
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Cristalización de proteínas en el diseño de fármacos en los últimos 50 años ; protein crystallization for drug design in the last 50 years

ARBOR Ciencia, Pensamiento y Cultura
Vol. 191-772, marzo-abril 2015, a222 ISSN-L: 0210-1963
CELEBRATING 100 YEARS OF MODERN CRYSTALLOGRAPHY / CIEN AÑOS DE CRISTALOGRAFÍA MODERNA
PROTEIN CRYSTALLIZATION FOR
CRISTALIZACIÓN DE PROTEÍNAS
DRUG DESIGN IN THE LAST 50
EN EL DISEÑO DE FÁRMACOS EN
LOS ÚLTIMOS 50 AÑOS
Enrico A. Stura
CEA, iBiTec-S, SIMOPRO, France
Citation/Cómo citar este artículo: Stura, E. A. (2015).
Copyright: 2015 CSIC. This is an open-access article distributed
"Protein Crystallization for Drug Design in the Last 50 Years".
under the terms of the Creative Commons Attribution-Non
Commercial (by-nc) Spain 3.0 License.
Received: September 12, 2014. Accepted: February 13, 2015.
ABSTRACT: We live in an era where we expect to be able to
RESUMEN: Vivimos en una época en la que esperamos ir al
visit our doctor and obtain a pill to cure any ailment from
médico y obtener una pastilla para curar cualquier dolencia que
which we suffer. Yet, this is still not the case. Many of the
padezcamos; por desgracia, esta expectativa no es real. Aunque
current cures are still derived from natural sources although
muchos de los remedios en uso provienen de fuentes naturales,
new drugs are increasingly the result of intelligent design. In
la mayoría de los nuevos medicamentos son el resultado de la
this process, X-ray protein crystallography now plays a major
investigación científica. En el proceso de diseño y descubrimiento
and effective role in the discovery of new treatments. The
de fármacos, la cristalografía de proteínas juega un papel central.
developments that have made this possible have evolved
Los conocimientos que han hecho esto posible han venido
during the past fifty years. The methods for crystallizing
evolucionando desde hace cincuenta años aproximadamente.
macromolecules and determining their structures by X-ray
Los métodos de cristalización de macromoléculas y la
crystallography have been automated and the speed for X-
determinación de sus estructuras a través de la cristalografía de
ray data acquisition is several orders of magnitude faster. Fif-
rayos X han sido automatizados y miniaturizados y la velocidad
ty years ago it took several years to solve a single structure.
de la adquisición de datos de difracción ha aumentado en varios
Now, several protein–ligand complexes can be determined
órdenes de magnitud. Si hace cincuenta años la resolución de una
in single day. High-throughput crystallography is considered
sola estructura podría llevar varios años, actualmente se pueden
to be a great asset to the drug discovery process, providing a
determinar las estructuras de varios complejos proteína-ligando
fast way to tailor drug candidates to their targets by analys-
en un solo día. La cristalografía de alto rendimiento hoy día es
ing their binding mode in detail. Crystallization remains the
un gran recurso en el proceso del descubrimiento de fármacos
main challenge.
pues proporciona una manera rápida y precisa de adaptar los
fármacos candidatos a las dianas mediante el análisis de su modo
de unión. La cristalización sigue siendo el principal desafío.
KEYWORDS: Drug design, crystallization.
PALABRAS CLAVE: diseño de fármacos, cristalización.
when Dorothy Hodgkin was offered a small sample
Protein crystallization pre-dates X-ray crystallogra-
of crystalline insulin by Robert Robinson (Howard,
2003). Unfortunately, X-ray crystallography at that
phy. Humans have practised this science since 1840
(Giegé, 2013), but other organisms have put protein
time could not cope with the complexity of the in-
sulin molecule. She was able to grow better crys-
crystallization to use much earlier. Bacillus thuring-
iensis, known for its insecticidal properties, produc-
tals by dialysing concentrated insulin against tap
water (containing traces of zinc), but failed to do so
es protein crystals during sporulation. Fifty years ago
the seminal ideas that would lead to modern protein
with distilled water. The 3-dimensional structure of
crystallization methods were already in place and as
insulin was eventually determined by X-ray crystal-
lography in her laboratory in 1969 (Crowfood and
or Drug Design in the Las
it became understood that it was possible to visu-
alize ligands in their binding sites, crystallographic
Riley, 1939). Zinc occupies the central position in a
pharmaceutical investigations started.
unit of six insulin molecules (hexamer). The insulin
hexamer is not a crystallization artefact, but this is
Protein crystals are themselves drugs (Figure 1).
the form produced and stored in the body. It is in-
Insulin is important in the treatment of diabetes,
active but has long-term stability. The monomer is
and to slow down the release of this hormone it is
the active form. The hexamer serves to keep the
delivered in crystalline form. NPH insulin, a suspen-
highly reactive monomeric insulin protected, yet
sion of crystalline zinc-insulin combined with a pos-
available through hexamer-monomer conversion.
itively charged polypeptide, was created in 1936
Insulin can aggregate and form fibrillar interdigitat-
by Nordisk, but the role of zinc, as an additive, to
ed β-sheets. This can cause injection amyloidosis,
induce protein crystallization was not known until
and prevents the storage of insulin for long periods
much later. The insulin crystal story began in 1934
(Ivanova et al., 2009).
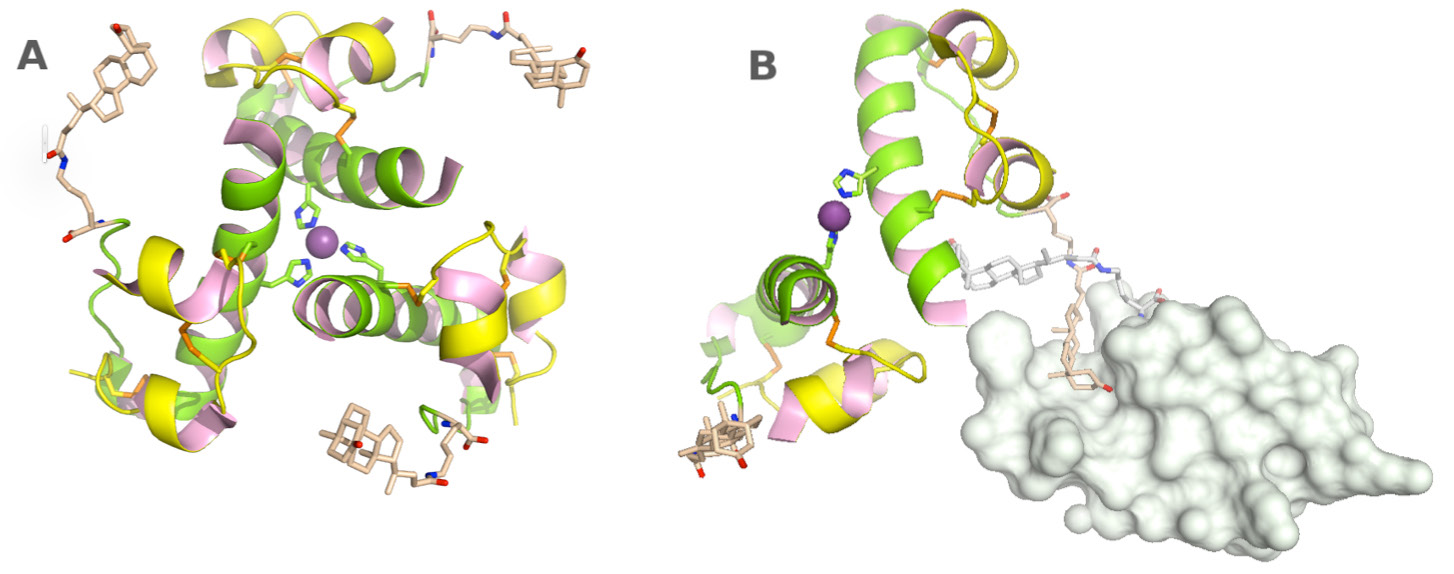
Figure 1. Crystals are drugs. Slow release insulin is designed with the addition of specific bulky hydrophobic
groups. These insulin crystals are more stable. In addition to zinc stabilization (A), the covalently linked litho-
cholyl group form specific van der Waals and hydrogen-bonding interactions with neighbouring molecules (B)
to strengthen the crystalline network and slow down dissolution of crystals. Lithocholic acid acylated insulin has
affinity for circulating serum albumin to ensure slow absorption into the blood stream and prolongation of its
half-life. The engineered insulin retains its affinity for its insulin receptor. (From PDB entry: 1UZ9; Whittingham
et al., 2004).
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963

Amyloid fibril formation and deposition can lead
salting-out). Transthyretin is a tetrameric protein in
to diseases, including spongiform encephalopathies,
dynamic equilibrium with a monomeric form that,
Alzheimer's and familial amyloidotic polyneuropa-
like insulin, has a tendency to aggregate. To prevent
thies. In familial amyloidotic polyneuropathy (FAP),
FAP, various potential inhibitors are being studied
the amyloid fibrils are mostly constituted by variants
to understand the structure–activity relationship
of transthyretin (TTR) (Quintas, Saraiva and Brito,
(SAR) (Nencetti and Orlandini, 2012). The relation-
1997). Protein instability, leading to aggregation can
ship between the chemical or 3D structure of a mol-
be a problem in protein crystallization for X-ray crys-
ecule and its biological activity. The objective is to
tallography, but not always. Transthyretin, previously
understand which chemical groups are responsible
known as prealbumin, is easily crystallized. The first
for its effect on the target protein. To achieve such
crystals were obtained fifty years ago by Purdy et
an understanding, 222 transthyretin structures, from
al. (1965) and by Haupt and Heide (1966) from 55%
human and other species, complexed and uncom-
saturated ammonium sulphate. The crystallization
plexed have been deposited in the Protein Data Bank
of insulin and transthyretin characterise the varia-
(PDB) (Berman et al., 2007). The inhibitors stabilize
tion in solubility of proteins, with respect to the salt
the tetrameric form shifting the equilibrium away
concentration in which they are bathed (salting-in/
from the amyloidogenic monomer.
Figure 2. Crystals and quaternary structure of transthyretin (TTR) a protein that transports thyroid hormones. The
morphology of transthyretin crystals is highly variable although the arrangement of the molecules in the lattice
remains constant (A-C). The crystals shape depends on the rate of growth of the crystals and not on the contacts
that the molecules make with one another. Four TTR molecules form a tetramer (D) and the tetramer packs
together with other tetramers always in the same manner. To reliably grow crystals streak seeding can be used.
The crystals grow in a straight line (E) where the seeds have been deposited by the cat whisker. TTR is studied to
develop amyloid inhibitors. Such inhibitor binds in the tetramer cavity in two independent binding sites (F). (From
PDB entry: 4PM1; Ciccone et al., 2015).
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963
Protein solubility in water is highly variable and
proline isomerase FKBP. The successful structure de-
the salting-in/salting-out concept applies only to
termination of the FKBP/FK506/calcineurin complex
soluble proteins. Crambin, a polypeptide from the
encourages others to follow the pathway. Amgen,
seeds of Crambe abyssinica, is not soluble in water.
another biotechnology start-up focuses on erythro-
It was dissolved in ethanol and crystallized in 1965
poietin (EPO), a glycoprotein hormone that controls
by adding water. It is resistant to denaturing agents
red blood cell production. In 1998 they solve the
like 3M urea, and only partially affected by guani-
structure of the EPO complex with the extracellular
dinium: It showed some solubility in 8 M guanidine
ligand-binding domain of its receptor (EPO binding
hydrochloride (Teeter and Hendrickson, 1979). Urea
protein EBP) (Syed et al., 1998), but they were pre-
and guanidine are now used to refold proteins ex-
ceded by a team at The Scripps Research Institute
or Drug Design in the Las
pressed in Escherichia coli and found in inclusion
(TSRI). The TSRI team succeeds, two years earlier,
bodies (Palmer and Wingfield, 2004), using similar
to determine the structure of the complex between
concentrations of these agents. High-level expres-
EBP and an EPO mimetic peptide (EMP) discovered
sion of many recombinant proteins in Escherichia
by Affymax (Livnah et al., 1996). The synthetic pep-
coli has revolutionized protein crystallization. Highly
tide, attached to polyethylene glycol, is approved the
aggregated protein (inclusion bodies) are formed in
FDA in 2012, and enters the market in 2013, only to
the bacteria cytoplasm when high-level expression
be recalled almost immediately, after 3 deaths, as
is induced. It is recovered from cell lysates by low
0.02% of patients suffer from severe hypersensitivity
speed centrifugation and the protein extracted from
reactions. Successes dominate over failures. Struc-
the washed pellets with guanidine·HCl in a soluble
tural studies have resulted in anti-HIV drugs that
but unfolded form that needs to be re-folded into
target the viral reverse transcriptase, integrase and
its native and biologically active form. Currently the
protease; and anti-cancer drugs aimed at various ty-
PDB contains 105,465 structures, of these 67,321
rosine kinases.
(64%) were expressed in Escherichia coli. In the
1970s, insulin and recombinant DNA technology al-
lows the birth of two biotech companies, Genentech
and Biogen. The challenge is to insert the DNA se-
The immunosuppressant drug, rapamycin, pro-
quence for human insulin into bacteria and let the
duced by the bacterium Streptomyces hygroscopicus,
bacteria produce the hormone.
and FK-506, produced by S. tsukubaensis, are used
to prevent organ rejection in transplantation. Their
The use of polyethylene glycol (PEG) to obtain crys-
action prevents activation of T cells and B cells by
tals for X-ray data analysis starts in 1975 with deoxy-
inhibiting the production of interleukin-2 (IL-2). FK-
hemoglobin crystals diffracting to 3.5 Å. Previously
506 gives the name to a family of FK-binding proteins
it had mainly been used for fractional precipitation
(FKBP). FK-506 binds to FKBP12 thus reducing the
(Ward et al., 1975). The steric exclusion mechanism
peptidyl-prolyl isomerase activity of this immunophi-
of this precipitant was correctly identified as being
lin. Most crystals of FKBPs have been obtained only in
similar to that of dextran reported by Torvard Lau-
complex with the immunosuppressant drugs.
rent (Laurent, 1963). Size exclusion chromatography,
using gels, typically made of dextran (Sephadex) and
In "The Billion Dollar Molecule: One Company´s
of other polymers, has become important as a pro-
quest for the perfect drug", Barry Werth describes
tein purification method prior to crystallization. PEG
the first few years of Vertex in its quest to create
is the single most successful precipitant, with cur-
drugs by rational drug design. Werth renders both
rently 38,976 macromolecular structures deposited
the science and the intricacies of the business deals
at Vertex. The narrative offers an insight at a critical
moment in the history of science when under the im-
While Genentech focuses mainly on proteins and
pulse of Joshua Boger, a researcher that leaves Merck
antibodies as their products, Vertex Pharmaceuticals
to found a new company, the focus of scientific re-
was founded in 1989 to pioneer an explicit strategy
search also shifts from screening soil samples and
of rational drug design rather than combinatorial
insect secretions to a new world where proteins and
chemistry. The aim is to understand the molecular
their inhibitor complexes can be crystallized and from
mechanisms of action of natural immunosuppres-
their structure using computing power new drugs
sants, FK506 and rapamycin, that act on peptidyl
can be designed. The founding of Vertex's is vision-
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963
ary, but the idea that the new drugs would be with-
helps crystallization because all the molecules can
out side effects because of the precision of the de-
shift in synchrony from one form to another, avoid-
sign, is still to be realised. The book describes Boger's
ing heterogeneity that would make crystallization
first target molecule, FKBP, important in preventing
more difficult. In absence of a cooperative process,
the host's body from rejecting transplanted organs.
heterogeneity is inevitable and the binding affinity of
The contrast between real science and the necessity
the ligand for the protein becomes important as it
of fund raising sees Boger going out to raise money,
will determine how many of the protein molecules
Vertex's researchers hunkered down in the laboratory
are complexed and how many are not. Higher affinity
benches to isolate and analyse FKBP, in a race against
ligands ensure that a higher portion of the protein
a tough team composed of Prof. Stuart L. Schreiber
molecules will be complexes. For low affinity ligands
at Harvard and Manuel Navia, the crystallographer.
the ligand is added in excess, up to ten times more
Manuel's parents went to the U.S. from Cuba, worked
compared to the protein. This strategy is not effec-
really hard and a strong value system that he tried to
tive to crystallize complexes of two or more proteins.
emulate. The advantage of academic science, where
In my laboratory we have investigated the use of bi-
collaborations are possible, contrasts with the world
functional inhibitors to bring together two proteases
of business where scientists need to hide proprietary
to change the manner in which crystallization occurs
results instead of presenting them at conferences or
(Antoni et al., 2013).
publishing them open to the criticism of reviewers.
The book is warmly recommended as a riveting tale
Ideas that the use of PEG instead of salts like am-
of human endeavour that shows how scientific antag-
monium sulphate would help maintaining complexes
onism can drive discovery. In this historical review we
have been abandoned as it has been realised that at
are more concerned on how crystallization methods
high salt concentrations, AS is as effective as PEG.
have evolved in the past 50 years than in discussing
academic in contrast to industrial research.
In crystallization, the inhibitors, substrates, modu-
Administration of folic acid (vitamin B9) worsens
lators and other ligands, be they other proteins or
leukemia. This led to the development of folic acid
small-molecules are the most important considera-
analogues, including methotrexate (MTX), to inhibit
tion in crystallization (Dale, Oefner and D'Arcy, 2003).
folic acid metabolism. MTX is used in the treat-
The various complexes have a different likelihood for
ment of cancer and autoimmune diseases. It acts
crystallization and different complexes will be able
by binding to dihydrofolate reductase (DHFR). MTX
to select a different polymorph (Vera et al., 2013)
was originally synthesised by an Indian biochemist
depending on how the ligand is able to change the
and in 1947. Sidney Farber and co-workers showed
properties of its target. One of the most fundamental
that the molecule could induce remission in chil-
changes that ligands can cause is the bringing togeth-
dren with acute lymphoblastic leukemia. The first
er of more that one molecule, in a manner that does
crystal structures of DHFR with MTX were deter-
not occur spontaneously. Even single atoms, like zinc,
mined for bacterial enzymes in 1982. In 1989, the
in the case of insulin, can bring together six or more
crystal structure of the chicken enzyme was solved
protein molecules. This has been recognized to be
and one year later the first complex of human DHFR
critical in the crystallization of insulin. A single atom,
with the folate and 5-deazafolate were solved. The
oxygen makes the difference between oxy- and de-
first crystal structure of human DHFR with MTX was
oxy- haemoglobin. When oxygen binds to the iron
obtained six years later, in 1995. The enzyme was a
complexed at the centre of the plane of the porphy-
MTX-resistant mutant with a single point mutation
rin ring it causes the iron atom to move back. This
in the drug binding pocket. The long delay between
triggers as series of cooperative changes that result
the demonstration of the effectiveness of the ligand
in a large scale movement of the whole assembly of
and the first crystal structure of the human enzyme
the four molecules that constitute the heamoglobin
complex gives an idea of the difficulty of obtain-
tetramer. The change in a single haemoglobin mol-
ing suitable crystals to carry out drug design. There
ecule is transmitted to the other three monomers
was great interest in DHFR and MTX on behalf of
in the tetramer, so that these too adopt a similar
pharmaceutical companies. The human DHFR-MTX
conformation in their hemes to facilitate the bind-
structural studies saw the involvement of research-
ing of oxygen to these sites. A cooperative process
ers from Gensia Pharmaceuticals. Agouron Pharma-
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963
ceuticals was founded in 1978 with the aim to find
DRUGS FROM STRUCTURAL STUDIES
selective inhibititors for DHFR and thymidate syn-
AIDS was first clinically observed in 1981 in the
thetase (TS). Hoffmann-La Roche had interests in an-
United States. In 1983, when the causative agent
tibacterial agents that target bacterial dihydrofolate
was identified the crystallization field was mature
reductases. The difficulties associated with protein
to face the challenge to design molecules for anti-
crystallization became evident. Dr. Villafranca from
retroviral therapy.
Agouron was a co-organizer of the fifth Interna-
tional Conference on the Crystallization of Biological
The inhibition of the HIV viral protease is regard-
ed as major success of structure-based drug design.
Macromolecules (ICCBM-5) in San Diego, in 1993.
Dr. D'Arcy from Hoffmann-La Roche was there too.
The protease inhibitors are highly effective against
or Drug Design in the Las
The National Aeronautics and Space Administration
the virus and since the 1990s have been a key com-
(NASA), interested in crystallization in microgravity,
ponent of anti-retroviral therapies for AIDS. Nelfi-
was a sponsor of the meeting.
navir (Viracept) (Figure 3), a protease inhibitor, was
developed by Agouron Pharmaceuticals as part of
Growing crystals in space was impractical, and re-
a joint venture with Eli Lilly and Company. Saquina-
searchers believed that convection free systems could
vir was developed by Roche, Ritonavir (Norvir) by
be developed to obtain the same effect on earth. A
AbbVie, Inc. Other companies focused on inhibiting
system where silica-gel methods can be used in both
HIV's reverse transcriptase. AZT, a nucleoside-based
vapour diffusion sitting drop and liquid-liquid diffu-
thymidine-analogue inhibitor, works by selectively
sion was proposed as an alternative to micrograv-
inhibiting transcription. Resistance against nucleo-
ity during the ICCBM-5 conference by Bob Cudney
tides developed, so non-nucleoside reverse-tran-
(Cudney, Patel and McPherson, 1994). Bob Cudney is
scriptase inhibitors were developed. To improve the
the current president of Hampton Research, a crys-
crystals of a clinically relevant double mutant HIV-1
tallization supply company. Until the introduction
of reverse transcriptase in complex with ATP and
of gels, three classical crystallization methods had
the non-nucleoside inhibitor HBY-097 (Das et al.,
dominated the field. These were batch, dialysis and
2007), streak seeding was used (Stura and Wilson,
vapour diffusion. The major advances consisted in
1990). One of the problems with protein crystalli-
the miniaturization of each of these methods. In the
zation is that as the crystal growth conditions are
batch method, the protein is mixed with the precipi-
optimized, nucleation can fail. Seeding is used to
tant, nucleation takes place on mixing and the crys-
stimulate nucleation. Various seeding techniques
tals are left to grow. By changing the temperature in
can be used (Stura and Wilson, 1991). In microseed-
a controlled manner, the degree of supersaturation
ing crystals are added to the protein solution before
can be changed. The miniaturization of this method,
full equilibration of the protein precipitant drop.
for crystallization under oil suitable for crystallization
This method can fail because the seeds dissolve or
with the IMPAX robot made by Douglas Instruments,
too many seeds are added. Macroseeding where
revived the method that was no longer used (Chay-
large seeds are added after equilibration, solves
en, Shaw Steward and Baldock, 1994). A generaliza-
both problems encountered with microseeding, but
tion of crystallization with oils was later proposed by
it is time consuming and manipulation errors can
Naomi Chayen during ICCBM-7 in Granada, Spain,
also lead to failure.
in 1998 (Chayen, 1999). Dialysis was miniaturized
Streak seeding where crystals are taken from an
more than forty years ago with microdots of 5-20 µL
existing crystal with a cat whisker and dispersed in
capacity made in plexiglas. New set-ups have been
a non-equilibrated or equilibrated experiment in
proposed using agarose gels to carry out dialysis ex-
straight line is fast. Since it is easily repeated if it fails,
periments in capillaries (Thiessen, 1994). Dialysis and
it is a practical method to find the best conditions to
batch account for only 60 and 766 structures in the
grow crystals. It can also be used to grow crystals in
PDB, respectively, while vapour diffusion counts for
a space group different from that of the seed crys-
66,887 entries. José Antonio Gavira has promoted
tals. This application is called: epitaxial jumps (Stura,
the use of free-interface diffusion (Otálora et al.,
Charbonnier and Taussig, 1999). The field has still
2009). Triana Science & Technology, Granada, Spain
many challenges. Over 50% of all modern medicinal
markets a kit to use the technique. The method ac-
drug targets are membrane proteins, proteins that
counts for only 12 PDB database entries.
interact with biological membranes (Figure 4).
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963

Figure 3. From crystals to drugs. The path towards a drug starts with the conception of an inhibitor (A) which
ignores the actual three dimensional structure of the compound once in the active site of the enzyme (B). Before
arriving at a formulation for the patient (C) it passes through a crystal structure. The results are often shown in
a simplified representation that shows only the secondary structure of the protein (D) and not all the atoms (E).
HIV protease inhibitors, including Viracept, are one of the great successes of structure-based drug design. (From
the PDB entry: 1OHR; Kaldor et al., 1997).
To crystallize these proteins, they must be extract-
tallization experiments using the vapour diffusion
ed from the membrane using a detergent, and then
technique as for soluble proteins. The method has in-
the crystallization can proceed in a manner analo-
creased the number of membrane proteins that have
gous to that used for soluble proteins. The problems
been crystallized.
arise when the protein is unhappy in the detergent.
The in meso method for crystallizing membrane pro-
Even if progress has been spectacular in the past
teins is growing in popularity (Li et al., 2014).
fifty years, the rapid progress is likely to continue in
the next fifty years. A crystal ball would be useful to
The method involves both lipids and detergents.
predict the future of this science.
The lipids are manipulated to generate a continuous
hard gel called lipid cubic phase (LCP). The protein
solubilized in detergent is added to the LCP so that
it can migrate into the lipid phase. The protein incor-
I am grateful to Natalia Stura for help with the Span-
porated into the LCP is dispensed and used in crys-
ish abstract.
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963

Figure 4. Membrane proteins. Membrane proteins are important targets for drug design. G-protein-coupled
receptors (GPCR) belong to the bateriorhodopsin (BR) family. They are of great interest to the pharmaceuti-
cal industry because they act as sensors to activate signal transduction pathways and cellular responses. Seven
transmembrane helices characterize GPCR. BR was the first membrane protein to be crystallized. Initially as two
dimensional crystals, but now after crystallization using lipidic cubic phases a three dimensional structure at
atomic resolution has been determined. The transmembrane helices are surrounded by lipids (green) (A). In the
centre there is a retinol (vitamin A) molecule (cyan). In mammals retinol is transported by retinol binding protein
which in plasma is found complexed with transthyretin (C). (From PDB entries: 1C3W and 1QAB; Luecke et al.,
1999; Naylor and Newcomer, 1999).
ation for Drug Design in the Las
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963
REFERENCES
Antoni, C., Vera, L., Devel, L., Catalani,
Giegé, R. (2013). A historical perspective on
son, I. A. (1996). Functional mimicry of
M. P., Czarny, B., Cassar-Lajeunesse,
protein crystallization from 1840 to the
a protein hormone by a peptide agonist:
E., Nuti, E., Rossello, A., Dive, V. and
present day. FEBS Journal, 280, pp. 6456–
the EPO receptor complex at 2.8 Å. Sci-
Stura, E. A. (2013). Crystallization of
ence, 273, pp. 464–71.
bi-functional ligand protein complexes.
Journal of Structural Biology, 182, pp.
Haupt, H. and Heide, K. (1966). Crystalliza-
tion of prealbumin from human serum.
Naylor, H. M. and Newcomer, M. E. (1999).
Experientia, 22, pp. 449–451.
The structure of human retinol-binding
protein (RBP) with its carrier protein
Berman, H., Henrick, K., Nakamura, H. and
transthyretin reveals an interaction with
Markley, J. L. (2007). The worldwide
Howard, J. A. K. (2003). Dorothy Hodgkin
the carboxy terminus of RBP. Biochem-
Protein Data Bank (wwPDB): ensuring
and her contributions to biochemis-
istry, 38, pp. 2647-2653.
a single, uniform archive of PDB data.
try. Nature Reviews Molecular Cell Bi-
Nucleic Acids Research, 35, pp. D301–
ology, 4, pp. 891-896.
Nencetti, S. and Orlandini, E. (2012). TTR
fibril formation inhibitors: is there a
Ivanova, M. I., Sievers, S., Sawaya, M. R., Wall,
SAR? Current Medicinal Chemistry,
Chayen, N. E., Shaw Stewart, P. D. and
J. S. and Eisenberg, D. (2009). Molecular
19, pp. 2356–2379.
Baldock, P. (1994). New develop-
basis for insulin fibril assembly. Proceed-
ments of the IMPAX small-volume
ings of National Academy of Sciences of
automated crystallization system.
the USA, 106, pp. 18990–18995.
Otálora, F., Gavira, J. A., Ng, J. D., García-Ruiz,
Acta Crystallographica, D50, pp.
J. M. (2009). Counterdiffusion meth-
ods applied to protein crystallization.
Kaldor, S. W., Kalish, V. J., Davies, J. F. 2nd.,
Progress in Biophysics and Molecular
Shetty, B. V., Fritz, J. E., Appelt, K., Bur-
Biology, 101, pp. 26–37.
Chayen, N. E. (1999). Crystallization with
gess, J. A.,, Campanale, K. M., Chir-
oils: a new dimension in macromolecu-
gadze, N. Y., Clawson, D. K., Dressman,
lar crystal growth. Journal of Crystal
B. A., Hatch, S. D., Khalil, D. A., Kosa, M.
Palmer, I. and Wingfield, P. T. (2004). Prepa-
Growth, 196, pp.
B., Lubbehusen, P. P., Muesing, M. A.,
ration and extraction of insoluble (in-
Patick, A. K., Reich, S. H., Su, K. S. and
clusion-body) proteins from Escherichia
Tatlock, J. H. (1997). Viracept (nelfina-
coli. Current Protocols in Protein Sci-
Ciccone, L., Tepshi, L., Nencetti, S. and Stu-
vir mesylate, AG1343): a potent, orally
ence, Chapter 6, Unit 6.3.
ra, E. A. (2015). Transthyretin complexes
bioavailable inhibitor of HIV-1 protease.
with curcumin and bromo-estradiol:
Journal of Medicinal Chemistry, 21, pp.
evaluation of solubilizing multicompo-
Purdy, R. H., Woeber, K. H., Holloway, M.
nent mixtures. New Biotechnology, 32,
T. and Ingbar, S. H. (1965). Preparation
of Crystalline Thyroxine-binding Preal-
Laurent, T. C. (1963). The interaction be-
bumin from Human Plasma. Biochem-
tween polysaccharides and other
istry, 4, pp. 1888–1895.
Crowfood, D. and Riley, D. (1939). X-Ray
macromolecules. 5. The Solubility of
Measurements on Wet Insulin Crystals.
Proteins in the presence of dextran.
Nature, 144, pp. 1011–1012.
Biochemical Journal, 89, pp. 253–257.
Quintas, A., Saraiva, M. J. and Brito, R.
M. (1997). The amyloidogenic po-
Luecke, H., Schobert, B., Richter, H. T.,
tential of transthyretin variants cor-
Cudney, R., Patel, S., and McPherson, A.
Cartailler, J. P. and Lanyi, J. K. (1999).
relates with their tendency to aggre-
(1994). Crystallization of macromole-
Structure of bacteriorhodopsin at 1.55
gate in solution. FEBS Letters, 418, pp.
cules in silica gels. Acta Crystallograph-
Å resolution. Journal of Molecular Biol-
ica, D50, pp. 479–483.
ogy, 291, pp. 899-911.
Stura, E. A. and Wilson, I. A. (1990). Analyti-
Dale, G. E., Oefner, C. and D'Arcy, A. (2003).
Li, D., Howe, N., Dukkipati, A., Shah, S.
cal and production seeding techniques.
The protein as a variable in protein
T. A., Bax, B. D., Edge, C., Bridges, A.,
Methods, 1, pp. 38–49.
crystallization. Journal of Structural Bi-
Hardwicke, P., Singh, O. M. P., Giblin,
ology, 142, pp. 88–97.
G., Pautsch, A., Pfau, R., Schnapp, G.,
Wang, M., Olieric, V. and Caffrey, M.
Stura, E. A. and Wilson, I. A. (1991). Applica-
(2014). Crystallizing Membrane Pro-
tions of the streak seeding technique in
Das, K., Sarafianos, S. G., Clark, A. D., Boyer,
teins in the Lipidic Mesophase. Experi-
protein crystallization. Journal of Crystal
P. L. Hughes, S. H. and Arnold, E. (2007).
ence with Human Prostaglandin E2 Syn-
Growth, 110, pp.
Crystal structures of clinically relevant
thase 1 and an Evolving Strategy. Crystal
Lys103Asn/Tyr181Cys double mutant
Growth & Design, 14, pp. 2034–2047.
HIV-1 reverse transcriptase in com-
Stura, E. A., Charbonnier, J. and Taus-
plexes with ATP and non-nucleoside
sig, M. J. (1999). Epitaxial jumps.
inhibitor HBY 097. Journal of Molecular
Livnah, O., Stura, E. A., Johnson, D. L., Mid-
Journal of Crystal Growth, 196, pp.
Biology, 365, pp. 77–89.
dleton, S. A., Mulcahy, L. S., Wrighton,
N. C., Dowr, W. J., Jolliffe, L. K. and Wil-
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963
Syed, R. S., Reid, S. W., Li, C., Cheetham,
Thiessen, K. J. (1994). The use of two novel
deoxyhemoglobin a crystals grown
J. C., Aoki, K. H., Liu, B., Zhan, H., Oss-
methods to grow protein crystals by
from polyethylene glycol solutions.
lund, T. D., Chirino, A. J., Zhang, J., Finer-
microdialysis and vapor diffusion in
Journal of Molecular Biology, 98, pp.
Moore, J., Elliot, S., Sitney, K., Katz, B. A.,
an agarose gel. Acta Crystallograph-
Matthews, D. J., Wendoloski, J. J., Egrie,
ica, D50, pp. 491–495.
J. and Stroud, R. M. (1998). Efficiency of
Whittingham, J. L., Jonassen, I., Havelund,
signalling through cytokine receptors
depends critically on receptor orienta-
Vera, L., Antoni, C., Devel, L., Czarny, B.,
S., Roberts S. M., Dodson E. J., Verma
Cassar-Lajeunesse, E., Rossello, A., Dive,
C. S., Wilkinson A. J. and Dodson G.
tion. Nature, 395, pp. 511–516.
V. and Stura, E. (2013). Screening Us-
G. (2004). Crystallographic and solu-
ing Polymorphs for the Crystallization
tion studies of N-lithocholyl insulin: a
Teeter, M. M. and Hendrickson, W. A. (1979).
of Protein–Ligand Complexes. Crystal
new generation of prolonged-acting
or Drug Design in the Las
Highly ordered crystals of the plant seed
Growth & Design, 13, pp. 1878-1888.
human insulins. Biochemistry, 25, pp.
protein crambin. Journal of Molecular
Biology, 127, pp.
Ward, K. B., Wishner, B. C., Lattman, E. E.
and Love, W. E. (1975). Structure of
ARBOR Vol. 191-772, marzo-abril 2015, a222. ISSN-L: 0210-1963
Source: http://arbor.revistas.csic.es/index.php/arbor/article/download/2025/2494
Egg Thaw Cycle Orientation Visit us online at www.NYUFertilityCenter.org Copyright 2008 – 2013 NYU Fertility Center – rev. 06/05/2013 Meet Our Physicians Dr. Frederick Licciardi Dr. James Grifo Dr. Nicole Noyes Dr. Alan Berkeley Dr. Lisa Kump-Checchio Dr. M. Elizabeth Fino Dr. David Keefe
Table des matières PATHOLOGIE RHINO-SINUSIENNE AIGUË .2 Rhinites aiguës.2 Sinusites aiguës .2 L'ethmoïdite aiguë de l'enfant .5 PATHOLOGIE RHINO-SINUSIENNE CHRONIQUE.7 Classification des pathologies rhinosinusiennes chroniques .7 Diagnostic de rhinosinusites chronique .7 Les sinusites antérieures de la face.7 Les pan-sinusites bilatérales et symétriques .8









