
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Effect of volcanic eruption on nutrients, light, and phytoplankton in oligotrophic lakes
Limnol. Oceanogr., 58(4), 2013, 1165–1175E 2013, by the Association for the Sciences of Limnology and Oceanography, Inc.
Effect of volcanic eruption on nutrients, light, and phytoplankton in oligotrophic lakes
Beatriz E. Modenutti,1 Esteban G. Balseiro,1,* James J. Elser,2 Marcela Bastidas Navarro,1Florencia Cuassolo,1 Cecilia Laspoumaderes,1 Maria S. Souza,1 and Vero´nica Dı´az Villanueva 1
1 Laboratorio de Limnologı´a, Instituto de Investigaciones en Biodiversidad y Medioambiente (INIBIOMA), Consejo Nacional de
Investigaciones Cientı´ficas y Te´cnicas–Universidad Nacional del Comahue, Bariloche, Argentina
2 School of Life Sciences, Arizona State University, Tempe, Arizona
Volcanic eruptions that shape the earth's surface can have major effect on ecosystems and, as natural
experiments, can yield insights into ecological dynamics. On 04 June 2011, a mega-eruption in the Puyehuevolcanic complex (Chile) discharged massive amounts of ash and pumice. Using long-term data from five NorthAndean Patagonian lakes (Espejo, Correntoso, Nahuel Huapi, Gutie´rrez, and Mascardi) that received differinglevels of ash, we show that, in Lakes Espejo, Correntoso, and Nahuel Huapi, these inputs resulted in 1.5- to 8-foldincreases in total suspended solids, light extinction, phosphorus concentrations, and phytoplankton biomassrelative to pre-eruption conditions. Although ashes affected light scattering, the ultraviolet : photosyntheticallyactive radiation ratio remained , 0.30–0.35 in all the lakes and no changes were seen in dissolved organic carbonin the affected lakes post-eruption. Thus, no differential specific absorption of the different light wavelengthsoccurred due to ash input. The results of multiple regression analysis identified light extinction coefficient of PAR(KPAR) as the primary variable that was associated with variation in phytoplankton biomass (chlorophyll).
Furthermore, incubation experiments demonstrated significant effects of photoinhibition on phytoplanktongrowth in these lakes at ambient pre-eruption light intensities. Thus, we infer that increased phytoplanktonbiomass following the eruption likely reflects nutrient (phosphorus) loading and attenuation of excessive lightintensities.
Volcanic eruptions have shaped much of Earth's surface
light intensities in the upper levels of the water column are
over geological time, but they also, in the shorter term,
known to reduce phytoplankton growth because of
affect ecosystems at local, regional, and even global scales
photoinhibition (Alderkamp et al. 2010; Gerla et al.
due to ejection and emission of gases, ashes, pumice, and
2011), consistent with the possibility that, in highly
lava. Thus, eruptions present unique opportunities for
transparent systems at least, increased light attenuation
scientific discovery though such studies are often hindered
by abiotic particles such as ash may positively affect
by a lack of pre-eruption and post-eruption data that allow
phytoplankton growth by reducing photoinhibition.
comprehensive assessment of their effects and the mecha-
Despite widespread recognition that pelagic ecosystem
nisms of those outcomes (Lindenmayer et al. 2010; Larson
function reflects the joint effects of dynamic light and
2011). Past studies of the effect of eruptions on aquatic
nutrient supplies modulated by water column physical
ecosystems have emphasized fertilization by ash-borne
structure and internal food web interactions (Sterner et al.
elements such as phosphorus and iron (Hamme et al.
1997; Falkowski and Raven 2007), the effect of light has
2010; Lin et al. 2011). Studies in marine environments have
been largely neglected in studies regarding volcanic
shown that, after volcanic eruptions, the concentrations of
eruption. The 04 June 2011 explosion of Puyehue-Cordo´n
chlorophyll, as a proxy of phytoplankton biomass, increase
Caulle (40u359S, 72u079W) in southern Chile (Fig. 1A)
(Hamme et al. 2010; Lin et al. 2011). Paleolimnological
provided a unique chance for assessing such dimensions, as
evidence from a lake in Iceland also shows that, after a
the event deposited massive amounts of ash into a set of
volcanic eruption that deposited considerable amounts of
nearby lakes in Argentine Patagonia that has been
tephra, there was an increase in chlorophyll-derived
extensively studied for 17 yr (Morris et al. 1995; Callieri
pigments in sediments, indicating an increase in phyto-
et al. 2007; Corno et al. 2009). These temperate Andean
plankton biomass following volcanic ash deposition
lakes (located in North-Patagonia around 41uS) are
(Einarsson et al. 1993). However, increased concentrations
characterized by high transparency and high ultraviolet
of suspended particles in the water column, such as
radiation (UVR) penetration (Morris et al. 1995), where
volcano-derived ashes, also increase light scattering and
planktonic organisms living in surface waters are chroni-
so decrease light penetration (Kirk 1994). In many
cally exposed to high light intensity and irradiation at
situations such shading would be expected to negatively
damaging wavelengths (Modenutti et al. 2004, 2005). Such
affect phytoplankton growth by reducing photosynthesis to
an eruption presents not only an opportunity to evaluate
levels where it does not exceed respiration (Huisman 1999;
how volcanic eruptions affect lakes but also serves as a
Huisman et al. 2002). However, in extremely transparent
‘‘natural experiment'' to test the roles of nutrient and light
oligotrophic and ultraoligotrophic aquatic systems, high
in the ecological functioning of large pelagic ecosystemsthat cannot otherwise be experimentally manipulated. To
* Corresponding author:
[email protected]
assess these effects, we documented optical, chemical, and
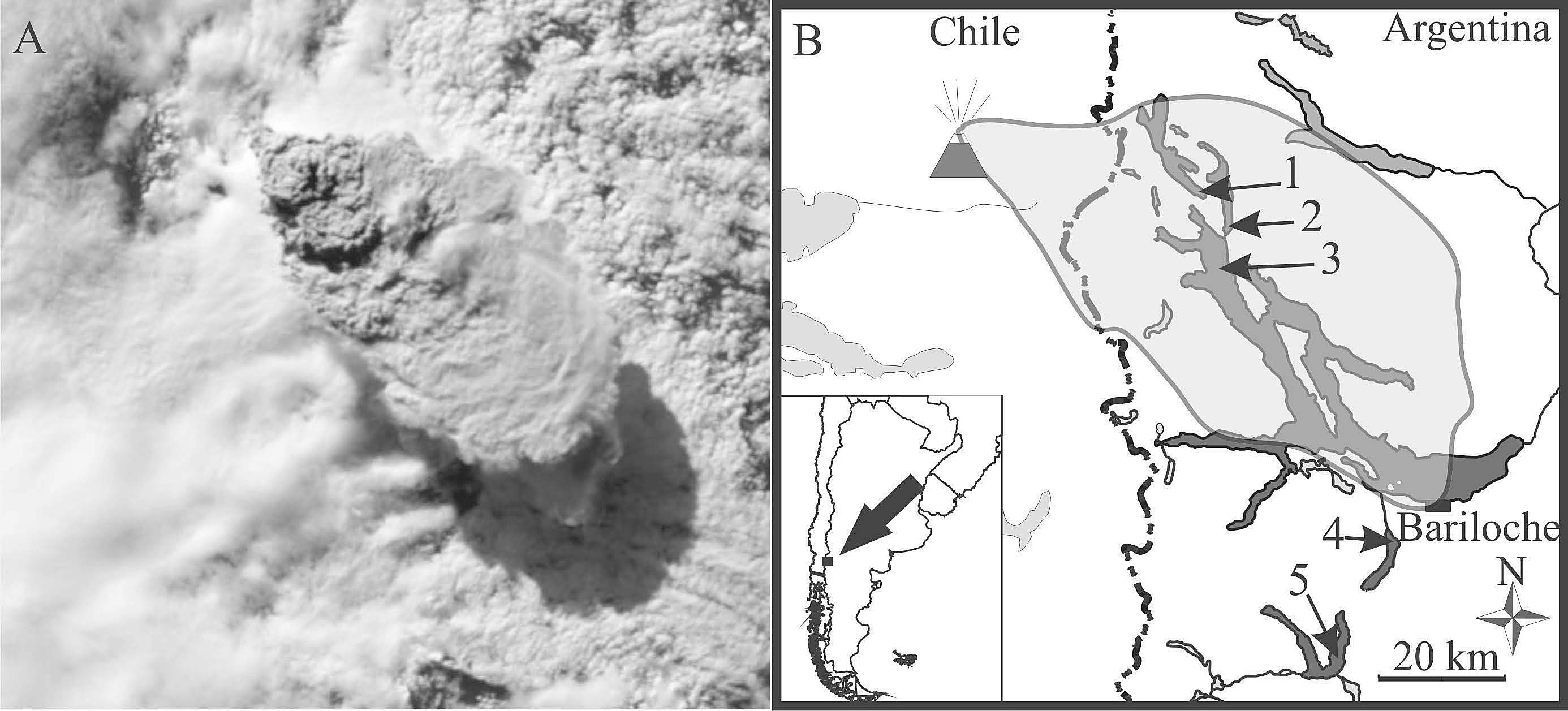
Modenutti et al.
(A) Satellite view of the eruption in the Puyehue-Cordo´n Caulle volcanic complex at
18:45 h Universal Time Coordinated (UTC) on 04 June 2011 (Source: http://earthobservatory.
nasa.gov). (B) The five main study lakes in relation to the eruption site; the silhouette indicatesthe cloud seen in (A). 1 5 Espejo; 2 5 Correntoso; 3 5 Nahuel Huapi (NW sampling siteindicated); 4 5 Gutie´rrez; 5 5 Mascardi.
biological properties in five lakes receiving different levels
water column using a PUV500B submersible radiometer
of ash input and compared these to values from pre-
(Biospherical InstrumentsTM). The profiler was lowered
eruption data. To test for the mechanisms involved, we also
at 0.2 m s21 and, at this descent rate, the temperature
performed incubation experiments for two lakes, one
resolution was . 0.1uC. The light profile included
strongly affected by the eruption (Lake Espejo) and one
ultraviolet (UV) bands (305, 320, 340, and 380 nm) as well
relatively unaffected (Lake Mascardi), assessing the relative
as photosynthetically active radiation (PAR; 400–700 nm).
effects of overall light intensity, light quality (e.g., UVR),
Water samples were taken using a closing sampler at 0, 10,
and nutrients on phytoplankton growth rate.
20, 30, and 45 m depth in the water column. Water sampleswere carried to the laboratory in thermally isolated
containers within 3 h after sampling, and processedimmediately after arrival to the laboratory. A volume of
Lake sampling—The field study comparing pre- and
200 mL was immediately fixed with acid Lugol's solution
post-eruption data was carried out in five lakes: Espejo,
for phytoplankton enumeration. A volume of 50 mL of
Correntoso, Nahuel Huapi, Gutie´rrez, and Mascardi
lake water was collected for enumeration of autotrophic
(Fig. 1B; Table 1), which are part of the glacial lakes
picoplankton in sterile tubes and fixed with 0.2 mm filtered
district of the North-Patagonian Andes. Lakes Espejo and
formaldehyde buffered with 0.1 mol L21 sodium cacodylate
Correntoso are closer to the eruption and were subjected to
(final concentration 2% vol : vol), stored in darkness at 4uC,
considerable ashfall during the initial explosion and via
and processed within 2 weeks (Callieri and Stockner 2002).
subsequent runoff (Fig. 1). Lake Nahuel Huapi is a large,
For chlorophyll determinations, a volume of 200 mL from
morphologically complex lake whose northwestern arm
each sampling depth was filtered onto Whatman GF/F
(hereafter: NW Nahuel Huapi) received direct surface
filters; in a more restricted set of samples, we also filtered
inputs of ash as well as inputs from inflow streams (Fig. 1).
onto 0.2 mm pore-size polycarbonate filters (Nuclepore).
Lakes Gutie´rrez and Mascardi received little ash and allow
All filters were frozen until extraction.
us to assess variability in the absence of eruption (Fig. 1).
Throughout the post-eruption study period, lakes were
Laboratory determinations—The readily available P
sampled at a standard deep-water station at approximately
content of ashes freshly collected at Bariloche during the
2–3 week intervals during the austral growing season
initial ash fall was measured by suspending 1 g of ashes in
(October 2011–March 2012). Vertical temperature and light
50 mL of MilliQTM water for 1 h and then evaluating
profile measurements were taken in the upper 50 m of the
soluble reactive phosphorus by the molybdate reaction
Location and morphometric characteristics of the studied North-Patagonian Andean lakes. Abbreviations: masl, meters
above sea level; Zmax, maximum depth of the lake.
Effect of volcanic eruption in lakes
(APHA 2005). For each sampling depth in routine
with epilimnetic lake water that was diluted 80% with
sampling, measurements of total phosphorus (TP; unfil-
filtered (GF/F glass-fiber filter) lake water to minimize
tered lake water) and total dissolved phosphorus (TDP;
grazing and allow for growth in the unenriched treatment.
Whatman GF/F–filtered lake water) concentrations were
During the experiment, temperature was maintained at ,
performed using the molybdate reaction after persulfate
18 6 1uC (similar to surface lake temperatures) and
digestion of the sample. Dissolved organic carbon (DOC)
incident solar irradiance was monitored with a photo-
was determined on filtered lake water (pre-combusted GF/
radiometer. Experiments were run for 72 h. Chl a was
F Whatman filters) using a ShimadzuTM total organic
measured at the start and end of the experiment.
carbon analyzer.
The Lake Mascardi experiment was completed from 25
Chlorophyll a (Chl a) was extracted in hot ethanol
to 28 January 2012, whereas the experiment for Lake
following Nusch (1980) and determined by fluorometric
Espejo took place from 02 to 05 February 2012. During this
analysis (Turner DesignsTM 10AU fluorometer) with acid
period the skies were generally cloudless; maximum daily
correction. Total suspended solids (TSS) were estimated by
incident irradiance for PAR was 2900 mmol photons
filtering 1 liter of lake water onto pre-weighed GF/F filters
m22 s21, whereas the daily irradiance dose was
that were then dried at 60uC for 48 h and reweighed. The
92 mol m22 for the 14 h day length. Maximum incident
two filter types (GF/F and 0.2 mm Nucleopore) gave very
intensities for UVR at 320 and 340 nm were 44 and
similar values in Chl a (no significant differences). Finally,
74 mW cm22 nm21, respectively, while their respective daily
filters for seston C analysis were prepared by filtering lake
doses were 11 kJ m22 and 20 kJ m22.
water onto pre-combusted Whatman GF/F glass-fiberfilters; these were dried and held in a desiccator until later
Calculations—For comparing pre- and post-eruption
analysis on a Thermo Finnigan EA1112 elemental analyzer.
data for TP and Chl a, the multiple depths in the water
Autotrophic picoplankton, mainly picocyanobacteria,
column were averaged. Note that because our sampling
were counted on black polycarbonate filters (Poretics,
depths are approximately evenly spaced across the upper
0.2 mm pore size) by autofluorescence of phycoerythrin
water layers and encompassed the deep chlorophyll
using an Olympus BH 50 epifluorescence microscope fitted
maximum, average chlorophyll concentration (per unit
with blue excitation cube (U-MWB) and green excitation
volume) is essentially equivalent to integrated chlorophyll
cube (U-MWG) light filters. Cells were counted using an
concentration from 0–45 m (per unit area). Long-term data
image analysis system (Image ProPlus; Media Cybernetics).
for all parameters were then analyzed by calculating an
Phytoplankton were enumerated to genus and/or species
overall, pre-eruption, average for each parameter for each
level using 50 mL settling chambers with an inverted
4 week interval during the October–February period. All
microscope. The four dominant phytoplankton types
individual data points were then normalized to that average
enumerated and analyzed reflect the species typically found
(by dividing) for each 4 week data bin; thus, a value in an
in Andean lakes over the past four decades (Thomasson
interval that does not differ from the overall pre-eruption
1963; Callieri et al. 2007). These are Chrysochromulina
dynamics would have a value of 1. We then calculated
parva (Haptophyceae), Rhodomonas lacustris (Cryptophy-
averages and 95% confidence intervals for these normal-
ceae), Aulacoseira granulata and Cyclotella stelligera
ized, pre-eruption data for each data bin. To plot the data
(Bacillariophyceae), and Gymnodimium paradoxum and
on a common graph for more than one lake (e.g., for the
Gymnodimium varians (Dinophyceae). The presence of
two unaffected lakes, Mascardi and Gutie´rrez), we plotted
Tabellaria flocculosa in the post-eruption samples was
the maximum confidence limits for each 4 week data
confirmed by microscopic analysis (Koppen 1975).
period. We normalized each post-eruption data point to itspre-eruption mean for the appropriate temporal bin and
Incubation experiments—To test the dependence of
plotted those data along with the normalized pre-eruption
phytoplankton growth rate on nutrients and light in both
confidence intervals; statistically significant deviations
Lake Mascardi (unaffected by ash inputs) and Lake Espejo
from historical dynamics were assessed by determining if
(affected by ash), we ran two outdoor incubation experi-
post-eruption values were outside of these 95% confidence
ments. A full 2 (unenriched, enriched) 3 2 (full solar
bands. The dependence of Chl a on light and TP
radiation [FSR]; PAR) 3 5 (100%, 75%, 33%, 11%, and
concentration was assessed with forward and backward
3% of solar radiation) factorial design (five replicates per
treatment) was carried out in 500 mL polypropylene bags
In addition, we analyzed light transparency and phyto-
arranged in a 1 m3 water bath. Nutrients were enriched
plankton composition of the pre- and post-eruption data
using the freshwater culture medium COMBO (Kilham et
set for the lake thermal stratified period (December–
al. 1998) to increase P by 15 mg L21; note that COMBO
February). Light attenuation coefficients (K) were estimat-
contains N (as NO3; N : P 5 44 by mass) as well as a mix of
ed from the vertical light profiles as the slope of loge-
trace metals. Light was manipulated in two different ways,
transformed irradiance data with depth. To evaluate
quality and quantity. The quality, or PAR treatment, was
possible UV wavelength–specific changes relative to PAR
produced using cut-off filters to remove wavelengths
absorption due to suspended ashes, we also analyzed the
shorter than 400 nm (CourtgardTM, CPFilms; Doyle et al.
ratio of the 1% depth of 320 nm UV relative to the 1%
2005). Light quantity was manipulated by reducing
depth of PAR following Rose et al. (2009).
irradiance with increasing layers of shade screens to achieve
For calculation of the mean light intensity in the mixed
the five levels of incident irradiance. Each bag was filled
layer (Im; Sterner et al. 1997) for all sampling dates, we used
Modenutti et al.
2011 of , 14 mg L21 in Lakes Espejo, Correntoso, andNW Nahuel Huapi, relative to values typically closer to ,0.5 mg L21 (maximum: , 1 mg L21) in these lakes beforeeruption and in Lakes Gutie´rrez and Mascardi both beforeand after the eruption. During summer 2011–2012, TSSlevels in the affected lakes (Espejo, Correntoso, and NahuelHuapi) were 2–8 times higher than typical pre-eruptionlevels (Fig. 2). Ash inputs had major effects on variousother limnological variables in the affected lakes (Fig. 3).
Prior to eruption, the five lakes were very transparent, withlight extinction coefficients (KPAR) generally 0.10–0.15 m21(Table 2); however, post-eruption data showed that KPARincreased 1.5- to 2.5-fold after the eruption in LakesEspejo, Correntoso, and Nahuel Huapi, while remaining atthe same historical levels in Mascardi and Gutie´rrez(Fig. 3A).
In two of the affected lakes (Lakes Espejo and
Post-eruption dynamics of TSS in the affected lakes,
Correntoso), TP concentrations increased up to . 3-fold
relative to typical pre-eruption values that never exceeded 1 mg L21
post-eruption (Table 2; Fig. 3B; increasing from values of
for a 15-yr record (horizontal line).
, 2.0–3.4 mg P L21 to , 4.2–8.4 mg P L21), a result thatlikely reflects direct contributions of suspended ash, as
light extinction coefficients (K
chemical analysis of fresh ash collected during the 04 June
PAR) obtained as described
above along with estimates of the depth of the mixing layer
2011 event indicated an available P content of 0.009% by
mass. There was no such increase in TP concentrations in
m) from the vertical temperature profile obtained at the
Lake Nahuel Huapi after the eruption (Table 2; Fig. 3B;
m was determined as the depth above the
temperature discontinuity (temperature difference . 1uC
from 3.9–4.5 mg P L21 to 5.6 mg P L21). The unaffected
m21) identified by direct inspection of the continuous
Lakes Gutie´rrez and Mascardi retained typical TP concen-
vertical temperature profiles obtained with the PUV
trations of 1.6–4.3 mg P L21 (Table 2; Fig. 3B). TDP
radiometer. Since the lakes were generally sampled on
showed a similar pattern to that of TP, including an
calm days in this windy region, small thermal gradients
absence of nutriclines in the vertical profiles. In two of the
(, 1uC) due to temporary diurnal microstratification were
affected lakes (Espejo and Correntoso), TDP increased
ignored in estimating Z
from 1.1–2.3 mg P L21 before the eruption to , 4.0–4.5 mg
m on a given day; note that most of
our sampling was relatively early in the morning so that
P L21 after the eruption, whereas TDP in Lake Nahuel
such temporary stratification events were uncommon in the
Huapi did not change appreciably (2.0–2.8 mg P L21 before
to 3.5 mg P L21 after). The unaffected Lakes Gutie´rrez and
Mascardi always had very low values of TDP (1.0–2.4 mg
m was then estimated as in previous work (Sterner et al.
Phytoplankton biomass as indicated by Chl a increased
strongly after the eruption in Lakes Espejo, Correntoso,
and NW Nahuel Huapi, with Chl a as much as four times
higher (Fig. 3C; Table 2), increasing from concentrations
Note that calculation of Im yields a value for the average
typically , 0.6 mg L21 for Correntoso and Espejo and
light experienced across the entire mixed layer as a fraction
, 0.9 mg L21 for NW Nahuel Huapi. However, Chl a in the
of surface irradiance; this Im varies from 0 to 1.
unaffected lakes (Gutie´rrez, Mascardi) did not deviate
The incubation experiment data were analyzed as growth
from pre-eruption dynamics, maintaining concentrations
rates based on Chl a data. Growth rate (GR) for each
, 0.85 mg L21 (Fig. 3C; Table 2).
replicate for the 3-d incubation was calculated as:
To assess the relative contributions of light attenuation
and nutrient loading to observed increases in lake
phytoplankton biomass, we performed stepwise multiple
regression. Both forward and backward algorithms relating
where Chl ai and Chl af are the initial and final
Chl a to KPAR and TP for all data from the five lakes
concentrations of Chl a. GR data were then analyzed with
indicated that TP was eliminated and only KPAR remained
a three-way factorial analysis of variance (ANOVA).
in the final model (r2 5 0.41) with a highly significant (p ,
Normality and homoscedasticity were confirmed prior to
0.001) and positive association with Chl a. The same
the ANOVA; the data did not require transformation.
overall result was obtained when the analysis was confinedto only the affected lakes following the eruption, during
which time KPAR would be dominated by light extinctioncontributed by ash particles. In addition, a similar result
In situ dynamics—Immediately after the eruption,
was obtained if TDP rather than TP was used in the
concentrations of TSS increased, reaching levels in July
multiple regression analysis; only KPAR was retained.
Effect of volcanic eruption in lakes
Post-eruption changes in (A) optical (light extinction coefficient [KPAR]), (B)
chemical (total phosphorus concentration [TP]), and (C) biological (phytoplankton biomass asindexed by chlorophyll a concentration [Chl a]) properties in the lakes, normalized to pre-eruption values from , 17 yr of monitoring (data in Table 2). Thus, a value of 1 indicates nochange relative to the corresponding pre-eruption interval. Dotted lines indicate 95% confidencelimits for each month's pre-eruption data.
More detailed analysis of vertical profiles shows that
increased only , 1.5-fold during the early part of the
light penetration changed up to 2.7-fold in the affected
growing season in the post-eruption period in this lake
lakes, particularly in Lakes Espejo and Correntoso (Fig. 4).
(Fig. 3A). In addition, we note that Lake Nahuel Huapi
As an average for the summer stratification period, in Lake
has a considerably deeper thermocline compared with the
Espejo the depth of 1% light penetration (Z1%) for PAR
other two affected lakes; this causes a different physical
decreased from , 40 m to 20 m (Fig. 4A,B) and in Lake
structure in which the euphotic zone is coincident with or
Correntoso from 42 m to 25 m (Fig. 4C,D). Because
near the upper limit of the mixolimnion (Fig. 4E,F).
thermal structure of the lakes did not change after the
The ratio between UV wavelength–specific changes
eruption, these increases in light attenuation also caused
relative to PAR showed that the ash inputs affected all
decreases in the mean irradiance of the mixing layer (Im),
wavelengths equally and thus no shift was found before and
from . 60% of surface irradiance in Espejo and
after the eruption, with the UV : PAR ratio remaining ,
Correntoso before the eruption to , 30% afterwards.
0.30–0.35 in all the lakes. This result likely reflects the lack
Notably, average Im in Lake Nahuel Huapi over the
of change in DOC in the affected lakes post-eruption; pre-
summer thermal stratification period did not decrease to
eruption DOC concentration did not exceed 0.6 mg L21
the same degree as in the other two lakes because mean Kd
(Morris et al. 1995) and these values were maintained after
Pre-eruption mean values of chlorophyll (Chl a; mg L21), total dissolved phosphorus (TP; mg L21), and light extinction
coefficient (KPAR; m21) for each month for each lake, used for standardization in Fig. 3. Data are from 1994–2010.
Modenutti et al.
Box plot of the chlorophyll a : sestonic C (Chl a : C)
ratio (mg : mg) for the study lakes during 2011–2012 samplingperiod. Box limits indicate 25th and 75th percentiles; horizontalline in the box represents the median.
average length 5 3.78 6 0.5 mm) with increased abundanc-es of both towards 45 m depth (Fig. 6A,B; see pre-eruptiongraphs). However, examination of post-eruption samplesindicated that, whereas picocyanobacteria remained thedominant component of the phytoplankton (Fig. 6B; seepost-eruption graphs), there was a noticeable change in thestructure of the phytoplankton community (Fig. 6A; seepost-eruption graphs). In Lakes Correntoso and Espejo weobserved an increase in the abundance of Cryptophyceae,in particular of Rhodomonas lacustris (cell average length 58.7 6 0.7 mm). In Lake Nahuel Huapi we noted a decreaseof C. parva with a concomitant increase in the abundanceof R. lacustris and diatoms (Tabellaria flocculosa andAulacoseira granulata; Fig. 6A; see post-eruption graphs).
Depth profiles for mean percent irradiance for 305,
After the volcanic event we observed that the increase in
320, 340, and 380 nm UV, and PAR and temperature for (A,C,E)
phytoplankton biomass occurred both in the deep chloro-
pre-eruption and (B,D,F) post-eruption periods. Pre-eruption
phyll maxima (DCM) and in the mixing layer, and that the
data are average of 2000–2008 summer (Dec–Feb). Post-eruption
mean depth of the maximum cell abundance of both
data are averages of December 2011–February 2012 sampling
phytoplankton components moved upwards to around 20 m
dates. References: A and B: Lake Espejo, C and D: Lake
depth, especially in Espejo and Correntoso (Fig. 6).
Correntoso, E and F: Lake Nahuel Huapi.
Incubation experiments—Our incubation experiments
the eruption (Espejo 5 0.52 6 0.15 mg L21, Correntoso 5
showed that, while nutrient fertilization modestly increased
0.51 6 0.14 mg L21, and Nahuel Huapi 5 0.51 6
phytoplankton growth, consistent with a role for ash-borne
0.11 mg L21).
nutrients in stimulating post-eruption chlorophyll concen-
The Chl a : C ratio (mg : mg) averaged 10–12 in the
trations in the affected lakes, there was also a large negative
affected lakes as well as in the unaffected Lakes Gutie´rrez
effect of overall light intensity on phytoplankton GR as
and Mascardi (Fig. 5). Furthermore, we found no signif-
well as an effect of UV removal (Fig. 7). GR declined at
icant differences in Chl a : C between lakes (ANOVA, F4,35
relatively low levels, when FSR intensity exceeded 10% of
5 1.43, p 5 0.24), although Lake Nahuel Huapi had more
incident irradiance (or even 3% for the unenriched
variable Chl a : C ratios (Fig. 5).
treatment in Lake Mascardi). Similar, though slightly more
Previous quantitative data on summer phytoplankton
modest, negative effects of light intensity were obtained
taxa in Lakes Espejo, Correntoso, and Nahuel Huapi
when UVR was removed. Notably, the difference between
indicated dominance by picocyanobacteria and the nano-
FSR and PAR treatments was only present in medium to
flagellate Chrysochromulina parva (Haptophyceae; cell
high light intensities, while at low light intensities there was
Effect of volcanic eruption in lakes
Vertical profiles of phytoplankton in Lakes Espejo, Correntoso, and NW Nahuel
Huapi pre-eruption and post-eruption. (A) Phytoplankton composition and cell abundance, (B)picocyanobacteria cell abundance. Pre-eruption data are average of 2000–2008 summer (Dec–Feb). Post-eruption data are averages of December 2011–February 2012 sampling dates.
little effect of UVR removal, especially in the Espejo
eruption, from , 62% (brighter than Lake Mascardi) to ,
experiment. For both lakes, ANOVA indicated highly
30%. This change was nearly entirely due to the increase in
significant main effects of light quality (p , 0.0001), light
KPAR post-eruption, as average mixing depth did not
intensity (p , 0.0001), and nutrients (p , 0.0001) on GR.
change appreciably following the eruption (Fig. 4A,B). In
We found statistically significant light intensity 3 nutrient
the Lake Espejo experiment (Fig. 7B), a decline in Im from
interactions in both experiments (p 5 0.006 in Espejo and
62% to 30% Im corresponds to a shift from light conditions
p 5 0.024 in Mascardi) but only the Espejo experiment had
that strongly inhibited phytoplankton growth (regardless of
significant two-way interactions involving light quality,
nutrients or UV) in the experiment to those at which GR
with intensity (p , 0.0001) and with nutrients (p 5 0.026)).
was near zero or positive.
No significant (p . 0.05) three-way interactions wereobserved.
To interpret these results in light of the Puyehue
eruption, we assessed the shading stimulation of phyto-
Overall, lake dynamics after eruption indicate that
plankton GR in light of the observed values of average
increased phytoplankton biomass was likely due to
relative mixed-layer irradiance (Im expressed as a percent-
combination of an increase in P supplies together with a
age of surface irradiance) before and after the eruption. Im
lowering of light intensities causes by suspended ashes. An
was , 42% in Lake Mascardi and did not change after the
indication that mechanisms other than nutrient loading
eruption. In the Lake Mascardi experiment (Fig. 7A),
alone appear to be involved was provided by the
phytoplankton had near-zero or slightly negative growth
observation that Chl a increased considerably in October
rates at this Im, even with nutrient fertilization and UVR
and November in NW Nahuel Huapi after the eruption,
removal. In Lake Espejo, Im declined markedly after the
despite no apparent change in P (Fig. 3; see October and
Modenutti et al.
and because of its location and the predominance ofwestern winds, has a considerable fetch (Fig. 1) that resultsin a very deep thermocline (Fig. 4E,F). Nevertheless,almost the whole epilimnion is exposed to UV-A whilethe upper 40% of the epilimnion is also exposed to UV-B(305 nm; Fig. 4). Indeed, in these North-PatagonianAndean lakes DOC concentrations are very low (Morriset al. 1995) and the lakes have elevated UV : PAR ratios (,0.35), indicating that light attenuation is not due to DOCbut instead is dominated by attenuation by suspendedparticles (Rose et al. 2009). The fact that suspended ashesincreased KPAR and light scattering at all wavelengths (nochanges in UV : PAR ratio were observed) implies anoverall reduction in total solar radiation received as well asamelioration of UVR exposure (Fig. 4).
The inference that increased post-eruption chlorophyll at
least partially reflects lower light intensities is supported bythe results of multiple regression analysis, which identifiedKPAR as the variable that is primarily associated withvariation in Chl a, both across all lakes throughout thestudy period as well as just in the affected lakes during thepost-eruption interval. Importantly, this correlation anal-ysis is bolstered by the experimental results (Fig. 7):nutrient enrichment had only modest effect on phytoplank-ton growth compared with the large positive effect oflowering solar radiation. We note that the modestresponses to nutrient enrichment we observed in theseexperiments are unlikely to reflect possible nutrient releasefrom cells damaged during preparation of filtered lakewater for the 80% experimental dilution because measure-ments of soluble (i.e., filtered) reactive phosphorus samplestypically are below our limit of detection of 1 mg L21,considerably lower than the 15 mg L21 experimental Penrichment. Removal of UVR also had a positive effect on
Results of incubation experiments testing the effects
GR, an understandable outcome given that ultraviolet
of overall light intensity (UVR+), removal of ultraviolet radiation
radiation is known to damage phytoplankton and reduce
(UVR2), and nutrients on growth rate of phytoplankton from
primary production (Holm-Hansen et al. 1993; Neale et al.
(A) Lake Mascardi (relatively unaffected by the eruption) and (B)
1998a). When we removed this damaging wavelength in our
Lake Espejo (strongly affected by the eruption). Error bars on
experiments, the overall photoinhibition effect was reduced
each symbol indicate 6 1 standard error. On each figure, vertical
but not eliminated. This result suggests that PAR itself is
lines indicate the historical 17 yr pre-eruption average mixed-layer
too high and sufficient on its own to induce photoinhibition
light intensity (Im, as a percentage of incident) as well as theaverage post-eruption value for 2011. For Lake Mascardi, the pre-
and that this effect is not counteracted by nutrient
and post-eruption values of I
m were essentially identical, but the
horizontal arrow in panel B indicates the post-eruption shift seen
Our inference that excess irradiance is an important
in Lake Espejo due to shading by suspended ash.
ecological factor in these lakes clarifies the observeddynamics in Lake Nahuel Huapi in spring and Lakes
November samples). Furthermore, Chl a decreased in early
Espejo and Correntoso in early summer, where phyto-
summer (December to January) in Lakes Espejo and
plankton biomass changed significantly despite no appar-
Correntoso with no decrease in P (Fig. 3A) but in concert
ent change in nutrient levels (Fig. 3) but in concert with
with declining KPAR (increasing light). One possible
shifts in light extinction. Consistent with this interpreta-
mechanism for these changes in Chl a despite no change
tion, previous studies in the same lakes have shown that
in TP is that suspended ash ameliorated exposure of
primary production at depths corresponding to 10% of
phytoplankton to excessive solar radiation, which previous
surface irradiance is 5–10 times higher than that in surface
studies have shown to be damaging in these highly
layers (Modenutti et al. 2004; Callieri et al. 2007), despite
transparent lakes (Morris et al. 1995; Modenutti et al.
vertical uniformity in nutrient concentrations.
2004; Villafan˜e et al. 2004). Previous to the eruption event,
Extrapolation of our experimental results to lake
wind-sheltered lakes like Espejo and Correntoso had their
conditions is complicated by the fact that we used a static
whole epilimnia illuminated and exposed to high PAR as
incubation but, under natural conditions, phytoplankton
well as hazardous UVR (including UV-B and UV-A;
cells in a lake's mixing layer are continuously brought in
Fig. 4). Lake Nahuel Huapi is the largest lake in the area,
and out of near-surface layers where solar radiation is high
Effect of volcanic eruption in lakes
and induces photoinhibition (Neale et al. 1998b; Modenutti
volcanic activity. However, diatoms would likely not be
et al. 2005). Relevant to such mechanisms, our experimen-
supported in Espejo and Correntoso because their shal-
tal results suggest a stronger negative effect of light on
lower mixing layers have low turbulent diffusivity com-
phytoplankton growth rate at irradiances exceeding Im
pared to Nahuel Huapi (Huisman et al. 2004).
than below (Fig. 6). This apparent nonlinearity suggests
Picocyanobacteria remained the dominant component in
that, compared to cells held at a constant value of Im (as in
the phytoplankton based on cell abundance both in pre-
our incubation experiment), overall photoinhibitory effects
and post-eruption periods (Fig. 6B). Picocyanobacteria
would be larger for circulating cells experiencing light . Im
have generally more relative abundance when nutrient
for part of the day and light , Im for part of the day.
concentrations are low (Callieri et al. 2007). For all three
Considering the differences in Im between Lakes Espejo and
affected lakes, the abundance of picocyanobacteria de-
Correntoso vs. Lake Nahuel Huapi, it seems that our static
clined after the eruption. Further, and consistent with a
experiments could be either under- or overestimating
decrease in nutrient limitation, the abundance of larger
photoinhibitory effects on phytoplankton experiencing in
nanoplankton increased. Picocyanobacteria have been
situ Im. Nevertheless, considering the in situ dynamics
reported to perform well under low-intensity, green light
following the volcanic natural experiment and our exper-
because of the presence of phycoerythrin (Stomp et al.
imental results together, photoinhibition emerges as a
2007). This helps explain increased abundances of phyco-
candidate factor contributing to dynamics of primary
erythrin-rich cells previously observed in the DCM of deep
production and phytoplankton biomass under normal
ultraoligotrophic Patagonian lakes (Callieri et al. 2007),
(pre-eruption) conditions in these lakes and suggests that
where blue-green light prevails (Pe´rez et al. 2002). Since no
excessive irradiance plays a similar role in other water
post-eruption changes were observed in absorption of
columns of high optical clarity.
different wavelengths (Fig. 4), dim, green light conditions
This inference is strengthened by previous studies in
after the eruption occurred at a shallower depth (see
these lakes, which also provide insight into how phyto-
increase around 20 m depth in Fig. 6B post-eruption
plankton vertical distributions shifted in response to
graphs). Thus, as the same light quality was achieved at
the eruption. Phytoplankton in clear North Andean-
shallower depth, picocyanobacteria moved upwards in the
Patagonian lakes often develop DCM, likely due to strong
water column. These shifts are consistent with previous
effects of photoinhibition (Modenutti et al. 2004). There-
documentation of the highly variable dynamics of pico-
fore, the possibility of a refuge against hazardous
cyanobacteria in forming DCM in the metalimnion or the
wavelengths in deep layers is important in these extremely
hypolimnion (Callieri et al. 2007).
clear lakes. Previous to the volcanic event, these lakes
Consistent with an overall view that high light intensities
regularly exhibited DCM, either in the hypolimnion
are an important ecological factor in these lakes are the low
(Espejo and Correntoso) or in the metalimnion (Nahuel
Chl a : C ratios we observed, as low Chl a : C ratios are
Huapi; Modenutti et al. in press). These DCM involve
generally considered to be indicative of high irradiance
mainly motile mixotrophic cells (Modenutti et al. 2004, in
conditions (Geider et al. 1997). While differences in average
press) that are able to move to deeper layers (Sommaruga
Chl a : C ratios between lakes were not significant, Chl a : C
and Psenner 1997), exploiting hypolimnetic levels of the
ratios in Lake Nahuel Huapi were quite variable (Fig. 5).
euphotic zone. Noticeably, after the eruption event,
This variability may be due to several factors. Since
phytoplankton composition changed, with an increase in
different taxonomic groups can differ considerably in the
the flagellate R. lacustris (Cryptophyceae) in Lakes Espejo
Chl a : C ratios (Chan 1980), one possible contributing
and Correntoso and an upward shift in the depth of its
factor is the greater variability in algal composition and cell
maximum abundance. This latter change is consistent with
size (including nanoflagellates and diatoms, from , 3 to
a community actively maintaining its position at a desired
45 mm in cell length) in Nahuel Huapi than seen in the other
light intensity. R. lacustris is a facultative mixotroph that is
lakes. Another possible contributing factor for the extreme
very common in less transparent lakes in the area (Balseiro
variability in Chl a : C ratios in Nahuel Huapi is its deeper
et al. 2004) and the flexibility of such mixotrophs has been
thermocline, its complex lake morphometry, and its greater
suggested as an adaptive advantage that allows them to
wind exposure than in the other lakes. Together, these may
dominate in plankton communities exposed to variation in
result in the whole euphotic zone being included in the
light (Laybourn-Parry et al. 1997). This change in flagellate
mixing layer, leading to the possibility of a weak photo-
species composition implies a change in cell size (domi-
acclimation because turbulence may drag cells all along the
nance from C. parva, cell average length 5 3.78 6 0.5 mm,
light gradient (Geider et al. 1997) or sporadically move
to R. lacustris, cell average length 5 8.7 6 0.7 mm), which
them out of the DCM into the mixed layer.
may help in understanding the observed increase in Chl a
Beyond documenting volcano effects on lake water
concentrations despite no significant change in cell
quality, our study suggests an unexpectedly contribution
numbers or Chl a : C ratio. On the other hand, the increase
of excess light in affecting phytoplankton growth and
of the diatoms A. granulata and T. flocculosa in Lake
production in these transparent Patagonian lakes. It has
Nahuel Huapi and their shift towards deeper levels suggest
recently been suggested that limiting light conditions are an
that these nonmotile cells accumulated at the thermocline
underappreciated factor in lake ecology (Karlsson et al.
because they cannot actively regulate their position in the
2009). However, the findings of Karlsson et al. are from
water column (Cullen 1982). The increase in diatoms may
small, highly colored lakes that, while numerous, do not
be a result of more silica in the water column from the
contain large volumes of surface freshwaters. In such
Modenutti et al.
shallow lakes, colored dissolved organic matter greatly
BEETON, A. M. 2002. Large freshwater lakes: Present state, trends,
reduces overall light intensity and acts as the ‘‘ozone of the
and future. Environ. Conserv. 29: 21
underwater world'' by selectively removing the shorter
wavelengths of light (Williamson and Rose 2010). However,
BOYCE, D. G., M. R. LEWIS, AND B. WORM. 2010. Global
in these clear North Patagonia lakes, dissolved organic
phytoplankton decline over the past century. Nature 466:
matter concentration is extremely low (, 0.6 mg L21; Morris
CALLIERI, C., B. MODENUTTI, C. QUEIMALIN˜OS, R. BERTONI, AND
et al. 1995; Corno et al. 2009) and high light has been
E. BALSEIRO. 2007. Production and biomass of picophyto-
recognized as an important factor structuring lacustrine
plankton and larger autotrophs in Andean ultraoligotrophic
plankton (Modenutti et al. 2004, 2005). The high-transpar-
lakes: Differences in light harvesting efficiency in deep layers.
ency, low-nutrient scenarios typical of our study lakes are
Aquat. Ecol. 41:
likely widespread in many large lakes worldwide; notably,
———, AND J. G. STOCKNER. 2002. Freshwater autotrophic
large lakes contain the majority (68%) of global unfrozen
picoplankton: A review. J. Limnol. 61: 1–14.
surface waters and provide a variety of key ecosystem
CHAN, A. T. 1980. Comparative physiological study of marine
services (Beeton 2002). Such high light scenarios may also be
diatoms and dinoflagellates in relation to irradiance and cell
common in many oceanic regions, leading us to suggest that
size. II. Relationship between photosynthesis, growth, andcarbon/chlorophyll a ratio. J. Phycol. 16:
increasing photoinhibition may be a factor contributing to
recently reported declines in oceanic phytoplankton biomass
CORNO, G., B. MODENUTTI, C. CALLIERI, E. BALSEIRO, R. BERTONI,
linked to warming surface layers during the last 30 yr (Boyce
AND E. CARAVATI. 2009. Bacterial diversity and morphology in
et al. 2010; but see also McQuatters-Gollop et al. 2011). For
deep ultraoligotrophic Andean lakes: The role of UVR on
lakes, future light environments will depend strongly on
vertical distribution. Limnol. Oceanogr. 54: 1098–111
possible changes in inputs of colored organic matter. While
some early assessments suggested reduced inputs of colored
CULLEN, J. J. 1982. The deep chlorophyll maximum: Comparing
organic materials and thus brighter surface mixed layers
vertical profiles of chlorophyll a. Can. J. Fish. Aquat. Sci 39:
during coming decades (Magnuson et al. 1997), more recent
studies highlight the possibility of increasing DOC inputs to
DOYLE, S. A., J. E. SAROS, AND C. E. WILLIAMSON. 2005.
lakes due to temperature stimulation of DOC mobilization
Interactive effects of temperature and nutrient limitation onthe response of alpine phytoplankton growth to ultraviolet
from peatlands (Freeman et al. 2001; Hansson et al. 2012).
radiation. Limnol. Oceanogr. 50:
According to the inferences we make here, such changes
might result in increased phytoplankton production in
EINARSSON, A., H. O
´ SKARSSON, AND H. HAFLIDASON. 1993.
highly transparent lakes by shielding phytoplankton from
Stratigraphy of fossil pigments and Cladophora and its
excessive irradiance. Regardless of the direction of such
relationship with deposition of tephra in Lake My´vatn,
DOC shifts, our work suggests that understanding the effects
Iceland. J. Paleolimnol. 8:
of shifts in DOC inputs to lakes will require assessment of
FALKOWSKI, P. G., AND J. A. RAVEN. 2007. Aquatic photosynthe-
how such changes might modulate photoinhibition of lake
sis, 2nd ed. Blackwell Science.
ecosystem production and interactions with nutrient supply.
FREEMAN, C., C. D. EVANS, D. T. MONTEITH, B. REYNOLDS, AND
Thus, our findings should motivate continued work on the
N. FENNER. 2001. Export of organic carbon from peat soils.
Nature 412:
role of optical conditions in affecting the functioning of the
GEIDER, R. J., H. L. MACINTYRE, AND T. M. KANA. 1997.
pelagic ecosystems that are fundamental in global water
Dynamic model of phytoplankton growth and acclimation:
supplies and biogeochemical cycles.
Responses of the balanced growth rate and the chlorophylla : carbon ratio to light, nutrient-limitation and temperature.
Mar. Ecol. Prog. Ser. 148:
J.J.E. was supported by the Fulbright Foundation and the
GERLA, D. J., W. M. MOOIJ, AND J. HUISMAN. 2011. Photoinhibition
National Science Foundation (DEB-0950179). We thank two
and the assembly of light-limited phytoplankton communities.
anonymous reviewers for their constructive comments on this
Oikos 120: 359–3
manuscript. Data were produced via grants to B.M. and E.B.
HAMME, R. C., AND OTHERS. 2010. Volcanic ash fuels anomalous
from the Fondo para la Investigacio´n Cientı´fica y Tecnolo´gica
plankton bloom in subarctic northeast Pacific. Geophys. Res.
(FONCyT, Argentina) and the National Geographic Society.
HANSSON, L.-A., AND OTHERS. 2012. Food-chain length alters
community responses to global change in aquatic systems.
Nature Clim. Change 3: 228–233.
ALDERKAMP, A.-C., H. J. W. DE BAAR, R. J. W. VISSER, AND K. R.
HOLM-HANSEN, O., E. W. HELBLING, AND D. LUBIN. 1993.
ARRIGO. 2010. Can photoinhibition control phytoplankton
Ultraviolet radiation in Antarctica: Inhibition of primary
abundance in deeply mixed water columns of the Southern
production. Photochem. Photobiol. 58:
Ocean? Limnol. Oceanogr. 55: 1248–126
HUISMAN, J. 1999. Population dynamics of light-limited phyto-
APHA. 2005. Standard methods for the examination of water and
plankton: Microcosm experiments. Ecology 80: 202–210
wastewater. American Public Health Association, AWWA.
BALSEIRO, E. G., C. P. QUEIMALIN˜OS, AND B. E. MODENUTTI. 2004.
———, J. SHARPLES, J. M. STROOM, P. M. VISSER, W. E. A.
Grazing impact on autotrophic picoplankton in two south
KARDINAAL, J. M. H. VERSPAGEN, AND B. SOMMEIJER. 2004.
andean lakes (Patagonia, Argentina) with different light : nutrient
Changes in turbulent mixing shift competition for light
ratios. Rev. Chil. Hist. Nat. 77:
between phytoplankton species. Ecology 85: 2960–297
Effect of volcanic eruption in lakes
———, AND OTHERS. 2002. Principles of the light-limited
MORRIS, D. P., AND OTHERS. 1995. The attenuation of solar UV
chemostat: Theory and ecological applications. Antonie van
radiation in lakes and the role of dissolved organic carbon.
Limnol. Oceanogr. 40:
KARLSSON, J., P. BYSTROM, J. ASK, P. ASK, L. PERSSON, AND M.
JANSSON. 2009. Light limitation of nutrient-poor lake ecosys-
NEALE, P. J., J. J. CULLEN, AND R. F. DAVIS. 1998a. Inhibition of
tems. Nature 460:
marine photosynthesis by ultraviolet radiation: Variable
KILHAM, S. S., D. A. KREEGER, S. G. LYNN, C. E. GOULDEN, AND
sensitivity of phytoplankton in the Weddell-Scotia Conflu-
L. HERRERA. 1998. COMBO—A defined freshwater culture
ence during the austral spring. Limnol. Oceanogr. 43:
medium for algae and zooplankton. Hydrobiologia 377:
———, R. F. DAVIS, AND J. J. CULLEN. 1998b. Interactive effects
KIRK, J. T. O. 1994. Light and photosynthesis in aquatic
of ozone depletion and vertical mixing on photosynthesis of
ecosystems. Cambridge Univ. Press.
Antarctic phytoplankton. Nature 392:
KOPPEN, J. D. 1975. A morphological and taxonomic consider-
ation of Tabellaria (Bacillariophyceae) from the northcentral
NUSCH, E. A. 1980. Comparison of different methods for
United States. J. Phycol. 11: 236–244.
chlorophyll and phaeopigment determination. Arch. Hydro-
biol. Beih. Ergebn. Limnol. 14: 14–36.
ARSON, D. W. 2011. Science after the volcano blew: Research
near Mount St. Helens proceeded despite bureaucratic
PE´REZ, G. L., C. P. QUEIMALIN˜OS, AND B. E. MODENUTTI. 2002.
hurdles, limited funding and an extremely hazardous envi-
Light climate and plankton in the deep chlorophyll maxima
ronment. Am. Sci. 98: 324–333.
in North Patagonian Andean lakes. J. Plankton Res. 24:
AYBOURN-PARRY, J., S. J. PERRISS, G. G. R. SEATON, AND J.
ROSE, K. C., C. E. WILLIAMSON, J. E. SAROS, R. SOMMARUGA, AND
OHOZINSKI. 1997. A mixotrophic ciliate as a major contrib-
utor to plankton photosynthesis in Australian lakes. Limnol.
J. M. FISCHER. 2009. Differences in UV transparency and
thermal structure between alpine and subalpine lakes:
Implications for organisms. Photochem. Photobiol. Sci. 8:
IN, I. I., AND OTHERS. 2011. Fertilization potential of volcanic dust
in the low-nutrient low-chlorophyll western North Pacific
subtropical gyre: Satellite evidence and laboratory study.
SOMMARUGA, R., AND R. PSENNER. 1997. Ultraviolet radiation in a
Global Biogeochem. Cycles
high mountain lake of the Austrian Alps: Air and underwater
measurements. Photochem. Photobiol.
LINDENMAYER, D. B., G. E. LIKENS, AND J. F. FRANKLIN. 2010.
Rapid responses to facilitate ecological discoveries from
TERNER, R. W., J. J. ELSER, E. J. FEE, S. J. GUILDFORD, AND T. H.
major disturbances. Front. Ecol. Environ.
HRZANOWSKI. 1997. The light : nutrient ratio in lakes: The
balance of energy and materials affects ecosystem structure
and process. Am. Nat. 150:
MAGNUSON, J. J., AND OTHERS. 1997. Potential effects of climate changes
STOMP, M., J. HUISMAN, L. VOROS, F. R. PICK, M. LAAMANEN,
on aquatic systems: Laurentian Great Lakes and Precambrian
T. HAVERKAMP, AND L. J. STAL. 2007. Colourful coexistence
Shield region. Hydrol. Processes 11: 825–87
of red and green picocyanobacteria in lakes and seas. Ecol.
MCQUATTERS-GOLLOP, A., AND OTHERS. 2011. Is there a decline in
THOMASSON, K. 1963. Araucanian lakes. Acta Phytogeogr. Suec.
marine phytoplankton? Nature 472: E6–E7
47: 1–139.
VILLAFAN˜E, V. E., A. G. J. BUMA, P. BOELEN, AND E. W. HELBLING.
MODENUTTI, B., E. BALSEIRO, M. BASTIDAS NAVARRO, C. LASPOUMA-
2004. Solar UVR-induced DNA damage and inhibition of
DERES, M. S. SOUZA, AND F. CUASSOLO. In press. Environmental
photosynthesis in phytoplankton from Andean lakes of
changes affecting light climate in oligotrophic mountain lakes:
Argentina. Arch. Hydrobiol. 161: 245–26
The deep chlorophyll maxima as a sensitive variable. Aquat.
WILLIAMSON, C. E., AND K. C. ROSE. 2010. When UV meets fresh
———, ———, C. CALLIERI, C. QUEIMALIN˜OS, AND R. BERTONI.
water. Science 329:
2004. Increase in photosynthetic efficiency as a strategy ofplanktonic organisms exploiting deep lake layers. Freshw.
Biol. 49:
Associate editor: John M. Melack
ODENUTTI, B. E., E. G. BALSEIRO, C. CALLIERI, R. BERTONI, AND
C. P. QUEIMALIN˜OS. 2005. Effect of UV-B and different PARintensities on the primary production of the mixotrophic
Received: 22 December 2012
planktonic ciliate Stentor araucanus. Limnol. Oceanogr. 50:
Accepted: 06 March 2013
Amended: 10 March 2013
Source: http://elserlab.asu.edu/pdf/Modenutti_2013.pdf
REPORT NR. 01/2016 FÜNDIGKEITSRISIKEN AUTOREN Allegra Seipp, Christine Grüning und Ulf Moslener * Die Studie stellt die persönliche Meinung der Autoren dar und nicht die der Institutionen, mit denen wir verbunden sind. Wir danken zahlreichen Interviewpartnern für die vielen Informationen und hilfreichen Kommentare. Ganz besonders: Kai Imolauer, Stephan A. Jacob, Matthias Kliesch, Christian Müller-Wagner, Kirsten Offermanns, Thorsten Schneider, Matthias Tönnis, Wesly Urena Vargas, Arndt Wierheim, und Jens Wirth.
We would like to thank the following colleagues for their help, advice and input into this report: David Bawden Care Quality Commission Kate Hall Policy Advisor, Monitor Dr. Gary Orr Consultant Psychiatrist, Hutt Valley Health Board Christine Boswell Chief Dr. Alasdair Honeyman Associate Director, Executive,Rotherham, Doncaster and Good Governance Institute Elaine Protheroe Board Secretary,





