
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Asian Transactions on Basic and Applied Sciences (ATBAS ISSN: 2221-4291) Volume 02 Issue 01
Development of Antidiabetic Active Compounds from Ethyl Acetate Extract of
Acorus calamus L.
Sri Hartati, Rizna T. Dewi, A. Darmawan and Megawati
diabetes mellitus in the World. In the year 2000, there are
Abstract—
In research development of herbal medicine from
around 5.6 million diabetics in Indonesia. However, in 2006
selected plants for antidiabetic, ethyl acetate extract of Acorus
the estimated numbers of diabetics in Indonesia increased
calamus L. plays on biological role in differentiation of
sharply up to 14 million people
.
preadiposites and posses powerful in diabetes mellitus (DM type
Acorus calamus L. (AC) also know as Calamus or
2). Two active compounds are already known are 3-
,22-
-
Sweet Flag have been used in the Indian and Chinese system
of medicine for hundreds years. The radix AC widely used for
and
-sitosterol-3-O-
-D-glucosidase. The proposed of this
the therapy of diabetes in traditionally folk medicine in
research is to isolate antidiabetic active compound with activity
America and Indonesia [4][5]. AC useful in cought, brochitiss,
as inhibitor
-glucosidase from ethyl acetate extract and modify
gout, inflammation, skin diseases, numbness, general debility,
the active compounds to obtained compound with higher activity
and lower side effect based on structure activity relationship
emetic and stomatic, and to treat dispepsia, colic pain,
(SAR). From the ethyl acetate extracts obtained four semi-polar
bronchitis, remittent fever and dysentery in children. Ethanol
fractions that has similar TLC spot (fraction 4, 5, 6 and 7) that
exract of AC demonstrated significant hypolipidemic activiy
was active as
-glucosidase inhibitor with IC
[5][6][7]. Previous study by Wu, et al., [8]-[9] showed that
50 values are 22.86,
13.54, 13.34, and 18.92 μg/mL, respectively, and three polar
ethyl acetate fraction of AC was found to enhance adipocytes
fractions (fractions 20, 21, and 22) were active as
-glucosidase
differentiation as did by roglitazone. AC has potential to be
inhibitor with IC50 values 13.55, 3.08, and 6.85 μg/mL,
useful for the treatment of diabetes and cardiovascular
respectively. SAR studies showed that
-sitosterol-3-O-
-D-
complication without body weight gain. Purpose of this study
glucosidase active compound have similarity with acarbose as
was to isolate the antidiabetic active compound from ethyl
positive standard of antidiabetic drug.
acetate extract of AC that acts as -glucosidase inhibitor and
Keywords— Antidiabetes,
-glucosidase, diabetes mellitus,
Acorus calamus L
sitosterol-3-
O--
D-glucosidase based on structure activity
relationship (SAR).
iabetes mellitus (DM) is a disease in which levels of glucose (simple sugar) in the blood is high because the
D body can not release or use insulin normally. Insulin is a
hormone secreted by the pancreas, which is responsible in
A. Isolation
maintaining normal blood sugar levels. Insulin incorporate
Acorus calamus L. Rhizome material dried at 50oC and
sugar into cells so that it can produce energy or stored as
made into powder. macerated with methanol for 2 x 24 hours
energy reserves. The number of diabetics worldwide currently
three times and concentrated using rotary evaporator to aford
is estimated at 150 million people, numbers will increase up to
methanol extract. Methanol extract partitioned with solvent
220 million by 2010 and 300 million by 2025 with 90% of
mixture
n-hexane:water (1:1). Water fraction further
them were DM type 2 diabetics[1][2][3]. According to WHO
partitioned with ethyl acetate and butanol to obtained ethyl
data, Indonesia ranks 4th largest in the number of patients with
acetate, butanol and water extracts. Ethyl acetate extracts
washed with
n-hexane to reduce - and -asarone contents.
Manuscript received February 12, 2012.
Ethyl acetate extract further isolated using gravitation column
Sri Hartati is with the Research Center for Chemistry, Indonesian Institute
chromatography method with G
of Science, Kawasan PUSPIPTEK Serpong, Tangerang Selatan, Banten,
60 silica gel as stationary phase
Indonesia. 15314. (corresponding author to provide phone: +62-21-7560929;
and n-hexane, ethyl acetate, and methanol as mobile phase is
fax: +62-21-7560549; e-mail:
[email protected]).
eluted in a gradient. The fraction obtained was evaporated,
Rizna T. Dewi is with the Research Center for Chemistry, Indonesian
collected and analyzed by SiGF254 thin layer chromatography
Institute of Science, Kawasan PUSPIPTEK Serpong, Tangerang Selatan,
(TLC) aluminum plates using appropriate eluent. Fractions are
Banten, Indonesia. 15314. (e-mail:
[email protected]).
A. Darmawan is with the Research Center for Chemistry, Indonesian
grouped according to the TLC spot patterns. Fractions were
Institute of Science, Kawasan PUSPIPTEK Serpong, Tangerang Selatan,
tested for α-glucosidase activity and compared with
Banten, Indonesia. 15314. (e-mail:
[email protected]).
nojirimicyn and quercetin as positive standard. Extract is
Megawati is with the Research Center for Chemistry, Indonesian Institute
considered active when the IC
of Science, Kawasan PUSPIPTEK Serpong, Tangerang Selatan, Banten,
50 value close to or smaller than
Indonesia. 15314.
IC50 value of the standards.
Asian Transactions on Basic and Applied Sciences (ATBAS ISSN: 2221-4291) Volume 02 Issue 01
semi-polar fractions (fraction 4, 5, 6, 7) and 3 polar fractions
B. -Glucosidase Test Methods
(fraction 22, 23, 24) with IC50 values are 22.86, 13.54, 13.34,
18.92, 13.55, 3.08 and 6,85 μg/mL, respectively. All fractions
α-glucosidase reaction mechanism is to catalyze the
have α-glucosidase inhibitor activity higher than quercetin
breakdown reaction of p-nitrophenyl-D-glucopyranosyde
(38.49 μg/mL) and only fraction 4 and 7 have α-glucosidase
(PNP) substrate to p-nitrophenol and glucose at 37oC
inhibitor activity lower than nojirimicyn (14.15 μg/mL) (Table
temperature (Fig. 1). The enzyme activity was measured by
uptake of p-nitrophenol generated. If the sample has the ability
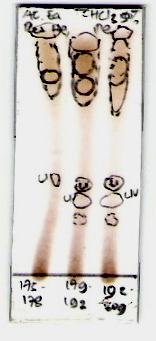
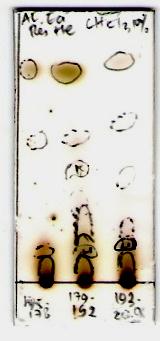
From the TLC profile spots of the active fractions showed
to inhibit the activity of the -glucosidase, p-nitrophenol
that fraction 4, 5, 6 and 7 (semi-polar fractions), as well as
generated will be reduced [10]. -glucosidase enzymatic
fraction 22, 23 and 24 (polar fractions) (Fig. 2).
method is a cheaper and faster alternatives in vitro method that
use as an initial screening test to determine the -glucosidase
inhibitor ability of a compound [10].
-glucosidase activity inhibition test performed according
to Kim Yong-Mu, et al. (2005) [11] (kit Waco Chemical Ltd.)
p-nitrophenyl-
Fig. 2. Thin layer chromatography (TLC) results, (a) semi-polar active
-glucosidase enzyme
fractions, eluted with n-hexane:ethyl acetate (9:1), (b) polar active fractions,
eluted with 5% methanol in chloroform, (c) polar active fractions, eluted with
α-GLUCOSIDASE INHIBITION ACTIVITY TEST RESULTS
p-nitrophenol α-D-glucose
IC50 (ug/mL)
-glucosidase reaction mechanism
C. SAR (Structure Activity Relationships)
To determine the relationship between structure and
activity (SAR) required several chemical computational
facilities such as virtual molecular docker (using Molegro
Virtual Docker/MVD), ChemDraw Ultra 10.0, Chem3D Ultra
10.0, α-glucosidase enzyme proteins (1LWJ) as receptors
taken from http://www.pdb.org and ligand (active compound
Based on literature study, majority antidiabetic active
or synthesis target compound).
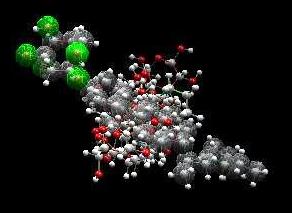
compounds contained in A. calamus are 3β,22α,23-
Data processing and analysis using a computer program,
by first making the chemical structure of the ligand compound
noside and -sitosterol-3-O-b-D-glucopyranoside (Fig. 3)
using ChemDraw Ultra 10.0, followed by the the most stable
conformation structures of the ligand using Chem-3D Ultra
10.0. α-glucosidase enzymes as a receptor docked with ligand compound using MVD in order to obtain bond energy value between them and compared with bond energy value from
acarbose as positive standard.
III. RESULT AND DISCUSSIONS
A. Fractionation Results and α-glucosidase inhibition
activity test
Fig. 3. Estimation of the chemical structure of the active compounds from A. calamus plants. (a) 3β,22α,23-trihydroxyolean-30-methoxycarbonyl-12-ene-
From 311 g of ethyl acetate extract of A. calamus obtained
(rhamnoside-acorus),
26 fractions. All fraction of ethyl acetate was tested its
glucopyranoside (-sitosterol-acorus)
antidiabetic activity using in vitro -glucosidase inhibition test
B. Structure Activity Relationship (SAR) Study
method and obtained 7 antidiabetic active fractions, there are 4
Asian Transactions on Basic and Applied Sciences (ATBAS ISSN: 2221-4291) Volume 02 Issue 01
The main purpose of doing chemical structure modification
For the first phase, we have to performed calculations using
by develop marker compounds that known have biological
MVD software to look for similarities between sulochrin and
activity is to produce new compounds that more effectively
α-glucosidase active compounds such as deoxynojirimicyn,
and safely used. This is based on the general assumption that a
miglitol, vasacine and vasacinol (isolated from Adthoda vasica
compound that have similar chemical structure backbone with
Ness), and salasinol (results isolated from Salacia oblonga).
the similar pharmacophore groups would have or show similar
Deoxynojirimicyn used as reference compounds because these
biological activity. Lead compounds guide is not intended
compounds are active as Glyset compound (oral drug α-
specifically as a clinical agent, but it is a starting point to
glucosidase inhibitor) with the competitive inhibitor action
develop a new compounds that have clinical function. Study
about structure and biological activity relationship of the lead
compound undertaken through a change or addition of
substituents [12] [13]. The biological activity of synthesize
LIGAND SIMILARITY CALCULATION SCORE RESULTS
USING MVD SOFTWARE
target compounds would be predicted by Molegro Virtual
Docking (MVD), HyperChem Pro-6.0, or compared with the
Similarity score
other lead compounds/drug (native ligand) or drugs such as
acarbose, deoxynojirimicyn, and miglitol with α-glucosidase
enzyme (Fig. 4), also with some natural product isolated
deoxinojirimicin
compounds that approved active as an α-glucosidase inhibitor
Rhamnosida -acorus
http://pubchem.ncbi.nlm.nih.gov) (Fig. 5).
Based on the results above, acarbose has the lowest
similarity value and close to the value of β-sitosterol-acorus,
or it can be said instead that β-sitosterol-acorus compound
with the acarbose (Fig. 6). The next step is to place (docking)
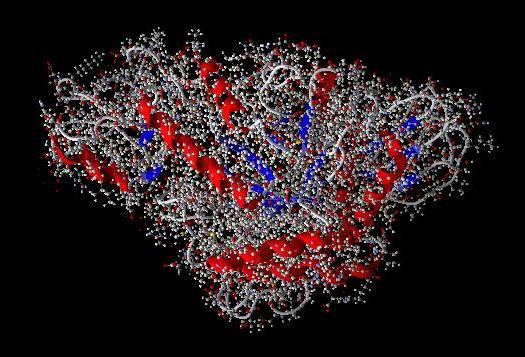
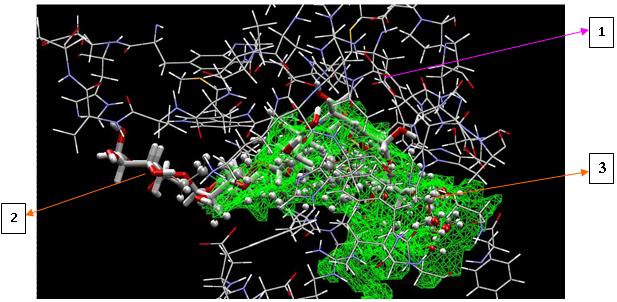
ligand on the target enzyme (1LWJ) to determine whether the
ligand has affinity towards the target (Fig.7).
Fig. 4. Some of chemical structure of α-glucosidase inhibitor active compounds. (1) acarbosa, (2) methyl, β-acarviocynida, (3) 1-deoxy-nojirimicyn
Fig. 6. Similarity alignment result of the lead compounds with comparable
Fig. 5. Some of chemical structure of α-glucosidase inhibitors isolated from plants. (4) vasicine, (5) vasicinol, (6) salacinol.
With the software we can determine pharmacophore
crystallographic
groups of the compounds that have been known active against
(http://www.pdb.org)
α-glucosidase enzyme. From the calculation results can be
known a few parameters that describe whether the compounds
After predicting the α-glucosidase enzyme binding site, the
have some similarities with standard active compounds
next step is to calculate the docking score of the ligand
(acarbose, deoxynojirimicyn, miglitol) (Table II) and
compound with deoxynojirimicyn and miglitol as a reference,
possibility to synthesize an analog or derivative compound.
Asian Transactions on Basic and Applied Sciences (ATBAS ISSN: 2221-4291) Volume 02 Issue 01
salacinol as comparator and synthesize target compounds as
[5] K. Heyri, T – H Han, S – G Lee, ―Anti-Inflammatory Activity of water
listed in Table III.
extract of Acorus calamus L. Leaves on Keratinocyte‖, HaCaT Cells‖, Journal of Ethnopharmcology, 122, 2009, pp. 149 –156.
[6] R. S. Parab, Sushma A. Mengi, ― Hypolipidemic activity of Acorus
calamus L. in rats‖, Fitoterapia, 73, 2002, pp. 451-455.
[7] S. Manikanda, R. Srikumar, and N. Jeva Parthasarathy, ―Protective
effect of Acorus calamus L. in free radical scavenger and lipid
DOCKING CALCULATION RESULTS SCORE
peroxidase in discrete regions of brain against noise stress‖. Biol.
USING MVD SOFTWARE
Pharm. Bul, (28), 2, 2005, pp. 2327-2330.
[8] H-S Wu, Y-Y Li, L-J Weng, C-X Zhou, Q-J He and Y-J Lou, ―A
fraction of Acorus calamus L. extract devoid of -asaron enhances
adipocyte differentiation in 3T4-Ll Cells‖, Phytotherapy Research, 21,
ACG_989 [Acarbose]
2007, pp.262 -264.
β-sitosterol acorus
[9] H-S Wu, D-F Zhu, C-H Zhou, C-R Feng, Y-J Lou, Yang Bo and Q-J
He, ―Insulin sensitizing activity of ethyl acetate fraction of Acorus
deoxynojirimicyn
calamus L. in vitro and in vivo", J. of Ethnopharmacology,123, 2009,
rhamnoside acorus
[10] N. Artanti, M. Hanafi and L.B.S. Kardono, ―Inhibition of α-glucosidase
Pose of the ligand compound in the binding site can be viewed
enzyme activity of Uncaria gambir Roxb. And Taxus sumatrana (Miquel) De Launbenfels. (Published Conference Proceedings style)‖, in
as shown in Fig. 8
Proc. 5th National Seminar of Chemistry. (in Bahasa Indonesia),
Yogyakarta, Indonesia, 2002, pp.483 -488.
[11] Y-M Kim, Y-K Jeong, M-H Wang, W-Y Lee, H-I Rhee, ―Inhibitory
Effect of Fine Extract on α-Glucosidase Activity and Postprandial Hyperglikemia‖, Nutrition, 21, 2005, pp.756-761.
[12] A. S. Cristoph, F. Wolfgang, H. W. Rudolf, M. R. Bernd, R. L. Klaus
and M. v. Janos, ― Automated Docking to Antibodies : Method and Aplications‖ Methode, 20, 2000, p. 280-291.
[13] S. Gisbert and B. Hans-Joachim, ― Vistual Screening and Fast automated
Docking Methods‖ DDT (Drug Discovery Today), vol 7, (1) Januaray 2002.
S. Hartati, Was born in Cilacap (Central Java) Indonesia, March 11,
1956, post graduated of Chemistry, Faculty of Mathematic and Sciences.
Padjadajaran University Bandung Indonesia, graduated in 1984. Master
Fig. 7. Pose of the ligand on α
degree Chemistry of Natural Product, faculty of mathematic and sciences
-glucosidase enzyme binding sites (MVD
docking). (1) amino acid residues on the side α
Indonesia University, graduated in 2000. Doctor Chemistry of Natural
-glucosidase enzyme bond, (2)
Product, faculty of mathematic and sciences Indonesia University,
acarbose native ligand, (3) docked ligand compounds (β-sitosterol,
graduated in 2007 .
rhamnosida, and acarbose).
Position Senior researcher at Natural Product Food and Farmaceutical
Division of Research Center for Chenistry LIPI. Since 2001 member of The Indonesian Society of Natural Products Chemistry. Since 2009
member of Indonesian Society for Cancer Chemoprevention.
-glucosidase activity test results showed that both
semi-polar and polar fractions can inhibit the action of the enzyme α-glucosidase with IC50 22.86, 13.54, 13.34, 18.92, 13.55, 3.08, and 6.85 μg/mL, compared with nojirimicyn (IC50
14.15 μg/mL) and quercetin (38.49 μg/mL). SAR study indicates that the -sitosterol-3-O--D-glucosidase compound is similar with acarbose as the antidiabetic drug with molecular docking score (MVD molecular docking score) -134.266 and -181.76, respectively.
[1] L. A. Collene, R. H. Steven, J. A Williams, and W. B. Wolf , ―Effects
of a Nutritional Supplement Containing Salacia oblonga Extract and Insulinogenic Amino Acids on Postprandial Glycemia, Insulinemia, and Breath Hydrogen Responses in Healthy Adults‖, Nutrition, 21, 2005, pp. 848-854.
[2] D-S Lee, and S-H Lee, ―Genistein, a Soy Isoflavon, is a Potent
α- Glucosidase Inhibitor‖, FEBS Letters, 501, 2001, pp. 84-86.
[3] Z. Dadan, F Isao, L. Changzheng, I, Kaori, G. Huashi, Z. Jien, ―The
Stimulatory Activities of Polysaccharide Compounds Derived from Algae Extracts on Insulin Secretion in vitro‖, Biol. Pharm. Bull., 31 (5), 2008, pp. 921-924.
[4] S.R. Yende, U.N. Harle, D. T. Raygure, T. A. Tuse and N. S. Vywahare,
―Pharmacological Profile of Acorus calamus : An Overview‖, Pharmacognosy Reviews [Phcog-Rev]-Suplement, 2 (4), pp. 22 – 26, July – Dec, 2008.
Source: http://www.asian-transactions.org/journals/vol02issue01/atbas/atbas-40226015.pdf
AGENZIA INTERNAZIONALE PER LA PREVENZIONE DELLA CECITÀSEZIONE ITALIANA Oftalmologia Sociale – Rivista di Sanità Pubblica Capo Redattoredott. Filippo CRUCIANI Comitato di redazioneprof. Luciano CERULLIdott.ssa Cristina MARTINOLIprof. Ugo MENCHINIprof. Giovanni SCORCIA COMITATO SCIENTIFICO NAZIONALE
Hernalser Hauptstraße 155 1170 Wien Tel./Fax: 01/486 24 04 [email protected] Neu! Liefe Neu! Lief rser erservice – Seite 3 vice – Seite 3 W ihnachtsmarkt – Seite 4 eihnachtsmarkt – Seite 4 Immer wieder Fieberbl Immer wieder Fieberb asen Über 90% der Bev









