
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Gutierrez-et-al-2011-jbc-rev-02
JBC Papers in Press. Published on May 31, 2011 as Manuscript M111.253674
OPTOGENETIC CONTROL OF MOTOR COORDINATION BY Gi/o PROTEIN-COUPLED
VERTEBRATE RHODOPSIN IN CEREBELLAR PURKINJE CELLS.
Davina V. Gutierrez2, Melanie D. Mark1, Olivia Masseck1, Takashi Maejima1, Denise Kuckelsberg1,
Robert A. Hyde2, Martin Krause1, Wolfgang Kruse1, and Stefan Herlitze1,2
From the 1Department of Zoology and Neurobiology, ND7/31, Ruhr-University Bochum, Universitätsstr.
150, D-44780 Bochum, Germany;
2Department of Neurosciences, Case Western Reserve University,
10900 Euclid Avenue, Cleveland, OH 44106-4975, USA.
Running head: Expression of vertebrate rhodopsin in Purkinje cells
Address correspondence to: Stefan Herlitze; Department of Zoology and Neurobiology, ND 7/31, Ruhr-
University Bochum, Universitätsstr. 150, D-44780 Bochum, Germany Phone: +49 234 32 25607
Fax:+49 234 32 14185 Email:
[email protected]
G protein-coupled receptors (GPCRs) are
inhibiting more or less specifically a certain
involved in the modulation of complex
GPCR pathway. Recently, we demonstrated that
neuronal networks in the brain. In order to
light-activated vertebrate rhodopsin (vRh) is a
investigate the impact of a cell-specific Gi/o
suitable alternative to control ion conductances
protein-mediated signalling pathway on brain
such as G protein-coupled inward rectifying K+
function, we created a new optogenetic mouse
channel (GIRK) and voltage-gated Ca2+
model in which the Gi/o protein-coupled
channels via pertussis toxin-sensitive Gi/o
receptor vertebrate rhodopsin (vRh) can be
protein-mediated signaling (3). Therefore, vRh
cell-specifically expressed with the aid of Cre
may allow for the precise spatio-temporal
recombinase. Here we use this mouse model
control of Gi/o-mediated pathway
in vivo, leading
to study the functional impact of Gi/o
to an investigation that focuses on the function
modulation in cerebellar Purkinje cells (PC).
of this pathway in animal behavior or brain
We show that in vivo light activation of vRh
functions such as motor coordination.
specifically expressed in PCs reduces simple
The cerebellum plays a central role in
spike firing that is comparable to the
overall motor coordination and motor learning.
reduction in firing observed for the activation
An extensive array of GPCRs are expressed
of cerebellar Gi/o-coupled GABAB receptors.
throughout the brain and are believed to be
Notably, the light exposure of the cerebellar
involved in the modulation of network activity
vermis in freely moving mice changes the
and synaptic plasticity. It has been recognized
motor behavior. Thus, our studies directly
that the code for motor coordination and balance
demonstrate that spike modulation via Gi/o-
lies within the firing cadence and output pattern
mediated signalling in cerebellar PCs affects
of cerebellar PCs, which are the sole-output
motor coordination and show a new
neurons from the cerebellar cortex (4,5). PCs
promising approach for studying the
integrate a range of cortical, vestibular and
physiological function of GPCR-mediated
sensory information via excitatory synaptic input
signalling in a cell-type specific manner.
from parallel and climbing fiber pathways and
inhibitory synaptic input originating from
The G protein-mediated signalling pathway
neighboring interneurons. The PC firing pattern
provides a pivotal module for the adjustment of
is determined by several factors that include, the
neuronal networks against physiological or
interplay between excitatory and inhibitory
behavioral tasks on a second to minute time
scale (1). Among G-proteins, the Gi/o-mediated
conductances that support intrinsic firing
signalling pathway is the primary role in which
properties and modulation by postsynaptic
GPCRs mediate their inhibitory action on
GPCRs like the GABAB receptor (GABABR) (6-
neuronal excitability (2). The processes and
8). GABABR activation by application of the
importance of such modulation in cellular and
selective agonist baclofen, leads to a reduction
network functions has mainly been investigated
in PC firing most likely due to membrane
with the application of drugs, activating or
hyperpolarization induced by GIRK channel
Copyright 2011 by The American Society for Biochemistry and Molecular Biology, Inc.
activation (9-12). The exact mechanism in which
conditions: 92oC for 30 s, 60oC for 45 s and
Gi/o mediated GPCR modulation may occur
72oC for 1 min run for 40 cycles or 95oC for 30
within PCs and how such modulation may
s, 55oC for 1 min and 72oC for 1 min 30 sec for
influence the single spike pattern and motor
40 cycles to detect vRh-GFP or Cre-
coordination has been difficult to address
in
Recombinase respectively. PCR products were
vivo, since GABABRs and other Gi/o coupled
analyzed on a 1% agarose gel utilizing standard
receptors are expressed in various cell-types in
the cerebellum and can only be activated by
identified
vRh-GFPPC mice expressed both the
slowly diffusing drugs.
vRh and Cre recombinase genes. Wild type
In order to overcome the kinetic and
littermates were distinguished as being negative
spatial issues that the pharmacological approach
for either vRh or Cre recombinase or both.
presents and to investigate the functional impact
β
-Galactosidase Staining- Animals
of Gi/o protein-mediated modulation on
were deeply anaesthetized with 0.2cc/g Avertin
cerebellar function via spike modulation in
(tribromoethanol; Sigma) and transcardially
cerebellar PCs, we created an optogenetic mouse
perfused with 1X PBS followed by a neutral
model for the cell-type specific expression of
vRh and demonstrated that spike modulation of
paraformaldehyde). Upon complete perfusion,
PCs affects motor cordination.
brains were isolated and post-fixed in the same
paraformaldehyde solution for 15 minutes.
EXPERIMENTAL PROCEDURES
Frozen, embedded brains (OCT, Tissue TEK)
were cut into 25-30 micron section on a rotary
Generation and Screening of transgenic Mice-
microtome, mounted onto Superfrost/Plus
In order to generate a colony of
vRh-GFPPC
Microscope Slides (Fisher), allowed to dry at
room temperature for 1 hour and permeabilized
Purkinje cell specific CRE (TgPcp2-cre) mice (13)
with PBST (0.2% Triton X-100) for 15 minutes.
Slices were incubated overnight with 1mg/ml X-
GFP(TgflvRh-GFP) mice. Routine screening of all
gal staining solution (200 mM ferricyanate;
transgenic mice was accomplished by adding
Sigma, 200 mM ferrocyanate; Sigma, X-gal (40
either tail or toe tissue to 0.3 ml of lysis buffer
mg/ml in DMSO); Sigma, 1 M MgCl2; Sigma,
containing 100 mM Tris (pH 8.5), 5 mM EDTA
0.02% NP40; Sigma, and 1X PBS) at 37oC in a
(disodium salt), 0.2% SDS and 200 mM NaCl.
Twenty microliters of proteinase K (20 mg/ml,
Immunohistochemistry- Animals were
Roche Diagnostics) was added to the lysis buffer
deeply anaesthetized with 0.2cc/g Avertin
and the mixture was shaken overnight at 55oC.
(tribromoethanol; Sigma) and transcardially
Following tissue dissolution, the mixture was
perfused with 1X PBS followed by a neutral
heated to 99oC for 10 minutes and then cooled to
room temperature. A PCR master mix contained
paraformaldehyde). Upon complete perfusion,
either of the following oligos: vRh-GFP (5'
brains were isolated and post-fixed in the same
CATGCTCACCACCGTCTGCT
paraformaldehyde solution for 1 hour followed
AAGATGGTGCGCTCCTGGAC)
by a 30% sucrose solution for 24-48 hours.
Frozen, embedded brains (OCT, Tissue TEK)
TCTCACGTACTGACGGTGG
were cut into 25-30 micron section on a rotary
ACCAGCTTGCATGATCTCC). The 50 ml
microtome, mounted onto Superfrost/Plus
final PCR reaction contained 1 ml gDNA, 1 ml
Microscope Slides (Fisher) and allowed to dry at
of each primer, 1 ml dNTP mix (10 mM each of
room temperature for 1 hour. Sections were
dATP, dTTP, dCTP, dGTP; New England
washed with 1X PBST for 15 minutes and
Biolabs (NEB)), 5 ml 10X Thermopol II
blocked with 2% goat serum (1X PBST, 2 ml
Reaction Buffer (NEB), 5 ml dimethyl
goat serum, Invitrogen) for 1 hour at room
sulfoxide, 0.5 ml Taq Polymerase (NEB) and
temperature. Primary antibodies (1:200 Anti-
35.5 ml dH20. PCR reactions were run on an
GFP, Synaptic Systems and 1:200 Anti-
Eppendorf thermocycler, using the following
Calbindin, Swant or 1:200 Anti-GFP, Millipore
and 1:200 Anti-GABAB1 R, Novus Biologicals)
The rodent's mouth was secured by using the
were incubated on the sections overnight at 4oC,
incisor adapter on the anterior mount of the
followed by three washes in 1X PBST for 15
apparatus. The nose was placed into the nose
minutes per wash. Anti-species specific
clamp and the head was checked for a level
secondary antibodies (anti-mouse Alexa 546 and
position (in regards to the apparatus). Fur from
anti-rabbit Alexa 488 or anti-rabbit Alexa 546
the top of the top of the head was removed and
and anti-mouse Alexa 488, Invitrogen) were
cleaned with 70% ethanol and 10% povidone-
incubated on the sections for 2 hours at room
iodine. A midline incision was made and all
temperature, followed by three rinses in 1X
soft tissue from the skull surface was removed.
PBST for 15 minutes per wash. Images were
One 1 mm wide hole was drilled through the
skull with a battery-operated drill at Bregma
microscopy and processed with Volocity
points -5.88mm to -6.24mm and two additional
1.5 mm, anterior-lateral holes were drilled for
Nissl Staining- Sagital sections (30 µm)
mounting screws. The dura was manually
of transcardially perfused brains were mounted
removed. The modified, flanged cannula guide
onto Superfrost/Plus Microsoft slides and
(Plastics One) and skull screws (Plastics One)
allowed to air dry for 24 hours. In order to stain
were cleaned in ethanol and saline and vertically
and remove the lipids and residual fixation
lowered into their correct coordinates. The
solutions from the tissue, slices were placed into
flanged cannula guide was kept in place by two
a 1:1 chloroform/ethanol solution for 45
2.4 mm long, 1.57 mm wide mounting screws.
minutes, 5% cresyl violet acetate for 3 minutes
A cap of dental cement (3M, Rely-X luting) was
applied on top of the head and surrounded the
(approximately 4 drops) for 3 minutes with each
cannula guide. The wound was permanently
step followed with a distilled water wash.
closed by applying a thin layer of Vetbond tissue
Following the initial stain, slices were
adhesive (3M) to each side of the scalp. Animals
dehydrated by placing them into a 70% ethanol
were subcutaneously administered 1 ml of sterile
solution for 3 minutes, 96% ethanol for 3
saline and recovered on a heating pad in
minutes, two isopropanol washes for 3 minutes
individual cages. In regards to the
in vivo
each. Two, 5 minute changes of xylene made
electrophysiology, data were obtained by
any unstained parts of the tissue transparent.
performing the above surgical procedure on
Finally, coverslips were mounted onto the slides
three to six-month old
vRh-GFPPC, vRh-
with DePeX mounting medium and allowed to
GFP(TgflvRh-GFP) and C57/B6 mice. Surgeries
dry overnight. Images were taken on an Zeiss
were identical except that only one hole was
Axiophot equipped with a CCD camera
drilled at Bregma points -5.88mm to -6.24mm.
(SensiCam, PCO, Kelheim, Germany).
Upon completion of the recording session, mice
Stereotaxic Surgeries and Cannula
were euthanized by cervical displacement.
Placement-Three to six-month old male
vRh-
Fiber Optics and Photostimulation- For
GFPPC and wild type littermates were the
blue-light photostimulation through the modified
subjects of these experiments. All surgeries
cannula guide, a diode pumped crystal laser (20
were performed under aseptic conditions.
mW, 473 nm, CrystaLaser, Reno, NV, BCL-
Rodents were anaesthetized using isoflurane for
473-020) was coupled into a multimode hard
one hour or less. Sedation was verified by
polymer-clad fiber (200 µm core diameter, 0.37
using the gentle toe pinch withdraw reflex. A
numerical aperture, Thorlabs BFL37-200). The
lubricating ophthalmic ointment was applied to
animal behavior photostimulation protocol
prevent corneal drying during surgery. Mice
involved applying a 26 second light pulse to the
were mounted into the stereotactic frame
cerebellar region located directly under the
(Narishige Group, Model SR-6M) by placing
cannula opening. Protocols for the
in vivo
non-rupture ear bars into the ear canals and
recordings included a 26 second light pulse
gently tightened into place. Confirmation of
applied 10-20 seconds into each sweep (total
correct ear bar placement was dependent upon
sweep time approximately 1 minute).
complete lateral immobilization of the head.
Optrode Construction- A cleaved
lowered into the vermis and recordings were
multimode glass optical fiber (50 mm core
taken from cells ranging in depth from 1100 to
diameter, 0.37 numerical aperature, Thorlabs
3200 mm below the surface. Activity was
AFS50/125Y) was stripped of the outer polymer
amplified and filtered (bandpass 0.5 to 9 kHz)
with a multi-channel spike sorter (Plexon Inc.,
microelectrode (Impedance 1-2.5MOhm) was
Austin, TX) and stored on a computer disk with
attached to the stripped end of the optical fiber
a sampling rate of 32 kHz. During off-line
with epoxy. The optrode was coupled to a blue
analysis, simple spikes and complex spikes were
laser (Crystal Laser BCL-473-020). Triggering
discriminated using custom made software
of the laser was controlled by a custom made
implemented in Matlab (MathWorks, Natick,
Matlab program and a corresponding D/A card.
MA). Single cell spike activity was used to
Electrophysiological Analysis- Brain
calculate mean firing rates and inter spike
Slice Recordings- Sagittal sections (250 µm
intervals. The coefficient of variation (CV) of
thick) were cut from the cerebellum of P21,
the simple spike interspike intervals was
C57/B6 mice. Mice were anaesthetized with
calculated to quantify the variability in spike
isoflurane and decapitated. The cerebellum was
dissected out, cooled and sliced in an ice-cold
Baclofen Application- A Union-40
solution containing 87 mM NaCl, 75 mM
iontophoresis pump (Kation Scientific) was used
sucrose, 2.5 mM KCl, 0.5 mM CaCl2, 7 mM
for the extracellular delivery of 1 mM baclofen
MgCl2, 1.25 mM NaH2PO4, 25 mM NaHCO3,
(dissolved in 150 mM NaCl, pH = 3.5) or saline
and 20 mM glucose bubbled with 95% O2 and
through a Carbostar-3 (Kation scientific) carbon
5% CO2 with a vibratome (VT1000S, Leica).
electrode, which includes 2 barrels for
Slices were kept for at least 1 hour at room
microiontophoresis. Baclofen was delivered by
temperature in a recording artificial cerebral
+ 50nA ejection pulses and retaining currents
spinal fluid composed of 124 mM NaCl, 3 mM
were -20nA. Baclofen or saline was applied
KCl, 2.5 mM CaCl2, 1.2 mM MgSO4, 1.23 mM
for 26 seconds to the Purkinje cells that had both
NaH2PO4, 26 mM NaHCO3, and 10 mM glucose
simple and complex spikes.
bubbled with 95% O2 and 5% CO2. Slices were
Lesion Studies- In order to confirm the
continuously perfused with an external solution
position of the recording electrodes electrical
containing 10 µM CNQX and 100 µM
microlesions were created at different sites
picrotoxin. Extracellular recordings from
following the completion of the
in vivo
Purkinje cells were made at (temperature) with
recordings. Lesion areas were at least 700 µm
10 mM baclofen being perfused during steady
apart. The designated regions received a 10 µA
state firing. Patch pipettes (2-4 megaohms)
anodic current for 1 minute via the recording
were filled with an internal solution comprised
electrode by using an A365 stimulus isolator
of 140 mM potassium methyl sulfate, 4 mM
(World Precision Instruments). Following
NaCl, 10 mM HEPES, 0.2 mM EGTA, 4 mM
lesions, mice immediately underwent a
Mg-ATP, 0.3 mM Na-GTP, and 10 mM Tris-
paraformaldehyde perfusion.
phosphocreatine, pH 7.3 (KOH). Membrane
voltages were recorded with an EPC10/2
Rotarod- Mice were placed on a 3.0 cm x 9.5
amplifier (HEKA). PatchMaster software
cm rotating drum of an accelerating rotarod
(HEKA) was utilized to control voltage and data
(Columbus Instruments, Rotamex-5 Rotarod).
acquisition. Data was further analyzed with Igor
The rod was elevated 44.5 cm above the floor of
Pro 6.0 software (Wavemetrics).
the apparatus. While the mice were allowed to
In Vivo Recordings- Extracellular
acclimate to the rotarod for 1 minute before
recordings were taken from vermal Purkinje cell
beginning the experimental protocol, no formal
layers of adult
vRh-GFPPC and wild type
prior training was introduced into the testing
littermates that underwent stereotactic surgery.
paradigm. Testing conditions included
Recordings from actual Purkinje cells were
application of either no light or a 26 second light
confirmed by the presence of both complex and
pulse. Upon receiving photostimulation, mice
simple spikes. The custom optrode was
were promptly placed onto the rotarod where the
duration and speed of the run were recorded.
light application and a 26 sec light pulse so that
Constant acceleration of 40 rotations/min was
each animal was measured twice.
applied until the mouse fell from the rod and
Balance Beam- In order to assess fine
activated the infrared beam. The running
motor coordination and balance abilities, the
duration and rotarod speed at time of fall were
capability to cross a narrow beam onto an
recorded. The runs were consecutively
enclosed platform was analyzed for each mouse.
measured, three times with a 5 min rest period
The horizontally placed, 70 cm long beam was 7
between each run. In the case of no light pulse,
mm in diameter and situated 50 cm above the
animals were allowed to rest for 1 min in
table surface. One end of the beam was mounted
between each run. If mice were unable to stay
to a small, illuminated supportive area while the
on the rotarod, they were assigned a baseline
other end was fastened to an enclosed (20 cm2)
value of 5 seconds. The latency to fall and
box. Mice underwent training on the beam for
speed were recorded for each mouse. Data was
three days (3 trials a day) before data collection.
averaged over 3 trials per mouse.
Briefly, the mouse was placed at an illuminated
Grip Strength Test- The muscle strength
end of the beam and the time required to traverse
of wild type and transgenic mice was assessed
the beam to the safety platform was recorded.
utilizing the Chatillon DFE Series Digital Force
In addition to recording the latency, hind feet
Gauge (AMETEK TCI Division - Chatillon
slips were also noted. Measurements were
Force Measurement Systems, Largo, Fla). The
taken both with and without light pulses. Data
instrument measures both fore- and hindlimb
was averaged over 3 trials per mouse.
grip strength in laboratory rodents by employing
an electronic digital force gauge that directly
calculates the animal's peak force value exerted
upon a pull bar. To measure forelimb grip
Creation of a new optogenetic mouse
strength, animals were held by the tail base and
line vRh-GFP(TgflvRh-GFP) for the controlled
lowered at an angle onto the flat wire mesh of
expression of vRh in a cell-type specific
the pull bar so that the forelimbs would be
manner. To investigate the cell-type specific
exclusively examined. The mouse was slowly
function of Gi/o pathway activation within
pulled away from the bar at approximately 2.5
neuronal networks
in vivo and to analyze the
cm/sec until release whereby the force gauge
functional impact of pathway activation on
recorded the peak tension. Hindlimb grip
mouse behavior, we created transgenic mice to
strength was determined by similar means
specifically activate the Gi/o coupled, light
except that the hindlimbs were solely in contact
activated GPCR vRh by Cre recombinases. We
with the pull bar. Measurements were
first identified positive pCZW-fl-Lac-Z-vRh-
averaged over 5 trials per mouse with and
GFP transgenic founders by genotypic analysis
without light pulses and are recorded as the peak
and examination of β-galactosidase expression
tension (g) and is calculated from the force
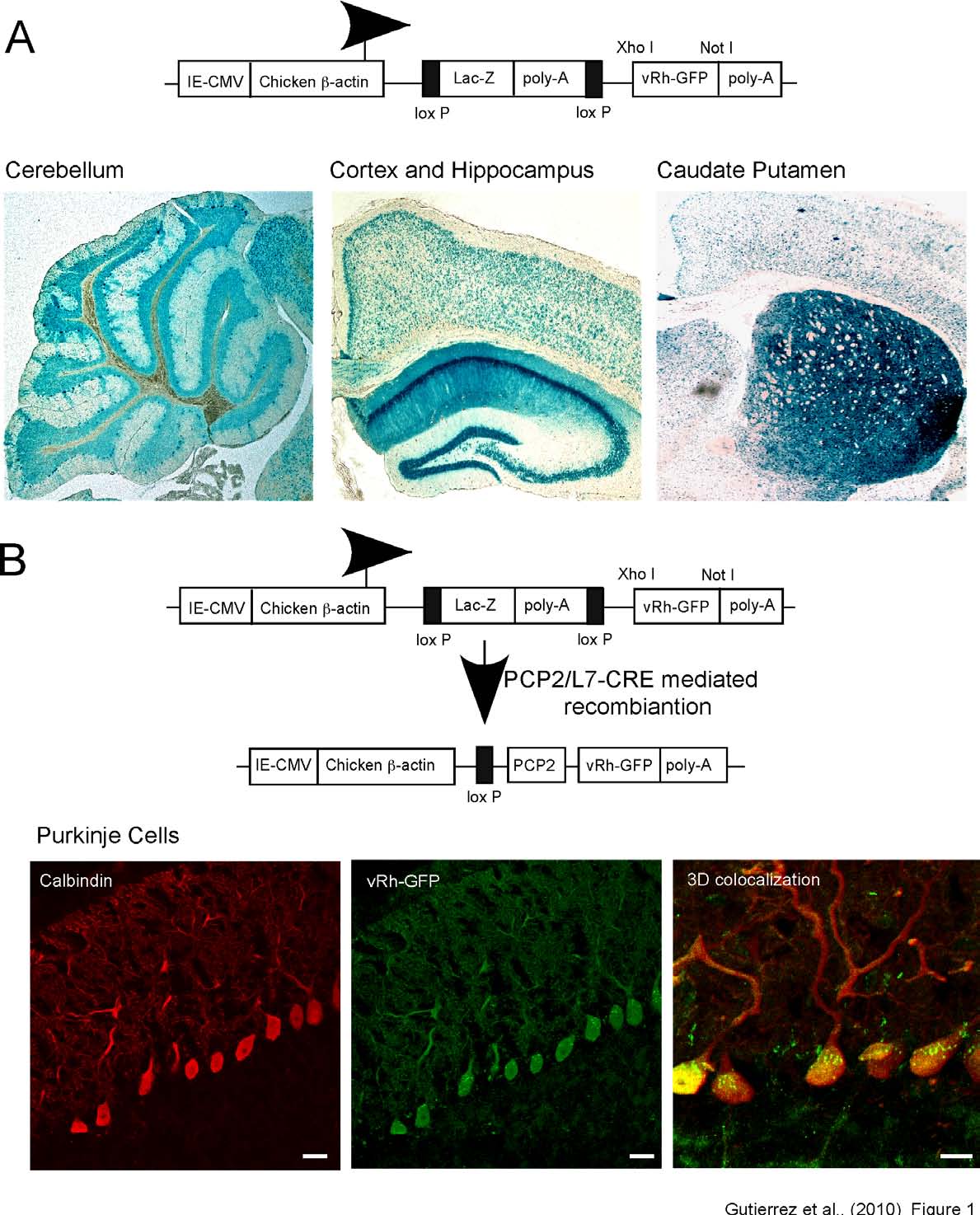
(Figure 1A). In this construct, Lac-Z is flanked
applied to the bar when grasp is released.
by loxP sites and followed by the vRh-GFP. The
Pole
expression of Lac-Z and vRh-GFP is under the
coordination were examined by calculating the
control of the ubiquitous chicken β-actin
capacity of the mice to navigate an angled pole.
promoter-cytomegalovirus enhancer. The vRh-
Mice were held by the tail and lowered, head-
GFP is only expressed when Lac-Z is excised by
upward onto the top of a vertical rough-surfaced
Cre recombinases, while LacZ is present
pole (diameter 8 mm; height 55 cm). The time
throughout the central nervous system (CNS)
required for descent to the base of the apparatus
when Cre is not expressed (Figure 1A) (14).
was recorded with a maximal duration of 120
By performing β-galactosidase staining of both
seconds. If the mouse was unable to descend
coronal and sagittal sections, we were able to
completely and fell off the pole, a maximal
visualize abundant LacZ expression throughout
default time of 120 sec was assigned to the
the CNS. Staining was especially robust in the
animal. Experimental conditions included no
cerebellum, hippocampus and caudate putamen (Figure 1A) and was also detected in other
tissues outside the CNS such as gut, pancreas
In vitro and in vivo application of
and stomach. To demonstrate that vRh-GFP
baclofen reduces the spontaneous firing rate of
expression can be induced cell-type specifically,
Purkinje neurons. We next investigated if the
we crossed mice that expressed Cre recombinase
activation of the Gi/o pathway within PC by an
under the PCP2/L7 promoter with pCZW-fl-
endogenously expressed Gi/o coupled GPCR
LacZ-vRh-GFP mice (Figure 1B) for the
such as GABAB-R would induce comparable
selective expression of vRh-GFP in cerebellar
modulation of PC firing as observed for the light
PCs (13). We call this mouse line
vRh-GFPPC.
activation of vRh. Because we are interested in
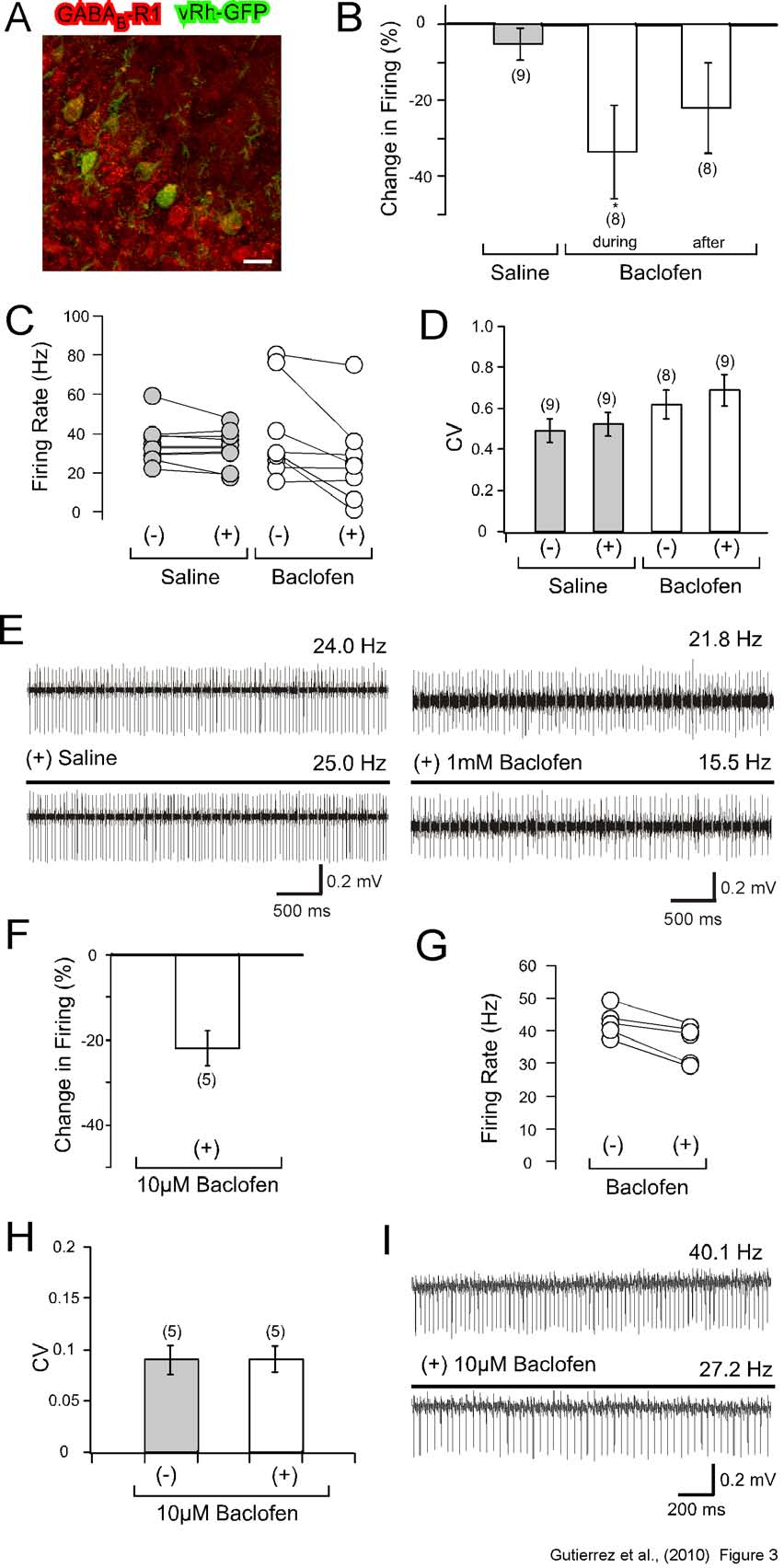
Immunohistochemical staining with GFP and
comparing the effects of GABAB-R mediated
calbindin antibodies verified that vRh expression
Gi/o activation to vRh, we first compared the
was exclusive to PCs in mice that had undergone
expression between vRh and GABAB1-R in
site-specific recombination (Figure 1B). Upon
cerebellar PCs. Immunohistochemical staining
closer examination, we detected vRh in the PC
of sagittal cerebellar sections revealed that
soma in a punctate pattern and in the proximal
B1-R expression is present in both granule
dendrites. Thus, the vRh-GFP(TgflvRh-GFP) mouse
and Purkinje cells and can be detected in cell
allows for the for cell-type selective, Cre
bodies, dendrites and spines of PCs (Figure 3A)
recombinase mediated expression of vRh-GFP.
(12,15). Overlay studies revealed colocalization
Gi/o pathway activation by vRh in vivo
between GFP and GABAB1-R expression in the
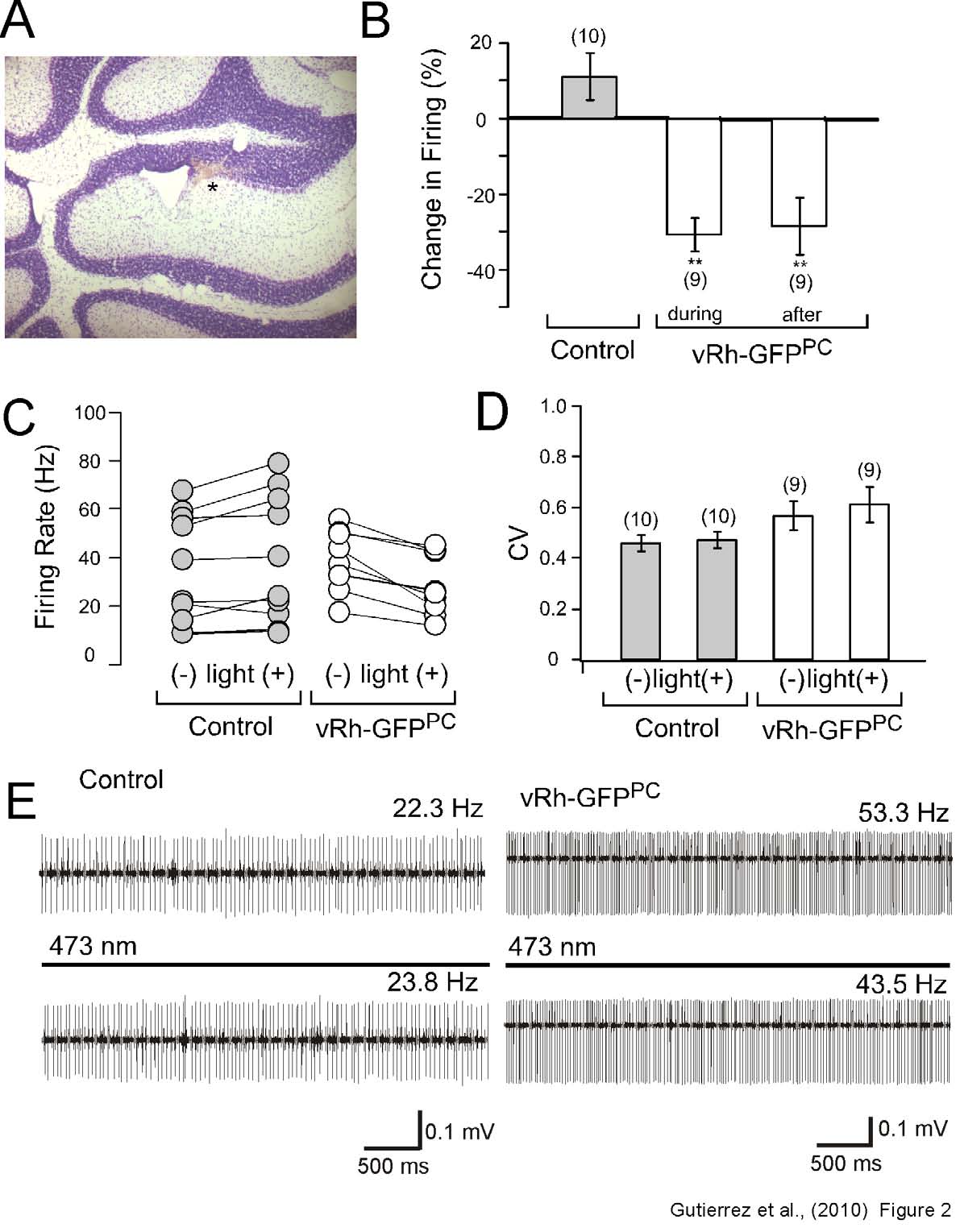
reduces the frequency of PC firing.We first
soma and proximal dendrites in PCs (Figure
examined if activation of vRh by light would
3A), suggesting the possibility that in these
modulate PC firing as would be expected from
subcellular PC regions, Gi/o pathway activation
GPCRs coupling to the Gi/o pathway. To test
by light could potentially activate GABAB-R
our hypothesis, an optrode coupled to a laser
downstream targets but this idea needs to be
delivered a 26 second 473 nm light pulse to
further investigated.
vermal PCs
in vivo. Throughout the experiments
In order to investigate how GABABR
PCs were selected by their characteristic regular
activation influences the firing properties of PCs
spiking pattern and by the occurrence of
in vivo, we iontophoretically applied the
complex and simple spikes. Additionally, we
GABABR agonist baclofen in 3 month old mice.
confirmed the location of the
in vivo recording
A 26s lasting iontophoretic application of 1 mM
site by an electrolytic lesion at the end of the
baclofen led to a reduction in the firing
experiments (Figure 2A). The recording
frequency by 33.6 ± 12.3% (n=8), which was
paradigm consisted of an initial 10 sec recording
significantly different from the 5.3 ± 4.1%
of simple and complex spikes followed by a 26
(n=10) reduction in firing frequency when saline
sec light pulse and a post-light recording of 30
was applied (Figure 3B and 3C). No change in
sec.
vRh-GFPPC mice exhibited an 30.8 ± 4.5 %
the CV was detected before and after application
(n=9) reduction in the spontaneous firing rate in
of baclofen or saline (Figure 3D; CV before and
comparison to a 10.9 ± 6.3 % (n=10) increase in
after saline application, 0.49 ± 0.06 and 0.52 ±
firing in control mice when the 26 sec light pulse
0.06 (n=10); CV before and after 1 mM baclofen
was applied (Figure 2B and 2C). No change in
application, 0.62 ± 0.07 and 0.69 ± 0.08 (n=8)).
the coefficient of variation (CV) was observed
A 22.0 ± 11.9 % (n=8) reduction in firing
before and after light stimulation (control before
frequency was still observed 30 sec after
and after light, 0.46 ± 0.03 and 0.47 ± 0.03
baclofen wash out (Figure 3B). In conclusion,
(n=10);
vRh-GFPPC before and after light, 0.57 ±
GABABR activation by baclofen in the PC layer
0.06 and 0.61 ± 0.07 (n=9), Figure 3D). Post-
of anaesthetized mice caused a reduction in the
light recordings indicated that reduction of firing
firing rate of PCs.
persisted for at least 30 sec after light was
In order to investigate if the reduction in firing
switched off (-28.4 ± 7.6% (n=9), Figure 2D).
frequency is caused by intrinsic or extrinsic PC
Thus, our data show that light-activation of vRh,
selectively expressed in PC, reduce the firing
recordings of PC firing in cerebellar slices from
frequency of PCs
in vivo.
4 week old mice and blocked the inhibitory as well as excitatory inputs into PCs with 10 µM
CNQX and 100 µM picrotoxin. We concentrated
type 93.25 + 9.63 seconds versus
vRh-GFPPC
on tonically firing PCs, and excluded PCs
mice 72.65 + 13.7 seconds; n=10, ANOVA
demonstrating a trimodal spiking activity.
**p<0.001). There was no significant difference
Application of 10 µM baclofen reduced the AP
in the time spent on the rotarod without any light
firing by 21.9 ± 4.1% (n=5) (Figure 3F and 3G).
application between the two groups of mice
Again, no change in the CV was detected before
(wild type 109.99 +10.57 seconds versus
vRh-
and after baclofen application (Figure 3H; CV
GFPPC mice 101.61 + 14.05 seconds n=10).
before and after baclofen application, 0.09 ±
Beam walk testing (Figure 4D) also revealed
0.014 and 0.09 ± 0.013 (n=5)). Thus, GABAB-R
that the modulation on motor behavior was
activation by baclofen in PC
in vivo induced a
dependent on light (wild type pre-pulse 13.27 +
reduction in firing frequency as observed by
2.14 seconds; post-pulse 10.72 + 1.38 seconds;
light activation of vRh, suggesting that vRh and
vRh-GFPPC pre-pulse 7.43 + 0.53 seconds; post-
GABAB-R activate a similar intracellular
pulse 17.36 + 4.37 seconds; n = 10, ANOVA
signalling pathway to modulate PC firing.
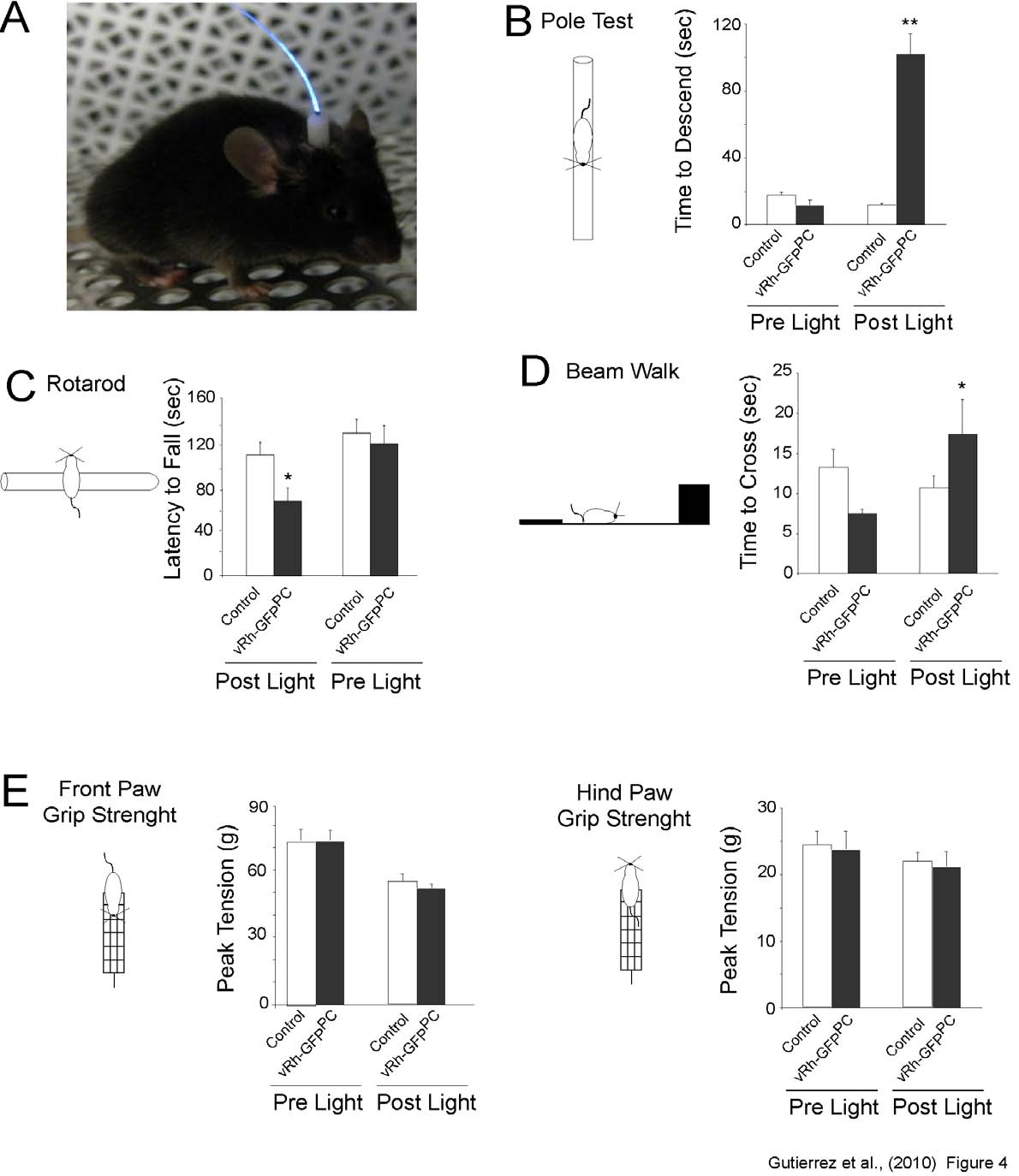
Photostimulation of vRh in Purkinje
As an additional control for each
cells alters motor behavior. In order to
behavioral test, grip strength for both hind and
investigate the functional consequence of Gi/o-
front paws was analyzed before and after light
mediated modulation of PC firing, we implanted
treatment. These tests were performed to
a laser guide positioned on top the cerebellum to
demonstrate that any significant differences
illuminate the cerebellar cortex (Figure 4A). We
between wild type and positive transgenic mice
chose the anterior vermis as the specific
detected throughout the behavioral tests were
illumination area because it is known to be
attributable to the photoactivation of vRh and
involved in balance, equilibrium and motor
are not a result of insufficient strength or muscle
execution (16-19). In all motor tests
ability. Measurements of front grip strength
administered, a significant difference was
revealed no significant difference between the
detectable between wild type and transgenic
two groups both before and after light
vRh-GFPPC adult mice after a 26s long light
application (Figure 4E; wild type pre-pulse 73.0
stimulus was applied to the vermis. Specifically,
+ 4.99 g; post-pulse 54.9 + 4.55 g;
vRh-GFPPC
vRh positive mice either fell off the pole after
pre-pulse 73.0 + 3.14 g; post-pulse 51.9 + 1.76
light delivery (scored as 120 sec) or took at least
g; n=10 ANOVA n.s.). There were also no
twice as long to descend to the bottom of the
indications of changes in hind grip strength
pole (Figure 4B, wild type pre-pulse 17.51 +
before and after light application (Figure 4F;
1.67 seconds; post-pulse 11.63 + 0.87 seconds;
wild type pre-pulse 24.5 + 1.9 g; post-pulse 22.0
vRh-GFPPC pre-pulse 18.85 + 2.87 seconds;
+ 1.26 g;
vRh-GFPPC pre-pulse 23.67 + 2.82 g;
post-pulse 102.1 + 12.4 seconds; n= 10,
post-pulse 21.1 + 2.31 g; n=10 ANOVA n.s.).
ANOVA ***p<0.0001). The accelerating
Thus our results indicate that Gi/o-mediated
rotarod test was administered by delivering a
modulation of PC firing is sufficient to alter
pulse of light at the beginning of the experiment,
motor coordination in behaving mice.
followed by a performance evaluation without
light application. This behavioral paradigm was
DISCUSSION
designed this way to control for the possibility
that the duration of time spent on the rotarod
vRh-GFP(TgflvRh-GFP) mouse for the cell-type
would increase because of the acquisition of
selective control of Gi/o signalling. The pursuit
motor skill learning, regardless of transgenic
to gain a more thorough understanding of the
expression, and could potentially mask any
physiological roles of cell-type specific GPCR
effects that light activation of vRh may have on
signalling
in vivo and
in vitro has resulted in the
firing and behavioral output (20). Accelerating
development of two new approaches that
rotarod testing revealed that the
vRh-GFPPC
circumvent the use of traditional receptor-
mice stay on the accelerating rod for a shorter
specific agonists and antagonists. The first
amount of time after light application in
consists of a chemical approach that utilizes
comparison to wild type mice (Figure 4C, wild
engineered GPCRs such as DREADDs, which
are activated by inert chemical compounds
optimized method for
in vivo expression but also
(21,22). The second technique is a physical
highlights the magnitude of influence that the
scheme that employs light-activated proteins to
Gi/o pathway has on motor control and the
evoke intracellular signalling pathways, like
PTX-sensitive Gi/o-coupled vRh in neurons
Numerous examinations of the cerebellum and
(3,23,24). The advantage of using light-activated
specifically the medial cerebellar region have
proteins is the guaranteed precise temporal
indicated that this area plays a pivotal role in
control, which cannot be achieved with
regulating extensor tone, sustaining upright
application of chemical compounds. To further
stance and dynamic balance control (18,19,25).
develop and utilize this tool for cell-type specific
It is thought that the cerebellum employs
applications, we created mice whose expression
anticipatory and feedback mechanisms to
of vRh-GFP was dependent upon the use of cell-
maintain balance during locomotion and that
type specific expression of Cre recombinase.
failure in these systems induce an ataxic-like
The vRh-GFP(TgflvRh-GFP) mice were crossed
with PCP2/L7-Cre recombinase (TgPcp2-cre) mice
revealed that the photostimulation of positive
for selective expression of vRh-GFP in PCs (13).
vermal PCs in
vRh-GFPPC mice induced
The vRh expression was induced one week after
changes in motor output. Specifically, an overall
birth following Cre-expression and was
lack of balance, coordination and performance
restricted to PCs of
vRh-GFPPC mice (Figure
was quite apparent with positive transgenic mice
1B). In order to visualize vRh-GFP after 1-3
that significantly differed from control
months of age, an antibody against GFP had to
littermates. These results are consistent with
be used, suggesting that the vRh-GPF
prior studies that have examined the correlation
concentration within PCs is low. Despite the
between vermal lesions and gait ataxia, postural
potential lower expression levels, light
defects and motor coordination difficulties and
stimulation of vRh
in vivo led to a significant
highlight the importance of Gi/o modulation of
reduction of AP firing in PCs that was
PC firing for motor control. As a side note, an
comparable to the effects induced by application
early examination of light delivery to positively
of GABABR agonist, baclofen. As shown by the
expressing vRh PCs indicated that the optimal
intense lacZ staining, especially within
length of activation was around 20 seconds.
hippocampus and basal ganglia (Figure 1A), the
Similar behavioral responses could be elicited
vRh-GFP(TgflvRh-GFP) mouse line is a promising
with longer light pulses but was ultimately
tool that could be used in the investigation of
found to be unnecessary. Furthermore, brief
Gi/o signalling in other neuronal populations.
pulses of light were unable to reliably evoke
According to our studies in PCs, vRh-
changes in the intrinsic firing properties of
GFP(TgflvRh-GFP) mice provide a new optogenetic
tool for the analysis of
in vivo function of
Considerations for controlling Gi/o signalling
in vivo by light. While we have provided an
Modulation of simple spikes in the medial
effective means to modulate the activity of a
cerebellar regions leads to changes in motor
single neuronal population and network, there
behavior. One of the surprising findings of our
are several concerns associated with this study
study was that a 20-30% reduction in vermal PC
that may be influenced by the overall methods
firing was sufficient to cause motor deficits in
utilized. These potential issues include the
freely behaving mice. This finding was
extent and range of light penetration within the
especially remarkable because the expression
cerebellum and the presence of any plausible
level of vRh appeared to be relatively low and
variables related to light delivery that may
limited throughout cerebellar PCs. While no
influence the
in vivo behavioral and/or
quantitative measurement was taken of
electrophysiological testing. Previous studies
expression levels, vRh was only visible with
investigating the feasibility of controlling
antibody application. The seemingly restricted
neuronal excitability in a noninvasive and light-
vRh concentration in vermal PCs not only
dependent manner revealed that vRh promoted
exhibits the necessity to create an alternative and
the modulation of GIRK and P/Q-type Ca2+
channels via a functional coupling to the
specific signalling properties of neurons (24),
pertussis toxin-sensitive, Gi/o protein pathway
further ingenuity is required to overcome the
(3). Because vRh couples to the G protein
intrinsic issues presented thus far. An additional
transducin, whereby the α subunit belongs to the
point of contention surrounds the idea that
Gi subfamily, these findings offer supporting
GABABRs in dissociated PCs have been
evidence that mammalian rhodopsins are
suggested to inhibit P/Q-type Ca2+ channels (31),
capable of coupling to other Gi/o family members
establish a heterodimeric functional coupling
in vitro. In order to examine the possibility that
with mGluR1 at postsynaptic sites of the PF-PC
vRh may also promote the precise spatio-
synapse (11,32) and are thought to be involved
temporal control of the Gi/o pathway
in vivo, we
in synaptic plasticity (33,34). Therefore, future
established an investigation that focused on the
studies should be focused on investigating which
function of this pathway in animal behavior and
downstream signalling pathway is activated and
system coordination such as motor control.
whether discrete motor learning tasks can be
Activation of the G
i/o pathway in a membrane
modulated by the photoactivation of vRh. An
delimited way is the main inhibitory action of
additional concern focuses on the delivery of a
GPCRs on neuronal excitability (2). Many
maximal but specific and controlled amount of
different transmitters, such as glutamate,
light to the brain tissue. To achieve this goal, a
acetylcholine (Ach), serotonin (5-HT) or GABA
stripped, multimode optical fiber (200 µm
couple via specific GPCRs to the Gi/o pathway,
diameter) was coupled to a blue laser light (20
which are expressed throughout the brain.
mW of power at 473 nm) and affixed above the
Among them, the GABAB receptor (GABABR)
cerebellar vermis. It is understood that the
is widely distributed throughout the brain
light scattering properties within the brain are
including the cerebellum (28) and is located in
influenced by species and age, incident
the granule cell, PC and molecular layer. Within
wavelength, and physiological characteristics of
the molecular layer GABABRs are found at the
the tissue (35-38). Specifically, the blue laser
presynaptic terminals of parallel fibers and at the
light utilized (473 nm) for this study has been
PC dendrites and spines (15,29,30). Taking all
described as having a high propensity for
of this into consideration, our
in vivo data seem
scattering within the brain and is also weakly
to suggest that the photoactivation of vRh in
absorbed (36-38). The specifics of this optrode
vermal PCs acts via the Gi/o mediated signalling
have been previously characterized in detail and
pathway in general. Up to this point, we have
it had been estimated that the fiber tip produces
not detected the activation of other G protein
a total tissue volume experiencing >1 mW mm-2
pathways using vRh such as Gq or Gs in cellular
light intensity to be 0.5 mm3 (36). These fiber
or neuronal culture systems. Our attempt to
optic specifics correlate with our data in that the
most significant decrease in the firing rate of
exogenously-expressed vRh still remains to be
vermal PCs was elicited in neurons located at
further developed. There are several matters to
more superficial tissue depths; thereby
consider that may influence the feasibility of
supporting the notion that increase tissue depth
controlling Gi/o signalling and include the
corresponds to a lower level of light intensity.
following: Firstly, Gi/o-coupled GPCRs have a
In summary, we generated a new mouse
variety of downstream signalling targets and
line that allows for the cell-type specific
have a binding preference to each of their
activation and modulation of the Gi/o pathway
respective targets. Secondly, more than one type
through vRh, and demonstrated the feasibility of
of Gi/o-coupled GPCR is expressed in a single
modifying the firing properties of a single
neuron and spreads in a specific distributing
neuronal population through the utilization of
pattern. Lastly, some Gi/o-coupled receptors can
light. Thus for the first time, our experimental
form heterodimers with other types of GPCRs.
results revealed that the
in vivo modulation of
Although we recently demonstrated the ability to
the Gi/o protein pathway in PCs has a significant
target and modify GPCRs by tagging vRh with
functional influence on motor control and
the C-terminal signalling domain of a specific
GPCR and were able to control 5-HT1A/Gi/o
REFERENCES
LeBeau, F. E., El Manira, A., and Griller, S. (2005)
Trends Neurosci 28, 552-561
Hille, B. (1994)
Trends Neurosci 17, 531-536
Li, X., Gutierrez, D. V., Hanson, M. G., Han, J., Mark, M. D., Chiel, H., Hegemann, P.,
Landmesser, L. T., and Herlitze, S. (2005)
Proc Natl Acad Sci U S A 102, 17816-17821
Thach, W. T., Goodkin, H. P., and Keating, J. G. (1992)
Annu Rev Neurosci 15, 403-442
Llinas, R., and Sasaki, K. (1989)
Eur J Neurosci 1, 587-602
Hausser, M., Raman, I. M., Otis, T., Smith, S. L., Nelson, A., du Lac, S., Loewenstein, Y.,
Mahon, S., Pennartz, C., Cohen, I., and Yarom, Y. (2004)
J Neurosci 24, 9215-9219
Walter, J. T., Alvina, K., Womack, M. D., Chevez, C., and Khodakhah, K. (2006)
Nat Neurosci 9, 389-397
Womack, M. D., and Khodakhah, K. (2004)
J Neurosci 24, 3511-3521
Billard, J. M., Vigot, R., and Batini, C. (1993)
Neurosci Res 16, 65-69
Curtis, D. R., Game, C. J., Johnston, G. A., and McCulloch, R. M. (1974)
Brain Res 70, 493-499
Tabata, T., Haruki, S., Nakayama, H., and Kano, M. (2005)
J Physiol 563, 443-457
Vigot, R., and Batini, C. (1997)
Neurosci Res 29, 151-160
Barski, J. J., Dethleffsen, K., and Meyer, M. (2000)
Genesis 28, 93-98
Braz, J. M., Rico, B., and Basbaum, A. I. (2002)
Proc Natl Acad Sci U S A 99, 15148-15153
Ige, A. O., Bolam, J. P., Billinton, A., White, J. H., Marshall, F. H., and Emson, P. C. (2000)
Brain Res Mol Brain Res 83, 72-80
Apps, R., and Hawkes, R. (2009)
Nat Rev Neurosci 10, 670-681
Ivry, R. B., Keele, S. W., and Diener, H. C. (1988)
Exp Brain Res 73, 167-180
Joyal, C. C., Meyer, C., Jacquart, G., Mahler, P., Caston, J., and Lalonde, R. (1996)
Brain Res 739, 1-11
Morton, S. M., and Bastian, A. J. (2007)
Cerebellum 6, 79-86
Shiotsuki, H., Yoshimi, K., Shimo, Y., Funayama, M., Takamatsu, Y., Ikeda, K., Takahashi, R.,
Kitazawa, S., and Hattori, N.
J Neurosci Methods 189, 180-185
Armbruster, B. N., Li, X., Pausch, M. H., Herlitze, S., and Roth, B. L. (2007)
Proc Natl Acad Sci
U S A 104, 5163-5168
Pei, Y., Rogan, S. C., Yan, F., and Roth, B. L. (2008)
Physiology (Bethesda) 23, 313-321
Masseck, O. A., Rubelowski, J. M., Spoida, K., and Herlitze, S. (2010)
Exp Physiol 96, 51-56
Oh, E., Maejima, T., Liu, C., Deneris, E., and Herlitze, S. (2010)
J Biol Chem 285, 30825-30836
Lalonde, R., and Strazielle, C. (2007)
Prog Neurobiol 81, 45-60
Horak, F. B., and Diener, H. C. (1994)
J Neurophysiol 72, 479-493
Timmann, D., and Horak, F. B. (1998)
Exp Brain Res 119, 73-84
Bettler, B., Kaupmann, K., Mosbacher, J., and Gassmann, M. (2004)
Physiol Rev 84, 835-867
Kulik, A., Nakadate, K., Nyiri, G., Notomi, T., Malitschek, B., Bettler, B., and Shigemoto, R.
(2002)
Eur J Neurosci 15, 291-307
Fritschy, J. M., Meskenaite, V., Weinmann, O., Honer, M., Benke, D., and Mohler, H. (1999)
Eur
J Neurosci 11, 761-768
Mintz, I. M., and Bean, B. P. (1993)
Neuron 10, 889-898
Hirono, M., Yoshioka, T., and Konishi, S. (2001)
Nat Neurosci 4, 1207-1216
Kamikubo, Y., Tabata, T., Kakizawa, S., Kawakami, D., Watanabe, M., Ogura, A., Iino, M., and
Kano, M. (2007)
J Physiol 585, 549-563
Kawaguchi, S., and Hirano, T. (2000)
Neuron 27, 339-347
Zhang, J., Laiwalla, F., Kim, J. A., Urabe, H., Van Wagenen, R., Song, Y. K., Connors, B. W.,
Zhang, F., Deisseroth, K., and Nurmikko, A. V. (2009)
J Neural Eng 6, 055007
Aravanis, A. M., Wang, L. P., Zhang, F., Meltzer, L. A., Mogri, M. Z., Schneider, M. B., and
Deisseroth, K. (2007)
J Neural Eng 4, S143-156
Bevilacqua, F., Piguet, D., Marquet, P., Gross, J. D., Tromberg, B. J., and Depeursinge, C. (1999)
Appl Opt 38, 4939-4950
Yaroslavsky, A. N., Schulze, P. C., Yaroslavsky, I. V., Schober, R., Ulrich, F., and
Schwarzmaier, H. J. (2002)
Phys Med Biol 47, 2059-2073
FOOTNOTES
We would like to thank Dr E.S. Deneris for reading the manuscript, Dr. Ron Conlon and the Case Transgenic and Targeting Facility for creating the mice and Dr. Gemma Casadesus and the CWRU Rodent Behavior Core for assistance in the behavior studies. Also we would like to thank Stephanie Krämer, Margareta Möllmann, Manuela Schmidt, Winfried Junke, Hermann Korbmacher and Volker
Rostek for excellent technical assistance. Supported by DFG HE2471/8-1, NIH MH081127 (SH), JSPS Postdoctoral Fellowships for Research Abroad (TM), R36MH086283 (DG).
The abbreviations used are: 5-HT, 5-Hydroxytryptamin (Serotonin); ANOVA, analysis of variance; CV, coefficient of variation; DREADD, designer receptors exclusively activated by a designer drug; GFP, green fluorescent protein; GIRK, G protein inwardly rectifying potassium channel; GPCR, G protein coupled receptor; PC, Purkinje cell; PTX, pertussis toxin; S.E.M., standard error of the mean; TEA, triethanolamine; vRh, vertebrate rhodopsin.
FIGURE LEGENDS
Fig. 1: Cell-type specific Cre recombinase-mediated expression of vertebrate rhodopsin in
cerebellar Purkinje cells. (A) Schematic description of the construct used to create the transgenic
animals expressing floxed vRh. vRh was cloned into the pCZW vector, which contains a CMV enhancer
and β-actin promoter and a lacZ expression cassette, flanked by two loxP sequences. X-gal staining of
sagittal brain slices from the vRh-GFP(TgflvRh-GFP) mouse line shows β-galactosidase expression
throughout the brain with robust expression localized in the cerebellum (left) and the hippocampus
(middle) and caudate putamen (right).
(B) Diagram revealing the results of Cre-mediated recombination
events indicates an excision of the lacZ expression cassette and cell type specific expression of vRh-GFP
driven in PCs. Cre recombinase-mediated induction of vRh-GFP expression in PCs was accomplished
by crossing vRh-GFP(TgflvRh-GFP) mice with Purkinje cell specific CRE (TgPcp2-cre) mice. PC specific
expression of vRh was verified by immunohistochemical staining for GFP (middle) and calbindin (a calcium binding protein associated with Purkinje cells, left). (Right) Three-dimensional reconstruction of confocal z-stack images revealing colocalization of vRh-GFP and calbindin in the soma and proximal dendrites of PCs. Scale bars (left and middle) 25 µm, (right) 10 µm.
Fig. 2: In vivo photostimulation of the cerebellar vermis of vRh-GFPPC induces a reduction in the
firing rate of Purkinje cells. (A) NISSL stain of sagittal cerebellum slices after electrolytic lesions
indicate that the
in vivo recordings and the application of baclofen (see Figure 3) were directed to the
Purkinje cell layer.
(B) PC firing rate recorded and calculated as percent change in firing before and after
light application. The vRh-GFPPC transgenic line demonstrated a significant reduction in firing during the
light pulse that persisted after 30 second once light was switched off.
(C) Representative firing rates
(Hz) of individual PCs from control and vRh-GFPPC mice with and without illumination reveal that only
neurons from the vRh-GFPPC line reveal decrease in the firing rate after light treatment.
(D) Analysis of
the coefficient of variation (CV) after light application indicates no significant difference between wild
type littermates and vRh-GFPPC mice.
(E) Raw traces of control littermates and vRh-GFPPC mice before
and during the 473 nm light pulse reveals a reduction in the PC firing rate only in vRh-GFP positive
transgenic mice. The presence of both simple and complex spikes as well as a comparative analysis of
depth with a standard mouse brain atlas confirmed that recordings were taken from Purkinje cells.
Statistical significance was evaluated with ANOVA. (** P < 0.01). Given values are mean ± S.E.M.
Fig. 3: In vivo and in vitro application of baclofen decreases the firing rate of cerebellar Purkinje
cells. (A-E) In vivo recordings of cerebellar PCs before and after baclofen application.
(A) Comparative
immunohistochemical staining of GABAB1R (red) and vRh-GFPPC (green) reveals the expression of
GABAB1R in both PCs and cerebellar cortical neurons. vRh-GFPPC expression is restricted to PCs and
colocalized partly (yellow) with GABAB1Rs. Scale bar 25 µm.
(B) Percentage change in number of
spikes for a 10 sec time interval during (t = 16-26 sec) and after (t = 20-30 sec) 1 mM baclofen
application compared to control (saline application). Bar graphs indicate a significant decrease in the
firing of Purkinje cells with baclofen application.
(C) Spike frequency (Hz) before and after saline or
baclofen application for each recorded PC demonstrates an overall reduction in firing that corresponds to
baclofen administration.
(D) Calculated values for the coefficient of variation (CV) between PCs treated
with saline or baclofen reveals no significant difference in data dispersion between the two treatment
groups.
(E) Raw traces of PCs before and during either saline or baclofen treatment indicates reduced
firing in the present of 1 mM baclofen. The presence of both simple and complex spikes as well as a
comparative analysis of depth with a standard mouse brain atlas confirmed that recordings were taken
from Purkinje cells.
(F-I) Cerebellar slice recordings of PCs before and after baclofen application.
(F)
Percentage change in spike number during the 10 mM baclofen bath application displays reduced firing.
(G) Modifications in the overall firing rate (Hz) for the recorded PCs during baclofen application.
(H)
Calculated CV values for extracellular PC recordings indicate no significant difference before and during
baclofen treatment.
(I) Raw data traces demonstrating a decrease in PC firing with baclofen. Statistical
significance was evaluated with ANOVA. (* P < 0.05). All values are mean ± S.E.M.
Fig. 4: Light activation of vertebrate rhodopsin expressed in Purkinje cells of the cerebellum
induces changes in motor behavior. (A) Photograph demonstrating the permanent placement of the
cannula light guide used for behavioral testing.
(B) Pole test performance of control and vRh-GFPPC
mice (n=10), before and after a 26 sec light pulse. The light activation of vRh in vRh-GFPPC mice results
in either a fall (scored as 120 sec) or an increase in the time required to descend from the pole. Light
application to control littermates did not initiate any significant difference in the time required to descend
the pole.
(C) Rotarod performance of wild type littermates (n=10) and vRh-GFPPC mice, before and after
a 26 sec light pulse. Light activation of vRh in vRh-GFPPC mice produces a significant decrease in rotarod
performance when compared to wild type littermates. Performance between the two groups when no
light pulse is applied reveals no significant difference.
(D) Beam walk analysis demonstrates an increase
in the time required to successfully cross the length of the beam after vRh activation in vRh-GFPPC mice.
Conversely, the time needed to cross the beam decreases in control littermates, regardless of the light
pulse. Falls were assigned a value of 120 sec. Additionally, a measurement of the number of paw slips
reveals a significant increase after light application for the left side of the vRh-GFPPC mice; whereas
control littermates experienced no significant increase in slips post-light application.
(E) Grip strength
assessment of wild type and vRh-GFPPC mice, before and after a 26 sec light illumination. No significant differences were observed for the grip strength of the front and hind paws between wild type littermates and vRh-GFPPC mice before and after light application. Statistical significance in all behavior
experiments was evaluated with ANOVA. (* P < 0.05; ** P < 0.01). Shown values are mean ± S.E.M.
Source: http://ko.cwru.edu/publications/Gutierrez.pdf
MIGRAÑA. TRATAMIENTO Y PREVENCIÓN MEDICINA (Buenos Aires) 2014; 74: 147-157 ACTUALIZACIÓN EN LA PREVENCIÓN Y TRATAMIENTO DE LA MIGRAÑA LAURA S. VISENS1 Departamento Médico, Janssen Cilag Farmacéutica S.A., Buenos Aires, Argentina Resumen La migraña es una afección sumamente frecuente y con importante repercusión socioeconómica.
Die Verbreitung von Impatiens glandulifera, Fal opia japo- nica, F. sachalinensis, F. ×bohemica und Heracleum mantegaz- zianum entlang der Hauptfließgewässer Luxemburgs Bob Glesener1, Manou Pfeiffenschneider1 & Christian Ries21 EFOR-ERSA ingénieurs-conseils, 7, rue Renert, L-2422 Luxemburg ([email protected], manou.pfeiffen-








