
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Domergues_20110148.vp
Significance of intermediate forms in phyletic reconstructionof ammonites: Early Jurassic
Phricodoceras case study
JEAN−LOUIS DOMMERGUES and CHRISTIAN MEISTER
Dommergues, J.−L. and Meister, C. 2013. Significance of intermediate forms in phyletic reconstruction of ammonites:Early Jurassic Phricodoceras case study.
Acta Palaeontologica Polonica 58 (4): 837–854.
This paper discusses the phyletic interpretation of the genus
Phricodoceras and its taxonomic classification at thesubfamily, family, and superfamily levels from an historical and critical perspective. First a review of the latest find−ings on this taxon is presented and the grounds for the attribution of
Phricodoceras to the Schlotheimiidae(Psiloceratoidea) are summarized and illustrated. This review is a synthesis grounded on evolutionary (e.g.,heterochronies, innovations), eco−ethological (e.g., assumed shell hydrodynamic capacities) and spatio−temporal pat−terns (e.g., bio−chronostratigraphy, palaeobiogeography). Then, the main stages of understanding the taxonomy of
Phricodoceras since the early nineteenth century are reviewed. Two main taxonomic concepts alternate over time. Thefirst is based on the "overall resemblance" of
Phricodoceras to some coeval Eoderoceratoidea leading to the genus be−ing included in its own family or subfamily (e.g., Phricodoceratinae) among the Eoderoceratoidea. The second hypoth−esis, recently confirmed by the discovery of an intermediate form (i.e.,
Angulaticeras spinosus), clearly includes
Phricodoceras within the Schlotheimiidae (Psiloceratoidea). Comparison of these two very different conceptions re−veals how "overall resemblance" can be misleading and shows that the discovery of intermediate forms is often the keyto phyletic reconstructions in ammonites.
K e y w o r d s : Cephalopoda, Ammonoidea, stratigraphy, paleobiogeography, taxonomy, character, homology, ontogeny,adaptation, Jurassic.
Jean−Louis Dommergues [Jean−Louis.Dommergues@u−bourgogne.fr], UFR Sciences Vie, Terre et Environnement,Université de Bourgogne, CNRS/uB, UMR 5561, Biogéosciences Dijon, 6 Boulevard Gabriel, F−21000 Dijon, France;Christian Meister [christian.meister@ville−ge.ch], Muséum d'Histoire Naturelle de Genève, Département de Géologie etde Paléontologie, 1 Rte de Malagnou, cp 6434, CH−1211 Geneva, Switzerland.
Received 18 September 2011, accepted 8 March 2012, available online 20 March 2012.
Copyright 2013 J.−L. Dommergues and C. Meister. This is an open−access article distributed under the terms of the Cre−ative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, pro−vided the original author and source are credited.
latest discoveries and their taxonomic implications; second,to recapitulate the main steps of the taxonomic practices in−
Phricodoceras is a homogeneous and unambiguously de−
volving
Phricodoceras since the early nineteenth century;
fined group among the late Sinemurian and Pliensbachian
and third, to examine the grounds for the major changes
ammonites. Although generally scarce,
Phricodoceras has
in the interpretation of the relationships of
Phricodoceras
been actively collected and studied since the early nine−
among the Sinemurian and Pliensbachian ammonites. Spe−
teenth century because of its attractive and unusual tubercu−
cial attention is also paid to why the misleading phyletic hy−
late ornamental pattern. As a result, despite its rarity, it is
pothesis by which the genus
Phricodoceras was ascribed to
discussed in a hundred or so publications. It is surprising
the Eoderoceratoidea should have proved so resilient in the
therefore that its relationships and consequently its taxo−
literature. The case of
Phricodoceras is discussed here to
nomic attribution should recently have been seriously
exemplify what is a common bias in ammonite taxonomic
questioned (Dommergues 1993, 2003; Dommergues and
practices. Taxonomic groupings grounded on some "over−
Meister 1999; Meister 2007; Dommergues et al. 2008) and
all resemblance" combined with stratigraphic control are
finally reconsidered at the superfamily level (Edmunds et
usually evidence. Unfortunately, later this may become
al. 2003; Meister et al. 2010, 2011; Blau and Meister 2011).
"coarse" evidence and/or may be found to be homeo−
This edifying late taxonomic revision illustrates the surpris−
morphy, as is shown here for
Phricodoceras. The impor−
ing immovability of questionable practices in ammonite
tance of transitional forms in convincingly defining the pri−
taxonomy. The aim of this work is, first, to summarize the
mary homologies is also clearly illustrated in the example
Acta Palaeontol. Pol. 58 (4): 837–854, 2013
ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
studied. Thus, beyond
Phricodoceras, the present work canbe viewed as a "case study" and a possible source of ideas
for ammonite taxonomy.
Institutional abbreviations.—UBGD, University of Burgundy.
Other abbreviations.—M, macroconch; m, microconch; t1,
latero−umbilical position; t2, latero−ventral position; t3, peri−
siponal position; us, umbilical seam; vb, ventral band (see also
Stratigraphic and geographic
The stratigraphic and paleobiogeographic frameworks of thegenus
Phricodoceras have been extensively and accurately
described by Meister (2007: figs. 12, 14, 16, 17). The results
of that key work are summarized here and supplemented
schematically by more recently published data (Figs. 1, 2).
The stratigraphic range of
Phricodoceras is objectively docu−
INEMURIAN
turneri
mented from the base of the
Echioceras raricostatum Chrono−
zone (
Crucilobiceras densinodulum Subchronozone) to the
top of the
Pleuroceras spinatum Chronozone (
Pleuroceras
hawskerense Subchronozone). In the Mediterranean Tethys
the last
Phricodoceras (
Phricodoceras aff.
cantaluppii Fantini
Sestini, 1978) are associated with
Emaciaticeras (Meister et
al. 2010). The earliest representatives of the genus
Phricodo−
NGIAN
Alsatites
ceras (
Echioceras raricostatum Chronozone) belong to the
group of
Phricodoceras gr.
taylori (Sowerby, 1826)–
P. lamel−
H
planorbis
losum (Orbigny, 1844). They exhibit from the outset all of the
impressive diagnostic features of the genus. Convincingly,
Phricodoceras roots among the genus
Angulaticeras and
A.
(
Angulaticeras)
spinosus Meister, Schlögl, and Rakús, 2010,
Fig. 1. Bio−chronostratigraphic framework of the six genera belonging tothe Schlotheimiidae as this family is understood in the present paper. The
a recently discovered species with a
Phricodoceras−like juve−
ranges are referred to the standard chronostratigraphic scale (stages and
nile stage, comes from a condensed Carpathian fauna suggest−
chronozones) so that relevant global comparisons can be made. The proba−
ing a period from the
Arietites bucklandi to the
Caenisites
ble age of
Angulaticeras spinosus is starred. Radiochronologic ages of the
turneri chronozones (Meister et al. 2010). The condensed con−
stage boundaries from Ogg et al. (2008). The height of the chronozone
text of this unusual fossiliferous locality must be underlined
boxes varies with the stage duration in Myr.
because the sedimentary processes often associated with con−densation can explain the presence of an episode that is usu−
Phricodoceras obviously reflects the persistence of only a few
ally missing at the regional level (e.g., long lasting submarine
but striking ornamental diagnostic traits (autapomorphies).
exposure and/or erosion) (Olóriz 2000; Cecca 2002; Olóriz
On the contrary, such flagrant features are missing among the
and Villaseñor 2010). Even if an age somewhere in the
Caeni−
earliest representatives of the family and the genus diagnoses
sites turneri Chronozone is plausible for
A. (
A.)
spinosus
are clearly less constrained as a result. So comparisons of ge−
(Fig. 1), there remains an undocumented stratigraphic gap in
nus durations are perhaps weakly significant in evolutionary
Phricodoceras history corresponding approximately to the
duration of the
Asteroceras obtusum–
Oxynoticeras oxynotum
In paleobiogeographic terms
Phricodoceras is a taxon
Chronozones. Fig. 1 shows that
Phricodoceras is clearly the
chiefly known in the Mediterranean and NW European con−
longest−surviving genus of the Family Schlotheimiidae. In
fines of the Western Tethys (Fig. 2). Thus, of the just over one
point of fact, the genus durations have a propensity to increase
hundred publications featuring, to some extent, the genus
throughout the history of the family, and this tendency appar−
Phricodoceras, 37 concern the Mediterranean faunas (includ−
ently peaks with
Phricodoceras. Obviously, like all taxo−
ing the Pontides, Northern Turkey), 41 discuss the NW Euro−
nomic groupings, genera are partly subjective and their dura−
pean faunas, and only 7 refer to other parts of the world. In
tion may be influenced by taxonomic practice, which widely
fact, very few specimens are cited outside the Mediterranean
depends on human perception. Thus the long duration of
Tethys, NW Europe and the Pontides (Fig. 2). Moreover, the
DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
approximate NW European vs. Tethyan faunal
Angulaticeras spinosus Asterocera
boundary during the Early Pliensbachian
Phricodoceras from the
Echioceras raricostatum Chronozone
Phricodoceras from the
Uptonia jamesoni Chronozone
Mediterranean Tethys
Phricodoceras from the
Tragophylloceras ibex Chronozone
Pontides (Northern Turkey)
Phricodoceras from the
Prodactylioceras davoei Chronozone
Phricodoceras from the
Amaltheus margaritatus Chronozone
Timor (Roti Island)
Phricodoceras from the
Pleuroceras spinatum Chronozone
Western North America (British Columbia, Oregon)
Phricodoceras from the Early Pliensbachian sensu lato
Fig. 2. Schematic distribution of
Angulaticeras spinosus Meister, Schlögl, and Rakús, 2010 and
Phricodoceras at the global scale. The approximate bound−ary between the NW European and Tethyan (Mediterranean) faunas is suggested by a dotted line. Paleogeographical reconstruction from Vrielynck andBouysse (2001), modified.
specimens from Western North America, Northern Chile and
More generally, the Mediterranean Tethys seems to be
the Eastern Himalayas are unconvincing or questionable. The
the only known sustained "hot spot" of
Phricodoceras di−
only reliable representative of the genus
Phricodoceras from
versity. By contrast, only a few species related to the group
outside the Mediterranean and NW European confines of the
of
P. taylori sensu lato are known in NW Europe and almost
Western Tethys is a finely preserved specimen from the Timor
all of the many specimens known in this area are associated
area close to the Australian Tethyan margin (Krumbeck
with a brief dramatic acme in the lower part of the
Uptonia
1922). Ideally, it would be best to consider the stratigraphic
jamesoni Chronozone. Paradoxically, the
Phricodoceras
sensu lato and sedimentological frameworks so as to counter−
are never common in the Mediterranean Tethys but both
balance this crude palaeobiogeographical data, which can
their taxonomic diversity and their morphological disparity
yield a partly biased picture of reality. Unfortunately, though,
remain persistently high in this area where the genus is
the present synthesis is grounded on such heterogeneous liter−
recurrently observed from the
Echioceras raricostatum
ature that the consideration of stratigraphic and sedimento−
Chronozone to the base of the
Pleuroceras spinatum
logical data is no more than an ideal. Nevertheless—as previ−
Chronozone (Figs. 1, 2). We must also emphasize that
ously demonstrated for the Early Pliensbachian by Dom−
Angulaticeras (
Angulaticeras)
spinosus, a possible ances−
mergues et al. (2009: fig. 6)—despite a similar study effort (at
tor of
Phricodoceras, is to date only known in the Mediter−
least in terms of number of publications), the Mediterranean
ranean Tethys (i.e., Austroalpine). In terms of diversity
Tethys palaeobiodiversity is clearly richer than that of NW
(i.e., comparison of the number of species during the
Echio−
Europe, although it is still undersampled in comparision.
ceras raricostatum and
Uptonia jamesoni chronozones) the
ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
Pontides area occupies an intermediate position between
ner whorls of the macroconch and microconch at all onto−
the Mediterranean Tethys and NW Europe.
genetic stages (e.g.,
P. taylori) are quite distinctive and pre−
In this paper, the binominal italicized names of chrono−
clude any confusion. At small diameters
Phricodoceras may
zones result from the policy of the journal that any names
display one of the most impressively tuberculate ornamenta−
derivative of biological species should be written in this
tions among the Early Jurassic ammonites, notably an excep−
tional peri−siphonal (t3) row of tubercles or spines (Figs. 4,5). The inner mould of the phragmocones exhibits only thebases of the spines, which in this case look like truncated tu−
Morphology, dimorphism,
bercles or bullae (Fig. 4), but some well−preserved speci−mens display prominent spines especially in peri−siphonal
ontogeny, and adaptation
(t3) and latero−ventral (t2) positions (e.g., Buckman 1911: pl.
33; Hoffmann 1982: pl. 14: 3; Edmunds et al. 2003: fig. 20.5)
The diagnostic features of
Phricodoceras and especially the
(Fig. 5E). Within the groups of
P. taylori (m)–
P. lamellosum
"juvenile" ornamental features are very unusual for Early Ju−
(M) and of P.
bettoni (m) Géczy, 1976–
P. urkuticum (M)
rassic ammonites and the genus has always been regarded as
(Géczy, 1959) at least, up to three rows of tubercles can be
forming both a highly distinctive and a homogeneous taxon.
observed, though briefly, during the most strongly orna−
Even the most morphologically derived forms (e.g., tiny Late
mented growth stage (Meister 2007: fig. 11). The positions
Pliensbachian microconchs or large Early Pliensbachian
of these three rows of tubercles are indicated in Fig. 4.
macroconchs) can be fairly easily attributed to the genus. As
Among the genus
Phricodoceras the latero−umbilical (t1)
a result, the synonymy of the genus is limited to a single
row of tubercles is often missing and the latero−ventral (t2)
taxon (i.e.,
Hemiparinodiceras Géczy, 1959) and there is no
row is sometimes absent, even in the group of
P. taylori
subgenus to suggest possible groupings within the twenty or
(m)–
P. lamellosum (M) (e.g.,
Phricodoceras aff.
cornutum
so nominal species. Despite its apparent homogeneity, the
[Simpson, 1843]) (Fig. 3D). Conversely the peri−siphonal
genus
Phricodoceras is not a simple lineage but, as evi−
(t3) row of tubercles remains visible, during a brief growth
denced by Meister (2007: fig. 15), a clade with a rather com−
stage at least. The permanence of this trait is strong evidence
plex internal structure. The concept of "species complex"
that the peri−siphonal tubercles or shoulder (t3 or s3) of
Phri−
might be helpful in putting the clade topology into words.
codoceras are homologous with the sudden peri−siphonal in−
Even if the phenomenon tends to decrease with time, a usu−
terruption of the ribs or shoulders (s3) of
Angulaticeras,
ally obvious microconch (m)/macroconch (M) dimorphism
which is also a very permanent juvenile trait (Figs. 4–6).
characterizes the
Phricodoceras as exemplified by the pair of
Although less distinctive, the suture lines of
Phricodoceras
nominal species
P. taylori (m)–
P. lamellosum (M) in Fig. 3.
also have informative features which can be contrasted with
Dimorphism seems to have peaked in this group close to the
Angulaticeras on the basis of a comparative study of septal
base of the Early Pliensbachian in NW Europe and therefore
suture ontogenies. The pointed, often slender and trifid
in a palaeobiogeographical context suggesting a briefly suc−
(sometimes sub−triangular) lateral lobe of
Phricodoceras is
cessful northward faunal ingression. The extent of this strik−
the most obvious similarity (Fig. 7), and despite many appar−
ing dimorphism is difficult to quantify because the largest
ent differences, the suture line of
Phricodoceras can be un−
known
P. lamellosum (M) are all incomplete phragmocones
derstood as a simplified version of that observable in
Angu−
(e.g., Fig. 3A), and their adult body chambers are unknown.
laticeras with wider saddles and chiefly without any clear re−
However, a ratio of about one to ten in diameter can be rea−
tracted suspensive lobe, as is usual in
Angulaticeras. Many
sonably suspected. The intermediate and outer whorls of the
of these differences and especially the lack of an obvious sus−
large macroconch forms have rather involute and compres−
pensive lobe are probably partially correlated with different
sed shells with slightly curved flanks and a rounded ventral
shell morphologies. At the same diameters, shells are clearly
area. The transition between the umbilical area and the base
more involute and compressed in
Angulaticeras than in
Phri−
of the flanks is rounded without shoulders, although faint
codoceras whose inner whorls, at least, often have sub−
peri−siphonal shoulders (s3), inherited from juvenile peri−
circular sections and barely overlap the successive whorls,
siphonal tubercles (t3), may persist at relatively large dia−
thus providing less space for the retraction of the umbilical
meters (e.g., Fig. 3A, B). The ornamentation of crowded,
lobes. Conversely, the suture lines of
Phricodoceras are very
fine, subdivided and slightly flexuous ribs is rather discreet
different from those of both the Lytoceratoidea and Eodero−
and often somewhat irregular (e.g., Fig. 3A). At large diame−
ceratoidea whose bifid or trifid lateral lobes are invariably
ters the ribs may cross the ventral area. Thus, the pre−adult
adapically broad but abapically often narrow (Fig. 8).
and probably also the adult (body chamber) habitus of the
The evolution of
Phricodoceras is, as demonstrated by
macroconch is coarsely comparable, at the same diameter, to
Meister (2007: fig. 11), basically controlled by ontogenetic
that of
Angulaticeras. Actually, at large diameters
Phricodo−
heterochronies in the "size−based" or "allometric" and not
ceras lamellosum (M) looks similar to
Angulaticeras al−
"age−based" sense of the term. Fig. 9 summarizes and sim−
though with a less compressed shell and a wider and more
plifies the model proposed by Meister (2007) for
Phricodo−
rounded ventral area. In contradistinction, the traits of the in−
ceras and extends it to a broader taxonomic framework

DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
Fig. 3. Microconch (m) / macroconch (M) dimorphism expressed by scholtheimiid ammonoid Phricodoceras exemplified by the NW Europe forms in the
Uptonia jamesoni to Tragophylloceras ibex chronozones. A. Phricodoceras lamellosum (Orbigny, 1844) (M), UBGD 277451, Mazenay, Saône et Loire,
France, probably early Uptonia jamesoni Chronozone, in apertural (A1), lateral (A2), and ventral (A3) views. B. Phricodoceras lamellosum (M), Kircheim unter
Teck, Baden−Würtemberg, Germany, Early Pliensbachian (from Schlegelmilch 1976: pl. 27: 4, modified; original from Quenstedt 1884: pl. 28: 24), in apertural
(B1), lateral (B2), and ventral (B3) views. C. Phricodoceras taylori (Sowerby, 1826) (m), Corbigny, Nièvre, France, Uptonia jamesoni Chronozone,
Phricodoceras taylori Subchronozone (from Dommergues 2003: pl. 1: 4), in lateral view. D. Phricodoceras aff. cornutum (Simpson, 1843) (m), Fresnay−
le−Puceux, Calvados, France, Early Pliensbachian (from Dommergues et al. 2008: pl. 3: 6, modified), in ventral (D1) and lateral (D2) views. E. Phricodoceras
taylori (m), Fresnay−le−Puceux, Calvados, France, Early Pliensbachian (from Dommergues et al. 2008: pl. 3: 5, modified), in ventral (E1) and lateral (E2) views.
The two specimens corresponding to A, B are incomplete phragmocones (juvenile or immature shells) but the three corresponding to C–E are adult microconchs
with the major part of the body chamber. The end of the phragmocone is starred. Notice the progressive ontogenetic transformation from tubercle (t3) to faint
shoulder (s3) in specimen B. Abbreviations: t2, tubercle in latero−ventral position; t3, tubercle in peri−siphonal position; s3, shoulder peri−siphonal position.
including Angulaticeras, with A. boucaultianum (Orbigny,
bachian). The first step (A. boucaultianum to A. spinosus)
1844) (Early Sinemurian) for comparison and A. spinosus
involves a "juvenile innovation" sensu Dommergues et al.
(Late Sinemurian) as a possible ancestor or at least the sister
(1986) and Dommergues (1987), a phenomenon that is not a
group of Phricodoceras (Late Sinemurian to Late Pliens−
heterochony sensu stricto but which immediately precedes
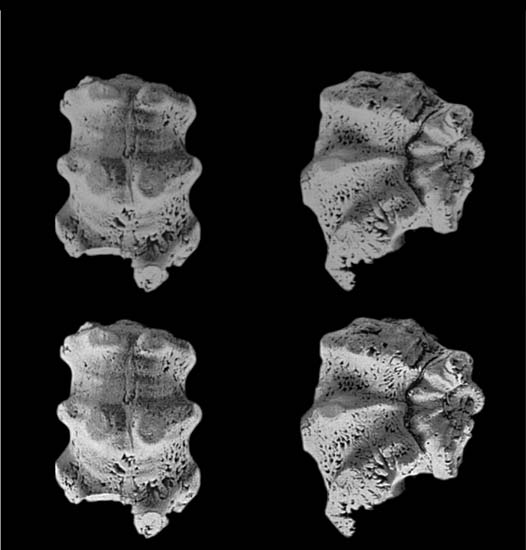
ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
case, retardation is accompanied by a dramatic enhance−ment of the juvenile features and the tuberculated ornamen−tation reaches a maximum in the group of P. taylori (m)–P.
lamellosum (M). The spines reach outstanding proportionsand three rows of tubercles are usual. The third (P. lamel−losum to P. urkuticum) and fourth (P. urkuticum to P.
paronai [Bettoni, 1900]) steps follow a reversal and an in−crease in complexity of the heterochronic pattern. Theselast two steps in Phricodoceras history witness a sustained
contraction and weakening of the juvenile tuberculate stageand a correlative progressive decline in adult size. Thiscomplex pattern suggests the combination of two distinctpolarities, one peramorphic (by acceleration of growth) andthe other paedomorphic (by hypomorphosis), although"phyletic dwarfism" is another possibility because size isnot necessarily a proxy of age. In palaeobiogeographicalterms the late tiny or at least smallish (possibly dwarf ?)Phricodoceras are rare, or even very rare, strictly Tethyanspecies; however, relations with the palaeoenvironmental
conditions remains obscure.
In terms of adaptation and traits of life history only as−
sumptions are possible. Nevertheless, the importance of pat−
Fig. 4. Position and terminology of the tubercles, spines and/or bullae on
terns chiefly related with juvenile stages (i.e., juvenile inno−
Phricodoceras shells (juvenile and/or microconch. A, B. Normal view. C, D.
vation and paedomorphosis by deceleration) suggests that
Shaded view with indication of the main ornamental structure outlines (white
the evolutionary history of Phricodoceras was a phenome−
lines). Abbreviations: t1, latero−umbilical position; t2, latero−ventral posi−
non partly associated with changes in juvenile living condi−
tion; t3, peri−siphonal position; us, umbilical seam; vb, ventral band.
tions (Fig. 10). It seems reasonable to assume that the spec−tacular tuberculate ornamentation ensured an effective pas−
evolutionary phenomena chiefly controlled by heterochro−
sive protection both for the juvenile macroconchs and for
nies. In the case of A. spinosus, the innovation is the possi−
the microconchs throughout their growth. In this sense, the
bly rapid emergence of an obviously tuberculated ornamen−
emergence of a tuberculate growth stage in Phricodoceras,
tation in the innermost whorls only. Conversely, the subse−
and therefore within the Schlotheiimidae, could be under−
quent and merely ribbed growth stages of this species are
stood as a convergence with the plentiful and diversified Late
usual for Angulaticeras. Truncated tubercles in (t2) posi−
Sinemurian and Early Pliensbachian tuberculated Eodero−
tion are clearly visible up to an umbilical diameter of 11 mm
ceratoidea (Fig. 11B, C). Conversely, it is possible that the
(Fig. 5A1, A2). They are similar to the tubercles in the same
living conditions of the post−juvenile macroconchs of Phri−
position and at the same diameter in Phricodoceras (Fig.
codoceras were little changed from those of Angulaticeras.
5B1) so, and although the ventral area is concealed by whorl
Differences in lifestyle between juvenile macroconchs and
overlap, it is plausible that tubercles also exist in peri−
microconchs (assumed to have been not very mobile but pas−
siphonal position in the inner whorls of A. spinosum. The
sively protected) and adult macroconchs (assumed to have
second step (A. spinosus to P. lamellosum) is chiefly a
had better hydrodynamic abilities and mobility, as suggested
paedomorpic pattern of heterochony with an obvious decel−
by the more compressed shell, with weaker and more flexu−
eration of growth sensu Reilley et al. (1997). As is often the
ous ornamentation) are therefore perhaps the key to the spe−
Fig. 5. Comparison of morphological and ornamental patterns of scholtheimiid ammonoid Angulaticeras spinosus Meister, Schlögl, and Rakús, 2010 and ®
Phricodoceras gr. taylori (Sowerby, 1826) (m)–Phricodoceras lamellosum (Orbigny, 1844) (M). A. Angulaticeras (Angulaticeras) spinosus (M?), holotype,
Chtelnica, Male Karpaty Mts., Western Carpathians, Slovakia, Sinemurian condensed bed (from Meister et al. 2010: fig. 34, a, b, modified), in lateral (A1, A2)
and apertural (A3) views. B. Phricodoceras taylori (m?), Corbigny, Nièvre, France, Uptonia jamesoni Chronozone, Phricodoceras taylori Subchronozone
(from Dommergues 2003: pl. 1: 2, modified), in lateral (B1, B2) and ventral (B3) views. C. Phricodoceras lamellosum (M), Hinterweiler, Baden−Würtemberg,
Germany, Early Pliensbachian (from Schlatter 1980: pl. 6: 6, modified), incomplete phragmocone showing the transition between the juvenile tuberculate stage
and the late merely ribbed stage, in lateral (C1) and ventral (C2) views. D. Phricodoceras taylori (m), Corbigny, Nièvre, France, Uptonia jamesoni Chronozone,
Phricodoceras taylori Subchronozone (from Dommergues 2003: pl. 1: 4), in lateral view. E. Phricodoceras taylori (m), Fresnay−le−Puceux, Calvados, France,
Early Pliensbachian (from Dommergues et al. 2008: pl. 3: 5, modified), in lateral (E1) and ventral (E2) views. To facilitate comparisons at small diameters,
A1 and B1, respectively corresponding to A2 and B2, are twice magnified. The three specimens corresponding to A–C are incomplete phragmocones (juvenile
or immature shells) but the two specimens corresponding to D, E are adult microconchs with the major part of the body chamber. The end of the phragmocone
is indicated by a star. Some noticeable ornamental elements are indicated by arrows: smooth ventral band (vb), tubercle in latero−ventral position (t2), tubercle
or shoulder in peri−siphonal position (t3 or s3).


DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
cific features of Phricodoceras. This hypothesis, summa−
1996). Such a pattern, however, is not rare among the extant
rized in Fig. 10, is partly speculative, though, because eco−
ethological considerations derived from shell type and sculp−ture with respect to "abilities" for swimming and/or maneu−
Geographic and stratigraphic range.—Chiefly NW Europe
verability are interesting but unfortunately limited for all
and Mediterranean Tethys including Pontides (Turkey). The
ectocochleate cephalopods (Westermann and Tsujita 1999).
presence of Phricodoceras is also attested in Timor (Indone−sia) but is doubtfull in British Columbia (Canada), Oregon
Systematic palaeontology
(USA), and Chile. Phricodoceras ranges from Late Sine−murien to Late Pliensbachian.
Class Cephalopoda Cuvier, 1798Subclass Ammonoidea Zittel, 1884
The phylogenetic and taxonomic
Order Phylloceratida Arkell, 1950
(sensu Hoffmann 2010)Suborder Psiloceratina Housa, 1965
Phricodoceras in the literature.—Since 1826, a hundred
(sensu Guex 1987 = Ammonitina Arkell, 1950,
or so publications have dealt, at least in part, with Phricodo−
sensu Hoffmann 2010)
ceras. Most of them contain illustrations (drawings or pho−
Superfamily Psiloceratoidea Hyatt, 1867
tographs). All these publications are considered in Fig. 12with a view to summarizing the taxonomic opinions of their
(sensu Guex 1995)
authors (Sowerby 1826; Zieten 1830; Orbigny 1844; Quen−
Family Schlotheimiidae Spath, 1923
stedt 1846, 1849, 1883; Oppel 1853, 1856; Hauer 1861;
Remarks.—In view of the close relationships between Angu−
Wright 1880; Fucini 1898, 1908; Bettoni 1900; Del Cam−
laticeras and Phricodoceras with A. spinosus as a convinc−
pana 1900; Hyatt 1900; Buckman 1911, 1921; Krumbeck
ing intermediate form, it appears convenient to include Phri−
1922; Schröeder 1927; Höhne 1933; Gérard and Théry
codoceras in the Schlotheimiidae and to abandon the sub−
1938; Roman 1938; Spath 1938; Otkun 1942; Venzo 1952;
family and family terms Phricododeratinae and Phricodo−
Fantini Sestini and Paganoni 1953; Donovan 1954; Arkell
ceratidae. This classification has already been adopted by
et al. 1957; Géczy 1959, 1979, 1998; Dean et al. 1961;
Meister et al. (2011). Its main advantage is that it is readily
Fantini Sestini 1962, 1978; Schindewolf 1962; Bremer
supported by the comparative anatomy within the Psilo−
1965; Cantaluppi and Brambillia 1968; Frebold 1970;
ceratoidea and is founded on an odd morpho−ornamental fea−
Wiedmann 1970; Tintant et al. 1975; Schlegelmilch 1976;
ture (i.e., the "Phricodoceras habitus") the complexity of
Schlatter 1977, 1980, 1990, 1991; Dommergues 1978,
which greatly reduces the risk of convergences.
1993, 2003; Dubar and Mouterde 1978; Alkaya 1979;Linares et al. 1979; Wiedenmayer 1980; Donovan et al.
1981; Hoffmann 1982; Venturi 1982; Braga 1983;
Genus Phricodoceras Hyatt in Zittel, 1900
Mouterde et al. 1983; Büchner et al. 1986; Meister and
= Hemiparinodiceras Géczy, 1959
Sciau 1988; Smith et al. 1988; Dommergues and Meister
Type species: Ammonites taylori Sowerby, 1826; Early Pliensbachian,
1990, 1999; Dommergues et al. 1990, 2000, 2008; Cope
from a boulder in glacial till at Happisburgh, Norfolk, England, by origi−
1991; Ferretti 1991; Sciau 1991; Tipper et al. 1991; Page
nal designation.
1993, 2008; Dommergues and Mouterde 1994; Mouterde
Remarks.—21 nominal species can be attributed to the genus
and Dommergues 1994, Alkaya and Meister 1995; El Hariri
Phricodoceras. Nine of them are based on NW European
et al. 1996; Faraoni et al. 1996; Smith and Tipper 1996;
specimens and 11 on Tethyan sensu lato forms. In a recent re−
Géczy and Meister 1998, 2007; Rakús 1999; Macchioni
vision of the genus, Meister (2007) retains only 11 valid spe−
2001; Venturi and Ferri 2001; Howarth 2002; Rakús and
cies, three of which are NW European while seven are
Guex 2002; Donovan and Surlyk 2003; Edmunds et al.
Tethyan. These proportions are representative of the high di−
2003; Meister et al. 2003, 2010, 2011; Hillebrandt 2006;
versity of the genus Phricodoceras in Tethyan and especially
Meister 2007; Yin et al. 2007; Venturi and Bilotta 2008;
Mediterranean faunas. According to Meister (2007), three
Venturi et al. 2010; Blau and Meister 2011).
m–M pairings can be suspected while four small or tiny spe−
In all, 162 specimens are figured in these publications, in−
cies (one NW European and three Mediterranean) cannot
cluding 78 for NW Europe and 84 for the Tethyan realm
readily be considered microconchs despite their small size.
sensu lato. Compared with other taxa, such a large number of
In fact, despite its indisputable success in the palaeonto−
illustrations is not in proportion to the relative scarcity of
logical literature, the m–M model is often far from evidence.
Phricodoceras in the fossil record but partly reflects the spe−
The possibility of small species without or at least without
cial interest shown by authors in this morphologically aston−
significant m–M dimorphism is rarely considered as a valu−
ishing and taxonomically challenging group. In fact, the il−
able alternative hypothesis for ammonites (Davis et al.
lustrated specimens correspond to a significant portion of the

DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
Fig. 6. Habitus of some specimens belonging to scholtheimiid ammonoid Agulaticeras, the genus which represents the root of Phricodoceras.
A. Angulaticeras (Sulciferites) charmassei (Orbigny, 1844), Stuttgart−Vaihingen, Baden−Würtemberg, Germany, Arietites bucklandi Chronozone,
Coroniceras rotiforme Subchronozone (from Bloos 1988: pl. 11, modified), in lateral (A1) and apertural (A2) views. B. Angulaticeras (Boucaulticeras)
boucaultianum (Orbigny, 1844), Chtelnica, Male Karpaty Mts., Western Carpathians, Slovakia, Sinemurian condensed bed (from Meister et al. 2010: fig.
42f, g, modified), in lateral (B1) and ventral (B2) views. C. Angulaticeras (Boucaulticeras) gr. deletum (Canavari, 1882), Jbel Bou Hamid, Central Hight At−
las (Rich), Morocco, Late Sinemurian (from Guex et al. 2008: pl. 4: 6, modified), in apertural (C1) and lateral (C2) views. D. Angulaticeras (Boucaulticeras)
gr. rumpens (Oppel, 1862), Chtelnica, Male Karpaty Mts., Western Carpathians, Slovakia, Sinemurian condensed bed (from Meister et al. 2010: fig. 40c, d,
modified), in ventral (D1) and lateral (D2) views. E. Angulaticeras (Sulciferites) chtelnicaense Meister, Schlögl, and Rakus, 2010, holotype, Chtelnica,
Male Karpaty Mts., Western Carpathians, Slovakia, Sinemurian condensed bed (from Meister et al. 2010: fig. 32d, e, modified), in ventral (E1) and lateral
(E2) views. A, C (and possibly B) are incomplete phragmocones (juvenile or immature shells) but the two specimens corresponding to D, E have a signifi−
cant part of the body chamber intact. The age of D is doubtful but E is probably an adult. The end of the phragmocone is indicated by a star. The ornamenta−
tion of Angulaticeras is chiefly constituted by usually crowded, fairly flexuous and divided ribs which suddenly break up just before reaching the venter. At
least at small diameters (juveniles, microconchs) the ventral area bears a narrow smooth and more or less depressed ventral band (vb). The abrupt endings of
the ribs look like shoulders in peri−umbilical position (s3). Shoulders may vanish progressively with growth (B). Moreover, some rare species may exhibit
unusual peri−umbilical projections from the ribs (ppr), which partially obstruct the umbilicus (E). Such projections are not true tubercles or spines.
samples collected in the NW European faunas and encom−
Hypotheses, discussions, and facts.—From Sowerby (1826)
pass almost all of the samples recovered in Tethyan sensu
to Hauer (1861), the early authors described and depicted
lato areas. In this context, the literature is probably very rep−
some convincing specimens belonging to the group of Phri−
resentative of the material collected over some two centuries,
codoceras taylori under the generic name Ammonites without
and largely housed in museums.
any indication of possible relationships within this huge genus
ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
A. martinschmidti (wh = 180 mm)
P. urkuticum (wh = 20 mm)
P. taylori (wh = 7 mm)
A. charmassei (wh = 40 mm)
P. taylori (wh = 6 mm)
A. densilobatum (wh = 32 mm)
P. taylori (wh = 5 mm)
A. lacunatum (wh = 4,5 mm)
P. gr. taylori (wh = 5 mm)
A. rumpens (wh = 3 mm)
Fig. 7. Septal suture lines of several Schlotheimiidae belonging to the genera Phricodoceras (A–E) and Angulaticeras (F–J). A. Phricodoceras urkuticum
(Géczy, 1959) (from Géczy 1976: fig. 49, modified). B. Phricodoceras taylori (Sowerby, 1826) (from Dommergues 2003: fig. 6A, modified).
C. Phricodoceras taylori (from Dommergues 2003: fig. 6B, modified). D. Phricodoceras taylori (from Schlegelmilch 1976: 61, modified). E. Phricodo−
ceras gr. taylori (Sowerby, 1826) (from Schlatter 1990: fig. 3, modified). F. Angulaticeras martinischmidti (Lange, 1951) (from Schlegelmilch 1976: 38,
modified). G. Angulaticeras charmassei (Orbigny, 1844) (from Schlegelmilch 1976: 38, modified). H. Angulaticeras densilobatum (Pompeckj, 1893)
(from Schlegelmilch 1976: 39, modified). I. Angulaticeras lacunatum (J. Buckman, 1844) (from Schlegelmilch 1976, 38, modified). J. Angulaticeras
rumpens (Oppel, 1862) (from Schlegelmilch 1976: 39, modified). For each suture line the whorl height (wh) is indicated, if known. The main elements of
the suture line are indicated by following abbreviations: E, external lobe; L, lateral lobe; U1, U2, umbilical lobes; I, internal lobe.
(Fig. 12). Publications during the subsequent period from
presence of tubercles and/or spines. At that same time, Hyatt
Wright (1880) to Del Campana (1900) still lack explicit infor−
(1900: 586–587) proposed the genus name Phricodoceras.
mation about the possible relationships of the Phricodoceras
Curiously this author included his new taxon in the "Cosmo−
at the family level. Nevertheless, the arrangement of the illus−
ceratidae" family with some Middle Jurassic forms (i.e., Kos−
trated specimens on the plates (e.g., Quenstedt 1883–1885)
moceras and Sigaloceras) and surprisingly, at an informal
and/or the use of genus names such as Aegoceras or Dero−
higher taxonomic level, in the "Cosmoceratida" with some
ceras (e.g., Wright 1880; Bettoni 1900) suggest that the au−
Cretaceous taxa (e.g., Douvillieiceras). The grouping at fam−
thors suspected possible relationships with certain taxa cur−
ily level proposed by Hyatt (1900) is based on obvious orna−
rently attributed to the Eoderoceratoidea (e.g., Liparocera−
mental convergences and it is currently rejected as strongly
tiadae). This pre−family position is clearly supported by the
polyphyletic. Only Gérard and Théry (1938) followed Hyatt's
DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
Zagouanites (wh = ?)
Epideroceras (wh = 30 mm)
Eolytoceras (wh = 18 mm)
Xipheroceras (wh = 15 mm)
Xipheroceras (wh = 8.5 mm)
Pleuroacantithes (wh = ?)
Analytoceras (wh = 10 mm)
Eoderoceras (wh = 8.6 mm)
Fig. 8. Septal suture lines of several Lytoceratoidea (A–D) and Eoderoceratoidea (E–H) for comparisons with those of the scholtheimiid ammonoids
Angulaticeras and Phricodoceras (Fig. 7). A. Zaghouanites arcanum (Wiedenmayer, 1977) (from Rakús and Guex 2002: fig. 54e, modified). B. Eolyto−
ceras tasekoi Frebold, 1967 (from Wiedmann 1970: text−fig. 9c, modified). C. Pleuroacanthites biformis (Sowerby in De La Beche, 1831) (from Canavari
1888: text–fig. 2.3, modified). D. Analytoceras gr. articulatum (Sowerby in De La Beche, 1831) (from Wiedmann 1970: text–fig. 8a, modified). E. Epi−
deroceras planarmatum (Quenstedt, 1856) (from Schlatter 1980: beil. 15a, modified). F. Xipheroceras rasinodum (Quenstedt, 1884) (from Schlegelmilch
1976: 57, modified). G. Xipheroceras ziphus (Zieten, 1830) (from Schlegelmilch 1976: 56, modified). H. Eoderoceras bisbinigerum (Buckman, 1918)
(from Schlegelmilch 1992: 62, modified). For each suture line the whorl height (wh) is indicated, if known. The main elements of the suture line are indi−
cated by following abbreviations: E, external lobe; L, lateral lobe; U1, U2, umbilical lobes; I, internal lobe.
(1900) proposal. On the contrary, Buckman (1911, 1921) ex−
superfamily level, the authors tend to conform to the position
plicitly includes Phricodoceras within the Liparoceratidae
of Arkell et al. (1957) even if the family and subfamily levels
thereby clarifying and formalizing the implicit hypothesis of
are sometimes challenged. For example, the grouping of Phri−
many previous authors. From that time until fairly recently—
codoceras and Epideroceras within the Phricodoceratinae
even if Spath (1938) creates the subfamily Phricodoceratinae
proposed by Arkell et al. (1957) is abandoned by several au−
(within the Eoderoceratidae)—Phricodoceras was under−
thors (e.g., Cope 1991; Schlatter 1991; Dommergues and
stood, usually unreservedly, as belonging to the Eoderocera−
Meister 1999). Nevertheless, it was not until 1991 that the in−
toidea. The single notable exception is Wiedmann (1970:
clusion of Phricodoceras in the Eoderoceratoidea was seri−
1002) who proposes that Phricodoceras is a possible relative
ously challenged by Kevin Page (personal communication to
of Adnethiceras within the Lytoceratoidea. In fact, at the
Dommergues 1993) and that convincing relationships with the

ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
late involute only ribbed stage
transition between the evolutetuberculated stage and theinvolute only ribbed stage
evolute coarse tuberculated stage,usually three rows of tuberclessensu lato (rows 1, 2, and 3)
juvenile evolute tuberculated stage,usually two rows of tubercles
Phricodoceras paronai (M)
approximate adult size
of the microconch
Phricodoceras urkuticum (M)
Phricodoceras lamellosum (M)
Angulaticeras spinosus (M)
Angulaticeras boucaultianum (M)
Fig. 9. Some illustrative steps—in terms of morphological ontogeny—in the intricate evolutionary trend from the Sinemurian scholtheimiid genus
Angulaticeras to the late Pliensbachian Phricodoceras (i.e., Phricodoceras paronai [Bettoni, 1900]). For simplicity, the complex and more or less gradual
ontogenetic transformations are reduced to just four stages (see A–C for an illustration of the last three). The length and the place of a given stage in the
ontogenetic cartouches depend on its duration and position during ontogeny. The overall length of the cartouche is proportional to adult size. Ontogenies of
the macroconchs (M) alone are depicted in the cartouches and the adult sizes (complete shells) of the microconchs (m) are suggested by black triangles (grey
if doubtful). A–C. Scholtheimiid ammonoid Phricododeras lamellosum (Orbigny, 1844), Rote Island, East Nusa Tenggara, Indonesia, probably Early
Pliensbachian (from Krumbeck 1922: pl. 17: 5, modified), in ventral (A), lateral (B), and apertural (C) views.
Schlotheimiidae within the Psiloceratoidea were considered
but with some reservations. Such an alternative was discussed
for the first time to be at least a plausible hypothesis. Despite
also by Venturi and Bilotta (2008) and Venturi et al. (2010),
this first serious challenge to the traditional taxonomic attribu−
and their choice of a doubtful superfamily classification for
tion, most authors until Yin et al. (2007) continued to consider
the Phricodoceratidae was due to the lack of decisive data. The
Phricodoceras as member of Eoderoceratoidea with no fur−
proof that Phricodoceras belongs to the Schlotheimiidae was
ther discussion. In spite of this taxonomic inertia, several pub−
ultimately provided by Meister et al. (2010), who described a
lications have understood Phricodoceras as an unresolved
new Angulaticeras (i.e., A. spinosus) whose inner whorls are
taxon and two to four credible but rival hypothesis have been
virtually indistinguishable from those of Phricodoceras gr.
suggested (Dommergues 1993, 2003; Dommergues and
taylori–P. lamellosum at the same diameter. Since this publi−
Meister 1999; Meister 2007; Dommergues et al. 2008). In all
cation, all subsequent works have placed the Phricodoceras
these papers, the possibility of the Schlotheimiidae and Phri−
within the Psiloceratoidea and close to or within the Schlo−
codoceras being closely related is seriously considered but
theimiidae (Blau and Meister 2011; Meister et al. 2011).
Edmunds et al. (2003) were clearly the first to propose this tax−onomic option unreservedly albeit unfortunately without any
Characters, assumed relationships, and taxonomic prac−
compelling evidence. Later, Page (2008) took up this position
tice.—The history of taxonomic practice is rarely considered
DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
for itself, especially for ammonites (Donovan 1994). This isregrettable because such historical approaches may help to re−
fine taxonomic practices empirically by highlighting some
misleading but consensual traditions. The case of Phricodo−ceras is particularly instructive in this respect because a
widely accepted hypothesis, herein rejected, has affected thetaxonomic understanding of this remarkable group of am−monites. This confusing but successful hypothesis is based ona dual argument grounded on both the concepts of "overall re−
semblance" and of "stratigraphic consistency". Indeed, Phri−
codoceras and especially the emblematic P. taylori, which islocally not rare in the Uptonia jamesoni and Tragophylloceras
ibex chronozones (Early Pliensbachian), can be roughly com−
pared with some Late Sinemurian and/or Early PliensbachianEoderoceratoidea (e.g., Eoderoceratidae, Polymorphitidae,Liparoceratidae). Some of these more of less markedly
tuberculated forms have subplatycone, subplanorbicone orsubsphaerocone shells with usually rounded and keelless ven−tral areas. The habitus of such Early Pliensbachian Eodero−
ceratoidea (Fig. 11) are not very close to those of Phri−
codoceras (Figs. 3–5) (e.g., lack of peri−siphonal tubercles but
usually presence of ventral secondary and intercalary ribs be−tween the ventro−lateral rows of tubercles in Eoderoceratoideabut not in Phricodoceras), but all these forms are roughly co−
juvenile growth stages
minor ontogenetic change
eval and the presence of tubercles and/or spines was long re−
post-juvenile growth stages
major ontogenetic change
garded as a diagnostic trait confined or pretty much so to theEoderoceratoidea among the Pliensbachian ammonites. Con−
late growth stages
trariwise, Schlotheimiidae were understood until recently as
Fig. 10. Schematic representation and comparison of the ontogenies of an
forms unable to produce true tubercles and/or spines. Thus, in
Angulaticeras macroconch (A. boucaultianum) and of a Phricodoceras
addition to the age (chiefly Early Pliensbachian), the presence
macroconch (P. lamellosum) in a simplified diagram taking into account the
of tubercles, the keelless smooth ventral area and the rather
assumed mobility (x−axis) and the assumed passive shell protection (y−axis).
evolute juvenile coiling pattern were all used as arguments
These parameters cannot be fully expressed quantitatively. Mobility de−
(taxonomic shoehorns) for placing Phricodoceras within the
pends mainly on hydrodynamic abilities, which are correlated with shell ge−ometry but also with some aspects of ornamentation. Marked ornamental
Eoderoceratoidea. This nearly universally or at least widely
traits may play an important role. For example a keel or a ventral groove may
accepted argument is in fact circular. It was ultimately over−
increase the hydrodynamic stability of the shell and thereby facilitate mobil−
turned by the recent discovery by Meister et al. (2010) of a
ity, but prominent tubercles and/or spines may significantly increase hydro−
clearly tuberculate juvenile growth stage in the inner whorls of
dynamic drag thereby reducing mobility. Conversely the prominence of or−
a typical Schlotheimiidae (i.e., Angulaticeras spinosus). From
namentation (chiefly of tubercles and/or spines) may be an effective passive
then on, it becomes easy to understand the genus Phricodo−
protection against predators. Although highly schematic and hypothetical,
ceras as a close relative of Angulaticeras within the Schlo−
such a diagram can be understood as an approximate representation of an"adaptative landscape" in which successive growth stages can be roughly
theimiidae and to fundamentally rethink the comparative anat−
situated. This "adaptative landscape" can be divided into four quadrants la−
omy of these forms. For example, it becomes possible to prove
beled A–D. The two studied species occupy only quadrants A (rather poor
the peri−siphonal shoulders of the Schlotheimiidae are homol−
mobility but good passive shell protection) and C (good mobility but poor
ogous with the peri−siphonal tubercles of Phricodoceras. In
passive shell protection). In fact, only the juvenile growth stages of Phri−
fact, the homologies (e.g., shell morphology, ornamentation,
codoceras lamellosum are situated in quadrant A but all the other growth
suture line if controlled by ontogenesis) with Angulaticeras
stages, of both species, are in quadrant C. This pattern underlines the
are so numerous and obvious, throughout the growth stages,
adaptative peculiarity of the juvenile growth stages of Phricodoceras.
that it seems unnecessary to use a distinct subfamily or familylevel name to separate the two genera.
they are stratigraphicaly well constrained. In addition, itshows how much an allegedly consensus−based formaliza−tion such as that proposed in the "Treatise of Invertebrate Pa−
leontology" (Arkell et al. 1957) may become sterilizing fortaxonomic research. The present synthesis suggests that the
The history of taxonomic practice with respect to Phri−
understanding of relationships between ammonites, and par−
codoceras is edifying because it clearly exemplifies the vul−
ticularly between clearly identified and distinct groups, de−
nerability of approaches based on "overall similarity" even if
pends largely on the discovery of transitional forms and/or

ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
Fig. 11. Habitus of some nodded, spined and/or tuberculate Lytoceratoidea (A) and Eoderoderatoidea (B–D). A. Analytoceras hermanni (Gümbel, 1861),
Kammerkaralpe, Waidring, Tyrol, Austria, probably Late Hettangian (from Wähner 1894: pl. 3: 3a, b, modified), in ventral (A1) and lateral (A2) views.
B. Epideroceras lorioli (Hug, 1899), St Peter's Field, Radstock, Somerset, UK, Echioceras raricostatum Chronozone, Paltechioceras aplanatum Sub−
chronozone (from Edmunds et al. 2003: fig. 21. 4, modified), in lateral (B1) and apertural (B2) views. C. Tetraspidoceras repentinum Edmunds, 2009, St Pe−
ter's Field, Radstock, Somerset, UK, Uptonia jamesoni Chronozone, Phricodoceras taylori Subchronozone (from Edmunds 2009: pl. 32: 1, modified), in
lateral (C1) and ventral (C2) views. D. Becheiceras bechei (Sowerby, 1821), Golden Cap, Seatown, Dorset, UK, Prodactylioceras davoei Chronozone,
Oistoceras figulinum Subchronozone (from Edmunds 2009: pl. 38: 1, modified), in lateral (D1) and apertural (D2) views. Tubercles and/or spines in (t1)
and/or (t2) positions of the Eoderoceratoidea (B–D) are not homologous with those of Phricodoceras, nevertheless this genus was long understood as a
(borderline) member of this superfamily. In the case of Lytoceratoidea (A) the ornamental features in peri−siphonal position (pn3) are parabolic nodes which
are morphologically clearly distinct from the tubercles or spines of both Eoderoceratoidea and Phricodoceras. The growth stage of the specimen is un−
known.
series in an acceptable stratigraphic context. If heterochroni−
tremely rich fossiliferous locality in the western Carpathians,
cal processes, possibly associated with innovation, are in−
Slovakia (Meister et al. 2010). This locality has yielded sev−
volved (as is the case for Phricodoceras), such transitional
eral thousand specimens including various Angulaticeras so
forms are often informative and easy to interpret in evolu−
Angulaticeras spinosus is obviously extremely rare. The sed−
tionary and phylogenetic terms. Unfortunately, intermediate
imentary context is certainly important. For example, con−
forms between obviously distinct groups are usually very
densed deposits are probably particularly favorable for the
rare and localized. For example, Angulaticeras spinosus, the
search of transitional forms. Nevertheless, and despite the
key species for the understanding of the relationship between
probable scarcity of many transitional forms, field studies
Angulaticeras and Phricodoceras, is known by only three
still appear to be the most reliable way to resolve many enig−
specimens (including the holotype) from a single but ex−
matic taxonomic problems and to clarify our knowledge of
DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
Mouterde et al.
Büchner et al.
Meister and Sciau
Smith et al.
Dommergues and Meister
Dommergues et al.
Page (personal commun.)
Tipper et al.
Dommergues and Mouterde
Alkaya and Meister
El Hariri et al.
Faraoni et al.
Geczy and Meister
Fantini and Paganoni
Dommergues and Meister
Arkell et al.
Dommergues et al.
Venturi and Ferri
Cantaluippi and Brambill
Donovan and Surlyk
Edmunds et al.
Meister et al.
Tintan et al.
Geczy and Meister
Dommergues et al.
Venturi and Bilotta
Meister et al.
Linares et al.
Venturi et al.
Meister et al.
Donovan et al.
Fig. 12. Historical synthesis of the taxonomic interpretation for the genus Phricodoceras from 1826 until today. Six options are considered: H?, no taxo−nomic attribution or attribution deliberately left undetermined; Eo, explicit attribution to Eoderoceratoidea or implicit proximity with some ammonites cur−rently attributed to the Eoderoceratidae; Ko, explicit attribution to the Kosmoceratidae; Ly, explicit attribution to the Lytoceratoidea (in the current sense);Ps, explicit attribution to the Psiloceratoidea and proximity with the Schlotheimiidae; La, enigmatic lazarus taxon. A cross indicates an absence of attribu−tion to a taxon. A single black dot suggests an implicit or explicit but very reserved attribution. Two black dots suggest an explicit but debatable attribution.
Three black dots suggest an unconditional explicit attribution. Four black dots suggest an explicit attribution based on ontogenetic evidence. For easy read−ing, the two columns corresponding to the two most frequent taxonomic interpretations (i.e., Eo and Ps) are shaded.
ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
palaeobiodiversity. In the absence of intermediate forms
Cecca, F. 2002. Paleobiogeography of Marine Fossil Invertebrates—Con−
and/or series, cladistic analysis can be a useful approach in
cepts and Methods. 273 pp. Taylor and Francis, London.
attempting to reconstruct phylogenies, but frequent homo−
Cope, J. 1991. Ammonite faunas of the Ammonitico Rosso of the Pontide
Mountains, northern Anatolia. Geologica Romana 27: 303–325.
plasies and the weakness of many primary homologies in the
Davis, R.A., Landman, N.H., Dommergues, J.−L., Marchand, D., and
absence of transitional forms mean that this type of approach
Bucher, H. 1996. Mature modifications and dimorphism in ammonoid
is often quite frustrating and nothing can replace the discov−
cephalopods. In: N.H. Landman, K. Tanabe, and R.A. Davis (eds.),
ery of a key intermediate form.
Ammonoid Paleobiology. Topics in Geobiology 13: 463–539.
Dean, W.T., Donovan, D.T., and Howarth, M.K. 1961. The Liassic ammonite
zones and subzones of the northwest European province. Bulletin of theBritish Museum (Natural History), Geology Series 4: 438–505.
Del Campana, D. 1900. I Cefalopodi del Medolo di Valtrompia. Bollettino
della Società Geologica Italiana 19: 555–644.
We are grateful to three reviewers, Massimiliano Bilotta (Perugia, It−
Dommergues, J.−L. 1978. Un cas de dimorphisme sexuel chez une ammonite
aly), Federico Olóriz (University of Granada, Spain) and Horacio Par−
carixienne Phricodoceras taylori (J. de C. Sowerby, 1826). Eodero−
ent (National University of Rosario, Argentina), for their very construc−
ceratidae Spath, 1929. Bulletin scientifique de Bourgogne 31: 41–45.
tive comments on the submitted version of the manuscript. We thank
Dommergues, J.−L. 1987. L'évolution chez les Ammonitina du Lias moyen
Christopher Sutcliffe (Quetigny, France) for the help with the English
(Carixien, Domérien basal) en Europe occidentale. Documents desLaboratoires de Géologie Lyon 98: 1–297.
version. This paper is a contribution by the team FED BioME "Bio−
Dommergues, J.−L. 1993. Les ammonites du Sinémurien supérieur de
diversité, Macroécologie, Evolution" of the "Biogéosciences" research
Bourgogne (France): Biostratigraphie et remarques paléontologiques.
unit (UMR 6282, CNRS/uB).
Revue de Paléobiologie 12: 67–173.
Dommergues, J.−L. 2003. Nouvelles données sur les ammonites du Carixien
basal (Jurassique inférieur) en Europe du Nord−Ouest: les faunes de
Corbigny (Nièvre, Bourgogne, France). Bulletin scientifique de Bour−gogne 51: 12–36.
Dommergues, J.−L. and Meister, C. 1990. Les faunes d'ammonites de
Alkaya, F. 1979. Lower Jurassic Ammonites from Northern Turkey. 320 pp.
l'Austroalpin Moyen dans les Alpes Rhétiques italiennes (région de
Unpublished Memoir, Ph.D. thesis, University of London, London.
Livigno); biostratigraphie et implications paléogéographiques. Revue
Alkaya, F. and Meister, C. 1995. Liassic ammonites from the central and
de Paléobiologie 9: 291–307.
eastern Pontides (Ankara and Kelkit areas, Turkey). Revue de Paléo−
Dommergues, J.−L. and Meister, C. 1999. Cladistic formalisation of rela−
biologie 14: 125–193.
tionships within a superfamily of lower Jurassic Ammonitina: Eodero−
Arkell, W.J., Kummel, B., and Wright, C.W. 1957. Mesozoic Ammonoidea.
cerataceae Spath, 1929. Revue de Paléobiologie 18: 273–286.
In: R.C. Moore (ed.), Treatise on Invertebrate Paleontology, Part L,
Dommergues, J.−L. and Mouterde, R. 1994. Phricodoceras taylori (J. de C.
Mollusca 4; Cephalopoda, Ammonoidea, 80–465. Geological Society
Sowerby, 1826). In: J.−C. Fischer (ed.), Révision critique de la Paléonto−
of America and The University of Kansas Press, Boulder.
logie française d'Alcide d'Orbigny, Volume 1, Céphalopodes Jurassi−
Bettoni, A. 1900. Fossili Domeriani della Provincia di Brescia. Mémoires de
ques, 90–91. Masson and Muséum national d'Histoire naturelle, Paris.
la Société paléontologique suisse 28: 1–88.
Dommergues, J.−L., Dugué, O., Gauthier, H., Meister, C., Neige, P., Raynaud,
D., Savary, X., and Trevisan, M. 2008. Les ammonites du Pliensbachien et
Bloos, G. 1988. Ammonites marmoreus Oppel (Schlotheimiidae) im unteren
du Toarcien basal dans la carrière de la Roche Blain (Fresnay−le−Puceux,
Lias (angulata−Zone, depressa−Subzone) von Württemberg (Südwest−
Calvados, Basse−Normandie, France). Taxonomie, implications strati−
deutschland). Stuttgarter Beiträge zur Naturkunde B 141: 1–47.
graphiques et paléontologiques. Revue de Paléobiologie 27: 265–329.
Braga, J.−C. 1983. Ammonites del Domerense de la zona subbetica (Cordil−
leras beticas, Sur de España). 410 pp. Tesis doctoral, Universidad de
Granada. España.
Bremer, H. 1965. Zur Ammonitenfauna und Stratigraphie des unteren Lias
(Sinemurium bis Carixium) in der Umgebung von Ankara (Turkei).
Neues Jahrbuch für Geologie und Paläontologie Abhandlungen 122:
Büchner, M., Hoffmann, K., and Jordan, R. 1986. Die Tongruben der
Ziegeleien im Unter−Pliensbachium (Lias gamma) der weiteren Umge−
bung von Bielefeld, ihre Geologie und Betriebsgeschichte: Ein Beitrag
für künftige Rohstoff−Erschliessungen. Veröffentlichungen aus dem
Naturkunde−Museum Bielefeld 1: 1–57.
Donovan, D.T. 1954. Synoptic supplement to T. Wright's "Monograph on
Buckman, S.S. 1909–1930. Yorkshire Type Ammonites. 2 volumes, 185 pp.
the Lias Ammonites of the British Islands" (1878–1886). Palaeonto−
Wesley and Son, London; followed by Type Ammonites. 5 volumes,
graphical Society, London 107: 1–54.
358 pp. Wheldon and Wesley, London.
Canavari, M. 1888. Contribuzione alla fauna del Lias inferiore di Spezia.
Memorie del Regio Comitato Geologico Italiano 3: 57–227.
Donovan, D.T. and Surlyk, F. 2003. Lower Jurassic (Pliensbachian) am−
Cantaluppi, G. and Brambilla, G. 1968. Le ammoniti del Ripiantino (Saltrio)
monites from Bornholm, Baltic Sea, Denmark. Geological Survey of
e della Breggia (Canton Ticino). Reflessi biostratigrafici sul Domeriano
Denmark and Greenland Bulletin 1: 555–583.
ed il suo limite inferiore. Atti della Società di Scienze naturali e del
Donovan, D.T., Callomon, J.H., and Howarth, M.K. 1981. Classification of
Museo civico di Storia naturale di Milano 107: 277–315.
the Jurassic Ammonitina. In: M.R. House and J.R. Senior (eds.), The
DOMMERGUES AND MEISTER—PHYLETIC RECONSTRUCTION OF EARLY JURASSIC AMMONOID
Ammonoidea. The Evolution, Classification, Mode of Life and Geological
führung des Unter−Pliensbachium (Carixium, Lias gamma) in Nordwest−
Usefulness of a Major Fossil Group, 101–155. Academic Press, London.
Deutschland. Geologisches Jahrbuch A 55: 3–439.
Dubar, G. and Mouterde, R. 1978. Les formations à ammonites du Lias
Hoffmann, R. 2010. New insights on the phylogeny of the Lytoceratoidea
moyen dans le Haut Atlas de Midelt et du Tadla. Notes et Mémoires du
(Ammonitina) from the septal lobe and its functional interpretation. Re−
Service géologique du Maroc 274: 1–112.
vue de Paléobiologie 29: 1–156.
Edmunds, M. 2009. A revision of the Lower Jurassic ammonite genus
Höhne, R. 1933. Beiträge zur Stratigraphie, Tektonik und Paläogeographie des
Eoderoceras Spath and its immediate descendants and other relatives.
südbaltischen Rhät−Lias, insbesondere auf Bornholm. Abhandlungen des
Monograph of the Palaeontological Society Publication 633: 1–89.
Geologisch−Paläontologischen Instituts der Universität Greifswald 12:
Edmunds, M., Varah, M., and Bentley, A. 2003. The ammonites biostrati−
graphy of the lower Lias "Armatum Bed" (Upper Sinemurian–Lower
Howarth, M.K. 2002. The Lower Lias of Robin Hood's Bay, Yorkshire, and
Pliensbachian) at St Peter's Field, Radstock, Somerset. Proceedings of
the work of Leslie Bairstow. Bulletin of the Natural History Museum 58:
the Geologists' Association 114: 65–96.
Hyatt, A. 1900. Cephalopoda. In: K.A. von Zittel (ed.), Textbook of Palae−
ontology, Vol. 1, Part 2, 502–592. Macmillan, London.
Krumbeck, L. 1922. Zur kenntnis des juras der insel Rotti. Jaarboek van het
mijnwezen in nederlandsch oost−indië 3: 107–119.
Fantini Sestini, N. 1962. Contributo allo studio delle ammoniti del Domeriano
Linares, A., Mouterde, R., and Rivas, P. 1979. Les Phricodoceras (Ammo−
di M. Domaro (Brescia). Rivista Italiana di Paleontologia e Stratigrafia
nitina) d'Andalousie. Cuadernos de Geologia 10: 259–265.
68: 483–554.
Macchioni, F. 2001. Ammonites of the Domerian–Early Toarcian in the
Fantini Sestini, N. 1978. Il genere Phricodoceras (Polymorphitidae, Ammo−
Subbetic Zone and the Umbria−Marche Apennines. Taxonomy, Tapho−
noidea) nel Pliensbachiano italiano. Rivista Italiana di Paleontologia e
nomy, Biostratigraphy and Palaeobiogeography. 186 pp. Unpublished
Stratigrafia 84: 327–348.
Memoir, Tesi di dottorato, Università degli Studi di Perugia, Italia.
Fantini Sestini, N. and Paganoni, C. 1953. Studi paleontologici sul Lias del
Meister, C. 2007. Les Phricodoceratidae Spath, 1938 (Mollusca, Cephalo−
Monte Albenza (Bergamo); Ammoniti del Lotharingiano e del Dome−
poda): ontogenèse, évolution et paléobiogéographie. Geodiversitas 29:
riano. Rivista Italiana di Paleontologia e Stratigrafia 59: 65–90.
Faraoni, P., Marini, A., Pallini, G., and Venturi, F. 1996. New Carixian
Meister, C. and Sciau, J. 1988. Une faune inédite d'ammonites du Carixien
ammonite assemblages of Central Apennines (Italy), and their impact
inférieur des Causses (France). Revue de Paléobiologie 7: 261–269.
on Mediterranean Jurassic biostratigraphy. Paleopelagos 6: 75–122.
Meister, C., Blau, J., Dommergues, J.−L., Feist−Burkhardt, S., Hart, M.,
Ferretti, A. 1991. Introduzione ad uno studio morfometrico degli Ammoniti
Hesselbo, S.P., Hylton, M., Page, K., and Price, G. 2003. A proposal for
pliensbachiani della catena del Catria (Appennino Marchigiano). Rivista
the Global Boundary Stratotype Section and Point (GSSP) for the base of
Italiana di Paleontologia e Stratigrafia 97: 49–98.
the Pliensbachian Stage (Lower Jurassic). Eclogae Geologicae Helvetiae
96: 275–297.
Meister, C., Dommergues, J.−L., Dommergues, C., Lachkar, N., and El
Fucini, A. 1898. Di alcune nuove Ammoniti di calcari rossi inferiori della
Hariri, K. 2011. Les ammonites du Pliensbachien du Jebel Bou Rharraf
Toscana. Palaeontographia italica 4: 239–250.
(Haut Atlas oriental, Maroc). Geobios 44: 117.e1–117.e60.
Fucini, A. 1908. Ammoniti medoliane dell'Appennino. Atti della Società
Toscana di Scienze naturali – Memorie 24: 79–95.
Géczy, B. 1959. Liparoceras (Hemiparinodiceras) urkuticum n. sg. n. sp.
(Ceph.) from the Middle Liassic of the Bakony Mountains, Trans−
danubia, Hungary. Földtani Közlöny (Bulletin de la Société géologique
Mouterde, R. and Dommergues, J.−L. 1994. Phricodoceras lamellosum
de Hongrie) 89: 143–147.
(d'Orbigny, 1844). In: J.−C. Fischer (ed.), Révision critique de la
Géczy, B. 1976. Les ammonites du Carixien de la montagne du Bakony, 220
Paléontologie française d'Alcide d'Orbigny, Volume 1, Céphalopodes
pp. Akadémiai Kiado, Budapest.
Jurassiques, 73. Masson and Museum national d'Histoire naturelle,
Géczy, B. 1998. Lower Pliensbachian ammonites of Villany (Hungary).
Hantkeniana 2: 5–47.
Mouterde, R., Dommergues, J.−L., and Rocha, R.B. 1983. Atlas des fossiles
Géczy, B. and Meister, C. 1998. Les ammonites du Domérien de la
caractéristiques du Lias portugais, 2 – Carixien. Ciências da Terra 7:
montagne du Bakony (Hongrie). Revue de Paléobiologie 17: 69–161.
Géczy, B. and Meister, C. 2007. Les ammonites du Sinémurien et du
Ogg, J.G., Ogg, G., and Gradstein, F.M. 2008. The Concise Geologic Time
Pliensbachien inférieur de la montagne du Bakony (Hongrie). Revue de
Scale. 177 pp. Cambridge University Press, Cambridge.
Paléobiologie 26: 137–305.
Olóriz, F. 2000. Time−averaging and long−term palaeoecology in macro−
Gérard, C. and Théry, A. 1938. Le Charmouthien de Meurthe−et−Moselle.
invertebrate assemblages with ammonites (Upper Jurassic). Revue de
Bulletin mensuel de la Société des Sciences de Nancy, nouvelle série
Paléobiologie, Volume Spécial 8: 123–140.
10–11: 167–191.
Olóriz, F. and Villaseñor, A.B. 2010. Ammonite biogeography: From de−
Guex, J. 1987. Sur la phylogenèse des ammonites du Lias inférieur. Bulletin
scriptive to dynamic, ecological interpretations. In: K. Tanabe, Y.
de Géologie Lausanne 292: 455–469.
Shigeta, T. Sasaki, and H. Hirano (eds.), Cephalopods−Present and
Guex ,J. 1995. Ammonites hettangiennes de la Gabbs Valley Range (Ne−
Past, 253–265. Tokai University Press, Tokyo.
vada, USA). Mémoires de Géologie Lausanne 27: 1–131.
Oppel, A. 1853. Der Mittlere Lias Schwabens. Württemberg Naturwissen−
Guex, J., Rakús, M., Morard, A., and Quartier−la−Tente, M. 2008. Ammonites
schaft Jahreshefte 10: 1–92.
sinémuriennes du Haut−Atlas marocain. Mémoires de Géologie Lausanne
Oppel, A. 1856–58. Die Juraformation Englands, Frankreichs und des
südwestlichen Deutschlands. Würtembergen naturwissenschaftlichen
Hauer, F.R. von 1861. Über die Ammoniten aus dem sogenannten Medolo der
Jahresheft 12–14: 1–857.
Berge Domaro und Guglielmo im val Trompia, Provinz Brescia. Sitzungs−
Orbigny A. d' 1844. Paléontologie française: Terrains jurassiques, I.
berichte der Mathematisch−Naturwissenschaftlichen Classe der Kaiser−
Céphalopodes, Livres 17–27, 193–312. Masson, Paris.
lichen Akademie der Weissenchaften 44: 403–422.
Otkun, G. 1942. Etude paléontologique de quelques gisements du Lias
Hillebrandt, A. von 2006. Ammoniten aus dem Pliensbachium (Carixium
d'Anatolie. Metae (Publications de l'Institut d'Etudes et de Recherches
und Domerium) von Südamerika. Revue de Paléobiologie 25: 1–403.
Minières de Turquie) Série B: Mémoires 8: 1–41.
Hoffmann, K. 1982. Die stratigraphie, Paläogeographie und Ammoniten−
Page, K. 1993. Mollusca: Cephalopoda (Ammonoidea: Phylloceratina,
ACTA PALAEONTOLOGICA POLONICA 58 (4), 2013
Lytoceratina, Ammonitina and Ancycloceratina). In: M.J. Benton (ed.),
Sowerby, J. de C. 1823–1829. The Mineral Conchiology of Great Britain;
The Fossil Record 2, 213–227. Chapman and Hall, London.
or Coloured Figures and Descriptions of Those Remains of Testaceous
Page, K. 2008. The evolution and geography of the Jurassic ammonoids.
Animals or Shells, which Have Been Preserved at Various Times and
Proceedings of the Geologists' Association 119: 35–57.
Depths in the Earth. 4 (fin)–7, pl. 384–648. London.
Quenstedt, F.A. 1845–1849. Petrefactenkunde Deutschlands. I. Cephalo−
Spath, L.F. 1938. A Catalogue of the Ammonites of the Liassic Family
poden. 580 pp. Fues, Tübingen.
Liparoceratidae in the British Museum (Natural History). 191 pp. Brit−
Quenstedt, F.A. 1883–1885. Die Ammoniten des Schwäbischen Jura. Bd. I.
ish Museum (Natural History), London.
Der Schwarze Jura (Lias). 440 pp. Schweizerbart, Stuttgart.
Tintant, H., Mouterde, R., and Enay, R. 1975. Esquisse de la phylognèse des
Rakús, M. 1999. Liassic ammonites from Hierlatz, Austria. Abhandlungen
ammonites du Jurassique. In: C. Pomerol (ed.), Stratigraphie et paléo−
der geologischen Bundesanstalt 56: 343–377.
géographie (ère Mésozoïque), 114–125. Doin, Paris.
Rakús, M. and Guex, J. 2002. Les ammonites du Jurassique inférieur et moyen
Tipper, H.W., Smith, P.L., Cameron, B.E.B., Carter E.S., Jakobs, G.K., and
de la dorsale tunisienne. Mémoires de Géologie Lausanne 39: 1–217.
Johns, M.J. 1991. Biostratigraphy of the Lower Jurassic formations of
the Queen Charlotte Islands, British Columbia. Geological Survey of
Canada, Paper 90–10: 203–235.
Venturi, F. 1982. Ammoniti liassici dell'Appennino centrale. 103 pp. Città
de Castello, Italia.
Roman, F. 1938. Les ammonites jurassiques et crétacées. 554 pp. Masson,
Venturi, F. and Bilotta, M. 2008. New data and hypotheses on early Jurassic
Schindewolf, O.H. 1962. Studien zur Stammesgeschichte der Ammoniten:
ammonite phylogeny. Revue de Paléobiologie 27: 859–901.
II, Psilocerataceae–Eoderocerataceae. Abhandlungen des Akademie des
Venturi, F. and Ferri, R. 2001. Ammoniti Liassici dell'Appennino Centrale,
Wissenschaften und der Literatur in Mainz, mathematisch−naturwissen−
III. 268 pp. Tibergraph, Città di Castello.
schatliche Klasse 8: 425–571.
Venturi, F., Rea, G., Silvestrini, G., and Bilotta, M. 2010. Ammoniti. Un
Schlatter, R. 1977. The Biostratigraphy of the Lower Pliensbachian at the
viaggio geologico nelle montagne appenniniche. 367 pp. Porzi editoriali
Type Locality (Pliensbach, Württemberg, SW−Germany). Stuttgarter
s.a.s, Perugia.
Beiträge zur Naturkunde B 27: 1–29.
Venzo, S. 1952. Nuove faune ad ammoniti del Domeriano–Aleniano dell'Alpe
Schlatter, R. 1980. Biostratigraphie und Ammonitenfauna des Unter−Pliens−
Turati e dintorni (Alta Brianza). Atti della Società di Scienze naturali di
bachium im Typusgebiet (Pliensbach, Holzmaden und Nürtingen; Wür−
Milano 91: 95–123.
temberg, SW−Deutschland). Stuttgarter Beiträge zur Naturkunde B 65:
Vrielynck, B. and Bouysse, P. 2001. Le visage changeant de la Terre.
L'éclatement de la Pangée et la mobilité des continents au cours des
Schlatter, R. 1990. Phricodoceras sexinodosum n. sp. (Ammonoidea) aus
dernièrs 250 millions d'années en 10 cartes. 32 pp. Commission de la
dem Lotharingium (Raricostatum zone) von Balingen (Baden−Würtem−
carte géologique du monde, Paris.
berg, Südwest−Deutschland). Stuttgarter Beiträge zur Naturkunde B
Wähner, F. 1882–1898. Beiträge zur Kenntniss der tieferen Zonen des
unteren Lias in nordöstlichen Alpen. I–VIII. Beiträge zur Paläontologie
Schlatter, R. 1991. Biostratigraphie und Ammonitenfauna des Ober−Lotha−
und Geologie Österreich–Ungarns und des Orients 2–11: 1–291.
ringium und Unter−Pliensbachium im Klettgau (Kanton Schaffausen,
Westermann, G.E.G. and Tsujita, C.J. 1999. Life habits of ammonoids. In:
Schweiz) und angrenzender Gebiete. Mémoires suisses de Paléonto−
E. Savazzi (ed.), Functional Morphology of the Invertebrate Skeleton,
logie 113: 1–133.
299–325. John Wiley and Sons, Chichester.
Schlegelmilch, R. 1976. Die Ammoniten des süddeutschen Lias. 212 pp.
Wiedenmayer, F. 1980. Die Ammoniten der mediterranen Provinz im
Gustav Fischer, Stuttgart.
Pliensbachian und unteren Toarcian aufgrund neuer Untersuchungen
Schröder, J. 1927. Die ammoniten der jurassischen fleckenmergel in den
im Generoso–Becken (Lombardische Alpen). Mémoires de la Société
bayrischen alpen. Palaeontographica 68: 111–232.
hélvétique de Sciences naturelles 93: 1–195.
Sciau, J. 1991. Coup d'śil sur les fossiles des Causses, 1 – Du Primaire au
Lias moyen. 78 pp. Association des amis du musée de Millau et Editions
Wiedmann, J. 1970. Über den Ursprung der Neoammonoideen. Das Prob−
du Castelet, Boulogne.
lem einer Typogenese. Eclogae Geologicae Helvetiae 63: 923–1020.
Smith, P.L. and Tipper, H.W. 1996. Pliensbachian (Lower Jurassic) Ammo−
Wright, T. 1878–1886. Monograph on the Lias Ammonites of the British Is−
nites of the Queen Charlotte Islands, British Columbia. Bulletins of
lands. 503 pp. Palaeontographical Society, London.
American Paleontology 108 (348): 1–122.
Yin, J.−R., Liu, G.−F., and Xie, Y.−W. 2007. Late Triassic and Early Jurassic
Ammonoids from Eastern Himalayas. Geoscience 21: 31–41.
Zieten, C.H. 1830–1834. Die Versteinerungen Württembergs. 102 pp. Ex−
pedition des Werkes unsere Zeitschrift. Schweizerbart, Stuttgart.
Source: https://www.app.pan.pl/archive/published/app58/app20110148.pdf
PERIODICUM BIOLOGORUM VOL. 117, No 1, 161–165, 2015 Non-pharmacological treatment of osteoporosis with Nuclear Magnetic Resonance Therapy (NMR-Therapy) Objectives: To demonstrate the long-term effects of the therapeutic use of nuclear magnetic resonance (NMR) on bone mineral density (BMD) parameters in patients with osteoporosis.
PROCTER & GAMBLE DESCRIPTION OF A CORPORATE SUCCESS ECOLE DE COMMERCE SOLVAY DES EN GESTION Daelemans Anneliese - Dekoninck Eric - Deschamps Dominique - Stevens Antoine Microeconomics - March 2002 1. Introduction. 3 2. Presentation of the company .4 Core values and basic principles of the Company .4











