
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
High diversity of hepatitis c viral quasispecies is associated with early virological response in patients undergoing antiviral therapy
High Diversity of Hepatitis C Viral Quasispecies Is
Associated with Early Virological Response in Patients
Undergoing Antiviral Therapy
Xiaofeng Fan,1,2 Qing Mao,3,4 Donghui Zhou,1,5 Yang Lu,1 Jianwei Xing,1 Yanjuan Xu,1 Stuart C. Ray,3 and
Adrian M. Di Bisceglie1,2
Differential response patterns to optimal antiviral therapy, peginterferon alpha plus ribavi-
rin, are well documented in patients with chronic hepatitis C virus (HCV) infection. Among
many factors that may affect therapeutic efficiency, HCV quasispecies (QS) characteristics
have been a major focus of previous studies, yielding conflicting results. To obtain a com-
prehensive understanding of the role of HCV QS in antiviral therapy, we performed the
largest-ever HCV QS analysis in 153 patients infected with HCV genotype 1 strains. A total
of 4,314 viral clones spanning hypervarible region 1 were produced from these patients
during the first 12 weeks of therapy, followed by detailed genetic analyses. Our data show an
exponential distribution pattern of intrapatient QS diversity in this study population in
which most patients (63%) had small QS diversity with genetic distance (d) less than 0.2. The
group of patients with genetic distance located in the decay region (d>0.53) had a signifi-
cantly higher early virologic response (EVR) rate (89.5%), which contributed substantially
to the overall association between EVR and increased baseline QS diversity. In addition, EVR
was linked to a clustered evolutionary pattern in terms of QS dynamic changes. Conclusion:
EVR is associated with elevated HCV QS diversity and complexity, especially in patients with
significantly higher HCV genetic heterogeneity.(HEPATOLOGY 2009;50:1765-1772.)
lic health concern worldwide. Over 2.7 million
mation may be valuable in improving the current antiviral
Americans are chronically infected with HCV,
which results in an estimated 10,000 deaths each year and
In this setting, HCV quasispecies (QS) characteris-
is a leading indication for liver transplantation.1 Cur-
tics have been a major focus of study in patients under-
rently, optimal antiviral therapy of chronic hepatitis C
going antiviral therapy. However, previous studies
with peginterferon alpha plus ribavirin cures up to 80% of
have generated conflicting data with regard to the role
patients infected with HCV genotypes 2 and 3. However,
of HCV QS in the determination of therapeutic effi-
the same treatment regimen is effective in only about 50%
ciency (see recent review3). Such results are to some
of patients infected with HCV genotype 1.2 It is thus
extent not surprising because the responses to antiviral
important to be able to identify the factor(s), either host
therapy represent a complex phenotype that is affected
Abbreviations: EVR, early virologic response; HCV, hepatitis C virus; HVR1, hypervariable region 1; QS, quasispecies; SOC, self-organized criticality; SVR, sustained
From the 1Division of Gastroenterology and Hepatology, Department of Internal Medicine, St. Louis University School of Medicine, St. Louis, MO; 2St. Louis University
Liver Center, St. Louis University School of Medicine, St. Louis, MO; 3Division of Infectious Diseases, Department of Medicine, Johns Hopkins University School ofMedicine, Baltimore, MD; 4Department of Infectious Diseases, Southwest Hospital, Third Military Medical University, Chongqing, China; 5Department of InfectiousDiseases, First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Received February 4, 2009; accepted August 6, 2009.
Supported by Roche, USA, and NIH grants R01 DK80711 (to X.F.) and R21 AI076834 (to A.M.D.).
Address reprint requests to: X. Fan or A.M. Di Bisceglie, Division of Gastroenterology and Hepatology, Department of Internal Medicine, St. Louis University School of
Medicine, 3635 Vista Avenue, St. Louis, MO 63110. E-mail: [email protected] or [email protected]; fax: (314) 577-8125.
Copyright 2009 by the American Association for the Study of Liver Diseases.
Published online in Wiley InterScience (www.interscience.wiley.com).
DOI 10.1002/hep.23290Potential conflict of interest: Dr. Di Bisceglie is a consultant for and received grants from Roche. Drs. Ray and Mao received grants from Roche.
Additional Supporting Information may be found in the online version of this article.
HEPATOLOGY, December 2009
by multiple factors from both the virus and host sides. The
quence. After the removal of primer sequences the tar-
involvement of these factors certainly interferes with the data
get domain for genetic analyses is 399 bp in length.
interpretation from HCV QS studies, especially when the
Nucleotide positions containing insertions or deletions
study population is small. In addition, techniques used to
within this domain were removed for the present anal-
assess HCV QS diversity may be another source for data
ysis and will be analyzed separately for their potential
discrepancy. The effect of mutations on gel mobility of a
influence on antiviral therapy. The HCV QS nature
given DNA molecule is sometimes unpredictable.4 Thus,
was characterized by measuring both genetic complex-
data from gel-based assays is not always consistent with the
ity and genetic diversity. The definitions and measure-
results from cloning/sequencing, which is thought to be the
ment of these genetic parameters are outlined in the
gold standard technique to assess viral diversity. In the cur-
rent study we performed a detailed QS analysis in 153 pa-
Phylogenetic analysis was used to verify HCV geno-
types and/or subtypes and potential sequence cluster-
(peginterferon alfa-2a plus ribavirin). Compared to many
previous studies, the current project has several unique fea-
constructed two phylogenetic trees, one with all 4,314
tures, such as being the largest study population, with an
clones (big tree) and the other with only 153 clones
exclusive focus on HCV genotype 1 and the application of
respectively representing the dominant HCV QS vari-
large-scale cloning and sequencing techniques. These char-
ant at the baseline from each patient (small tree). Both
acteristics allow a thorough dissection of the potential effect
trees were computed using the program MEGA 4 with
of HCV QS during antiviral therapy.
Neighbor-Joining approach, using the maximum com-posite likelihood model. We also assumed a rate varia-
Patients and Methods
tion among sites with an experienced value of gammaparameter (␣ ⫽ 0.5). Forty-five reference HCV se-
Samples. This was an ancillary study of a large clinical
quences, representing different HCV genotypes and
trial that aimed to compare the therapeutic efficiency of
subtypes, were included.
peginterferon alpha-2a and alpha-2b in treatment-naı¨ve
Statistical Analysis. Values of genetic parameters
patients with chronic HCV infection.5 Of 380 patients
from comparative analyses were tested for statistical
enrolled in the trial, 189 patients were treated with pegin-
significance using two-tailed Student's
t test. Categor-
terferon alpha-2a and are the subjects of the present study.
ical data from cross analyses were tested for statistical
Patient recruitment was restricted to HCV genotype 1.5
significance using the 2 test with Yate's correction or
Serum samples were collected at multiple timepoints dur-
Fisher's exact test. With regard to HCV QS diversity at
ing the early phase of antiviral therapy, including baseline
baseline, we explored its potential distribution pattern
(w00), w04, w08, and w12. Deidentified specimens were
in this study population (n ⫽ 153). In doing so, a
shipped to St. Louis University (SLU) and Johns Hopkins
one-sample Kolmogorov-Smirnov test was first used to
University (JHU) and stored at ⫺80°C until use. For eachpatient, molecular cloning was planned for two serum
test common distribution patterns. Next, a newly de-
samples, one at the baseline and the other at the latest
veloped procedure was applied to see if the data fit a
timepoint during the early phase of antiviral therapy be-
power-law or power-law-like distribution, such as ex-
fore week 12 with a minimum HCV viral load of more
ponential distribution, in which the given quantities
than 1,000 copies/mL, approximately equal to 1,111
are tightly clustered around their average values with
IU/mL when the HCV RNA level is quantified with
much reduced probability far from the mean in a one-
Roche Amplicor HCV Monitor, v. 2.0 (lower limit of
way direction.8 In this setting the term "decay region"
quantification, 600 IU/mL).
is used to denote the low-boundary phase of the distri-
Molecular Cloning of HCV Hypervariable Region 1
butions and the value at the proposed low-bound point
(HVR1). A 442-basepair (bp) fragment covering HCV
(min) on the curve was calculated.8 All statistical anal-
HVR1 was amplified by reverse transcription-polymerase
yses were done with SPSS (v. 13.0) except for the HCV
chain reaction (RT-PCR), followed by gel purification
QS distribution analysis, run in MatLab (http://www-
and TA cloning. About 15 independent clones for each
sample were sequenced. Detailed experimental proce-
Nucleotide Sequence Accession Numbers. A total of
dures are provided in the Supporting Material.
3,909 distinct HCV QS sequences generated in this study
Genetic Analysis. Raw sequences were edited with
were deposited in GenBank under accession numbers
the programs ClustalW6 and BioEdit7 in which HCV
FJ688411 through FJ692319. The entire dataset includ-
H77 strain (AF009606) served as the reference se-
ing 4,313 sequences is available on request.
HEPATOLOGY, Vol. 50, No. 6, 2009
Experimental Performance and Sample Compila-
tion. We had a 100% success rate for the amplification of
a 442-bp target by using the protocol described above.
This high success rate mainly resulted from our efforts to
optimize PCR primers, including their positions and
composition. In some samples with low viral loads near
1,000 copies/mL (n ⫽ 15, 5.7%), we found that it was
necessary to increase the input amount of serum RNA for
reverse transcription (RT). This was achieved by using
280 L of serum, instead of the regular 140 L, for RNA
extraction, followed by the elution into the same volume
of Tris buffer (60 L). Most experiments resulted in a
single visible band with the expected size on agarose gel.
In the molecular cloning experiments the positive rate of
recombinant clones was ⬇95%.
For each patient, HCV QS profiles were generated at
two timepoints, the baseline and the latest timepoint dur-ing the early phase of antiviral therapy (ⱕweek 12) with a
Fig. 1. Neighbor-joining tree of 153 dominant clones representing
minimum HCV viral load more than 1,000 copies/mL.
individual patients and 45 reference sequences from GenBank. A boot-
Based on this standard we finally identified 110 patients
strap test was done with 500 replicates and is shown at major branches.
Among 153 patients, 38 were infected with HCV genotype 1b and 115
with two timepoints and 43 patients with only one time-
were genotype 1a. The latter was further clustered into three subgroups
point (baseline), which resulted in 263 serum samples to
with more than 90% bootstrap support.
be studied. A total of 4,314 clones were generated andsequenced from these samples, an average of 16.4 clonesper sample. Among 153 patients, 104 (68%) achieved
HCV II Line Probe assay mistyped 16 of 17 HCV 1a
early virological response (EVR), defined as more than a 2
patients as HCV genotype 1b, suggesting the existence of
log decrease of HCV RNA level at week 12 compared to
an intrinsic bias to HCV genotype 1b.
the baseline.9 Potential differences between these two
There was no significant difference between HCV sub-
groups were examined in terms of viral factors, including
types with regard to early response patterns, EVR versus
baseline viral load, HCV subtypes, QS diversity, com-
non-EVR, 1a, 76.9% versus 23.1%, and 1b, 71.4% ver-
plexity, and dynamics.
sus 28.6% (Fig. 1). In the phylogenetic tree, HCV geno-
Lack of Association Among Viral Load, Subtypes,
type 1a strains were further clustered into three
and Early Response Patterns. The phylogenetic tree
subgroups, supported by the bootstrap test (Fig. 1).
constructed with all 4,314 clones (big tree) displayed sin-
Again, no statistical significance was detected with respect
gle patient-based clusterings, indicating the lack of HCV
to the relationship between HCV 1a subgroups and early
coinfection with different subtypes or strains. This obser-
response patterns in terms of current treatment regimen
vation further excluded any contamination during the
experimental performance. We verified the HCV geno-
We also investigated the potential relationship and in-
type/subtype for all patients through the phylogenetic
teractions between pretreatment HCV RNA levels and
analysis of 153 viral sequences representing dominant QS
early response patterns, HCV genotypes, and subgroups.
variant from each patient. Thus, 115 patients were in-
As shown in Table 1, pretreatment HCV viral load was
fected with HCV genotype 1a and 38 with HCV geno-
not associated with early virological response patterns
type 1b (Fig. 1). HCV genotype 1a isolates further formed
(
P ⫽ 0.137), HCV genotypes (
P ⫽ 0.489), or HCV 1a
three clusters, named subgroups 1, 2, and 3 (Fig. 1).
subgroups (
P ⫽ 0.171).
Among 109 patients with HCV subtypes based on Inno-
Effect of HCV QS Diversity on Antiviral Therapy.
LiPA HCV II Line Probe assay, 17 appeared to have been
We separated our amplified region into two domains,
mistyped based on our phylogenetic analysis (Fig. 1).
HVR1 (81 bp) and non-HVR1 (318 bp), to avoid possi-
Thus, the Inno-LiPA HCV II Line Probe assay seems to
ble masking of statistical significance due to apparently
have an error rate of HCV subtyping at 15.6%, consistent
unequal nucleotide substitution rates between these two
with a previous report.10 More interesting, the Inno-LiPA
domains. Next, we performed the analyses at two levels,
HEPATOLOGY, December 2009
Table 1. Lack of Correlation Between HCV Viral Load at Baseline and Response Patterns, HCV Genotypes, and HCV 1a
HCV Subtype
HCV 1a Subgroup
P value†
HCV viral load was determined by using Roche Amplicor HCV Monitor Test, version 2.0 (Roche Diagnostics) and is expressed as average log10 values and standard
†Two-tailed
t test under the assumption of two-sample equal variance. NP, not performed.
pretreatment genetic diversity and its early dynamic
population-based genetic diversity without the consider-
changes during antiviral therapy.
ation of sequence differences between two populations,
With regard to pretreatment genetic diversity, subjects
which is reflected by the net change. In other words, the
with EVR had overall higher values than non-EVR group
net change estimates the extent of "clustered evolution"
in most parameters measured. Thus, for HVR1, d 0.253
in terms of its phylogenetic representation.11
versus 0.1723; dS 0.0849 versus 0.0810; dN 0.1451 ver-
The average change in genetic diversity was calculated
sus 0.1033, and for Non-HVR1, d 0.0333 versus 0.0265;
based on either HVR1 (81 bp) or non-HVR1 region (318
dS 0.0528 versus 0.0528; and dN 0.0064 versus 0.0051.
bp). The net change was determined with HVR1 only.
However, only HVR1 dN reached statistical significance
Both EVR and non-EVR groups displayed a trend toward
(
P ⫽ 0.039) (Fig. 2).
decreasing genetic diversity over time, shown as all posi-
The criteria for sample selection identified 110 patients
tive values of genetic parameters. However, the average
with serum samples to be cloned and sequenced at two
change of non-HVR1 dN increased in the EVR group
timepoints, one at baseline and the other at the latest
(Fig. 3). Because the absolute values of dN change of
timepoint during the early phase of antiviral therapy
non-HVR1 are actually very minimal in both EVR and
(ⱕweek 12) with a minimum HCV viral load more than
non-EVR groups (0.0013 versus 0.0018), such an in-
1,000 copies/mL. Again, these patients were separated
crease may not be biologically significant. The EVR group
into two groups, EVR (n ⫽ 66) and non-EVR (n ⫽ 44).
had a more prominent decrease of genetic diversity over
We conducted two kinds of group-based analyses, the
time than the non-EVR group (Fig. 3). However, the
average change and net change of genetic diversity be-
difference between the two groups was not statistically
tween two timepoints. Average change simply compares
significant (Fig. 3).
Fig. 2. The correlation between EVR or non-EVR patterns and genetic
diversity at baseline. Although the EVR group showed a higher genetic
Fig. 3. The correlation between early virologic response patterns (EVR
diversity than the non-EVR group, the difference between the two groups
or Non-EVR) and dynamic changes of genetic diversity. Both the average
did not reach statistical significance (two-tailed
t test) except for HVR1
and net changes were more apparent in the EVR group than the non-EVR
dN, which indicated a higher pressure for the positive selection in the EVR
group. The difference between two groups did not reach statistical
significance (two-tailed
t test).
HEPATOLOGY, Vol. 50, No. 6, 2009
The net change of genetic diversity had similar patterns
as the average change. The EVR group was associatedwith more apparent decrease of net genetic diversity.
Again, the difference between the EVR and Non-EVRgroup was not supported statistically (Fig. 3).
Genetic complexity was estimated by measuring aver-
age Shannon entropy in the HVR1 domain. Like geneticdiversity, the EVR group had a higher pretreatment ge-netic complexity than the non-EVR group at either thenucleotide or amino acid level (Fig. 4). The latter reachedstatistical significance (P ⫽ 0.0499). The average changeof genetic complexity was also higher in the EVR groupthan that in the non-EVR group, although this differencewas not statistically supported (Fig. 4).
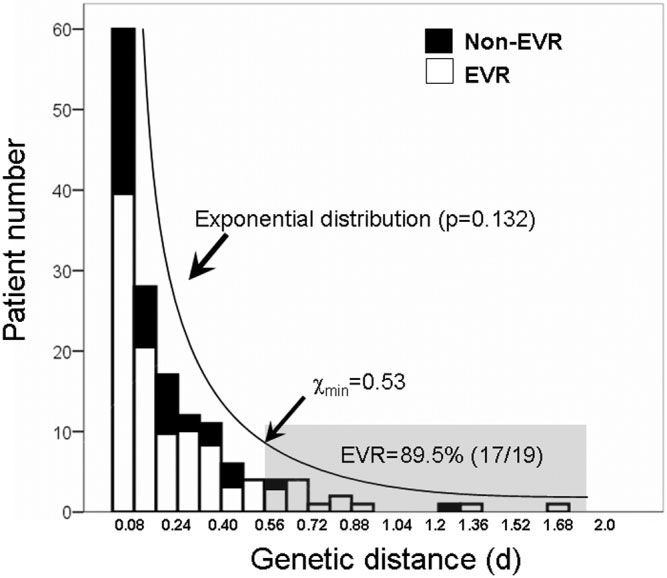
Distribution of QS Diversity in Patients Infected
with HCV Genotype 1 Isolates. The current project,
the largest-ever QS study yet to focus on HCV genotype
1, investigated possible distribution patterns of QS diver-
Fig. 5. Histogram of baseline QS diversity in the study population. The
sity. Using HVR1 genetic distance (d), we first plotted its
number of patients in each category of genetic distance (d) based onHVR1 was calculated. The distribution of QS diversity in this study
histogram in this study population. The distribution pat-
population fit an exponential distribution (P ⫽ 0.132 by KS test) in which
terns were subsequently estimated by a one-sample Kol-
most patients (about 63%) had a small d value less than 0.2. The min,
estimated using the described formula in MatLab, is about 0.53, whichput the genetic distance from 19 patients in the decay region (shaded
exponential distribution (P ⫽ 0.132) with the exclusion
area). This group of patients had a much higher EVR rate (89.5%)
of normal (P ⬍ 0.001), uniform (P ⬍ 0.001), and Poi-
compared to the overall EVR rate (68%, P ⫽ 0.032) or patients with
sson (P ⬍ 0.001) distributions (Fig. 5). We further cal-
genetic distance less than 0.53 (65%, P ⫽ 0.016). KS test, one-sampleKolmogorov-Smirnov test.
culated the lower bound (min) using described formulasunder the hypothesis of either continuous power-law orexponential distribution.8 The power-law distributionwas not favored due to the short tail, only 15 patients
located in the power-law region (min ⫽ 0.58), which is
We evaluated HCV QS heterogeneity at baseline and
too few to be meaningful. The min for exponential dis-
its dynamic changes during the early therapeutic period.
tribution was equal to 0.53, putting genetic distance from
In this type of study, sampling bias is often a concern due
19 patients in the decay region (Fig. 5).
to the lack of normalization of the entry HCV RNAamount used for RT-PCR.12 Thus, viral QS heterogene-ity may be to some extent dependent on viral titers. How-ever, under our experimental procedure and studyprotocol we consider the potential for sampling bias to beminimal, and its role on our observations and conclusionsunimportant. First, we previously failed to detect a statis-tical relationship between QS diversity and viral titers.13Second, for a given viral region used to measure QS di-versity, such as HVR1 in this study, QS diversity is main-tained by sequencing an adequate number of clones,usually ⬎10, and the use of fixed PCR primers.14,15 Fi-nally, we focused on comparative HCV QS analyses be-tween EVR and non-EVR groups. There is no statisticaldifference with regard to their average HCV viral loads ineither group, which may further reduce the sampling bias,assuming it exists. An additional limitation, by the nature
Fig. 4. Comparisons of genetic complexity between EVR and non-EVR
of the PEAK study that only followed patients through
groups. Pretreatment HCV genetic complexity (left side) and its average
the first 12 weeks of therapy, is the inability to correlate
change over time (right side) were compared between two groups ateither the amino acid or nucleotide level. AA, amino acid; Nu, nucleotide.
HCV QS heterogeneity with sustained virologic response
HEPATOLOGY, December 2009
(SVR). Rather, we chose to correlate it with EVR. The
versity or complexity in the EVR group, this trend may be
number of patients with rapid virologic response (RVR),
only the reflection of the elimination of drug-sensitive
defined as undetectable viral RNA at week 4, was only 11
HCV QS variants. However, this explanation cannot be
patients (7.2%), making it difficult to analyze. Nonethe-
applied to the sequence diversification (net change) in
less, we actually did not find any viral factors analyzed in
which a similar trend has been featured. Thus, consistent
this study that were specifically associated with RVR (data
with previous reports, HCV QS diversification is more
not shown). Thus, we focused on the EVR rather than
likely associated with early response patterns.11
RVR. EVR is also a response pattern that appears to re-
By taking the advantage of this largest-ever HCV QS
flect intrinsic sensitivity of HCV to combination therapy.
study, we explored potential distribution patterns of in-
It has been reported that EVR is a very good predictor of
trapatient HCV QS diversity at the population level. In
SVR.16 Data interpretation from our study may have gen-
contrast to viral load that displayed a typical normal
eral applicability in terms of HCV antiviral therapy.
(Gaussian) distribution among 153 patients we studied
HCV genotype is a well-documented independent fac-
(data not shown), the QS diversity showed a best fit to an
tor affecting the efficiency of antiviral therapy.2 The large
exponential distribution (Fig. 5).23 This finding has sev-
size of this study population allowed us to examine
eral important implications. First, the exponential distri-
whether or not such an observation can be extended to
bution of QS diversity may explain long-existing
HCV subtype level. Statistically, we demonstrated that
controversies with regard to the role of QS diversity in
pretreatment HCV viral loads, HCV subtypes, and HCV
HCV antiviral therapy.11,24-40 Although the overall EVR
subgroups within HCV genotype 1a are not determinants
rate in this study population is about 68%, the group of
of early response patterns. Previous studies suggest that a
patients with HVR1 genetic distance beyond the low
low baseline HCV viral load is an independent predictor
bound (min ⫽ 0.53) has a significantly higher EVR rate
to SVR (reviewed17). In our study, the EVR group even
(89.5%, P ⫽ 0.032) (Fig. 5). In fact, when taking out the
had a higher average viral load, although the differences
patient group with large QS diversity (d ⬎0.53), the sta-
between groups were not statistically supported (Table 1).
tistical significance of dN values was lost in terms of its
The discordance may be attributed to multiple factors,
association with the EVR (EVR, 0.106 versus non-EVR,
such as different therapeutic regimens, patient selection,
0.092, P ⫽ 0.194). Thus, assuming a potential role of QS
and various stages of disease progress.18,19 Alternatively, it
diversity in the antiviral therapy, we can conclude that
may simply suggest that pretreatment HCV viral load is
such a role may become dominant only in patients with
not an independent factor to predict therapeutic effi-
large QS diversity (d ⬎0.53). This conclusion was also
true in the similar analysis using dN values (data not
An important finding came from our QS analysis. Due
shown). Because the QS diversity in most patients, as
to known differences in mutation frequency between
measured by genetic distance (d), is less than 0.53 (Fig. 5),
HVR1 and non-HVR1 domains, our analysis focused on
intrinsic uncertainty is actually accompanied with any
HVR1. EVR was associated with increased baseline QS
HCV genetic studies to explore the role of QS diversity in
diversity, shown as higher values of genetic diversity and
the antiviral therapy, especially when the study popula-
complexity. Especially, dN reached statistical significance
tion size is small. For example, in our previous study there
(P ⫽ 0.039). The dN reflects the strength of evolutionary
was only one patient confirmed with genetic distance
selection that is frequently interpreted as immune pres-
larger than 0.53 among 29 patients studied (36).
sure.20 Because HVR1 contains putative B and T cell
Second, since the introduction of QS theory into virol-
epitopes,21,22 our observation suggests a role of pretreat-
ogy, most viral genetic studies simply use this term to
ment immune status in the determination of early viro-
describe viral genome heterogeneity. Its original defini-
logical response patterns. Compared with previous
tion is largely ignored.41,42 According to this theory, all
studies, it should be emphasized that our conclusion is
viral variants in infected individuals form a network and
more solid in terms of statistical power given the large
act as a unit in response to internal or external interfer-
number of patients studied. Additionally, two aspects of
ence, such as antiviral therapy. In this study we found that
HCV QS dynamics have been assessed, including genetic
the EVR is associated with high QS diversity. Such an
diversity/complexity (average change) and sequence di-
observation is not easily understood with classical popu-
versification (net change). In the former aspect, both EVR
lation biology because high QS diversity indicates an in-
and non-EVR groups display a general trend of reduced
creased possibility to contain drug-resistant viral variants.
genetic diversity and complexity after the start of antiviral
In this setting, QS theory may provide a plausible expla-
therapy. Such a trend seems more apparent in the EVR
nation by treating the entire viral population as an acting
group. Given the fact of higher pretreatment genetic di-
unit presumably in a status of the self-organized criticality
HEPATOLOGY, Vol. 50, No. 6, 2009
(SOC), which is a prevailing hypothesis to explain many
9. Di Bisceglie AM, Fan X, Chambers T, Strinko J. Pharmacokinetics, phar-
complex phenomena in nature, including exponential
macodynamics, and hepatitis C viral kinetics during antiviral therapy: thenull responder. J Med Virol 2006;78:446-451.
distribution.43 Consequently, high QS diversity may im-
10. Ross RS, Viazov S, Roggendorf M. Genotyping of hepatitis C virus isolates
ply a critical status in which HCV reaches its maximum
by a new line probe assay using sequence information from both the 5'
capability to maintain the QS network. Such a status, as
untranslated and the core regions. J Virol Methods 2007;143:153-160.
11. Abbate I, Lo Iacono O, Di Stefano R, Cappiello G, Girardi E, Longo R, et
assumed by the SOC hypothesis, would be extremely sen-
al. HVR-1 quasispecies modifications occur early and are correlated to
sitive to any external stimulus, such as antiviral therapy,
initial but not sustained response in HCV-infected patients treated with
resulting in the collapse of the entire QS network (virus
pegylated- or standard-interferon and ribavirin. J Hepatol 2004;40:831-836.
extinction). Thus, our data provide indirect evidence for
12. Shan-Lu Liu SL, Rodrigo AG, Shankarappa R, Learn GH, Hsu L, Davidov
the support of QS theory in virology and the SOC hy-
O, et al. HIV quasispecies and resampling. Science 1996;273:413-417.
pothesis should be an important addition to this theory.
13. Fan X, Solomon H, Poulos JE, Neuschwander-Tetri BA, Di Bisceglie AM.
Comparison of genetic heterogeneity of hepatitis C viral RNA in liver
Third, the formation of QS diversity results from the
tissue and serum. Am J Gastroenterol 1999;94:1347-1354.
complicated interaction between virus and host. The ex-
14. Fan X, Lyra AC, Tan D, Xu Y, Di Bisceglie AM. Differential amplication
ponential distribution of QS diversity suggests the lack of
of hypervariable region 1 of hepatitis C virus by partially mismatchedprimers. Biochem Biophys Res Commun 2001;284:694-697.
an ideal genetic distance that may favor HCV infection.
15. Torres-Puente M, Bracho MA, Jimenez N, Garcia-Robles I, Moya A,
In other words, the QS nature is not necessarily the only
Gonzalez-Candelas F. Sampling and repeatability in the evaluation of hep-
prerequisite for HCV to establish or maintain its persis-
atitis C virus genetic variability. J Gen Virol 1003;84:2343-2350.
16. Ferenci P, Fried M, Shiffman M, Smith C, Marinos G, Gonc¸ales F, et al.
tent infection. Indeed, most RNA viruses, such as dengue
Predicting sustained virological responses in chronic hepatitis C patients
and West Nile virus, share the QS nature.44,45 However,
treated with peginterferon alfa-2a (40KD)/ribavirin. J Hepatol 2005;43:
only a few of them result in a persistent infection in hu-
17. Mihm U, Herrmann E, Sarrazin C, Zeuzem S. Review article: predicting
response in hepatitis C virus therapy. Aliment Pharm Therap 2006;23:
Finally, our data showed the large variability of HCV
QS diversity in infected patients. With the fact of a con-
18. Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M,
siderable relatedness between the EVR and high HCV QS
Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared withinterferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C:
diversity, the modulation of HCV QS diversity may rep-
a randomised trial. Lancet 2001;358:958-965.
resent a novel strategy for antiviral therapy. The underly-
19. Shiffman ML, Di Bisceglie AM, Lindsay KL, Morishima C, Wright EC,
ing mechanism responsible for the formation of high QS
Everson GT, et al. Peginterferon Alfa-2a and ribavirin in patients withchronic hepatitis C who have failed prior treatment. Gastroenterology
diversity in HCV patients therefore warrants further in-
20. Nei M. Phylogenetic analysis in molecular evolutionary genetics. Annu
Rev Genet 1996;30:371-403.
We thank Aaron Clauset (Santa Fe
21. Mondelli MU, Cerino A, Meola A, Nicosia A. Variability or conservation
Institute) for assistance with statistical analysis.
of hepatitis C virus hypervariable region 1? Implications for immune re-sponses. J Biosci 2003;28:305-310.
22. Mondelli MU, Cerino A, Segagni L, Meola A, Cividini A, Silini E, et al.
Hypervariable region 1 of hepatitis C virus: immunological decoy or bio-logically relevant domain? Antivir Res 2001;52:153-159.
1. National Institutes of Health. NIH Consensus Development Conference
23. Bak P. How Nature Works: The Science of Self-organized Criticality. New
Statement: management of hepatitis C. HEPATOLOGY 2002;36(Suppl 1):
York: Springer; 1999:1-31.
24. Gonzalez-Peralta RP, Qian K, She JY, Davis GL, Ohno T, Mizokami M,
2. Feld, JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in
et al. Clinical implications of viral quasispecies heterogeneity in chronic
treatment of hepatitis C. Nature 2005;436:967-972.
hepatitis C. J Med Virol 1996;49:242-247.
3. Wohnsland A, Hofmann WP, Sarrazin C. Viral determinants of resistance
25. Hino K, Yamaguchi Y, Fujiwara D, Katoh Y, Korenaga M, Okazaki M, et
to treatment in patients with hepatitis C. Clin Microbiol Rev 2007;20:23-
al. Hepatitis C virus quasispecies and response to interferon therapy in
patients with chronic hepatitis C: a prospective study. J Viral Hepat 2000;
4. Upchurch DA, Shankarappa R, Mullins JI. Position and degree of mis-
matches and the mobility of DNA heteroduplexes. Nucleic Acids Res
26. Kanazawa Y, Hayashi N, Mita E, Li T, Hagiwara H, Kasahara A, Fusamoto
H, et al. Influence of viral quasispecies on effectiveness of interferon ther-
5. Di Bisceglie AM, Ghalib RH, Hamzeh FM, Rustgi VK. Early virologic
apy in chronic hepatitis C patients. HEPATOLOGY 1994;20:1121-1130.
response after peginterferon alpha-2a plus ribavirin or peginterferon al-
27. Koizumi K, Enomoto N, Kurosaki M, Murakami T, Izumi N, Marumo F,
pha-2b plus ribavirin treatment in patients with chronic hepatitis C. J Viral
et al. Diversity of quasispecies in various disease stages of chronic hepatitis
C virus infection and its significance in interferon treatment. HEPATOLOGY
6. Higgins DG, Sharp PM. CLUSTAL: a package for performing multiple
sequence alignment on a microcomputer. Gene 1988;73:237-244.
28. Moribe T, Hayashi N, Kanazawa Y, Mita E, Fusamoto H, Negi M, et al.
7. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and
Hepatitis C viral complexity detected by single-strand conformation poly-
analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 1999;
morphism and response to interferon therapy. Gastroenterology 1995;
8. Clauset A, Shalizi CR, Newman MEJ. Power-law distributions in empiri-
29. Nagasaka A, Hige S, Tsunematsu I, Yoshida J, Sasaki Y, Matsushima T, et
cal data. 2007; http://arxiv.org/abs/0706.1062.
al. Changes in hepatitis C virus quasispecies and density populations in
HEPATOLOGY, December 2009
patients before and after interferon therapy. J Med Virol 1996;50:214-
variable during pegylated interferon therapy of chronic hepatitis C virus
infection. J Virol 2005;79:3071-3083.
30. Okada S, Akahane Y, Suzuki H, Okamoto H, Mishiro S. The degree of
37. Farci P, Strazzera R, Alter HJ, Farci S, Degioannis D, Coiana A, et al. Early
variability in the amino terminal region of the E2/NS1 protein of hepatitis
changes in hepatitis C viral quasispecies during interferon therapy predict
C virus correlates with responsiveness to interferon therapy in viremic
the therapeutic outcome. Proc Natl Acad Sci U S A 2002;99:3081-3086.
patients. HEPATOLOGY 1992;16:619-624.
38. Sarrazin C, Bruckner M, Herrmann E, Ru¨ster B, Bruch K, Roth WK, et al.
31. Shindo M, Hamada K, Koya S, Arai K, Sokawa Y, Okuno T. The clinical
Quasispecies heterogeneity of the carboxy-terminal part of the E2 gene
significance of changes in genetic heterogeneity of the hypervariable region
including the PePHD and sensitivity of hepatitis C virus 1b isolates to
1 in chronic hepatitis C with interferon therapy. HEPATOLOGY 1996;24:
antiviral therapy. Virology 2001;289:150-163.
39. Torres-Puente M, Cuevas JM, Jimenez-Hernandez N, Bracho MA, Gar-
32. Le Guen B, Squadrito G, Nalpas B, Berthelot P, Pol S, Brechot C. Hepa-
cia-Robles I, Wrobel B, et al. Genetic variability in hepatitis C virus and its
titis C virus genome complexity correlates with response to interferon
role in antiviral treatment response. J Viral Hepatitis 2008;15:188-199.
therapy: a study in French patients with chronic hepatitis C. HEPATOLOGY
40. Morishima C, Polyak SJ, Ray R, Doherty MC, Di Bisceglie AM, Malet PF,
et al. Hepatitis C virus-specific immune responses and quasi-species vari-
33. Lopez-Labrador FX, Ampurdanes S, Gimenez-Barcons M, Guilera M,
ability at baseline are associated with nonresponse to antiviral therapy
Costa J, Jimenez MT, et al. Relationship of the genomic complexity of
during advanced hepatitis C. J Infect Dis 2006;193:931-940.
hepatitis C virus with liver disease severity and response to interferon in
41. Domingo E. Quasispecies theory in virology. J Virol 2002;76:463-465.
patients with chronic HCV genotype 1b infection. HEPATOLOGY 1999;29:897-903.
42. Biebricher CK, Eigen M. What is a quasispecies? Curr Top Microbiol
34. Nakazawa T, Kato N, Ohkoshi S, Shibuya A, Shimotohno K. Character-
ization of the 5' noncoding and structural region of the hepatitis C virus
43. Bak P, Tang C, Wiesenfeld K. Self-organized criticality. Phys Rev 1998;
genome from patients with non-A, non-B hepatitis responding differently
to interferon treatment. J Hepatol 1994;20:623-629.
44. Wang WK, Lin SR, Lee CM, King CC, Chang SC. Dengue type 3 virus in
35. Sandres K, Dubois M, Pasquier C, Payen JL, Alric L, Duffaut M, et al.
plasma is a population of closely related genomes: quasispecies. J Virol
Genetic heterogeneity of hypervariable region 1 of the hepatitis C virus
(HCV) genome and sensitivity of HCV to alpha interferon therapy. J Virol
45. Jerzak G, Bernard KA, Kramer LD, Ebel GD. Genetic variation in West
Nile virus from naturally infected mosquitoes and birds suggests quasispe-
36. Chambers TJ, Fan X, Droll DA, Hembrador E, Slater T, Nickells MW, et
cies structure and strong purifying selection. J Gen Virol 2005;86:2175-
al. Quasispecies heterogeneity within the E1/E2 region as a pretreatment
Source: http://livercenter.slu.edu/uploads/1_article.pdf
2008_PM_Umschlag.qxd 27.03.2008 19:39 Uhr Seite 1 Gebrauchsanleitung! Bitte aufmerksam lesen! Instructions for use! Please Ücretsiz yedek parçalar (Garanti) read carefully. Mode d'emploi. Veuillez lire attentivement avant utilisation ! Reservdelar kostnadsfritt (garanti) Wir wünschen Ihnen viel Freude Istruzioni per l'uso. Leggere attentamente prima dell'uso! Instrucciones de
Preclinical Studies on the Mechanism of Action and theAnti-Lymphoma Activity of the Novel Anti-CD20 Antibody GA101 Stephane Dalle, Lina Reslan, Timothee Besseyre de Horts, et al. 2011;10:178-185. Published online January 10, 2011. Mol Cancer Ther Access the most recent version of this article at: This article cites 39 articles, 24 of which you can access for free at:





