
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Untitled
International Journal of Systematic and Evolutionary Microbiology (2013), 63, 893–899
Pseudonocardia antitumoralis sp. nov., adeoxynyboquinone-producing actinomyceteisolated from a deep-sea sediment
Xin-Peng Tian,1 Li-Juan Long,1 Su-Mei Li,1 Jing Zhang,1 Ying Xu,2Jie He,2 Jie Li,1 Fa-Zuo Wang,1 Wen-Jun Li,2 Chang-Sheng Zhang1and Si Zhang1
1Key Laboratory of Marine Bio-resources Sustainable Utilization, CAS; RNAM Center for Marine
Microbiology, CAS; Guangdong Key Laboratory of Marine Materia Medica; South China Sea
Institute of Oceanology, Chinese Academy of Sciences, Guangzhou, 510301, PR China
2Key Laboratory of Microbial Diversity in Southwest China, Ministry of Education and Laboratory for
Conservation and Utilization of Bio-resources, Yunnan Institute of Microbiology, Yunnan University,Kunming, Yunnan, 650091, PR China
An aerobic actinomycete, designated SCSIO 01299T, was isolated from a deep-sea sedimentcollected from the northern South China Sea at a depth of 3258 m. The isolate was found to be anatural producer of the synthesized antitumour agent deoxynyboquinone and its three newderivatives, pseudonocardians A, B and C. A BLAST search based on almost-complete 16S rRNAgene sequences showed that strain SCSIO 01299T had high sequence similarities with membersof the genus Pseudonocardia. The 16S rRNA gene sequence phylogenetic tree revealed thatstrain SCSIO 01299T was a member of the genus Pseudonocardia. Phenotypic analysis,chemotaxonomy and DNA–DNA relatedness could readily distinguish the isolate from establishedmembers in this genus. It was concluded that strain SCSIO 01299T represents a novel species,for which the name Pseudonocardia antitumoralis sp. nov. is proposed. The type strain is SCSIO01299T (5DSM 45322T 5CCTCC M 2011255T).
Actinomycetes are well known as the largest group (45 %) of
actinomycetal taxa is very important for gaining new sources
bioactive secondary metabolite producers
of pharmaceutical potential.
Over 50 years of research on terrestrial actinomycetes, the
rate of discovery of new compounds has decreased because
resources in the South China Sea, a deep-sea sediment
of the high rate of reisolation of producers of known
sample (120u 0.9759 E 19u 0.6649 N) at a depth of 3258 m
compounds ). For the discovery of
(pH 7.8, 2 uC) was collected. The sampling process and
structurally diverse natural products in drug discovery
isolation procedure were done as described by
programmes, novel screening procedures and high-quality
One grey-white aerial mycelial colony with an
biological materials from new sources are two critical factors
obvious inhibition zone after 3 weeks at 28 uC on selective
(). So, extremophiles have been
isolation medium ISP 5 prepared with natural seawater,
focused on increasingly by chemists, including actinomyce-
designated strain SCSIO 01299T, was selected. By the
tal resources in marine environments. Recent studies have
bioactivity-guided isolation methods, we found that the
revealed that marine actinomycetes are still a robust source
isolate could produce deoxynyboquinone (15 mg l21), a
of excellent natural products, such as the genera Salinispora
synthetic potential antitumour agent, and its three new
salinosporamide A (NPI-0052), sporolides, saliniquinone
derivatives, pseudonocardians A (0.66 mg l21), B (1.5 mg
A-F, salinosporamide K), Verrucosispora (abyssomicins),
l21) and C (1.3 mg l21) Strain SCSIO
Micromonospora [diazepinomicin (ECO-4601)]
01299T was maintained on ISP 2 or stored at 280 uC as a
and ‘Marinispora' (marinomycins, marinisporolides) (
20 % (w/v) glycerol suspension.
Therefore, the discovery of novel marine
Morphological observation and cultural characteristics
The GenBank/EMBL/DDBJ accession number for the 16S rRNA gene
were performed on Difco media ISP 2, 3, 4 and 5,
sequence of strain SCSIO 01299T is JN204514.
Czapek's agar nutrient agar and potato
Two supplementary figures are available with the online version of this
agar at 28 uC for 28 days. Cellular morphology was
examined by light microscopy and scanning electron
037135 G 2013 IUMS
Printed in Great Britain
X.-P. Tian and others
Table 1. Culture characteristics of strain SCSIO 01299T and its
as described by Catalase activity was
closest phylogenetic neighbours in the genus Pseudonocardia
evaluated by the production of oxygen bubbles in 3 % (v/v)H2O2 and oxidase activity was determined using oxidase
Strains: 1, Pseudonocardia antitumoralis sp. nov. SCSIO 01299T; 2, P.
reagent (bioMe´rieux). Growth at 4, 10, 15, 20, 28, 35, 40,
ammonioxydans CGMCC 4.1877T; 3, P. kongjuensis DSM 44525T; 4, P.
45 and 50 uC, at pH 4.5–11 (in increments of 0.5 pH unit)
autotrophica BCRC 12444T; 5, P. endophytica YIM 56035T. All data
and with 0–25 % (w/v) NaCl (at intervals of 2.5 %) was
were taken from this study after incubation at 28 uC for 21 days.
studied as described by The buffer
Colours were taken from ISCC–NBS colour charts All
solutions used to adjust the pH were acetate/citrate (pH 4–
strains form white aerial mycelium on ISP 2, 3 and 4. None of the
6), KH2PO4/NaOH (pH 6–8), NaHCO3/Na2CO3 (pH 9–
strains forms soluble pigment on ISP 2, 3, 4 and 5, nutrient agar or
10) and Na2HPO4/NaOH (pH 11). Sole carbon source
potato agar. DOY, Dark orange yellow; LOY, light orange yellow; MOY,
utilization tests (final concentration 0.5 %, w/v) were
moderate orange yellow; MYB, moderate yellowish brown; POY, pale
performed as described by
orange yellow; PW, pinkish white; W, white; YW, yellowish white; 2, no
Antibiotic susceptibility was examined as described by
using antibiotic discs on ISP 2.
Biomass for chemotaxonomic and molecular systematicstudies was obtained at the same physiological age by
Substrate mycelium
cultivation in ISP 2 with shaking at 150 r.p.m. at 28 uC for
Substrate mycelium
1 week. Cell-wall diaminopimelic acid and whole-cell
Substrate mycelium
sugars, menaquinones and phospholipids were analysed
Substrate mycelium
according to previously established procedures
Czapek's agar Aerial mycelium
). Cellular fatty acid analysis was
Substrate mycelium
performed according to the protocol of the Sherlock
Microbial Identification System (MIDI) by GC (model
Nutrient agar Aerial mycelium
6890; Agilent), based on biomass obtained by culture in
Substrate mycelium
TSB (Difco) for 4 days at 28 uC. The results were compared
with the TSBA 6 database of fatty acids in Sherlock version
Substrate mycelium
6.1. The G+C content of genomic DNA was determinedusing the HPLC method
Extraction of genomic DNA and PCR amplification of 16S
microscopy (JSM 5600LV; JEOL) using cells incubated for
rRNA gene were carried out according to
14 and 28 days on ISP 2. Diffusible pigments were
Similarity calculations were performed based on EzTaxon-e
observed by comparing the cultures with the most suitable
and MEGA version 5.0
colour chips from the ISCC-NBS colour charts
with parameters of pairwise deletion for gap treat-
All physiological and biochemical tests were
ment. Multiple alignments were created using CLUSTAL X
performed at 28 uC, such as diffusible pigment, hydrolase
Phylogenetic trees were con-
and H2S and melanin production, according to methods
structed using MEGA version 5.0 with the neighbour-joining
described by Gram-reaction was done
method of with the parameter of
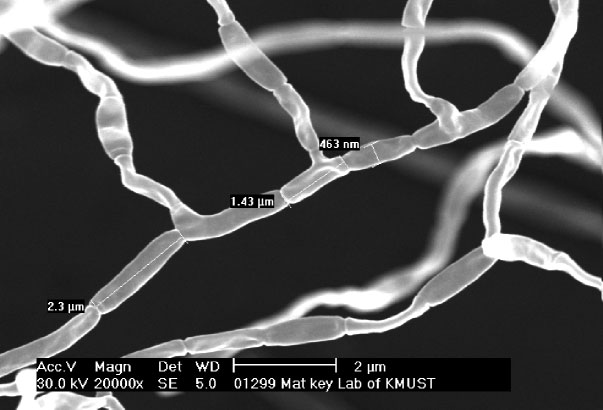
Fig. 1. Scanning electron micrograph of aerialmycelia and spore chains of strain SCSIO01299T after incubation for 14 days on ISP 2at 28 6C. Bar, 2 mm.
International Journal of Systematic and Evolutionary Microbiology 63
Pseudonocardia antitumoralis sp. nov.
Table 2. Physiological characteristics of strain SCSIO 01299T and its closest phylogenetic neighbours in the genus Pseudonocardia
Strains: 1, Pseudonocardia antitumoralis sp. nov. SCSIO 01299T; 2, P. ammonioxydans CGMCC 4.1877T; 3, P. kongjuensis DSM 44525T; 4, P.
autotrophica BCRC 12444T; 5, P. endophytica YIM 56035T; All data were taken from this study under the same culture conditions. All strains utilizearabinose, cellobiose*, D-galactose, glycerol, glucose, maltose and sodium oxalate as sole carbon sources, and are susceptible to chloramphenicol(30), ciprofloxacin (5), gentamicin (10), netilmicin (30), tobramycin (10) and vancomycin (30). +, Positive; 2, negative.
Diffusible pigment
Milk peptonization
Nitrate reduction
Utilization of urea
Sole carbon sources
Antibiotic susceptibility (mg per disc)
Erythromycin (15)
Penicillin G (10)
Sulfamethoxazole (24)
Tetracycline (30)
*For columns 2 and 5, not consistent with the original data.
DNot consistent with the original data.
X.-P. Tian and others
Pseudonocardia zijingensis 6330 T (AF325725)
Pseudonocardia adelaidensis EUM 221T (FJ805427)
Pseudonocardia petroleophila ATCC 15777 T (X80596)
Pseudonocardia xinjiangensis AS 4.1538 T (AF325728)
Pseudonocardia yunnanensis IFO 15681T (D85472)
Pseudonocardia aurantiaca DSM 44773 T (FR749916)
Pseudonocardia rhizophila YIM 67013T (GU322368)
Pseudonocardia thermophila IMSNU 20112T (AJ252830)
Pseudonocardia chloroethenivorans SL-1T (AF454510)
Pseudonocardia saturnea IMSNU 20052T (AJ252829)
Pseudonocardia babensis VN05A0561T (AB514449)
Pseudonocardia asaccharolytica DSM44247T (Y08536)
Pseudonocardia mongoliensis MN08-A0270T (AB521671)
Pseudonocardia ailaonensis YIM45505T (DQ344632)
Pseudonocardia oroxyli D10 T (DQ343154)
Pseudonocardia halophobica DSM 43089 T (Y08534)
Pseudonocardia tetrahydrofuranoxydans K1T (AJ249200)
Pseudonocardia benzenivorans B5 T (AJ556156)
Pseudonocardia sulfidoxydans DSM 44248 T (Y08537)
Pseudonocardia spinosispora LM 141T (AJ249206)
Pseudonocardia eucalypti EUM 374 T (FJ805426)
GMKU095 T (EU921261)
Pseudonocardia carboxydivorans Y8 T (EF114314)
Pseudonocardia antarctica DVS 5a1T (AJ576010)
99 Pseudonocardia alni IMSNU 20049T (AJ252823)
Pseudonocardia tropica YIM 61452T (GQ906587)
Pseudonocardia parietis 04-St-002T (FM863703)
Pseudonocardia nitrificans IFAM 379 T (X55609)
Pseudonocardia antitumoralis SCSIO 01299TP (JN204514)
Pseudonocardia ammonioxydans H9T (AY500143)
Pseudonocardia endophytica YIM 56035T (DQ887489)
Pseudonocardia kongjuensis LM 157 T (AJ252833)
Pseudonocardia autotrophica IMSNU 20050 T (AJ252824)
IMSNU 20111T (AJ252825)
Actinophytocola burenkhanensis MN08-A0203T (AB535095)
Actinokineospora riparia NRRL B-16432 T (AF114802)
Allokutzneria albata DSM 44149 T (AJ512462)
Fig. 2. Neighbour-method phylogenetic tree based on 16S rRNA gene sequences, showing the position of strain SCSIO01299T in the genus Pseudonocardia. Bootstrap values (.50 %) based on 1000 resamplings are shown at branch nodes.
Filled circles indicate that the corresponding nodes were also recovered in trees generated with the minimum-evolution andmaximum-likelihood methods. Bar, 0.5 % sequence divergence.
complete deletion gaps and minimum-evolution, max-
pH 7.0–8.0, at 20–30 uC and with 3–8 % (w/v) NaCl. The
imum-likelihood and maximum-parsimony methods, and
isolate could use most of the sole carbon sources tested in
confidence analysis was undertaken using 1000 bootstrap
this study. The detailed physiological characteristics are
replicates DNA–DNA hybridization was
done according to the optical renaturation method
An almost-complete 16S rRNA gene sequence (1435 bp) of
) using a UV-VIS spectropho-
strain SCSIO 01299T was obtained. BLAST search results in
tometer (model UV1601; Shimadzu).
EzTaxon-e showed that strain SCSIO 01299T had the highest
Strain SCSIO 01299T had abundant white aerial mycelia,
sequence similarities with Pseudonocardia ammonioxydans
spores and yellowish white to moderate yellowish brown
H9T (98.5 %) P. kongjuensis LM 157T
substrate hyphae. The isolate grew well on ISP 2, 3, 4 and 5
(98.3 %) P. autotrophica IMSNU 20050T
and Czapek's agar at 28 uC and grew weakly on potato
(97.8 %) P. nitrificans IFAM 379T
agar, but did not grow on nutrient agar. Dark orange
(97.7 %) (), P. tropica YIM 61452T
yellow pigment was produced on Czapek's agar. The
(97.7 %) (P. endophytica YIM 56035T
detailed cultural characteristics of this strain and its closest
(97.6 %) and P. compacta IMSNU 20111T
neighbours are listed in The organism formed an
(97.6 %) (The neighbour-joining
extensively branched substrate mycelia and aerial hyphae
phylogenetic tree (showed that the isolate formed
that differentiated into long spore chains. Spores were rod-
a distinct branch that was most closely related to P. ammo-
like with smooth surfaces (0.4–0.5 mm wide and 1.2–
nioxydans H9T and more loosely related to P. kongjuensis LM
2.6 mm long) Cells of strain SCSIO 01299T were
157T, P. autotrophica IMSNU 20050T, and P. endophytica
aerobic and Gram-positive. Good growth occurred at
YIM 56035T, with high bootstrap support. These findings
International Journal of Systematic and Evolutionary Microbiology 63
Pseudonocardia antitumoralis sp. nov.
Table 3. Chemotaxonomic characteristics of strain SCSIO 01299T and its closest phylogenetic neighbours in the genusPseudonocardia
Strains: 1, Pseudonocardia antitumoralis sp. nov. SCSIO 01299T; 2, P. ammonioxydans CGMCC 4.1877T; 3, P. kongjuensis DSM 44525T; 4, P.
autotrophica BCRC 12444T; 5, P. endophytica YIM 56035T; Data were taken from this study unless otherwise stated. All strains contain Araand Gal as the diagnostic cellular sugars. DAP, Diaminopimelic acid; DPG, diphosphatidylglycerol; PC, phosphatidylcholine; PE,phosphatidylethanolamine; PG, phosphatidylglycerol; PI, phosphatidylinositol; PIM, phosphatidylinositol mannosides; PL, unknown phospholipid;PME, phosphatidylmethylethanolamine; ND, not detected.
anteiso-C17 : 1v9c
C17 : 0 10-methyl
Amino acids/peptides in
meso-DAP, Asp, Gly,
meso-DAP, Asp, Gly,
DPG, PC, PE, PMEc
*Data were obtained from cells incubated in TSB with shaking at 28 uC for 5 days. Fatty acids representing ,0.5 % of the total in all taxa are notshown. Summed features represent two or three fatty acids that cannot be separated by the Microbial Identification System. Summed feature 3consisted of C16 : 1v7c and/or C16 : 1v6c. Summed feature 9 consisted of iso-C17 : 1v9c and/or C16 : 0 10-methyl.
DPhospholipid data were taken from: a, b, c,
were supported with the minimum-evolution and max-
genus Pseudonocardia. These cumulative data confirmed
imum-likelihood analyses ) and the maximum-
that the isolate should be assigned to the genus
parsimony analysis (Fig. S1, available in IJSEM Online).
The chemotaxonomic data clearly showed the
The cultural and micromorphological characteristics in
differences between strain SCSIO 01299T and its four
and including dark orange yellow soluble
closest phylogenetic neighbours. The fatty acid composi-
pigment on Czapek's agar, no growth on nutrient agar,
tion of strain SCSIO 01299T contained a small amount of
show the differences between strain SCSIO 01299T and its
iso-C11 : 0 3-OH (1.03 %) and moderate amounts of
four closest neighbours. As shown in the isolate
anteiso-C17 : 0 (10.21 %) and summed feature 9 (compris-
could use inositol, L-rhamnose, sodium oxalate and
ing iso-C17 : 1v9c and/or C16 : 0 10-methyl; 10.85 %). The
sodium citrate easily as sole carbon source for growth,
polar lipids (Fig. S2) were diphosphatidylglycerol, phos-
but not fucose, D-fructose or D-xylose. Strain SCSIO
01299T was susceptible to lincomycin and ampicillin, but
not to erythromycin, rifampicin, sulfamethoxazole or
nositol and three unknown phospholipids. There were no
neomycin. The isolate could use cellulose for growth, was
phosphatidylinositolmannosides present in the isolate. The
positive for hydrolysis of starch and Tween 60 and milk
type IV cell-wall composition of meso-diaminopimelic
peptonization and was negative for utilization of urea,
acid, arabinose and galactose
production of H2S and hydrolysis of gelatin. Moreover,
the predominant menaquinone MK-8(H4), and the
strain SCSIO 01299T could grow at 4 uC and with up to
major fatty acid iso-C16 : 0 were also consistent with the
15 % (w/v) NaCl. In conclusion, all of the above data could
X.-P. Tian and others
distinguish strain SCSIO 01299T easily from its closest
The type strain, SCSIO 01299T (5DSM 45322T 5CCTCC
relative, P. ammonioxydans CGMCC 4.1877T, and other
M 2011255T), was isolated from a deep-sea sediment
members of the genus Pseudonocardia.
sampled at a depth of 3258 m in the northern South ChinaSea. The G+C content of the genomic DNA is 73.2 mol%.
DNA–DNA hybridization showed that the isolate exhibited
The type strain produces the previously synthesized
low DNA–DNA relatedness with its closest phylogenetic
antitumour agent deoxynyboquinone and its novel deri-
neighbours (13 % with P. ammonioxydans CGMCC 4.1877T,
vatives, pseudonocardians A–C.
25 % with P. autotrophica BCRC12444T and 18 % with P.
kongjuensis DSM 44525T), values which are below the 70 %cut-off point recommended for the delineation of genomic
species (). It has been reported that,although many species in the genus Pseudonocardia share
This research was supported by the National Basic Research Programof China (2010CB833801), the Knowledge Innovation Program of
98.5–99.6 % 16S rRNA gene sequence similarity, DNA–
Chinese Academy of Sciences (KSCX2-YW-G-065, KSCX2-EW-G-12,
DNA relatedness between them are below the 70 % cut-off
KZCX2-YW-JC202), the National Natural Science Foundation of
point ; These findings and
China (40906075, 40906076, 41006089), the Natural Science Funds of
results confirm that strain SCSIO 01299T represents a
South China Sea Institute of Oceanology for Young Scholar
genetically distinct species.
(SQ200901) and South China Sea Open Cruise by R/V Shiyan 3,South China Sea Institute of Oceanology, CAS.
The phenotypic, physiological and chemotaxonomic char-acteristics and phylogenetic analysis of strain SCSIO 01299Tand its closest neighbours in the genus Pseudonocardia
suggest that it can be readily differentiated from species withvalidly published names. Thus, we propose that strain
Be´rdy, J. (2005). Bioactive microbial metabolites. J Antibiot (Tokyo)
SCSIO 01299T represents a novel species of the genus
Pseudonocardia, Pseudonocardia antitumoralis sp. nov.
Chen, H.-H., Qin, S., Li, J., Zhang, Y.-Q., Xu, L.-H., Jiang, C.-L., Kim,C.-J. & Li, W.-J. (2009). Pseudonocardia endophytica sp. nov., isolated
Description of Pseudonocardia antitumoralis
from the pharmaceutical plant Lobelia clavata. Int J Syst EvolMicrobiol 59, 559–563.
Felsenstein, J. (1985). Conference limits on phylogenies: an approach
Pseudonocardia antitumoralis (an.ti.tu.mo9ria.lis. Gr. prep.
using the bootstrap. Evolution 39, 783–791.
anti against; L. n. tumour a swelling; L. fem. suff -alis suffix
Fenical, W., Baden, D., Burg, M., deGoyet, C. V. & Grimes, J. D.
used with the sense of pertaining to; N.L. fem. adj.
(1999). Marine-derived pharmaceuticals and related bioactive com-
pounds. In From Monsoons to Microbes: Understanding the Ocean'sRole in Human Health, pp. 71–86. Edited by W. Fenical. Washington,
Gram-positive, aerobic actinomycete forming abundant
DC: National Academies Press.
white aerial mycelia, spores and yellowish white to
Goodfellow, M. & Fiedler, H. P. (2010). A guide to successful
moderate yellowish brown substrate hyphae on ISP 2, 3,
bioprospecting: informed by actinobacterial systematics. Antonie van
4 and 5 and Czapek's agar at 28 uC. Dark orange yellow
Leeuwenhoek 98, 119–142.
soluble pigment is produced on Czapek's agar. Fragments
Groth, I., Rodrı´guez, C., Schu¨tze, B., Schmitz, P., Leistner, E. &
are observed on aerial mycelia and spore chains after
Goodfellow, M. (2004). Five novel Kitasatospora species from soil:
incubation at 28 uC for 14–21 days. Spores are smooth-
Kitasatospora arboriphila sp. nov., K. gansuensis sp. nov., K.
surfaced and rod-like (0.4–0.5 mm wide and 1.2–2.6 mm
nipponensis sp. nov., K. paranensis sp. nov. and K. terrestris sp. nov.
long). The pH, NaCl concentration and temperature ranges
Int J Syst Evol Microbiol 54, 2121–2129.
for growth are pH 6.0–8.5, 0–15 % NaCl and 4–40 uC,
Henssen, A., Happach-Kasan, C., Renner, B. & Vobis, G. (1983).
respectively (optima pH 7.5, 3 % NaCl and 28 uC). Positive
Pseudonocardia compacta sp. nov. Int J Syst Bacteriol 33, 829–
for hydrolysis of starch, cellulose, Tweens 20, 40 and 60,
milk coagulation and peptonization and catalase. Negative
Huss, V. A. R., Festl, H. & Schleifer, K. H. (1983). Studies on the
for hydrolysis of gelatin, casein and Tween 80, production
spectrophotometric determination of DNA hybridization from
renaturation rates. Syst Appl Microbiol 4, 184–192.
2S and melanin, reduction of nitrate, utilization of
urea and oxidase. Most sugars are utilized as sole carbon
Jahnke, K. D. (1992). BASIC computer program for evaluation of
sources for growth, but fucose is not. Susceptible to
spectroscopic DNA renaturation data from Gilford System 2600spectrophotophotometer on a PC/XT/AT type personal computer.
ampicillin, amikacin, chloramphenicol, ciprofloxacin, gen-
J Microbiol Methods 15, 61–73.
tamicin, lincomycin, netilmicin, norfloxacin, tetracycline,tobramycin and vancomycin, and resistant to erythromy-
Kelly, K. L. (1964). Inter-Society Color Council – National Bureau ofStandards Color Name Charts Illustrated with Centroid Colors.
cin, neomycin, penicillin G, rifampicin and sulfamethox-
Washington, DC: US Government Printing Office.
azole. The cell-wall chemotype is type IV. The predominant
Kim, O.-S., Cho, Y.-J., Lee, K., Yoon, S.-H., Kim, M., Na, H., Park, S.-C.,
menaquinone is MK-8 (H4). The phospholipid type is type
Jeon, Y. S., Lee, J.-H. & other authors (2012). Introducing EzTaxon-
III. The major fatty acids are iso-C16 : 0, iso-C16 : 1 H,
e: a prokaryotic 16S rRNA gene sequence database with phylotypes
anteiso-C17 : 0 and summed feature 9 (comprising iso-
that represent uncultured species. Int J Syst Evol Microbiol 62, 716–
C17 : 1v9c and/or C16 : 0 10-methyl.
International Journal of Systematic and Evolutionary Microbiology 63
Pseudonocardia antitumoralis sp. nov.
Kwon, H. C., Kauffman, C. A., Jensen, P. R. & Fenical, W. (2009).
Smibert, R. M. & Krieg, N. R. (1981). General characterization. In
Marinisporolides, polyene-polyol macrolides from a marine actino-
Manual of Methods for General Microbiology, pp. 409–443. Edited
mycete of the new genus Marinispora. J Org Chem 74, 675–684.
by P. Gerhardt, R. G. E. Murray, R. N. Costilow, E. W. Nester,
Lam, K. S. (2006). Discovery of novel metabolites from marine
W. A. Wood, N. R. Krieg & G. B. Phillips. Washington: American
actinomycetes. Curr Opin Microbiol 9, 245–251.
Society for Microbiology.
Lechevalier, M. P. & Lechevalier, H. A. (1970). Chemical composition
Tamura, K., Peterson, D., Peterson, N., Stecher, G., Nei, M. & Kumar,
as a criterion in the classification of aerobic actinomycetes. Int J Syst
S. (2011). MEGA5: Molecular Evolutionary Genetics Analysis using
Bacteriol 20, 435–443.
maximum likelihood, evolutionary distance, and maximum par-simony methods. Mol Biol Evol 28, 2731–2739.
Lee, S. D., Kim, E. S., Min, K. L., Lee, W. Y., Kang, S. O. & Hah, Y. C.
(2001). Pseudonocardia kongjuensis sp. nov., isolated from a gold mine
Thompson, J. D., Gibson, T. J., Plewniak, F., Jeanmougin, F. &
cave. Int J Syst Evol Microbiol 51, 1505–1510.
Higgins, D. G. (1997). The CLUSTAL_X windows interface: flexible
Li, W.-J., Xu, P., Schumann, P., Zhang, Y.-Q., Pukall, R., Xu, L.-H.,
strategies for multiple sequence alignment aided by quality analysis
Stackebrandt, E. & Jiang, C.-L. (2007). Georgenia ruanii sp. nov., a
tools. Nucleic Acids Res 25, 4876–4882.
novel actinobacterium isolated from forest soil in Yunnan (China),
Tian, X.-P., Zhi, X.-Y., Qiu, Y.-Q., Zhang, Y.-Q., Tang, S.-K., Xu, L.-H.,
and emended description of the genus Georgenia. Int J Syst Evol
Zhang, S. & Li, W.-J. (2009). Sciscionella marina gen. nov., sp. nov., a
Microbiol 57, 1424–1428.
marine actinomycete isolated from a sediment in the northern South
Li, S. M., Tian, X. P., Niu, S. W., Zhang, W. J., Chen, Y. C., Zhang, H. B.,
China Sea. Int J Syst Evol Microbiol 59, 222–228.
Yang, X. W., Zhang, W. M., Li, W. J. & other authors (2011).
Tindall, B. J., Sikorski, J., Smibert, R. A. & Krieg, N. R. (2007).
Pseudonocardians A–C, new diazaanthraquinone derivatives from
Phenotypic characterization and the principles of comparative
Pseudonocardia sp. SCSIO 01299. Mar Drugs 9, 1428–1439.
systematics. In Methods for General and Molecular Microbiology,
Liu, Z.-P., Wu, J.-F., Liu, Z.-H. & Liu, S.-J. (2006). Pseudonocardia
pp. 330–393. Edited by C. A. Reddy, T. J. Beveridge, J. A. Breznak,
ammonioxydans sp. nov., isolated from coastal sediment. Int J Syst
G. A. Marzluf, T. M. Schmidt & L. R. Snyder. Washington: American
Evol Microbiol 56, 555–558.
Society for Microbiology.
Mesbah, M., Premachandran, U. & Whitman, W. B. (1989). Precise
Waksman, S. A. (1961). The Actinomycetes. In Classification,
measurement of the G+C content of deoxyribonucleic acid by high-
Identification and Descriptions of Genera and Species, vol. 2, pp. 1–
performance liquid chromatography. Int J Syst Bacteriol 39, 159–167.
363. Baltimore: Williams & Wilkins.
Murray, R. G. E., Doetsch, R. N. & Robinow, F. (1994). Determinative
Warwick, S., Bowen, T., McVeigh, H. & Embley, T. M. (1994). A
and cytological light microscopy. In Methods for General and Molecular
phylogenetic analysis of the family Pseudonocardiaceae and the genera
Bacteriology, pp. 21–41. Edited by P. Gerhardt, R. G. E. Murray,
Actinokineospora and Saccharothrix with 16S rRNA sequences and a
W. A. Wood & N. R. Krieg. Washington, DC: American Society for
proposal to combine the genera Amycolata and Pseudonocardia in
an emended genus Pseudonocardia. Int J Syst Bacteriol 44, 293–
Park, S. W., Park, S. T., Lee, J. E. & Kim, Y. M. (2008). Pseudonocardia
carboxydivorans sp. nov., a carbon monoxide-oxidizing actinomycete,
Wayne, L. G., Brenner, D. J., Colwell, R. R., Grimont, P. A. D., Kandler,
and an emended description of the genus Pseudonocardia. Int J Syst
O., Krichevsky, M. I., Moore, L. H., Moore, W. E. C., Murray, R. G. E. &
Evol Microbiol 58, 2475–2478.
other authors (1987). International Committee on Systematic
Qin, S., Zhu, W.-Y., Jiang, J.-H., Klenk, H.-P., Li, J., Zhao, G.-Z., Xu,
Bacteriology. Report of the ad hoc committee on reconciliation of
L.-H. & Li, W.-J. (2010). Pseudonocardia tropica sp. nov., an endophytic
approaches to bacterial systematics. Int J Syst Bacteriol 37, 463–
actinomycete isolated from the stem of Maytenus austroyunnanensis.
Int J Syst Evol Microbiol 60, 2524–2528.
Xu, P., Li, W.-J., Tang, S.-K., Zhang, Y.-Q., Chen, G.-Z., Chen, H.-H.,
Saitou, N. & Nei, M. (1987). The neighbor-joining method: a new
Xu, L.-H. & Jiang, C.-L. (2005). Naxibacter alkalitolerans gen. nov., sp.
method for reconstructing phylogenetic trees. Mol Biol Evol 4, 406–
nov., a novel member of the family ‘Oxalobacteraceae' isolated from
China. Int J Syst Evol Microbiol 55, 1149–1153.
Source: http://t2.mcp.cn/oshpgngl/642334/papers/Pseudonocardia%20antitumoralis%20sp.%20nov.,%20a%20deoxynyboquinone-producing%20actinomycete%20isolated%20from%20a%20deep-sea%20sediment..pdf
El Zenzontle Número 84 ENERO 2011 COOPERACIÓN VOLUNTARIA NOS HACE LIBRES Jastal ‘oj ka' jb ajtikb'a slekilal ja jmojtiki‘oj ch'ay b'a jk'ujoltikspetzanil jab'akechan jb'aj ke' ntik ¿Cómo nos comprometemos los unos con los otros,para el bien de nuestros compañeros?Que se pierda en nuestro corazóntodo lo que es sólo para nosotros.
Hernalser Hauptstraße 155 1170 Wien Tel./Fax: 01/486 24 04 [email protected] Neu! Liefe Neu! Lief rser erservice – Seite 3 vice – Seite 3 W ihnachtsmarkt – Seite 4 eihnachtsmarkt – Seite 4 Immer wieder Fieberbl Immer wieder Fieberb asen Über 90% der Bev





