
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Suszenierozpylowe.pl
www.buchi.com Information Bulletin Number 59/2010
Laboratory Scale Spray Drying Of Inhalable Drugs:
A Review

Mini Spray Dryer B�290
Figure 1: Mini Spray Dryer B-290
The Mini Spray Dryer B-290 from Büchi
a compact spray dryer. The residence
The adjustable process parameters
Labortechnik AG is a laboratory scale
time of the drying air within the spray
instrument to perform spray drying
chamber is about 1.5 seconds. The
processes down to 30 mL batch volume
powder col ection is provided by a
• inlet and outlet temperature,
and up to 1 litre of water or organic
glass-made cyclone separator, which
• sample feed rate,
solvent per hour. Thanks to the glass-
is internal y coated with a thin nanosize
• drying gas flow rate and
ware, the complete drying process
antistatic film to reduce powder
• spray gas flow
from the two-fluid nozzle down to the
adhesion to the glass wall. The
powder col ection vessel is visible.
separation works by centrifugal forces
Fine particles are produced because
by virtue of inertia of the solid
of the short residence time in such
Features and benefits
Mini Spray Dryer B-290
Main benefit
for traditional spray drying, established process
Max. inlet temperature
1.0 kg/h, higher for solvents
Nozzle types
two-fluid nozzle, three-fluid nozzle
typical y around 50% - 70%
Min. sample volume
Max. sample viscosity
300 cps (viscous samples and juices possible)
possible to scale-up to kg- and tons-scale
Table 1: Features and benefits of the Mini Spray Dryer B-290

Nano Spray Dryer B�90
Figure 2: Nano Spray Dryer B-90
The new Nano Spray Dryer B-90 is
The liquid sample is fed to the spray
membrane to vibrate, ejecting mil ions
based on a new spray drying concept.
nozzle via a peristaltic pump in a re-
of precisely sized droplets per second
The drying gas enters the apparatus
circulation mode.
with a very narrow distribution. These
from the top where it is heated to the set
The generation of droplets is based on
extremely fine droplets are dried
inlet temperature, flows then through
a piezoelectric driven actuator, vibrating
into solid particles and col ected by
the drying chamber, and exits the spray
a thin, perforated, stainless steel
electrostatic charging and subsequent
dryer at the bottom outlet. The gas is
membrane in a small spray cap. The
deflection to the collecting electrode.
additional y fine filtered before leaving the
membrane (spray mesh) features an
Final y the resulting powder is col ected
instrument. The inlet temperature and
array of precise, micron-sized holes
using a rubber spatula.
outlet temperature are measured just
(4.0, 5.5 or 7.0 μm). The actuator is
after the heater and before the fine filter.
driven at around 60 kHz, causing the
Features and benefits
Nano Spray Dryer B-90
Main benefit
for small quantities, finest particles, highest yields
Max. inlet temperature
Nozzle type
piezoelectric driven vibrating mesh
electrostatic particle col ector
Min. sample volume
Max. sample viscosity
10 cps (diluted samples)
limited by spray head and electrical particle col ector
Table 2: Features and benefits of the Nano Spray Dryer B-90
Laboratory Scale Spray Drying Of Inhalable Drugs: A Review
Author: Dr. Cordin Arpagaus, Dr. Nina
[3, 4]. It has the potential to generate
particles, based on the available RDD
Schafroth and Marco Meuri
highly dispersible powders for inhalation
online proceedings database. The liter-
in the range from 1 to 5 μm size with a
ature review showed breakthrough R&D
particle morphology that can more eas-
innovations in the field of respiratory
The pharmaceutical industry addresses
ily be influenced compared to for exam-
drug delivery with key information about
a number of demands on novel respira-
ple jet mil ing [5].
available spray drying parameters and
ble particulates, which from a process
technology perspective can be broadly
This study reports a review, regarding
Spray drying applications focused
categorized into the areas of: perfor-
research work on particles for inhalation
especial y on anti-asthmatic drugs
mance (e.g. total/local lung deposition,
that have been published in the RDD
[2, 5-9], antibiotics [1, 9-12], proteins,
immediate versus controlled release),
proceedings database, using laboratory
such as insulin [13-15], bovine serum
processing (e.g. achieve flow properties)
scale Büchi Mini Spray Dryer models
albumin [16] or human serum albumin
and stability (e.g. physical/chemical sta-
B-190, B-191 and B-290
[17], antibodies [18] and tuberculosis
bility and activity).
A new trend in pulmonary drug delivery
Literature Review
Various excipients were applied to
is to move from the liquid or pressurised
stabilize drugs during formulation,
formulations to dry powder inhalation
A search query in the RDD online data-
predominately mannitol [13, 14, 17, 18,
formulations. This, in part, is due to the
base with the key word "spray drying"
20], poly(lactic-co-glycolic-acid) PLGA
advantages of dry powder systems, in-
revealed 53 hits. Figure 3 visualizes the
[8, 10, 19, 21], lactose [5, 8, 16] and
cluding breath-actuated inhalation, lim-
distribution of these published papers
chitosan [7]. SEM photographs of
ited coordination requirements, no pro-
over the last several years. It seems
the spray dried powders exhibited
pel ant requirement and short treatment
that the ful potential of the spray drying
mostly spherical shapes with corrugated
process for dry powder aerosols has
surfaces, resin-like or even hol ow
Spray drying is a simple, rapid, repro-
not been ful y exploited yet. Spray
structures, depending on the sub-
ducible, economic and easy to scale-up
drying has become a well established
stance material and drying conditions
production process [2] that has been in-
technology in pulmonary drug delivery.
(Table 3A and 3B).
tensively studied for pharmaceuticals
The produced particles were in the respi-
and excipients for pulmonary drug de-
Table 3A and 3B reviews the spray
rable size range with roughly 1 - 5 μm
livery in dry powder inhalation systems
drying research with regard to inhalable
aerodynamic diameters. High fine parti-
online database
D
ber of abstracts
ord "spray drying" 4
ith keyw
w 0
published in R
Figure 3: Number of abstracts published in the RDD online database (www.rddonline.com, visited January 8, 2010) with key word "spray drying" (total 53 abstracts)
Particle size, shape,
Carrier and
Spray drying
yield, fine particle
Reference and
application
fraction (FPF) and
emitted dose (ED)
Terbutaline
Spherical particles
Cook et al. 2004
sulphate
University of London
Matrix forming exipients
throat impaction 23.9 % Dryer B-191
School of Pharmacy, UK
4% w/w terbutaline
1 - 15 μm particle size
Terbutaline
Learoyd et al. 2006a
sulphate
6-36% w/w leucine and
gas spray 600 L/min
Aston University,
25-50% w/w chitosan
Hol ow to porous
Salbutamol
particles, reduced
phosphatidylcholine,
Brandes et al. 2004
sulphate
agglomeration tendency Mini Spray
Christian Albrecht
compared to jet-mil ed
calcium chloride
University, Germany
powders, 40% drug
dihydrate, Solkane 227
Spherical particles
Salbutamol
Learoyd et al. 2006b
PLGA, beclomethasone
sulphate
spray gas 600 L/min
Aston University,
dipropionate, PVA,
feed rate 3.2 mL/min
Dryer B-290 Birmingham, UK
Spherical particles
Weiler et al. 2008
Salbutamol
3.2 μm , FPF around
Johannes Gutenberg-
sulphate
Lactose monohydrate
70 %, dispersion
University Mainz,
Boehringer Ingelheim,
Cabral Marques and
Spherical particles
University of Lisbon,
feed rate 5 - 11 mL/min
Raisin-like particles
Hydrochloric acid,
Cagnani et al. 2004
T in < 140 °C
sodium hydroxide,
University of Parma,
respirable particles
polyalcohols, mannitol
Particle diameter < 5.8 μm
Najafabadi et al. 2007
sponge-like morphology
Aqueous solution of
University of Medical
T out 62 - 65°C
suitable for respiratory
insulin and additives
Sciences, Tehran, Iran
spray gas 550 NL/h
(mannitol, polymer)
Pasteur Institute of Iran,
feed rate 3 mL/min
ED 59 - 81% dispersability 57 - 60 %
Drying air humidity <20%
Maltensen and van de
Aqueous solution
T in 75 - 220°C
Resin-like morphology
spray gas 7 - 17 L/min
particle size of 4 μm
University of Copenhagen,
feed rate 2 - 5 mL/min
suitable for inhalation
aspirator 80 - 100%
Spherical to corrugated
Gentamicin
shape particles of
Lechuga-Ballesteros et al.
Gentamicin with small
amounts of trileucine
Nektar Therapeutics, USA
Table 3A: Literature review of spray dried inhalable products using the laboratory scale Büchi Mini Spray Dryer models B-190, B-191 and B-290. "Part 1"
Particle size, shape,
Carrier and
Spray drying
yield, fine particle
Reference and
application
fraction (FPF) and
emitted dose (ED)
Doxycycline
Traini et al. 2007
Corrugated particles
University of Sydney,
Monash University,
Victoria, Australia
Bain et al. 2002 Quintiles (UK) Ltd,
Rifampicin
Spherical particles
Poly(D,L-lactide) (PDLLA)
University of Strathclyde,
Glasgow, Scotland
John Moores University, Liverpool, England
Cefotaxime
Spherical particles
Najafabadi et al. 2005
University of Medical
T out 87 - 89 °C
better aerolisation
Sciences, Tehran, Iran
compared to jet mil ing
Spherical particles with
Tobramycin
corrugated surfaces
Parlati et al. 2008
0.25 - 2.0 % sample
< 3.0 μm size yield 60 %
University of Sydney,
in vitro drug deposition
Corrugated particles
Bovine Serum
Lactose / Brij 76
5.4 / 12.8 μm size
Li and Sevil e 2008
Aston University,
recovery of drug after
spray gas 600 L/h
inhalation >95 %
Schüle et al. 2004
No change in secondary Mini Spray
University of Munich,
spray gas 670 L/h
proteins structure
Dryer B-290 Boehringer Ingelheim,
feed rate 3 mL/min
Protein/mannitol ratio
Zimontkowski et al. 2005
(antibody) and
Spherical particles
University of Bonn,
Human Serum
spray gas 700 L/h
Boehringer Ingelheim,
feed rate 9 mL/min
Garcia-Contreras et al.
Proteins secreted Poly
1.95 μm particle size
(lactic-co-glycolic acid)
feed rate 7 ml/min
University of North
activity > 93 %
Spherical particles
Lysozyme
200 mL solutions of
5 μm particle size
Shoyele et al. 2008
(enzyme for
University of Bradford, UK
T out 50 ± 2°C
3M Drug Delivery
spray gas 500 L/h
phosphate buffer
12 weeks storage
feed rate 5 mL/min
66% retained enzymatic
Mean size 4 - 10 μm
Colombo et al. 2008
Morphine
concentration of morphine
satisfactory morphine
University of Parma
spray gas 600 L/h
HCl, mannitol and lecithin
stability in agglomerated Dryer B-191
University of Salerno
feed rate 3.2 mL/min
University of Ferrara, Italy
Mannitol
concentrations up
Morton et al. 2008
Mannitol with 1 - 10%
to 35 ppm in air
Monash University,
w/w different additives
feed rate 0.1 mL/s
mean particle size
Victoria, Australia
Table 3B: Literature review of spray dried inhalable products using the laboratory scale Büchi Mini Spray Dryer models B-190, B-191 and B-290. "Part 2"


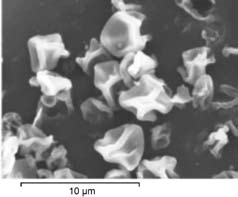
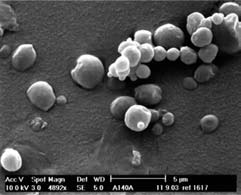
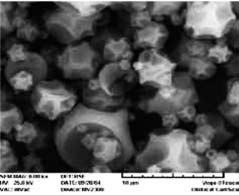
cle fractions were achieved, ranging
sulphate nanoparticles (an anti-asthma-
acting inhaled drug particles (about
from 30 - 60% [6-8, 16] to over 85%
tic drug) into microparticles [2].
0.5 - 3.3 μm which represents
[13]. Inhaler emitted powder doses of
Physically and chemically stable
deposition in the lung alveoli).
over 90% were reported [2, 7, 8]. Amor-
non-cohesive spray dried particles,
The key benefits of this technology are
phous powders were typical y generated
with smal aerodynamic diameters
the possibilities to control the size and
due to the short drying time in the labo-
were designed to be efficiently delivered
morphology of the particles under a
ratory scale spray dryers [3, 22]. Aero-
as a dry powder aerosol [11]. Spray
relatively gentle processing method.
solized powder clouds with maximal
drying produced powders with superior
Indeed, this method has been proven
volume concentrations of up to 35 ppm
biochemical stability upon formulation
for the preparation of heat-sensitive
particles in air were achieved [20].
compared to spray freeze drying;
materials such as protein based drugs.
Cefotaxime sodium
(Bain et al. 2002)
(Cagnani et al. 2004)
(Najafabadi et al. 2005)
(Traini et al. 2007)
(Brandes et al. 2004)
(Cook et al. 2004)
(Learoyd et al. 2004)
(Weiler et al. 2008)
Figure 3: SEM photographs of inhalable spray dried powder from literature.
Compared to jet mil ed samples, higher
although with less efficient aerosol
While the traditional bench-top spray
fractions of potential y inhalable aerosol
properties [24]. Sustained release of
dryers have been shown capable
particles of antibiotic cefotaxime
highly dispersible amino acid leucine
tools for the laboratory aim generation
sodium were measured for spray dried
incorporated PLGA powders was
of respiratory sized particles, the area
formulations [23]. Deagglomeration
exhibited over several days [8].
of process technology is ever-evolving.
of spray dried protein formulations
The Nano Spray Dryer B-90 offers new
was possible [17]. Higher powder
possibilities in the field of laboratory
dispersibility of spray dried powders
scale spray drying and eliminates
compared to jet mil ed particles was
Spray drying is a very useful technique
some weak points of traditional spray
explained by their spherical shape
to produce inhalable dry powders with
dryers; including increased recovery
and therefore smaller surface contact
predetermined specifications. There
(up to 90%), smal quantity production
is significant research activity in dry
(100 mg amounts) and highly definable
powder aerosol formulation to treat
particle size ranges (300 nm - 5 μm) [25].
Particularly, high values of respirable
several diseases including asthma,
fractions were found for insulin because
tuberculosis, diabetes and bacterial
of the spray dried particle size [13]. The
infection in the lung. Spray drying offers
capability for inhalation with relatively
great potential to these applications
high drug loading was shown, for
because of the easy achievement of the
example by incorporation of terbutaline
accepted optimum size range for local y
[10] Bain, D. F. et al. (2002),
formulations for spray drying",
"Biodegradable microspheres for
RDD 4 , 377-380.
[1] Parlati, C. et al. (2008), "In vitro
control ed intra-pulmonal delivery of
evaluation of co-processed antibiotic
Rifampicin to treat tuberculosis",
[19] Garcia-Contreras, L. et al. (2004),
for inhalation", RDD, 907-910.
RDD 8, 561-563.
"Formulation strategies for a novel
inhaled tuberculosis vaccine",
[2] Cook, R. O. et al. (2004), "Sustained
[11] Lechuga-Bal esteros, D. et al.
RDD 4, 877-880.
release microparticles containing
(2004), "Designing stable and high
drug nanoparticles for pulmonary
performance respirable particles of
[20] Morton, D.A.V. et al. (2008),
administration", RDD 4, 777-780.
pharmaceuticals", RDD IX, 565-568.
"Investigating Effects of Surface
modifications on an Mannitol Dry
[3] Dem, C. et al. (2006),
[12] Traini, D. et al. (2007), "Co-spray
Powder Inhaler Plume by Laser
"Understanding the spray dry design
dried antibiotics for dry powder
Diffraction", RDD, 649-653.
process through single droplet
inhalation delivery", RDD Europe,
investigations", RDD, 257-265.
[21] Arpagaus, C. and Schafroth, N. (2009), "Laboratory scale spray
[4] Hickey, A.J. et al. (1996),
[13] Cagnani, S. et al. (2004), "A novel
drying of biodegradable polymers",
Pharmaceutical inhalation aerosol
spray dried formulation for
RDD Europe 2009, 269-274.
technology, Marcel Dekker,
pulmonary administration of Insulin",
RDD 4, 813-816.
[22] Colombo, P. et al. (2006), "Nasal powders of morphine microcrystal
[5] Weiler, C. et al. (2008), "Dispersibility
[14] Najafabadi, A.R. et al. (2007), "The
agglomerates", RDD, 885-887.
of jet mil ed vs. spray dried powders ",
effect of polymer on the properties
RDD Europe, 571-575.
of insulin/mannitol spray dried
[23] Najafabadi, A.R. et al. (2005),
powder for inhalation", RDD Europe,
"Evaluation of cefotaxime sodium
[6] Brandes, H.G. et al. (2004),
microparticles for respiratory drug
"Particle design to improve
delivery", RDD Europe, 265-268.
pulmonary delivery of powdered
[15] Maltesen, M.J. and Van de Weert,
medications," RDD 4, 229-231.
M. (2008), "Particle size of spray
[24] Shoyele, A.S. et al. (2008), "A
dried insulin evaluated by NIR
comparative study on the bio-
[7] Learoyd, T.P. et al. (2006a),
spectroscopy", RDD, 827-830.
chemical stability and pharmaceutical
"Sustained drug delivery from
performance of excipient-free
chitosan-based respirable spray-
[16] Li, H.Y. and Sevil e, P.C. (2008),
spray dried and spray freeze dried
dried powders ", RDD, 441-444.
"Preparation of pMDI protein
protein pMDIs", RDD, 823-826.
formulations using surfactant-
[8] Learoyd, T.P. et al. (2006b),
coated spray dried powders", RDD,
[25] Schmid, K. et al. (2009),
"Leucine-modified PLGA-based
"Evaluation of a vibrating mesh
respirable spray-dried powders for
spray dryer for preparation of
sustained drug delivery", RDD,
[17] Zimontkowski, S. et al. (2005),
submicron particles", RDD Europe,
"Dispersion characteristics of spray
dried protein powder formulations
[9] Cabral Marques, H. M. and Almeida
assessed by laser diffraction and
Coimbra, R. N. M. A. (2009),
SEM", RDD Europe, 273-276.
"Preparation and in vitro evaluation
of cyclodextrin/beclomethasone
[18] Schüle, S. et al. (2004),
complexes as dry powder inhaler
"Determination of the secondary
formulations", RDD Europe, 413-417.
protein structure of IgG1 Antibody
BÜCHI Labortechnik AG
BUCHI UK Ltd.
BUCHI Hong Kong Ltd.
Nihon BUCHI K.K.
BUCHI Canada Ltd.
CH – 9230 Flawil 1
GB – Oldham OL9 9QL
J – Tokyo 110-0008
CDN – Thornhil , Ontario L4J 6Z2
T +41 71 394 63 63
T +44 161 633 1000
T +81 3 3821 4777
T +1 416 277 7407
F +41 71 394 65 65
F +44 161 633 1007
F +81 3 3821 4555
F +1 905 764 5139
BÜCHI Labortechnik GmbH
BÜCHI Labortechnik GmbH
BUCHI India Private Ltd.
D – 45127 Essen
RC – 500052 Shanghai
IND – Mumbai 400 055
USA – New Castle,
e subject to change without notice/ Quality Systems ISO 9001
Freecall 0800 414 0 414
T +86 21 6280 3366
T +91 22 667 18983 / 84 / 85
T +49 201 747 490
T +31 78 684 94 29
F +86 21 5230 8821
F +91 22 667 18986
Toll Free: +1 877 692 8244
F +49 201 237 082
T +1 302 652 3000
F +1 302 652 8777
Technical data ar
BUCHI Sarl
BÜCHI Italia s.r.l.
BUCHI (Thailand) Ltd.
F – 94656 Rungis Cedex
I – 20090 Assago (MI)
T – Bangkok 10600
T +39 02 824 50 11
T +66 2 862 08 51
F +66 2 862 08 54
11592334 The English version is the original language version and serves as basis for all translations into other languages.
We are represented by more than 100 distribution partners worldwide. Find your local representative atwww.buchi.com
Source: http://suszenierozpylowe.pl/wp-content/uploads/2015/09/B-290_BB_59_Laboratory_Scale_Spray_Drying_of_inhalable_drugs_en_01.pdf?4db01d
Asian Transactions on Basic and Applied Sciences (ATBAS ISSN: 2221-4291) Volume 02 Issue 01 Development of Antidiabetic Active Compounds from Ethyl Acetate Extract of Acorus calamus L. Sri Hartati, Rizna T. Dewi, A. Darmawan and Megawati diabetes mellitus in the World. In the year 2000, there are Abstract— In research development of herbal medicine from
HEAVY DUTY SUBMERSIBLE TRANSCEIVER VERTEX STANDARD CO., LTD.4-8-8 Nakameguro, Meguro-Ku, Tokyo 153-8644, JapanVERTEX STANDARDUS Headquarters10900 Walker Street, Cypress, CA 90630, U.S.A.YAESU EUROPE B.V.P.O. Box 75525, 1118 ZN Schiphol, The NetherlandsYAESU UK LTD.Unit 12, Sun Valley Business Park, Winnall CloseWinchester, Hampshire, SO23 0LB, U.K.VERTEX STANDARD HK LTD.Unit 5, 20/F., Seaview Centre, 139-141 Hoi Bun Road,Kwun Tong, Kowloon, Hong Kong












