
Postnote template

January 2010 Number 352
COUNTERFEIT MEDICINES
Counterfeiting of medicines is increasing, is often linked
to other criminal activities and poses risks to public
health. It exposes people to medicines of unverified
quality, safety and efficacy. This POSTnote considers
the extent of the global counterfeit medicine trade, its
impact in the UK and the technologies and policy
options available to combat it. It also examines the risks
and benefits of online pharmacy, one of the main ways
in which counterfeits are distributed.
Background
Counterfeiting is a high-volume, high-profit business
which poses health risks, infringes intellectual property rights, medicines legislation and other aspects of criminal
law. Indirect impacts are lost revenue for pharmaceutical
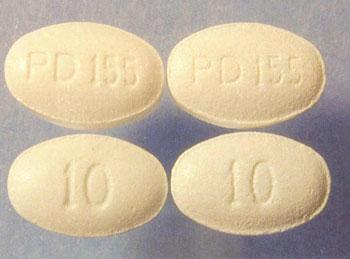
Figure 1. Distinguishing genuine (right hand side) and counterfeit tablets
companies, brand damage and decreased public
(and packaging) apart on visual inspection is difficult (image courtesy of Pfizer).
confidence. Counterfeit medicines and devices circulate
In 2005, a counterfeit version of this cholesterol-lowering drug was found in the
globally via unregulated channels (including unauthorised
UK. 120,000 packs were recalled from 240 pharmacies; 60% were counterfeit.1
online pharmacies) but can also enter legitimate drug
supply chains. It can be difficult to distinguish them from
pharmaceutical industry activities, often in collaboration.
genuine products (Fig 1). The World Health Organisation
Counterfeits pose a greater problem in countries where
(WHO) classes medicines as counterfeit if they:
manufacture and supply of drugs are less regulated and
where enforcement is weak. Estimates indicate that:
are fraudulently packaged or mislabelled with respect
to identity and/or source (for example if they are not
in wealthy countries like the UK with strong
made by the genuine manufacturer);
regulatory frameworks (Box 1), counterfeits are likely
to account for 1% of the total medicines market,
contain no active ingredient, incorrect quantities or an
undeclared active ingredient;
but an estimated 50% of drugs sold online are fake;
are contaminated with other materials (chalk, boric
in emerging economies, the proportion is 10% but in
acid, lead and rat poison are typical examples);
the former Soviet republics it can be as high as 20%;
are past their expiry date;
in Latin America, South East Asia and Sub-Saharan
Africa an estimated 30% of medicines are counterfeit.2
contain no or incorrect patient information leaflets.
Counterfeits often display more than one of these
features. A study of 286 incidents showed that 67% had
Data collated by the Pharmaceutical Security Institute
both counterfeited drug and packaging, 28% had
report that the number of unique incidents increased
counterfeit drug and 5% had counterfeit packaging only.
annually from 196 in 2002 to 1,834 in 2008.3 The
global reach of a counterfeit made in one location can be
Extent of Counterfeit Medicines
significant: drugs made by a producer in China were
While counterfeiting of branded and generic (copies of
found in 42 countries.4 EU borders and customs agencies
out-of-patent drugs) medicines is increasing in volume
have targeted illegal medicines, seizing 2.7m items in
and range, there are few comprehensive analyses of
2006. A two-month action in 2008 (MEDI-FAKE) led to
global statistics due to varied definitions of counterfeits
seizure of 34m illegal medicines, including antibiotics,
between legislatures, and the level and frequency of
erectile dysfunction tablets and chemical precursors used
monitoring. Data are collected by governments and
in manufacturing.5
postnote January 2010 Number 352 Counterfeit medicines Page 2
Box 1. Safeguarding the UK Drug Supply Chain
Box 2. Counterfeits in the UK Legal Supply Chain
The UK government‟s Medicines and Healthcare products
The MHRA recalls suspect medicines, alerting hospitals and
Regulatory Agency (MHRA) is responsible for ensuring that
pharmacies to remove the items, or to retrieve them from
medicines are safe and effective. Its Enforcement and
patients once dispensed. It has issued nine recalls since
Intelligence Group investigates suspected counterfeiting in
2004 for counterfeits that reached pharmacies and patients
the regulated and unregulated supply chains. If the MHRA
through the legitimate supply chain.1 Four batches were
identifies a public health risk, it issues an alert (classified
intercepted at wholesale level and one in a clinical trial.
from 1 (most critical) to 4 depending on risk) and the drug is
Medicines often contained insufficient active ingredients and
withdrawn. Class 1 alerts arise from medicines being
had been repackaged into English cartons via parallel trade
contaminated, mislabelled or containing incorrect ingredients
(Box 3). They included:
and require immediate recall. The MHRA has a 24 hour
cardio-vascular disease - counterfeit Plavix (inhibits
hotline for reporting counterfeits and encourages all agencies
blood clots) was recalled from pharmacies in 2007.
(police, pharmaceutical companies, customs, trading
prostate cancer - counterfeits of Astra Zeneca‟s Casodex
standards and pharmacies) and individuals (health
reached patients in 2007. The packets bore genuine
professionals and the public) to report suspect drugs.
Astra Zeneca lot numbers; the original lots bearing
these numbers were supplied to France.
anti-psychotics - counterfeits of Eli Lilly‟s drug Zyprexa
Manufacture of Counterfeits
bore genuine lot numbers and reached patients
Recent seizures at EU borders show that 60% of drugs
triggering a Class 1 recall.
originated from China, where counterfeiters focus on
cholesterol - counterfeits of Pfizer‟s drug Lipitor reached
high-value, in-demand drugs. Raids on premises show
pharmacies in 2005 and 2006. The Class 2 recalls
issued also had to apply to authentic products since
that production often occurs in unsanitary conditions,
genuine lot numbers were used on the fakes.
using rudimentary equipment and cheap labour to mass-
obesity - counterfeits of Abbott‟s anti-obesity drug
produce tablets and packaging. The UK is not a major
Reductil reached patients in 2004.
location for manufacturing counterfeits, but is a large market for sale and transit with seizures traced to
production in the Far East and Indian sub-continent.
Using online pharmacies poses indirect health risks. Men
are less likely to consult a doctor than women and more
Types of Counterfeit Medicines in Circulation
likely to buy medicines online. Men buying POMs (such
The classes of drugs most commonly counterfeited are
as for erectile dysfunction) from illegal online pharmacies
genito-urinary, anti-infectives and central nervous system
may risk their health in two ways. Firstly, the drug may
medicines but piracy of other categories is increasing. A
be counterfeit. Secondly, a man consulting a GP about
variety of counterfeit medicines has been seized in the
erectile dysfunction would be assessed for the risk of
UK including treatments for erectile dysfunction, hair
cardiovascular disease and stroke, since the conditions
loss, obesity and pain relief (Box 2). Life-saving drugs for
may be linked. A man buying online would forgo this
blood pressure and cancer, and also anti-psychotics and
assessment and so miss out on opportunities for other
statins are now appearing. Medicines seized in the UK
diagnoses, with consequences for long-term health.
often contain reduced amounts of the active ingredient
and all included impurities and failed to meet standards
Non-health Related
required by Good Manufacturing Practice regulations.
Indirect impacts of counterfeiting include intellectual
The UK has had nine known cases of counterfeit
property infringements and economic impacts on industry
prescription-only medicines (POMs) reaching the legal
from lost sales and brand damage; such commercially
supply chain since 2004. In an incident in 2008,
sensitive information is not available. There are few
72,000 packs of counterfeit heart and cancer medicines
analyses of economic losses, but estimates of the market
led to four recalls. The MHRA points out that this is in
value of seized medicines in the UK since 2004 amount
the context of 800 million prescriptions issued annually.
to £6.5m. Sales from unauthorised online sources exceed
There are no reliable statistics on the number of
this figure by a significant margin, estimated by one
counterfeits reaching consumers through unregulated
survey at $12bn a year worldwide in 2008.
sources, such as illegal online pharmacies.
Incentives to Counterfeit Medicines
Consequences of Counterfeit Medicines
Several factors make this activity attractive to criminals:
Public Health and Patient Safety
free trade zones, globalisation and complex medicine
Counterfeits containing sub-therapeutic levels of active
supply chains make it easy to introduce counterfeits
ingredients pose a significant health risk to patients
into legitimate supply channels, especially in areas
taking medicines to manage life-threatening conditions.
with weak drug regulatory controls.
Counterfeit formulations may be metabolised differently,
it is a lucrative activity, especially for drugs that
so even if an active ingredient is present, it may not be
command high prices or are required in large volumes.
taken up by the body. Health can also be compromised if
technology to make constituent ingredients and
counterfeits contain toxic substances such as heavy
packaging is cheap and readily available.
metals. No fatalities have been attributed officially to
the Internet provides counterfeiters with ready access
counterfeits in the UK but have been reported elsewhere
to markets outside regulated medicine supply chains.
(including Canada, but mostly in the developing world). It
Current legislation and regulation do not provide a
is unclear whether the UK coroners‟ system is well-
strong enough deterrent, through both enforcement
placed to detect the involvement of counterfeits.
and penalties, to discourage counterfeiters.4
postnote January 2010 Number 352 Counterfeit medicines Page 3
Complex Medicine Supply Chains
Medicine distribution in the EU is complex. Historically,
Box 4. Anti-Counterfeiting Technologies
medicines passed from the manufacturers to patients via
Packaging technologies deter counterfeiters and alert users
wholesalers but now many legitimate intermediates are
to medicines of dubious origin. The optimum place for overt
security measures is at the point of use by the patient. Some
involved, including wholesalers, brokers and parallel
technologies currently used include specialised printing and
traders. Parallel trade (Box 3) is international import of
security inks, blister packs, hi-tech holograms, watermarks
goods, occurring when versions of drugs are made for
and tamper-evident packaging. Even the most sophisticated
sale in different markets or when the same drug is priced
holograms can be copied to look convincing to consumers.
differently (UK prices are among the highest in the EU).
However, importing and repackaging medicines through
legal parallel trade often renders such measures ineffective.
It stimulates competitive pricing of patented medicines
but has been blamed for shortages. The British
Supply chain management such as track and trace
Association of Pharmaceutical Wholesalers (10
technologies use unique identification numbers (for batches
wholesalers supply 90% of the UK‟s medicines)
and individual packets) to register product authenticity
estimates that parallel traded medicines account for 13%
against a database. Other technologies include 2-D
barcodes, similar to standard black and white barcodes but
of total UK drug sales, valued at £9-10bn. Such a large,
with greater data storage capacity to include origin, lot
complex, supply chain is difficult to secure. It is almost
number and expiration date. Radio Frequency Identification
impossible to track a medicine (in the case of a recall for
(RFIDs) tagging, used by retailers for stock control has been
example) to its origins when it has passed through
trialled by the US Food and Drug Administration. It
multiple handlers in numerous countries. Some
advocates phasing them into the medicine supply chain.
Some US companies tag „at-risk‟ products. Others supply
companies believe that EU regulatory and enforcement
pharmacies directly, which is complex and costly, but may
capability has not kept pace with increasingly complex
be necessary to protect revenue. There are concerns that this
medicine trading mechanisms. Pfizer has responded to
may lead to shortages. Where several wholesalers provide
this by supplying UK pharmacies directly. Such schemes
medicines, one can step in if another cannot fulfil an order.
bypass intermediaries; other companies may follow suit.
strategies (on legislation, enforcement, use of technology and sharing intelligence). It runs a web-based counterfeit
Box 3. Parallel Trading in Medicines
reporting system and works with international agencies,
Paral el traded medicines are purchased in one country and
notably Interpol, the Permanent Forum on International
then legally repackaged and/or relabelled and sold elsewhere
Pharmaceutical Crime and the World Customs
at a higher price. A single box of tablets may be handled by
Organisation. They co-ordinate operations and train
numerous intermediates before reaching the end user.
Paral el trading is regulated by the European Medicines
police and officials from customs and drug regulatory
Agency and the MHRA. Repackaging and re-labelling is
agencies in counterfeit sample handling and
inspected by the MHRA but import and distribution takes
identification. Initiatives between IMPACT and Interpol
place outside the original manufacturer‟s supply chain. Pfizer
have included "Internet days of action" to target illegal
estimates that the number of parallel importing licences
online sales as well as longer operations in 2008:
granted for one of its products rose from 62 to 660 in 2006.
Operation Mamba raided 45 premises in Tanzania
and Uganda and seized 100 types of suspect
Measures to Tackle Counterfeiting
medicines including anti-malarials and anti-fungals.
Pharmaceutical Industry
Operation Storm in south-east Asia targeted
Industry tackles counterfeiting in three main ways:
manufacturers/distributors of medicines identified as a
investing in overt and covert technologies to secure
health risk (for pneumonia and childhood-related
medicine packaging and supply chains (see Box 4);
illnesses). After 200 raids, 16m tablets were seized.
investigating suspected counterfeit rackets and
working with government agencies in and outside the
The European Policy Response
UK to bring civil claims or criminal prosecutions;
The European Commission outlined proposals to tackle
funding awareness campaigns to educate healthcare
counterfeiting in 2006, and is likely to approve a
professionals and the public about the dangers.
Directive on falsified medicines in 2010. Proposals aim
Adopting the technologies described in Box 4 is widely
to prevent counterfeits entering the supply chain by:
supported, but raises issues of costs and agreement on a
using mandatory safety features (such as individual
harmonised system for use in the global supply chain.
product codes or seals) for medicines deemed „high
Pharmaceutical companies test drugs to identify
risk‟, affixed by authorised manufacturers and replaced
counterfeiting of their brands. This involves examining
under strict conditions, allowing identification and
packaging and forensic analysis of suspicious products.
authentication by all involved in distribution.
Companies bring prosecutions under civil law, to recover
more rigorous certification and inspection of
assets acquired through criminal activity. Others work
wholesalers, including audits and listing in a database
with national agencies to instigate criminal proceedings.
kept by the European Medicines Evaluation Agency.
tightening requirements for import of medicines and
The International Policy Response
active pharmaceutical ingredients from outside the EU
The WHO‟s International Medical Products Anti-
and improvement of inspections and enforcement.
Counterfeiting Taskforce (IMPACT) is a coalition of 193
The Directive is welcomed by industry although there is
countries set up to promote international collaboration
no agreement on which security features should be used
and to publish guidelines for developing national
or on how a system could be harmonised in the EU. It is
postnote January 2010 Number 352 Counterfeit medicines Page 4
unlikely that any system would be operational before 2012 and unclear which medicines will be classed as
Box 5. Consumer Attitudes to Online Pharmacy
"high risk" and whether this will cover all generic
Pfizer, which produces the branded medicine Viagra,
medicines. The European Federation of Pharmaceutical
surveyed 935 men aged over 35. Men were surveyed since
they are much less likely than women to access healthcare
Industries and Associations is concerned that introducing
services and more likely to purchase the types of medicines
variable standards will result in less protected medicines
that are commonly counterfeited. Key survey findings were:
being targeted and wants the same standards applied to
1 in 10 bought POMs online without a prescription;
all POMs. Critics argue that the proposals will succeed
50% purchasing medicines without a prescription did
only if member states allocate adequate resources to
so online (67% for those buying erectile dysfunction
medicines) from sites in the UK and further afield;
enforce them and there are steps to tackle counterfeiting
men did not see this as a high risk activity;
outside Europe, where most production occurs. The EC
60% agreed that if it were possible that their medicine
will expect member states to "monitor" online sales and
was counterfeited it would influence their decision to
take legal action against those selling illegal products.
purchase POMs through the internet.
The UK's Policy Response
The MHRA‟s anti-counterfeiting strategy aims to make
credit card companies could bar unauthorised sites from
the UK a less attractive market to counterfeiters.6 The
search results and impose extra checks on transactions.7
agency is seeking to raise tariffs for offences and a
There is concern that health professionals lack awareness
consultation on strengthening policy closes in March
of the risk from counterfeits and cannot advise patients
2010. It keeps a watch list of high-risk medicines to
on how to protect themselves from disreputable online
inform intelligence and participates in international
pharmacies or auction sites. The MHRA runs public
initiatives. It has prosecuted offenders for crimes ranging
awareness campaigns but has not yet targeted this group
from illegal advertising to global counterfeiting rings.
and there is little guidance for the medical profession
Investigations are complex, especially when international
from professional bodies. The Royal Pharmaceutical
networks target weaknesses in national and international
Society of Great Britain‟s accreditation scheme identifies
supply chains. Recently, several prosecutions for
legitimate pharmacy websites with a logo. Pfizer‟s
distribution have been brought. Sentences ranged from a
ongoing publicity campaign warns of the dangers of
£1,000 fine to 6 years‟ imprisonment. The MHRA co-
buying from unlicensed websites. The impact of these
operates with police, Customs and Excise and Trading
activities has not been assessed, but it is hoped that the
Standards to prosecute using:
public will undermine the counterfeits market by buying
from reputable sources and reporting suspect medicines.
the Medicines Act, where the maximum sentence is 2
years imprisonment and/or an unlimited fine;
Overview
the Trade Marks Act and Proceeds of Crime Act with
maximum sentences of 10 and 14 years respectively.
A wide variety of counterfeit medicines circulates
globally, largely through unauthorised channels such
Internet Pharmacies and Counterfeits
as online pharmacies, posing a public health risk.
Online pharmacy offers improved access, choice and
The European Commission intends to strengthen
convenience. Some value online anonymity over the
medicine supply chains though a proposed Directive.
possible risks of buying a counterfeit, particularly for
This is unlikely to be enforced before 2012. Its impact
embarrassing conditions (Box 5). The European Alliance
on counterfeit production outside the EU is uncertain.
for Access to Safe Medicines (EAASM) estimates that
The UK‟s Medicines and Healthcare products
62% of websites concealing their physical address supply
Regulatory Agency is consulting on policies to improve
counterfeits. It warns that buying from unauthorised
the security of the regulated national supply chain.
sources increases the risk of being supplied with them.
Progress is being made to raise public awareness of
Several UK chains have legitimate online services to
the risks of illegal online pharmacies and counterfeits.
supply over-the-counter and POMs but counterfeiters
Endnotes
exploit the online marketplace by producing sophisticated
1 www.mhra.gov.uk//index.htm
websites that appear legitimate. They use search engine
2 World Health Organisation, www.who.int/en/
advertising and spam emails to increase web traffic to
3 Pharmaceutical Security Institute, www.psi-inc.org/ 4 OECD, The Economic Impact of Counterfeiting and Piracy, 2007
online stores. Dubious sites sell counterfeit POMs without
5 Community Customs Activities on Counterfeit and Piracy: Results
professional advice or checks on their quality and
at the European Border, EC Taxation & Customs Union, 2007
effectiveness. There is no legal recourse should problems
6 Anti-Counterfeiting Strategy 2007-2010, MHRA
arise. Surveys in the US of brand infringement for six
7 The Counterfeiting Superhighway, 2008, European Al iance for
POMs found 110,000 fraudulent sites and 2,986 online
Access to Safe Medicines
pharmacies. Most were hosted in the US, China and
POST is an office of both Houses of Parliament, charged with providing
Russia (12% in the UK). Estimates of annual sales
independent and balanced analysis of public policy issues that have a basis in
through such sites increased from $4bn to $12bn from
science and technology.
2007 to 2008. Sites rarely required a prescription,
POST is grateful to all contributors and reviewers. For further information on this subject, please contact Dr Sarah Bunn, at POST.
declared fake accreditation or sold drugs at significant
Parliamentary Copyright 2010
discounts compared with genuine sources. Users‟ details
The Parliamentary Office of Science and Technology, 7 Millbank, London,
were at risk since many sites did not secure transactions.
SW1P 3JA; Tel: 020 7219 2840; email: [email protected]
Websites hosted outside the UK are not regulated by the
MHRA. The EAASM proposes that internet search and
Source: http://www.parliament.uk/documents/post/postpn352.pdf
Information and Recommendations for the Engineer Fan Bearing Maintenance & Troubleshooting LubricationProper lubrication and maintenance are essential for long possible, the proper level with the fan on must be bearing life. An adequate supply of clean lubricant must marked on the level also. Oil can be blown out of bear- be present at all times to prevent damaging, metal-to-
A SURVEY OF TIME-OF-USE (TOU) PRICING AND DEMAND-RESPONSE (DR) PROGRAMS July 2006 Submitted By: Energy & Environmental Economics 353 Sacramento Street, Suite 1700 San Francisco, CA 94111 (415) 391-5100 The U.S. Energy Environmental Protection Agency (U.S. EPA) commissioned this report to support state public utility commissions in their expanding use of energy efficiency (EE) in their states. The report is part of the EPA-State Energy Efficiency and Renewable Energy (EERE) Project index.htm). The EPA-State EERE Projects are a joint initiative between the U.S. EPA, the National Association of Regulatory Utility Commissioners, and individual state utility commissions designed to explore approaches that deliver significant energy cost savings and other benefits through greater use of energy efficiency, renewable energy, and clean distributed generation.






