weeks, respectively (Biswas et
1.2.2.12 Cinnamomum verum
Scientific name: Cinnamomum verum Presl.
Synonym: Cinnamomum zeylanicum Blume.
Common name: Cinnamon, Ob choey
Family name: Lauraceae
Botanical description: Trees; young branches dark brown, terete, glabrous.
Leaves opposite or subopposite, coriaceous, ovate to broadly ovate, 10-15 cm long, 4-8 cm wide,tripliveined, glabrous, apex blunt or slightly acute, petioles stout, ca. 1 cm long. Flowers graypubescent, in axil ary, sparsely strigose inflorescences as long as or longer than leaves; tepals 6,equal, erect; fertile stamens 9. Fruit an el ipsoid berry, ca. 1 cm long, subtended by a cupule withpersistent tepals at ached to the rim. (www. hear.org/pier/species/cinnamomum_verum.htm)
Figure 1.15 Cinnamomum verum Presl.
(www. toptropicals.com)
Part used: Bark (Figure 1.15)
Traditional uses: Antidysentery, antiseptic, antifungal agents, stimulant,
astringent and carminative, as an antidote for diar hoea and stomach upsets(9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539)
Chemical constituents: Eugenol, benzaldehyde, linalool and α-perpineol
(9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539)
Biological activity: It has been reported that C. verum possessed an anti-
nociceptive ef ect against both acetic acid-induced writhing and hot plate-induced thermalstimulation (At a and Alkofahi, 1998). The essential oil from C. verum exhibited antibacterialactivities against Staphylococcus aureus, Streptococcus pneumoniae, Enterococcus spp.,
Escherichia coli, Serratia marcescens, Enterobacter cloacae, Klebsiella pneumoniae strainsisolated from pediatric patients severly infected (Hersch-Martinez et al., 2005).
1.2.2.13 Plumbago zeylanica
Scientific name: Plumbago zeylanica Linn.
Synonym: Plumbago viscosa Blanco.
Common name: White leadwort, Jet amoonplerng-khaw
Family name: Plumbaginaceae
Botanical description: This is a spreading or somewhat climbing, half-woody
plant, 1 to 2 m in length, and smooth except for the glandular calyces (Figure 1.16). The leavesare oblong-ovate to ovate, 4 to 10 cm long, and pointed at the tip; the base of the stalk is dilatedand clasps the stem. The spikes are 5 to 25 cm in length. The calyx is green, about 1 centimeterlong, and covered with long-stalked, glandular hairs. The corol a is white or very pale blue, about1.5 cm in diameter; it has a slender tube, about 2 cm long, and spreading limb (Farnsworth andBunyapraphatsara, 1992).
Figure 1.16 Plumbago zeylanica Linn.
Traditional uses: Effective in intestinal disorders, cures wounds, fever, skin
disorders, rheumatism and white spots of skin and dental diseases (9:9;<:9 =>?@ABCAD:EC FGAHC9>I JIKI:@LMCNOPC, 2539)
Chemical constituents: Plumbagin (Paiva et al., 2003)
Biological activity: It has been reported that plumbagin isolated from roots of
white leadwort had antimicrobial activity against Staphylococcus aureus and Candida albicanswith minimum inhibitory concentrations of 1.56 µg/ml and 0.78 µg/ml, respectively (Paiva et
1.2.2.14 Dioscorea membranacea
Scientific name: Dioscorea membranacea Pier e
Common name: Hua-khaw-yen
Family name: Dioscoreaceae
Botanical description: The rhizome is a wide running, perhaps even to 2 m. It
is dark brown with white flesh. The stem is slightly ridged and unarmed. Leaves are deeplytrifid above a cordate base with the short acuminate 9 nerved, two primary nerves reaching theforerunner tip along with the midrib and the second pair reaching the tips of the letheral lobes(Figure 1.17). The petioles are 1/2-2/3 of the length of the blade. Male flowers have smalsubsessile cymes with up to 4 flowers, sepals 1 mm long, and long-ovate. Stamens, alike thefilaments insert just below the sepals 0.3 mm long. The anther is smal and introse. Femaleflowers are on downwardly directed spike-like racemes. Outer sepals are obovate, inner ones arelanceolate, and the inner are a lit le shorter than the outer. Style is short. Capsules are apart, about1 -2 cm (Burkil , 1951).
Figure 1.17 Dioscorea membranacea Pierre
Part used: Rhizome
Traditional uses: Treatments of dermopathy, lymphopathy, inflammation,
cancers, veneral diseases, and leprosy (Tewtrakul and Itharat, 2006)
Chemical constituents: Isoflavone, 7,6′-dihydroxy 3′-methoxy isoflavone,
taxifolin and astilbin (Yijun et al., 1998), naphthofuranoxepins, dioscorealides A and B, and 1,4-phenanthraquinone, dioscoreanone (Itharat et al., 2003).
Biological activity: It has been reported that the ethanolic extract of D.
membranacea roots showed cytotoxic activity against lung cancer cel lines (IC50= 4.6 µg/ml),prostate cancer cel lines (IC50= 17.55 µg/ml) and normal cel lines (IC50= 66.05 µg/ml)(Saetung et al., 2005).
In addition, the ethanolic extract had antimicrobial activities against
Staphylococcus aureus, Bacillus subtilis, Escherichia coli and E. floccosum with MIC values of<1.25, <1.25, 2.5 and < 1.25 mg/ml, respectively (Itharat, 2002). The ethanolic extract of D.
membranacea exhibited potent inhibitory activity against β-hexosaminidase release as a markerof degranulation in RBL-2H3 cel s, with an IC50 value of 37.5 µg/ml. Dioscorealide B possessedthe highest antial ergic activity with an IC50 value of 5.7 µM, fol owed by dioscoreanone
(IC50 = 7.7 µM), dioscorealide A (IC50 = 27.9 µM), and diosgenin (IC50 = 29.9 µM) (Tewtrakuland Itharat, 2006).
1.2.2.15 Syzygium aromaticum
Scientific name: Syzygium aromaticum (Linn.) Mer & Per y.
Synonym: Eugenia caryophyllus (Sprengel) Bul ock & Har ison
Common name Clove, Kan pluu, Chan-jee
Family name: Myrtaceae
Botanical description: The plant is an evergreen tree 5-10 m high; having dark
grey or light brown; al parts glabrous. Leaves are simple, opposite, el iptic or oblongobovate, 7-12 cm long, 3-5 cm wide, shiny above, paler beneath, pel ucid-dot ed; consisting of tip acuminate,margin entire, base cuneate, dark green. Petioles are 2-3 cm long with swol en reddish base.
Flowers are bisexual, 3-20 in paniculate cymes, having angled peduncles and short pedicels,about 5 mm long. Each flower consists of 4 freshly triangular sepals; petals 4, implicate, rounded,tinced red, fal ing as flower opens; stamens numerous, smal , filaments slender, anthers pale-yel ow; ovary inferior, 2-3 cel ed with several ovules; style about 3 mm; stigma 2-lobed. Fruit is afreshy drupe, obovoid-el ipsoid or oblong obovoid, about 2-3 by 1.2 cm, containing 1, rarely 2seeds. Seeds are oblong, about 1.5 cm long, groovedon one surface (Farnsworth andBunyapraphatsara, 1992).
Figure 1.18 Syzygium aromaticum (Linn.) Mer &Per y.
Part used: Flowers (Figure 1.18)
Traditional uses: For masking alcoholic smel on the breath, stomachache,
fainting, toothache, gastrointestinal disturbances, antiseptic, antiflatulence, treatment of beri beri(STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO, 2537)
Chemical constituents: Eugenol, β-caryophyl ene, acetyl eugenol, methyl amyl
ketone, chavicol, eugenol acetate (9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539)
Biological activity: It has been reported that the volatile oil of clove exhibited
antibacterial activity against Bacillus subtilis, Enterobacter aerogenes, Escherichia coli,
Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus faecalis (Umehara et al.,1992; Dean et al., 1995), S. paratyphi and S. dysenteriae (Ahmad and Beg, 2000). In additionboth clove oil and eugenol exhibited inhibitory action against Trichophyton, Achorion, and
Epidermophyton. They were found to be ef ective at dilutions of 1:8000 to 1:16000 and themicrobes failed to become resistant to the oil. The oil showed no ir igation action (Farnsworthand Bunyapraphatsara, 1992).
1.2.2.16 Andrographis paniculata
Scientific name: Andrographis paniculata (Burm.f.) Nees
Synonym: Justicia paniculata Burm. f.
Common name: Fa thalaai (Bangkok), Fa thalaai joan, Yaa kannguu
(Songkhla), Khee-pang-hee (Chinese)
Family name: Acanthaceae
Botanical description: The plants are annual herbs about 30-100 cm high,
having stem erect, 4-angled, much branches (Figure 1.19). Leaves are simple, opposite, sessile orshort petioled, el iptic or lanceolate, 2.5-8 cm long, 1-3 cm wide, glabrous on both surfaces.
Flowers are in racemes, 2.5-10 cm long, consisting of flowers distant; frequently 1-sided; bractsmal , linear; pedicel 0-6 mm; calyx 1, green, about 3 mm long, connate at the base, divided into5 linear segments, hairy; corol a white, tubular, divided into 2 lips, upper lip 3-lobed, rose-purplespot ed, hairy; lower lip smal , 2-lobed; stamens 2, filaments hairy, upwards, anther dark-purple;ovary 1, style slender, tip minutely bifid. Fruit is a linear-oblong capsule about 1.5 cm long, 3-5
mm wide, loculicidal, nearly glabrous. Each capsule contains 6-12 seeds which are subquadrate,bony, yel ow or deep brown, slightly translucent (Farnsworth and Bunyapraphatsara, 1992).
Figure 1.19 Andrographis paniculata (Burm.f.) Nees
Part used: Leaves
Traditional uses: Curing flu and sore-throat, treatment of abscesses,
antidysentery, wound healing, anti-inflammation and antidiarrhoeal (STJ9I9U <TST99;U FGALPVO9DT ;C:P@ULMCNO, 2537; Farnsworth and Bunyapraphatsara, 1992)
Chemical constituents: Andrographolide, diterpene lactone, andrographiside,
paniculide, diterpenoids, farnesol (9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539;Farnsworth and Bunyapraphatsara. 1992)
Biological activity: It has been reported that the aqueous extract,
andrographolides and arabinogalactan proteins from A. paniculata showed antimicrobial activityagainst Candida albicans, Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis(Singha et al., 2003).
It has been demonstrated that 95% ethanol extract of A. paniculata showed
antibacterial activity against Staphylococcus aureus while the hot water extract showed no ef ect.
However, both extracts were active against E. coli (Farnsworth and Bunyapraphatsara, 1992).
1.2.2.17 Cymbopogon citratus
Scientific name: Cymbopogon citratus Stapf.
Synonym: Andropogon citratus
Common name: Lemon grass, Lapine, Takhrai, Khaa-hom (Shan-Mae Hong
Son), Khrai (Peninsular), Cha khrai (Northern), Soet-kroei, Loe-kroei (Khmer-Surin), Ho-wo ta-po (Karen-Mae Hong Son), Hua-sing-khai (Khmer-Prachin-buri)
Family name: Gramineae
Botanical description: C. citratus is a perennial aromatic herb with tufted
culms, erect, up to 1 m high, terete and hard (Figure 1.20). It is scarcely flowered. Leaves arearomatic when crushed due to essential oils; consisting of terete and glabrous leaf sheaths; linearblade, nar ow base, acute apex, up to 100 cm long and 2 cm wide; chartaceous ligule, about 2 mmlong, truncate, minutely ciliate. Inflorescence is a large false panicle; racemes paired, subtendedby spathes. Spikelets are paired; consisting of the upper pedicel ate; the lower sessile, about 4 mmlong, lanceolate, as long as the spikelet, acute, having margins inrol ed, 2-keeled, hispid alongkeels, 5-veined; upper glume is lanceolate, chartaceous, about 4 mm long, 1-keeled, havingmargin inrol ed and fimbriate, inconspicuously 3-vained; lower lemma is membranous,lanceolate, margins broadly inrol ed and fimbrate, cuspidate, 1-veined; upper lemma is lanceolate,having membranous, about 3 mm long, having margins fimbrate, 1-veined, aristate (Farnsworthand Bunyapraphatsara, 1992).
Figure 1.20 Cymbopogon citratus Stapf.
Part used: Stalks, whole plant
Traditional uses: Anti-flatulance, treatment of disorders of urination, Tinia
versicolor, strangury, as an appetite stimulant (STJ9I9U <TST99;U FGA LPVO9DT ;C:P@ULMCNO,2537).
Chemical constituents: Citral, myrcene, citronel al, geraniol, menthol,
citronel ol, eugenol, borneol (9:9;<:9 =>?@ABCAD:EC FGA HC9>I JIKI:@LMCNOPC, 2539).
Biological activity: It has been reported that C. citratus exhibited antimicrobial
activities against Salmonella spp., Escherichai coli O157, Campylobacter jejunii and Clostridium
perferingens (Wannissorn et al., 2005). Pure lemongrass oil from C. citrates posessedantibacterial activity against P. acnes with MIC and MBC values of 0.6 µl/ml (Lertsatit hanakorn
et al., 2006).
1.2.2.18 Rhinacanthus nasutus
Scientific name: Rhinacanthus nasutus (Linn.) Kurz.
Synonym: Justicia nasuta L.
Common name: Thong phan chang, Yaa man kai (Central)
Family name: Acanthaceae
Botanical description: The plant is a smal shrub, up to 1.5 m high; the stem is
obtusely quadrangular, when young it is covered with fine, up curved hairs (Figure 1.21). Leavesare simple opposite; elipptic or lanceolate; 4-6 by 2-3 cm; chartaceous; entire; light green; shortlypubescent having acute base and apex. Flowers are white, in short axil ary clusters; denselyappressed pubescent. The calyx is divided into 5 deeply acute parted, light green, 5-6 mm long.
The corol a-tube is about 2 cm, having brownish purple spots at the throat of the tube, bilabiate,upper lip erect, bifid, lower lip 3-lobed; stamens 2, inserted in the throat; ovary 2-loculed.
Capsule is loculicidal y 2-valved pulp (Farnsworth and Bunyapraphatsara, 1992).
Figure 1.21 Rhinacanthus nasutus (Linn.) Kurz.
Part used: Fresh leaves
Traditional uses: To cure eczema, pruritis, abscess pain, skin diseases, anti-
inflammatory, Tinea versicolor, ringworm and rash (9:9;<:9 =>?@ABCAD:EC FGAHC9>I JIKI:@LMCNOPC, 2539; Farnsworth and Bunyapraphatsara, 1992)
Chemical constituents: 4-acetonyl-3, 5-dimethoxy-p-quinol, rhinacanthin-A,
rhinacanthin-D, rhinacanthin-Q, rhinacanthone, ρ-hydroxybenzaldehyde, methyl vanil ate,syrengaldehyde, lupeol, wogonin, oroxylin A, (+)-praeruptorin, al antoin, β-amyrin, stigmasterol,
sitosterol, stigmasterol-4-en-3-one, 2-methylanthraquinone, 2, 4-dimethoxybenzoquinone, 2-methoxy-4-propionylphenol, syringic acid, vanil ic acid (Sat ar et al., 2004)
Biological activity: It has been reported that aqueous ethanolic extract of R.
nasutus exhibited a potent dose dependent anti-fungal activity against Candida albicans and
Trichophyton. In addition, anti-bacterial activity of the plant is also observed against gram-positive bacteria (Bacillus subtilis, B. cereus, B. globigii). However, it was inef ective againstgram-negative bacteria (Proteus morgani, P. mirabilis, Samonella typhi, Pseudomonas
aeruginosa, Escherichia coli (Sat ar et al., 2004).
1.2.3 Alpinia galanga
1.2.3.1 Chemical constituents of A. galanga
Rhizomes: (1′S)-1′-acetoxychavicol acetate, (1′S)-1′-acetoxyeugenol acetate,
1′-acetoxychavicol acetate, 1′-acetoxyeugenol acetate, D-camphor, chavicol, chavicol acetate,1,8-cineole, trans-coniferyl diacetate, tran- p -coumaryl diacetate, di-(p-hydroxyl-cis-styryl)methane, essential oil, eugenol, eugenol acetate, trans-β-farnesene, galangin, 7-hydroxy-3, 5-dimethoxy
4-hydroxybenzaldehyde,
1′-hydroxy-chavicol acetate, p-
hydroxycinnamaldehyde, isorhamnetin, kaempferol, kaempferol-4′-methyl ether, kaempferol-7-methyl ether, methylcinnamate, methyleugenol, pinenes, quercetin, quercetin-3-methyl ether,resins, sesquiterpenoids (Farnsworth and Bunyapraphatsara, 1992)
Fruits: 1d-acetoxychavicol acetate, 1′-acetoxyeugenol acetate (Farnsworth and
Bunyapraphatsara, 1992)
Seeds: galanal A, galanal B, (E)-8,17-epoxylabd-12-ene-15, 16-dial (Farnsworth
and Bunyapraphatsara, 1992)
Not specified part used: D,L-1′-acetoxychavicol acetate, dl-1′-acetoxyeugenol
acetate, anethol, benzaldehyde, benzyl benzoate, benzyl salicylate, camphor, caryophyl ene, 1,8-cineole, cinnamic aldehyde, p-cymene, dilupiol, elemicin, essential oil, eugenol, isosafrole,limonene, lialool, methylchavicol, methyleugenol, nerolidol, phel andrene, ∝-pinene, β-pinene,safrole (Farnsworth and Bunyapraphatsara, 1992; Yang and Eilerman, 1999)
1.2.3.2 Pharmacological activities of A. galanga
Antimicrobial activityIt has been reported that galangal extract exhibited inhibitory ef ect against
Staphylococcus aureus 209Ps with MIC value of 0.325 mg/ml and MBC value of 1.3 mg/ml. Themajor compound of the extract was D,L-1′-acetoxychavicol acetate (Oonmet a-aree et al., 2005).
Antifungal activityIt has been reported that chloroform extract of A. galanga possessed antifungal
activity against Cryptococcus neoformans and Microsporum gypseum, but exhibited weak activityagainst Cantida albicans (Phongpaichit et al., 2005).
The ethanolic extract of A. galanga rhizome had inhibitory activity against a
variety of human pathogen fungi, including Cryptococcus neoformans, Wangiellia dermatitidis,
Alternaria alternate, Aspergillus fumigatus, Fusarium oxysporum, Microsporum gypseum,
Pseudallescheria, Rhizopus species and Tricophyton mentagrophytes (Ficker et al., 2003).
Antitumor activityIt has been reported that 1′-acetoxychavicol acetate (ACA) isolated from A.
galanga had antitumor activity. It suppressed chemical y induced carcinogenesis in Ehrlichascites tumor cel s. The anticarcinogenic ef ects of ACA might be partly due to perturbation ofthe polyamine metabolic pathway and triggering of caspase-3-like activity, which result inapoptosis (Moffat et al., 2000).
Cytotoxic activityIt has been reported that A. galanga exhibited cytotoxic activity. 1′-
Acetoxychavicol acetate, the major compound had cytotoxic activity against COR L23 lungcancer cel line and MCF7 breast cancer cel line with IC50values of 7.8 µM and 23.9 µM,respectively (Lee and Houghton., 2005).
Enzyme inhibition activityIt has been reported that xanthine oxidase inhibitors were isolated and identified
as trans-p-coumaryl diacetate, trans-coniferyl diacetate, (1′S)-acetoxychavicol acetate, (1′S)-acetoxyeugenol acetate and 4-hydroxybenzaldehyde from A. galanga. The inhibitory action of thefirst two compounds was mediated through a non-competitive reaction with the substratexanthine. It was also reported that A. galanga rhizomes elicited slight amylase-inhibitory activity(Farnsworth and Bunyapraphatsara, 1992).
Smooth muscle stimulating activityAn hydroalcoholic (1:1) extract of rhizomes was reported to have smooth muscle
stimulating effect on the isolated guinea pig ileum (Farnsworth and Bunyapraphatsara, 1992).
Hypoglycaemic activityIt has been reported that A. galanga powdered rhizome and its methanol and
aqueous extracts significantly lowered blood glucose in normal rabbits but A. galanga and itsmethanol and aqueous extracts did not produce significant reduction of blood glucose in al oxandiabetic rabbits (Akhtar et al., 2002).
Antiallergic activityIt has been reported that 80% aqueous acetone extract of the rhizomes of A.
galangal inhibited release of beta-hexosaminidase, a marker of antigen-IgE-mediateddegranulation in RBL-2H3 cel s. 1′S-1′-acetoxychavicol acetate and 1′S-1′-acetoxyeugenolacetate isolated from A. galanga exhibited potent inhibitory activity with IC50values of 15 and 19µM, respectively, and inhibited ear passive cutaneous anaphylaxis reactions in mice and theantigen-IgE-mediated TNF-α and IL-4 production (Matsuda et al., 2003a).
Anti-inflammationIt has been reported that 80% aqueous acetone extract of A. galanga rhizomes
showed nitric oxide (NO) production inhibitory activities in mouse peritoneal macrophages.
Galanganal (IC50=68 µM), galanganols B (88 µM) and C (33 µM), 1′S-1′-acetoxychavicolacetate (2.3 µM), 1′S-1′-acetoxyeugenol acetate (11 µM), trans-p-hydroxycinnamaldehyde (ca.
20 µM), trans-p-coumaryl alcohol (72 µM), and trans-p-coumaryl diacetate (19 µM) isolatedfrom the aqueous acetone extract were found to show inhibitory activity (Toshio et al., 2005).
Immunostimulating activityIt has been reported that hot water polysaccharide extracts of A. galanga had
immunostimulating activity. It showed a stimulate effect on the reticulo-endothelial system (RES)and increased the number of peritoneal exudate cel s (PEC), and spleen cel s of mice. Theoptimum dose was 25 mg/kg. On the other hand, polysaccharide extract of A. galanga enhancedthe proliferation of the murine spleen cel s in vitro (Bendjeddou et al., 2003).
Antioxidative activityIt has been reported that ethanolic extract of A. galanga had antioxidant activity.
It exhibited strong superoxide anion scavenging activity, Fe2+ chelating activity. The antioxidantactivity of the extract cor elated wel with reducing power. Furthermore, ethanolic extract A.
galanga acted as radical scavenger and also as lipoxygenase inhibitor (Juntachote and Berghofer,2005).
Gastroprotective effectIt has been reported that 1′S-1′-acetoxychavicol acetate and 1′S-1′-
acetoxyeugenol acetate isolated from A. galanga rhizomes inhibited the ethanol-induced gastricmucosal lesions (ED500.61 and ca. 0.90 mg/kg). In addition, 1′S-1′-acetoxychavicol acetateinhibited the lesions induced by 0.6 M HCl (ED500.73 mg/kg) and aspirin (ED500.69 mg/kg). Inaddition 1′-acetoxyl group of 1′S-1′-acetoxychavicol acetate and 1′S-1′-acetoxyeugenol acetatewas found to be essential for their strong activity (Matsuda et al., 2003b).
The aims of the present study were as fol ows:1. To investigate the antibacterial activity of the selected plants against P. acnes2. To select the plant extract that exhibited the strongest antibacterial activity against P.
acnes3. To isolate the active compound from the selected plant using a bioassay-guidedisolation4. To establish HPLC system for quantitative determination of the activecompound5. To study on preliminary formulation study on formulation of anti-acne cream using theextract of the selected plant as an active ingredient
Source: http://kb.psu.ac.th/psukb/bitstream/2553/1465/7/283406_ch1.pdf
NFATc1 Balances Quiescence andProliferation of Skin Stem CellsValerie Horsley,Antonios O. Aliprantis,Lisa Polak,Laurie H. Glimcher,and Elaine Fuchs,1Howard Hughes Medical Institute, Laboratory of Mammalian Cell Biology and Development, The Rockefeller University,New York, NY 10065, USA2Department of Infectious Diseases and Immunology, Harvard School of Public Health, Boston, MA 02115, USA3Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA*Correspondence: DOI 10.1016/j.cell.2007.11.047
CONSEILS ET PRINCIPES DE TRAITEMENT POUR UN DIABÈTE DE TYPE 2 Le diabète est une maladie chronique, il n'y a pas de « petit - Les ongles doivent être coupés régulièrement en diabète ». C'est une maladie qui expose à des complications évitant toute blessure (préférer des soins de pédicurie +++). Ne mettez pas vos pieds en danger : ne marchez pas pieds nus,












