
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Noninsulindoctor.ibog.steno.dk
Metformin: An Update
Dmitri Kirpichnikov, MD; Samy I. McFarlane, MD; and James R. Sowers, MD
Metformin is an insulin-sensitizing agent with potent antihyper-
Although the precise mechanism of hypoglycemic action of met-
glycemic properties. Its efficacy in reducing hyperglycemia in
formin remains unclear, it probably interrupts mitochondrial oxi-
type 2 diabetes mellitus is similar to that of sulfonylureas, thia-
dative processes in the liver and corrects abnormalities of intra-
zolidinediones, and insulin. Metformin-based combination therapy
cellular calcium metabolism in insulin-sensitive tissues (liver,
is often superior to therapy with a single hypoglycemic agent. The
skeletal muscle, and adipocytes) and cardiovascular tissue.
antihyperglycemic properties of metformin are mainly attributed to
suppressed hepatic glucose production, especially hepatic glu-
Ann Intern Med. 2002;137:25-33.
coneogenesis, and increased peripheral tissue insulin sensitivity.
For author affiliations, see end of text.
Insulin resistance contributes greatly to development of arytoweightlossorfatredistribution)mayhaveadditional
cardiovascular disease in patients with the metabolic syn-
cardiovascular benefits in insulin-resistant persons treated
drome and its extreme presentation, type 2 diabetes melli-
with metformin (13, 14). Weight loss during metformin
tus. Therefore, treatment with an insulin-sensitizing agent,
treatment has been attributed to decreased net caloric in-
such as metformin, in patients with type 2 diabetes melli-
take (15), probably through appetite suppression, an effect
tus may correct several of the primary pathophysiologic
that is largely independent of gastrointestinal side effects of
abnormalities of the metabolic syndrome. In diabetic pa-
metformin (such as nausea and diarrhea) (10). Reduction
tients, metformin appears to provide cardiovascular protec-
in hyperinsulinemia related to reduced insulin resistance
tion that cannot be attributed only to its antihyperglycemic
may have an additive effect on weight reduction in obese
effects. These additional cardioprotective effects in these
insulin-resistant persons (13, 14).
patients may be related to the favorable actions of met-
At doses of 500 to 1500 mg, metformin has an abso-
formin on lipid metabolism, vascular smooth-muscle and
lute oral bioavailability of 50% to 60% (16). The drug is
cardiomyocyte intracellular calcium handling, endothelial
not protein bound and therefore has a wide volume of
function, hypercoagulation, and platelet hyperactivity. We
distribution (8), with maximal accumulation in the small-
discuss known mechanisms by which metformin exerts its
intestine wall (17). Metformin undergoes no modifications
beneficial glycemic and cardiovascular actions.
in the body and is secreted unchanged by rapid kidneyexcretion (through glomerular filtration and, possibly, tu-
CLINICAL ROLE OF METFORMIN
bular secretion) (8). Impaired kidney function slows elim-
Metformin, an insulin-sensitizing biguanide used to
ination and may cause metformin accumulation (18). The
treat type 2 diabetes, has been shown to be as effective as
H2-blocker cimetidine competitively inhibits renal tubular
insulin or sulfonylureas when used as monotherapy (1–5).
secretion of metformin, significantly decreasing its clear-
In conjunction with diet, metformin reduces fasting glu-
ance and increasing its bioavailability (16, 19).
cose concentration by 2.78 to 3.90 mmol/L (50 to 70mg/dL), which corresponds to a 1.3% to 2.0% reductionin hemoglobin A1c values (1, 2, 4, 6 – 8). The magnitude of
METFORMIN AS A PART OF COMBINATION THERAPY
plasma glucose reduction is related to pretreatment glucose
Metformin has been shown to be effective in combi-
levels (7, 9). The efficacy of metformin monotherapy has
nation with insulin, sulfonylureas (2, 10, 20, 21), and thia-
been shown to be independent of age, body weight, eth-
zolidinediones (22). This finding is important because
nicity, duration of diabetes, and insulin and C-peptide lev-els (1, 2).
single-drug therapy often fails to maintain normoglycemia,
Metformin may have special benefits in overweight
particularly as diabetes progresses (23, 24). As seen in the
patients with type 2 diabetes. Unlike sulfonylureas, insulin,
United Kingdom Prospective Diabetes Study (UKPDS),
and thiazolidinediones, metformin does not affect body
50% of patients treated with diet or a single antidiabetic
mass index (1) or decreases body weight in obese patients
drug achieved the target hemoglobin A1c value of less than
with (4, 10) and without (11, 12) diabetes. Significant
7% after 3 years of follow-up; after 9 years, only 25%
reductions in total body fat and visceral fat have been ob-
maintained this goal (24). As diabetes progresses and treat-
served in women with preexistent abdominal or visceral
ment with maximal doses of sulfonylurea fails, addition of
obesity who are treated with metformin (11). Excessive fat
metformin significantly improves glycemic control (2). In
localized to the paraintestinal region is a major contributor
the UKPDS trial, combination therapy tended to control
to the pathogenesis of the cardiovascular metabolic syn-
glycemia more effectively than monotherapy (hemoglobin
drome (13, 14), and the reduction in visceral fat (second-
A1c value, 0.075 [7.5%] versus 0.081 [8.1%]) (23).
2002 American College of Physicians–American Society of Internal Medicine
E-25
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
Update Metformin: An Update
PRACTICAL CONSIDERATIONS IN METFORMIN THERAPY
feres with mucosal-cell intracellular calcium handling, thus
The ideal patient for initiation of metformin treatment
disrupting calcium-dependent absorption of vitamin B12 in
would be an obese person with type 2 diabetes mellitus who
the ileum (30). Such decreases in vitamin B12 levels rarely
has normal kidney function (creatinine concentration ⬍133
have clinical significance (2, 9).
m d/L [⬍1.5 mg/dL] in men and ⬍124 m d/L in women,
Development of hypoglycemia during metformin
or creatinine clearance ⬎1.17 mL/s without coexistent symp-
monotherapy is rare because metformin only partially sup-
tomatic congestive heart failure or a hypoxic respiratory con-
presses gluconeogenesis in the liver and does not stimulate
dition) (9, 16, 25). Contraindications to metformin therapy
insulin production (9, 31).
are liver failure, alcoholism, and active moderate to severe in-
Lactic acidosis is a life-threatening complication of bigua-
fection (9, 25); these conditions predispose to development of
nide therapy that carries a mortality rate of 30% to 50% (28).
lactic acidosis, either by increased production or decreased
Metformin therapy may increase blood lactate levels (1) and is
metabolism of lactic acid (9, 16 –18, 25). Administration of
occasionally associated with development of lactic acidosis (2,
radiocontrast material to a patient with diabetes may worsen
28). The estimated incidence of metformin-associated lactic
already-compromised kidney function and cause accumula-
acidosis is 0.03 cases per 1000 patient-years (25), which is 10
tion of metformin, leading to toxic levels of drug. Further-
to 20 times lower than that seen with phenformin therapy
more, administration of general anesthesia may cause hypo-
(28). Development of lactic acidosis appears to be unrelated to
tension, which leads to renal hypoperfusion and peripheral
plasma metformin concentrations (28), and even in persons
tissue hypoxia with subsequent lactate accumulation (25–28).
with chronic renal insufficiency, metformin accumulation
Therefore, if administration of radiocontrast material is re-
does not necessarily lead to lactic acidosis (18). Developmentof lactic acidosis is almost always related to coexistent hypoxic
quired or urgent surgery is needed, metformin should be
conditions that are probably responsible for the associated
withheld and hydration maintained until preserved kidney
high mortality rate. In one report, 91% of patients who de-
function is documented at 24 and 48 hours after the interven-
veloped lactic acidosis while being treated with metformin had
tion (9, 26 –28). Metformin should be used with caution in
a predisposing condition, such as congestive heart failure, re-
elderly patients, whose reduced lean body mass may lead to
nal insufficiency, chronic lung disease with hypoxia, or age
misleading low creatinine concentrations that fail to reflect
older than 80 years (26). Thus, patients with compromised
decreased glomerular filtration rates (9, 25–28).
renal function or coexistent hypoxic conditions should not be
Metformin therapy should be initiated with a single
given metformin. Chronic or acute intake of large amounts of
dose of medication (usually 500 mg) taken with the pa-
alcohol may potentiate the effect of metformin on lactate me-
tient's largest meal to prevent gastrointestinal symptoms.
tabolism. A careful history of alcohol use is therefore impor-
Gastrointestinal symptoms generally disappear within 2
tant before starting metformin therapy (26, 27).
weeks of treatment (10, 11). Medication doses may beincreased by 500-mg increments every 1 to 2 weeks, asindicated by glycemic control, until a desirable blood glu-
MECHANISMS OF ANTIHYPERGLYCEMIC ACTION OF
cose level or the maximal recommended daily metformin
dose of 2550 mg is reached (2, 25). The hypoglycemic
The glucose-lowering effects of metformin are mainly a
effect of metformin is dose related, and a plateau of hypo-
consequence of reduced hepatic glucose output (primarily
glycemic action is achieved at a daily dose of 2000 mg (6).
through inhibition of gluconeogenesis and, to a lesser extent,
Side effects of metformin are mostly limited to diges-
glycogenolysis) and increased insulin-stimulated glucose up-
tive tract symptoms, such as diarrhea, flatulence, and ab-
take in skeletal muscle and adipocytes (25, 27, 31–35) (
Figure
dominal discomfort (1, 6, 8 –10). These symptoms are
1). Its major mode of action is to reduce hepatic glucose pro-
dose dependent and can usually be avoided by slow titra-
duction, which is increased at least twofold in patients with
tion and, in some cases, reduction of the dose (9). About
type 2 diabetes (32, 36). In a recent study of the mechanism
5% of patients cannot tolerate treatment because of gastro-
by which metformin decreases endogenous glucose produc-
intestinal side effects (6, 9, 10). The mechanisms of these
tion in patients with type 2 diabetes, the increased plasma
gastrointestinal side effects remain unclear but probably are
glucose level was attributed to a threefold increase in the rate
related to accumulation of high amounts of metformin in
of gluconeogenesis, as assessed by nuclear magnetic resonance
the intestinal tissue (17), with subsequent elevation of local
spectroscopy (32). Metformin treatment decreased fasting
lactate production. Histologic examination has not re-
plasma glucose concentrations by 25% to 30% and reduced
vealed changes in the intestinal mucosa in metformin-
glucose production (32), findings that are consistent with
treated animals (29), indicating a functional rather than a
those of other investigators (27, 35). The decrease in glucose
structural basis for gastrointestinal symptoms. Ten percent
production was attributable to a reduction in the rate of glu-
to 30% of patients receiving long-term metformin therapy
coneogenesis (32).
develop vitamin B12 malabsorption, as indicated by de-
Data from in vivo studies (27, 32, 36) are consistent with
creased concentrations of total vitamin B12 and its bioavail-
those of in vitro studies demonstrating an inhibitory effect of
able form, holotranscobalamin (2, 30). Metformin inter-
metformin on gluconeogenesis (37, 38) (
Figure 1). For exam-
E-26 2 July 2002 Annals of Internal Medicine Volume 137 • Number 1
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
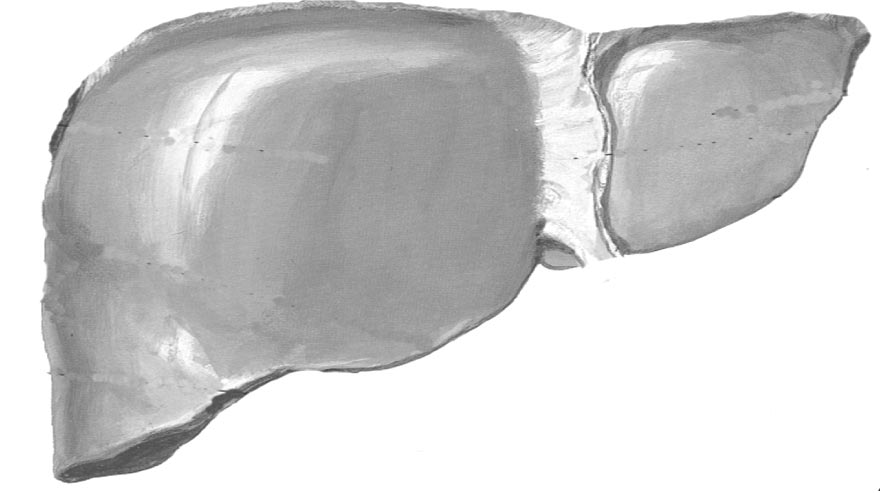
Metformin: An Update Update
ple, metformin was observed to decrease gluconeogenesis in
Figure 1. Mechanisms of metformin action on hepatic glucose
perfused liver, primarily through inhibition of hepatic lactate
production and muscle glucose consumption.
uptake (37). Others reported that metformin therapy de-
creased concentrations of adenosine triphosphate in isolated
rat hepatocytes (38). Because adenosine triphosphate is an
allosteric inhibitor of pyruvate kinase, the investigators sug-
gested that the metformin-mediated reduction in hepatic glu-
cose production resulted from increased pyruvate kinase flux.
Metformin also decreases gluconeogenic flux through inhibi-
tion of pyruvate carboxylase–phosphoenolpyruvate carboxyki-
nase activity and possibly through increased conversion of
pyruvate to alanine (34). Metformin also facilitates insulin-
induced suppression of gluconeogenesis from several sub-
stances, including lactate, pyruvate, glycerol, and amino acids
(31), and opposes the gluconeogenic actions of glucagon (39)
(Figure 1).
The exact mechanism through which metformin re-
duces hepatic glucose production remains unclear, but itsprimary site of action appears to be hepatocyte mitochon-dria, where it disrupts respiratory chain oxidation of com-
Metformin decreases hepatic gluconeogenesis by interfering with respi-
plex I substrates (for example, glutamate) (15, 39). Inhibi-
ratory oxidation in mitochondria. It suppresses gluconeogenesis fromseveral substrates, including lactate, pyruvate, glycerol, and amino acids.
tion of cellular respiration decreases gluconeogenesis (39)
In addition, metformin increases intramitochondrial levels of calcium
and may induce expression of glucose transporters and,
(Ca⫹⫹), a modulator of mitochondrial respiration. In insulin-sensitive
therefore, glucose utilization (40). It is not clear whether
tissues (such as skeletal muscle), metformin facilitates glucose transportby increasing tyrosine kinase activity in insulin receptors and enhancing
metformin acts on mitochondrial respiration directly by
glucose transporter (GLUT) trafficking to the cell membrane. ADP ⫽
slow permeation across the inner mitochondrial membrane
䢇䢇䢇; ATP ⫽ 䢇䢇䢇; Ca⫹⫹ ⫽ intracellular calcium levels; OAA ⫽
(39) or by unidentified cell-signaling pathways (15). It has
䢇䢇䢇; PEP ⫽ phosphoenolpyruvate; Pi ⫽ 䢇䢇䢇; TK ⫽ 䢇䢇䢇.
been suggested that biguanides bind specifically and com-petitively to divalent cation sites on proteins, thus interfer-
cogen synthesis (47) (Figure 1). Thus, metformin has met-
ing with intracellular handling of calcium ([Ca2⫹]i) (41,
abolic effects on insulin-sensitive tissues that may
42) especially in the mitochondria (41). Davidoff and col-
contribute to its glucose-lowering effect.
leagues (41) showed that even small doses of biguanides
Metformin has been shown to reduce free fatty acid
increase the rates of [Ca2⫹]i uptake in isolated hepatic
oxidation by 10% to 30% (25, 31–33). Elevated levels of
mitochondria, where [Ca2⫹]i serves as a potent activator of
free fatty acid are commonly seen in diabetes and obesity
mitochondrial respiration (Figure 1). This effect was
(48), and they contribute to increased hepatic glucose pro-
shown at biguanide concentrations as low as 5 to 10 m
duction and development of insulin resistance (49, 54)
(41), levels that are expected in the liver with antihypergly-
(Figure 2). Increased fatty acid oxidation inhibits key en-
cemic doses of the drug and are 20- to 50-fold lower than
zymes of the glycolytic pathway by accumulation of acetyl
those that inhibit mitochondrial respiration. In several tis-
coenzyme A and citrate, by-products of free fatty acid ox-
sues, including skeletal muscle and adipocytes, metformin
idation (51). Increased glucose 6-phosphate concentra-
facilitates trafficking of glucose transporters 4 and 1 to the
tions, in turn, inhibit the hexokinase enzyme, resulting in
plasma membrane (25, 31, 43). Moreover, metformin may
reduced glucose uptake and oxidation (51). In addition,
increase the glucose transport capacity of glucose trans-
free fatty acid independently inhibits insulin receptor sub-
porter 4, and to some extent, glucose transporters 1 (31).
strate-1–associated PI3-kinase activity (52) and subse-
The effects of metformin on peripheral insulin-sensi-
quently attenuates transmembrane glucose transport (48)
tive tissues require the presence of insulin for its full action.
(Figure 2). By decreasing free fatty acid levels, metformin
Metformin enhances most of the biological actions of in-
not only improves insulin sensitivity but may also help
sulin, including glucose transport and glycogen and lipid
correct impaired insulin secretion by -cells (53). Met-
synthesis, in persons with preexisting insulin resistance
formin has no direct effect on -cell function (9), but it
(31). It facilitates glucose transport in cultured skeletal
can improve insulin secretion that has been altered by
muscle in the absence of insulin (44, 45). Metformin acti-
long-term exposure to free fatty acid or hyperglycemia
vates insulin and tyrosine kinase activity in insulin-like
(glucose toxicity) (53).
growth factor-1 receptor of vascular smooth-muscle cells
Metformin may also improve hyperglycemia by attain-
independently of insulin action (46). The drug activates
ing high concentrations in the small intestine (17, 31) and
tyrosine kinase in Xenopus oocytes, with subsequent stim-
decreasing intestinal absorption of glucose (29, 54), an ac-
ulation of inositol 1,4,5-triphosphate production and gly-
tion that may contribute to decreased postprandial blood
2 July 2002 Annals of Internal Medicine Volume 137 • Number 1 E-27
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
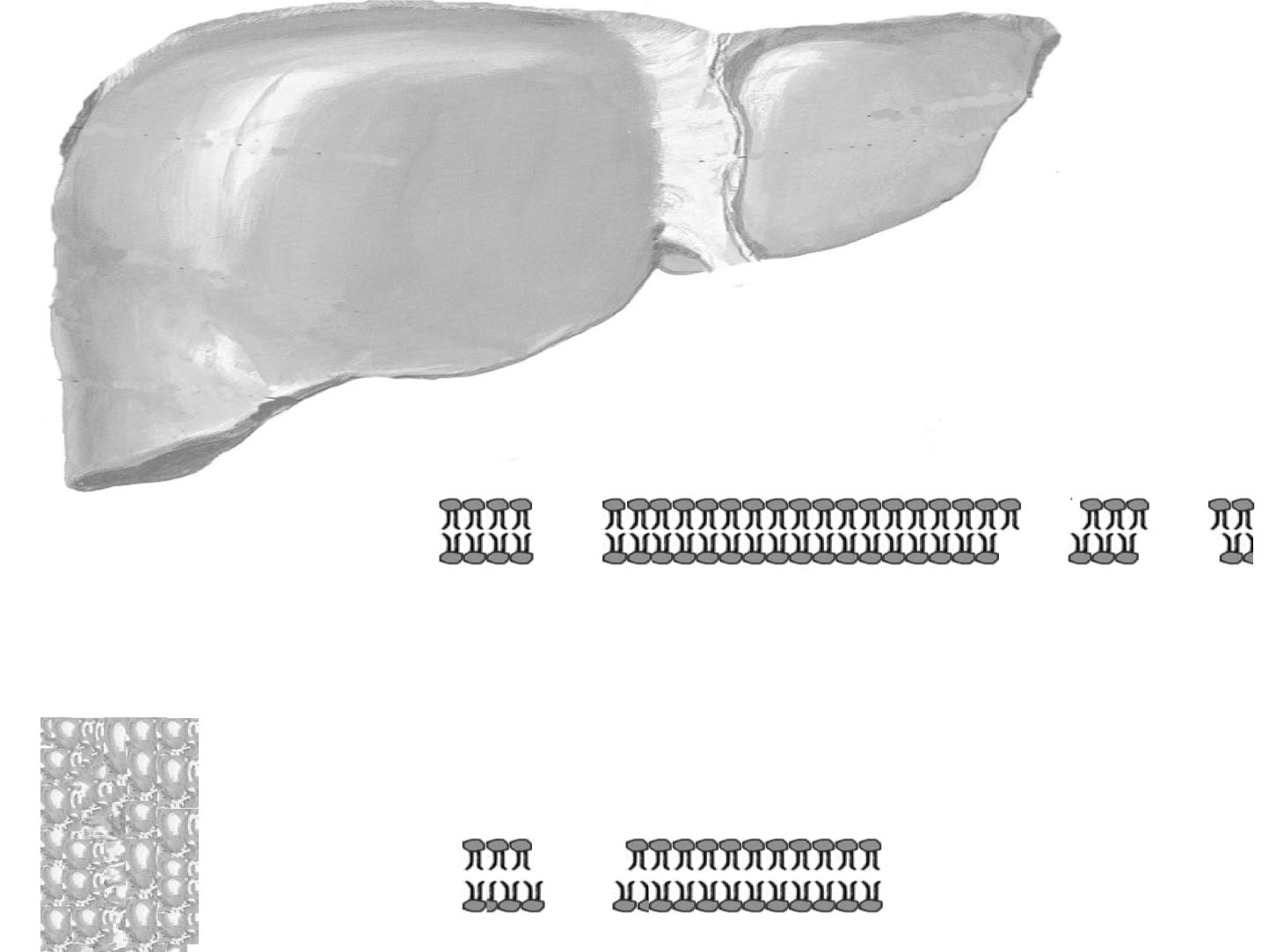
Update Metformin: An Update
Figure 2. Metformin and fatty acids.
Metformin inhibits fatty acid (FA) production and oxidation, thereby reducing fatty acid–induced insulin resistance and hepatic glucose production.
CoA ⫽ coenzyme A; CPT ⫽ 䢇䢇䢇; FFA ⫽ free fatty acid; GLUT ⫽ glucose transporter; IGF-1 ⫽ 䢇䢇䢇; IRS-1 ⫽ 䢇䢇䢇; OAA ⫽ 䢇䢇䢇; PDH ⫽
䢇䢇䢇; PFK ⫽ 䢇䢇䢇; PI-3 ⫽ 䢇䢇䢇.
glucose levels (55). It has been speculated that increased
Clinically, administration of metformin improves hirsutism
glucose consumption in the small intestine of metformin-
(11), normalizes menstrual cycles (11, 12, 57, 59), and in-
treated patients may prevent further glucose transport to
duces ovulation (57, 59) in a substantial number of patients
the hepatic circulation (29).
with the polycystic ovary syndrome.
In summary, metformin decreases hepatic glucose pro-
duction through inhibition of gluconeogenesis and possi-
EFFECT OF METFORMIN TREATMENT ON
bly glycogenolysis and improves peripheral insulin sensitiv-
CARDIOVASCULAR MORBIDITY AND MORTALITY
ity. In addition, metformin decreases gastrointestinalglucose absorption and indirectly improves pancreatic
In the UKPDS 34, metformin therapy was compared
-cell response to glucose by reducing glucose toxicity and
with conventional treatment or treatment with sulfonyl-
free fatty acid levels.
urea or insulin (5). In this trial, which was designed toachieve fasting plasma glucose levels less than 6 mmol/L(⬍108 mg/dL), 342 patients with newly diagnosed type 2
EFFECT OF METFORMIN IN THE POLYCYSTIC OVARY
diabetes were allocated to receive metformin treatment and
951 patients were allocated to receive either chlorpropam-
Hyperinsulinemia reflecting insulin resistance is a com-
ide, glibenclamide, or insulin. The control group included
mon feature in lean and obese patients with the polycystic
411 overweight diabetic patients who were randomly as-
ovary syndrome (11, 56, 57). Hyperinsulinemia contributes
signed to conventional therapy, primarily with diet alone,
directly to excessive testosterone production by the ovaries
which resulted in suboptimal glycemic control. During 10
(56) and decreased synthesis of sex hormone– binding globu-
years of follow-up, both drug-treated groups achieved
lin in the liver (11, 58), thereby increasing levels of total and
equal degrees of glycemic control (median hemoglobin A1c
free testosterone. Metformin therapy increases insulin sensitiv-
value of 0.074 [7.4%]), whereas the conventionally treated
ity and decreases insulin levels in patients with the polycystic
group had a median hemoglobin A1c value of 0.08 (8.0%)
ovary syndrome (56, 57, 59). Improvement of hyperinsulin-
(5). Compared with the conventionally treated group,
emia is associated with decreased levels of total and free tes-
metformin-treated patients had a risk reduction of 32%
tosterone (11, 12, 57, 59) and increased estradiol (12) levels.
any diabetes-related end point, 39% for myocardial infarc-
E-28 2 July 2002 Annals of Internal Medicine Volume 137 • Number 1
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
Metformin: An Update Update
tion, 42% for diabetes-related death, and 36% for all-cause
poorly controlled diabetes (48) contribute not only to de-
mortality (5). These differences may be partially explained
velopment of insulin insensitivity but also to increased syn-
by differences in the degree of glycemic control between
thesis and secretion of very low-density lipoprotein (69).
the metformin and diet groups. In the UKPDS 35 (60),
Elevated triglyceride levels inhibit degradation of apopro-
the risk for cardiovascular events, stroke, and all-cause
tein B in the liver and lead to increased assembly of very
death was closely related to the degree of glycemia in dia-
low-density lipoprotein and smaller, denser LDL particles
betic patients. In that study, each 1% reduction in the
(69). Excessive generation of reactive oxygen species and
hemoglobin A1c value during treatment of type 2 diabetes
free radicals (such as peroxynitrates) by cardiovascular tis-
was associated with a reduction of 21% in diabetes-related
sue, in combination with increased nonenzymatic glycation
deaths, 14% in the incidence of myocardial infarction,
of lipoproteins (glycooxidation), leads to formation of
12% in fatal and nonfatal strokes, and 16% in heart failure
atypical glycooxidized LDL particles. These particles bind
(60). Nevertheless, metformin was more effective than sul-
poorly to classic LDL receptors but have high affinity for
fonylureas or insulin in reducing rates of any diabetes-
"scavenger" receptors, which are located predominantly on
related end point, all-cause mortality, and stroke, even
macrophages (63). Accumulation of glycooxidized small,
though both agents decreased hemoglobin A1c values
dense LDL particles converts macrophages into foam cells,
equally (5). These observations suggest that metformin
which are essential participants in the early steps of athero-
might have additional cardiovascular protective actions be-
sclerotic plaque formation (63). Compared with the gen-
yond its antihyperglycemic properties. However, data indi-
eral population, diabetic persons have a twofold to fourfold
cate that metformin in combination with sulfonylurea
increased risk for cardiovascular disease at any cholesterol
might increase cardiovascular mortality in patients with
level (70), which indicates a more aggressive type of dys-
type 2 diabetes (5, 61). In those studies, metformin was
lipidemia. Furthermore, decreasing cholesterol and triglyc-
not used as an initial therapy but rather was added to
eride levels has been shown to be particularly beneficial in
treatment when sulfonylurea therapy failed. Patients taking
patients with diabetes (70, 71). In addition, hypertriglyc-
combination therapy with metformin and sulfonylurea
eridemia may be an independent risk factor for cardiovas-
tended to have long-standing poorly controlled diabetes
cular disease in patients with type 2 diabetes (72). Met-
before addition of the biguanide (62). Moreover, they had
formin has major effects on lipid metabolism in patients
greater obesity (61), which could independently increase
with insulin resistance. It decreases plasma levels of free
mortality. Therefore, the reported increase in risk for car-
fatty acid (20, 73) and oxidation of these acids by tissue
diovascular disease in patients treated with combination
(25, 28, 32); it decreases levels of triglycerides (2, 10, 20,
therapy might reflect selection bias attributable to the natural
55, 74) and, therefore, very low-density lipoprotein (20).
history of long-standing diabetes rather than to adverse effects
Metformin therapy decreases levels of total cholesterol (2,
of this combination.
68, 74) and LDL cholesterol (2, 68, 74) while maintaining(68, 74) or increasing (2, 20, 55, 57, 67) levels of high-density lipoprotein cholesterol. Metformin decreases oxida-
MECHANISM OF THE CARDIOPROTECTIVE ACTION OF
tive stress and reduces lipid oxidation (75) by lowering
plasma glucose levels (2). Taken together, these observa-
Insulin resistance, a cornerstone of type 2 diabetes and
tions suggest that the beneficial effects of metformin on
the metabolic cardiovascular syndrome, is commonly asso-
lipoprotein metabolism may contribute to its protective
ciated with hypertension, abdominal obesity, atherogenic
effects against cardiovascular disease.
dyslipidemia, and vascular dysfunction, all of which con-
Metformin has also been shown to lessen hypercoagula-
tribute greatly to the development of accelerated athero-
tion and increase fibrinolysis in insulin-resistant states by de-
sclerosis (63). Hyperinsulinemia reflects insulin resistance
creasing levels of plasminogen activator inhibitor-1 (76, 77)
and may be an independent risk factor for coronary artery
and increasing tissue plasminogen activator activity (74).
disease (64 – 66). Metformin, an insulin-sensitizing agent,
Therapy with metformin also reduces thrombogenic propen-
decreases insulin resistance in patients with (20, 31, 55)
sity by decreasing levels of tissue plasminogen activator anti-
and without (11, 12, 57, 67) diabetes, thus effectively re-
gen (78) and von Willebrand factor (78). In the Biguanides
ducing baseline and glucose-stimulated insulin levels (12,
and the Prevention of the Risk of Obesity study, 457 nondi-
20, 55, 57, 67).
abetic patients with visceral obesity (body mass index of 32.5
Several studies have shown that metformin improves
kg/m2) were randomly assigned to treatment with diet or met-
lipoprotein profiles in diabetic patients (2, 10, 20, 55, 68).
formin (850 mg twice daily) (78). Weight loss was associated
Dyslipidemia in diabetes is characterized by hypertriglycer-
with a 30% to 40% decrease in plasminogen activator inhib-
idemia (increased levels of very low-density lipoprotein
itor-1 activity, regardless of the method used, whereas met-
cholesterol); decreased levels of high-density lipoprotein
formin produced significantly larger decreases in von Wille-
cholesterol; and elevated levels of small, dense atherogenic
brand factor levels than did diet therapy (78). Furthermore,
low-density lipoprotein cholesterol (LDL) particles. The
metformin therapy decreased platelet aggregation in diabetic
increased levels of free fatty acid that occur in obesity and
patients treated with 1700 mg/d (79). Thus, metformin ther-
2 July 2002 Annals of Internal Medicine Volume 137 • Number 1 E-29
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
Update Metformin: An Update
Table. Direct and Indirect Cardiovascular Protective Effects of
over, insulin is responsible for the normal handling of di-
valent cations in vascular smooth muscle (92). Those pro-cesses are altered in insulin resistance. Impaired vascular
Decreases hyperglycemia
insulin action may result in impaired nitric oxide–
Improves diastolic functionDecreases total cholesterol levels
dependent vascular relaxation, decreased sodium pump ac-
Decreases very low-density lipoprotein cholesterol levels
tivity, and increased levels of [Ca2⫹]i in vascular smooth
Decreases low-density lipoprotein cholesterol levels
muscle in patients with type 2 diabetes (14, 63, 92). These
Increases high-density lipoprotein cholesterol levelsDecreases oxidative stress
abnormalities in divalent cation and nitric oxide metabo-
Improves vascular relaxation
lism appear to play a role in the increased vascular resis-
Decreases plasminogen activator inhibitor-1 levels
tance and impaired vasorelaxation that characterize hyper-
Increases tissue plasminogen activator activityDecreases von Willebrand factor levels
tension, which frequently occurs in diabetic patients (92).
Decreases platelet aggregation and adhesion
Several reports indicate an antihypertensive effect of
metformin in animals (88, 93–95) and humans (74, 96).
In contrast, no effect of metformin on blood pressure was
apy appears to lessen the hypercoagulability and exaggerated
reported in other human studies (1, 2, 23). Careful 24-
platelet aggregation and adhesion in diabetic patients (Table).
hour ambulatory studies may better characterize the effectsof metformin on blood pressure in diabetic patients (89).
Potential mechanisms of antihypertensive action of met-
ETFORMIN AND DIABETIC CARDIOMYOPATHY
formin are complex and include both insulin-dependent
Persons with diabetes have a high prevalence of con-
and insulin-independent vasodilatory actions (Figure 3).
gestive heart failure (80) secondary to diabetic, hyperten-
Acute administration of metformin to rat tail arteries in-
sive, and ischemic changes in the myocardium. Diabetic
creases repolarization and causes subsequent artery relax-
cardiomyopathy, a unique clinical entity, is characterized
ation (97) through reduction in agonist-induced increase
by structural changes in the myocardium (fibrosis) and
in intracellular levels of [Ca2⫹]i vascular smooth muscle
functional alterations in diastolic relaxation and ventricular
(46, 94). This attenuation of [Ca2⫹]i responses may be
compliance (81– 84) (Figure 3). Delayed diastolic relax-
secondary to increased nitric oxide production by vascular
ation in diabetic cardiomyopathy is related to diminished
smooth muscle during exposure to metformin (94). In-
removal of [Ca2⫹]i from cardiomyocytes after systolic con-
deed, nitric oxide has been shown to decrease vascular
traction (82, 83). Hyperglycemia has been shown to con-
smooth muscle [Ca2⫹]i responses to vasoconstrictor ago-
tribute to these functional changes (82– 84), and insulin
nists through activation of the cyclic guanosine monophos-
resistance also directly contributes to these abnormalities
phate pathway (98). Metformin may also reduce [Ca2⫹]i
(85). Metformin treatment of streptozotocin diabetic ratscorrects these functional cardiac abnormalities (84, 86),perhaps through tyrosine kinase– dependent increases in
Figure 3. Proposed cellular mechanisms of metformin action in
intracellular [Ca2⫹]i removal after systole (84). This car-
the vascular smooth-muscle cells and cardiomyocytes.
dioprotective action of metformin was shown to be insulinindependent (84). Moreover, treatment of spontaneouslyhypertensive rats with metformin has been reported to de-crease heart rate (a sympathoinhibitory effect) more thanplacebo (87, 88). Although these findings are of interest,no clinical trials to date have investigated the effect of met-formin on the development and course of congestive heartfailure in diabetic patients.
METFORMIN AND VASCULAR REACTIVITY
Hypertension is often associated with insulin resis-
tance (89). Diabetic patients have a higher incidence ofhypertension compared with the general population, andhypertensive persons are more prone to develop diabetes(90, 91). Recently, investigators demonstrated that defec-tive insulin signaling may contribute to increased vascularresistance (92), which is the hallmark of hypertension in
In vascular smooth-muscle cells, metformin decreases vasoconstriction by
type 2 diabetes (89). Insulin normally acts through the
enhancing sodium pump activity and nitric oxide (NO) production,
PI3-kinase pathway to activate nitric oxide synthase, en-
causing a decrease in intracellular calcium levels (Ca⫹⫹). Metforminimproves diastolic relaxation by enhancing calcium removal from cardio-
hance sodium pump activity in vascular smooth muscle,
myocytes after systole. ATP ⫽ 䢇䢇䢇; CGMP ⫽ 䢇䢇䢇; GTP ⫽
and increase glucose transmembrane transport (63). More-
䢇䢇䢇; K-ATP ⫽ 䢇䢇䢇.
E-30 2 July 2002 Annals of Internal Medicine Volume 137 • Number 1
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
Metformin: An Update Update
by increasing the activity of the sodium–adenosine triphos-
Grant Support: By the National Institutes of Health (RO1-HL-63904-
phatase pump (99) and enhancing adenosine triphosphate–
01), the Veterans Affairs Merit Review, and the American Diabetic As-
sensitive K⫹channels (100) (Figure 3). The ability of met-
formin to stimulate sodium pump activity is probably
Requests for Single Reprints: James R. Sowers, MD, State University
linked to increased lactate production in vascular smooth
of New York Health Science Center at Brooklyn, 450 Clarkson Avenue,
muscle (99, 101). Metformin may have central antihyper-
Box 1205, Brooklyn, NY 11203; e-mail, [email protected].
tensive actions, because infusion of this drug into lateralcerebral ventricles of spontaneous hypertensive rats pro-
Current Author Addresses: Drs. Kirpichnikov, McFarlane, and Sowers:
duced dose-dependent decreases in mean arterial pressure,
State University of New York Health Science Center at Brooklyn, 450
heart rate, and renal sympathetic nerve activity (88).
Clarkson Avenue, Box 1205, Brooklyn, New York 11203.
Even a small elevation in blood pressure significantly
Current author addresses are available at www.annals.org.
increases death from cardiovascular disease and risk formyocardial infarction, stroke, and congestive heart failurein diabetic persons (102). Each 10 –mm Hg increase in
systolic blood pressure produces a 15% increase in the rate
1. Hermann LS, Scherste´n B, Bitze´n PO, Kjellstro¨m T, Lindga¨rde F, Melander
of death related to diabetes and an 11% increase in inci-
A. Therapeutic comparison of metformin and sulfonylurea, alone and in various
combinations. A double-blind controlled study. Diabetes Care. 1994;17:1100-9.
dence of myocardial infarction, 19% in stroke, and 12% in
congestive heart failure (101). Therefore, even a minimal
2. DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-
reduction in blood pressure during treatment with met-
insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group.
formin may contribute to a significant decrease in diabetes-
N Engl J Med. 1995;333:541-9. [PMID: 7623902]
related morbidity and mortality.
3. United Kingdom Prospective Diabetes Study (UKPDS). 13: Relative efficacyof randomly allocated diet, sulphonylurea, insulin, or metformin in patients withnewly diagnosed non-insulin dependent diabetes followed for three years. BMJ.
1995;310:83-8. [PMID: 7833731]
4. Johansen K. Efficacy of metformin in the treatment of NIDDM. Meta-anal-
ysis. Diabetes Care. 1999;22:33-7. [PMID: 10333900]
In summary, metformin, the only biguanide available
5. Effect of intensive blood-glucose control with metformin on complications in
in the United States, is a potent insulin-sensitizing agent
overweight patients with type 2 diabetes (UKPDS 34). UK Prospective Diabetes
that acts primarily on hepatic glucose production and has
Study (UKPDS) Group. Lancet. 1998;352:854-65. [PMID: 9742977]
6. Garber AJ, Duncan TG, Goodman AM, Mills DJ, Rohlf JL. Efficacy of
additional effects on peripheral insulin sensitivity. Its major
metformin in type II diabetes: results of a double-blind, placebo-controlled, dose-
antihyperglycemic effects are mediated through reduction
response trial. Am J Med. 1997;103:491-7. [PMID: 9428832]
in hepatic gluconeogenesis, perhaps by affecting mitochon-
7. Selby JV, Ettinger B, Swain BE, Brown JB. First 20 months' experience with
drial [Ca2⫹]i handling. Metformin has an excellent safety
use of metformin for type 2 diabetes in a large health maintenance organization.
profile and is effective as monotherapy or in combination
Diabetes Care. 1999;22:38-44. [PMID: 10333901]
8. Davidson MB, Peters AL. An overview of metformin in the treatment of type
with sulfonylureas, insulin, and thiazolidinediones. Unlike
2 diabetes mellitus. Am J Med. 1997;102:99-110. [PMID: 9209206]
insulin, sulfonylurea, and thiazolidinediones, metformin
9. DeFronzo RA. Pharmacologic therapy for type 2 diabetes mellitus. Ann Intern
does not promote weight gain and may even cause weight
Med. 1999;131:281-303. [PMID: 10454950]
reduction in obese patients. It appears to have substantial
10. Haupt E, Knick B, Koschinsky T, Liebermeister H, Schneider J, Hirche H.
beneficial effects on lipid metabolism, clotting factors, and
Oral antidiabetic combination therapy with sulphonylureas and metformin. Di-abetes Metab. 1991;17:224-31. [PMID: 1936481]
platelet function. In laboratory animals, metformin has
11. Pasquali R, Gambineri A, Biscotti D, Vicennati V, Gagliardi L, Colitta D,
been shown to correct diabetes-induced cardiac diastolic
et al. Effect of long-term treatment with metformin added to hypocaloric diet on
dysfunction. Metformin improves vascular relaxation and
body composition, fat distribution, and androgen and insulin levels in abdomi-
probably decreases blood pressure in selected patients.
nally obese women with and without the polycystic ovary syndrome. J Clin
These effects may contribute to improved cardiovascular
Endocrinol Metab. 2000;85:2767-74. [PMID: 10946879]
12. Glueck CJ, Wang P, Fontaine R, Tracy T, Sieve-Smith L. Metformin-
mortality rates during monotherapy. Observations from
induced resumption of normal menses in 39 of 43 (91%) previously amenorrheic
the UKPDS of increased mortality during combination
women with the polycystic ovary syndrome. Metabolism. 1999;48:511-9.
therapy with metformin plus sulfonylurea are probably
attributable to the natural course of type 2 diabetes
13. Sowers JR. Obesity and cardiovascular disease. Clin Chem. 1998;44:1821-5.
[PMID: 9702991]
rather than to the effect of therapy itself and require further
14. McFarlane SI, Banerji M, Sowers JR. Insulin resistance and cardiovascular
disease. J Clin Endocrinol Metab. 2001;86:713-8. [PMID: 11158035]
15. Yki-Ja¨rvinen H, Nikkila¨ K, Ma¨kimattila S. Metformin prevents weight gain
by reducing dietary intake during insulin therapy in patients with type 2 diabetes
From State University of New York Health Science Center at Brooklyn
mellitus. Drugs. 1999;58 Suppl 1:53-4; discussion 75-82. [PMID: 10576526]
and Veterans Affairs Medical Center, Brooklyn, New York.
16. Scheen AJ. Clinical pharmacokinetics of metformin. Clin Pharmacokinet.
1996;30:359-71. [PMID: 8743335]
Acknowledgments: The authors thank Jun Ren, PhD; Amy Davidoff,
17. Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal
PhD; and Jacob Peuler, PhD, for their seminal contribution to work on
and diabetic mouse. Xenobiotica. 1994;24:49-57. [PMID: 8165821]
the cardiovascular effects of metformin.
18. Lalau JD, Andrejak M, Morinie re P, Coevoet B, Debussche X, Westeel PF,
2 July 2002 Annals of Internal Medicine Volume 137 • Number 1 E-31
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
Update Metformin: An Update
et al. Hemodialysis in the treatment of lactic acidosis in diabetics treated by
40. Ebert BL, Firth JD, Ratcliffe PJ. Hypoxia and mitochondrial inhibitors
metformin: a study of metformin elimination. Int J Clin Pharmacol Ther Toxi-
regulate expression of glucose transporter-1 via distinct Cis-acting sequences.
col. 1989;27:285-8. [PMID: 2500402]
J Biol Chem. 1995;270:29083-9. [PMID: 7493931]
19. Somogyi A, Stockley C, Keal J, Rolan P, Bochner F. Reduction of met-
41. Davidoff F, Bertolini D, Haas D. Enhancement of the mitochondrial Ca2⫹
formin renal tubular secretion by cimetidine in man. Br J Clin Pharmacol. 1987;
uptake rate by phenethylbiguanide and other organic cations with hypoglycemic
23:545-51. [PMID: 3593625]
activity. Diabetes. 1978;27:757-65. [PMID: 658623]
20. Reaven GM, Johnston P, Hollenbeck CB, Skowronski R, Zhang JC, Gold-
42. Davidoff F, Carr S. Calcium-like action of phenethylbiguanide and related
fine ID, et al. Combined metformin-sulfonylurea treatment of patients with
compounds: inhibition of pyruvate kinase. Proc Natl Acad Sci U S A. 1972;69:
noninsulin-dependent diabetes in fair to poor glycemic control. J Clin Endocrinol
1957-61. [PMID: 4505673]
Metab. 1992;74:1020-6. [PMID: 1569149]
43. Kozka IJ, Holman GD. Metformin blocks downregulation of cell surface
21. Riddle M. Combining sulfonylureas and other oral agents. Am J Med. 2000;
GLUT4 caused by chronic insulin treatment of rat adipocytes. Diabetes. 1993;
108 Suppl 6a:15S-22S. [PMID: 10764846]
42:1159-65. [PMID: 8325447]
22. Fonseca V, Rosenstock J, Patwardhan R, Salzman A. Effect of metformin
44. Klip A, Guma A, Ramlal T, Bilan PJ, Lam L, Leiter LA. Stimulation of
and rosiglitazone combination therapy in patients with type 2 diabetes mellitus: a
hexose transport by metformin in L6 muscle cells in culture. Endocrinology.
randomized controlled trial. JAMA. 2000;283:1695-702. [PMID: 10755495]
1992;130:2535-44. [PMID: 1572281]
23. UKPDS 28: a randomized trial of efficacy of early addition of metformin in
45. Hundal HS, Ramlal T, Reyes R, Leiter LA, Klip A. Cellular mechanism of
sulfonylurea-treated type 2 diabetes. U.K. Prospective Diabetes Study Group.
metformin action involves glucose transporter translocation from an intracellular
Diabetes Care. 1998;21:87-92. [PMID: 9538975]
pool to the plasma membrane in L6 muscle cells. Endocrinology. 1992;131:
24. Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet,
1165-73. [PMID: 1505458]
sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus:
46. Dominguez LJ, Davidoff AJ, Srinivas PR, Standley PR, Walsh MF, Sowers
progressive requirement for multiple therapies (UKPDS 49). UK Prospective
JR. Effects of metformin on tyrosine kinase activity, glucose transport, and intra-
Diabetes Study (UKPDS) Group. JAMA. 1999;281:2005-12. [PMID:
cellular calcium in rat vascular smooth muscle. Endocrinology. 1996;137:113-21.
25. Bailey CJ, Turner RC. Metformin. N Engl J Med. 1996;334:574-9. [PMID:
47. Stith BJ, Goalstone ML, Espinoza R, Mossel C, Roberts D, Wiernsperger
N. The antidiabetic drug metformin elevates receptor tyrosine kinase activity and
26. Misbin RI, Green L, Stadel BV, Gueriguian JL, Gubbi A, Fleming GA.
inositol 1,4,5-trisphosphate mass in Xenopus oocytes. Endocrinology. 1996;137:
Lactic acidosis in patients with diabetes treated with metformin [Letter]. N Engl
2990-9. [PMID: 8770923]
J Med. 1998;338:265-6. [PMID: 9441244]
48. Shulman GI. Cellular mechanisms of insulin resistance in humans. Am J
27. Cusi K, DeFronzo RA. Metformin: a review of its metabolic effects. Diabetes
Cardiol. 1999;84:3J-10J. [PMID: 10418851]
49. Clore JN, Glickman PS, Nestler JE, Blackard WG. In vivo evidence for
28. Lalau JD, Race JM. Lactic acidosis in metformin therapy. Drugs. 1999;58
hepatic autoregulation during FFAfree fatty acid-stimulated gluconeogenesis in
Suppl 1:55-60; discussion 75-82. [PMID: 10576527]
normal humans. Am J Physiol. 1991;261:E425-9. [PMID: 1928334]
29. Ikeda T, Iwata K, Murakami H. Inhibitory effect of metformin on intestinal
50. Sindelar DK, Chu CA, Rohlie M, Neal DW, Swift LL, Cherrington AD.
glucose absorption in the perfused rat intestine. Biochem Pharmacol. 2000;59:
The role of fatty acids in mediating the effects of peripheral insulin on hepatic
887-90. [PMID: 10718348]
glucose production in the conscious dog. Diabetes. 1997;46:187-96. [PMID:
30. Bauman WA, Shaw S, Jayatilleke E, Spungen AM, Herbert V. Increased
intake of calcium reverses vitamin B12 malabsorption induced by metformin.
51. Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin
Diabetes Care. 2000;23:1227-31. [PMID: 10977010]
resistance: a reexamination. Diabetes. 2000;49:677-83. [PMID: 10905472]
31. Wiernsperger NF, Bailey CJ. The antihyperglycaemic effect of metformin:
52. Dresner A, Laurent D, Marcucci M, Griffin ME, Dufour S, Cline GW, et
therapeutic and cellular mechanisms. Drugs. 1999;58 Suppl 1:31-9; discussion
al. Effects of free fatty acids on glucose transport and IRS-1-associated phospha-
75-82. [PMID: 10576523]
tidylinositol 3-kinase activity. J Clin Invest. 1999;103:253-9. [PMID: 9916137]
32. Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V,
53. Patane G, Piro S, Rabuazzo AM, Anello M, Vigneri R, Purrello F. Met-
et al. Mechanism by which metformin reduces glucose production in type 2
formin restores insulin secretion altered by chronic exposure to free fatty acids or
diabetes. Diabetes. 2000;49:2063-9. [PMID: 11118008]
high glucose: a direct metformin effect on pancreatic beta-cells. Diabetes. 2000;
33. Perriello G, Misericordia P, Volpi E, Santucci A, Santucci C, Ferrannini E,
49:735-40. [PMID: 10905481]
et al. Acute antihyperglycemic mechanisms of metformin in NIDDM. Evidence
54. Wilcock C, Bailey CJ. Reconsideration of inhibitory effect of metformin on
for suppression of lipid oxidation and hepatic glucose production. Diabetes.
intestinal glucose absorption. J Pharm Pharmacol. 1991;43:120-1. [PMID:
1994;43:920-8. [PMID: 8013758]
34. Large V, Beylot M. Modifications of citric acid cycle activity and gluconeo-
55. Wu MS, Johnston P, Sheu WH, Hollenbeck CB, Jeng CY, Goldfine ID, et
genesis in streptozotocin-induced diabetes and effects of metformin. Diabetes.
al. Effect of metformin on carbohydrate and lipoprotein metabolism in NIDDM
1999;48:1251-7. [PMID: 10342812]
patients. Diabetes Care. 1990;13:1-8. [PMID: 2404714]
35. Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, et al.
56. Pugeat M, Ducluzeau PH. Insulin resistance, polycystic ovary syndrome and
Efficacy and metabolic effects of metformin and troglitazone in type II diabetes
metformin. Drugs. 1999;58 Suppl 1:41-6; discussion 75-82. [PMID: 10576524]
mellitus. N Engl J Med. 1998;338:867-72. [PMID: 9516221]
57. Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al.
36. Jeng CY, Sheu WH, Fuh MM, Chen YD, Reaven GM. Relationship be-
Metformin effects on clinical features, endocrine and metabolic profiles, and
tween hepatic glucose production and fasting plasma glucose concentration in
insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, pla-
patients with NIDDM. Diabetes. 1994;43:1440-4. [PMID: 7958496]
cebo-controlled 6-month trial, followed by open, long-term clinical evaluation.
37. Radziuk J, Zhang Z, Wiernsperger N, Pye S. Effects of metformin on lactate
J Clin Endocrinol Metab. 2000;85:139-46. [PMID: 10634377]
uptake and gluconeogenesis in the perfused rat liver. Diabetes. 1997;46:1406-13.
58. Nestler JE, Powers LP, Matt DW, Steingold KA, Plymate SR, Rittmaster
RS, et al. A direct effect of hyperinsulinemia on serum sex hormone-binding
38. Argaud D, Roth H, Wiernsperger N, Leverve XM. Metformin decreases
globulin levels in obese women with the polycystic ovary syndrome. J Clin En-
gluconeogenesis by enhancing the pyruvate kinase flux in isolated rat hepatocytes.
docrinol Metab. 1991;72:83-9. [PMID: 1898744]
Eur J Biochem. 1993;213:1341-8. [PMID: 8504825]
59. Velazquez EM, Mendoza S, Hamer T, Sosa F, Glueck CJ. Metformin
39. Dominguez LJ, Davidoff AJ, Srinivas PR, Standley PR, Walsh MF, Sowers
therapy in polycystic ovary syndrome reduces hyperinsulinemia, insulin resis-
JR. Effects of metformin on tyrosine kinase activity, glucose transport, and intra-
tance, hyperandrogenemia, and systolic blood pressure, while facilitating normal
cellular calcium in rat vascular smooth muscle. Endocrinology. 1996;137:113-21.
menses and pregnancy. Metabolism. 1994;43:647-54. [PMID: 8177055]
60. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, et al.
E-32 2 July 2002 Annals of Internal Medicine Volume 137 • Number 1
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
Metformin: An Update Update
Association of glycaemia with macrovascular and microvascular complications of
82. Ren J, Davidoff AJ. Diabetes rapidly induces contractile dysfunctions in
type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:
isolated ventricular myocytes. Am J Physiol. 1997;272:H148-58. [PMID:
405-12. [PMID: 10938048]
61. Olsson J, Lindberg G, Gottsa¨ter M, Lindwall K, Sjo¨strand A, Tisell A, et al.
83. Ren J, Dominguez LJ, Sowers JR, Davidoff AJ. Troglitazone attenuates
Increased mortality in Type II diabetic patients using sulphonylurea and met-
high-glucose-induced abnormalities in relaxation and intracellular calcium in rat
formin in combination: a population-based observational study. Diabetologia.
ventricular myocytes. Diabetes. 1996;45:1822-5. [PMID: 8922371]
2000;43:558-60. [PMID: 10855529]
84. Ren J, Dominguez LJ, Sowers JR, Davidoff AJ. Metformin but not gly-
62. Turner RC, Holman RR. Metformin and risk of cardiovascular disease.
buride prevents high glucose-induced abnormalities in relaxation and intracellular
Cardiology. 1999;91:203-4. [PMID: 10516415]
Ca2⫹ transients in adult rat ventricular myocytes. Diabetes. 1999;48:2059-65.
63. Kirpichnikov D, Sowers JR. Diabetes mellitus and diabetes-associated vas-
cular disease. Trends Endocrinol Metab. 2001;12:225-30. [PMID: 11397648]
85. Ren J, Sowers JR, Walsh MF, Brown RA. Reduced contractile response to
64. Ruige JB, Assendelft WJ, Dekker JM, Kostense PJ, Heine RJ, Bouter LM.
insulin and IGF-I in ventricular myocytes from genetically obese Zucker rats.
Insulin and risk of cardiovascular disease: a meta-analysis. Circulation. 1998;97:
Am J Physiol Heart Circ Physiol. 2000;279:H1708-14. [PMID: 11009458]
996-1001. [PMID: 9529268]
86. Verma S, McNeill JH. Metformin improves cardiac function in isolated
65. Haffner SM. Epidemiology of insulin resistance and its relation to coronary
streptozotocin-diabetic rat hearts. Am J Physiol. 1994;266:H714-9. [PMID:
artery disease. Am J Cardiol. 1999;84:11J-14J. [PMID: 10418852]
66. Despre´s JP, Lamarche B, Maurie ge P, Cantin B, Dagenais GR, Moorjani S,
87. Muntzel MS, Hamidou I, Barrett S. Metformin attenuates salt-induced
et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease.
hypertension in spontaneously hypertensive rats. Hypertension. 1999;33:1135-
N Engl J Med. 1996;334:952-7. [PMID: 8596596]
40. [PMID: 10334800]
67. Semplicini A, Del Prato S, Giusto M, Campagnolo M, Palatini P, Rossi
88. Petersen JS, DiBona GF. Acute sympathoinhibitory actions of metformin in
GP, et al. Short-term effects of metformin on insulin sensitivity and sodium
spontaneously hypertensive rats. Hypertension. 1996;27:619-25. [PMID:
homeostasis in essential hypertensives. J Hypertens Suppl. 1993;11 Suppl
5:S276-7. [PMID: 8158383]
89. Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovas-
68. Robinson AC, Burke J, Robinson S, Johnston DG, Elkeles RS. The effects
cular disease: an update. Hypertension. 2001;37:1053-9. [PMID: 11304502]
of metformin on glycemic control and serum lipids in insulin-treated NIDDM
90. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an
patients with suboptimal metabolic control. Diabetes Care. 1998;21:701-5.
angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in
high-risk patients. The Heart Outcomes Prevention Evaluation Study Investiga-
69. Howard BV. Insulin resistance and lipid metabolism. Am J Cardiol. 1999;
tors. N Engl J Med. 2000;342:145-53. [PMID: 10639539]
84:28J-32J. [PMID: 10418856]
91. Gress TW, Nieto FJ, Shahar E, Wofford MR, Brancati FL. Hypertension
70. Steiner G. Lipid intervention trials in diabetes. Diabetes Care. 2000;23 Suppl
and antihypertensive therapy as risk factors for type 2 diabetes mellitus. Athero-
2:B49-53. [PMID: 10860191]
sclerosis Risk in Communities Study. N Engl J Med. 2000;342:905-12. [PMID:
71. Management of dyslipidemia in adults with diabetes. American Diabetes
Association. Diabetes Care. 1998;21:179-82. [PMID: 9538989]
92. Sowers JR, Draznin B. Insulin, cation metabolism and insulin resistance. J
72. Taskinen MR. Triglyceride is the major atherogenic lipid in NIDDM. Dia-
Basic Clin Physiol Pharmacol. 1998;9:223-33. [PMID: 10212836]
betes Metab Rev. 1997;13:93-8. [PMID: 9222120]
93. Katakam PV, Ujhelyi MR, Hoenig M, Miller AW. Metformin improves
73. Abbasi F, Kamath V, Rizvi AA, Carantoni M, Chen YD, Reaven GM.
vascular function in insulin-resistant rats. Hypertension. 2000;35:108-12.
Results of a placebo-controlled study of the metabolic effects of the addition of
metformin to sulfonylurea-treated patients. Evidence for a central role of adipose
94. Bhalla RC, Toth KF, Tan E, Bhatty RA, Mathias E, Sharma RV. Vascular
tissue. Diabetes Care. 1997;20:1863-9. [PMID: 9405908]
effects of metformin. Possible mechanisms for its antihypertensive action in the
74. Landin K, Tengborn L, Smith U. Treating insulin resistance in hypertension
spontaneously hypertensive rat. Am J Hypertens. 1996;9:570-6. [PMID:
with metformin reduces both blood pressure and metabolic risk factors. J Intern
Med. 1991;229:181-7. [PMID: 1900072]
95. Verma S, Yao L, Dumont AS, McNeill JH. Metformin treatment corrects
75. Tessier D, Maheux P, Khalil A, Fu¨lo¨p T. Effects of gliclazide versus met-
vascular insulin resistance in hypertension. J Hypertens. 2000;18:1445-50.
formin on the clinical profile and lipid peroxidation markers in type 2 diabetes.
Metabolism. 1999;48:897-903. [PMID: 10421233]
96. Giugliano D, Quatraro A, Consoli G, Minei A, Ceriello A, De Rosa N, et
76. Grant PJ, Stickland MH, Booth NA, Prentice CR. Metformin causes a
al. Metformin for obese, insulin-treated diabetic patients: improvement in glycae-
reduction in basal and post-venous occlusion plasminogen activator inhibitor-1 in
mic control and reduction of metabolic risk factors. Eur J Clin Pharmacol. 1993;
type 2 diabetic patients. Diabet Med. 1991;8:361-5. [PMID: 1713132]
44:107-12. [PMID: 8453955]
77. Nagi DK, Yudkin JS. Effects of metformin on insulin resistance, risk factors
97. Chen XL, Panek K, Rembold CM. Metformin relaxes rat tail artery by
for cardiovascular disease, and plasminogen activator inhibitor in NIDDM sub-
repolarization and resultant decreases in Ca2⫹ influx and intracellular [Ca2⫹].
jects. A study of two ethnic groups. Diabetes Care. 1993;16:621-9. [PMID:
J Hypertens. 1997;15:269-74. [PMID: 9468454]
98. Trovati M, Anfossi G. Insulin, insulin resistance and platelet function: sim-
78. Charles MA, Morange P, Eschwe ge E, Andre´ P, Vague P, Juhan-Vague I.
ilarities with insulin effects on cultured vascular smooth muscle cells. Diabetolo-
Effect of weight change and metformin on fibrinolysis and the von Willebrand
gia. 1998;41:609-22. [PMID: 9662040]
factor in obese nondiabetic subjects: the BIGPRO1 Study. Biguanides and the
99. Ofenstein JP, Dominguez LJ, Sowers JR, Sarnaik AP. Effects of insulin and
Prevention of the Risk of Obesity. Diabetes Care. 1998;21:1967-72. [PMID:
metformin on glucose metabolism in rat vascular smooth muscle. Metabolism.
1999;48:1357-60. [PMID: 10582541]
79. Marfella R, Acampora R, Verrazzo G, Ziccardi P, De Rosa N, Giunta R, et
100. Peuler JD, Miller JA, Bourghli M, Zammam HY, Soltis EE, Sowers JR.
al. Metformin improves hemodynamic and rheological responses to L-arginine in
Disparate effects of antidiabetic drugs on arterial contraction. Metabolism. 1997;
NIDDM patients. Diabetes Care. 1996;19:934-9. [PMID: 8875085]
46:1199-205. [PMID: 9322807]
80. Shindler DM, Kostis JB, Yusuf S, Quinones MA, Pitt B, Stewart D, et al.
101. Campbell JD, Paul RJ. The nature of fuel provision for the Na⫹, K(⫹)-
Diabetes mellitus, a predictor of morbidity and mortality in the Studies of Left
ATPase in porcine vascular smooth muscle. J Physiol. 1992;447:67-82. [PMID:
Ventricular Dysfunction (SOLVD) Trials and Registry. Am J Cardiol. 1996;77:
1017-20. [PMID: 8644628]
102. Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al.
81. Fiordaliso F, Li B, Latini R, Sonnenblick EH, Anversa P, Leri A, et al.
Association of systolic blood pressure with macrovascular and microvascular com-
Myocyte death in streptozotocin-induced diabetes in rats in angiotensin II-depen-
plications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ.
dent. Lab Invest. 2000;80:513-27. [PMID: 10780668]
2000;321:412-9. [PMID: 10938049]
2 July 2002 Annals of Internal Medicine Volume 137 • Number 1 E-33
Downloaded From: http://annals.org/ by a Novo Nordisk A/S (USD) User on 10/06/2015
Source: https://noninsulindoctor.ibog.steno.dk/fileadmin/indhold/Required_reading/D4_Metformin_an_Update_Ann_Intern_Med_2002.pdf
DefenDers unDer Promoting sexual and reProductive rights amnesty International is a global movement of more than 7 million people who campaign for a world where human rights are enjoyed by all. Our vision is for every person to enjoy all the rights enshrined in the universal Declaration of Human rights and other international human rights standards.
NFATc1 Balances Quiescence andProliferation of Skin Stem CellsValerie Horsley,Antonios O. Aliprantis,Lisa Polak,Laurie H. Glimcher,and Elaine Fuchs,1Howard Hughes Medical Institute, Laboratory of Mammalian Cell Biology and Development, The Rockefeller University,New York, NY 10065, USA2Department of Infectious Diseases and Immunology, Harvard School of Public Health, Boston, MA 02115, USA3Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115, USA*Correspondence: DOI 10.1016/j.cell.2007.11.047












