
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Lawreview.law.pitt.edu
GREEN MEDICINE: USING LESSONS FROM TORT LAW AND
ENVIRONMENTAL LAW TO HOLD PHARMACEUTICAL
MANUFACTURERS AND AUTHORIZED DISTRIBUTORS LIABLE
FOR INJURIES CAUSED BY COUNTERFEIT DRUGS
Stephanie Feldman Aleong*
The majority of the American public would be astonished by the
frequency with which counterfeit prescription drugs appear on reputable drugstore shelves. In 2004, the Food and Drug Administration (FDA) noted thatthose who counterfeit prescription drugs "deny ill patients the therapies thatcan alleviate suffering and save lives."1 In 2006, the World HealthOrganization (WHO) estimated that there exists a $30 billion market in fakedrugs.2 Although the FDA has tried to characterize the incidence of
Stephanie Feldman Aleong is an Assistant Professor of Law and the Director of the Masters of
Science in Health Law Program at Nova Southeastern University, Shepard Broad Law Center, in Ft.
Lauderdale, Florida. Prior to becoming an academic, Professor Aleong was Florida's Health Care FraudPriority Leader in the Office of the Statewide Prosecutor. She also serves on the Advisory Board for SecureSymbology, Inc., a technology company aimed at bar-coding many products to enhance inventory controland safety. Professor Aleong first presented the concept of this Article at the American Society of Law,Medicine and Ethics' 30th Annual Health Law Professors' Conference and later made the same presentationas a Young Scholar at the Southeastern Association of Law Schools' Annual Conference. Professor Aleongwould like to thank Professor Joel A. Mintz, Professor Michael Flynn, and Professor Kathy Cerminara, hercolleagues at NSU, for their review and discussion of this article. She also thanks her SEALS mentor,Professor Steven Ellman, New York Law School, for his guidance. Finally, Professor Aleong would liketo thank students Michael Pascucci and Laura Cancilla for their efforts as her research assistants.
U.S. FOOD & DRUG ADMIN., COMBATING COUNTERFEIT DRUGS: A REPORT OF THE FOOD AND
DRUG ADMINISTRATION i (2004),
available at http://www.fda.gov/oc/initiatives/counterfeit/report02_04.pdf[hereinafter FDA REPORT 2004]. The FDA even noted that, in some countries, the problem was sooverwhelming that many patients had a better chance of getting fake medicine than authentic drugs.
Id.
In some of the more impoverished states, these fake "medicines" constitute 30% of the
prescription drug market. MSNBC.com, WHO to Crack Down on Fake Drugs, http://www.msnbc.msn.com/id/15730101 (last visited Jan. 23, 2008). In late 2006, the WHO created a taskforce entitled"IMPACT" (International Medical Products Anti-Counterfeiting Taskforce) to "put a stop to the deadlytrade in fake drugs, which studies suggest kill thousands of people every year." William Burns,
WHOLaunches Taskforce to Fight Counterfeit Drugs, 84 BULL. WORLD HEALTH ORG. 689, 689 (2006),
available at http://www.who.int/entity/bulletin/volumes/84/9/news.pdf. In 2003, the WHO conducted astudy and determined that global sales of counterfeit drugs amounted to an estimated $32 billion,constituting 10% of all medicines sold worldwide. Ronald W. Buzzeo,
Counterfeit Pharmaceuticals and
UNIVERSITY OF PITTSBURGH LAW REVIEW
counterfeit medications in the United States prescription drug marketplace as"rare,"3 numerous instances of counterfeit drugs reaching consumers from theshelves of large, retail pharmacy chains have been well-documented.4 As aresult, the FDA has lifted the stay on a nearly fifteen-year-old regulation thatrequires distributors of prescription drugs to document the sources of thedrugs they peddle.5 In fact, the high risk of receiving fake or diverted drugsin the United States has been referred to as "pharmaceutical roulette" formillions of American patients.6
Moreover, the incidence of counterfeit drugs is a growing problem in the
United States. The Pharmaceutical Security Institute reported that the valueof seized, counterfeit, and diverted7 drugs in this country approached $200million in 2003, a sevenfold increase from 2002.8 Further, the United StatesDrug Intelligence Center (NDIC) reports that in 2006, 78.8% of state and locallaw enforcement agencies reported moderate to high availability of illicitpharmaceuticals in their jurisdictions.9
the Public Health, WALL ST. J., Oct. 4, 2005, at A20.
FDA REPORT 2004,
supra note 1, at i. The FDA claims that a "relatively comprehensive system
of laws, regulations, and enforcement by [f]ederal and state authorities has kept drug counterfeiting rare"in the United States, but did acknowledge the growing problem of counterfeit drugs.
Id. The FDA furtherremarked that the "exact prevalence rates [of counterfeit drugs] in the U.S. are not known."
Id. at 2.
See, e.g., KATHERINE EBAN, DANGEROUS DOSES: HOW COUNTERFEITERS ARE CONTAMINATING
AMERICA'S DRUG SUPPLY (2005). Eban details how counterfeit drugs emanating from hot trailers, cartrunks, a Miami strip club and other questionable sources arrived on the shelves of drugstores like CVS.
See id. at 359. In the interest of full disclosure, I was one of the prosecutors described in Ms. Eban's bookas I worked on counterfeit drug prosecutions in my capacity as Florida's Health Care Fraud Priority leader.
NBC's
Dateline has also done investigative reporting on the prevalence of counterfeit drugs in thelegitimate United States marketplace.
Dateline NBC: Inside the World of Counterfeit Drugs (NBCtelevision broadcast June 9, 2006) (transcript on file with the
University of Pittsburgh Law Review).
Anna Wilde Mathews & Heather Won Tesoriero,
FDA to Order Tracking of Medicines, WALL
ST. J., June 9, 2006, at A2. The FDA lifted the stay on the "pedigree" requirement provided in 21 C.F.R.
§ 203.50 (2007), which requires non-authorized distributors of pharmaceuticals to provide a documentdetailing the distribution history of a pharmaceutical.
Gilbert M. Gaul & Mary Pat Flaherty,
U.S. Prescription Drug System Under Attack, WASH.
POST, Oct. 19, 2003, at A15. Middlemen divert expensive and popular drugs from the legitimate marketinto a shadow market where criminals introduce counterfeit medication and then move the drugs back intothe mainstream distribution network.
Id.
The term "diverted" indicates drugs that have strayed out of the distribution channel for which
they were originally intended. Diverted drugs include those that were originally purchased by a governmentprogram such as Medicare, Medicaid, or the Veterans' Administration, and then resold into the legitimatemarket. U.S. FOOD & DRUG ADMIN., FDA'S COUNTERFEIT DRUG TASK FORCE INTERIM REPORT (2003),
available at http://www.fda.gov/oc/initiatives/counterfeit/report/interim_report.html [hereinafter FDAINTERIM REPORT 2003].
Buzzeo,
supra note 2.
CENTER, U.S. DEPARTMENT OF JUSTICE, NATIONAL DRUG
THREAT ASSESSMENT 2007, at 18 (2007),
available at http://www.usdoj.gov/ndic/pubs21/21137/
The drug distribution system in the United States is porous and
vulnerable. Drugs are usually not sold directly from a manufacturer to adispensing pharmacy.10 Drugs weave their way through a complicateddistribution chain that includes large and small distributors, authorized andnon-authorized distributors, and even criminal hands before reachingdispensing pharmacies which might, in turn, sell unused product back into thedistribution chain.11
This Article will consider the following: (1) How prescription drugs are
distributed in the United States; (2) whether the law currently containsadequate safeguards to protect the integrity of the drug supply fromcounterfeiters; (3) how courts could hold manufacturers and authorizeddistributors vicariously liable for injuries caused by counterfeit drugs whensuch entities fail to take special precautions to avoid breaching a nondelegableduty of safe distribution; and (4) whether Congress should go beyond existingtort principles by imposing strict liability on manufacturers and distributorsof pharmaceutical drugs when counterfeit drugs injure patients—much like thestrict liability schemes for hazardous waste imposed by environmental lawssuch as the Resource Conservation and Recovery Act (RCRA)12 and theComprehensive Environmental Response, Compensation and Liability Act(CERCLA).13 Much like the problem of hazardous waste, the occurrence of
Gaul & Flaherty,
supra note 6, at A15. The "gold standard" for safe distribution existed when
manufacturers sold directly to the three largest distributors in the country, which in turn sold directly to drugdispensing entities, thereby involving no other wholesalers in distribution.
Id.
Regarding the seizure of more than 70,000 doses of counterfeit Viagra, Aaron Graham, who had
been an investigator for both the government and for the pharmaceutical industry, commented to
Dateline,
You might think medicines go straight from the factory to your pharmacy. But there's actuallya complex network of wholesalers who buy and sell surplus medicines. All it takes is somephony paperwork and some realistic packaging to let fake medicine slip into the system, andbe shipped to local pharmacies nationwide. And criminals know this.
Dateline NBC: Inside the World of Counterfeit Drugs,
supra note 4.
42 U.S.C. §§ 6901-6992k (2000).
42 U.S.C. §§ 9601-9675 (2000).
UNIVERSITY OF PITTSBURGH LAW REVIEW
counterfeit drugs is both foreseeable14 and the social byproduct of otherwiselegitimate pharmaceutical manufacture and distribution.
II. HOW DRUGS ARE DISTRIBUTED IN THE UNITED STATES
Drugs in the United States generally do not travel straight from the line
of production to the dispensing pharmacy. Rather, a serpentine maze providesa ripe environment for the infiltration of counterfeit, adulterated, and diverteddrugs.15
The distribution system is primarily tiered among manufacturers, the "Big
3" distributors/drug wholesalers, secondary wholesalers,16 and repackagers.
The FDA has identified three primary routes for drug sales in the UnitedStates, and each involves drugs passing through multiple hands, demonstratingthe vulnerability of the distribution system to counterfeit, adulterated, anddiverted products.17 The "Big 3" wholesalers—Cardinal Health,18 McKesson19
On May 6, 2005, Cardinal Health promised to discontinue its pharmaceutical arbitrage desk
which bought and sold drugs among secondary wholesalers because of the high incidence of counterfeitdrugs in the distribution network in the United States. Heather Won Tesoriero,
Cardinal Health Ends DrugTrading, WALL ST. J., May 6, 2005, at B2. Additionally, in its SEC filing on September 23, 2005,AmerisourceBergen Corporation promised that, effective October 1, 2005, it would purchase all of itsbranded and generic pharmaceuticals for distribution in the United States only from manufacturers.
AmerisourceBergen Corp., Current Report, (Form 8-K), at Exhibit 99.1 (Sept. 23, 2005). "In the rareinstances in which a manufacturer requires [AmerisourceBergen] to purchase products from an exclusivedistributor, AmerisourceBergen [would] follow the manufacturer's requirements. This change [was] notexpected to have a material impact on the company's fiscal year 2006 earnings."
Id.
Corrupt wholesalers often solicit members of the distribution chain to resell discounted drugs
to them for re-introduction into the nation's drug supply and often buy drugs from criminal enterprises.
See EBAN,
supra note 4, at 19-92.
Secondary wholesalers buy selected drug products from wholesalers, and then resell to other
wholesalers, including large wholesalers, and pharmacies. FDA INTERIM REPORT 2003,
supra note 7.
However, some fifteen regional distributors, which represent billions of dollars in drug sales, do exist inaddition to the "Big 3" distributors. EBAN,
supra note 4, at 90.
The figure pictured below from the
FDA Interim Report 2003 depicts the chains of drug
distribution in the United States:
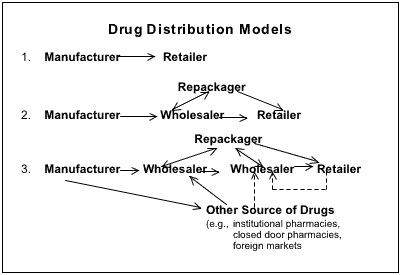
and AmerisourceBergen,20 which collectively account for nearly 90% of theprimary wholesale market21—sell drugs into a distribution web containinglarge governmental agencies, secondary wholesalers, and criminal actors.22"Repackagers" of drugs further obscure the origin of a particular drug whenthey break wholesale drugs in bulk containers into smaller units for sale topharmacies or, conversely, re-aggregate smaller units purchased as overstock
[T]hree models showing the movement of drugs through the U.S. drug distribution system.
(The dotted lines indicate potential illegal sales.) In the simplest situation, the manufacturersells directly to a retailer. However, in many instances, there can be one or more wholesalers,or even a repackager, who handles the drug before it reaches the retailer. It is in theseintermediate steps, particularly when the wholesaler(s) and/or repackager(s) obtain productsfrom sources other than the original manufacturer, that the greatest opportunities forcompromising the security of the U.S. distribution system exist.
FDA INTERIM REPORT 2003, supra note 7.
For fiscal year 2007, Cardinal Health reported $87 billion in revenue and $1.4 billion in
operating earnings. CardinalHealth Inc., Annual Report (Form 10-K), at 27 (Aug. 24, 2007).
McKesson Corp. reported $88.1 billion in operating revenue for fiscal year 2006. McKesson
Corp., Annual Report (Form 10-K), at 25 (May 16, 2006).
AmerisourceBergen reported $56.7 billion in operating revenue for fiscal year 2006.
AmerisourceBergen Corp., Annual Report (Form 10-K), at 26 (Dec. 8, 2006).
FDA INTERIM REPORT 2003, supra note 7, § 2.
UNIVERSITY OF PITTSBURGH LAW REVIEW
from pharmacies into larger bundles for resale to wholesalers.23 Because ofthe multiple distributors and the repackaging, the true origin of drugs in thisnet remains obscure.24
Although the government and wholesalers have maintained that legitimate
reasons exist for so many entities to be involved in the distribution of drugs,25the primary factor fueling diversion that was identified in the legislativehistory of the Prescription Drug Marketing Act (PDMA) is the cycling ofdrugs from one entity to another before reaching dispensing pharmacies.26The high cost of drugs, "virtually anonymous distribution channels,"27 andhigh consumer demand compound the desire of counterfeiters to flood themarketplace with tainted drugs. The riches from such an illegal enterprise aregreat.28 One FDA official opined that "some of the experts are telling us it'smore lucrative to sell a counterfeit drug than it is [to sell] a narcotic such asheroin."29
This complex maze of distribution allows tainted medicine to penetrate
the legitimate marketplace at multiple points. In all-too-familiar patterns,criminals sell counterfeit, diverted, and adulterated drugs to small,unscrupulous wholesalers which, in turn, introduce the drugs into thedistribution network. As the FDA itself has recognized, criminals can now
Even the New York State Attorney General has become concerned with the integrity and source
of drugs that circulate in this web. His concern was triggered after a Long Island, New York, teenager andliver transplant recipient purchased from a local CVS adulterated medication that had traveled through aMiami strip club, a trailer, a laundry room, and then ten other facilities before reaching the shelves at CVS.
EBAN, supra note 4, at 358 chart. Shortly after his mother injected him with the adulterated Epogen, Faganconvulsed at home and suffered radiating pain throughout his body. Id. at 122-24. He continued havingthese reactions after each injection until a staff person at CVS warned his mother that several vials ofcounterfeit Epogen bearing the same lot number as Fagan's drug had been found. Id.
The FDA stated that legitimate price differentials available because of temporary overstocks of
drugs and quick turnaround for the temporary needs of pharmacies were among reasons to include multiplewholesaling entities in the distribution chain. FDA INTERIM REPORT 2003, supra note 7, § 2(A). Thelobbyist organization for wholesale distributors, Healthcare Distribution Management Association,maintains that wholesale distributors play a critical role in making sure that patients do not suffer fromshortfalls of medication, although they admit that wholesalers have no responsibility for ensuring thatsufficient quantities of drugs are manufactured initially. Healthcare Distribution Management Association,Product Availability/Drug Shortage, http://www.healthcaredistribution.org/issues_in_dist/product.asp (lastvisited Jan. 23, 2008).
S. REP. NO. 100-303, at 1-2 (1988); see also Prescription Drug Marketing Act of 1987, Pub. L.
No. 100-293, 102 Stat. 95 (1988) (codified as amended at 21 U.S.C. §§ 301-399 (2000 & Supp. IV 2004)).
Buzzeo, supra note 2, at A20.
introduce counterfeit drugs at any point in the distribution process.30 A singlecounterfeiter can contaminate a nation's drug supply at multiple levels ofdistribution.31
Some may argue that the complicated system of distribution is "no one's
fault." Nonetheless, manufacturers and major drug wholesalers areresponsible for the establishment of the overly complex and unnecessarilyobtuse web. The activities that occur in this web are not "beyond thecontrol"32 of manufacturers. Very clearly, manufacturers have voluntarilychosen not to engage in the business of distributing drugs to dispensingpharmacies. Further, they have chosen the group of distributors to which theysell.33 In addition, the three major wholesalers decide for themselves fromwhom they buy and to whom they sell drugs. Until recently, the largestwholesalers maintained pharmaceutical arbitrage divisions that scavenged themarketplace, buying drugs from nearly all comers at the lowest expense
FDA INTERIM REPORT 2003, supra note 7, § 2(C).
In fact, Neil Spence, a high-level vice president of Cardinal Health, one of the three largest drug
wholesalers in the United States, conspired with a drug counterfeiter and allowed thousands of doses ofcounterfeit plasma medicine into the back door of Cardinal Health, which were then distributed throughoutthe country. Indictment at 2-4, United States v. Carlow, No. 3:06-00047 (M.D. Tenn. Mar. 29, 2006)available at http://www.dangerousdoses.com/pdf/Robert_Neil_Spence_Indictment%5B1%5D.pdf. Spencewas buying, on behalf of Cardinal Health, fragile plasma medicine from Michael Carlow, who traffickedin counterfeit and diverted medicine. Id. Another of America's largest distributors, AmerisourceBergen,was the subject of an FDA and FBI investigation regarding its illegal diversion of drugs into the secondarymarket to boost profits. Heather Won Tesoriero & Gary Fields, FBI, FDA Investigate Big DrugWholesaler, WALL ST. J., Sept. 19, 2003, at B1. That same drug counterfeiter, Michael Carlow, was notonly indicted in Tennessee regarding his transactions with Cardinal Health but also pled guilty in Missourifor dealing in $42 million of diverted Lipitor. News Release, Bradley J. Schlozman, United States Attorney,Western District of Missouri, Florida Business Owner Pleads Guilty to $42 Million Lipitor Conspiracy(Nov. 3, 2006), available at http://www.usdoj.gov/usao/mow/news2006/carlow.ple.htm. The Departmentof Justice announced that the District Court for the Western District of Missouri could impose a sentenceof up to five years on Michael Carlow for his involvement in the multi-million dollar counterfeit Lipitorscheme. Id. Carlow was also indicted in Florida for repackaging millions of dollars' worth of adulterateddrugs in the laundry room of his home. Grand Jury Indictment No. 1, State v. Carlow, No. SC02-2645 (Fla.
July 21, 2003), available at http://myfloridalegal.com/IndictmentCarlowetal.pdf (alleging numerousoffenses relating to his trade in counterfeit, diverted, and adulterated drugs, including, inter alia,racketeering, conspiracy to commit racketeering, organized scheme to defraud, and fraud).
A lawyer at deKieffer & Horgan argued that the "byzantine" system of pharmaceutical
distribution is almost wholly beyond the control of manufacturers. Donald deKieffer, Trojan Drugs:Counterfeit and Mislabeled Pharmaceuticals in the Legitimate Market, 32 AM. J.L. & MED. 325, 327(2006).
EBAN, supra note 4, at 90. Manufacturers and large pharmacy retailers profit greatly from this
web of a distribution system that "allows them to centralize their selling and purchasing and save billionsin distribution costs." Id.
UNIVERSITY OF PITTSBURGH LAW REVIEW
possible.34 Clearly, the largest stakeholders in the pharmaceutical industrydetermine how medicine is distributed.
III. THE LAW CURRENTLY DOES NOT CONTAIN ADEQUATE SAFEGUARDS
PROTECT THE INTEGRITY OF THE DRUG SUPPLY FROM COUNTERFEITERS
A. Ineffective and Unimplemented Federal Law: The Prescription DrugMarketing Act of 1987
In 1987, the federal government specifically enacted the Prescription
Drug Marketing Act (PDMA)35 to curb diversion of prescription drugs whichendangers public health.36 With the passage of the PDMA, Congress soughtto end the recycling of drugs through the complex distribution system.37 In thetext of the public law itself, Congress lamented that the American public couldnot trust the integrity of the drugs marketed in the United States.38 Althoughit created no new effective provisions itself, the Act amended numerous
See supra note 14. In fact, when Cardinal Health announced it would stop purchasing products
from secondary sources in May 2005, the company acknowledged the risks posed by these sources:
Ridding itself of a profitable but problematic business Interest, Cardinal Health will shut downits Cardinal Health Pharmaceutical Trading operation, which buys and sells discounted andoverstocked pharmaceuticals in the secondary distribution market. The move—announced ina letter to employees and suppliers May 6—follows recent legal action from New York StateAttorney General Elliot Spitzer, who last month subpoenaed Cardinal and its two largest wholecompetitors as part of a high-profile investigation of drug sourcing, counterfeiting and thepharmaceutical supply chain.
deKiefer, supra note 32, at 328 n.24 (citing James Frederick, Cardinal latest wholesaler to curb secondarydealing, DRUG STORE NEWS, May 23, 2005, at 8). McKesson has made no such pledge to discontinuepurchasing drugs from the secondary market.
Prescription Drug Marketing Act of 1987, Pub. L. No. 100-293, 102 Stat. 95 (1988).
See S. REP. NO. 100-303, at 1-2 (1988). "The purpose of the legislation is to curb operation of
the diversion market for prescription drugs that operates outside of normal channels of distribution andmakes it difficult to protect American consumers from mislabeled, subpotent, adulterated, expired, orcounterfeit pharmaceuticals." Id. at 2.
EBAN, supra note 4, at 107.
In its statutory findings, Congress stated that:
(1) American consumers cannot purchase prescription drugs with the certainty that the productsare safe and effective.
(2) The integrity of the distribution system for prescription drugs is insufficient to prevent theintroduction and eventual retail sale of substandard, ineffective, or even counterfeit drugs.
(3) The existence and operation of a wholesale submarket, commonly known as the "diversionmarket," prevents effective control over or even routine knowledge of the true sources ofprescription drugs in a significant number of cases.
Prescription Drug Marketing Act, § 2.
provisions of the Federal Food Drug and Cosmetics Act (FFDCA).39 ThePDMA sought to curtail numerous ills that Congress felt were contributing tothe proliferation of tainted drugs in the United States drug supply, including:(1) the existence of the wholesale diversion market;40 (2) the resale in largevolume of wholesale-priced drugs by entities other than the manufacturer orinitial purchaser;41 and (3) the illegal sale of adulterated drugs that began asphysicians' samples.42
Among its amendments to the FFDCA, the PDMA required drug
wholesalers which were not "authorized distributors of record"43 to submit astatement identifying each sale of a drug in its lifetime before reaching adispenser or being returned to a distributor.44 This identifying statement iscommonly referred to as a "pedigree."45 Pedigrees provide a transparenthistory of the source of a drug along with leads to investigators who want totrace the source of bad drugs.46 The pedigree requirement was Congress'sattempt to "restore accountability to the wholesale sector of thepharmaceutical market."47
Regrettably, the imagined accountability in the drug supply chain
promised by the PDMA was short-lived. Then-President Ronald Reagan'sless-than-enthusiastic PDMA signing statement foreshadowed a sluggish FDAeffort to implement such accountability.48 Predictably, in response to cries
See 21 U.S.C. §§ 301, 331, 333, 353, 381 (2000). See generally 21 U.S.C. §§ 301-399 (2000
& Supp.VI 2006).
"The existence and operation of a wholesale submarket, commonly known as the ‘diversion
market,' prevents effective control over or even routine knowledge of the true sources of prescription drugsin a significant number of cases." Prescription Drug Marketing Act, § 2(3).
"The bulk resale of below wholesale priced prescription drugs by health care entities, for
ultimate sale at retail, helps fuel the diversion market and is an unfair form of competition to wholesalersand retailers that must pay otherwise prevailing market prices." Id. § 2(7).
"The existing system of providing drug samples to physicians through manufacturer's
representatives has been abused for decades and has resulted in the sale to consumers of misbranded,expired, and adulterated pharmaceuticals." Id. § 2(6).
Id. § 6(e)(1). The problematic elasticity of the definition of an authorized distributor is
discussed below. See infra pp. 256-57.
Prescription Drug Marketing Act, § 6(e)(1).
William McConagha, Associate General Counsel, U.S. Food & Drug Admin., Brief Overview
of Prescription Drug Marketing Act, Address at the Counterfeit Task Force Public Workshop/VendorDisplay 19 (Feb. 9, 2006), available at http://www.fda.gov/oc/meetings/rfid/0209FDA1.pdf; see alsoEBAN, supra note 4, at 163.
See McConagha, supra note 45, at 19-20. Eban notes that even dog breeders and car dealers
must provide pedigrees of their wares. EBAN, supra note 4, at 163.
H.R. REP. NO. 100-76, at 16-17 (1987).
In his signing statement, the President said:
Finally, although the lack of traceability of drug products in the diversion market is a valid
UNIVERSITY OF PITTSBURGH LAW REVIEW
from wholesalers49 and manufacturers,50 both claiming that forcingwholesalers to provide histories of drugs would cripple the industry out ofexistence, the FDA reduced the pedigree requirement51 to a mere guidanceopinion,52 in effect staying the implementation of pedigrees. Through aprolonged ten-year rulemaking process and the following decade, the FDA
concern that I share, the magnitude of the public health problem created by diverted drugs isstill not clear. I am therefore also concerned by the provisions of the bill requiring use ofsubstantial amounts of scarce Federal public health resources to police these practices.
Statement on Signing the Prescription Drug Marketing Act of 1987, 24 WEEKLY COMP. PRES. DOC. 519(Apr. 25, 1988).
In a Senate hearing before the passage of the PDMA, a representative of one of the wholesalers'
lobbies likened having to source medicine to being required to source real estate, a process which wouldmake the cost of drugs dramatically higher and would be operationally impossible for secondarywholesalers. Prescription Drug Marketing Act of 1987: Hearing on S. 368 and H.R. 1207 Before theSubcomm. on Int'l Trade of the S. Comm. on Finance, 100th Cong. 49 (1987) (statement of Ronald J.
Streck, Vice President, National Wholesale Druggists Association).
The manufacturer Smith Kline & French protested the pedigree requirement in an October 3,
1988, letter to the FDA. EBAN, supra note 4, at 385 nn.162-63.
Pedigree requirements were explicitly defined by regulation by the FDA in 21 C.F.R.
§§ 203.1-.60.
On August 1, 1988 the FDA issued a guidance letter setting forth the agency's preliminary views
regarding the industry's responsibilities under the PDMA. The letter stated that to be an "authorizeddistributor," the type of ongoing relationship that needed to exist was only a "continuing businessrelationship in which it is intended that the wholesale distributor engage in wholesale distribution of amanufacturer's prescription drug product or products." Letter from Daniel L. Michels & Thomas S. Bozzo,Directors, Food & Drug Administration Office of Compliance, to Regulated Industry and Other InterestedPersons (Aug. 1, 1988), available at http://www.fda.gov/oc/pdma/report2001/attachment-e.pdf. By statingthat drug wholesalers only had to comply with existing recordkeeping practices of the industry, the FDAstayed the enforcement of a pedigree requirement, which wholesalers had never made an industry practice:
The new law's requirements for State licensing of wholesale drug distributors and the
attendant requirements for minimum standards for recordkeeping, storage, and handling ofprescription drugs pose potential economic implications for wholesale drug distributors.
Although the statute itself does not specify these minimum standards, Congress clearly intendedthat these standards match currently recommended practices within the wholesale drug sector.
The recommendation by the House of Representatives' Committee on Energy and Commercefor FDA to consider the Guidelines for the Inspection of Wholesales issued by the NABPprovided explicit guidance on the specifications for these standards. FDA sought to conformthe proposed minimum standards with the NABP guidelines and NWDA's-related proposeduniform standards by limiting modifications to conformance with current language and toclarifications required for consistency with existing drug regulations. Thus, the proposedminimum standards are intended to mirror recommended practices already existing among drugwholesalers.
The agency is not aware of the degree to which drug wholesalers comply with the various
existing guidelines, but the agency believes that these represent the norm of current practicesand procedures among drug wholesalers.
Guidelines for State Licensing of Wholesale Drug Distributors, 53 Fed. Reg. 35325, 35327 (Sept. 13, 1988)(to be codified at 21 C.F.R. pt. 205).
administratively negated the full pedigree requirement five times over nearlytwenty years.53 On June 9, 2006, the FDA announced that it was lifting thestay on implementation of pedigrees, effective December 6, 2006.54 Eventhough numerous documented cases of counterfeit drugs had come to light,55the agency claimed it was lifting the stay because of the availability oftechnology to help distributors comply with pedigree requirements andbecause small wholesalers were no longer voicing complaints aboutcompliance with the pedigree regulation.56
Sadly, a fully implemented federal pedigree requirement is still not a
reality. In RxUSA Wholesale, Inc. v. Department of HHS, secondarywholesalers, which would not qualify as "authorized distributors" under thePDMA, alleged that implementation of the pedigree requirement by FDAregulations at section 203.50, the PDMA, or the two in conjunction, was anunconstitutional violation of the Equal Protection and Due Process clauses ofthe Fourteenth Amendment.57 The wholesalers argued that the pedigreeregulation constituted disparate treatment in violation of the Equal ProtectionClause in as much as it arbitrarily and capriciously required non-authorizeddistributors to trace products back to the manufacturer while exemptingauthorized distributors, thus preventing non-authorized distributors fromconducting business.58 In addition, the wholesalers argued that FDAregulations at section 203.50 (interpreting the PDMA) violated the DueProcess Clause by requiring only non-authorized distributors to source59 adrug to the manufacturer. They contended that the regulation had no rationalrelation to any legitimate state interest and was an erroneous interpretation of
EBAN, supra note 4, at 386 n.165. The FDA finally promulgated a final rule regarding
implementation of the pedigree in 1994, which required non-authorized distributors to include the nameof each business who sold the drug starting with the manufacturer. 21 C.F.R. § 203.50 (2007).
In its press release, the FDA stated that in an effort to protect the public from counterfeit drugs,
"the FDA will fully implement regulations related to the Prescription Drug Marketing Act of 1987, whichrequires drug distributors to provide documentation of the chain of custody of drug products—the so-called‘pedigree'—throughout the distribution system." Press Release, U.S. Food & Drug Administration, FDAAnnounces New Measures to Protect Americans from Counterfeit Drugs (June 9, 2006), available athttp://www.fda.gov/bbs/topics/NEWS/2006/NEW01386.html [hereinafter FDA News].
Katherine Eban's book, Dangerous Doses, had been published in 2005, and Dateline had done
several reports in 2005 and 2006. See supra note 4.
The FDA claimed it had not received many complaints from small wholesalers about the impact
of requiring pedigrees and finally recognized it could no longer justify delaying the requirement. FDANews, supra note 54.
Complaint at 14, RxUSA Wholesale, Inc. v. Dep't of Health & Human Servs., 467 F. Supp. 2d
285 (E.D.N.Y. 2006) (No. 06-506).
Id. at 14-16.
To "source" means to identify the sales history of a drug.
UNIVERSITY OF PITTSBURGH LAW REVIEW
the PDMA, which requires that wholesale customers be informed of "all priorsales of the product."60
On November 30, 2006, seven days before the long-anticipated effective
date of a federal pedigree requirement, the District Court for the EasternDistrict of New York issued a preliminary injunction staying theimplementation of the federal pedigree requirement.61 The court disregardedthe government's anemic laches claim (that it would suffer prejudice if thecourt delayed the implementation of the pedigree rule) given that the FDAitself had delayed the rule's implementation for so long.62 Regrettably, noother federal legislation currently requires pedigrees in the distributionchain.63 The need for a fully implemented PDMA is apparent.64
If federal pedigrees become mandatory, the definition of what qualifies
as an exempt "authorized distributor" under the PDMA is nevertheless toobroad to provide real protection to consumers. Under the PDMA, an"authorized distributor" is exempt from having to produce any pedigree whentransacting pharmaceuticals.65 In 1999, the FDA promulgated its final ruleand deemed that, without receiving any further governmental scrutiny, an"authorized distributor" is anyone with an "ongoing relationship" with a
According to the complaint, because the FDA had maintained a nearly twenty-year "status quo"
of not requiring non-authorized distributors to produce pedigrees sourcing a drug back to the manufacturer,to now require wholesalers to do that would be erroneous. Complaint at 17-18, RxUSA, 467 F. Supp. 2d285.
The court reasoned that the preliminary injunction was merely a "temporary reprieve of the Rule
taking effect." RxUSA Wholesale, Inc. v. Dep't of Health & Human Servs., 467 F. Supp. 2d 285, 307(E.D.N.Y. 2006).
On May 12, 2005, Representative Steve Israel (D-NY) introduced "Tim Fagan's Law" which
would have made pedigrees on the federal level mandatory and given the FDA investigatory subpoenapower as well as the authority to increase criminal penalties for counterfeiting. H.R. 2345, 109th Cong.
(2005). The bill continues to languish in the House Energy and Commerce Subcommittee on Health. TheLibrary of Congress, THOMAS, http://thomas.loc.gov (select "109th Congress" and search bill number "H.R.
2345"; then view "All congressional actions") (last visited Jan. 23, 2008).
The known threat of counterfeit drugs that spawned only a handful of investigations in the 1990s
now has jumped nearly five-fold. Afia K. Asamoa, Not as Easy As It May Appear: Using Radio FrequencyIdentification Technology to Fulfill the Prescription Drug Marketing Act's Elusive Pedigree Requirement,61 FOOD & DRUG L.J. 385, 385 (2006) (citing FDA REPORT 2004, supra note 1).
21 C.F.R. § 203.50(a) (2007) provides, in part:
Identifying statement for sales by unauthorized distributors. Before the completion of anywholesale distribution by a wholesale distributor of a prescription drug for which the seller isnot an authorized distributor of record to another wholesale distributor or retail pharmacy, theseller shall provide to the purchaser a statement identifying each prior sale, purchase or tradeof such drug.
The regulation goes on to specify what information shall be contained in that identifying statement. Seeid.
manufacturer.66 Thus, no governmental entity exercises oversight in certifyingwhich entities are "safe enough" to be trusted without a traceable history ofdistribution. Even when the FDA issued guidance documents for the drugindustry regarding compliance with the PDMA in 2006, the nonbindingrecommendations provided no additional procedures to clearly identify anauthorized distributor of record.67 Ironically, even the Healthcare DistributionManagement Association (HDMA)—the lobbyist organization for wholesaledistributors—noted that the definition of an authorized distributor under thePDMA should be enhanced to make any current pedigree requirementsmeaningful.68
The regulations define an "authorized distributor" as a "distributor with whom a manufacturer
has established an ongoing relationship to distribute such manufacturer's products." Id. § 203.3(b). An"ongoing relationship" is further defined in § 203.1(u) as the following:
Ongoing relationship means an association that exists when a manufacturer and a distributorenter into a written agreement under which the distributor is authorized to distribute themanufacturer's products or a period of time or for a number of shipments. If the distributor isnot authorized to distribute a manufacturer's entire product line, the agreement must identifythe specific drug products that the distributor is authorized to distribute.
In responding to the question, "What information should be in the written agreement between
a manufacturer and an ADR?," the FDA merely reiterated the definition of "ongoing relationship" contained§ 203.3(u). U.S. FOOD & DRUG ADMIN., GUIDANCE FOR THE INDUSTRY: PRESCRIPTION DRUG MARKINGACT (PDMA) REQUIREMENTS , QUESTIONS AND ANSWERS 4 (2006), available at http://www.fda.gov/OHRMS/DOCKETS/98fr/06d-0226-gdl0003.pdf.
In a press release posted on its website on June 9, 2006, following the FDA lifting the stay on
the federal pedigree requirement, the HDMA suggested changing the definition of "authorized distributor"and "distributor" in general to the following:
"Authorized Distributor of Record" means a drug distributor with whom a manufacturer hasestablished an ongoing relationship to distribute the manufacturer's product. An ongoingrelationship is deemed to exist when a drug distributor, including any affiliated group, asdefined in Section 1504 of the Internal Revenue Code, of which the distributor is a member:a) Is listed on the manufacturer's list and the list is updated monthly, or b) Has a written agreement currently in effect with the manufacturer, orc) Has a verifiable account with the manufacturer and minimal transaction or volume
requirement thresholds as follows: 5,000 sales units per company within twelve (12) monthsor twelve (12) purchases (invoices) from the manufacturer within twelve (12) months.
"Distribution or wholesale distribution" means the distribution of prescription drugs to personsother than a consumer or patient, but does not include:a) Intracompany sales;b) The purchase or other acquisition by a hospital or other health care entity that is a member
of a group purchasing organization of a drug for its own use from the group purchasingorganization or from other hospitals or health care entities that are members of suchorganizations;
c) The sale, purchase, or trade of a drug or an offer to sell, purchase, or trade a drug by a
charitable organization to a nonprofit affiliate of the organization to the extent otherwisepermitted by law;
d) The sale, purchase, or trade of a drug or an offer to sell, purchase, or trade a drug among
UNIVERSITY OF PITTSBURGH LAW REVIEW
B. State Law Attempts to Source the Drug Distribution Chain
Faced with a federal pedigree requirement effectively stuck in purgatory,
some states did undertake the burden of attempting to craft meaningfulprotection for consumers against counterfeit, adulterated, and fake medicines.
Florida,69 Nevada,70 California,71 and New York72 led the nation in attempting
hospitals or other health care entities that are under common control;
e) The sale, purchase, or trade of a drug or an offer to sell, purchase, or trade a drug for
emergency medical reasons;
f) The sale, purchase, or trade of a drug, an offer to sell, purchase, or trade a drug, or the
dispensing of a drug under a prescription executed in accordance with section of thischapter;
g) The distribution of drug samples by manufacturers' and authorized distributors'
h) The sale, purchase, or trade of blood or blood components intended for transfusion;i) Drug returns, when conducted by a hospital, health care entity, or charitable institution in
accordance with section of this chapter or the Boards'/Departments' regulations;or
j) The sale of minimal quantities of drugs by retail pharmacies to licensed practitioners for
"Distributor or wholesale distributor" means any person engaged in the distribution ofprescription drugs, including, but not limited to, manufacturers; repackers; own-labeldistributors; private-label distributors; jobbers; brokers; warehouses, including manufacturers'and distributors' warehouses, chain drug warehouses, and drug warehouses; and retailpharmacies that conduct drug distributions as defined in this section.
Healthcare Dist. Mgmt. Assoc., Recommendations for Enhancing the Domestic Prescription Drug SupplyChain 2-3 (2004), available at http://www.healthcaredistribution.org/gov_affairs/pdf_anti/HELP_Com_alternatives.doc.
See Prescription Drug Protection Act of 2003, 2003 Fla. Laws 155.
Nevada enacted the toughest controls in the country regarding who can qualify as a wholesaler
in the state, thereby attempting to squeeze out the criminal element in the entire shadow market. GilbertM. Gaul & Mary Pat Flaherty, Nevada Gets Tough, With Mixed Results, WASH. POST, Oct. 22, 2003, atA16. An owner of a wholesaler must submit his own fingerprints, employ an authorized representative with6,000 hours of experience, and provide a complete list of employees/agents. Id. Only if a corporation ispublicly traded is the owner exempt from the fingerprinting and employee list requirements. NEV. ADMIN.
CODE § 639.593(7) (2007). Although the regulation shoved many wholesalers out of state, Nevada by itselfcannot clean up the nation's distribution problems. Wholesalers merely moved across state lines andcontinued to distribute bad medicine into the porous national drug supply. Gaul & Flaherty, supra note 6.
However, Nevada did enact a stringent pedigree requirement, requiring wholesalers to pass pedigrees whenthe wholesaler is either (1) not an authorized distributor of a medicine or (2) when a wholesaler bought adrug from a source other than the manufacturer. See NEV. ADMIN. CODE § 639.603(1) (2007).
California has repeatedly delayed implanting a comprehensive state pedigree law, having just
this year pushed the state law's implementation date back to January 1, 2011. Press Release, California Bd.
of Pharmacy, Decision of the California State Board of Pharmacy Pursuant to Business & Professions CodeSection 4163.5 (Mar. 25, 2008), available at http://www.pharmacy.ca.gov/laws_regs/delay_implementation.pdf. The Board acquiesced to widespread industry complaints regarding the difficulty of
to pass laws and administrative regulations cracking down on the secondarywholesale market. Among all of these states, Florida initially appeared to bea shining example of regulation.
Florida's legislative experience, however, proved disappointing and
ineffective. In 2003, the Seventeenth Statewide Grand Jury in Florida issueda scathing report summarizing the great danger posed when counterfeiters anddiverters infiltrate the legitimate distribution stream of prescription drugs.73The Florida legislature embraced the Grand Jury's recommendation for astrong state pedigree law.74 It passed the Prescription Drug Protection Act of
meeting the state law's requirements. California had accepted the same industry argument concerning theindustry's inability to meet California's requirement that every drug carry an electronic pedigree (a pedigreethat could be tracked and verified through electronic means) when it delayed implementation of the lawuntil 2009 from its prior implementation date of 2007. See Beth Bacheldor, Cardinal Health DeployingE-Pedigree System, RFID J., May 8, 2007, available at http://www.rfidjournal.com/article/articleview/3295/1/1; see also CAL. BUS. & PROF. CODE § 4163 (West 2007), as amended by 2006 Cal. Legis. Serv.
658 (West). In their presentation to the FDA in 2006, Patricia Harris, Executive Director, and Judi Nurse,Pharm D Supervising Inspector, indicated that the California Board of Pharmacy may delay implementationof the wholesale drug pedigree until January 1, 2008. Patricia Harris & Judi Nurse, Cal. Bd. Pharmacy,California Prescription Drug Pedigree (Feb. 27, 2006) (presentation slides available at http://www.fda.gov/oc/meetings/rfid/nurse.ppt).
On May 31, 2006, New York State Assemblywoman Amy Paulin and Senator Nick Spano
introduced companion legislation to increase the penalties for counterfeiting medication. Press Release,Amy Paulin & Nick Spano, Assemblywoman Amy Paulin & Senator Nick Spano Call for Tougher Lawsto Prevent the Sale of Counterfeit Drugs (May 31, 2006), available at http://www.turkewitzlaw.com/Counterfeit-Drug-2.pdf. On May 31, 2006, the bill was amended and recommitted to the higher educationsubcommittee where it died once the legislative session ended. Assemblywoman Paulin has since proposedsimilar legislation to help stop the sale and distribution of counterfeit drugs. New York State Assembly,Bill Summary of A07810, http://assembly.state.ny.us/leg/?bn=A07810 (last visited Jan. 23, 2008).
Companion legislation has also been introduced in the New York State Senate. New York State Assembly,Bill Summary of S05290, http://assembly.state.ny.us/leg/?bn=S05290 (last visited Jan. 23, 2008).
The Grand Jury noted that tainted drugs move easily through the national drug distribution chain
because of the failure of state and federal agencies to enforce the law and because of the "complicity of thewholesalers who turn a blind eye to the corrupt practices of other wholesalers that supply them with someof their pharmaceuticals." SUPREME COURT OF THE STATE OF FLORIDA, FIRST INTERIM REPORT OF THESEVENTEENTH STATEWIDE GRAND JURY 3 (2003), available at http://myfloridalegal.com/grandjury17.pdf[hereinafter FIRST INTERIM REPORT OF GRAND JURY]. The Grand Jury also noted that the 422 wholesaledistributors of drugs in Florida who bought and sold drugs amongst themselves before passing them on todispensing entities permitted an "alarming percentage" of illegally purchased drugs from the black market.
Id. at 2. The Grand Jury further noted that many of the wholesalers in Florida were "unqualified,inexperienced, irresponsible and incompetent." Id.; see also SUPREME COURT OF THE STATE OF FLORIDA,SECOND INTERIM REPORT OF THE SEVENTEENTH STATEWIDE GRAND JURY (2003), available athttp://myfloridalegal.com/interimjury17.pdf (illustrating the millions of dollars the Medicaid program waslosing as Medicaid recipients and corrupt infusion clinics resold Medicaid medication to the black market,all of which was illegally reintroduced into the wholesale distribution system).
FIRST INTERIM REPORT OF GRAND JURY, supra note 73.
UNIVERSITY OF PITTSBURGH LAW REVIEW
2003,75 which amended many provisions of the Florida Drug and CosmeticAct.76 In addition to strengthening licensing requirements for wholesalers77and increasing felony penalties for counterfeiter-diverters78 and non-compliantwholesalers,79 the state expanded its administrative rule requiring pedigreepapers to travel with the most "vulnerable drugs" in the state80
2003 Fla. Laws 155.
FLA STAT. ANN. §§ 499.001-.081 (West 2007).
The legislative history of this section evidences an intent to severely curtail who might qualify
as a wholesaler in the state and refers in its preamble to the changes made by the 2003 Prescription DrugProtection Act:
Legislative findings and intent.—Based on the report of the Seventeenth Statewide Grand Juryin its First Interim Report the Legislature finds that prescription drugs brought into the state bywholesalers are being relabeled and falsely represented as being of a higher dosage by otherwholesalers in order to charge higher prices for those drugs and that counterfeit substanceslabeled as genuine pharmaceuticals are being distributed, thereby causing an extreme dangerthat persons eventually receiving the drugs by prescription are receiving ineffective drugs innontherapeutic doses, or even receiving dangerous or unwholesome substances, with the resultthat the health and well-being of the public is at risk. The Statewide Grand Jury also found thatthe lack of an effective pedigree paper requirement has resulted in the inability of prescriptiondrug users to have confidence in the purity and efficacy of the drugs they use. The StatewideGrand Jury further noted that present laws do not allow effective criminal prosecution ofpersons involved in such false representations. It is the intent of the Legislature that thestatutory changes and recommendations outlined in the Statewide Grand Jury's report beimplemented as provided by this act.
Id. § 2.
The legislature elevated all criminal offenses relating to counterfeit and adulterated drugs to
felonies of the second degree or higher. See FLA. STAT. ANN. § 499.0691 (West 2007).
See id. § 499.005. Revisions to the Prescription Drug Protection Act made unlawful both the
failure to obtain a pedigree paper and the receipt of prescription drugs without pedigree papers. See id.
§ 499.005(27)-(28). The good-faith defense (set forth below), previously contained in § 499.005(7), wasalso eliminated:
The giving of a false guaranty or false undertaking with respect to a drug, device, or cosmetic,except by a person who relied on a guaranty or undertaking to the same effect signed by, andcontaining the name and address of, the person residing in this state from whom she or hereceived in good faith the drug, device, or cosmetic.
See Prescription Drug Protection Act of 2003, 2003 Fla. Laws 155, § 4.
Before recent amendments, the Florida Department of Health had the power to require
wholesalers to provide complete pedigrees, including all prior sales and unique identifying lot numbers, ofdrugs on a "specified list." FLA. STAT. ANN. § 499.0121(6)(e) (West 2005) (current version at FLA. STAT.
ANN. § 499.0121(6)(d) (West 2007)). The department could place a drug "on the list of specified drugsif the department [had] seized or issued a stop sale notice on the . . drug because of adulteration,counterfeiting or diversion of the prescription drug from" legal distributions channels, or if the FDA oranother government regulator "responsible for the sale or distribution of prescription drugs in another statehas notified the department" of such contamination of a drug in the legitimate marketplace, so long as theprescription drug satisfied one of the following criteria:
(I) The prescription drug is included among the top 150 prescription drugs for which the statehas incurred the highest amount of Medicaid claims in the most recently ended state fiscal year;(II) The prescription drug is available for normal prescription use in dosages or strengths that
have a wholesale cost of $200 or more;(III) The prescription drug is used extensively for patients with human immune deficiency virus,acquired immune deficiency syndrome, cancer, or other serious, life-threatening conditions,where drug non-responsiveness would not be considered to be medically unusual;(IV) The prescription drug is an injectible drug;(V) The prescription drug is subject to a special, limited distribution process and is notgenerally old to wholesale distributors by the manufacturer of the prescription drug;(VI) The department has found not less than five instances where statements required pursuantto paragraph (d) for the prescription drug were not passed on other than because ofunintentional oversight, or have been passed on by or to a wholesale distributor and suchstatements were fraudulent; or(VII) A shipment of a prescription drug has been reported to a law enforcement agency ashaving been stolen or as missing.
Id. § 499.0121(6)(e)(3)(a) (West 2005) (current version at § 499.0121(6)(d)(5)(b)(1)(I)-(IV) (West 2007)).
The Department of Health, after consultation with the Drug Wholesaler Advisory Council, wouldpromulgate a rule placing drugs on the "specified drug" list. Id. § 499.0121(6)(e)(3)(b) (West 2005)(current version at § 499.0121(6) (West 2007)). The list of specified drugs appears in Florida'sAdministrative Code. FLA. ADMIN. CODE ANN. r. 64F-12.001(2)(y) (2007). That list includes:
1. Bextra (valdecoxib);2. Celebrex (celecoxib);3. Combivir (lamivudine/zidovudine);4. Crixivan (indinavir sulfate);5. Diflucan (fluconazole);6. Epivir (lamivudine);7. Epogen (epoetin alfa);8. Gamimune (globulin, immune);9. Gammagard (globulin, immune);10. Immune globulin;11. Lamisil (terbinafine);12. Lipitor (atorvastatin calcium); (fully effective 3/29/2004)13. Lupron (leuprolide acetate);14. Neupogen (filgrastim);15. Nutropin AQ (somatropin, e-coli derived);16. Panglobulin (globulin, immune);17. Procrit (epoetin alfa);18. Retrovir (zidovudine);19. Risperdal (risperidone);20. Rocephin (ceftriaxone sodium);21. Serostim (somatropin, mannalian derived);22. Sustiva (efavirenz);23. Trizivir (abacavir sulfate/lamivudine/zidovudine);24. Venoglobulin (globulin, immune);25. Viagra (sildenafil citrate);26. Videx (didanosine);27. Viracept (nelfinavir mesylate);28. Viramune (nevirapine);29. Zerit (stavudine);30. Ziagen (abacavir sulfate);31. Zocor (simvastatin);
UNIVERSITY OF PITTSBURGH LAW REVIEW
by requiring pedigree papers to accompany all drugs.81 The term "pedigree"under Florida law was strictly defined as a document containing the salesinformation and lot number of the prescription drug.82 The document was tobegin with the sale from the manufacturer and was to include all subsequentsales information until the drug was dispensed to a patient.83
Unfortunately, Florida's stringent pedigree requirement never went into
effect. The effective date of the newly defined pedigree was to be July 1,2006,84 but pharmaceutical manufacturers and distributors mounted an assaulton Florida's 2006 legislative session to make sure that the requirement wouldnot become effective.85 At their urging, the Florida legislature amended thedefinition of what qualified as a pedigree, tacking that alternate definition toa free-cancer-drug donation bill.86 The new provision alternatively defined a
32. Zofran (ondansetron);33. Zoladex (goserelin acetate); and34. Zyprexa (olanzapine).
FLA. STAT. § 499.0121(6)(e)(1) (West 2005) (current version at § 499.0121(6)(d) (West 2007)).
"Pedigree paper" means:
Effective July 1, 2006, a document in a form approved by the Department of Health andcontaining information that records each distribution of any given legend drug, from sale by apharmaceutical manufacturer, through acquisition and sale by any wholesaler or repackager,until final sale to a pharmacy or other person administering or dispensing the drug. Theinformation required to be included on a legend drug's pedigree paper must at least detail theamount of the legend drug, its dosage form and strength, its lot numbers, the name and addressof each owner of the legend drug and his or her signature, its shipping information, includingthe name and address of each person certifying delivery or receipt of the legend drug, and acertification that the recipient has authenticated the pedigree papers. It must also include thename, address, telephone number and, if available, e-mail contact information of eachwholesaler involved in the chain of the legend drug's custody. The department shall adopt rulesand a form relating to the requirements of this paragraph no later than 90 days after the effectivedate of this act.
Id. § 499.003(31)(b) (West 2005) (current version at § 499.003(31)(a)-(b) (West 2007)).
When I spoke in front of the Florida Senate Judiciary Committee on April 25, 2006, I was the
only person present who testified against changing the law. Meanwhile, representatives from McKesson,Cardinal Health, and AmerisourceBergen sat with a bevy of lobbyists for other wholesalers andRepresentative Homan to register their objection to the pedigree requirement. The drug industryrepresentatives stayed in Tallahassee until the pedigree law was changed some time after 11 p.m. on thevery last day of the 2006 legislative session.
After H.R. 1397, 2006 Leg., Reg. Sess. (Fla. 2006) (regarding just pedigrees and drug
distribution), H.R. 685, 2006 Leg., Reg. Sess. (Fla. 2006) (regarding the distribution of prescription drugsby veterinarians), and S. 926, 2006 Leg., Reg. Sess. (Fla. 2006) (regarding just pedigrees and distributionof prescription drugs), the drug industry, through House and Senate representatives, tacked the change inthe pedigree requirement on to a free drug donation program for cancer patients in H.R. 371, 2006 Leg.,Reg. Sess. (Fla. 2006) and S. 1310, 2006 Leg., Reg. Sess. (Fla. 2006).
"pedigree" as merely a document which states the name and strength of a drugaccompanied by a promise by the manufacturer that "this wholesale distributorpurchased the specific unit of the prescription drug directly from themanufacturer."87 This change eliminates the ability of law enforcementofficers, customers, and agencies to find the source of counterfeit drugs usingthe unique lot number from the pedigree paper.88
Despite its steady support for the free-drug donation program throughout
the legislative session, the American Cancer Society made a last-minuterequest to Florida Governor Jeb Bush to veto House Bill 371, because "therisks to cancer patients through any weakening of the state's toughprescription drug safety law appear to outweigh the benefits of a new program
The alternate definition of "pedigree" reads:
A statement, under oath, in written or electronic form, confirming that a wholesale distributorpurchases and receives the specific unit of the prescription drug directly from the manufacturerof the prescription drug and distributes the prescription drug directly, or through anintracompany transfer, to a chain pharmacy warehouse or a person authorized by law topurchase prescription drugs for the purpose of administering or dispensing the drug, as definedin § 465.003. For purposes of this paragraph, the term "chain pharmacy warehouse" means awholesale distributor permitted pursuant to § 499.01 that maintains a physical location forprescription drugs that functions solely as a central warehouse to perform intracompanytransfers of such drugs to a member of its affiliated group as described in § 499.0121(6)(h)1.
1. The information required to be included pursuant to this subparagraph must include:
a. The following statement: "This wholesale distributor purchased the specific unit ofthe prescription drug directly from the manufacturer."b. The manufacturer's national drug code identifier and the name and address of thewholesaler and the purchaser of the prescription drug.
c. The name of the prescription drug as it appears on the label.
d. The quantity, dosage form, and strength of the prescription drug.
2. The wholesale distributor must also maintain and make available to the department, uponrequest, the point of origin of the prescription drugs, including intracompany transfers; thedate of the shipment from the manufacturer to the wholesale distributor; the lot numbers ofsuch drugs; and the invoice numbers from the manufacturer.
FLA. STAT. ANN. § 499.003(31) (West 2007).
The Seventeenth Statewide Grand Jury noted that the requirement that a wholesaler maintain
receipts of sale and present them if asked for them was meaningless as the entire state of Florida had so fewdrug inspectors. See FIRST INTERIM REPORT OF GRAND JURY, supra note 73, at 2, 18 ("There exists inFlorida approximately 422 licensed wholesalers in the prescription drug industry. In addition, there areapproximately 977 wholesalers outside of the state of Florida that are licensed by the State to shipprescription drugs into Florida."). Most states have only a handful of inspectors to monitor the practicesof thousands of wholesalers that exist nationally. Gaul & Flaherty, supra note 6, at A15.
UNIVERSITY OF PITTSBURGH LAW REVIEW
whose merits are not yet established."89 Sadly, Governor Bush signed the billinto law in June of 2006.90
As well-intentioned as they might be, individual state efforts alone will
not secure the drug supply in the United States.91 Drugs move seamlesslyamong billion-dollar wholesalers, smaller wholesalers, and retailersthroughout the United States. Drugs that originate in one state easily reach allparts of the country.92 A patchwork of conflicting state regulations will becumbersome for the industry without ever uniformly ensuring the safety ofmedicine.93 A uniform legal theory, as well as a codified federal legal duty,
In a letter dated May 17, 2006, Michael Kasper, M.D., Chairman of the Board and President of
the American Cancer Society wrote the following to Governor Bush:
The promise of a lesser financial burden to particularly needy cancer patients means little
if the prescription drugs provided to them could put their lives in jeopardy. While the cancerdrug donation program is a viable concept that could ease some financial worries for somecancer patients, the greater concern to our organization is overall patient safety. In other words,Governor, we feel that it is more important to save lives than to save money.
It truly disappoints us to be in a position to recommend that you veto a bill that includes a
positive new tool in the fight against cancer, but we feel the greater good would be served bystaying the course with the drug pedigree process in current statute.
Letter from Michael Kasper, M.D., Chairman of the Board/President, American Cancer Society, to JohnEllis Bush, Governor of Florida (May 17, 2006) (on file with the American Cancer Society).
Florida House of Representatives, Bill Summary for H.R. 371, http://www.flhouse.gov/
Sections/Bills/billsdetail.aspx?BillId=32048&SessionId=42 (last visited Jan. 23, 2008).
As of 2007, a number of states have begun to pass legislation regulating wholesalers in an
attempt to curb counterfeiting. Such states include: Alabama, Illinois, Iowa, Kansas, Kentucky, Maryland,Massachusetts, Missouri, Montana, Nevada, New York, Oklahoma, Tennessee, Utah, Washington, andWyoming. NAT'L CONFERENCE OF STATE LEGISLATURES, 2007 PRESCRIPTION DRUG STATE LEGISLATION5 http://www.ncsl.org/programs/health/drugbill07.htm#CA (last visited Jan. 23, 2008). Many states havealready passed some measure of wholesaler reform in 2007, including Arkansas, Colorado, Georgia,Indiana, North Dakota, South Dakota, Texas, and Wyoming. Id. Nevada remains a beacon of hope againstcounterfeiting as it has retained its pedigree requirement. Since January 1, 2007, Nevada has requiredwholesalers to utilize electronically transmitted pedigrees for all sales of drugs. NEV. REV. STAT. ANN.
§ 639.540(3) (West 2007).
The FDA Counterfeit Drug Task Force noted the ability of bad drugs to reach all parts of the
distribution system:
Investigations performed by Federal and State authorities have repeatedly shown the existenceof illicit nationwide networks designed to capitalize on the inadequate due diligence performedby members of the drug distribution system in order to introduce potentially unsafe diverted andcounterfeit drugs in the U.S. drug distribution system.
FDA INTERIM REPORT 2003, supra note 7, § 2(C). While manufacturers themselves may sell only to ahandful of large distributors, the distributors buy drugs from other sources such as other distributors andsmall wholesalers. "[T]hese secondary market sales are the primary, if not exclusive means by which[tainted] drugs enter the bloodstreams of the unwary." deKieffer, supra note 32, at 329. CounterfeiterMichael Carlow, who lived in Florida and repackaged drugs in his laundry room, infected the drug supplyof Missouri, Tennessee, and the national wholesaler Cardinal Health (at the very least). See supra note 31.
The gaps among conflicting state and federal regulations and the complexity of the labyrinth of
is necessary to hold all parts of the drug distribution system accountable forpatient injuries caused by circulating tainted medicine.
IV. THE "GOOD HUMOR" OF PRESCRIPTION DRUGS: THE NON-DELEGABLE
DUTY OF SAFE DISTRIBUTION WHICH MANUFACTURERS AND ALL
DISTRIBUTORS SHOULD BEAR
Generally, manufacturers and distributors that are part of a business
enterprise may escape liability for negligence occurring in their enterprise bymaking use of independent contractors.94 However, some courts haveconcluded that, as a matter of public policy,95 all the employers in anenterprise should be responsible for the torts of independent contractors "whoare carrying out the work of the enterprise."96 A nondelegable duty may ariseby statute, contract, or common law.97 The courts' imposition of anondelegable duty presupposes that, in certain circumstances, the employerof the independent contractor is "in the best position to identify, minimize,and administer the risks involved in the contractor's activities."98
An employer of an independent contractor has a nondelegable duty of
care for delegated work negligently performed by the contractor99 when thebusiness enterprise engaged in is either (1) inherently dangerous100 or
those patchy regulations provide opportunities to introduce tainted drugs in areas with less stringentregulation. These drugs then flow undetected through the tributaries of the national distribution system.
See deKieffer, supra note 32, at 330-31.
An employer who hires an independent contractor is generally not liable for the contractor's
negligence. RESTATEMENT (SECOND) OF TORTS § 409 (1965); see also Pusey v. Bator, 762 N.E.2d 968,972 (Ohio 2002) (stating that "an employer is generally not liable for the negligent acts of an independentcontractor"); Sloan v. Atl. Richfield Co., 552 P.2d 157 (Alaska 1976) (holding that no wrongful deathrecovery should be imposed in favor of an employee of an independent contractor against thepossessor/owner of land upon a theory of vicarious liability). But cf. Privette v. Super. Ct., 854 P.2d 721,724 (Cal. 1993) (observing that the nonliability rule is "now primarily important as a preamble to thecatalog of its exceptions" (citing Van Arsdale v. Hollinger, 437 P.2d 508 (Cal. 1968))).
Policy reasons have been the basis for creation of exceptions to the general premise of
nonliability. See Privette, 854 P.2d at 724.
DAN B. DOBBS, THE LAW OF TORTS § 337 (2000).
41 AM. JUR. 2D, Independent Contractors § 43 (1995); see also Majorowicz v. Allied Mut. Ins.
Co., 569 N.W.2d 472, 476 (Wis. Ct. App. 1997). In fact, Congress could codify a nondelegable duty ofsafe distribution for both manufacturers and all distributors, adopting the same rationale articulated bycourts in this section.
Wilson v. Good Humor Corp., 757 F.2d 1293, 1301 (D.C. Cir. 1985).
41 AM. JUR. 2D, Independent Contractors § 43 (2005).
100. DOBBS, supra note 96, at § 337. "Inherently dangerous" has also been defined as "unusually
hazardous." See, e.g., Wilson, 757 F.2d at 1303.
UNIVERSITY OF PITTSBURGH LAW REVIEW
generically hazardous activity,101 or (2) poses a peculiar risk of substantialharm to the public absent special precautions.102 Although some courts haveblurred the boundaries of nondelegable duties by defining an inherentlydangerous or generally hazardous activity as an activity which poses a peculiarrisk of substantial harm in the absence of special precautions,103 betterreasoning keeps this category distinct.104 When an activity poses a great riskof harm to the public against which the public has little ability to protect itselfbecause of the specialized knowledge involved in an industry, that activityshould not need to be "inherently dangerous" to trigger a nondelegable duty.
A business enterprise is inherently dangerous if danger exists in doing the
activity, regardless of the method used or how skillful the independentcontractor may be at the given activity.105 Conversely, an activity is notinherently dangerous if proper precautions can minimize the risk of injury.106Typically associated with strict liability, inherently dangerous work does not
101. Wilson, 757 F.2d at 1303-04 (holding that the nondelegable duty of care for inherently
dangerous activity clearly applies to generically hazardous work, but is not limited to that category).
102. The Restatement (Second) of Torts clarifies the concept of a nondelegable duty when enterprise
activity poses the special risk of physical harm:
One who employs an independent contractor to do work which the employer should recognizeas likely to create during its progress a peculiar risk of physical harm to others unless specialprecautions are taken, is subject to liability for physical harm caused to them by the failure ofthe contractor to exercise reasonable care to take such precautions, even though the employerhas provided for such precautions in the contract or otherwise.
RESTATEMENT (SECOND) OF TORTS § 416 (1965).
103. "Work is inherently dangerous when it creates a peculiar risk of harm to others unless special
precautions are taken." Pusey v. Bator, 762 N.E.2d 968, 973 (Ohio 2002) (holding that hiring armedguards to protect property was inherently dangerous activity as the employer could foresee that an armedconfrontation might be required to do the job). Some courts have blurred the distinction between work ofa peculiar risk and inherently dangerous work when discussing the finding of a nondelegable duty. See,e.g., Privette v. Super. Ct., 854 P.2d 721, 723 (Cal. 1993) ("Under the peculiar risk doctrine, a person whohires an independent contractor to perform work that is inherently dangerous can be held liable for tortdamages when the contractor's negligent performance of the work causes injuries to others.").
104. See Wilson, 757 F.2d at 1303 (reasoning that cases should be read to suggest that two distinct
types of independent contractor activity can bring an employer within the nondelegable duty exception tovicarious liability).
105. See RESTATEMENT (SECOND) OF TORTS § 427 cmt. b (1965). The comment reads:
It is not, however, necessary to the employer's liability that the work be of a kind which cannotbe done without a risk of harm to others, or that it be of a kind which involves a high degreeof risk of such harm, or that the risk be one of very serious harm, such as death or serious bodilyinjury. It is not necessary that the work call for any special skill or care in doing it. It issufficient that work of any kind involves a risk, recognizable in advance, of physical harm toothers which is inherent in the work itself.
106. See id. § 427.
require negligence on the part of the independent contractor to render theprincipal directly liable.107
An activity represents a peculiar risk in situations where the activity will
likely cause injury to others unless special precautions are taken.108 A peculiarrisk includes a danger resulting directly from the work done and not from thecollateral negligence of the contractor in the operative, easily controlleddetails of the work.109 However, no bright-line rule exists to define when anactivity represents a peculiar risk.110 The peculiar risk must be foreseeable tothe employer at the time of contracting,111 and the activity must present aprobability of harm to others, not just a possibility.112 The peculiar riskdoctrine differs from the general concept of a nondelegable duty in that apeculiar risk does not require abnormally great risk to render an employervicariously liable for the negligence of a contractor.113
Because counterfeit medicine poses a peculiar risk of harm to patients,
courts could hold that manufacturers of pharmaceuticals that sell to authorizeddistributors—who, in turn, buy and sell from other wholesalers—bear anondelegable duty to ensure the safety and integrity of the medicines theypeddle.114 Manufacturers and authorized distributors have contractual
107. Privette, 854 P.2d at 723.
108. Restatement (Second) of Torts provides that a "peculiar risk" arises when a risk is "peculiar to
the work to be done and aris[es] out of its character, or out of the place where it is to be done, against whicha reasonable [person] would recognize the necessity of taking special precautions." RESTATEMENT(SECOND) OF TORTS § 413 cmt. b (1965).
109. Routine, predictable dangers are not peculiar risks, as employers can expect the contractor to
follow recommended safety procedures to avoid routine dangers. In Sievers v. McClure, the court refusedto hold an employer liable for the death of a contractor's employee, declining to characterize the danger offalling off of a sloped roof during a roofing contract as a "peculiar risk," because no reasonable mind couldfind that the risk of falling was abnormal for such a contract. 746 P.2d 885, 888-90 (Alaska 1987).
110. See Wilson v. Good Humor Corp., 757 F.2d 1293, 1304 n.10 (D.C. Cir. 1985); Red Roof Inns,
Inc. v. Purvis, 691 N.E.2d 1341, 1344-46 (Ind. Ct. App. 1998) (analyzing the nature of the work and theconditions under which it was performed to determine whether the risk of a roofer falling off a roof is anordinary or peculiar risk).
111. See E. Airlines v. Joseph Guida & Sons Trucking Co., 675 F. Supp. 1391, 1395-96 (E.D.N.Y.
1987) (holding that the job of driving through an airport at night in order to deliver top soil was not aninherently dangerous activity for a truck driver).
112. See Pusey v. Bator, 762 N.E.2d 968, 973 (Ohio 2002) ("The exception does not apply, however,
where the employer would reasonably have only a general anticipation of the possibility that the contractormay be negligent in some way and thereby cause harm to a third party.").
113. See Privette v. Super. Ct., 854 P.2d 721, 725 n.2 (Cal. 1993).
114. Authorized distributors and secondary wholesalers that buy directly from a contaminated source
would be negligent themselves and thus bear direct liability.
UNIVERSITY OF PITTSBURGH LAW REVIEW
relationships with one another,115 and distributors frequently buy and sellmedicines among themselves.116
Both inter-relationships appear very similar to the long-term,
independent-contractor relationship between Good Humor Corporation andthe vendors that sold its ice cream,117 providing an interesting analogy forcourts to examine when considering vicarious liability claims againstmanufacturers and authorized distributors. In Wilson v. Good Humor Corp.,a three-year-old child was struck and killed by traffic when she attempted tocross the street to a Good Humor truck that was vending ice cream by the sideof the road.118 The court held that Good Humor (the employer) could be suedfor the child's death given the peculiar risk involved in vending ice cream tochildren on busy, car-laden streets by trucks playing music to attractcustomers.119 Because Good Humor knew or had special reason to know ofthe risks to children that were likely to arise from selling ice cream in thestreet, it could be sued directly.120 Just as the vendors in Wilson performed thebulk of Good Humor's business in a setting of foreseeable peculiar risk—acurbside littered with children121—drug distributors now perform the bulk ofthe drug manufacturers' business in a market filled with counterfeit,adulterated, and diverted drugs.
Just as Good Humor chose not to inform its vendors or take precautions
against the known and specific dangers to children who purchase ice creamfrom its vendors,122 drug manufacturers and large distributors have chosen todisclaim responsibility and failed to take adequate precautions against theknown and specific danger posed by fake drugs in the market.123
115. In order to be an "authorized distributor" under federal law, the distributor must have a
contractual relationship with the manufacturer. 21 C.F.R. §§ 203.3(b), (u) (2007).
116. See supra note 17 and accompanying text.
117. Wilson v. Good Humor Corp., 757 F.2d 1293, 1306 (D.C. Cir. 1985); Contra Rivera v. Flav-O-
Rich, 876 F. Supp. 373 (D.P.R. 1995). In Contra Rivera, the court held that an ice cream truck owner wasnot the ice cream manufacturer's "independent contractor," but merely a purchaser of its products. 876 F.
Supp. at 383-84. Thus, the manufacturer could not be liable for the alleged negligence of the truck owner'semployee in connection with a child's fall from the truck. Id. The court reasoned that, despite the fact thatthe owner regularly purchased significant amounts of the manufacturer's products, the manufacturer hadno control over the manner, means, or results of the owner's work, since no forward sales contract existedbetween the parties and the truck owner was not restricted from selling other manufacturers' products. Id.
118. Id.
119. Id. at 1305.
120. Id.
121. Wilson, 757 F.2d at 1306.
122. Id.
123. Instead, manufacturers and distributors have fought the establishment of a reasonable federal
pedigree requirement for nearly twenty years and have engaged only in brief forays over the last two years
Undoubtedly, given their substantial financial resources, manufacturers andlarge distributors have the best knowledge of the dangers of drug distribution.
They are also in the best position to identify the precautions that are necessaryto safely perform the job.124 This "top-down" approach to vicarious liabilitytherefore ensures that those manufacturers and authorized distributors thatunscrupulously barter for the lowest-cost drugs without taking precautions toensure drug integrity and safety do not escape liability.
To protect themselves against vicarious liability and to satisfy their
nondelegable duty of care, manufacturers and distributors would only need totake those special safety precautions that are readily available to them.125Numerous such precautions exist to keep counterfeit, adulterated, and diverteddrugs out of the legitimate stream of commerce. First, manufacturers anddistributors could merely acquiesce to a pedigree requirement, eitherelectronic or paper, beginning with the manufacturer and exempting no partyin the chain of distribution.126 Many technologies—including lower costsolutions such as bar coding127 and covert encoded inks,128 as well as highercost solutions like Radio Frequency Identification Technology (RFID)129 and
into "track and trace" technology that might record the distribution of drugs. Michael A. Gips, DrugTracking Heats Up, SEC. MGMT., May 2007, at 18, 18-19.
124. Wilson, 757 F.2d at 1306.
125. Although I do sit on the Advisory Board for Secure Symbology, this article does not advocate
that any one of the listed options for improving the security of the drug supply should be mandatory orpreferred by the courts, the FDA, or Congress. Companies should exercise their autonomy and select thesafety procedure that meets their own corporate needs so long as it adequately safeguards patients' health.
126. This would stand in contrast to the current requirements which, if enforced, would still exempt
manufacturers and all authorized distributors from a pedigree requirement. See 21 C.F.R. § 203.50 (2007).
127. Secure Symbology provides unique, multi-dimensional bar codes that help track a drug to the
point of sale for use on individual units of drugs, cases of drugs, pallets of drugs, and larger containers ofdrugs. Describing one of its products, the ESC System, the company states that:
Secure Symbology Inc.'s ESC System provides "track and trace" reliability from production topharmacist, assuring consumer protection, while meeting the stringent compliance requirementsof federal and state regulatory agencies. It accomplishes this by using globally acceptedmachine-readable bar code symbologies, and/or the emerging RFID technology, within thecontext of SSI's patented and novel utilization of construction, deconstruction, andreconstruction of databases.
Secure Symbology, Secure Health, http://securesymbology.com/health.asp (last visited Jan. 23, 2008).
128. Inksure Technologies stated that it offers a covert, encoded, machine-readable unique ink that
a companion scanner detects during inventory control. Inksure Technologies, Inksure's Security Approachfor Our Customers, http://www.inksure.com/default.asp?psys=solutions (last visited Jan. 23, 2008).
129. RFID is defined as the following:
[A] generic term for technologies that use radio waves to automatically identify people orobjects. There are several methods of identification, but the most common is to store a serialnumber that identifies a person or object, and perhaps other information, on a microchip thatis attached to an antenna (the chip and the antenna together are called an RFID transponder or
UNIVERSITY OF PITTSBURGH LAW REVIEW
DNA-embedding technologies130—are now available for drug industryparticipants, enabling them to "track and trace" the source and distribution ofmedication and produce electronic pedigrees.131 A multi-pronged, layered useof multiple technologies and a mandatory pedigree requirement (withoutexempt parties) would provide the most security.132
Manufacturers and distributors are well aware of these technologies. The
pharmaceutical companies even choose to dabble in technology pilotprojects.133 Nonetheless, those same parties have failed to use any of the
an RFID tag). The antenna enables the chip to transmit the identification information to areader. The reader converts the radio waves reflected back from the RFID tag into digitalinformation that can then be passed on to computers that can make use of it.
RFID Journal, Frequently Asked Questions, http://www.rfidjournal.com/faq/16/49 (last visited Jan. 23,2008). Hundreds of companies market one form of RFID product or another. See RFID Journal, FrequentlyAsked Questions, http://www.rfidjournal.com/faq/16/53 (last visited Jan. 23, 2008).
130. Applied DNA Sciences offers markers for implementation that uniquely identify a real product
from a counterfeit. Describing its patented SigNature markers, the company stated:
[W]e use DNA segments from one or more botanical sources, rearrange them into uniqueencrypted sequences, and then implement one or more layers of anti-counterfeit techniques.
Because the portion of DNA in a SigNature DNA Marker used to identify the marker is sominute, it cannot be detected unless it is replicated billions of times over, or amplified. Thisamplification can only be achieved by applying matching strands of DNA, or a primer, andPCR techniques to the SigNature DNA Marker. The sequence of the relevant DNA in aSigNature DNA Marker must be known in order to manufacture the primer for that DNA.
Applied DNA Sciences, The SigNature Program, http://www.adnas.com/signature (last visited Jan. 23,2008).
131. Electronic pedigrees can be produced once a manufacturer or distributor employs a tracker, such
as a bar code or RFID, a data management base, and a communications mechanism to relay informationto partners. See VeriSign, Supply Chain Information Center, http://www.verisign.com/global-consulting/supply-chain-services/supply-chain-information/ (last visited Jan. 23, 2007). VeriSign markets anElectronic Pedigree Service that it states can manage these three components efficiently. Id. SupplyScapesells a computer software application, Supplyscape E-pedigree, which works in conjunction with othertechnology to produce pedigree papers electronically, cutting down the cost and amount of human hoursnecessary to provide source documentation for drugs. SupplyScape, Product Security e-Pedgree Overview,http://www.supplyscape.com/products/security/pedigree/index.html (last visited Jan. 23, 2008).
132. Purdue Pharma's model for tracking Oxycontin provides a useful model for tracing drugs:
Purdue Pharma's system consists of ultrahigh-frequency (UHF) tags and readers, devicemanagement software, and data management software (Class 0 UHF tags and readers, SymbolTechnologies, Holtsville, NY; device-management software, Northern Apex Software, FortWayne, IN; "Auto-ID" and "Event Manager" software from SAP AG, Walldorf, Germany).
This system interacts with the company's enterprise resource-planning system ("R/3 ERP," SAPAG) and makes it possible to link each bottle of OxyContin to a specific case and pallet.
Hallie Forcinio, Tagging Tools to Provide E-Pedigree, PHARMACEUTICAL TECH., Sept. 2, 2006, at 44, 44,available at http://www.pharmtech.com/pharmtech/article/articleDetail.jsp?id=371131.
133. Cardinal Health has begun a pilot project but expressed that until stakeholders across the
industry achieve a global standard, it would have to rely on "direct distribution," which Cardinal did notfurther define. Pharmaceutical RFID Pilot Finds Promise, Problems, RFID UPDATE, Nov. 15, 2006,http://www.rfidupdate.com/articles/index.php?id=1246 (last visited Jan. 23, 2008). AmerisourceBergen
technologies in a widespread manner. Even the FDA has scolded drugmanufacturers and distributors for not implementing readily availabletechnological advances.134
The risk of counterfeit drugs reaching patients has long surpassed a mere
possibility.135 In the wake of this reality, wholesalers still resist pedigreelaws,136 and manufacturers continue to view wholesale distributors as theirprimary concern137 rather than worrying about the safety of patients. Patientsshould begin suing manufacturers and distributors for injuries caused bycounterfeit drugs until the drug industry satisfies its nondelegable duty of careby implementing technological security measures and acquiescing to the fullimplementation of pedigrees. The drug manufacturers and distributors cansatisfy their obligation to protect the public and cut off liability by merelyimplementing the special precautions that exist.
The drug industry is unique and well suited for the application of the
peculiar risk doctrine. No other industry serves a population already
announced it would begin an RFID pilot project in December 2006. Innovative Track and Trace Program,INSIGHTS (AmerisourceBergen, Valley Forge, PA), Dec. 2006, at 1, 1, available at http://www.amerisourcebergen.com/cp/1/docs/related_resources/retail_pharmacies/INSIGHTS/Dec_06_Insights_FINAL.pdf. Purdue Pharma exclaims that it is the only pharmaceutical company to "implementfully" an RFID "track and trace" program. Michael A. Gips, supra note 123, at 18, 18-19. However,Purdue Pharma has only fully implemented that system for a single drug, Oxycontin. Forcinio, supra note132, at 44. In 2004 and 2005, Pfizer also conducted a year-long bar code and RFID pilot on the single drugViagra. Tim Marsh, Viagra RFID: One Year Later, RFID UPDATE, May 23, 2007,http://www.rfidupdate.com/articles/index.php?id=1366 (last visited Jan. 23, 2008). Even though the pilotwas successful, Pfizer only announced plans to further implement the technology by tracking the single drugViagra in pallet and case quantities. Id. Pfizer markets over 50 different name-brand medications. Pfizer,Pfizer Pharmaceutical Products, http://www.pfizer.com/products/rx/rx.jsp (last visited Jan. 23, 2008).
134. A recent Information Week article reported the following:
[P]harma's use of RFID today remains limited to a handful of test projects. An FDA report thismonth, while calling those efforts encouraging, chastises drugmakers, wholesalers, and retailersfor being slow to implement "e-pedigree" systems such as bar codes or RFID to prevent drugcounterfeiting. The report singled out RFID adoption in particular: "We are disappointed withthe lack of overall progress across the drug supply chain."
Time for the pharmaceutical industry to pick up the pace, amid a growing number of FDA
and state government mandates for drug pedigree systems.
Rick Whiting, FDA Scolds Drug Industry for Anemic RFID Adoption, INFO. WEEK, June 19, 2006, at 28,28 available at http://www.informationweek.com/story/showArticle.jhtml?articleID=189500084.
135. See supra Section I.
136. See, e.g., RxUSA Wholesale, Inc. v. Dep't of Health & Human Servs., 467 F. Supp. 2d 285
(E.D.N.Y. 2006), discussed supra pp. 255-56.
137. After I testified to the Senate Judiciary Committee in opposition of amending Florida's pedigree
definition in 2006, a representative from Astra Zeneca said that, although she understood my concern aboutdrug integrity, the manufacturer could not publicly oppose the will of distributors who wanted to waterdown the pedigree since "the distributors were [their] consumers."
UNIVERSITY OF PITTSBURGH LAW REVIEW
compromised by health conditions and so manifestly vulnerable. The dangersposed by counterfeit drugs range from death by poisoning, at the worst, to theingestion of non-active ingredients by sick consumers, which itself can leadto a patient's decline, eventual illness, and even death. Additionally, the inter-relationship of manufacturers—which as patent holders stand in a uniqueposition as sole suppliers of a medicine or a group of medicines—and thedistributors—which serve them further—illustrates the need for a specialliability standard.
V. "GOING GREEN": WHY CODIFYING A STRICT LIABILITY STANDARD FOR
THE INCIDENCE OF COUNTERFEIT DRUGS MAKES SENSE
Beyond the vicarious liability option outlined above, another approach to
secure the drug supply chain would be for Congress to enact legislationimposing strict liability on manufacturers and distributors for injuries causedby counterfeit drugs. Environmental laws already impose strict liability forinjuries caused by the distribution of hazardous chemical byproducts oflegitimate industry.138 These laws, driven by a concern for the public health,provide both an analogy and a blueprint for Congress to create a strict liabilityscheme for injuries caused by the distribution of counterfeit drugswhich—like hazardous waste—are harmful chemicals representing thebyproduct of a legitimate industry.139
138. See, e.g., Resource Conservation and Recovery Act, 42 U.S.C. §§ 6901-6992k (2000);
Comprehensive Environmental Response Compensation and Liability Act, 42 U.S.C. §§ 9601-9675 (2000).
139. The RCRA allows private citizens to bring suit against a past or present generator, operator, or
owner who has contributed or is contributing to the handling, storage, treatment, transportation, or disposalof waste which may present an imminent and substantial endangerment to health or the environment. Id.
§ 6972(a)(1)(B). The EPA Administrator can bring similar suits under the RCRA when health isendangered. Id. § 6973(a). CERCLA also focuses on the threat to public health as the trigger for action,including when the President may abate activity which poses such a threat:
[W]hen the President determines that there may be an imminent and substantial endangermentto the public health or welfare or the environment because of an actual or threatened release ofa hazardous substance from a facility, he may require the Attorney General of the United Statesto secure such relief as may be necessary to abate such danger or threat.
42 U.S.C. § 9606(a) (2000).
A. Strict Liability for Injury Caused by the Distribution of HarmfulChemical Byproducts
The RCRA governs the treatment, handling, and disposal of solid and
hazardous waste.140 The purpose of the RCRA was to "empower[] [the] FDAto regulate hazardous wastes from cradle to grave, in accordance with . .
rigorous safeguards."141 The RCRA is largely a "forward looking" statute inthat most of its provisions attempted to create a regulatory scheme for futurestorage, treatment, transport, and disposal of waste.142
In addition to promulgating a pro-active regulatory scheme, however, the
RCRA establishes strict liability for past or present waste generators,transporters, owners, and treatment plant operators whose handling of wastemay present an imminent and substantial endangerment to public health or theenvironment.143 The RCRA gives both the government and private citizens theright to seek judicial relief to avert imminent and substantial threats to thepublic health.144 The RCRA also allows the EPA or a private citizen to obtainan injunction when any person shows an endangerment to the public health.145Congress intended the RCRA, as initially enacted and then as later amendedin 1984, to impose strict liability without reference to fault or negligence.146
140. See Meghrig v. KFC W., Inc., 516 U.S. 479, 483 (1996) (citing Chicago v. Envtl. Def. Fund,
511 U.S. 328, 331-32 (1994)). The definition of what constitutes hazardous waste in the environmentalcontext is extensively governed by the RCRA itself and by EPA Guidance documents. See OFFICE OFENFORCEMENT & COMPLIANCE ASSURANCE, U.S. ENVTL. PROT . AGENCY , GUIDANCE ON THE USE OFSECTION 7003 OF RCRA 14-16 (1997), available at http://www.epa.gov/compliance/resources/policies/cleanup/rcra/971020.pdf (superseding the Final Revised Guidance Memorandum on the Use andIssuance of Administrative Orders Under Section 7003 of the Resource Conservation and Recovery Act,which was issued by the E.P.A. on Sept. 26, 1984). This article presumes that counterfeit, adulterated, ordiverted medicine is hazardous to human health because it does not, at the very least, meet FDArequirements for human consumption in its most innocuous form. Id.
141. Chicago v. Envtl. Def. Fund, 511 U.S. 328, 331 (1994).
142. ROGER W. FINDLEY & DANIEL A. FARBER, ENVIRONMENTAL LAW IN A NUTSHELL 191 (5th ed.
143. See 3 SUSAN M. COOKE, THE LAW OF HAZARDOUS WASTE: MANAGEMENT, CLEANUP, LIABILITY,
AND LITIGATION § 15.01[3][a], at 15-6. (2001) ("[L]iability under Sections 7003 and 7002(a)(1)(B) attachesto any person who (1) has contributed or is contributing to (2) the past or present handling, storage,treatment, transportation, or disposal of (3) any solid or hazardous waste which (4) presents an imminentand substantial endangerment to health or the environment.").
144. The EPA would bring suit on these grounds under 42 U.S.C. § 6973, while private citizens
would bring such a strict liability suit under § 6972. See 42 U.S.C. §§ 6972-73 (2000).
145. Id.; see also United States v. Bliss, 667 F. Supp. 1298, 1313 (E.D. Mo. 1987).
146. Bliss, 667 F. Supp. at 1313.
UNIVERSITY OF PITTSBURGH LAW REVIEW
CERCLA is "backwards looking," as the provisions are not regulatory in
nature and instead focus on creating broad civil liability for the cleanup ofleaking sites of waste treatment, transport, storage, and disposal.147 CERCLAprovides that the federal government, states, or private citizens may bringactions to recover costs incurred in responding to the release of hazardoussubstances.148 CERCLA joins the RCRA in imposing liability without regardto a liable party's fault or state of mind.149 Responsible parties may be jointlyand severally liable, although liability may also be apportioned amongdefendants in appropriate cases.150 The intent of including a strict liabilitystandard in CERCLA and not allowing parties to contract out of that liabilitywas to ensure that economic power did not enable manufacturers to shiftliability for harm to those transporting, distributing, or treating the hazardousmaterial.151
147. See United States v. Ne. Pharm. & Chem. Co., 810 F.2d 726, 740 (8th Cir. 1986) (holding that
CERCLA applies retroactively and that the RCRA imposes strict liability upon past off-site generators andtransporters of hazardous substances); see also Bliss, 667 F. Supp. at 1313 (stating that the RCRA imposesliability without fault or negligence and applies to present conditions resulting from the past activities ofpast off-site generators and transporters); COOKE, supra note 143, § 15.01[4], at 15-13 n.55 (citing thecongressional discussion of the standard "regardless of fault or negligence" and the statement that "non-negligent generators whose wastes are no longer being deposited at a particular site may be ordered to abatethe hazard to health or the environment posed by the leaking of the wastes they once generated and whichhave been deposited at the site." (citing H.R. REP. NO. 98-198, pt. 1, at 47-49 (1983), as reprinted in 1984U.S.C.C.A.N. 5576, 5609-09)).
148. FINDLEY & FARBER, supra note 142, at 191.
149. See COOKE, supra note 143, at § 14.01[1], 14-10.1-19.
150. Bliss, 667 F. Supp. at 1313 (holding that the generators of waste, the company that arranged for
the transportation of the waste, and the company which stored and sprayed oil on the container of wastewere all jointly and severally liable for the expense of the government's cleanup of dioxin and TCP to abatethe release of hazardous substances). But cf. United States v. A & F Materials Co., 578 F. Supp. 1249,1258 (S.D. Ill. 1984) (holding that Congress intended courts to apply the equities of each case whenapportioning fault amongst defendants); see also COOKE, supra note 143, at § 14.01[2][c], at 14-20.
151. In United States v. Price, the court noted:
[T]he strict liability standard fits most closely with the legislative aims of CERCLA whichinclude goals such as cost-spreading and assurance that responsible parties bear their cost ofthe clean up. The fulfillment of these Congressional goals is more likely to be effectuated if thedefendants who allegedly contributed to the environmental mess are now held to a verystringent standard of liability. Though strict liability may impose harsh results on certaindefendants, it is the most equitable solution in view of the alternative—forcing those who bearno responsibility for causing the damage, the taxpayers, to shoulder the full cost of the cleanup.
577 F. Supp. 1103, 1114 (D.C.N.J. 1983) (citations omitted). With Senator Randolph concurring, SenatorCannon discussed why section 107(e)(1) was enacted, stating:
It is my understanding that this section is designed to eliminate situations where the owner oroperator of a facility uses its economic power to force the transfer of its liability to otherpersons, as a cost of doing business, thus escaping its liability under the act all together.
Utilizing the same public-health rationale that motivated the imposition
of strict liability in both the RCRA and CERCLA, Congress could impose astrict liability scheme for manufacturers and distributors when counterfeitdrugs pass through the legitimate distribution system and injure patients. Thesimilarities between the two situations are manifold. The hazardous wastecontemplated by the environmental statutes and fake medicine are bothchemical compounds. Manufacturers of pharmaceutical drugs are similar tothe generators of industrial waste which, when making their legitimate andvaluable products, produce hazardous chemical byproducts. In the context ofgenerators, the chemical product is the hazardous waste itself. In thepharmaceutical context, the high drug prices set by manufacturers enticeprofit-seeking criminals to pollute the drug stream, creating the socialbyproduct of counterfeit drugs. Just as Congress did not allow generators ofhazardous waste to use their economic might to contract out of liability forhazards later caused by the disposal of that waste, so too should Congress holdmanufacturers responsible for injuries caused by counterfeit drugs rather thanallowing them to escape liability through the hiring of distributors.
Drug distributors should also be held jointly and severally liable for
injuries caused by counterfeit drugs since those distributors are similar to the"transporters" of hazardous waste covered by the environmental statutes.152Drug distributors can choose to buy medicine solely from manufacturers andthereby avoid sources which open doors for criminals to penetrate the drugsupply. Instead, distributors try to increase their profits by engaging inpharmaceutical arbitrage, in some cases buying medicines at costs even lowerthan those offered by the manufacturers of those drugs.153 Like transporterswho continually hauled waste to a contaminated landfill,154 distributors havecontinuously bought bad drugs from cheap suppliers, thereby reaping millionsof dollars and perilously endangering lives. Moreover, distributors choose not
126 CONG. REC. 30,984 (1980), reprinted in MARK E. ANTHONY REISCH, A LEGISLATIVE HISTORY OF THECOMPREHENSIVE ENVIRONMENTAL RESPONSE, COMPENSATION, AND LIABILITY ACT OF 1980, at 22 (1983).
In discussing both the Senate and the House's three key concerns with hazardous substance legislation,Representative Florio stated that the "absence of negligence is not a defense to liability," as CERCLAwould create a standard of strict liability qualified only by the narrow defenses contained within the statuteitself. Id. at 24 (quoting 126 CONG. REC. 31,965 (1980)).
152. Section 107(a) of CERCLA clearly imposes liability on transporters who select a treatment and
disposal site which later becomes hazardous. 24 U.S.C. § 9607(a)(4) (2000); United States v. Ne. Pharm.
& Chem. Co., 579 F. Supp. 823, 839, 844 (W.D. Mo. 1984) (confirming liability of transporters).
153. See supra Section II for discussion of authorized distributors' purchase practices.
154. See, e.g., United States v. Waste Indus., Inc., 734 F.2d 159 (4th Cir. 1984) (granting permanent
injunctive relief after it was shown that Waste Industries' operation of a landfill over several years led tothe burial of unknown quantities of solid and hazardous waste that contaminated the area's water supply).
UNIVERSITY OF PITTSBURGH LAW REVIEW
to employ new security measures such as bar coding or RFID, which makestheir inventory susceptible to penetration by criminals if even oneunscrupulous employee of a distributor allows a felon to introduce counterfeitdrugs into a warehouse.155
A federal statute holding drug manufacturers and distributors jointly and
severally liable could provide for both government-directed and private citizensuits. Following the RCRA's equitable remedy model, when the FDA or statepharmaceutical agencies discover that counterfeit drugs have been releasedinto the legitimate drug supply, the statute could enable the FDACommissioner or a private citizen to seek an injunction directing themanufacturer of the drug and all distributors to remove the lot number (or aseries of lot numbers) from the marketplace. Additionally, followingCERCLA's paradigm of seeking clean-up costs, a federal statute could allowthe FDA and private citizens to recoup, when relevant, the costs ofinvestigation, prosecution, and personal injury related to the counterfeit drugs.
B. Defining an Imminent and Substantial Endangerment to Health
Of critical importance to any proposed federal drug statute would be
defining a trigger event indicating when counterfeit, adulterated, or divertedmedicine presents an imminent and substantial endangerment to health. TheRCRA's provisions allow the EPA or a private citizen to seek injunctive reliefwhen hazardous waste "may present" an imminent and substantialendangerment to the environment.156 The inclusion of the terms "maypresent" signified Congress's intent to provide courts with broad equitablepowers to abate any risk posed by toxic waste.157 However, the RCRA doesnot allow the EPA or a private citizen to enjoin hazardous waste activity when"the risk of harm is remote in time, completely speculative in nature, or deminimus in degree."158
155. See, e.g., Indictment, United States v. Carlow, No. 3:06-00047 (M.D. Tenn. Mar. 29, 2006),
available at http://www.dangerousdoses.com/pdf/Robert_Neil_Spence_Indictment%5B1%5D.pdf (allegingthat Neil Spence, a single employee at Cardinal Health, enabled felon Michael Carlow to sell thousands ofdollars worth of counterfeit drugs to the distributor which in turn sent the drugs out nationally to retailers).
156. The RCRA authorizes suit against those who handle hazardous waste in a manner which "may
present an imminent and substantial endangerment to health or the environment." See 42 U.S.C.
§§ 6972(a)(1)(B), 6973(a) (2000).
157. COOKE, supra note 143, § 15.01[3][e], at 15-11 (citing United States v. Waste Indus., Inc., 734
F.2d 159, 166 (4th Cir. 1984)); see also United States v. Price, 688 F.2d 204, 213 (3d Cir. 1982); UnitedStates v. Valentine, 856 F. Supp. 621, 626 (D. Wyo. 1994)).
158. H.R. REP. NO. 93-1185, at 36 (1974), as reprinted in 1974 U.S.C.C.A.N. 6454, 6488.
What constitutes an imminent and substantial endangerment under the
RCRA is an inherently fact-specific question.159 The Supreme Court notedthat an endangerment is "imminent" if it threatens to occur immediately, andthe term implies that the danger must be a present threat, "although the impactof the threat may not be felt until later."160 This "lenient standard"161 allowsgovernment intervention to prevent hazardous waste contamination fromhappening, although the latent harm to public health caused by thecontamination might not be evident for a substantial period of time.162Imminence must be construed with consideration of the time it takes foradministrative and judicial action, including the preparation, issuance, andenforcement of administrative court orders to achieve the legislative purposeof protecting public health.163
CERCLA does not define within its own provisions what constitutes an
"imminent and substantial" risk to public health.164 Actual harm need nothave occurred before the government or a private citizen can seek relief;165instead, a threatened or potential harm is sufficient to qualify as anendangerment.166 Under CERCLA, "a finding of endangerment requires theCourt to evaluate the nature and degree of the risk posed by the hazardoussubstances."167
For the proposed federal statute, Congress could implement the same
standard to trigger strict liability that it wrote into the RCRA andCERCLA—circumstances which may present an imminent and substantialendangerment to health. In its guidance memoranda, the FDA could propose
159. COOKE, supra note 143, § 15.01[3][e], at 15-11 (noting that the standard has not been
thoroughly explored by the courts). The difficulties of interpreting this statutorily undefined standard werecontemplated by my colleague, Joel A. Mintz. See Joel A. Mintz, Abandoned Hazardous Waste Sites andthe RCRA Imminent Hazard Provision: Some Suggestions for a Sound Judicial Construction, 11 HARV.
ENVTL. L. REV. 247 (1987).
160. Meghrig v. KFC W., Inc., 516 U.S. 479, 485-86 (1996).
161. Price, 688 F.2d at 211.
162. See id.; Cox v. Dallas, 256 F.3d 281, 299 (5th Cir. 2001).
163. H.R. REP. NO. 93-1185, at 36 (1974), as reprinted in 1974 U.S.C.C.A.N. 6454, 6488.
164. United States v. Conservation Chem. Co., 619 F. Supp. 162, 192 (W.D. Mo. 1985) (lamenting
that Congress gave no guidance as to the specifics of the factual circumstances that would constitute sucha danger under CERCLA).
165. Id. at 193 ("Because endangerment entails only a threat of harm, the endangerment provisions
‘have enhanced the courts' traditional equitable powers by authorizing the issuance of injunctions whenthere is but a risk of harm, a more lenient standard tha[n] [the] traditional requirement of threatenedirreparable harm.'" (quoting United States v. Price, 688 F.2d 204, 211 (3d Cir. 1982))).
166. Id. at 192 (citing Ethyl Corp. v. EPA, 541 F.2d 1 (D.C. Cir. 1976); Reserve Mining Co. v. EPA,
514 F.2d 492, 528 (8th Cir. 1975)).
167. United States v. Bliss, 667 F. Supp. 1298, 1314 (E.D. Mo. 1987).
UNIVERSITY OF PITTSBURGH LAW REVIEW
a list of non-exclusive factual scenarios which would highlight circumstanceswhere an individual or the government should know that counterfeit drugs"may present" such an endangerment to public health, although no actual harmto health has yet occurred. During the course of federal or state investigations,the discovery of counterfeit, adulterated, or diverted medicine should be asufficient trigger to enable the government or an individual to seek injunctiverelief to abate future harm to consumers. Discovery of counterfeit drug labelsshould also trigger liability and action. Finally, when breaches of security ateither manufacturing or distributing facilities are uncovered, the FDA or acitizen should have the power to ask a court to issue a recall of the tainteddrug by petitioning for injunctive relief.
Certainly, a patient suffering actual harm from counterfeit drugs would
satisfy the imminent and substantial endangerment standard. At that point,using CERCLA as the "clean up" model, a federal statute could allow thepatient to recoup her or his personal injury damages and likewise permit theFDA to recover the costs of conducting a recall of the drugs pursuant to acourt-issued injunction.
The FDA's Anti-Counterfeiting Task Force reports168 and state
experiences with the outbreak of drug counterfeiting, contamination, anddiversion169 demonstrate the risks posed to the public by tainted drugs. Theuse of independent contractors and the economic might of manufacturers andauthorized distributors are no longer adequate justifications for exemptingthese parties from liability for injuries caused by tainted products. ThisArticle suggested two paradigms of liability: Under one approach, vicariousliability would be judicially imposed based on manufacturers' and authorizeddistributors' nondelegable duty of safe distribution. Under another approach,Congress would espouse the commitment to protecting public healthembodied in environmental laws by imposing strict liability in situationswhere tainted drugs may present an imminent and substantial endangermentto health. Either of these paradigms would enhance patient safety. Thepurchase of drugs from a multiplicity of sources because of low prices and thefailure to take adequate precautions in an industry of peculiar risk should nolonger be economical for corporations. The liability system should favor
168. See supra Section I.
169. See supra Sections I-III(B).
protecting innocent, dependent patients over any corporate profit margin.
Mahatma Gandhi best articulated the need for the prioritization of the public'swell-being over monetary gain: "It is health which is real wealth and notpieces of gold and silver."170
170. See Mem'l Hosp. v. Maricopa County, 415 U.S. 250, 259 n.14 (quoting President Nixon's use
of Gandhi's quote in RICHARD NIXON, HEALTH, MESSAGE FROM THE PRESIDENT, H.R. Doc. No. 92-49, at18 (1st Sess. 1971)).
Source: https://lawreview.law.pitt.edu/ojs/index.php/lawreview/article/download/113/113
This article was originally published in a journal published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, fornon-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific
THE PAST HISTORY,PRESENT USES, ANDFUTURE OF DRUGS Chapter 1Introduction to Pharmacology and the History of Drugs Chapter 2Drug Design, Testing, Manufacturing, and Marketing Chapter 3Drug Forms Chapter 4Routes of Administration and the Drug Cycle Chapter 5Using Drugs Therapeutically





