
Ldh-buct.com
Ind. Eng. Chem. Res. 2009, 48, 5590–5597
Synthesis and Controlled Release Properties of Prednisone Intercalated Mg-
Al
Layered Double Hydroxide Composite
Fusu Li,† Lan Jin,† Jingbin Han,† Min Wei,*,† and Congju Li*,‡
State Key Laboratory of Chemical Resource Engineering, Beijing UniV
ersity of Chemical Technology,Beijing 100029, P. R. China, and College of Material Science and Engineering, Beijing Institute of FashionTechnology, Beijing 100029, P. R. China
A drug-inorganic composite involving prednisone-cholate ion micelles intercalated Mg-Al layered doublehydroxide (LDH) has been assembled by a coprecipitation method. Powder X-ray diffraction (XRD), Fouriertransform infrared (FT-IR), and UV-vis absorption spectroscopy indicate a successful intercalation ofprednisone-containing micelles into galleries of the LDH matrix. The in vitro drug release studies show thatno burst release phenomenon was observed at the beginning of release tests, and the pH value imposes verylittle influence on the release performance of prednisone in the studied pH range 4.8-7.6. It is, therefore,concluded that the MgAl-LDH can be used as an excellent inorganic drug carrier for prednisone in a widerange of pH values. Four kinetic models (first-order equation, Higuchi equation, Bhaskas equation, andRitger-Peppas equation) were chosen to study the release kinetics of prednisone from the LDH carrier, andit was found that this process can be described by the Ritger-Peppas equation satisfactorily based on adirecting Excel-based solver (DEBS). Moreover, the mechanism for drug release was also discussed.
therapeutics such as ibuprofen in controlled release systems.7,8Owing to the intercalation property of LDHs, many LDH
Many drugs have poor water solubility, leading to difficulties
compounds with intercalated beneficial organic anions, such as
in efficient dose delivery and unwanted side effects. An example
DNA,9-11 amino acid,12-16 anti-inflammatory drugs17,18 and
is prednisone, a drug of adrenocorticotro, which has been
plant growth regulators19 have also been prepared. Many reports
investigated as a possible therapeutic treatment for several forms
were focused on the study of drug-LDH hybrid materials to
of cancer. However, systemic drug administration results in
increase the bioavailability of poorly water-soluble, negatively
distribution of the drug throughout the patient's body through
charged, anti-inflammatory drugs. These drugs can be directly
blood circulation. This can lead to elevated drug concentrations
intercalated into the LDH galleries and then be released in
in undesired parts of the body with severe side effects.
molecular form. The composites have high chemical stability
Additionally, there are many cases where conventional drug
and can be maintained as long as 4 years.20
administration methods do not provide satisfactory pharmaco-
Although this scheme provides an interesting route to deliver
kinetic profiles because the drug concentration rapidly falls
negatively charged drugs based on LDH materials, the delivery
below desired levels. Therefore, a drug delivery and controlled
of nonionic, poorly water-soluble drugs remains a challenge.
release system is a more sophisticated drug administration
Tyner et al., who reported a new method, utilized LDHs to
method designed to overcome such problems.1 These systems
control nonionic, poorly water-soluble drug delivery.21,22 This
utilize carriers that slowly release their contents in order to
process involves first encapsulating the hydrophobic molecules
maintain drug concentrations at the desired levels for a longer
in an anionic micelle derived from a biocompatible surfactant,
period of time. At present, much attention has been paid to
and then the negative charge on the surfactant allows the uptake
polymers or various types of lipid vesicles and liposomes, as
of the drug-loaded micelle between the sheets of the LDHs by
drug carriers that form micro- or nanoparticles.2-5
an ion exchange process.
Recently, biocompatible inorganic materials, such as layered
In the present study, prednisone was selected as a model drug
double hydroxides (LDHs), are being used in drug delivery and
for nonionic, poorly water-soluble drugs. It was first encapsu-
controlled release systems. These materials are more stable and
lated in cholate ions micelles and then intercalated into MgAl-
less toxic than conventional drug carriers. LDHs consist of layers
LDH galleries by the method of coprecipitation. The physi-
of magnesium hydroxide, with aluminum isomorphically sub-
ological and biological importance of bile salts (sodium cholate,
stituted to give the layers a net positive charge. This charge is
for instance) lies in their ability in delivery systems for
balanced by interlayer hydrated anions, resulting in multiple
medicines, to solubilize and emulsify cholesterol, dietary lipids,
layers of alternating host layers and gallery anions. There has
and fatsoluble vitamins in the gastrointestinal tract.23 In this
been interest in the preparation of biomolecule-LDH complexes
work, cholate was chosen to form prednisone-containing mi-
for delivery systems. This approach has been used to success-
celles and then was further intercalated into the LDH matrix as
fully deliver pharmaceutically active molecules, such as grami-
a drug carrier. X-ray diffraction (XRD), Fourier transform
cidin, amphotericin B, ampicillin, and nalidixic acid.6 Similar
infrared (FT-IR), and UV-vis spectroscopy indicate a successful
systems based on LiAl-LDH have been studied to deliver
intercalation of this prednisone-cholate micelles. The releasebehavior of the resulting composite at different pH buffers has
* To whom correspondence should be addressed. Phone: +86-10-
been studied, demonstrating that this drug-micelle-LDH
64412131. Fax: +86-10-64425385. E-mail:
[email protected]
composite can be used as an excellent controlled release
(M.W.);
[email protected] (C.L.).
formulation in a wide range of pH values from 4.8 to 7.6.
Beijing University of Chemical Technology.
‡ Beijing Institute of Fashion Technology.
Moreover, four kinetic models (first-order equation, Higuchi
10.1021/ie900043r CCC: $40.75
2009 American Chemical Society
Published on Web 05/18/2009
Ind. Eng. Chem. Res., Vol. 48, No. 12, 2009
equation, Bhaskas equation, and Ritger-Peppas equation) were
2.4. Determination of Prednisone Loading. A known
chosen respectively to study the release kinetics of prednisone
weight of the LDH-cholate-prednisone composite (typically
from LDH carrier, and it was found that this process can be
10 mg) was dissolved by 5.0 mL of 1.0 M HCl solution and
described by the Ritger-Peppas equation satisfactorily based
then diluted to 10.00 mL by alcohol. The concentration of
on a directing Excel-based solver (DEBS). It can be therefore
prednisone was measured by UV-vis spectroscopy (
λmax: 244
expected that the method in this work provides a potential
nm) based on a multipoint working curve. Runs were performed
application in the field of controlled release for nonionic and
in triplicate.
2.5. In Vitro Drug Release Study. To measure the release
performances of prednisone from LDH-cholate-prednisone,
2. Materials and Methods
0.4 g composite powder was added in 900 mL of phosphate
2.1. Reagents. Prednisone (98% purity) was purchased from
buffer solutions (pH 4.8, 6.8, and 7.6, respectively) and was
J&K Chemical Ltd. and used as received; sodium cholate (98%
stirred at 37 °C. At specified time intervals, 5 mL of solution
purity) was purchased from Shanghai Sangon Biological
was removed and filtered through a 0.2
µm syringe filter. The
Engineering Technology Co., Ltd. Other inorganic chemicals
absorbance of the filtrate, at the
λ
max of prednisone, was
3)2 6H2O, Al(NO3)3 9H2O, NaOH, etc., were
measured and plotted as the relative release percentages of
of analytical grade and used without further purification.
Phosphate buffer solutions were used at 37 °C.
prednisone against time.
2.2. Determination of the Critical Micellar Concentra-
2.6. Characterization. Powder X-ray diffraction data were
tion (CMC) Value of Sodium Cholate. The formation of
recorded by a Shimadzu XRD-6000 power X-ray diffractometer
micelles was confirmed by using prednisone as a fluorescence
using Cu KR radiation (
λ ) 0.154 nm) at 40 kV, 30 mA, a
probe. Prednisone and sodium cholate were suspended in
scanning rate of 10° min-1, and a 2
θ angle ranging from 2° to
distilled water, and the pH of the stock solution was found to
70°. The sample of LDH-cholate-prednisone was also char-
be ∼8. The fluorescence spectra were measured with the
acterized on a Rigaku D/MAX2500 VB2+/PC X-ray diffrac-
concentration of sodium cholate varying from 8 to 32 mM (the
tometer under air condition, using Cu KR radiation (0.154184
prednisone concentration was 1.06 × 10-6 M). The excitation
nm) at 40 kV and 200 mA with a scanning rate of 5°/min, a
wavelength (
λex) is 283 nm.
step size of 0.02°/s, and a 2
θ angle ranging from 1.5° to 10°.
2.3. Synthesis of LDH-
Cholate-
Prednisone Composite.
UV-vis absorption spectra were performed on a Shimadzu UV-
Synthesis of LDH-cholate-prednisone by a coprecipitation
2501PC spectrometer. The Fourier transform infrared (FT-IR)
method was carried out as follows. Prednisone (30 mg, as a 2
spectra were recorded using a Vector 22 (Bruker) spectropho-
mg/mL solution in chloroform) was added to an aqueoussolution (200 mL) of sodium cholate (1.72 g, 20 mM) and stirred
tometer using the KBr pellet technique in the range 4000-400
cm-1 with 2 cm-1 resolution. Fluorescence measurements were
2 to allow for the evaporation of chloroform. When the
cholate micelles containing prednisone molecules have been
carried out with Shimadzu RF-5301PC spectrofluorimeter.
formed, an aqueous solution (25 mL) containing NaOH (0.32
Thermogravimetry and differential thermal analysis (TG-DTA)
g) and a solution (25 mL) containing Mg(NO
were carried out on a PCT-1A thermal analysis system under
3)3 9H2O (0.500 g) (initial Mg/Al ) 2.0) were
ambient atmosphere with a heating rate of 10 °C/ min. Elemental
simultaneously added dropwise into the micellar solution under
analyses were performed by inductively coupled plasma (ICP)
N2 atmosphere with vigorous stirring until the final pH of ca.
atomic emission spectroscopy using solutions prepared by
10 was obtained. The resulting slurry was aged at 70 °C for
dissolving the samples in dilute HCl. Carbon, hydrogen, and
60∼70 h. The product was filtered, washed thoroughly with
nitrogen analyses were carried out using a Perkin-Elmer
CO2-free water, and finally dried at 70 °C for 12 h. The product
Elementarvario elemental analysis instrument.
was denoted as LDH-cholate-prednisone.
Figure 1. (A) Emission spectra of prednisone with different concentrations of sodium cholate. (B) Intensity of prednisone at emission maximum (364 nm)
in the emission spectra as a function of concentration of sodium cholate at room temperature. [prednisone] ) 1.06 × 10-6 M; [sodium cholate] ) 8-32 mM;
λ
Ind. Eng. Chem. Res., Vol. 48, No. 12, 2009
Figure 4. UV-vis spectra of (a) prednisone solution, (b) prednisone-cholate
Figure 2. Powder X-ray diffraction patterns for (a) LDH-cholate and (b)
micelle, (c) LDH-cholate-prednisone after dissolution in solution (VHCl:
LDH-cholate-prednisone (for clarity, the XRD pattern in the 2θ range of
1:1), and (d) sodium cholate solution.
1.5-10° was displayed in the inset).
Figure 5. UV-vis spectra of the LDH-cholate-prednisone with the
concentration of (a) [cholate] ) 20 mM and (b) [cholate] ) 10 mM.
Figure 3. FT-IR spectra of (a) sodium cholate, (b) prednisone, (c)
3.2. Characterization of the LDH-Cholate-Prednisone
LDH-cholate-prednisone, and (d) LDH-cholate.
Composite. The powder XRD patterns of LDH-cholate and
LDH-cholate-prednisone are shown in Figure 2. The interlayer
3. Results and Discussion
distance d003 value, representing the combined thickness of the
3.1. Determination of CMC Value of Sodium Cholate. The
brucitelike layer (0.48 nm) and the gallery height, is a function
formation of cholate ion micelles was verified by a fluorescence
of the size and the orientation of intercalated anions.26 Compared
probe technique using prednisone.24 Although the reported CMC
with the LDH-cholate (Figure 2a, 2θ ) 2.299, d
values of sodium cholate range from 10 to 19 mM,24 it is
the basal reflection (003) of LDH-cholate-prednisone com-
necessary to determine the CMC value for this system first. The
posite (Figure 2b, 2θ ) 2.236, d
39.5 Å) shifts to a lower
fluorescence emission spectra of prednisone in the presence of
2θ angle. This may indicate the intercalation of prednisone-cholate
sodium cholate at a fixed λex of 283 nm are shown in Figure
micelle into galleries of LDH, which will be further confirmed
1A. It can be seen that the peak intensity decreases with the
in the next section.
increase of the concentration of sodium cholate, especially at364 nm. This is due to the fact that prednisone in water has a
Scheme 1. Possible Representation for the Structure of
strong emission peak at 364 nm, which decreases significantly
when it transfers into a hydrophobic environment. This phe-nomenon indicates the formation of micelles, which is inagreement with Small's model of micelle formation.25 Figure1B shows the effect of the sodium cholate concentration on theintensity of 364 nm for the emission spectrum. The intersectionpoint of the horizontal line and the bias can be defined as theCMC value, which was found to be 19 mM. As a result it canbe concluded that prednisone-containing cholate micelle isformed in aqueous solution with the CMC value of 19 mM.
Ind. Eng. Chem. Res., Vol. 48, No. 12, 2009
respectively. The spectrum of prednisone (Figure 3b) shows thestretching vibration of CdO at 1700 cm-1 and CdC fromcyclohexadiene at 1660 cm-1. The other absorption bands below1000 cm-1 are attributed to δ(C-H) deformation modes. Thespectrum of LDH-cholate (Figure 3d) displays characteristicbands of sodium cholate at 1584 and 1404 cm-1, confirmingthe intercalation of cholate ions. For the spectrum of LDH-cholate-prednisone (Figure 3c), both of the characteristic bandsof prednisone at 1700 and 1660 cm-1 and those of sodiumcholate at 1584 and 1404 cm-1 were observed. It was foundthat the IR absorption of prednisone is weak for the sample ofLDH-cholate-prednisone composite, which can be attributedto two possible reasons: (1) According to previous reports,27,28the movement of a drug molecule from a micelle core will beslower in comparison to the movement of drug out of core thatis more mobile, which could lead to the low IR absorption ofprednisone.(2)TheactualdrugconcentrationintheLDH-cholate-prednisone composite is low. This will be further discussed
Figure 6. Release profiles of prednisone from the composite in buffer
solutions at 37 °C with different pH values.
UV-vis spectroscopy was used to investigate whether
The FT-IR spectra of pristine sodium cholate, pristine
intercalation of prednisone into the LDH host was associated
prednisone, LDH-cholate-prednisone, and LDH-cholate are
with any change in its chemical composition or environment.
displayed in Figure 3. For the sake of clarity, only the main
Figure 4 shows the UV-vis spectrum of prednisone released
absorption bands were listed. In the spectrum of sodium cholate
from LDH-cholate-prednisone composite after dissolution in
(Figure 3a), the strong absorption bands at 1584 and 1404 cm-1
HCl-ethanol solution (Figure 4c), with the pristine prednisone
are characteristic of the stretching vibrations of CdO and OsH,
(Figure 4a), prednisone/cholate micelle (Figure 4b), and sodium
Figure 7. Plots of different kinetic models for the release of prednisone from the composite at pH 7.6.
Ind. Eng. Chem. Res., Vol. 48, No. 12, 2009
Figure 8. Plots of different kinetic models for the release of prednisone from the composite at pH 6.8.
cholate (Figure 4d) as comparison samples. It can be seen that
UV-vis spectroscopy based on a multipoint working curve. This
pristine prednisone exhibits a strong absorption band at 244 nm,
result is a little higher than other reports6,21 on encapsulating
while pristine sodium cholate displays no absorption from 200
hydrophobic drug molecules into LDH. Elemental analysis gave
to 400 nm. A band at 244 nm (Figure 4b) is noted for the
Mg 8.192%, Al 4.339%, C 48.95%, and H 7.684%. The
prednisone/cholate micelle. In the case of prednisone released
chemical composition for the LDH-cholate-prednisone com-
from the LDH-cholate-prednisone composite, an absorption
posite can be obtained: [Mg0.68Al0.32(OH)2](C21H26O5)0.022-
band at 245 nm (Figure 4c) was observed. Combined with the
24H39O5 )0.32 0.12H2O, based on the results of elemental
results obtained by fluorescence probe technique using pred-
analysis, ICP, TG-DTA, and UV-vis spectroscopy.
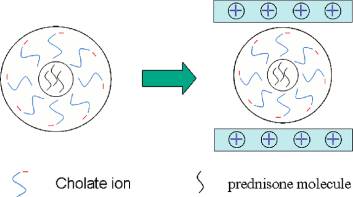
nisone (3.1 part), the XRD and FT-IR results mentioned above,
On the basis of the basal spacing d
the UV-vis results indicate that the prednisone-cholate micelle
003 of 39.5 Å for the
LDH-cholate-prednisone composite observed by XRD, the gal-
was successfully intercalated into the LDH host.
lery height was calculated to be 34.7 Å by subtracting the
It should be noted that the micellization of cholate is very
thickness of the inorganic layer (4.8 Å). The host-guest
important for prednisone to intercalate into LDH. The following
interactions for the composite consist of the electrostatic
experiments can verify it. For comparison, two composite
attraction between the positively charged LDH layers and the
samples of LDH-cholate-prednisone were synthesized by the
negatively charged micelles, as well as the hydrogen bonding
same method with the sodium cholate concentrations of 10 and20 mM, respectively, i.e., one concentration is lower than the
formed among the host layers, the guest anions and the interlayer
CMC value, and the other is higher than it. Equal weights of
water molecules. Taking into account the molecular dimensions
two samples were dissolved in solution (V
of cholate and prednisone (11.66 and 9.93 Å, respectively,
measured by UV-vis spectrometer, respectively (Figure 5). The
determined by the ChemWindow 6.0 software) and the existence
spectrum of LDH-cholate-prednisone ([cholate] ) 20 mM,
of the prednisone-cholate micelle, a schematic supramolecular
Figure 5a) displays a band at 245 nm attributed to prednisone,
structure of the LDH-cholate-prednisone composite was
while it is inconspicuous in the spectrum of LDH-cholate-
tentatively proposed and presented in Scheme 1.
prednisone ([cholate] ) 10 mM, Figure 5b). The comparison
3.3. In Vitro Drug Release Behavior. The drug release
study were indicates that the formation of prednisone-cholate
properties of prednisone from the LDH-cholate-prednisone
micelle is crucial for the preparation of LDH-based drug
composite have been investigated at a constant temperature of
37 °C. Figure 6 shows the release profiles of composite in
The drug loading for the LDH-cholate-prednisone com-
solution at pH 4.8, 6.8, and 7.6, respectively. It was found that
posite was determined to be 3.82% (w/w) by the method of
the rapid release during the first 40 min is followed by a slower
Ind. Eng. Chem. Res., Vol. 48, No. 12, 2009
Figure 9. Plots of different kinetic models for the release of prednisone from the composite at pH 4.8.
release of the drug, and equilibrium was achieved after ca. 150
from the LDH-cholate-prednisone composite is very compli-
min. In the case of pH 7.6 (Figure 6a), the released percentages
cated and not completely understood. According to the literature,
of 65% and 90% were obtained after 40 and 140 min,
a first-order equation (eq 1),32 the Higuchi equation (eq 2),33,34
respectively, and prednisone was completely released at ∼175
the Bhaskas equation (eq 3),32 and the Rigter-Peppas (RP)
min. For pH 6.8 and 4.8 (Figure 6b and c), the release rates are
equation (eq 4)35,36 with modification were chosen to study the
a little higher than that of pH 7.6, and complete release of
release dynamics of this system:
prednisone was observed at ∼105 min. Compared with the
release behavior based on LDH-drug composites reported
X ) 1 - e k(t-R)
previously,21 it is worth noticing that there is no burstphenomenon occurring at the beginning of all the release tests.
X ) k(t - R)1/2
It was also found that the pH value of the medium imposesvery little influence on the release performances of prednisone.
X ) 1 - e k(t - R)0.65
This is rather different from the release behavior of drugintercalated LDHs reported previously, in which lower pH leads
X ) k(t - R)n
to faster release of pharmaceutically active components fromLDH.19 In this work, prednisone is double protected from the
where �, t, k, and R are the release percentage, release time,
physiological environment in the LDH-cholate-prednisone
kinetic constant, and modified parameter, respectively. Here, n
composite, first by the organic environment of the micelles and
is an exponent, which is normally used to describe different
second by the durability of the LDH. Therefore, the release
release mechanisms. The value of n < 0.45 corresponds to the
process of the LDH-cholate-prednisone is controlled by the
drug diffusion control; n > 0.89 is attributed to the dissolution
synergistic effect of both the cholate micelle and LDH host,
of LDH particles; 0.45 < n < 0.89 is due to the cooperation of
demonstrating almost 100% release of prednisone and ap-
drug diffusion and LDH dissolution. A directing Excel-based
plicability in a wide pH range of 4.8-7.6.
solver (DEBS) was used in this work, and the equations were
The drug release based on the LDH-cholate-prednisone
evaluated by residual sum of squares (SUM, SUM ) Σ(X -
composite could be controlled by any of the following steps:
X′)2) and r (coefficient).
(1) dissolution of LDH particles;29,30 (2) ion-exchange reaction
On the basis of the four different kinetic models, the fitting
between prednisone-containing micelles and phosphate anions
results of drug release profiles at pH 7.6, 6.8, and 4.8 are given
in buffer solution;31 (3) disaggregation of the cholate micelle
in Figures 7, 8, and 9, respectively. The parameters of SUM,
and release of prednisone. The release mechanism of prednisone
R, n, and r are tabulated in Table 1. It can be seen from Table
Ind. Eng. Chem. Res., Vol. 48, No. 12, 2009
Table 1. Fitting Parameters of Drug Release Profiles to Different
(1) Kidane, A.; Bhatt, P. P. Recent advances in small molecule drug
parameter first-order eq Higuchi eq Bhaskas eq
delivery. Curr. Opin. Chem. Biol. 2005, 9, 347.
(2) Pojarova, M.; Ananchenko, G. S.; Udachin, K. A.; Daroszewska,
3.35 × 10-2 4.79 × 10-2
M.; Perret, F.; Coleman, A. W.; Ripmeester, J. A. Solid lipid nanoparticles
of p-hexanoyl calix[4]arene as a controlling agent in the photochemistry of
a sunscreen blocker. Chem. Mater. 2006, 18, 5817.
(3) Yoshida, R.; Sakai, K.; Okano, T.; Sakurai, Y. Drug release profiles
2.08 × 10-2 2.13 × 10-2
in the shrinking process of thermoresponsive poly (N-isopropylacrylamide-
co-alkyl methacrylate) gels. Ind. Eng. Chem. Res. 1992, 31, 2339.
(4) Ananchenko, G. S.; Udachin, K. A.; Pojarova, M.; Dubes, A.;
Ripmeester, J. A.; Jebors, S.; Coleman, A. W. Van der waals nanocapsular
7.29 × 10-3 1.33 × 10-2
complexes of amphiphilic calixarenes. Cryst. Growth Des. 2006, 6, 2141.
(5) Zhang, L.; Qian, Y.; Long, C.; Chen, Y. Systematic procedures for
formulation design of drug-loaded solid lipid microparticles: selection of
carrier material and stabilizer. Ind. Eng. Chem. Res. 2008, 47, 6091.
(6) Trikeriotis, M.; Ghanotakis, D. F. Intercalation of hydrophilic and
1 that the release of prednisone from LDH-cholate-prednisone
hydrophobic antibiotics in layered double hydroxides. Int. J. Pharm. 2007,
follows the RP equation very well at different pH values, with
332, 176.
satisfactory coefficients of 0.9977 (pH 7.6), 0.9923 (pH 6.8),
(7) Khan, A. I.; Lei, L.; Norquist, A. J.; O'Hare, D. Intercalation and
and 0.9937 (pH 4.8). The value of n is 0.277 (n < 0.45) at pH
controlled release of pharmaceutically active compounds from a layered
7.6, so the release mechanism corresponds to the drug diffusion
double hydroxide. Chem. Commun. 2001, 22, 2342.
(8) Ambrogi, V.; Fardella, G.; Grandolini, G.; Perioli, L. Intercalation
control. At pH 6.8 and 4.8, the values of n are 0.528 and 0.631
compounds of hydrotalcite-like anionic clays with anti-inflammatory agents:
(0.45 < n < 0.89), respectively, indicating that the drug release
I. Intercalation and in vitro release of ibuprofen. Int. J. Pharm. 2001, 220,
mechanism depends on the combination behavior control,
including dissolution of the composite, ion-exchange, and
(9) Kwak, S. Y.; Jeong, Y. J.; Park, J. S.; Choy, J. H. Bio-LDH
nanohybrid for gene therapy. Solid State Ionics 2002, 151, 229.
diffusion of prednisone. Because of the noncorrelative relation-
(10) Xu, Z. P.; Walker, T. L.; Liu, K.; Cooper, H. M.; Lu, G. Q. M.;
ship between the release behavior and pH value, it can be
Bartlett, P. F. Layered double hydroxide nanoparticles as cellular delivery
speculated that the synergistic effect of both cholate micelles
vectors of supercoiled plasmid DNA. Int. J. Nanomed. 2007, 2, 163.
and LDH host plays an important role in determining the drug
(11) Choy, J. H.; Kwak, S. Y.; Park, J. S.; Jeong, Y. J.; Portier, J.
release properties.
Intercalative nanohybrids of nucleoside monophosphates and DNA in
layered double hydroxide. J. Am. Chem. Soc. 1999, 121, 1399.
(12) Hibino, T.; Tsunashima, A. Synthesis of paramolybdate intercalates
of hydrotalcite-like compounds by ion exchange in ethanol/water solution.
Chem. Mater. 1997, 9, 2082.
A new delivery system for prednisone has been demonstrated
(13) Yuan, Q.; Wei, M.; Evans, D. G.; Duan, X. Preparation and
in this work. The drug was loaded into micelles first and then
investigation of thermolysis of L -aspartic acid-intercalated layered double
intercalated into the MgAl-LDH galleries by the method of
hydroxide. J. Phys. Chem. B 2004, 108, 12381.
coprecipitation. XRD, FT-IR, and UV-vis absorption spec-
(14) Hibino, T. Delamination of layered double hydroxides containing
troscopy indicate a successful intercalation of prednisone-
amino acids. Chem. Mater. 2004, 16, 5482.
(15) Aisawa, S.; Takahashi, S.; Ogasawara, W.; Umetsu, Y.; Narita, E.
containing micelles between the LDH layers. The in vitro release
Direct intercalation of amino acids into layered double hydroxides by
studies show that there is no burst phenomenon occurred at the
coprecipitation. J. Solid State Chem. 2001, 162, 52.
beginning of release tests at different pH buffers. Because of
(16) Nakayama, H.; Wada, N.; Tsuhako, M. Intercalation of amino acids
the synergistic effect of both cholate micelle and LDH host,
and peptides into Mg-Al layered double hydroxide by reconstruction method.
Int. J. Pharm. 2004, 269, 469.
the solution pH imposes very little influence on the release
(17) Mohanambe, L.; Vasudevan, S. Anionic clays containing anti-
performance of prednisone in the studied pH range 4.8-7.6.
inflammatory drug molecules: comparison of molecular dynamics simulation
Four kinetic models (first-order equation, Higuchi equation,
and measurements. J. Phys. Chem. B 2005, 109, 15651.
Bhaskas equation, and Ritger-Peppas equation) were used to
(18) Gu, Z.; Thomas, A. C.; Xu, Z. P.; Campbell, J. H.; Lu, G. Q. In
study the release dynamics of this system. The kinetic studies
vitro sustained release of LMWH from MgAl-layered double hydroxide
nanohybrids. Chem. Mater. 2008, 20, 3715.
by a directing Excel-based solver (DEBS) show that the release
(19) Hussein, M. Z. B.; Zainal, Z.; Yahaya, A. H.; Foo, D. W. V.
of prednisone from LDH-cholate-prednisone follows the RP
Controlled release of a plant growth regulator, a-naphthaleneacetate from
equation satisfactorily. At pH 7.6, the release mechanism
the lamella of Zn-Al-layered double hydroxide nanocomposite. J. Controlled
corresponds to drug diffusion control. At pH 6.8 and 4.8, the
Release 2002, 82, 417.
drug release depends on the combination behavior control,
(20) Ambrogi, V.; Fardella, G.; Grandolini, G.; Nocchetti, M.; Perioli,
L. Effect of hydrotalcite-like compounds on the aqueous solubility of some
including dissolution of the composite, ion-exchange, and
poorly water-soluble drugs. J. Pharm. Sci. 2003, 92, 1407.
diffusion of prednisone. As a result, this drug-containing micelle
(21) Tyner, K. M.; Schiffman, S. R.; Giannelis, E. P. Nanobiohybrids
intercalated LDH composite in this work provides a novel drug
as delivery vehicles for camptothecin. J. Controlled Release 2004, 95, 501.
release formulation with potential application for nonionic and
(22) Han, B. H.; Winnik, M. A.; Bourlinos, A. B.; Giannelis, E. P.
water-insoluble drugs, taking advantage of applicability in a
Luminescence quenching of dyes by oxygen in core-shell soft-sphere ionic
liquids. Chem. Mater. 2005, 17, 4001.
wide range of solution pH (4.8-7.6) and almost complete
(23) Sverdlov, M.; Shajahan, A. N.; Minshall, R. D. Tyrosine phos-
release (100%).
phorylation-dependence of caveolae-mediated endocytosis. J. Cell. Mol.
Med. 2007, 11, 1239.
(24) Subuddhi, U.; Mishra, A. K. Micellization of bile salts in aqueous
medium: A fluorescence study. Colloids Surf. B 2007, 57, 102.
This project was supported by the National Natural Science
(25) Stephenson, B. C.; Goldsipe, A.; Beers, K. J.; Blankschtein, D.
Foundation of China, the 111 Project (Grant No.: B07004), the
Quantifying the hydrophobic effect. 2. a computer simulation-molecular-thermodynamic model for the micellization of nonionic surfactants in
863 project (Grant no.: 2007AA021900), and the Program for
aqueous solution. J. Phys. Chem. B 2007, 111, 1045.
Changjiang Scholars and Innovative Research Team in Uni-
(26) Wei, M.; Pu, M.; Guo, J.; Han, J. B.; Li, F.; He, J.; Evans, D. G.;
versity (Grant No.: IRT0406).
Duan, X. Intercalation of L-Dopa into layered double hydroxides: enhance-
Ind. Eng. Chem. Res., Vol. 48, No. 12, 2009
ment of both chemical and stereochemical stabilities of a drug through host-
of captopril-intercalated Mg-Al layered double hydroxides. J. Solid State
guest interactions. Chem. Mater. 2008, 20, 5169.
Chem. 2006, 179, 1792.
(27) Wei, H.; Zhang, X. Z.; Zhou, Y.; Cheng, S. X.; Zhuo, R. X. Self-
(33) Chern, J.; Lee, W.; Hsieh, M. Absorption isotherm of caffeine and
assembled thermoresponsive micelles of poly(N-isopropylacrylamide-b-
release kinetics from swollen NIPAAm hydrogels: experiments and model-
methyl methacrylate). Biomaterials 2006, 27, 2028.
ing. Ind. Eng. Chem. Res. 2004, 43, 6150.
(28) Allen, C.; Maysinger, D.; Eisenberg, A. Nano-engineering block
(34) Choudary, B. M.; Madhi, S.; Chowdari, N. S.; Kantam, M. L.;
copolymer aggregates for drug delivery. Colloids Surf. B 1999, 16, 3.
Sreedhar, B. Layered double hydroxide supported nanopalladium catalyst
(29) Kameshima, Y.; Yoshizaki, H.; Nakajima, A.; Okada, K.
for heck-, suzuki-, sonogashira-, and stille-type coupling reactions of
Preparation of sodium oleate/layered double hydroxide composites with
chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127.
acid-resistant properties. J. Colloid Interface Sci. 2006, 298, 624.
(35) Serra, L.; Domenech, J.; Peppas, N. A. Drug transport mechanisms
(30) Berber, M. R.; Minagawa, K.; Katoh, M.; Mori, T.; Tanaka, M.
and release kinetics from molecularly designed poly (acrylic acid-g-ethylene
Nanocomposites of 2-arylpropionic acid drugs based on Mg-Al layered
glycol) hydrogels. Biomaterials 2006, 27, 5440.
double hydroxide for dissolution enhancement. Eur. J. Pharm. Biopharm.
(36) Scott, R. A.; Peppas, N. A. Kinetics of copolymerization of PEG-
2008, 35, 354.
containing multiacrylates with acrylic acid. Macromolecules 1999, 32, 6149.
(31) Yang, J. H.; Han, Y. S.; Park, M.; Park, T.; Hwang, S. J.; Choy,
J. H. New inorganic-based drug delivery system of indole-3-acetic acid-
ReceiVed for reView January 10, 2009
layered metal hydroxide nanohybrids with controlled release rate. Chem.
ReVised manuscript receiVed March 30, 2009
Mater. 2007, 19, 2679.
Accepted May 2, 2009
(32) Zhang, H.; Zou, K.; Guo, S.; Duan, X. Nanostructural drug-
inorganic clay composites: structure, thermal property and in vitro release
Source: http://www.ldh-buct.com/wp-content/uploads/2015/03/2009-2.pdf
La voz de los protagonistas Publicación Oficial de DAECPU Distribución Gratuita Diciembre de 2011 Año 7 Nº 278 Una casa en construcción y de puertas abiertas. / d / José AriSi. 14º Encuentro de Murga Joven / d / Adrián Baseda. Ya están las "reinitas" de Promesas.
AQUEL QUE NUNCA ME DEJA A fines del siglo XVIII, la oscura conciencia de sí, de su a raíz de la toma de su ciudad por los británicos y que, por otras mo- en las más sobresalientes sensibilidades de la Isla: las de sus poetas. SilvestredeBalboaensudiscutido Espejode paciencia. Se enca- está, claro, la famosa «Oda a la piña», del habanero Manuel de ción de la Sociedad Patriótica, luego Real Sociedad Económica de





