
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Somnomedics.de

Author's personal copy
Psychiatry Research 189 (2011) 62–66
Contents lists available at ScienceDirect
Psychiatry Research
Schizophrenia patients with predominantly positive symptoms have more disturbed
sleep–wake cycles measured by actigraphy
Pedro Afonso a,⁎, Sofia Brissos a, Maria Luísa Figueira b, Teresa Paiva ba Lisbon's Psychiatric Hospitalar Center (CHPL), Lisbon, Portugalb Hospital Santa Maria, Faculty of Medicine, University of Lisbon, (FMUL), Lisbon, Portugal
Sleep disturbances are widespread in schizophrenia, and one important concern is to determine the impact of
Received 28 February 2010
this disruption on self-reported sleep quality and quality of life (QoL). Our aim was to evaluate the sleep–wake
Received in revised form 20 December 2010
cycle in a sample of patients with schizophrenia (SZ), and whether sleep patterns differ between patients with
Accepted 31 December 2010
predominantly negative versus predominantly positive symptoms, as well as its impact on sleep quality andQoL. Twenty-three SZ outpatients were studied with 24 h continuous wrist-actigraphy during 7 days. The
quality of sleep was assessed with the Pittsburgh Sleep Quality Index (PSQI), and the self-reported QoL was
Pittsburgh Sleep Quality Index
evaluated with the World Health Organization Quality of Life — Abbreviated version (WHOQOL-Bref). About
half of the studied population presented an irregular sleep–wake cycle. We found a trend for more disrupted
Negative symptoms
sleep–wake patterns in patients with predominantly positive symptoms, who also had a trend self-reported
worse quality of sleep and worse QoL in all domains. Overall, patients with worse self-reported QoLdemonstrated worse sleep quality. Our findings suggest that SZ patients are frequently affected with sleep andcircadian rhythm disruptions; these may have a negative impact on rehabilitation strategies. Moreover, poorsleep may play a role in sustaining poor quality of life in SZ patients.
2011 Elsevier Ireland Ltd. All rights reserved.
Although sleep architecture improves with antipsychotic treatment,
sleep remains mostly fragmented and fails to establish its normal
Sleep is an important restorative physiologic process (Suresh Kumar
pattern (Kupfer et al., 1970). This suggests that sleep physiology might
et al., 2007). The sleep–wake cycle is a circadian rhythm generated and
share a common substrate with SZ symptoms (Boivin, 2000; Poulin
regulated by the suprachiasmatic nucleus of the hypothalamus,
et al., 2003; Cohrs, 2008). Furthermore, worse sleep quality has been
synchronized by internal (body) and external (environment) stimuli
associated with poorer quality of life (QoL), even after correcting for
(Albrecht, 2002; Van Gelder, 2004).
depression and drug effects (Hofstetter et al., 2005; Ritsner et al., 2004).
Patients with schizophrenia (SZ) frequently experience sleep
Positive and negative symptoms, neurocognitive impairment and brain
problems (Keshavan et al., 1990; Taylor et al., 1991; Tandon et al.,
structure may also correlate with important sleep variables such as sleep
1992; Monti and Monti, 2004; Cohrs, 2008), like advanced sleep phase
latency, sleep efficiency, SWS, and REM sleep parameters (Cohrs, 2008).
syndrome and hypersomnia with short naps (Wirz-Justice et al.,
REM density was inversely correlated with positive, cognitive, and
2001). Reduced sleep efficiency and total sleep time, increased sleep
emotional discomfort symptoms as well as the total score on the Positive
latency, decrease in slow wave sleep (SWS) and rapid eye movement
and Negative Syndrome Scale (Yang and Winkelman, 2006).
(REM) latency have also been reported in most patients with SZ
However, because of the limited number of methodologically
(Tandon et al., 1992; Zarcone et al., 1987; Keshavan et al., 1998). This is
rigorous studies, no clear statement can be made about the influence
especially true during psychotic episodes (Kupfer et al., 1970), but also
of these variables on sleep structure (Cohrs, 2008). Actigraphy has been
in the prodromal phase (Donlon and Blacker, 1975; Cohrs, 2008).
used in SZ to study sleep and circadian rhythms (Haug et al., 2000;
It remains unclear whether sleep problems in SZ are secondary
Poyurovsky et al., 2000; Shamir et al., 2000; Hofstetter et al., 2005;
to social withdrawal and reclusive behavior, to medication, or to an
Martin et al., 2005; Wulff et al., 2006), but studies have been limited,
abnormality of the neuroendocrine systems regulating sleep and
probably for methodological feasibility reasons (Vanelle, 2009).
wakefulness (Wulff et al., 2006).
One of the most important goals in SZ treatment is social and
professional rehabilitation. To accomplish this, physiologic sleep,compatible with work routines and timetables is necessary.
We aimed to evaluate the sleep–wake cycle in a sample of SZ
⁎ Corresponding author. Lisbon Psychiatry Hospital Center, Av. do Brasil n°. 53, 1749-
002 Lisbon, Portugal. Tel.: +351 217917000; fax: +351 217 952 989.
patients, and whether sleep patterns differed between patients with
E-mail address: [email protected] (P. Afonso).
predominantly negative versus predominantly positive symptoms.
0165-1781/$ – see front matter 2011 Elsevier Ireland Ltd. All rights reserved.
doi:10.1016/j.psychres.2010.12.031
Author's personal copy
P. Afonso et al. / Psychiatry Research 189 (2011) 62–66
Moreover, we hypothesized that worse quality and sleep patterns,
would be associated with poorer self-reported QoL.
Sociodemographic and clinical characteristics.
2. Material and methods
positive symptoms)
negative symptoms)
2.1. Participants
Gender (men:women)
Data herein reported is based on an ongoing investigation on sleep characteristics
of SZ patients at Lisbon's Psychiatric Hospital Center. Twenty-three patients with SZ,aged 19–52 yrs, were diagnosed according to DSM-IV criteria (APA, 2000), ascertained
from interview with a psychiatrist and medical chart review. Patients were evaluated
with the Positive and Negative Syndrome Scale — PANSS (Kay et al., 1989). Based on
Educational level (yrs)
the PANSS scores, patients were divided into two groups according to symptom
Illness duration (yrs)
preponderance: Group 1 (n = 11) included patients with predominant positive
Number of admissions
symptoms, and Group 2 (n= 12) included patients with predominant negative
Legend: yrs: years; S.D.: standard deviation.
⁎ p b0.05.
At the time of evaluation all patients had been on a stable medication regimen for at
least 1 month. Patients taking benzodiazepines in daily doses lower than the equivalentof diazepam 15 mg (never taken after 18h00) were accepted. A minimum washoutperiod of 72 h was obligatory for hypnotic medication. The daily dosage of
(iii) mean duration (s) of uninterrupted immobility (MIP)(activity =0). The mean
antipsychotics and diazepam used in each treatment group was as follows. (1) Group
duration of uninterrupted immobility periods (MIP) provides a global measure
1; olanzapine (n=4), mean 18.8 mg; quetiapine (n=1), mean 600 mg; risperidone
of the distribution and number of immobility periods.
(n=3), mean 6 mg; clozapine (n=3), mean 367 mg; diazepam (n=2) mean 7.5 mg;(2) Group 2: olanzapine (n=4), mean 17.5 mg; quetiapine (n=1), mean 400 mg;
2.4. Statistical analysis
risperidone (n=2), mean 4.5 mg; clozapine (n=4), mean 362.5 mg; ziprasidone(n=1), mean 120 mg; diazepam (n=3) mean 8.3 mg.
Both groups were compared in sociodemographic, and clinical variables using
Schizoaffective disorder, organic impairment, previous head trauma or neurolog-
nonparametric Mann–Whitney test, Chi-Square and contingency (symmetry). Correla-
ical disorders, or substance abuse/dependence were considered exclusion criteria.
tions of sociodemographic and clinical variables, WHOQOL-BREF, PSQI, and actigraphy
Patients working night-shifts were also excluded.
data were calculated through Spearman correlation. We used SPSS 17.0 (SPSS Inc.,
The local Ethics committee approved the study, and all participants provided
Chicago, IL, USA).
written informed consent.
2.2. Assessment instruments
The quality and patterns of sleep were measured with the Pittsburgh Sleep Quality
Social, demographic, and clinical characteristics of our sample are
Index (PSQI) (Buysse et al., 1989). This self-report questionnaire rates sleep quality and
presented in Table 1. Significant statistical differences were found
patterns during the previous month, and evaluates 7 components of sleep: subjective
only in professional activity, patients with preponderant negative
quality, latency, duration, usual efficiency, sleep disturbances, medication use, anddaytime dysfunction. The score is given through a Likert-like scale, between 0 and 3,
symptoms being all inactive.
with a cut-off value of 5, higher scores meaning worse sleep quality.
There was no difference in PANSS total (Group 1, mean 89.4, S.D.
Subjective quality of life (QoL) was assessed using the WHO Quality of Life Measure —
19.3; Group 2, mean 82, S.D. 22.6) or general psychopathology results
Abbreviated version (WHOQOL–BREF–PT), Portuguese version. The 26-item version
between both groups (Group 1, mean 48.5, S.D. 10.5; Group 2, mean
WHOQOL–BREF (W.H.O.Q.O.L. Group, 1994) provides measurement on four domains:
44, S.D. 11.0). Because of the patients' allocation, the positive
physical, psychological, social relationships, and environment. Higher scores meanbetter QoL.
symptom's PANSS subscale results were higher in Group 1 (mean
Wrist-actigraphy was used for sleep–wake cycle assessment. Actigraphy provides
24.2, S.D. 5.4) than in Group 2 (mean 12.3, S.D. 3.2). On the other
the recording of continued limb motor activity during 24 h or longer periods and is a
hand, negative symptom's PANSS subscale results were higher in the
useful and validated instrument for sleep studies (Ancoli-Israel et al., 2003). Actimeters
latter (mean 26.1, S.D. 9.2) than in Group 1 (mean 16.6, S.D. 5.2). For
(SomnoWatch® actigraphy system) were strapped on the patients' nondominantwrists. Movement counts of the actigraph were stored in 1 second interval, with the
these results, there was statistical significance.
signal sampled at 32 Hz with 12 Bit ADC, allowing continuous recording for 168 h
Both groups presented a score above of 5 on the PSQI, which is
(7 days) on the 16 MB memory. We chose the smallest possible interval to achieve
considered as poor sleep quality (Table 2). Group 2 subjects self-
maximum temporal resolution. Since actigraphy cannot distinguish between sleep and
reported better QoL in all domains, compared to Group 1, but the
sedentary activities, subjects recorded in a diary, bed and wake times, awakenings
differences were not statistically significant (Table 2).
at night, day naps, and other activities. During the study period all participants usedthe equipment at all times, except when bathing. In these situations we asked
No significant differences were found in motor activities measure-
the participants to mark this period by pressing a button to mark events on the
ments during wakefulness (day) and sleep (night) between the two
SomnoWatch® (before and after bathing). Data during these periods were treated as
groups (Table 3).
2.3. Sleep/wake variables and actigraphic parameters
Table 2Quality of sleep and quality of life in both patient groups.
Sleep latency was considered as the time period between turning-off the light and
falling asleep. The actigraph contains a light sensor that measures the time between the
instant when the light goes off and the reduction of motor activity (typical of sleep
onset). To score sleep onset we used the 5 min of actigraphic immobility criterion (Chae
positive symptoms) negative symptoms) Test
et al., 2009). To assess sleep–wake cycles the actogram was visually analysed and
confronted with the sleep log information given by the patients. The sleep–wakerhythm was considered regular when there was a clear distinction between sleep/
inactivity occurring at night and wake/activity periods occurring during the day, and
regular occurrences of both during the week.
Using the sleep log information, and the light sensor information, we divided the
WOQOL — physical
data between night (period between lights off and wake) and day period. Data were
transferred to our own EXCEL® templates for further analyses. We chose to extract
WOQOL — psychological
three variables from the data that had previously been described (Middelkoop et al.,
1997). Actigraphy measures were calculated as follows:
WOQOL — social domain 40.4 (20.7)
WOQOL — environmental 59.7 (17.5)
(i) movement index (MI) (%), indicating the percentage of epochs with an activity
Legend: PSQI: Pittsburgh Sleep Quality Index; WHOQOL: World Health Organization
(ii) activity level (AL), number of activity counts per hour.
Quality of Life.
Author's personal copy
P. Afonso et al. / Psychiatry Research 189 (2011) 62–66
Actigraphy results between the patient groups.
Our results show that there is a trend for more disrupted sleep–
wake patterns and circadian activity rhythms in SZ patients with
positive symptoms)
negative symptoms)
predominant positive symptoms. In fact, Group 1 patients self-
reported worse quality of sleep as compared to Group 2, except forone item (hypnotic use). Moreover, Group 1 patients self-reported
Sleep latency (min)
Movement index (%)
worse QoL in all domains as compared to patients with predominant
negative symptoms, indicating that worse sleep patterns may lead
to worse subjective quality of sleep and QoL. Our results support
Activity level (number of counts/hour)
previous findings that SZ patients show more disrupted sleep–wake
patterns and circadian activity compared to healthy subjects (Martin
Uninterrupted immobility duration (s)
et al., 2005).
An important aspect in actigraphy results was sleep latency; in both
groups sleep latency proved to be quite long (usually, sleep latency
Regular sleep–wake
should take about 30 min), as confirmed by the patients' subjective
opinion of worse quality of sleep.
Legend: min: minutes; s: seconds; S.D.: standard deviation.
We found differences, although not statistically significant,
b Chi-square test.
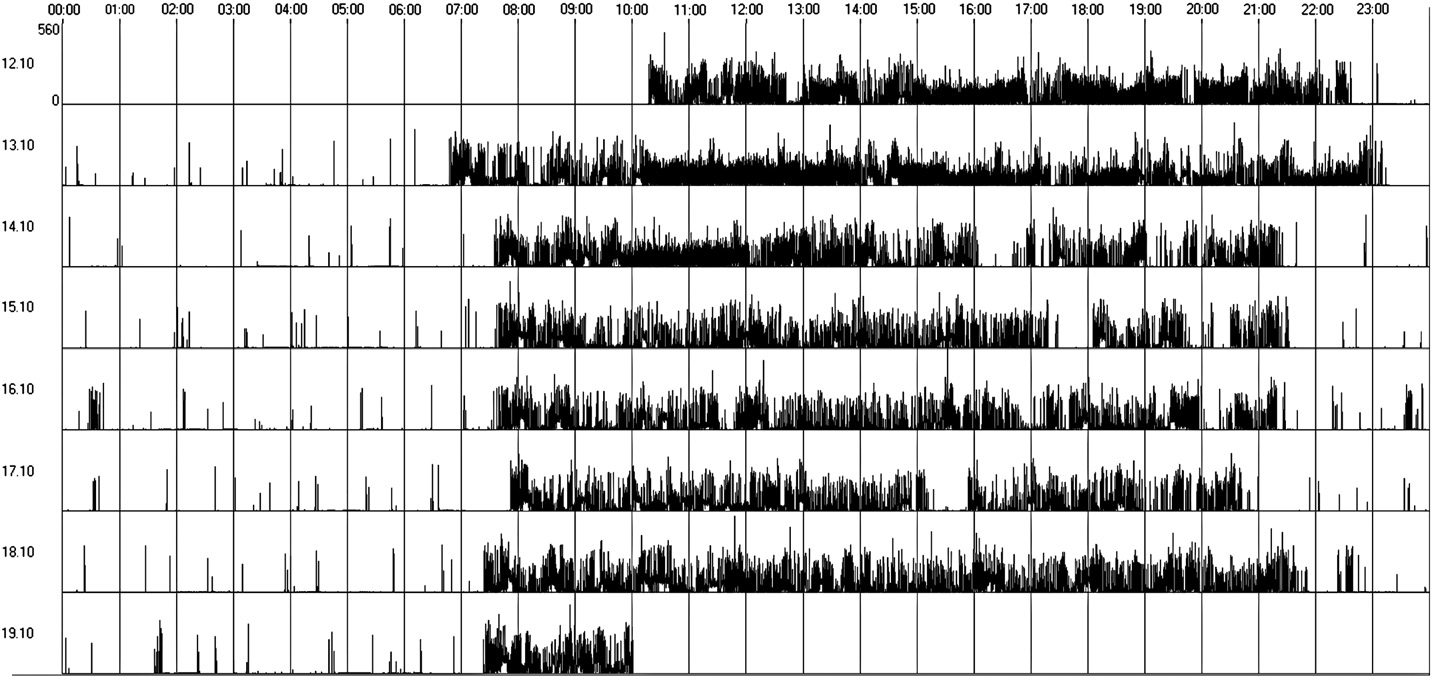
between the groups regarding regular sleep–wake rhythms; 11 outof 23 subjects showed an irregular sleep–wake rhythm. We verified arelative absence of a circadian pattern of the sleep–wake cycle. The
Group 1 presented worse actigraphy patterns with 7 patients (64%)
actogram and the sleep log showed that sleep times were randomly
presenting an irregular sleep–wake cycle (Fig. 2) and only 4 patients
distributed throughout the day and night and sleep duration and
showing regular sleep–wake cycles (Table 3 and Fig. 1). Sleep latency
wake-up episodes were variable and unpredictable during the 24 h
was higher in Group 1 (Table 3), but these differences were not
period. Actigraphy confirmed daytime napping and nighttime
statistically significant. Only 36% of Group 1 patients presented
fragmentation, a common finding in SZ patients (Yamadera et al.,
normal alternation between sleep/wake states, as compared to 67%
in Group 2; however, these differences were not statistically significant.
We found a significant association between psychopathology and
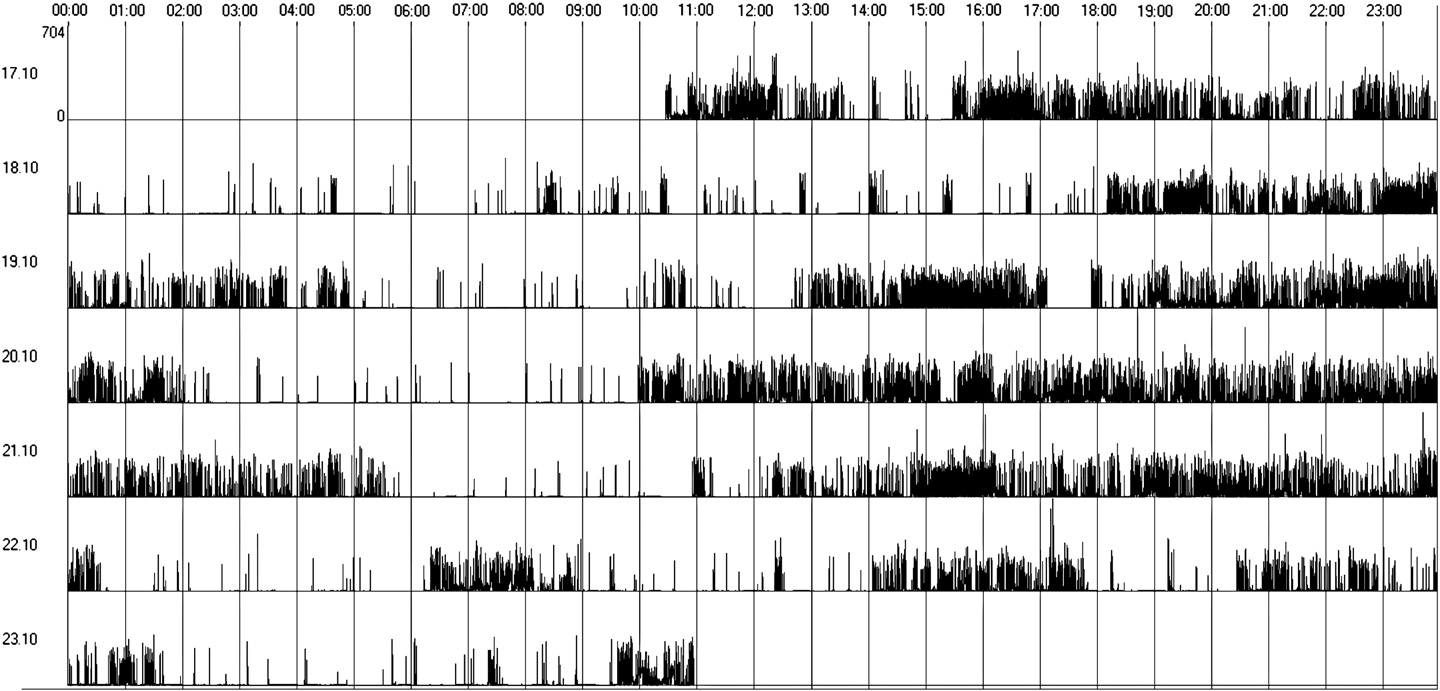
In Group 2, 4 patients (33%) presented irregular sleep–wake pattern,
general symptoms, and quality of sleep, but no significant correlations
while 8 showed regular sleep–wake cycles; these differences were
with positive and negative symptoms, and quality of sleep. Other
not significant (Chi2=2.112; p=0.220), but the contingency analysis
symptoms, namely depressive, could possibly influence more the
showed symmetry among them (p=0.146).
quality of sleep, than positive or negative symptoms. We were able to
Significant negative correlations were found between PANSS sub-
replicate earlier findings showing that in schizophrenia self-reported
scales' scores and WHOQOL-BREF and PSQI scores, indicating that
QoL is associated with quality of sleep (Ritsner et al., 2004; Hofstetter
higher symptom levels correlate with lower self-reported QoL and
et al., 2005; Xiang et al., 2009). In our sample, patients with
worse quality of sleep (Table 4). However, we found no significant
predominant negative symptoms reported better quality of sleep
correlations between sleep/wake variables and quantitative motor
and better QoL. Patients with negative syndrome present low motor
activity on actigraphy, and scores on any PANSS subscales, and/or any
activity levels (Walther et al., 2009), possibly facilitating sleep. On the
QoL domain (data not shown).
other hand, hallucinations and delusions may cause difficulty in falling
Fig. 1. Regular sleep–wake rhythm (actogram obtained by actigraphy over a 7-day period).
Author's personal copy
P. Afonso et al. / Psychiatry Research 189 (2011) 62–66
Fig. 2. Irregular sleep–wake rhythm (actogram obtained by actigraphy over a 7-day period).
asleep, and thereby having a negative impact on both sleep quality and
fetal organs such as the brain and pineal gland (Richardson-Andrews,
2009). Moreover, lesions of the suprachiasmatic nucleus have been
Evidence shows that when circadian rhythm sleep is disturbed,
postulated to play an important role in the aetiology of SZ (Trbovic,
daytime sleepiness, insomnia, and displacement of social timetables
2010). Therefore, future studies should explore the relation between
occur (APA, 2000). This contributes negatively to rehabilitation
sleep–wake cycles and melatonin levels in SZ patients.
interventions. Interestingly, we found an association between having
The inclusion of patients who were taking benzodiazepines before
a daily occupation, and better sleep quality and lower sleep latency,
18h00 is a study limitation, since activity levels are significantly
indicating that social and professional rehabilitation could reflect
reduced following an acute administration of lorazepam 2.5 mg
positively in the patients' circadian rhythms.
(Dawson et al., 2008). Despite that, in our sample all the 4 patients
Unexpectedly, we found no significant correlations between
had been taking diazepam (medium dose=8 mg) for long-term. Our
psychopathology, self-reported QoL and quality of sleep, and sleep/
patients presented elevated symptom levels, and therefore our results
wake variables registered by actigraphy, possibly due to the small
may not be applicable to patients in remission.
sample size. Also, our sample consisted of rather symptomatic patients,
Previous studies reported lower quantitative motor activity
possibly with low insight into their quality of sleep and QoL.
parameters than those of the present study (Walther et al., 2009;
Atypical antipsychotics tend to improve sleep induction and/or
Farrow et al., 2005; Farrow et al., 2006). This can probably be explained
sleep maintenance in SZ patients (Monti and Monti, 2004), and most
by differences in algorithms used for calculation of activity and by
atypical antipsychotics demonstrate an increase in total sleep time
differences between the actigraphs, since they have different sensor
and/or sleep efficiency in SZ patients, with the exception of risperidone
types, sampling rates and storage rates. Furthermore, the American
(Cohrs, 2008). To disentangle the effects of schizophrenia itself
Academy of Sleep Medicine (AASM) referred some problems when
from the influence of medication on sleep is difficult (Cohrs, 2008),
comparing actigraphy data. According to the "AASM Standards and
but it seems unlikely that treatment only could explain the differences
Practice", additional research is needed which compares results from
between the groups.
different actigraphy devices and the variety of algorithms used to
The causes for the present findings remain unknown. The tendency
evaluate actigraphy data in order to further establish standards of
for more daytime inactivity sleepiness periods in Group 2 patients
actigraphy technology (Morgenthaler et al., 2007).
may be explained by social withdrawal, reclusive behavior, and
Quantitative motor activity parameters during wakefulness were
low motor activity levels associated with the negative syndrome
related to the clinically assessed negative syndrome in schizophrenia
(Walther et al., 2009). On the other hand, positive symptoms, namely
(Walther et al., 2009). In our study we didn't replicate these findings,
suspiciousness, hallucinations and hyperactivity, could make it harder
probably because of the small sample size. Furthermore, we didn't
for the patients to fall asleep, due to an increased neurophysiologic
find a relation between motor activity parameters and sleep–wake
arousal, explaining the longer sleep latency, and lower total sleep time
rhythm. This could be explained by the fact that schizophrenia
found in this group.
is a heterogeneous disease with significant differences in activity or
Another good candidate to explain changes in the sleep–wake
immobility periods. Therefore, the volume of specific executive brain
rhythm is melatonin, a hormone produced by the pineal gland, and an
structures may affect motor behaviors. Cumulative motor activity
endogenous synchronizer of circadian rhythms. Nocturnal plasma
over a 20 h period was correlated with the volume of left anterior
melatonin levels have been reported to be reduced in SZ patients as
cingulate cortex in schizophrenia patients (Farrow et al., 2005).
compared with normal controls (Shamir et al., 2000; Viganò et al., 2001;
Finally, our results are preliminary and due to the small sample
Mann et al., 2006; Suresh Kumar et al., 2007), probably due to changes
size, and lack of a control group, need further replication.
of the pineal gland (Sandyk et al., 1990). Some theories have proposed
In summary, our findings show a trend for more disrupted sleep–
that SZ is caused by a damage produced in utero, to zinc dependent
wake patterns and circadian activity rhythms in SZ patients with
Author's personal copy
P. Afonso et al. / Psychiatry Research 189 (2011) 62–66
predominant positive symptoms; these patients also self-reported
Martin, J.L., Jeste, D.V., Ancoli-Israel, S., 2005. Older schizophrenia patients have more
worse quality of sleep and worse QoL in all domains as compared to
disrupted sleep and circadian rhythms than age-matched comparison subjects.
Journal of Psychiatric Research 39, 251–259.
patients with predominant negative symptoms.
Middelkoop, H.A., van Dam, E.M., Smilde-van den Doel, D.A., Van Dijk, G., 1997. 45-hour
These circadian abnormalities may reinforce the altered sleep
continuous quintuple-site actimetry: relations between trunk and limb move-
patterns, cognitive problems, and social engagement, with a negative
ments and effects of circadian sleep–wake rhythmicity. Psychophysiology 34,199–203.
impact on rehabilitation strategies. Moreover, our results support the
Monti, J.M., Monti, D., 2004. Sleep in schizophrenia patients and the effects of
hypothesis that poor sleep may worsen QoL in SZ patients. In clinical
antipsychotic drugs. Sleep Medicine Reviews 8, 133–148.
practice, psychiatrists should give more attention to sleep complaints
Morgenthaler, T.I., Lee-Chiong, T., Alessi, C., Friedman, L., Aurora, R.N., Boehlecke, B.,
Brown, T., Chesson Jr., A.L., Kapur, V., Maganti, R., Owens, J., Pancer, J., Swick, T.J.,
in schizophrenia since that can have a negative impact in the quality
Zak, R., 2007. Standards of Practice Committee of the American Academy of Sleep
of life of these patients.
Medicine Practice parameters for the clinical evaluation and treatment of circadianrhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep 30,1445–1459.
Conflict of interest
Poulin, J., Daoust, A.M., Forest, G., Stip, E., Godbout, R., 2003. Sleep architecture and its
clinical correlates in first episode and neuroleptic-naive patients with schizophrenia.
The authors report no conflict of interest. Dr. Sofia Brissos is consultant for Janssen-
Schizophrenia Research 62, 147–153.
Cilag Portugal.
Poyurovsky, M., Nave, R., Epstein, R., Tzischinsky, O., Schneidman, M., Barnes, T.R.,
Weizman, A., Lavie, P., 2000. Actigraphic monitoring (actigraphy) of circadianlocomotor activity in schizophrenic patients with acute neuroleptic-induced
akathisia. European Neuropsychopharmacology 10, 171–176.
Richardson-Andrews, R.C., 2009. The sunspot theory of schizophrenia: further evidence,
The authors thank Dr. Gama Marques for his contribution in patient recruitment.
a change of mechanism, and a strategy for the elimination of the disorder. MedicalHypotheses 72, 95–98.
Ritsner, M., Kurs, R., Ponizovsky, A., Hadjez, J., 2004. Perceived quality of life in
schizophrenia: relationships to sleep quality. Quality of Life Research 13, 783–791.
Sandyk, R., Kay, S.R., Schizophrenia, Bulletin, 1990. Pineal melatonin in schizophrenia:
Albrecht, U., 2002. Regulation of mammalian circadian clock genes. Journal of Applied
a review and hypothesis. Schizophrenia Bulletin 16, 653–662.
Physiology 92, 1348–1355.
Shamir, E., Laudon, M., Barak, Y., Anis, Y., Rotenberg, V., Elizur, A., Zisapel, N., 2000.
American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental
Melatonin improves sleep quality of patients with chronic schizophrenia. The
Disorders. Washington, DC, 4th ed. (DSM IV-TR).
Journal of Clinical Psychiatry 61, 373–377.
Ancoli-Israel, S., Cole, R., Alessi, C., Chambers, M., Moorcroft, W., Pollak, C.P., 2003. The
Suresh Kumar, P.N., Andrade, C., Bhakta, S.G., Singh, N.M., 2007. Melatonin in
role of actigraphy in the study of sleep and circadian rhythms. Sleep 26, 342–392.
schizophrenic outpatients with insomnia: a double-blind, placebo-controlled
Boivin, D.B., 2000. Influence of sleep–wake and circadian rhythm disturbances in
study. The Journal of Clinical Psychiatry 68, 237–241.
psychiatric disorders. Journal of Psychiatry & Neuroscience 25, 446–458.
Tandon, R., Shipley, J.E., Taylor, S., Greden, J.F., Eiser, A., DeQuardo, J., Goodson, J., 1992.
Buysse, D.J., Reynolds, C.F., Monk, T.H., Berman, S.R., Kupfer, D.J., 1989. The Pittsburgh
Electroencephalographic sleep abnormalities in schizophrenia: relationship to
Sleep Quality Index: a new instrument for psychiatric practice and research.
positive/negative symptoms and prior neuroleptic treatment. Archives of General
Psychiatry Research 28, 193–213.
Psychiatry 49, 185–194.
Chae, K.Y., Kripke, D.F., Poceta, J.S., Shadan, F., Jamil, S.M., Cronin, J.W., Kline, L.E., 2009.
Taylor, S.F., Tandon, R., Shipley, J.E., Eiser, A.S., 1991. Effect of neuroleptic treatment on
Evaluation of immobility time for sleep latency in actigraphy. Sleep Medicine 10,
polysomnographic measures in schizophrenia. Biological Psychiatry 30, 904–912.
Trbovic, S.M., 2010. Schizophrenia as a possible dysfunction of the suprachiasmatic
Cohrs, S., 2008. Sleep disturbances in patients with schizophrenia: impact and effect of
nucleus. Medical Hypotheses 74, 127–131.
antipsychotics. CNS Drugs 22, 939–962.
Van Gelder, R.N., 2004. Recent insights into mammalian circadian rhythms. Sleep 27,
Dawson, J., Boyle, J., Stanley, N., Johnsen, S., Hindmarch, I., Skene, D.J., 2008.
Benzodiazepine-induced reduction in activity mirrors decrements in cognitive
Vanelle, J.M., 2009. Schizophrenia and circadian rhythms. L'Encéphale 35, 80–83.
and psychomotor performance. Human Psychopharmacology 23, 605–613.
Viganò, D., Lissoni, P., Rovelli, F., Roselli, M.G., Malugani, F., Gavazzeni, C., Conti, A.,
Donlon, P.T., Blacker, K.H., 1975. Clinical recognition of early schizophrenic decom-
Maestroni, G., 2001. A study of light/dark rhythm of melatonin in relation to cortisol
pensation. Diseases of the Nervous System 36, 323–327.
and prolactin secretion in schizophrenia. Neuro Endocrinology 22, 137–141.
Farrow, T.F., Hunter, M.D., Wilkinson, I.D., Green, R.D., Spence, S.A., 2005. Structural
W.H.O.Q.O.L. Group, 1994. The Development of the World Health Organization Quality
brain correlates of unconstrained motor activity in people with schizophrenia. The
Of Life assessment instrument (the WHOQOL). In: Orley, J., Kuyken, W. (Eds.),
British Journal of Psychiatry 187, 481–482.
Quality of Life Assessment: International Perspectives. Springer Verlag, Heidelberg,
Farrow, T.F., Hunter, M.D., Haque, R., Spence, S.A., 2006. Modafinil and unconstrained
pp. 41–60.
motor activity in schizophrenia: double-blind cross-over placebo-controlled trial.
Walther, S., Koschorke, P., Horn, H., Strik, W., 2009. Objectively measured motor activity
The British Journal of Psychiatry 189, 461–462.
in schizophrenia challenges the validity of expert ratings. Psychiatry Research 169,
Haug, H.J., Wirz-Justice, A., Rössler, W., 2000. Actigraphy to measure day structure as a
therapeutic variable in the treatment of schizophrenic patients. Acta Psychiatrica
Wirz-Justice, A., Haug, H.J., Cajochen, C., 2001. Disturbed circadian rest-activity cycles in
Scandinavica 407, 91–95.
schizophrenic patients: an effect of drugs? Schizophrenia Bulletin 27, 497–502.
Hofstetter, J.R., Lysaker, P.H., Mayeda, A.R., 2005. Quality of sleep in patients with
Wulff, K., Joyce, E., Middleton, B., Dijk, D.J., Foster, R.G., 2006. The suitability of
schizophrenia is associated with quality of life and coping. BMC Psychiatry 3, 5–13.
actigraphy, diary data, and urinary melatonin profiles for quantitative assessment
Kay, S.R., Opler, L.A., Lindenmayer, J.P., 1989. The Positive and Negative Syndrome Scale
of sleep disturbances in schizophrenia: a case report. Chronobiology International
(PANSS): rationale and standardization. The British Journal of Psychiatry 7, 59–67.
23, 485–495.
Keshavan, M.S., Reynolds, C.F., Kupfer, D.J., 1990. Electroencephalographic sleep in
Xiang, Y.T., Weng, Y.Z., Leung, C.M., Tang, W.K., Lai, K.Y., Ungvari, G.S., 2009. Prevalence
schizophrenia: a critical review. Comprehensive Psychiatry 31, 34–47.
and correlates of insomnia and its impact on quality of life in Chinese schizophrenia
Keshavan, M.S., Reynolds III, C.F., Miewald, M.J., Montrose, D.M., Sweeney, J.A., Vasko Jr.,
patients. Sleep 32, 105–109.
R.C., Kupfer, D.J., 1998. Delta sleep deficits in schizophrenia: evidence from
Yamadera, H., Takahashi, K., Okawa, M., 1996. A multicenter study of sleep–wake
automated analyses of sleep data. Archives of General Psychiatry 55, 443–448.
rhythm disorders: clinical features of sleep–wake rhythm disorders. Psychiatry and
Kupfer, D.J., Wyatt, R.J., Scott, J., Snyder, F., 1970. Sleep disturbance in acute
Clinical Neuroscience 50, 195–201.
schizophrenic patients. The American Journal of Psychiatry 126, 1213–1223.
Yang, C., Winkelman, J.W., 2006. Clinical significance of sleep EEG abnormalities in
Mann, K., Rossbach, W., Müller, M.J., Müller-Siecheneder, F., Pott, T., Linde, I., Dittmann,
chronic schizophrenia. Schizophrenia Research 82, 251–260.
R.W., Hiemke, C., 2006. Nocturnal hormone profiles in patients with schizophrenia
Zarcone Jr., V.P., Benson, K.L., Berger, P.A., 1987. Abnormal rapid eye movement
treated with olanzapine. Psychoneuroendocrinology 31, 256–264.
latencies in schizophrenia. Archives of General Psychiatry 44, 45–48.
Source: http://somnomedics.de/fileadmin/SOMNOmedics/Dokumente/PsychiatryReseach_Schizophrenia_Patients-Akti.pdf
& about . National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS)National Institutes of HealthPublic Health Service • U.S. Department of Health and Human Services For Your Information This publication contains information about medicationsused to treat the health condition discussed in this booklet.When this booklet was printed, we included the most up-to-date (accurate) information available. Occasionally,new information on medication is released.
COMPOSANTES BIOLOGIQUES Quelques fondements de biologie moléculaire et de génie génétique intéressants pour approfondir le sujet, malgré le parti pris instructionniste qui sous-tend la présentation. Source : Centre Scientifique de la Biotechnologie/Industrie Canada http://strategis.ic.gc.ca/. Qu'est-ce qu'une cellule ? La cellule est l'unité du monde vivant et les millions de types différents d'organismes qui







