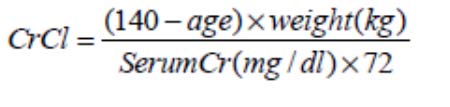Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Biogen.ca
PRODUCT MONOGRAPH
Pr
FAMPYRA™
10 mg Sustained Release Tablet
Potassium Channel Blocker
Biogen Idec Canada Inc.
Date of Revision:
90 Burnhamthorpe Road West, Suite 1100
November 26, 2014
Mississauga, Ontario L5B 3C3
Submission Control No: 177177
Page 1 of 32
TABLE OF CONTENTS
PART I: HEALTH PROFESSIONAL INFORMATION.3 SUMMARY PRODUCT INFORMATION .3 INDICATIONS AND CLINICAL USE .3 CONTRAINDICATIONS .3 WARNINGS AND PRECAUTIONS .4 ADVERSE REACTIONS .9 DRUG INTERACTIONS .14 DOSAGE AND ADMINISTRATION .15 OVERDOSAGE .16 ACTION AND CLINICAL PHARMACOLOGY .17 STORAGE AND STABILITY .19 SPECIAL HANDLING INSTRUCTIONS .19 DOSAGE FORMS, COMPOSITION AND PACKAGING .19 PART II: SCIENTIFIC INFORMATION .20 PHARMACEUTICAL INFORMATION .20 CLINICAL TRIALS .21 DETAILED PHARMACOLOGY .24 TOXICOLOGY .25 REFERENCES .27 PART III: CONSUMER INFORMATION .28
Page 2 of 32
PART I: HEALTH PROFESSIONAL INFORMATION
SUMMARY PRODUCT INFORMATION
Dosage Form / Strength
Clinically Relevant Nonmedicinal
Ingredients
Sustained Release Tablet, 10 mg of
For a complete listing see Dosage
fampridine per tablet
Forms, Composition and Packaging section.
INDICATIONS AND CLINICAL USE
PrFAMPYRA™ (fampridine) sustained release tablets are indicated for the symptomatic
improvement of walking in adult patients with multiple sclerosis (MS) with walking disability
(EDSS 3.5-7). The initial prescription should be for no more than 4 weeks, and assessment for
improvement in walking should be carried out within that timeframe (see DOSAGE AND
ADMINISTRATION).
FAMPYRA should only be prescribed by (or following consultation with) clinicians who are experienced in the management of multiple sclerosis and who are knowledgeable of the efficacy and safety profile of FAMPYRA and are able to discuss the benefits/risks with patients.
Geriatrics (> 65 years of age):
Renal function should be checked in elderly patients before starting treatment with FAMPYRA
and monitored regularly. Use in patients with any degree of renal impairment (mild, moderate or
severe) is contraindicated (see CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS,
Special Populations).
Pediatrics (< 18 years of age):
Safety and efficacy of FAMPYRA in patients younger than 18 years of age have not been
evaluated. FAMPYRA is not indicated for patients younger than 18 years of age.
CONTRAINDICATIONS
PrFAMPYRA™ (fampridine) sustained release tablets are contraindicated:
Hypersensitivity reactions, including anaphylaxis and angioedema, have been observed in
patients treated with fampridine. Therefore, FAMPYRA is contraindicated in patients with a known hypersensitivity to the drug or to any ingredient in the formulation or component of the container. For a complete listing, see the DOSAGE FORMS, COMPOSITION AND
Page 3 of 32
PACKAGING. See also WARNINGS AND PRECAUTIONS, and ADVERSE REACTIONS, Post Market Adverse Drug Reactions.
In patients taking concurrent compounded 4-aminopyridine or other forms of fampridine. In patients with mild, moderate or severe renal impairment (creatinine clearance <80mL/min)
(see WARNINGS AND PRECAUTIONS: Renal Impairment).
In patients with a prior history or current presentation of seizure (see WARNINGS AND
PRECAUTIONS, Seizure Risk).
In patients taking medicinal products that are inhibitors of the renal Organic Cation
Transporter 2 (OCT2), such as cimetidine and quinidine (see DRUG INTERACTIONS, ORGANIC CATION TRANSPORTER 2 (OCT2)).
WARNINGS AND PRECAUTIONS
SUMMARY OF IMPORTANT PRECAUTIONS TO BE TAKEN PRIOR TO
INITIATING AND DURING TREATMENT WITH FAMPYRA
PrFAMPYRA™ (fampridine) should be used under the supervision of a clinician experienced
in the treatment of multiple sclerosis and familiar with the safety and efficacy of FAMPYRA.
Recommended dose should not be exceeded due to an increased risk of seizure (see
DOSAGE AND ADMINISTRATION).
Renal impairment and certain concomitant medications are among the factors that can result
in increased fampridine plasma levels, and therefore result in increased risk of seizure (see CONTRAINDICATIONS; WARNINGS AND PRECAUTIONS, General).
Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylaxis and angioedema, have been observed in patients treated with fampridine. In several cases, these reactions occurred after the first dose. These hypersensitivity reactions included: anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, and urticaria. Patients should be informed of the signs and symptoms of a serious allergic reaction (e.g. itching, swelling of the face, tongue, throat, difficulty breathing, rash etc.). Patients should be instructed to seek immediate emergency assistance if they develop any of these signs and symptoms.
General
PrFAMPYRA™ (fampridine) sustained release tablets should not be administered at doses higher
than the recommended dose of 10 mg twice daily. One 10 mg tablet should be taken in the
morning and one 10 mg tablet should be taken in the evening. The doses should be taken 12
hours apart. Treatment with fampridine increases seizure risk. A dose-dependent increase in
risk of seizures has been observed in clinical studies with FAMPYRA at doses above the
recommended dose of 10 mg taken twice daily. In open label extension trials in MS patients, the
Page 4 of 32
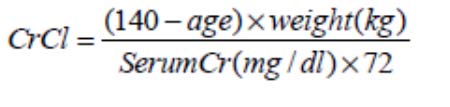
incidence of seizures during treatment with FAMPYRA 15 mg twice daily (1.7/100 patient years) was over 4 times higher than the incidence during treatment with 10 mg twice daily (0.4/100 patient years) (see WARNINGS AND PRECAUTIONS, Seizure Risk).
Renal Impairment
FAMPYRA is primarily excreted unchanged through the kidneys. Patients with renal
impairment have higher plasma concentrations (see ACTION AND CLINICAL
PHARMACOLOGY, Special Populations, Renal Insufficiency), which are associated with
increased adverse drug reactions, in particular neurological effects. Because patients with renal
impairment would require a dose lower than 10 mg twice daily and dosage strengths less than 10
mg are not available, FAMPYRA is contraindicated in patients with mild, moderate and severe
renal impairment [Creatinine Clearance (CrCl) ≤80 mL/min] (see CONTRAINDICATIONS).
Determining renal function before treatment, and regular monitoring during treatment, is recommended in all patients. Creatinine clearance can be estimated using the Cockroft-Gault formula (multiply by 0.85 for women):
Caution is required when FAMPYRA is prescribed concurrently with drugs or medicinal products that can significantly impact renal function. These include substrates of OCT2, such as beta blockers (carvedilol, pindolol, propranolol), procainamide, metformin, ranitidine and varenicline. Inhibitors of OCT2 (including cimetidine and quinidine are contraindicated (see DRUG INTERACTIONS, CONTRAINDICATIONS).
Seizure Risk
A dose-dependent increase in risk of seizures has been observed in clinical studies with
FAMPYRA at doses above the recommended dose. The recommended daily dose of
FAMPYRA, 10mg twice daily, taken 12 hours apart, should not be exceeded.
Treatment in patients with a prior history or current presentation of seizure is contraindicated.
Prior to starting FAMPYRA, all patients should be assessed for their risk of seizure, by taking a full patient history. Patients who are considered by the physician to be at high risk of seizure should be excluded from treatment (see CONTRAINDICATIONS).
The risk of seizures is also increased with renal impairment, due to reduced clearance of fampridine (see ACTION AND CLINICAL PHARMACOLOGY, Special Populations, Renal Insufficiency). Therefore, assessment of the risk of seizure should also include assessment of renal function prior to initiating treatment with FAMPYRA. Treatment of patients with mild, moderate or severe renal impairment (creatinine clearance < 80 mL/min) is contraindicated (see CONTRAINDICATIONS).
Page 5 of 32
FAMPYRA should be administered with caution in the presence of factors which may lower seizure threshold.
FAMPYRA should be discontinued immediately in patients who experience a seizure while on treatment and not restarted.
Published epidemiological studies indicate that the MS population has a higher background prevalence of seizures than the general population (2 to 4% vs. 0.5 to 1%). This rate increases with age and progressive disease. In the MS population, the background incidence rate of first seizures has been reported to be in the range of 0.2 to 0.6 cases per 100 person-years. The seizure incidence observed during over 1,200 person-years of exposure in open-label treatment of MS patients with FAMPYRA tablets 10 mg twice daily is consistent with this expected background rate (0.41 cases per 100 person-years).
In placebo controlled studies in MS, the incidence of seizure was not higher in the patients treated with fampridine 10 mg twice daily than in the placebo-treated patients (1/532 [0.19%] versus 1/249 [0.4%], respectively).
As seizures have been seen with the compounded form of fampridine and the immediate release formulations, the clinical trials were designed to exclude patients with a history of seizure or epileptiform activity. The safety data from the controlled trials has shown that at the recommended therapeutic dose (fampridine 10 mg twice daily), the risk of seizure is no higher than the placebo group.
Concurrent Treatment with Other Forms of 4-Aminopyridine
Concurrent treatment with other forms of 4-aminopyridine (4-AP, fampridine) is contraindicated
since the active ingredient is the same (see CONTRAINDICATIONS). Patients should
discontinue use of any product containing 4-aminopyridine prior to initiating treatment with
FAMPYRA in order to reduce the potential for dose-related adverse reactions.
Carcinogenesis and Mutagenesis
See Part II, Toxicology
Cardiovascular
Cardiac Conduction Disorders
FAMPYRA is a potassium channel blocker and, therefore, should be administered with caution to patients with cardiovascular symptoms of rhythm and sinoatrial or atrioventricular conduction cardiac disorders (these effects are seen in overdose). There is limited safety information in patients with cardiovascular disease as they were excluded from the clinical trials.
Immune
In clinical studies low white blood cell counts were seen in 2.1% of FAMPYRA patients versus
1.9% of patients on placebo. Infections were seen in the clinical studies as stated below
(Table 1). An increased infection rate and impairment of the immune response cannot be
excluded.
Page 6 of 32
Infections and Infestations
Placebo Controlled Studies 202/203/204
FAMPYRA TEAEsa with Incidence
≥1% in FAMPYRA vs
Infections and Infestations
59 (24.8%)
Gastroenteritis viral
Upper respiratory tract infection
Urinary tract infection
a TEAEs - Treatment Emergent Adverse Events
Neurological
Dizziness and Balance Disorder
The increased incidence of dizziness and balance disorder seen with FAMPYRA may result in an increased risk of falls. Patients who are using walking aids should continue to use these aids as needed.
Exacerbation of Trigeminal Neuralgia
Exacerbation of Trigeminal neuralgia has been reported in MS patients with history of trigeminal neuralgia treated with fampridine during postmarketing experience (see ADVERSE REACTIONS, Post market Adverse Drug Reactions). In the majority of cases, onset was within 1 month of initiating treatment with FAMPYRA and symptoms improved or resolved following discontinuation of FAMPRYA, with or without pharmacological treatment of the trigeminal neuralgia. Some patients that received pharmacological treatment for adverse events of worsening trigeminal neuralgia required higher doses of previously effective treatments to manage symptoms.
Occupational Hazards
Since dizziness or fatigue may occur with the use of this drug, sensitive patients should be
cautioned against activities requiring mental alertness and physical coordination until their
response to the drug has been well-established.
Genitourinary
A higher incidence of urinary tract infections (UTI) was reported with FAMPYRA (12%) than
with placebo (8%), during controlled clinical trials. The underlying mechanism is not fully
Page 7 of 32
understood but may involve the effect of fampridine on the sensory and or motor innervation of the bladder. Adverse events of UTI were frequently reported based on symptoms of UTI without confirmation from urinalysis or culture results. Reported UTIs are usually moderate and transient.
Patient Counselling Information
Patients should be informed of the following:
FAMPYRA should be taken exactly as prescribed, one 10 mg tablet in the morning and
one 10 mg tablet in the evening. Doses should be taken 12 hours apart.
Do not take an extra dose after missing a dose. There is a dose-dependent risk of seizure. FAMPYRA must be discontinued if they
experience a seizure.
Renal impairment increases plasma concentration of fampridine, which may lead to an
increased risk of seizure. Advise patients that co-administration of certain drugs or medicinal products, such as beta blockers (carvedilol, pindolol, propranolol), procainamide, metformin, ranitidine and varenicline, can impact renal function.
The assessment of improvement in walking should be done within 4 weeks of starting
FAMPYRA treatment. If there is no benefit to the patient seen within that time frame, treatment should be stopped.
Patients should be informed of the signs and symptoms of a serious allergic reaction (e.g.
itching, swelling of the face, tongue, throat, difficulty breathing, rash etc.). Patients should be instructed to seek immediate emergency assistance if they develop any of these signs and symptoms.
Special Populations
Pregnant Women: There are no adequate and well-controlled studies of FAMPYRA in
pregnant women. The use of FAMPYRA during pregnancy should only be considered if the
potential benefit to the mother justifies the potential risk to the fetus.
Administration of fampridine to animals during pregnancy resulted in decreased offspring viability and growth at doses 6.8 times the maximum recommended human dose (MRHD) of 20 mg/day (see TOXICOLOGY).
Nursing Women: It is not known whether fampridine is excreted in human milk. Because
many drugs are excreted in human milk and because of the potential for serious adverse reactions
in nursing infants from fampridine, FAMPYRA is not recommended during breast feeding.
Pediatrics (<18 years of age): The safety and efficacy of FAMPYRA in patients younger than
18 years of age have not been evaluated. FAMPYRA is not indicated for patients younger than
18 years of age.
Geriatrics (>65 years of age): Clinical studies of FAMPYRA did not include sufficient
numbers of subjects aged 65 and over to determine whether they respond differently from
younger subjects (see ACTION AND CLINICAL PHARMACOLOGY). Because elderly
Page 8 of 32
patients are more likely to have decreased renal function, renal function should be determined in elderly patients before starting treatment with FAMPYRA and monitored regularly.
Monitoring and Laboratory Tests
Clearance of fampridine is decreased in patients with renal impairment and is significantly
correlated with creatinine clearance. Therefore, determining renal function before treatment and
its regular monitoring during treatment is recommended in all patients who may be at risk of
reduced renal function (see CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS -
Renal Impairment, Special Populations, and ACTION AND CLINICAL PHARMACOLOGY).
ADVERSE REACTIONS
Adverse Drug Reaction Overview
Pre-market clinical trials in multiple sclerosis included 1,075 patients treated with FAMPYRA
for at least 12 weeks, 819 patients for 6 months, 628 patients for at least one year and 526
patients for at least two years.
Adverse reactions identified are mostly neurological and relate to nervous system excitation, including seizure, insomnia, anxiety, balance disorder, dizziness, paraesthesia, tremor, headache and asthenia. This is consistent with fampridine's pharmacological activity. The highest incidence of adverse reactions identified from placebo-controlled trials in multiple sclerosis patients with FAMPYRA given at the recommended dose, are reported as urinary tract infection (in approximately 12% of patients, and 8% in patients given placebo).
Clinical Trial Adverse Drug Reactions
Because clinical trials are conducted under very specific conditions the adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice and should not be compared to the rates in the clinical trials of another drug. Adverse drug reaction information from clinical trials is useful for identifying drug-related adverse events and for approximating rates.
Table 2 lists treatment emergent adverse events that occurred during active treatment in ≥1% of FAMPYRA-treated MS patients and more frequent compared to placebo in controlled clinical trials.
Treatment-Emergent Adverse Events with an incidence of ≥1% of
FAMPYRA treated MS patients and at ≥ 1% higher rate than for placebo
Adverse Event
FAMPYRA 10 mg twice daily
Urinary tract infection
Page 9 of 32
Adverse Event
FAMPYRA 10 mg twice daily
Balance Disorder
Pharyngolaryngeal pain
White blood cell count decreased
Hypertriglyceridemia
Other Adverse Events Observed During Clinical Trials
The following is a list of treatment-emergent adverse events reported by patients treated with
fampridine any dose and any formulation in the safety population (n=1,510). This population
includes patients receiving fampridine during clinical pharmacology studies, placebo-controlled
studies in patients with multiple sclerosis, placebo-controlled studies in patients with spinal cord
injury and uncontrolled studies.
Events that have already been included in Table 2 have been excluded. Although the events reported occurred during treatment with fampridine, they were not necessarily caused by fampridine.
Events are listed by system organ class and frequency as defined as follows:
Frequent: occurring on 1 or more occasions in at least 1/100 patients
Infrequent: occurring in less than 1/100 but at least 1/1,000 patients
Rare: occurring in less than 1/1,000 patients.
Blood and lymphatic system disorders:
Infrequent: anaemia, lymph node pain
Rare: leukopenia, neutropenia
Page 10 of 32
Cardiovascular:
Frequent: palpitations, tachycardia
Infrequent: atrioventricular block first degree, bundle branch block right, chest pain, coronary artery disease, ventricular extrasystoles, ventricular hypertrophy
Rare: bundle branch block left, dilatation ventricular
Ear and labyrinth disorders:
Frequent: tinnitus, vertigo
Infrequent: deafness bilateral, ear pain
Endocrine Disorders:
Infrequent: goitre
Rare: thyroid cyst
Eye Disorders:
Frequent: vision blurred, visual disturbance
Infrequent: blepharospasm, blindness, conjunctivitis, diplopia, eye haemorrhage, eye movement disorder, lacrimation increased, ocular hyperaemia, photopsia, scotoma
Rare: eyelid ptosis
Gastrointestinal disorders:
Frequent: abdominal discomfort, dry mouth, flatulence, stomach discomfort, toothache
Infrequent: abdominal hernia, abdominal pain lower, abdominal tenderness, dysphagia, epigastric discomfort, gastritis, haemorrhoidal haemorrhage, hypoaesthesia oral, irritable bowel syndrome
Rare: colitis, haematemesis
General disorders and administrative site conditions:
Frequent: chest discomfort, chest pain, chills, feeling hot, gait disturbance, influenza like
illness, irritability
Infrequent: catheter related complication, cyst, gravitational oedema, injection site erythema,
pitting oedema, suprapubic pain, tenderness
Immune System Disorders:
Frequent: hypersensitivity, seasonal allergy
Infections and Infestations:
Frequent: bronchitis, cystitis, ear infection, fungal infection, herpes simplex, tooth abscess,
vulvovaginal mycotic infection
Infrequent: bacterial infection, candidiasis, escherichia urinary tract infection, eye infection,
folliculitis, herpes virus infection, infection, labyrinthitis, laryngitis, localised infection, oral
Page 11 of 32
candidiasis, otitis externa, pharyngitis, pharyngitis streptococcal, rhinitis, sepsis, skin infection, subcutaneous abscess, tooth infection
Rare: abscess oral, bacterial pyelonephritis, clostridial infection, gingival abscess,
paronychia, vaginal infection
Injury Poisoning and procedural complications:
Frequent: back injury, joint sprain, muscle strain, procedural pain, skin laceration, thermal burn
Infrequent: arthropod bite, arthropod sting, corneal abrasion, epicondylitis, eschar, fibula fracture, hand fracture, joint injury, laceration, ligament injury, neck injury, patella fracture, skeletal injury, sunburn, tendon injury, tooth fracture, wrist fracture
Rare: fracture, ligament sprain
Investigations:
Frequent: blood cholesterol increased, blood creatine phosphokinase increased, blood triglycerides increased, body temperature increased, white blood cell count increased
Infrequent: aspartate aminotransferase increased, blood creatinine increased, blood lactate dehydrogenase increased, blood phosphorus increased, blood potassium decreased, blood potassium increased, blood urea increased, cardiac murmur, carotid bruit, crystal urine present, electrocardiogram T wave inversion, electrocardiogram abnormal, full blood count abnormal, heart rate decreased, heart rate increased, heart rate irregular, hepatic enzyme increased, lymphocyte count decreased, monocyte count decreased, neutrophil count decreased, platelet count decreased, red blood cell count decreased, red blood cell count increased, red blood cells urine, red blood cells urine positive, weight increased, white blood cells urine
Rare: blood cholesterol abnormal, right ventricular systolic pressure increased, thyroxine increased, urine cytology abnormal
Metabolic and nutritional disorders:
Frequent: decreased appetite, hypercholesterolaemia
Infrequent: diabetes mellitus, hypokalaemia
Rare: polydipsia
Musculoskeletal and connective tissue disorders:
Frequent: bursitis, chest wall pain, muscle tightness, musculoskeletal discomfort, osteoporosis
Infrequent: bone pain, cervical spasm, groin pain, joint instability, limb discomfort, muscle twitching, musculoskeletal chest pain, osteoarthritis, osteopenia, pain in jaw, sensation of heaviness
Rare: trigger finger
Neoplasms benign, malignant and unspecified (including cysts and polyps):
Infrequent: breast cancer, uterine leiomyoma
Page 12 of 32
Rare: lentigo
Nervous system disorders:
Frequent: migraine, neuropathic pain, somnolence, trigeminal neuralgia
Infrequent: amnesia, dysaesthesia, dysgeusia, lethargy, Lhermitte's sign, motor dysfunction, myoclonus, neuralgia, nystagmus, peroneal nerve palsy, sciatica, sinus headache, syncope
Rare: anticholinergic syndrome, head titubation
Psychiatric disorders:
Frequent: abnormal dreams, confusional state, nervousness, sleep disorder
Infrequent: hallucination, panic attack, paranoia
Renal and urinary disorders:
Frequent: dysuria, micturition urgency, urinary incontinence, urinary retention
Infrequent: bladder spasm, nephrolithiasis, nocturia, polyuria, pyuria, terminal dribbling, urinary hesitation
Reproductive system and breast disorders:
Infrequent: menorrhagia
Respiratory, thoracic and mediastinal disorders:
Frequent: nasal congestion, sinus congestion
Infrequent: asthma, atelectasis, epistaxis, hiccups, pharyngeal erythema, rhinorrhea, wheezing
Rare: nasal dryness, sinus disorder
Skin and subcutaneous tissue disorders:
Frequent: blister, ecchymosis, hyperhidrosis, skin ulcer
Infrequent: alopecia, cold sweat, dry skin, ingrown nail, livedo reticularis, purpura, rash macular, scab, skin lesion
Rare: drug eruption, hypotrichosis, skin fissures, telangiectasia
Vascular disorders:
Frequent: hot flush, hypertension, peripheral coldness
Infrequent: deep vein thrombosis, flushing, haematoma, hypotension, phlebitis
Rare: thrombosis
Seizures: Cases of seizure were reported infrequently during controlled clinical trials and open
label extension studies with fampridine (5/532, 0.9 % and 5/660, 0.76%, respectively). Most of
these incidences were associated with uncontrolled overdose, high systemic doses, or high
plasma levels of fampridine (see WARNINGS AND PRECAUTIONS, Seizure Risk).
Page 13 of 32
Post-Market Adverse Drug Reactions
The following adverse reactions have been identified during post marketing experience with
fampridine: seizures, exacerbations of trigeminal neuralgia (TN) in patients with a history of TN
(see WARNINGS AND PRECAUTIONS, Trigeminal Neuralgia) and hypersensitivity reactions
(including anaphylactic/anaphylactoid reactions such as swollen tongue and swollen throat
(pharyngeal edema) (see CONTRAINDICATIONS; WARNINGS AND PRECAUTIONS,
Hypersensitivity Reactions). For the majority of cases of anaphylaxis a relationship to fampridine
could not be excluded.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
DRUG INTERACTIONS
Overview
Patients should be advised to inform their physicians if they are taking, or plan to take, any
prescription or over-the-counter medications.
Because fampridine is actively excreted unchanged by the kidneys; there is the potential for interactions with other drugs that are renally excreted (see PHARMACOKINETICS).
No pharmacokinetic drug interactions were observed between FAMPYRA™ (fampridine) and interferon or baclofen. There was evidence of direct inhibition of CYP2E1 by fampridine at 30μM (approximately 12% inhibition) which is approximately 100 times the average plasma fampridine concentration measured for the 10mg tablet.
Interferon
No pharmacokinetic drug-drug interaction of interferon beta-1b was observed on fampridine
plasma levels. Under single dose and steady-state conditions 7.5 mg t.i.d. of fampridine as an
immediate release formulation was given alone and with subcutaneous injections of 8 million
units interferon beta-1b. Immediate release fampridine was used to more closely approximate
the time to peak plasma levels for interferon. Fampridine kinetics were comparable following
administration of fampridine alone (steady state Cmax of 56.7 ng/mL and AUC0–∞ of 216.0
ng·hr/mL) or following co-administration of fampridine (steady state Cmax of 50.1 ng/mL and
AUC0–∞ of 207.2 ng·hr/mL) and Betaseron® in 3 male and 6 female MS patients.
Baclofen
No pharmacokinetic drug-drug interactions were observed on fampridine and baclofen plasma
levels. Following a single oral dose of 15 mg fampridine (controlled release capsule formulation)
administered to 12 healthy male volunteers, the mean Cmax was 47.2 ng/mL and the mean AUC0–
∞ was 399.8 ng·hr/mL and after a 10 mg dose of baclofen, the Cmax was 199.5 ng/mL and the
AUC0–∞ was 1055.0 ng·hr/mL. When the same doses of fampridine and baclofen were co-
administered, a similar Cmax and AUC0–∞ were found: the fampridine Cmax was 46.2 ng/mL and
the AUC0–∞ was 402.5 ng·hr/mL and the baclofen Cmax was 201.0 ng/mL and the AUC0–∞ was
1024.4 ng·hr/mL.
Page 14 of 32
CYP Enzymes
In vitro data with human liver microsomes showed that fampridine was not a direct or time-dependent inhibitor of CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, or CYP3A4/5 at concentrations up to 30 μM. There was evidence of direct inhibition of CYP2E1 by fampridine at 30 μM (approximately 12 % inhibition), which is approximately 100 times the average plasma fampridine concentration measured for the 10 mg FAMPYRA tablet.
Potential for Fampridine to Affect Other Drugs
The potential for 4-aminopyridine to induce human hepatocytes at therapeutic concentrations is
remote. Other in vitro studies with cultured human hepatocytes with 0.025 μM, 0.25 μM, 2.5
μM and 25 μM fampridine had little or no effect on CYP1A2, CYP2B6, CYP2C9, CYP2C19,
CYP2E1 or CYP3A4/5 enzyme activities.
P-glycoprotein Transporter
In vitro, fampridine is not a substrate or an inhibitor for the P-glycoprotein transporter. The pharmacokinetics of FAMPYRA are unlikely to be affected by drugs that inhibit the P–glycoprotein transporter, and fampridine is not likely to affect the pharmacokinetics of drugs that are substrates of the P-glycoprotein transporter.
Organic Cation Transporter 2 (OCT2)
Fampridine is eliminated mainly via the kidneys with active renal secretion accounting for about
60% of elimination. In vitro studies have shown that the Organic Cation Transporter (OCT2) is
the main transporter responsible for the active secretion of fampridine. Therefore, the
concomitant use of fampridine with medicinal products that are inhibitors of OCT2 for example,
cimetidine and quinidine, is contraindicated and concomitant use of fampridine with medicinal
products that are substrates of OCT2 for example, beta blockers (carvedilol, pindolol,
propranolol), procainamide, metformin, ranitidine and varenicline is cautioned (see
CONTRAINDICATONS and WARNINGS AND PRECAUTIONS, Renal Impairment).
DOSAGE AND ADMINISTRATION
Recommended Dose and Dosage Adjustment
The recommended dose of PrFAMPYRA™ (fampridine) sustained release tablets is one 10 mg
tablet twice daily. One 10 mg tablet should be taken in the morning and one 10 mg tablet should
be taken in the evening. The doses should be taken 12 hours apart.
Administration
Tablets should only be taken by swallowing whole with a glass of water. Doses should be taken
without food.
Patients should be advised to not divide, crush, dissolve, suck or chew the tablet because broken
tablets can release too much of the drug at one time and increase the risk of seizure adverse
events. Patients should also be advised not to take an extra dose if a dose is missed, due to the
increased risk of seizure adverse events.
Page 15 of 32
Starting FAMPYRA Treatment: Initial assessment of benefit
The initial prescription for FAMPYRA should be for no more than 4 weeks of therapy as
clinical benefits should generally be identified within 4 weeks after starting FAMPYRA.
The assessment for evaluation of improvement should be conducted prior to starting
treatment and again within 4 weeks.
FAMPYRA should be discontinued if benefit is not reported by patient.
Ongoing confirmation of positive benefit/risk profile
Physicians should continue to actively review the benefit/risk of FAMPYRA for the
individual patient, to ensure continued positive benefit/risk.
In all cases, FAMPYRA should be discontinued if patients no longer report benefit, or if seizure occurs.
Missed Dose
The dosing regimen of one tablet in the morning and one tablet in the evening taken 12 hours
apart should always be followed. Patients should be advised to not take an extra dose if a
dose is missed.
No additional benefit was demonstrated at doses greater than 10 mg twice daily and adverse
events and discontinuations were more frequent at higher doses. In particular, the risk of seizure
may increase with doses greater than 10 mg twice daily.
Dosing in Special Populations
Elderly: Renal function should be determined in elderly patients before starting treatment with FAMPYRA. Monitoring renal function to detect any renal impairment is recommended in elderly patients (see ACTION AND CLINICAL PHARMACOLOGY, Pharmacokinetics, Special Populations and Conditions).
Renal impairment: FAMPYRA is contraindicated in patients with mild, moderate or severe renal impairment (Creatinine Clearance ≤80 mL/min) (see CONTRAINDICATIONS).
Hepatic impairment: No dose adjustment is required for patients with hepatic impairment.
Pediatric population: The safety and efficacy of FAMPYRA in patients younger than 18 years have not been evaluated.
OVERDOSAGE
For management of a suspected drug overdose, contact your regional Poison Control Centre.
Page 16 of 32
Acute symptoms of overdose with FAMPYRA were consistent with central nervous system excitation and included confusion, tremulousness, diaphoresis, seizure, and amnesia. Central nervous system side effects at high doses of 4-aminopyridine include confusion, seizures, status epilepticus, involuntary and choreoathetoid movements. Other side effects at high doses include cases of cardiac arrhythmias (for example, supraventricular tachycardia and bradycardia) and ventricular tachycardia as a consequence of potential QT prolongation. Reports of hypertension have also been received.
Several cases of overdose are found in the scientific literature in which various formulations of fampridine were used, resulting in adverse events including seizure, confusion, tremulousness, diaphoresis and amnesia. In some instances, patients developed status epilepticus, requiring intensive supportive care and were responsive to standard therapy for seizures.
Three cases of overdose were reported in controlled clinical trials with PrFAMPYRA™ (fampridine) sustained release tablets, involving two MS patients. The first patient took six times the currently recommended dose (60 mg) and was taken to the emergency room with altered mental state. The second patient took 40 mg doses on two separate occasions. In the first instance, the patient experienced a complex partial seizure and, in the second instance, a period of confusion was reported. Both patients recovered by the following day without sequelae.
Patients with repeated seizure activity should be treated with benzodiazepine, phenytoin, or other appropriate acute anti-seizure therapy.
ACTION AND CLINICAL PHARMACOLOGY
Mechanism of Action
The mechanism by which fampridine exerts its therapeutic effect has not been fully elucidated.
Fampridine is a broad spectrum potassium channel blocker. In animal tissue preparations,
fampridine has been shown to increase conduction of action potentials in demyelinated axons
through inhibition of potassium channels.
Pharmacodynamics
Based on studies in animals, by blocking potassium channels, FAMPYRA is thought to reduce
the leakage of ionic current through these channels and enhance action potential formation in
demyelinated axons. It is thought that by enhancing action potential formation, more impulses
might be conducted in the central nervous system.
Absorption:
Orally administered fampridine is rapidly and completely absorbed from the gastrointestinal
tract. Absolute bioavailability of sustained-release FAMPYRA tablets has not been assessed, but
relative bioavailability is 96% when compared to an aqueous oral solution. The sustained release
tablet delays absorption of fampridine relative to the solution formulation manifested by slower
rise to a lower peak concentration (Cmax), with no effect on the extent of absorption (AUC).
When fampridine tablets are taken with food, the reduction in the area under the plasma
concentration-time curve (AUC0-∞) of fampridine is approximately 2-7% (10 mg dose). The
small reduction in AUC is not expected to cause a reduction in the therapeutic efficacy.
Page 17 of 32
Distribution:
Fampridine is largely unbound to plasma proteins (97–99%). The apparent volume of
distribution is 2.6 L/kg.
Metabolism:
Fampridine is metabolized by oxidation to 3-hydroxy-4-aminopyridine and further conjugated to
the 3- hydroxy-4-aminopyridine sulfate. No pharmacological activity was found for the
fampridine metabolites against selected potassium channels in vitro.
In vitro studies with human liver microsomes indicate that CYP2E1 was the major enzyme responsible for the 3-hydroxylation of fampridine based on correlation analysis, chemical inhibition studies and incubations with recombinant human CYP enzymes. The identity of the CYP enzymes suspected of playing a minor role in the 3-hydroxylation of fampridine could not be established unequivocally.
Excretion:
Fampridine and metabolites are eliminated nearly complete after 24 hours with 95.85% of the
dose recovered in the urine and 0.51% recovery in feces. Most of the excreted radioactivity in
the 0–4 hour pooled urine was parent drug (90.3%). Two metabolites were identified: 3-
hydroxy-4-aminopyridine (4.3%) and 3-hydroxy-4-aminopyridine sulfate (2.6%).
The elimination half-life of fampridine following administration of the sustained-release tablet formulation of FAMPYRA is 5.2 to 6.5 hours. The plasma half-life of the sulfate conjugate is approximately 7.6 hours and the half-life of 3-hydroxy-4-aminopyridine could not be calculated because concentrations for most subjects were close to or below the limit of quantitation.
Special Populations and Conditions
Pediatrics:
The pharmacokinetics of fampridine in the pediatric population has not been studied.
FAMPYRA is not indicated for patients younger than 18 years of age.
Geriatrics:
Clinical studies of FAMPYRA did not include sufficient numbers of subjects aged 65 and over
to determine whether they respond differently from younger patients. Because FAMPYRA is
primarily excreted unchanged by the kidneys, and creatinine clearance decreases with age,
monitoring of renal function in elderly patients is recommended (see WARNINGS AND
PRECAUTIONS, Special Populations; and Renal Impairment).
Gender:
A population pharmacokinetic analysis suggested that female patients would be expected to have
higher maximum fampridine plasma concentration than male patients. The magnitude of these
relationships is small and does not necessitate any dose modifications.
Renal Insufficiency:
The pharmacokinetics of fampridine was studied in 9 male and 11 female subjects with varying
degrees of renal function. Elimination of the drug is significantly correlated with the creatinine
clearance. Total body clearance of fampridine was reduced in patients with impaired renal
Page 18 of 32
function by 42.7% in mild (CLcr ≥50–80 mL/min), 50.3% in moderate (CLcr = 30–50 mL/min), and 72.7% in severe (CLcr ≤30 mL/min). The terminal half-life of fampridine is prolonged by 3.3-fold in severe renal impairment but not prolonged in mild or moderate impairment.
Fampridine is contraindicated in patients with renal impairment (see CONTRAINDICATIONS).
Hepatic Impairment:
The pharmacokinetics of fampridine have not been studied in subjects with hepatic impairment.
Since fampridine is primarily excreted unchanged in the urine, hepatic insufficiency is not
expected to significantly affect fampridine pharmacokinetics or recommended dosing.
Race:
There was an insufficient number of non-Caucasians to evaluate the effect of race.
STORAGE AND STABILITY
Store PrFAMPYRA™ (fampridine) sustained release tablets between 15 to 30° C in the original
bottle to protect from light and moisture.
SPECIAL HANDLING INSTRUCTIONS
Not applicable.
DOSAGE FORMS, COMPOSITION AND PACKAGING
PrFAMPYRA™ (fampridine) sustained release tablets are film-coated, white to off-white,
biconvex, oval shaped, non-scored tablets with flat edge, debossed with ‘A10' on one side
containing 10 mg fampridine. Non-medicinal ingredients: hydroxypropyl methylcellulose USP,
microcrystalline cellulose USP, colloidal silicon dioxide NF, magnesium stearate USP,
hydroxypropyl methylcellulose/hypromellose USP, titanium dioxide USP, and macrogol/PEG
400 NF.
Bottles containing 14 tablets and a silica gel desiccant. Four bottles in a carton.
Page 19 of 32
PART II: SCIENTIFIC INFORMATION
PHARMACEUTICAL INFORMATION
Drug Substance
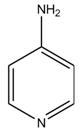
Proper name: fampridine
Chemical name: 4‐aminopyridine
CAS: 504‐24‐5
Molecular formula C5H6N2 and molecular mass: 94.1
Structural formula:
Physicochemical properties:
Fine white powder
At ambient temperature, fampridine is soluble in water, methanol, acetone, tetrahydrofuran, isopropanol, acetonitrile, N, N‐dimethylformamide, dimethylsulfoxide and ethanol.
Page 20 of 32
CLINICAL TRIALS
The efficacy of PrFAMPYRA™ (fampridine) sustained release tablets in improving walking in
patients with multiple sclerosis was evaluated in two adequate and well controlled trials
involving 540 patients (MS-F203 and MS-F204). Patients in these two clinical trials had a mean
disease duration of 13 years and a median Kurtzke Expanded Disability Status Scale (EDSS)
score of 6. Patient inclusion criteria included the ability to walk 25 feet in 8 to 45 seconds.
Patient exclusion criteria included a history of seizures or evidence of epileptiform activity on a
screening EEG, and onset of an MS exacerbation within 60 days.
MS-F203 was a randomized, placebo-controlled, parallel group, 21-week study (one week post screening, two-week, single-blind placebo run-in, 14-week double-blind treatment, and 4-week no treatment follow-up) in 301 patients with multiple sclerosis at 33 centers in the U.S. and Canada: 229 patients assigned to FAMPYRA 10 mg b.i.d. and 72 patients assigned to placebo. A total of 283 patients (212 FAMPYRA and 71 placebo) completed all study visits.
MS-F204 was a randomized, placebo-controlled, parallel group, 14-week study (one week post-screening, two weeks of single-blind, placebo run-in, nine weeks of double-blind treatment, and two weeks of no-treatment follow-up) in 239 patients with multiple sclerosis at 39 centers in the U.S. and Canada: 120 patients assigned to 10 mg twice daily and 119 assigned to placebo. A total of 227 patients (113 FAMPYRA and 114 placebo) completed all study visits.
The primary measure of efficacy in both trials was walking speed (in feet per second) as
measured by the Timed 25-foot Walk (T25W), using a responder analysis. A responder was
defined as a patient who showed faster walking speed for a least three visits out of a possible
four during the double-blind period than the maximum value achieved in the five non-double-
blind no treatment visits (four before the double-blind period and one after).
Study results
A significantly greater proportion of patients taking FAMPYRA 10 mg twice daily were responders, compared to patients taking placebo, as measured by the T25FW (MS-F203: 34.8% vs. 8.3%; MS-F204: 42.9% vs. 9.3%). The increased response rate in the FAMPYRA group was observed across all four major types of MS disease course.
During the double-blind treatment period, a significantly greater proportion of patients taking FAMPYRA 10 mg twice daily had increases in walking speed of at least 10%, 20%, or 30% from baseline, compared to placebo (Figure 1 and Figure 2).
Page 21 of 32
Figure 1:
Average walking speed change (%) from baseline during the double-blind
phase of MS-F203.
Page 22 of 32
Figure 2:
Average walking speed change (%) from baseline during the double-blind
phase of MS-F204.
Beneficial response to FAMPYRA was found to be independent of MS disease course (relapsing or progressive, including primary progressive), independent of concomitant treatment with the major immunomodulatory drugs approved for this condition, and similar in magnitude across the full range of baseline ambulatory deficits examined, including patients with Expanded Disability Status Scores (EDSS) from 2.5 to 7. No differences in efficacy based on degree of impairment, age, gender, or body mass index were detected. There were too few non-Caucasians in the patient population to evaluate the effect of race.
In both studies, consistent improvements in walking speed were shown to be associated with improvements on a patient self-assessment of ambulatory disability, the 12-item Multiple Sclerosis Walking Scale (MSWS-12), for both drug and placebo treated patients.
Page 23 of 32
DETAILED PHARMACOLOGY
Mechanism of Action
Fampridine (4-aminopyridine, 4-AP) blocks multiple potassium channels. When the axon is
demyelinated the internodal membrane and its ion channels become exposed to larger electrical
transients during the passage of an action potential. Leakage of ionic current through the
potassium channel, under these conditions, then contributes to impairment of action potential
conduction through the axon. In animal tissue preparations, 4-AP has been shown to increase
conduction of action potentials in demyelinated axons through inhibition of potassium channels.
Fampridine is thought to reduce the leakage of ionic current through these channels and enhance
action potential formation in demyelinated axons.
Safety Pharmacology
Fampridine was shown to inhibit hERG (human ether-à-go-go related gene) channel current in
vitro but only at very high concentrations, e.g. hERG IC50 equals 3.83 mM (360,000 ng/mL), a
value that is >15,000 times the clinical Cmax of 0.23 μM ( 22 ng/mL).
In Purkinje fibers isolated from dogs ex vivo, 4-AP significantly (P<0.05) prolonged action
potential duration of all three repolarization levels (APD30, APD50, APD90) only at the highest
concentration tested, 500 μM (47 μg/mL), which corresponds to >2,000 times the clinical Cmax of
0.23 μM ( 22 ng/mL).
In dogs in vivo, at fampridine doses that resulted in peak systemic exposure of 13–21 μM (1.25–
2.0 μg/mL), >50 times the clinical Cmax, no changes in ECG parameters were observed.
Non-Clinical Pharmacokinetics
Pharmacokinetic (PK) parameters of 4-AP have been determined in rats, guinea pigs and dogs
and toxicokinetic (TK) parameters of 4-AP have been determined in mice, rats, rabbits and dogs.
The primary routes of administration in rats and dogs were intravenous (i.v.) or oral (p.o.). In the guinea pig, the administration routes were i.v. and intramuscular (i.m.). When adjusted for differences in body surface area, the doses studied were similar to those used in humans. Of the nonclinical species tested, the ADME properties of 4-AP have been most comprehensively studied in rats. Overall, the absorption, distribution, metabolism and excretion of 4-AP are similar across all species examined including humans.
Following oral administration, 4-AP is rapidly absorbed with peak systemic exposure occurring within 1.5 hours. In rats, the absolute bioavailability is >50% and, in humans, it is 95%. With repeated doses, 4-AP does not accumulate systemically. Systemic exposure to 4-AP increases with increasing dose; although the increase is less than dose proportional.
The volume of distribution of 4-AP in rats, guinea pigs, dogs and in humans is high, exceeding body water. 4-AP does not bind appreciably to plasma proteins (< 25% bound in rats, dogs and humans). In rats, 4-AP distributes into most tissues including the brain.
4-AP is partially metabolized, more extensively in rats and dogs than in humans. 4-AP is metabolized primarily by hydroxylation, followed by sulfate conjugation, and in rats, approximately one-third of the dose is cleared by hepatic first-pass metabolism. In humans, the predominant cytochrome P450 (CYP) isozyme responsible for 3-hydroxylation is CYP2E1. In rats and dogs, the primary metabolites identified through radiolabeled studies are 3-hydroxy-4-
Page 24 of 32
aminopyridine (3-OH-4-AP) and 3-OH-4-AP sulfate. These are also the predominant metabolites in mice, rabbits and humans. By 24 hours post radiolabeled drug administration, only 1% of the dosed radioactivity is still present in rats. In radiolabeled studies in rats and dogs, between 75 and 92% of the dose is excreted in urine within the first 12 hours, approximately 40% of which is the unchanged parent compound. In rats and dogs, versus humans, the clearance rate is higher (>20 versus 9 mL/min/kg) and the elimination half-life (t1/2) is shorter (1–2 versus 4 hours). CYP inhibition studies in human microsomes demonstrated that 4-AP does not inhibit activity of the major metabolizing CYP isozymes. Studies with hepatocytes showed that 4-AP cultured human hepatocytes had little or no effect on CYP1A2, 2B6, 2C9, 2C19, 2E1 or 3A4/5 activity. The likelihood of 4-AP-mediated CYP dependent drug-drug interactions taking place through induction or inhibition of CYP activity in humans appears to be remote. 4-AP is renally excreted.
TOXICOLOGY
The preclinical safety of 4-AP was assessed in mice, rats, rabbits and dogs. The dosing regimen
greatly affected the rate of mortality and incidence of adverse clinical signs in all species studied.
In general, higher rates of mortality and adverse clinical signs were noted when 4-AP was
administered in a single large dose as compared to administration by multiple (two, three, or
four) equally divided sub-doses, or when administered through dietary admixture. This
suggested that peak plasma levels may be more important than total exposure when considering
toxicity of 4-AP.
Toxic responses to orally administered 4-AP were rapid in onset and included tremors, convulsions, ataxia, dyspnea, dilated pupils, prostration, abnormal vocalization, increased respiration, excess salivation, gait abnormalities, and hyper- and hypo-excitability. These clinical signs were not unexpected and represent exaggerated pharmacology of 4-AP. In single-dose studies in rats and in repeated-dose studies in dogs, gross necropsy findings observed in animals that died prematurely included discolorations of the kidney, lung and liver, thymus and spleen. In a 1 year repeated-dose study in dogs, these lesions were evaluated histologically, and were characterized as congestion and hemorrhage secondary to convulsion. In repeated-dose studies, no histological evidence of target organ toxicity was observed in either rats or dogs that survived to scheduled termination, aside from glandular dilation of the stomach in treated rats. Exposures associated with the no adverse effect levels (NOAEL) in these species were between 2- (rat, glandular dilation of the stomach) and 10-fold above those achieved in humans at the MRHD of 10 mg administered twice daily (b.i.d).
No evidence of carcinogenicity was observed in either of the 2-year bioassays conducted in mice and rats when administered via dietary admixture, at the maximally tolerated doses of 80 and 18 mg/kg/day, respectively. In mice receiving 80 mg/kg/day, mean plasma exposures in males were approximately 17-fold above the anticipated peak clinical exposure of 21.6 ng/mL at the MRHD of 10 mg b.i.d. Mean exposures in surviving females, euthanized during week 100 due to reduced survival at the 80 mg/kg/day dose level, were approximately 11-fold above the anticipated peak clinical exposure at the MRHD of 10 mg b.i.d.
Similar exposures were obtained during the 104-week carcinogenicity study in rats. Mean exposures in males at the 18 mg/kg/day dose level were approximately 17-fold above the peak exposure of 21.6 ng/mL at the MRHD of 10 mg b.i.d, and approximately 12-fold in females. A
Page 25 of 32
slight, non-dose-related increase in uterine polyps was observed in female rats at 18 mg/kg/day. Microscopically, an increased incidence of inflammation of the foot with secondary reaction in the regional lymph nodes and hypercellularity of the bone marrow was seen, particularly at 18 mg/kg/day.
4-Aminopyridine was not mutagenic in either the Ames bacterial mutagenicity test or in the L5178Y mouse lymphoma cell line, when tested in vitro. No clastogenic effects were observed either in vitro, when tested in Chinese Hamster Ovary (CHO) cells or in vivo, when tested in mice at oral doses of 9 mg/kg, or in Sprague Dawley rats at oral doses of 15 mg/kg.
No adverse effects were noted on fertility or copulatory indices in rats, and no treatment-related variations in estrous cyclicity, were attributed to 4-aminopyridine in surviving animals at doses of up to 9 mg/kg. There were no indications of developmental toxicity and no test article-related fetal malformations or developmental variations at any dosage level tested when pregnant dams were exposed to oral doses of up to 10 mg/kg/day (rat) or 5 mg/kg/day (rabbit) during the period of fetal organogenesis. Based upon data from bridging toxicokinetic studies, peak plasma exposures in pregnant rats and rabbits were greater than 23-fold above those achieved in humans at the MRHD of 10 mg b.i.d.
Effects on parturition and lactation, as evidenced from neonatal behavior, viability, growth and offspring (F1) reproductive performance, were evaluated in rats at doses of up to 6 mg/kg/day. Doses of 3 and 6 mg/kg/day were maternally toxic, as evidenced by reduced maternal food consumption and body weight during both gestation and lactation, and fewer live births were observed in pregnant dams in the 6 mg/kg dose group. Administration of 4-AP to offspring (F1) through lactation also resulted in fewer live pups per litter and reduced weight gain during and beyond lactation for animals in the 6 mg/kg dose group; however, no effects were observed at any dose level, with respect to behavior and development. Based upon data from a bridging toxicokinetic study, peak exposure levels in lactating dams were greater than 28-fold above those achieved in humans at the MRHD of 10 mg b.i.d.
Secretion of fampridine in milk has not been studied in animals.
Page 26 of 32
REFERENCES
1. Albrecht H,Wötzel C, Erasmus LP, Kleinpeter M, König N, Pöllmann W. Day-to-day
variability of maximum walking distance in MS patients can mislead to relevant changes in the Expanded Disability Status Scale (EDSS): average walking speed is a more constant parameter. Multiple Sclerosis 2001; 7: 105–109.
2. Cohen JA, Fischer JS, Bolibrush DM, Jak AJ, Kniker JE, et al. Intrarater and interrater
reliability of the MS functional composite outcome measure. Neurology 2000;54: 802—806.
3. Goodman AD, Brown TR, Krupp LB, Schapiro RT, Schwid SR, Cohen R, Marinucci LN,
Blight AR. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet 2009;373:732-738.
4. Goodman AD, Brown TR, Edwards KR, Krupp LB, Schapiro RT, Cohen R, Marinucci
LN, Blight AR. A Phase 3 Trial of Extended Release Oral Dalfampridine in Multiple Sclerosis. Ann Neurol 2010;68:494–502.
5. Graham RC, Hughes RAC. Clinimetric properties of a walking scale in peripheral
neuropathy. J. Neurol. Neurosurg. Psychiatry 2006;77: 977–979.
6. Heesen C, Böhm J, Reich C, Kasper J, Goebel M, et al. Patient perception of bodily
functions in multiple sclerosis: gait and visual function are the most valuable. Multiple Sclerosis 2008;14: 988–991.
7. Hobart JC, Riazi A, Lamping DL, Fitzpatrick R, Thompson AJ. Measuring the impact of
MS on walking ability: the 12-Item MS Walking Scale (MSWS-12). Neurology 2003:60: 31–36.
8. Hoogervorst ELJ, Kalkers NF, Cutter GR, Uitdehaag BMJ, Polman CH. The patient's
perception of a (reliable) change in the Multiple Sclerosis Functional Composite. Mult Scler 2004;10: 55–60.
9. Kragt JJ, van der Linden FAH, Nielsen JM, Uitdehaag BMJ, Polman CH. Clinical impact
of 20% worsening on Timed 25-foot Walk and 9-hole Peg Test in multiple sclerosis. Multiple Sclerosis 2006;12: 594–598.
10. McGuigan C, Hutchinson M. Confirming the validity and responsiveness of the Multiple
Sclerosis Walking Scale-12 (MSWS-12). Neurology 2004;62: 2103–2105.
11. Paltamaa J, Sarasoja T, Leskinen E, Wikström J, Mälkiä E. Measures of physical
functioning predict self-reported performance in self-care, mobility, and domestic life in ambulatory persons with multiple sclerosis. Arch Phys Med Rehabil. 2007;88:1649-1657.
12. Uges DRA, Sohn YJ, Greijdanus B, Scaf AH, Agoston S. 4-Aminopyridine kinetics. Clin
Pharmacol Ther 1982; 31(5):587–593.
Page 27 of 32
ABOUT THIS MEDICATION
PART III: CONSUMER INFORMATION
Fampridine sustained release tablets
What the medication is:
This leaflet is part III of a three‐part "Product
FAMPYRA may help adults (18 years and over) with
Monograph" published when FAMPYRA was
multiple sclerosis (MS) related walking disability to
approved for sale in Canada and is designed
specifically for Consumers. This leaflet is a
summary and will not tell you everything
FAMPYRA can be used alone or with other
about FAMPYRA. Contact your doctor or
medicines used to treat MS.
pharmacist if you have any questions about
the drug.
Multiple sclerosis is an autoimmune disease that affects the central nervous system (CNS). The CNS
Make sure you read and understand the
is made up of the brain, nerves and spinal cord. The
section, PROPER USE OF THIS
nerves carry electrical and chemical messages from
MEDICATION. Follow the instructions. Ask
your doctor or pharmacist to explain the
our brain to the rest of our body – giving us the
proper use of FAMPYRA if you do not
ability to think, speak and move. When nerves are
understand these instructions.
damaged by MS, the normal ability for these
Take only one 10 mg tablet of
messages to move along the nerves may be lost. This
FAMPYRA in the morning and one 10
leads to the neurological symptoms such as walking
mg tablet in the evening, 12 hours
apart.
difficulties, numbness, vision problems, and
Take the tablet whole and do not
divide, crush, dissolve, suck or chew
What it does:
the tablet.
You must always leave 12 hours
FAMPYRA contains the active substance fampridine,
between each tablet. Do not take two
which belongs to a group of medicines called
tablets at once to make up for a missed
potassium channel blockers. The way that
dose. Take your next tablet when you
would normally take it.
FAMPYRA works in MS patients is not fully
Taking more than one tablet at a time
understood. FAMPYRA is thought to work by
or more often than every 12 hours can
blocking potassium channels, which may help
increase the risk of having a serious
messages to pass down the damaged nerve.
side effect, such as a seizure.
If you have taken more FAMPYRA than
When it should not be used:
the prescribed dose, get emergency
medical help immediately by
Do not take FAMPYRA if you:
contacting your regional Poison
Have an allergy or are sensitive to fampridine or
Control center or by calling 911. Tell
any ingredients in this medicine (listed below).
them you are at risk of having a seizure
after taking too much FAMPYRA.
Are taking 4-aminopyridine (4-AP) compounded
by your pharmacist.
Have ever had a seizure (also referred to as a fit
Have kidney problems.
Page 28 of 32
Are taking any other medicine containing
You have kidney disease. FAMPYRA should
fampridine. This may increase your risk of
not be used by patients who have kidney
serious side effects.
Are taking medicines that will reduce the
You have heart rhythm or conduction problems.
elimination of FAMPYRA from your body,
You have a history of nerve pain in the face
which may increase your risk of serious side
(trigeminal neuralgia).
effects. Some of these medicines include cimetidine, and quinidine.
You are pregnant or planning to become
Are taking other medicines that are known to
increase the risk of seizures, such as bupropion,
You are breastfeeding or plan to breastfeed.
tramadol, tapentadol, or preparations used for
Tell your doctor if you have any other medical
colon cleansing.
FAMPYRA should not be used in children and
Before starting treatment and regularly during
adolescents under 18 years, because it has not been
treatment, your doctor should check that your
studied in MS patients younger than 18 years of age.
kidneys are working properly.
What the medicinal ingredient is:
Serious allergic reactions have been observed in
The active ingredient in FAMPYRA is called
patients treated with Fampyra. Signs of allergic
reaction may include rash, itching, difficulty breathing, swelling of the face, lips, tongue or
What the nonmedicinal ingredients are:
throat. In several cases, these reactions occurred
The non-medicinal ingredients of FAMPYRA tablets
after the first dose. Seek immediate emergency
are: hydroxypropyl methylcellulose USP,
assistance if you develop any of these signs or
microcrystalline cellulose USP, colloidal silicon
dioxide NF, magnesium stearate USP, hydroxypropyl
This medicine may make you feel dizzy or
methylcellulose/hypromellose USP, titanium dioxide
unsteady and this may increase the risk of falling.
USP, and macrogol/PEG 400 NF.
If you use a walking aid, such as a cane, you should
What dosage forms it comes in:
continue to use it as needed.
FAMPYRA comes as a 10 mg sustained release
Risk of Seizure
It is important that you take only one 10 mg tablet of
WARNINGS AND PRECAUTIONS
FAMPYRA in the morning and one 10 mg tablet in the evening, 12 hours apart. Do not divide, crush,
BEFORE you use FAMPYRA talk to your doctor or
dissolve, suck or chew the tablet. Taking more than
one tablet at a time or more often than every 12
You have ever had a seizure. FAMPYRA
hours, or taking a broken tablet can increase the risk
should not be used by patients who have had
of having a serious side effect, such as a seizure.
seizures. FAMPYRA can increase the risk of
If you have taken more FAMPYRA than the
prescribed dose, get emergency medical help
Ask your doctor if you have any factors or if you
immediately by contacting your regional
are taking any medicines that affect your risk of
Poison Control center or by calling 911. Tell
them you are at risk of having a seizure after taking too much FAMPYRA.
Page 29 of 32
If you think you missed a dose, do not take
more often than every 12 hours. The tablets are to
two tablets at once to make up for a missed
be taken without food.
dose. Take your next tablet when you would
Swallow each tablet whole, with a drink of water. If
normally take it. You must always leave 12
you cannot swallow FAMPYRA tablets whole, tell
hours between each tablet.
INTERACTIONS WITH THIS MEDICATION
Do not divide, crush, dissolve, suck or chew the tablet. A broken tablet can release too much of the
Tell your doctor about all of the medicines you take
drug at one time. This can increase your risk of
now or have taken recently, including prescription
having a seizure.
and non-prescription medicines. This includes any vitamin or mineral supplement, or herbal products.
Your doctor should assess your walking ability before you start FAMPYRA and again within the
Do not start any new medicines until you talk to your
first 4 weeks of treatment. If you and your doctor
doctor who prescribed FAMPYRA.
decide there has not been benefit to you in this
4-aminopyridine and other medicines containing
period, treatment should be stopped.
fampridine: Do not take FAMPYRA if you are
If the decision is to continue treatment, it is important
taking 4-aminopyridine (4-AP, fampridine)
that you and your doctor continue to periodically
compounded by your pharmacist. These
reassess whether you are experiencing benefit, and to
medicines contain the same active ingredient as
stop taking the drug if you are not.
FAMPYRA and should be discontinued before starting FAMPYRA, to reduce the risk of serious
Take only the dose your doctor has prescribed you.
Medicines that affect the kidneys: Your doctor
Do not change your dose of FAMPYRA. If you take
will be especially careful if FAMPYRA is given
more than your prescribed dose, there is a risk of
at the same time as any medicine that may affect
your kidney function. Tell your doctor if you are taking medicines such as beta blockers
If you have taken more FAMPYRA than your
(carvedilol, pindolol, propranolol),
doctor has prescribed, seek emergency help
procainamide, metformin, ranitidine, and
immediately by contacting your regional Poison
Control Centre or by calling 911, even if there are
Some medicines that affect kidney function should
no symptoms. Tell them you are at risk of having a
not be taken with FAMPYRA. Examples of these
seizure after taking too much FAMPYRA. Take the
medicines include cimetidine and quinidine.
medication package with you if you go to the hospital.
PROPER USE OF THIS MEDICATION
Always follow your doctor's instructions for taking FAMPYRA. You should check with your doctor or
pharmacist if you are not sure. Do not take more
If you forget to take a tablet, do not take two tablets
than the prescribed dose.
at once to make up for a missed dose. You must
always leave 12 hours between each tablet.
Take one 10 mg tablet of FAMPYRA in the morning
Taking more than your prescribed dose can
and one tablet in the evening. You must leave 12
increase the risk of serious side effects.
hours between each tablet. Do not take a tablet
Page 30 of 32
SIDE EFFECTS AND WHAT TO DO ABOUT
SERIOUS SIDE EFFECTS, HOW OFTEN THEY
HAPPEN AND WHAT TO DO ABOUT THEM
Like all medicines, FAMPYRA can have side effects.
Symptom / effect
Talk with your Stop taking
If you have any worrying side effects including any
that are not included here, contact your doctor or
Seizures: Some patients have had seizures while
taking FAMPYRA, including patients who have
never had seizures before. If you have a seizure
while taking FAMPYRA get emergency help right
away and do not take any more FAMPYRA.
Serious allergic reactions have been observed in
patients treated with Fampyra Signs of allergic reaction may include rash, itching, difficulty
breathing, swelling of the face, lips, tongue or
loss of consciousnes
throat. In several cases, these reactions occurred
after the first dose. Seek immediate emergency
assistance if you develop any of these signs or
1 and 10 in every
FAMPYRA can cause side effects. Please review
reaction (symptoms
the information in the table carefully.
Very common side effects (affect more than 1 in 10
difficulty breathing,
Urinary tract infection
swelling of the face, lips,
Common side effects (affect between 1 and 10 in
every 100 patients).
Feeling unsteady, dizziness, headache, feeling
This is not a complete list of side effects. For any
weak and tired, difficulty sleeping, anxiety,
unexpected effects while taking FAMPYRA, contact
tremor (minor shaking), numbness or tingling of
your doctor or pharmacist.
the skin, sore throat, shortness of breath, feeling sick (nausea), being sick (vomiting),
HOW TO STORE IT
constipation, upset stomach, back pain.
Store FAMPYRA at room temperature (between 15 to 30° C). Store the tablets in the original bottle to
Uncommon side effects (affect between 1 and 10 in
protect them from light and moisture. Each bottle
every 1000 patients).
contains 14 tablets and a silica gel desiccant. Do not
Worsening of nerve pain in the face (trigeminal
take your medicine after the expiry date shown on the
bottle or carton.
If any of these side effects affects you severely, tell
Keep out of reach and sight of children.
your doctor right away.
Page 31 of 32
Medicines should not be disposed of in waste water
Ireland Limited (APIL). FAMPYRA™ is a
or household garbage. Ask your pharmacist how to
trademark of Acorda Therapeutics, Inc.
dispose of medicines you no longer need.
This document plus the full Product Monograph,
REPORTING SUSPECTED SIDE EFFECTS
prepared for health professionals can be obtained by contacting Biogen Idec Canada Inc. at:
You can report any suspected adverse reactions
associated with the use of health products to the
Canada Vigilance Program by one of the following
This leaflet was prepared by Biogen Idec Canada Inc.
Last revised: November 26, 2014
Report online at
www.healthcanada.gc.ca/medeffect
Call toll-free at 1-866-234-2345
Complete a Canada Vigilance Reporting Form and:
‐ Fax toll‐free to 1‐866‐678‐6789, or
‐ Mail to: Canada Vigilance Program
Health Canada
Postal Locator 0701D
Ottawa, Ontario
Postage paid labels, Canada Vigilance
Reporting Form and the adverse reaction
reporting guidelines are available on the
MedEffect™ Canada Web site at
www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to the
management of side effects, contact your health
professional. The Canada Vigilance Program does
not provide medical advice.
MORE INFORMATION
FAMPYRA™ is marketed under license from Acorda Therapeutics, Inc. and is manufactured for Acorda under license from Alkermes Pharma Ireland Limited (APIL), utilizing APIL's MatriX Drug Absorption System (MXDAS™) technology. MXDAS™ is a trademark of Alkermes Pharma
Page 32 of 32
Source: https://www.biogen.ca/content/dam/corporate/en_CA/pdfs/products/FAMPRYA/28November2014-Fampyra-PM-E.pdf
HIGHLIGHTS OF PRESCRIBING INFORMATION ---------------------------DOSAGE FORMS AND STRENGTHS------------------ These highlights do not include all the information needed to use VIVELLE-DOT Transdermal system: 0.025 mg/day, 0.0375 mg/day, 0.05 mg/day, 0.075 safely and effectively. See full prescribing information for VIVELLE-DOT.
Journal of Cardiac Failure Vol. 14 No. 6 2008 Pioglitazone and Heart Failure: Results From a Controlled Study in Patients With Type 2 Diabetes Mellitus and Systolic Dysfunction THOMAS D. GILES, ALAN B. MILLER, MD,URI ELKAYAM, MONDIRA BHATTACHARYA, MD,AND ALFONSO PEREZ, New Orleans, Louisiana; Jacksonville, Florida; Los Angeles, California; Deerfield, Illinois Background: Thiazolidinediones are associated with fluid retention, often interpreted as worsening car-diac function, limiting their use in patients with heart failure (HF). We compared the effects of pioglita-zone and glyburide on cardiac function in patients with type 2 diabetes, systolic dysfunction, and NewYork Heart Association (NYHA) functional Class II/III HF.Methods and Results: Participants received pioglitazone or glyburide (6insulin) for 6 months in thisdouble-blind, randomized, multicenter study. The primary end point was time to HF, a composite ofcardiovascular mortality and hospitalization or emergency room (ER) visit for HF. Secondary endpointsincluded echocardiographic and functional classification assessments. An earlier time to onset and higherincidence of the primary endpoint was noted with pioglitazone (13%) versus glyburide (8%) (P 5 .024).Hospitalization or ER visit occurred in 30 pioglitazone and 15 glyburide participants, 19 and 12 of whom,respectively, continued treatment. Cardiac mortality (5 versus 6 participants, respectively) and cardiacfunction, as measured by change in ventricular mass index (P 5 .959), ejection fraction (P 5 .413), orfractional shortening (P 5 .280), were similar between treatments.Conclusions: Pioglitazone was associated with a higher incidence of hospitalization for HF without anincrease in cardiovascular mortality or worsening cardiac function (by echocardiography). (J CardiacFail 2008;14:445e452)Key Words: Cardiovascular disease, thiazolidinediones, left ventricular dysfunction.