
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
D: archiwum vol21fasc1 vol21fasc1.vp
Arch. Pol. Fish. (2013) 21: 29-39DOI 10.2478/aopf-2013-0004
Transferable drug-resistant coliforms in fish exposed to sewage
Sutapa Sanyal, Samir Banerjee
Received – 21 October 2012/Accepted – 19 February 2013. Published online: 31 March 2013; Inland Fisheries Institute in Olsztyn, PolandCitation: Sanyal S., Banerjee S. 2013 – Transferable drug-resistant coliforms in fish exposed to sewage – Arch. Pol. Fish. 21: 29-39.
Abstract. In this study, the thermotolerent fecal coliform (Th
FC) bacterial population (n = 81) in a waste-fed aquaculturesystem was examined for multiple antibiotic resistance and
Wastewater-fed aquaculture provides an opportu-
the possession of transferable drug resistance factors (Rfactors). Multiple antibiotic resistant (MAR) coliforms were
nity to treat wastewater with integrated material-flow
found to be common in the sewage-fed pond environment,
recycling. Several goals are achieved simultaneously:
with 83% of the screened MAR isolates harboring plasmids of
on the one hand valuable goods such as foodstuffs,
> 10 kilo base pair (kb). The transfer of resistance was
animal feeds, raw materials, ornamental plants and
confirmed by mating experiments in 92% of plasmid-positive
animals, mainly fish, are produced, and on the other
MAR coliforms with a nalidixic acid (NA) resistant strain,
utilizable gray water, or purified and hygienized
Escherichia coli ATCC (American Tissue Type Cell Culture)14948
wastewater, is produced. Nowadays, recycling and
(Deoxyribonuclease). Antibiotic resistance profiles of some
reusing wastes through aquaculture present several
mated progenies (70.83%) indicated that transfer was
concerns that are mainly related to health hazards
unidirectional. DNase-treated cell-free supernatants did not
generated by the use of water recovered from sewer-
transform, which excluded transduction. A DNase-resistant
age systems. This reclaimed water contains parasites,
conjugation-like mechanism probably plays a major role in
bacteria, viruses, various types of pharmaceuticals
the transfer of resistance factors. Physical evidence oftransmissible resistance factors in fish suggests a potential
such as antibiotics, estrogens, and active ingredients
health risk to humans and animals, including farmed fish.
of drugs at relatively low concentrations. Althoughindividual antibiotic concentrations are low, there
Keywords: fish, health risk, multiple-antibiotic-resistant
are so many different ones that when combined they
isolates, transferable drug resistance factors
can pose serious health and environmental problems(Koplin 2002). These lower concentrations of antibi-otics are sufficient to affect susceptible bacteria(Al-Ahmad et al. 1999). Therefore, the occurrence ofsuch antibiotic concentrations in sewage has the po-tential to select for antibiotic resistance.
In the field of aquaculture, both environmental
Department of Zoology, Bethune College, 181 Bidhan SaraniKolkata - 700006, West Bengal, India
and therapeutic problems are addressed since
Tel. + 91 9874075312; e-mail:
[email protected],
antimicrobial agents are released into surroundingwaters of waste-fed fish ponds through domestic
S. BanerjeeDepartment of Zoology, University of Calcutta, 35-Ballygunge
sewage (Aoki 1992). Some of the major concerns
Circular Road, Kolkata 700019, West Bengal, India
presented by antibiotics in the aquatic environment
Sutapa Sanyal, Samir Banerjee
are that entire trophic levels of bacteria can be elimi-
bacterial population (more than one antibiotic) ex-
nated in some ecosystems, or that multiple
hibits R factors, and which portion of them is capable
drug-resistant bacteria flourishes and make their
of transferring R factors and at what rates to sensitive
way into the food chain. Commercial fishes residing
in wastewaters can act as carriers of antibiotic resis-tant bacteria prompting health risks to consumers(Schwartz et al. 2003, Pathak and Gopal 2005,
Materials and Methods
Newaj et al. 2008). The occurrence of antibiotic resis-tance might signal the occurrence of plasmid transferin the microbial milieu of sewage systems (Schluter
Study area
et al. 2003). The high concentrations of bacteria, nu-trients, and suspended solids in sewage-fed ponds
A wetland located in Bandipur, Rahara, North 24
are all factors that enhance horizontal gene transfer
Parganas, (22°44' N and 88°24' E), where untreated
(Lorenz and Wackernagel 1994). Wastewaters and
domestic wastewater is utilized for fish farming, was
the fishes inhabiting them are, therefore, a potent
the study site. The raw sewage is entirely of domestic
source of antibiotic-resistant bacteria, which, in turn,
origin, and comes from the adjacent town of Titagarh,
can transfer their resistance genes to nonresistant
a municipal area in North 24 Parganas, West Bengal,
bacteria. Today, transferable drug resistance repre-
sents a major threat to the treatment of infectious dis-eases in both humans and animals, including farmed
Sampling and dissection
fish (Schwartz et al. 2003).
The potential for antibiotic exposure and resis-
The fish samples were caught with a net and were
tance development in human and animal gastroin-
immediately transferred to the laboratory in contain-
ers with pond water. The major Indian carps
Labeo
abundance in waters contaminated with human and
rohita (Hamilton),
Cirrhinus mrigala (Hamilton), and
animal wastes, make the thermotolerent fecal
Oreochromis niloticus (Peters) were subjected to bac-
coliform bacteria a logical focal group for studies of
teriological assays. Ten live fish of each of the species
antibiotic resistance and transfer in waste-fed
were selected randomly from the catch at each sam-
aquatic environments. Sewage-fed fisheries play
pling time (bimonthly from March 2010 to Novem-
a great role in improving water quality in tropical
ber 2010). The fish were dissected according to
Asian countries, and it can be compared to an effi-
Buras et al. (1987). Muscles and digestive tract con-
cient stabilization pond as far as fish production is
tents were isolated and placed in sterile glass vessels.
concerned. Moreover, potential public health haz-
The tissues were weighed under sterile conditions,
ards have never been considered although good fish
ground in a mortar, and suspended in a sodium chlo-
production has been achieved (Strauss 1997). In
ride (NaCl) physiological solution (9 ml of the solu-
view of the public health concern about the safety of
tion for each 1 g of muscle or digestive tract content).
fish raised in wastewater ponds, the aim of this study
The suspensions were homogenized using a Univer-
was to analyze the antibiotic resistance patterns in
sal Laboratory Aid Type MPW-309 homogenizer, at
thermotolerent fecal bacteria collected from fish and
1000 rpm for 10 min. The homogenates were then
the waste-fed aqueous environments they are reared
serially diluted (10-1 to 10-8 for muscles and 10-1 to
in. The investigation was also undertaken to study
10-10 for digestive tract contents) and inoculated into
the transfer of R factors and the frequencies of trans-
culture media. The time lag from fish collection to
fer under standardized laboratory conditions. Much
analyses did not exceed 6 h. Water from the sew-
of the work dealing with the transfer of R factors has
focused on which portion of an antibiotic-resistant
sub-surface, i.e., 15 to 20 cm below the water
Transferable drug-resistant coliforms in fish exposed to sewage
surface, to avoid surface contamination and analyzed
Plasmid profiles of drug-resistant
simultaneously with the fish sampling.
Drug resistant isolates were tested for the availability
of plasmids. A rapid alkaline extraction procedureproposed by Sambrook et al. (1989) was followed for
The pond water and fish samples were examined for
screening plasmid DNA. Electrophoresis was per-
thermotolerent fecal coliforms using the three-tube
formed using a 0.8% and 1% agarose gel system
fermentation technique (APHA 1998). Representa-
(Bangalore Genei, India) in tris acetate buffer with
tives of typical thermotolerent coliform isolates were
molecular weight markers (Hi Media, Mumbai, In-
selected randomly by colony morphology from the
dia). The gels were stained with ethidium bromide
selective culture medium (as described by APHA
(0.5 µg ml-1 Sigma). The resolved bands were visual-
1998 and Sanyal et al. 2011). They were streaked
ized on a UV-transilluminator at a wavelength of 360
aseptically several times on freshly prepared Nutri-
nm, and photographed using a UV gel documenta-
ent agar plates to obtain pure isolates for antibiotic
tion system (Alpha Imager, Innotech Corporation,
resistance tests. Pure fecal isolates were subjected to
USA). The concentrations of agarose noted above
several biochemical tests which included indole pro-
duction, methyl-red, and Voges Proskauer reactions,
high-molecular-weight plasmid DNA (up to 25 kb).
citrate utilization, and reaction in motility and ureamedium. Eighty-one purified fecal bacterial isolateswere tested for anti-microbial sensitivity using the
Mating experiments on the transfer of
disc diffusion method (Bauer et al. 1966). Antibiotic
resistance factors in bacteria
impregnated discs of 8 mm diameter were used for
A nalidixic acid (NA) resistant strain,
E. coli ATCC
the test, and the disks contained the following anti-
14948, was used as the recipient in all the mating ex-
bacterial agents: ampicillin (AMP 10 µg); amikacin
periments. All of the environmental isolates that were
not resistant to nalidixic acid were tested as donor
cotrimoxazole (CO 25 µg); gentamicin (GEN 30 µg);
strains in individual mating assays. Isolates demon-
kanamycin (KAN 30 µg); streptomycin (STR 10 µg);
strating multiple-drug resistance were processed and
tetracycline (TET 25 µg). Resistance was estimated
tested separately for the ability to transfer each type of
by measuring the inhibition zone as per standards
drug resistance factor. Twenty-four plasmid-positive
(NCCLS 2002). Reference strains
E. coli ATCC
25922 and
Pseudomonas aeruginosa ATCC 27853,
coliforms isolated from the fish and the water of the
recommended by NCCLS (1997), were used as con-
waste-fed ponds were tested for transferable resis-
trol organisms for verification of the disks on
Tryptone soya agar plates. All the culture media andantibiotic discs were obtained from Hi Media Labo-ratories Ltd., Mumbai, India. The rationale for choos-
Transfer of resistance factors in liquid
ing these compounds as target antibiotics was based
on previous studies, (Andersen 1993, Goñi-Urriza etal. 2000, Kim and Diana 2007, Jury et al. 2010) that
Prior to mating, individual bacterial cultures were
reported the occurrence of AMP, AK, C, CO, GEN,
grown overnight at 30°C in Tryptone Soya Broth
KAN, STR, and TET in municipal wastewater treat-
(TSB) containing 30 ìg ml-1 of the appropriate anti-
ment plants. This made it likely that the microbial
biotic (NA for
E. coli ATCC 14948 and either AMP,
biomass in the Bandipur waste-fed ponds also in-
C, STR, or TET for the environmental isolates). The
cluded bacteria resistant to these antibiotics.
bacterial cells were pelleted by centrifugation for 5
Sutapa Sanyal, Samir Banerjee
min at 8,500 g
, and resuspended in fresh TSB lack-
STR or TET, all at 30 ìg ml-1) and incubated for 24 h
ing antibiotic. Mating experiments were conducted
at 30°C. When mating progeny was achieved, the an-
as described by Bell et al. (1983). Briefly, equal vol-
umes (0.1 ml) of donor cells and recipient cells were
recombinants were determined with multi tipped
mixed in a 1.5 ml tube with 0.8 ml of antibiotic-free
disks. Additional mating experiments were also per-
TSB. Mated cells in broth were supplemented with
formed in the presence of DNase I following the
DNase I and MgCl2 at final concentrations of 200 µg
method described above with cell-free supernatants
ml-1 and 2 mM, respectively (Omar et al. 2007). The
of donors that were not filtered. Further experiments
mixtures were incubated at 30°C for 24 h. After incu-
were performed in the presence of DNase I with
bation the mixtures of donor and recipient cells were
treated supernatants from co-cultures of donors and
diluted 10-fold in 0.9% saline to a 10-7 dilution.
recipient cells.
Samples (0.1 ml) from each dilution were plated ontoTSA containing dual antibiotics (NA and either AMP,C, STR or TET, all at 30 ìg ml-1) and incubated for
Measurement of transfer frequency
24 h at 30°C to select for recombinants. When mat-ing progeny was achieved, the antibiotic-resistance
Transfer frequency was estimated by dividing the
patterns of representative recombinants were deter-
number of recombinants per milliliter by the number
mined with multi tipped disks. Negative mating con-
of donors per milliliter in the mating mixture (Yutaka
trol experiments lacking either the donor or recipient
et al. 2004). A number of transferable resistant
bacteria were included in all mating experiments to
coliforms were characterized biochemically to detect
check for the mutation of either strain to antibiotic re-
possible effects on strain distribution caused by the
sistance. No mutation to specific antibiotic resistance
discharge of domestic waste effluent in sewage-fed
was noted with any donor strains or the universal
E.
ponds. Biochemical characterizations of the isolates
coli ATCC 14948 recipient strain (West et al. 2011).
were performed to identify genus by a standard pro-cedure used for
Enterobacteriaceae (Kelly et al.
1995). In the present study, the presence of coliforms
Transfer of resistance factors using cell
was confirmed using multiplex PCR for the specific
amplification of the lacZ gene that encodes â-Dgalactosidase and a portion of the uid A gene for
E.
Experiments using cell-free culture supernatants as
coli detection. For multiplex PCR, a pair of primers
a DNA source were done as described below. After
the harvested cells of donors and recipients were cen-
5'-CACCATGCCGTGGGTTTCAATATT-3', Bej et al.
trifuged for 5 minutes at 8,500
g, the supernatants
1990) located within the coding region of the lacZ
were passed through a filter with a 0.2 µm pore size
gene of
E. coli and a pair of primers (5'-
(Life Sciences Products, Inc.) and then centrifuged
for an additional 3 min at 11,500
g. Aliquots of recip-
5'-ACGCGTGGTTACAGTCTTGCG-3', Bej et al.
ient cells (0.1 ml) were mixed in 0.8 ml of antibi-
1991) located within the uid A structural gene of
E.
otic-free TSB broth tubes together with 0.1 ml of the
coli were used. The primers were obtained from
treated supernatants of the donor cells. These experi-
Messers Bangalore Genei, Bangalore, India. During
ments were done in the presence of DNase I, as de-
the development of the PCR amplification procedure
scribed above. The mixtures were incubated at 30°C
for coliform detection, total genomic DNA was ex-
for 24 h. After incubation, the mixtures were diluted
tracted from the cultures with the procedure by
10-fold in 0.9% saline to a 10-7 dilution. Samples
Ausubel et al. (1996). In each PCR amplification,
(0.1 ml) from each dilution were plated onto TSA
a buffer control, to which no DNA template was
containing dual antibiotics (NA and either AMP, C,
added, was included as an internal control. DNA
Transferable drug-resistant coliforms in fish exposed to sewage
Table 1
Antibiotic resistance patterns in thermotolerent fecal coliforms from different sources at a waste-fed farm. Numbers of isolates in
brackets. NA= not analyzed, -ve = negative for plasmid, AMP = ampicillin, AK = amikacin, C = chloramphenicol, CO =
cotrimoxazole, GEN = gentamicin, KAN = kanamycin, STR = streptomycin and TET = tetracycline, MAR= Multiple antibiotic
resistance
Single antibiotic
Patterns and number of MAR No. of plasmid positive MAR
resistant isolates
AMP-CO-STR-TET (1)
AMP-CO-STR-TET (1)
Labeo sp.
AMP-TET (3, 1-ve)
templates of coliform bacteria and
E. coli extracted
associated with multiple resistances. Twelve differ-
ent MAR patterns were observed with a wide range of
pneumoniae ATCC 27736,
E. coli ATCC 25922)
multi resistance distribution, to as many as five anti-
were included as positive controls.
biotics (Table 1). Most of the isolated strains shared
resistance to ampicillin and tetracycline. Of 29 MAR
isolates screened, 24 (83%) harbored plasmids (Ta-
ble 1). Single high molecular weight plasmid (> 10
kb) possessed by isolates was clearly observed in 0.8
A substantial portion of bacteria isolated from the
and 1% agarose gel electrophoresis (Fig. 1a and 1b),
fish and pond water were resistant to ampicillin,
while 92% of the MAR isolates that harbored
chloramphenicol, and tetracycline (except
Cirrhinus
plasmids were able to transfer all or part of their de-
sp.), whereas bacteria resistant to kanamycin (except
terminants of antibiotic resistance to
E. coli ATCC
14948 (Table 2). However, in none of the successful
co-trimoxazole were infrequent (Table 1). No resis-
mating experiments was the presence of DNase
tance was found for amikacin and gentamicin. Resis-
I able to prevent the development of recombinants.
tance to ampicillin alone was very common, whereas
The addition of treated, cell-free culture supernatant
resistance to each of the other antibiotics was usually
of donors in the presence of DNase I did not
Sutapa Sanyal, Samir Banerjee
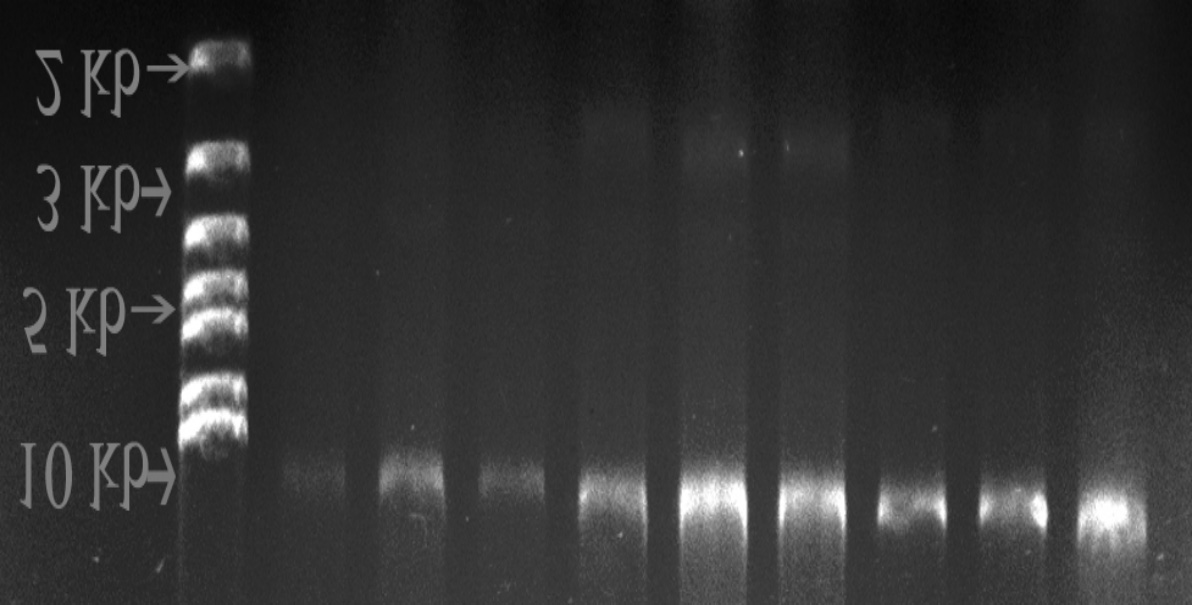
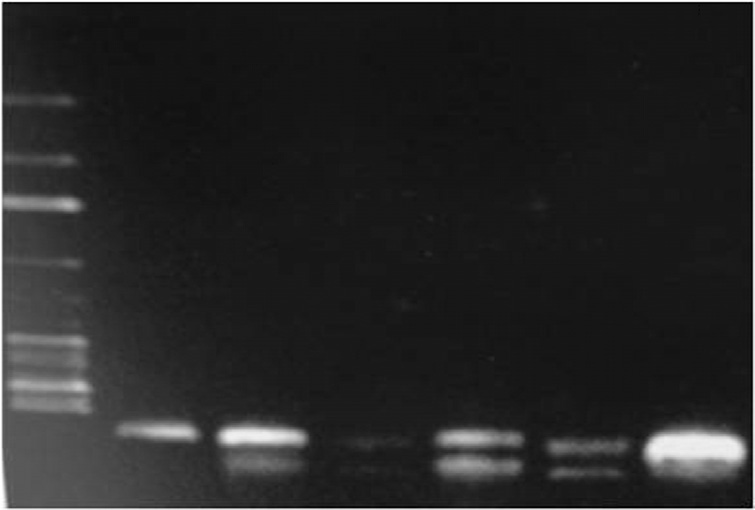
Figure 1. Plasmids of Drug Resistant Coliform isolates. 1a) 0.8% agarose gel (Plasmids of > 10 kilo base pair (kb)). 1b) 1% agarose gel(Plasmids of > 10 kilo base pair (kb)).
Table 2
Resistance pattern observed after gene transfer for non-nalidixic acid isolates.* Marks indicate mated progeny with unidirectional
transfer. AMP = ampicillin, AK = amikacin, C = chloramphenicol, CO = cotrimoxazole, GEN = gentamicin, KAN = kanamycin,
STR = streptomycin and TET = tetracycline
No. of iso-lates trans-
Resistance pattern
genetically trans-
Antibiotic resistance pattern
Treated cell-free supernatant of donors
Untreated cell-free supernatant of donors
Supernatant from co-culture of donors and
Transferable drug-resistant coliforms in fish exposed to sewage
transform the recipient cells (Table 2). Additional ex-
periments were performed with supernatants thatwere not filtered. The results were the same as thoseobserved with the filtered supernatants. Mating ex-
periments were performed with supernatants fromco-cultures of donors and recipients, and the results
were the same again (Table 2), with 45% of the iso-lates (10 out of 22) capable of achieving antibiotic re-
sistance transfer rates greater than 10-3 per donor
organism (Table 2). The following numbers of com-binations of fecal coliforms with transferable R+ de-
terminants were found: 13 in the E. coli populations,4 in the Enterobacter populations, 4 in the Klebsiellapopulations, and 1 in the Citrobacter populations(Table 2).
The PCR amplification of oligonucleotide prim-
ers yielded a detectable DNA fragment of an ex-pected molecular weight (876 bp for coliformbacteria; 876 bp and 147 bp for E. coli) only in the
presence of their respective DNA templates. How-
ever, the amplified products of Klebsiella spp. with
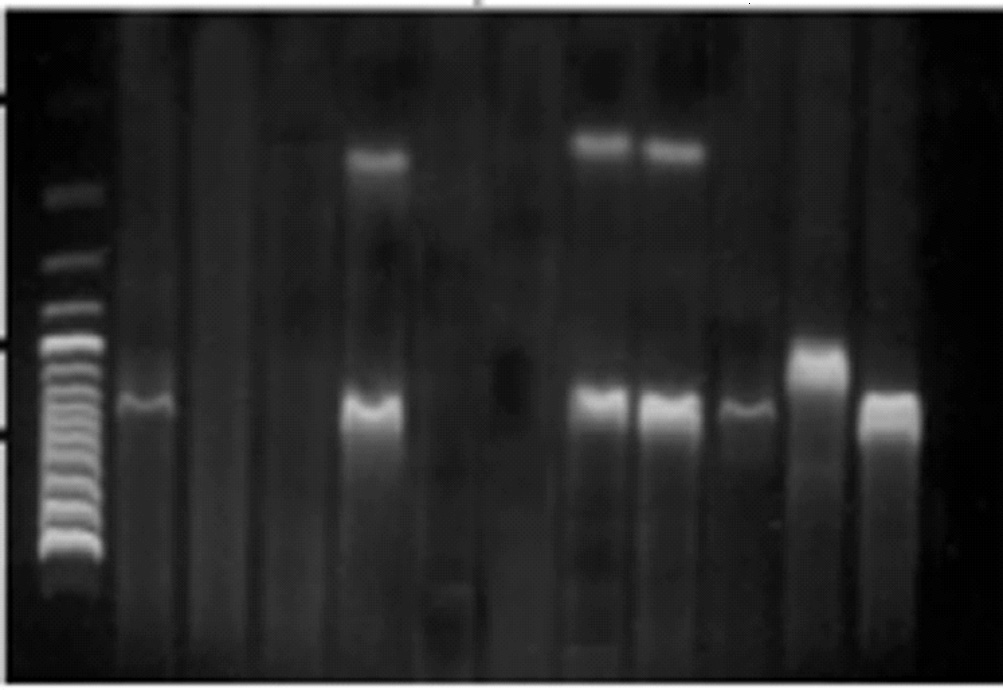
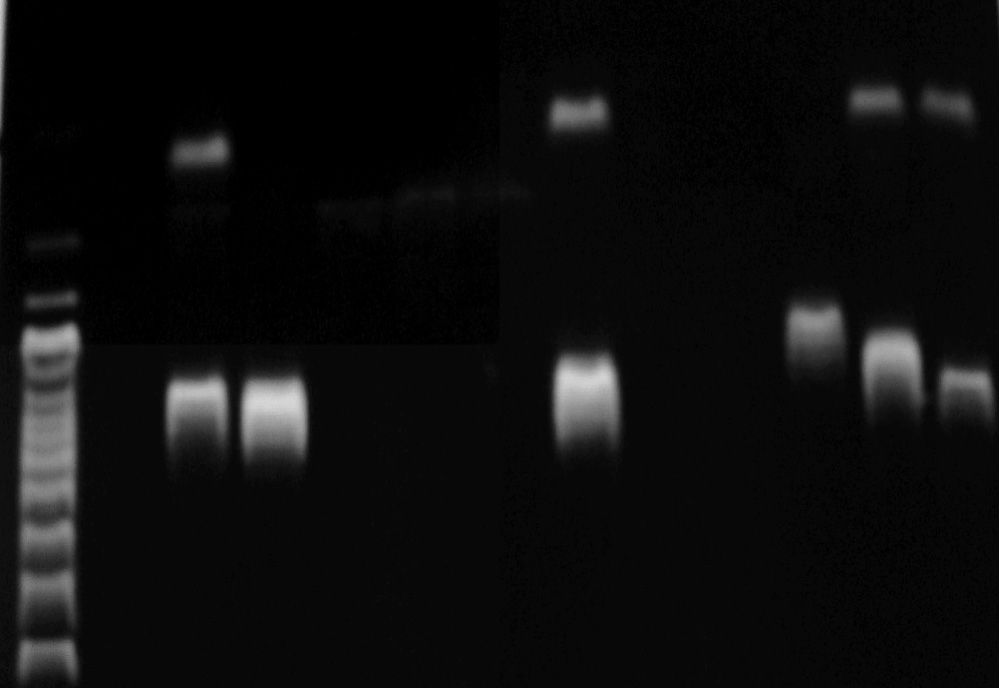
Figure 2. Agarose gel (2%) electrophoresis of PCR amplified prod-
lacZ primer were larger than those of E. coli,
ucts from various pure bacterial DNAs, using optimized multi-
Citrobacter spp., and Enterobacter spp., indicating
plex PCR. 2a) Lane M: molecular size marker (100 base pairs
a difference between the target lacZ among these or-
{bp} DNA ladder); lane 1 Klebsiella spp., lanes 4, 7 and 8 E. coli,lane 9, Klebsiella spp., lanes 10 and 11 Citrobacter spp. respec-
ganisms (Fig. 2).
tively. 2b) Lane M: molecular size marker (100 bp DNA ladder);lane 2 positive control, lane 3 Enterobacter spp., lane 7 E. coli,Lane 10 Citrobacter spp., lanes 11 and 12 E. coli.
chloramphenicol because of its genotoxicity, em-
A high resistance to ampicillin reflects the influence
bryo- and fetotoxicity, carcinogenic potential, and the
of humans on the environment (Andersen 1993). In-
lack of a dose response relationship for aplastic ane-
terestingly, in the present study the fish exhibited
mia in humans. In addition, the Aquaculture Author-
high antibiotic resistance to chloramphenicol and
ity of India banned 19 antibiotics, including
chloramphenicol, from aquaculture (Aquaculture
aquaculture. There are no reports available on the
News 2003). On the other hand, even when there is
use of these drugs applied either as feed supple-
high cotrimoxazole resistance in the water, low resis-
ments or as drugs used in aquaculture practice in the
tance was reported in fish (Table 1). This indicates
Bandipur waste-fed farm. It can be presumed that
that although the drug is effective, it exerts low selec-
tion pressure (Ogbondeminu and Olayemi 1993).
antimicrobial-resistant bacteria enter the fish ponds
The sensitivity exhibited for gentamicin is a signal of
with the sewage effluents, thus, these anthropogenic
the effectiveness of broad-spectrum antibiotics of the
factors might have influenced the fecal coliforms in
present generation (Pathak and Gopal 2005).
their acquisition of resistance. Fish and pond water
The high recovery rates of thermotolerent fecal
are not expected to have high resistance to
coliforms from Oreochromis sp. and Labeo sp. noted
Sutapa Sanyal, Samir Banerjee
in the present study, with the simultaneous resis-
completely destroyed extracellular DNA and pre-
tance to three to four antibiotics, suggest changes in
nutritionally beneficial microflora with unexpected
transduction, at least for the isolates studied (Table 2).
Zemelman 2001). The high recovery rate of antibi-
To confirm that the DNase-resistant recombination
otic-resistant bacteria from all the three fish species
was not due to protein-coated DNA or DNA present in
has immense ecological and public health implica-
membrane blebs, experiments were performed with
tions; specially, if the resistance is plasmid mediated,
cell-free supernatants that were not filtered. The re-
then there could be a problem associated with the
sults were the same as those observed with filtered
transfer of resistance determinants to human patho-
supernatants. To exclude the possibility that the re-
genic bacteria that could enter the human population
sults were because of a phage induced by the
through fish consumption (Miranda and Zemelman
co-culturing of strains, experiments were also per-
2001). The high proportion of multiple antibi-
formed with supernatants from co-cultures of donors
otic-resistance in Oreochromis sp. in comparison
and recipients. The results were the same again (Table
with the detritivorous Cirrhinus sp. suggests that the
2); the transfer was not affected by the presence of
fecal bacterial populations in Oreochromis sp. were
deoxyribonuclease I, which suggested the conjugative
subjected to conditions that fostered the acquisition
nature of it. The unidirectional process of DNA trans-
of multiple-resistance determinants.
fer was confirmed in 70.83% plasmid positive resis-
The simultaneous resistance of thermotolerent
tant progeny in the presence of DNase I by resistance
to secondary antibiotic markers, which is consistent
aminoglycoside could be the result of the dissemina-
with natural conjugation (Table 2). Mating experi-
tion of antibiotic-resistant plasmids in the aquatic en-
ments were not performed to confirm that the devel-
vironment (Miranda and Zemelman 2001). Of the
opment of resistant progeny in the presence of DNase
multidrug-resistant isolates, most exhibited resistance
required cell-to-cell contact. A similar process of
to combinations of antimicrobial drugs that included
DNase-resistant transformation not involving conjugal
ple-resistance genes may coexist on one plasmid
gonorrhoeae (Catlin 1981).
(Sayah et al. 2005), a single conjugative transposon
The results for the frequencies of transfer for the
(Pembroke et al. 2002), or an integron (Mazel 2006).
different mating experiments were in general agree-
This condition is particularly disconcerting given that
ment with those of Bell et al. (1983). Most of the iso-
exposure to one antibiotic agent may result in resis-
lates were capable of achieving resistance transfer
tance to others without previous exposure (Sayah et al.
rates within a range of 10-1 to 10-4 (Table 2), which is
2005) or cost to bacterial fitness (Aminov et al. 2002).
consistent with the results of Mach and Grimes
Plasmids, conjugative transposons, and integrons
(1982). Transfer rates varied between biotypes of the
make it possible for new antibiotic resistance genes to
same genus used as donors and between different
spread through bacterial populations by the process of
genera (Walter and Vennes 1985). Intragenetic
lateral gene transfer (Scott 1993). High rates of suc-
transfer might be the reason for the high transfer po-
cess of resistance gene transfer, as was observed in the
tentiality of E. coli noted in the present investigation
present assay (92% overall), suggests that regardless
(Table 2); however, the limited number of strains
of the physical location of resistance genes, i.e. chro-
used in the mating experiments does not permit
mosome, plasmid or integrons within transposons,
drawing broad conclusions on the frequency of such
these environmental isolates have the ability to move
transfers occurring in nature. In the development of
copies of themselves from one bacterial cell to another
a resistant clone under natural conditions, the
(Jury et al. 2010). The concentration of DNase I (200
amount of R plasmid donor cells would be substan-
µg ml-1) was so high in the present experiments that it
tially smaller than that in the present mating
Transferable drug-resistant coliforms in fish exposed to sewage
mixtures. However, the number of donor cells would
Banerjee for his guidance and invaluable constructive
increase over time under continued antimicrobial
criticism during manuscript preparation.
pressure. While in present investigation transferswere achieved with only a few strains, more strains
Author contributions. S.S. conceived of and designed
might be positive under different conditions or with
the experiments, performed the experiments, and ana-lyzed the data, S.S. and S.B. interpreted the data and
other recipients.
wrote the paper.
The mating experiments were performed at
30°C, a temperature that is common in tropicalaquatic systems, thus, their transfer in voided ex-creta, sewage, and polluted rivers is a definite possi-
bility. However, the intent was to use a method inwhich laboratory manipulation has the least impact
Al-Ahmad A., Daschner F.D., Kümmerer K. 1999 –
on both resistance genes and bacteria, and which
Biodegradability of cefotiam, ciprofloxacin, meropenem,penicillin G, and sulfamethoxazole and inhibition of
provides a better indication of what can happen in
waste water bacteria – Arch. Environ. Contam. Toxicol.
the environment. Coliforms, which are generally re-
37: 158-163.
garded as harmless indicators, can transfer drug re-
Aminov R.I., Chee-Sanford J.C., Garrigues N., Teferedegne B.,
sistance to pathogens with detrimental consequences
Krapac I.J., White B.A. 2002 – Development, validation,
for both fish and humans (Grabow and Prozesky
and application of PCR primers for detection of tetracy-cline efflux genes of gram-negative bacteria – Appl. Envi-
1973). The fish used in the current investigation con-
ron. Microbiol. 68: 1786-1793.
tained high levels of fecal coliforms with transferable
Andersen S.R. 1993 – Effects of waste water treatment on the
drug resistance factors when eaten raw or insuffi-
species composition and antibiotic resistance of coliform
ciently cooked; this could result in gut microflora be-
bacteria – Curr. Microbiol. 26: 97-103.
coming resistant to many drugs without any
Aoki T. 1992 – Present and future problems concerning the
development of resistance in aquaculture – In: Chemo-
symptoms of disease. During a subsequent illness,
therapy in Aquaculture from Theory to Reality (Eds) C.
the pathogen could become resistant by transfer from
Michel, D. Alderman, Office International des Epizooties,
existing gut microflora (Cooke 1976). The uncon-
Paris, France: 254-262.
trolled use of antibiotics and the common practice of
APHA 1998 – Standard methods for the examinations of
self-medication typical in India could place selection
water and wastewaters, 20th ed. – American PublicHealth Association, American Water Works Association
pressure on the wastewater and fishes in favor of or-
and Water Environment Federation, Washington DC.
ganisms possessing genes that code for resistance
Aquaculture News 2003 – List Antibiotics Banned in India,
(Sanyal et al. 2011). Thus, the R factors in the fecal
Marine Products Exports Authority of India – Cochin:
coliforms of the present study can spread among op-
portunistic pathogens originating in humans, ani-
Ausubel F.M., Brent R., Kingston R.E. 1996 – Current proto-
cols in molecular biology – New York, John Wiley and
evolutionarily or ecologically and constitute a signifi-
Bauer A.W., Kirby W.M.M., Sherris J.C., Turck M. 1966 –
cant public concern.
Antibiotic susceptibility testing by a standard single diskmethod – Am. J. Clin. Pathol. 45: 493.
Acknowledgments. This investigation received finan-
Bej A.K., Steffan R.J., Dicesare J., Haff J., Atlas R.M. 1990 –
cial assistance from the Minor Research Project in Sci-
Detection of coliform bacteria in water by polymerase
ence scheme (Project No. PSW/054/09-10) from the
chain reaction and gene probes – Appl. Environ.
Microbiol. 56: 307-314.
University Grants Commission, New Delhi, Govern-
Bej A.K., McCarty S.C., Atlas R.M. 1991 – Detection of
ment of India). The authors are thankful to the Head of
coliform bacteria and Escherichia coli by multiplex poly-
the Department of Zoology of University of Calcutta
merase chain reaction: comparison with defined sub-
who permitted the research work to be conducted in the
strate and plating methods for water quality monitoring –
department. The first author is grateful to Prof. Samir
Appl. Environ. Microbiol. 57: 2429-2432.
Sutapa Sanyal, Samir Banerjee
Bell J.B., Elliott G.E., Smith D.W. 1983 – Influence of Sewage
NCCLS 1997 – Performance standards for antimicrobial disk
treatment and urbanization on selection of multiple resis-
susceptibility tests. Approved standard M2-A6 –
tance in fecal coliform populations – Appl. Environ.
National Committee for Clinical Laboratory Standards.
Microbiol. 46: 227-232.
NCCLS 2002 – Performance standards for antimicrobial disk
Buras N., Duek L., Niv S., Hepher S., Sandbank S. 1987 –
susceptibility tests – National Committee for Clinical
Microbiological aspects of fish grown in treated
wastewater – Water Res. 21: 1-10.
Newaj F.A., Mutani A., Ramsubhag A., Adesiyun A. 2008 –
Catlin B.W. 1981 – Cell-to-cell transmission of chromosomal
loci in Neisseria gonorrhoeae – In: Genetic exchange:
anti-microbial resistance in tilapia and their pond water
a celebration and a new generation – Proceedings of the
in Trinidad – Zoonoses. Public Health 55: 206-213.
25th Wind River Conference. New York, N.Y: Marcel
Ogbondeminu F.S., Olayemi A.B. 1993 – Antibiotic resistance
in enteric bacterial isolates from fish and water media – J.
Dekker: 310-325.
Aqua. Trop. 8: 207-212.
Cooke M.D. 1976 – Antibiotic resistance in coliform and fecal
Omar A.O., Ronald R., Steffen B. 2007 – Conjugative transfer
coliform bacteria from natural waters and effluents – N.
of chromosomally encoded antibiotic resistance from
Z. J. Mar. Freshwat. Res. 10: 391-397.
Helicobacter pylori to Campylobacter jejuni – J. Clin.
Goñi-Urriza M., Capdepuy M., Arpin C., Raymond N.,
microbiol. 45: 402-408.
Caumette P., Quentin C. 2000 – Impact of an urban efflu-
Pathak S.P., Gopal K. 2005 – Occurrence of antibiotic and
ent on antibiotic resistance of riverine Enterobacteriaceae
metal resistance in bacteria from organs of river fish –
and Aeromonas spp. – Appl. Environ. Microbiol. 66:
Environ. Res. 98: 100-103.
Pembroke J.T., MacMahon C., McGrath B. 2002 – The role of
Grabow W.K., Prozesky O.W. 1973 – Drug resistance of
conjugative transposons in the Enterobacteriaceae – Cell.
coliform bacteria in hospital and city sewage – Antimicro.
Mol. Life Sci. 59: 2055-2064.
Agents Chemother. 3: 175-180.
Sambrook J., Fritsch E.F., Maniatis T. 1989 – Molecular clon-
Jury K.L., Vancov T., Stuetz R.M., Khan S.J. 2010 – Antibiotic
ing – Cold Spring Harbor Laboratory Press, Cold Spring
resistance dissemination and sewage treatment plants –
Harbor, New York.
Current Research, Technology and Education Topics in
Sanyal S., Basu A., Banerjee S. 2011 – Drug resistance pro-
Applied Microbiology and Microbial Biotechnology:
files of coliforms from sewage exposed fish – World J.
fish Marine Sc. 3: 275-282.
Sayah R.S., Kaneene J.B., Johnson Y., Miller R. 2005 – Pat-
Enterobacteriaceae – In: Manual of Clinical Microbiology
terns of antimicrobial resistance observed in Escherichia
4th ed. (Eds) W.J. Hausler, K.L. Herrmann, H.D.
coli isolates obtained from domestic and wild animal
Isenberg, H. Shadomy, American Society for Microbiol-
fecal samples, human septage, and surface water – Appl.
ogy, Washington, DC: 347-352.
Environ. Microbiol. 71: 1394-1404.
Kim S.S., Diana S.A. 2007 – Potential ecological and human
Schluter A., Heuer H., Szczepanowski R., Forney L.J.,
health impacts of antibiotics and antibiotic-resistant bac-
Thomas C.M., Puhler A., Top E.M. 2003 – The 64 508 bp
teria from wastewater treatment plants – J. Toxicol. Envi-
IncP-1 â antibiotic multi resistance plasmid pB10 iso-
ron. Health. 10: 559-573
lated from a waste-water treatment plant provides evi-dence for recombination between members of different
Koplin D.W. 2002 – Pharmaceuticals, hormones, and other
branches of the IncP-1 â group – Microbiology 149:
Organic wastewater contaminates in U.S. Streams,
1999-2000. A National Reconnaissance – Environ. Sci.
Schwartz T., Kohnen W., Jansen B., Obst U. 2003 – Detection
Technol. 36: 1202-1211.
of antibiotic-resistant bacteria and their resistance genes
Lorenz M.G., Wackernagel W. 1994 – Bacterial gene transfer
in wastewater, surface water, and drinking water biofilms
by natural genetic transformation in the environment –
– FEMS Microbiol. Ecol. 43: 325-335.
Microbiol Rev. 58: 563-602.
Scott J.R. 1993 – Conjugative transposons – In: Bacillus
Mach P.A., Grimes D.J. 1982 – R-plasmid transfer in
subtilis and other gram positive bacteria (Ed.) A.
a wastewater treatment plant – Appl. Environ. Microbiol.
Sonnenshein, J.A. Hoch, R. Losick, American Society for
44: 1395-1403.
Microbiology, Washington, DC: 597-614.
Mazel D. 2006 – Integrons: agents of bacterial evolution – Nat.
Strauss M. 1997 – Health (pathogen) considerations regard-
Rev. Microbiol. 4: 608-620.
ing the use of human waste in aquaculture – Environ.
Miranda C.D., Zemelman R. 2001 – Antibiotic resistant bacte-
Res. Forum. 5: 83-98.
ria in fish from the Concepción Bay, Chile – Marine
Walter M.V., Vennes J.W. 1985 – Occurrence of multi-
Pollut. Bull. 42: 1096-1102.
ple-antibiotic-resistant enteric bacteria in domestic
Transferable drug-resistant coliforms in fish exposed to sewage
sewage and oxidation lagoons – Appl. Environ.
wastewater treatment facilities – Water Air Soil Pollut.
Microbiol. 50: 930-933.
217: 473-479.
West B.M., Liggit P., Clemans D.L., Francoeur S.N. 2011 –
Yutaka S., Naohiro S., Yohei D., Yoshichika A. 2004 – Esche-
Antibiotic resistance, gene transfer, and water quality
richia coli producing CTX-M-2 beta lactamase in cattle,
patterns observed in waterways near CAFO farms and
Japan – Emer. Infect. Dis. 10: 69-75.
Source: http://www.infish.com.pl/wydawnictwo/Archives/Fasc/work_pdf/Vol21Fasc1/Vol21Fasc1_w04.pdf
Takeaways Toolkit Tools, interventions and case studies to help local authorities develop a response to the health impacts of fast food takeaways A London Food Board and Chartered Institute of Environmental Health publication Based on a consultancy report by Food Matters Updated June 2014 In 2010 the issue of fast food takeaways hit the headlines when a number of London
Annual Management Report 2007-2008 An environment focused on caring for clients! TABLE OF CONTENTS MESSAGE FROM THE PRESIDENT AND THE DIRECTOR GENERAL . 2 DECLARATION BY THE DIRECTOR GENERAL . 4 ORGANIZATIONAL STRUCTURE . 5 A PROFILE OF THE CSSS POPULATION AND TERRITORY. 8 BOARD AND COMMITTEE MEMBERS . 11








