
Cialis ist bekannt für seine lange Wirkdauer von bis zu 36 Stunden. Dadurch unterscheidet es sich deutlich von Viagra. Viele Schweizer vergleichen daher Preise und schauen nach Angeboten unter dem Begriff cialis generika schweiz, da Generika erschwinglicher sind.
Introduction
Alcohol studies in translational models:
behavioural consequences of adolescent
exposure and novel approaches to reduce the
propensity to relapse.
The research described in this thesis was conducted at the department of Anatomy and Neurosciences, Neuroscience Campus Amsterdam, VU University Medical Center, Amsterdam, The Netherlands and at the department of Molecular and Cellular Neurobiology, Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University, Amsterdam, The Netherlands. My research was supported by ZONMW Topgrant 912-06-148. Cover: artwork by Martijn C.L. Van Roovert. Layout by Esger Brunner. Printed by GVO drukkers & vormgevers B.V. P+L ISBN: 978-90-6464-703-1 Copyright J.A. Wouda (
[email protected]), 2013. Al rights reserved. No part of this thesis may be reproduced, stored in a retrieval system, transmitted in any form without prior permission from the author.
VRIJE UNIVERSITEIT
Alcohol studies in translational models:
behavioural consequences of adolescent exposure and
novel approaches to reduce the propensity to relapse
ACADEMISCH PROEFSCHRIFT
ter verkrijging van de graad Doctor aan
de Vrije Universiteit Amsterdam,
op gezag van de rector magnificus
prof.dr. F.A. van der Duyn Schouten,
in het openbaar te verdedigen
ten overstaan van de promotiecommissie
van de Faculteit der Aard- en Levenswetenschappen
op donderdag 7 november 2013 om 13.45 uur
in de aula van de universiteit,
De Boelelaan 1105
Jelte Aerjen Wouda
geboren te Zaanstad
prof.dr. T.J. de Vries
Lees commissie:
Dr. H.B.M Lesscher
Dr. R.O. Stiedl
prof. dr. W. van den Brink
prof. dr. M. Kindt
prof. dr. A.N.M. Schoffelmeer
prof. dr. S. Spijker
prof. dr. R.W.H.J. Wiers
Table of Contents
Chapter 1
An introduction to alcohol-use, adolescence and rat models for human behaviour
A history of adolescent binge-like alcohol exposure increases alcohol self-administration in adulthood, but leaves visuospatial attention and inhibitory response control unaffected. Chapter 3
Peri-adolescent alcohol exposure brings on long-lasting spatial memory deficits.
Varenicline attenuates cue-induced relapse to alcohol-, but not nicotine-seeking, while reducing inhibitory response control Chapter 5
Disruption of long-term alcohol-related memory reconsolidation: Role of β-adrenoceptors and NMDA receptors Chapter 6
General Discussion
7. Summary in Dutch, Nederlandse samenvatting 155 8. Acknowledgements/ Dankwoord
Table of Figures
Figure 1. Water and alcohol are polar molecules
Figure 2. Mesolimbic dopamine (reward) system
Box 3. DSM-IV criteria alcohol-use disorders
Figure 3. Adolescent brain development
Box 2. Translating human adolescence
Box 3. Alcohol treatment
Box 4. Self-administration-reinstatement paradigm
Box 5. 5-choice serial reaction time task
Box 6. Spatial memory paradigms
Figure 4. Alcohol-intake during CSA treatment
Figure 5. Blood alcohol levels BLI treatment
Figure 6. SA performance of CSA-treated animals
Table 1 . Overview statistical analysis SA data CSA
Figure 7. SA performance in BLI-treated animals
Table 2 . Overview statistical analysis SA data BLI
Figure 8. 5CSRTT performance of post-adolescent CSA rats
Figure 9. 5-CSRTT performance of peri-adolescent CSA rats
Table 3. Overview statistical analysis 5CSRTT Data CSA
Figure 10. 5-CSRTT performance of post-adolescent BLI rats
Figure 11. 5-CSRTT performance of peri-adolescent BLI rats
Table 4 . Overview statistical analysis 5CSRTT Data BLI
Table 5 . Preclinical studies of adolescent alcohol exposure
Figure 12. Experimental design of the RAM paradigm
Figure 13. Experimental design of the OPR paradigm
Figure 14. Performance test phase RAM
Figure 15. Performance test phase OPR
Figure 16. Varenicline effects on self-administration
Figure 17. Varenicline effects on cue-induced reinstatement
Figure 18. Varenicline effects on 5-CSRTT performance
Figure 19. Varenicline effects under increased attentional load
Figure 20. Acquisition of alcohol self-administration.
Figure 21. Memory retention after propranolol treatment
Figure 22. Memory retention after MK801 treatment
Figure 23. BLI alcohol treatment
Chapter 1
An introduction to alcohol-use, adolescence
and rat models for human behaviour
1.1. Rationale
This thesis revolves around alcohol-(ab)use, adolescence and translational rat
models for human behaviour. Alcohol is the world's most popular addictive
substance and is said to be the cause of and solution to all of life's problems
(Simpson 1997). Indeed, excessive alcohol consumption poses serious health,
domestic and economic problems. Associated with approximately 2.5 million
deaths a year and accounting for 4.5% of the global disease burden, alcohol-
(ab)use claims more lives than HIV and costs up to 3.3% of the gross domestic
product in western societies (WHO 2010). Considering this, it is not surprising
that independent expert panels in the Netherlands and the United Kingdom
classified alcohol as the most harmful drug to users and to others (Nutt et al.
2010; RIVM et al. 2009). Of course, the putative detrimental consequences of
alcohol-(ab)use depend on many factors, including the quantity and pattern of
intake. Binge drinking, is an example of a very popular but hazardous type of
alcohol-consumption. It involves episodic consumption of large quantities of
alcohol in a short period of time, often with the aim to get intoxicated. The
National Institute on Alcohol Abuse and Alcoholism recently defined binge
drinking as "
a pattern of drinking alcohol that brings blood alcohol concentration
(BAC) to 0.08 gram percent or above. For the typical adult, this pattern
corresponds to consuming five or more drinks (male), or four or more drinks
(female), in about two hours" (NIAAA 2004). Demographically, binge drinking
and other forms of excessive alcohol consumption are most abundant among
adolescents and young adults (van Laar et al. 2010). In the Netherlands this has
contributed to an alarming escalation of the number of hospitalizations of
adolescents suffering from alcohol intoxication, from 237 individuals in 2007 to a
massive 762 in 2011 (van der Lely et al. 2011).
Problems related to alcohol-(ab)use, have been on both political and scientific
agenda's for decades. Nonetheless, our understanding of the long-term
consequences of adolescent alcohol-use remains insufficient and studies aiming
to counter the wide occurrence of (relapse to) alcohol-misuse have yielded few
pharmacological treatments for alcohol-use disorders (AUDs) and these few
have only limited efficacy (Anton et al. 2006a). Consequently, there is a
compelling need for the development of novel strategies to aid alcoholics in
recovery and to achieve a better understanding of the aetiology of AUDs.
Employing experimental rodent behavioural models, this thesis aims to extend
the current knowledge on these subjects by approaching alcohol-(ab)use from
two different angles. One set of experiments explores adolescent vulnerability to
alcohol exposure. Investigating how a history of adolescent alcohol-use affects
performance of rats in behavioural models of cognition and alcohol-taking and
seeking in later life. Using the same behavioural models, a second set of
experiments studies the feasibility of novel approaches to aid alcohol-abusers
during cessation and to prevent relapse. This introduction is therefore shaped
according to three overarching themes that are consecutively considered: (1)
alcohol, alcohol-(ab)use and putative pharmacotherapies; (2) the adolescent
period; (3) behavioural paradigms that model cognition and alcohol-taking and
seeking in rats.
1.2. Alcohol
Alcohol is a well known, but poorly understood outsider among addictive
substances. Chemists use the term alcohol for a group of organic molecules that
all feature a carbon atom that is bound to a hydroxyl group. However, in this
thesis the term refers to one specific acyclic alcohol that consists of a 2-
hydrocarbon backbone bound to a single hydroxyl group (C2H5OH). This
molecule bears the chemical name ethanol, yet to the general public it is better
known as alcohol (
Figure 1).
1.2.1. Alcohol-(ab)use
In nature, alcohol is formed by fermentation of starch or sugar containing fruits
and plants. Although it is unknown when the first humans drank alcohol, the
unearthing of late Stone Age beer jugs indicates fermented beverages were
already produced over 12.000 years ago (Patrick, 1952). Since then, alcohol has
grown into one of the most widely (ab)used drugs in the world. Figures from the
Dutch Trimbos-Institute indicate that approximately 80% of the population over
twelve years of age occasionally drinks alcohol, 10% of these are considered
heavy drinkers. In total, the consumption of alcohol in the Netherlands adds up
to roughly 7.9 litres pure alcohol, per person per year (van Laar et al. 2010).
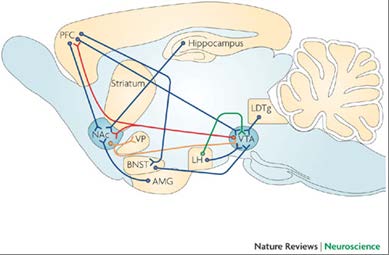
Figure 1. Water is a polar
molecule due to the positively
charged hydrogen atoms that are
bound to one side of the
negatively charged oxygen
molecule. Alcohol owes its
amphiphilic nature to the
combination of non-polar
hydrocarbon chain bound to a
polar hydroxyl group.
Figure 2. Simplified schematic of the circuitry of the mesolimbic dopamine (reward)
system in the rat brain highlighting the major inputs to the nucleus accumbens (NAc) and
ventral tegmental area (VTA) (glutamatergic projections, blue; dopaminergic projections,
red; GABAergic projections, orange; orexinergic projections, green). AMG, amygdala;
BNST, bed nucleus of the stria terminalis; LDTg, laterodorsal tegmental nucleus; LH,
lateral hypothalamus; PFC, prefrontal cortex; VP, ventral pal idum.(Kauer and Malenka
2007)
Upon ingestion, this alcohol is predominantly absorbed in the stomach and small
intestine. Through the gastrointestinal tract it enters the bloodstream, spreads
throughout the body and eventually penetrates the brain by crossing the blood
brain barrier. The enjoyable and addictive properties of alcohol are general y
attributed to modulation of neural signalling in this organ.
With alcohol-use, the pattern of intake is an important factor determining how
alcohol affects individuals. Moderate consumption, i.e. one (females) or two
(males) daily alcoholic consumptions, is considered low-risk drinking and may
actually have positive cardiovascular effects (Li 2008). High-risk drinking, on the
other hand, is often associated with hangover or mild withdrawal symptoms.
Moreover, high-risk drinking is associated with detrimental effects on cognitive
functioning (Li 2008;Tapert et al. 2004b). High-risk drinking also increases the
risk of developing alcohol-use disorders (AUDs). The classification method of
mental health disorders (DSM-IV) distinguishes two classes of AUDs: alcohol-
abuse and alcohol-dependence. Both are characterized by persistent
maladaptive patterns of alcohol-use with adverse consequences. A diagnosis is
based on compliance to the criteria described in Box 1. In general these criteria
reflect either some form of tolerance to alcohol's effects and aspects that
indicate the loss of control over alcohol-intake.
Different variants of high-risk drinking patterns can be distinguished. Heavy
drinking is defined as frequent consumption of alcohol (Li 2008). Binge drinking,
has an intermittent pattern and is particularly common in adolescents (Tapert et
al. 2004a;van Laar et al. 2010;WHO 2004; 2010). It entails consuming more
than three (females) or over four (males) units within a short period. Binge
drinking induces blood alcohol concentrations of over 0.8 mg/dl (Li 2008;NIAAA
2004) and often results in withdrawal symptoms. Both high blood alcohol
concentrations and withdrawal periods are suggested to induce neuronal
damage (Guerri and Pascual 2010) and dysfunction of brain regions that control
cognitive functions, such as memory, decision making, attention and inhibitory
response control (which is involved in impulsive behaviour) (Crews and Boettiger
2009).
1.2.2. The complex effects of alcohol
Among addictive substances, alcohol has rather unique properties. It mediates
its reinforcing, addictive properties and neurotoxic effects differently. For
Box 1. DSM-IV criteria alcohol-use disorders (American Psychiatric Association 2001)
A. Criteria: DSM-IV alcohol-abuse (1 or more criteria for over 1 year)
1. Role Impairment (e.g. failed work or home obligations) 2. Hazardous use (e.g. Driving, swimming or operating machinery while
3. Legal problems related to Alcohol-use 4. Social or interpersonal problems due to Alcohol
B. Criteria: DSM-IV alcohol-dependence (3 criteria for over 1 year)
1. Tolerance (increased drinking to achieve same effect) 2. Alcohol Withdrawal signs or symptoms 3. Drinking more than intended 4. Unsuccessful attempts to cut down on use 5. Excessive time related to Alcohol (obtaining, hangover) 6. Impaired social or work activities due to Alcohol 7. Use despite physical or psychological consequences
instance, nicotine binds nicotinic acetylcholine receptors to mediate its rewarding effects (Foulds 2006). Similarly, the active ingredient of cannabis is a ligand for cannabinoid receptors (Matsuda et al. 1990). Curiously, there are no known receptors that directly mediate the effects of alcohol. Instead, alcohol seems to indirectly influence nearly every neurotransmitter system known to date (as well as a broad range of other signalling proteins and enzymes!) (Chastain 2006). Exactly how alcohol influences these molecular targets remains elusive, although allosteric modulation or water displacement is most likely involved. Allosteric modulation occurs when a compound binds to regulatory sites rather than the primary binding pocket of receptors, resulting in changed protein conformation that may influence receptor function. Similarly, water displacement is basically an indirect form of allosteric modulation (Klemm 1998). Because water and alcohol share the capacity to form hydrogen bonds with organic molecules, they compete with each other to interact with membrane molecules. Alcohol, however, is amphiphilic, i.e. it is attracted to both hydrophobic and hydrophilic membrane targets. As a result, the replacement of water by alcohol can change membrane properties and thereby the conformation and functionality of proteins that are embedded in the membrane (Klemm 1998).
Thus alcohol can influence various signalling systems in the brain, which in turn
may impact various behavioural modalities.
1.2.3. Alcohol and neuronal signalling
Among the neuronal signalling systems affected by alcohol is the glutamatergic
system, the most prominent excitatory neurotransmitter system in the brain.
The N-methyl-D-aspartate (NMDA) variant of glutamate receptors plays an
important role in executive and mnemonic functions, such as learning and
memory. Several lines of evidence indicate that alcohol reduces NMDA signalling
and glutamate release (Fadda and Rossetti 1998), which may be involved in the
memory impairing and general intoxicating effects of alcohol. Furthermore, upon
prolonged alcohol exposure, the number of NMDA-receptors increases (Diamond
and Gordon 1997), possibly explaining the seizures and hyper-excitability seen
during withdrawal from alcohol-abuse. Alcohol's modulatory activities are not
restricted to excitatory neurotransmission. For instance, by facilitating gamma-
amino-butyric acid (GABA) signalling it increases inhibitory communication in the
brain. Repeated alcohol exposure, on the other hand, reduces GABA-induced
hyper-polarization. The latter may be involved in behavioural tolerance to the
effects of alcohol (Chastain 2006).
Several other neurotransmitter systems suggested to be involved in the effects
of alcohol are related to its rewarding effects. Here activation of the mesolimbic
dopamine (DA) pathway plays a prominent role (Figure 2). DA signalling in this
"reward" pathway is considered to be involved in the reinforcing effects of all
drugs of abuse, including alcohol (Di Chiara and Imperato 1988). Accordingly,
modulation of the DA system affects alcohol consumption (Samson et al. 1992).
Alcohol may also affect cholinergic neurotransmission. For instance, it enhances
the function of several nicotinic acetyl-cholinergic receptor (nAchR) subtypes,
including the α4β2-nAchR, while inhibiting the function of others, such as the
α7-subtype (Davis and de Fiebre 2006). Finally, alcohol's reinforcing effects may
additionally depend on interactions with several other neurotransmitter systems
that are known to modulate neuronal signalling in the reward pathway, including
the opioid, cannabinoid, serotonergic, and histaminergic systems (Chastain
2006).
In summary, alcohol is an ancient and widely used addictive substance with
unique molecular properties that set it apart from other drugs of abuse.
Alcohol's amphiphilic nature enables it to alter the functional properties of a wide
variety of neural signalling molecules through indirect (allosteric) modulation.
Consequently, the neurobiological effects of alcohol-misuse are complex. This
may be one of the reasons for the scarcity of effective pharmacological
treatments for AUDs.
1.3. Pharmacotherapies for alcohol-related disorders
Only three pharmacological treatments for alcohol-dependence are approved by
the United States Food and Drug Association (FDA) (Mason and Heyser 2010).
The oldest, Disulfiram, prevents metabolism of the toxic alcohol metabolite
acetaldehyde by inhibiting the enzyme acetaldehyde dehydrogenase. As a result,
alcohol consumption while using Disulfiram leads to severely aversive
acetaldehyde-induced symptoms, such as headache, nausea and vomiting
(Chastain 2006). Despite its long track record, scientific evidence for
Disulfiram's efficacy is restricted. Its relapse-preventing effects are limited and
due to Disulfiram's aversive effects patient compliance is low (Williams 2005).
Chronologically, the opioid receptor antagonist Naltrexone is the second FDA
approved medication for the treatment of alcohol-dependence. It tempers the
reinforcing effects of alcohol, most likely by modulating DA signalling in the
mesolimbic pathway (Chastain 2006;Tambour and Quertemont 2007;Williams
2005). Although Naltrexone's long-term effectiveness is much debated, in
combination with behavioural counselling it has been shown to have positive
effects on drinking outcome (Anton et al. 2006). Furthermore, specific
polymorphisms in µ-opioid receptors have been suggested to predict clinical
response to Naltrexone in alcohol-dependent individuals (Oslin et al. 2006). Next
to Naltrexone, several other drugs targeting the opioid system are being pursuit
as alcoholism therapeutics and some have been suggested to effectively reduce
heavy drinking, craving and relapse (Tambour and Quertemont 2007).
Acamprosate is the final and most widely prescribed FDA approved
pharmacological treatment for AUDs in the USA (Mason and Heyser 2010). Its
mechanism of action is poorly understood, but it is believed to normalize
alcohol-induced dysregulation of glutamatergic and GABAergic signalling (Anton
et al. 2006;Mason and Heyser 2010). Acamprosate is well tolerated together
with additional medication. In combination with behavioural therapy, it was
shown to reduce intake and to prolong abstinence (Mason and Heyser
2010;Williams 2005). Interestingly, other drugs acting on glutamatergic
signalling have not made it past preclinical studies (Tambour and Quertemont
2007). GABAergic agents, such as Baclofen or the sedative Diazepam, have been more successful (Tambour and Quertemont 2007). Other putative pharmacotherapies have been explored. Topiramate, a drug that was originally marketed as an antiepileptic, has yielded some success in the treatment of alcoholism. Although the exact mechanisms are unknown, this mono-saccharin interferes with glutamatergic and GABAergic signalling and is believed to inhibit mesolimbic DA release (Tambour and Quertemont 2007;Williams 2005). Given the prominent role of DA in drug reinforcement, direct modulation of DA signalling system in the mesolimbic system is arguably a promising strategy in alcohol pharmacotherapy. However, due to the complex interaction of different DA-receptors and the variety of behavioural functions that are subserved by DA signalling, many clinical studies using DA-antagonists have been unsuccessful (Tambour and Quertemont 2007). Nonetheless, in Europe Tiapride, a DA D2-receptor antagonist, has been used to reduce withdrawal symptoms, and to promote abstinence (Chastain 2006). Other interesting pharmacological targets are neurotransmitter systems that are involved in the modulation of the DA system, such as the cannabinoid and serotonin (5-HT) systems. Until recently, the cannabinoid receptor antagonist Rimonabant, marketed for its anti-obesity properties, was also studied in addiction research. Despite promising initial results, Rimonabant was banned due to its severe side-effects, such as depression and suicidal thoughts (Cahill and Ussher 2011). The 5-HT system has also been targeted because of its role in anxiety. It was hypothesized that alcohol in some cases may be used as self-medication. That is, to alleviate anxiety and depression, for instance by increasing 5-HT levels in the brain. For that reason selective serotonin reuptake inhibitors, such as Citalopram or Fluoxetine, that act by facilitating the availability of serotonin have been prescribed to aid alcoholics suffering from comorbid depression. Also other antidepressants and sedatives, aiming to improve mood, may reduce the need to use alcohol (Chastain 2006;Tambour and Quertemont 2007;Williams 2005). Whether these approaches are truly effective remains to be examined. In summary, different pharmacotherapies have been explored for the treatment AUDs. Most aim at reducing the positive effects of alcohol-intake, ameliorating craving or restoring homeostasis, often by modulating the function of specific receptors or enzymes. A drawback of all types of pharmacotherapies for AUDs is
their limited efficacy compared to placebo. As is the case with many disorders in
DSM-IV, alcohol-dependence and alcohol-abuse are complex non-unitary
disorders. This may explain the limited efficacy of pharmacotherapies that are
often only effective in subgroups of AUD patients.
1.3.1. Novel treatment Strategies
Considering the
limited efficacy of
pharmacotherapeutics for AUDs, exploring novel strategies is of key importance.
A favourable and cost effective strategy is to use existing pharmacological
agents for new purposes. Recent examples of drugs that, based upon their
pharmacological profile, were reinvented to treat addiction are Topiramate and
Baclofen as described in more detail in the previous section. In line with this
reasoning, the novel smoking cessation aid varenicline deserves further
investigation.
Varenicline acts on nicotinic acetylcholine receptors (nAChRs) that are well
known for their ability to modulate the reinforcing effects of alcohol (Ericson et
al. 1998; 2000;Soderpalm et al. 2000). It belongs to a novel class of
therapeutics, aiming not to block or stimulate receptors, but rather to act as a
partial agonist. Such drugs are believed to have a dual action on DA release in
the mesolimbic pathway. On the one hand they block the stimulating effects of
drugs of abuse by shielding the nAChR receptor; on the other hand they reduce
withdrawal symptoms by moderately stimulating DA release (Coe et al.
2005;Niaura et al. 2006). This potent blend of activities has been proposed to
offer superior efficacy. Indeed, recent studies indicate that varenicline may be
effective in reducing alcohol self-administration (Steensland et al. 2007).
Varenicline's effects on relapse to alcohol-use are yet to be explored. As will be
discussed later, this was one of the aims of this thesis.
1.3.2. Manipulating alcohol-related memories
An entirely different approach in the treatment of addiction has been derived
from fear literature. In fear conditioning, rats are trained to associate a tone
with an electric foot shock. If rats are subsequently presented with this tone,
they express fear-related behaviour. A seminal paper published at the turn of
the century demonstrated that this fear expression was abolished when a
protein syntheses inhibitor was injected shortly after the memory that was
mediating fear expression was reactivated by presenting the animals with the
fear-associated tone (Nader et al. 2000). This paper redefined our
understanding of memory maintenance and renewed interest in the reconsolidation theory. This theory proposes that retrieval of a consolidated memory induces a transient period of plasticity, during which memories can be updated, strengthened or integrated in different memory traces. To be maintained after retrieval, active stabilization is required (Misanin et al. 1968;Nader and Hardt 2009). This process of reactivation and stabilization of memories was termed reconsolidation (Sara 2000). Recently, researchers have started to manipulate the reconsolidation process to affect fear memories in humans (Kindt et al. 2009). Specific roles for different brain regions have been identified, such as (basolateral) amygdala, hippocampus and nucleus accumbens, as well as molecular mechanisms, including protein synthesis, neurotransmission and downstream signalling pathways in reconsolidation memory traces (among others reviewed by Alberini 2005;Sorg 2012;Tronson and Taylor 2007). The fact that maladaptive memories can be manipulated during reconsolidation poses the question whether a similar approach may potential y be effective in addiction. In addiction environmental stimuli that have been associated with drug-use are involved in continuation of drug-use. Exposure to these stimuli can induce relapse even after long periods of abstinence (Everitt et al. 2001;Le and Shaham 2002). As such, (alcohol) addiction can be characterized as a chronic, relapsing disease in which maladaptive, drug-related memories play a crucial role. Therefore, over the last five years the putative therapeutic effect of reconsolidation blockade in addiction has been studied extensively in animal models. Moreover, a reconsolidation disruption-based therapy was recently shown to attenuate craving in heroin addicts (Xue et al. 2012). Of particular interest for the work presented here, is the accumulating evidence that suggests a pivotal role for glutamatergic and adrenergic neurotransmission in reconsolidation of drug-related memories (for review, see Sorg 2012). Different groups have reported that expression of conditioned place preference (a model frequently used in addiction research, for details see Section 1.6.2) for amphetamine and cocaine can be disrupted by post-reactivation administration of the NMDA-receptor antagonist dizocilpine (MK801) (eg. Brown et al. 2008b;Itzhak and Anderson 2007;Sadler et al. 2007) and the β-adrenergic receptor antagonist propranolol (eg. Bernardi et al. 2006;Fricks-Gleason and Marshall 2008). Recently, reconsolidation blockade using propranolol and dizocilpine was also shown to reduce cocaine-seeking in the Self-Administration-
Reinstatement paradigm (the behavioural model that is also used in Chapters 2,
4 and 5, for details see Section 1.6.2 and Box 4) (Milton et al. 2008a;Milton et
al. 2008b). Because AUD patients seeking treatment usually have a long history
of drug-use, they have been exposed to drugs for a long time and have
associated drug-use to numerous cues and potentially long periods of abstinence
before treatment, which perhaps induces stronger drug-related memories. In
order to develop a successful reconsolidation-based treatment for AUD patients,
these issues require further investigation in animal models. In Chapter 5 the
effect of post-reactivation treatment with propranolol or dizocilpine on relatively
old alcohol-related memories was explored.
1.4. Hallmarks of adolescence
The previous sections described what makes alcohol stand out among other
addictive substances, how it affects neuronal signalling and discussed existing
and putative new treatments for AUDs. The following section introduces the
stage of life during which most people start drinking alcohol, i.e. adolescence
(Grant et al. 2006;WHO 2010).
Adolescents abundantly indulge in excessive drinking aiming to become
intoxicated, with Dutch adolescents being among the heaviest drinkers in Europe
(van Laar et al. 2010;WHO 2010). Add to this that the number of
hospitalizations of adolescents suffering from alcohol intoxication in the
Netherlands is rapidly increasing (van der Lely et al. 2011), and it is clear why it
is important to explore how alcohol-misuse affects adolescents. But before
elaborating on this issue, the unique developmental period of adolescence
requires further introduction.
Adolescence, as it is witnessed in many different species, can be characterized
as a gradual period of transition from childhood to adulthood (Spear 2000). In
addition to noticeable physical development, adolescents go through extensive
behavioural changes that include a shift in social orientation from parents to
intensified peer relationships, expression of impulsive behaviour and increased
risk-taking (Spear 2000). This behavioural development coincides and may be
driven by hormonal changes and widespread neuronal maturation. It would be
short sighted, however, to consider adolescent development simply as infants
maturing into better functioning adults. The behavioural and neurobiological
changes that characterize adolescence also promote the necessary skil s for a
shift towards independence, such as openness to new challenges, an unmatched
ability to adapt, and a drive to invest in relationships with peers that will shape
the future (Spear 2000;Spear and Varlinskaya 2010).
1.4.1. Neuroanatomical maturation
Our understanding of adolescent brain development has greatly advanced with
the introduction of Magnetic Resonance Imaging (MRI). Studies using this
technology indicate that cortical development correlates with cognitive
development. During childhood, sensory systems mature before association
cortices, which are responsible for language skills and spatial attention, while
higher-order association areas, including the prefrontal cortex (PFC), continue to
develop into adolescence (Casey et al. 2005; 2008). Although MRI studies do
not allow for any conclusions regarding causality, it is interesting to note that
while the frontal lobes are developing, working memory, inhibitory response
control, planning, decision making and processing speed continuously improve
during adolescence (Bava and Tapert 2010;Chambers et al. 2003;Crews et al.
2007). By the time we reach adolescence, the total volume of our brain changes
little (Figure 3), yet significant morphological changes are ongoing. Grey matter
volume develops according to an inverted U-shape, with peak values at the
beginning of adolescence, followed by a gradual decline into adulthood (Bava
and Tapert 2010;Casey et al. 2005; 2008). In contrast to grey matter changes,
white matter volume seems to progress linearly into adulthood, with maximum
volumes being reached in adulthood (Bava and Tapert 2010;Casey et al.
2005;Paus et al. 2008). These changes, which are seen in humans and in
rodents, are an indication of ongoing remodelling of neuronal connections
throughout the brain, including PFC, limbic and hippocampal regions. These
brain areas are involved in reward processing, development of emotional,
analytical as well as executive functions. As such, their maturation may
determine the behavioural characteristics of adolescence, such as impulsivity,
risk-taking and increased peer interaction (Crews et al. 2007;Guerri and Pascual
2010).
Figure 3. Adolescent brain development. By the time we reach adolescence brain volume
changes little. Nevertheless, major changes are seen in neuronal connectivity and chemical
maturation.
1.4.2. Neurochemical maturation
Alongside morphological development, major neurochemical changes are
progressing during adolescence (Crews et al. 2007). Ontogenetic changes in
neurotransmitter receptor levels seem to reflect overproduction followed by
pruning of (particularly excitatory) synapses in mid and late adolescence (Spear
2000). For instance, glutamate binding to NMDA-receptors peaks during early
adolescence and declines into adulthood, with a loss of 30% of these glutamate
receptors in young adult rats (Crews et al. 2007;Guerri and Pascual 2010;Spear
2000). Development of GABA neurotransmission in rats mostly occurs from birth
into early adolescence, but regional receptor subunit levels as wel as GABAergic
connectivity continue to develop through adolescence into adulthood (Crews et
al. 2007;Spear 2000).
Interestingly, brain regions receiving DA input are also remodelled during
adolescence (Bava and Tapert 2010;Guerri and Pascual 2010;Spear and
Varlinskaya 2010). In the PFC, DA fibre density increases during adolescence,
and regional changes are seen in DA-transporter and receptor levels (Bava and
Tapert 2010;Spear 2000). Maturation of DA synthesis and turnover progresses
into adulthood and in vivo microdialysis studies suggest ontogenetic changes of
basal DA levels in the nucleus accumbens (NAc) during adolescence (Bava and
Tapert 2010;Chambers et al. 2003;Maldonado-Devincci et al. 2010b;Paus et al.
2008). Given their role in the reinforcing effects of addictive substances, the
maturation of DA signalling in these areas is a strong indication that the reward
pathway is being reshaped during adolescence (Bava and Tapert
2010;Chambers et al. 2003;Crews et al. 2007;Maldonado-Devincci et al.
2010b;Paus et al. 2008).
In conclusion, adolescence is a unique developmental period characterized by
marked neurobiological and behavioural changes (for review see Spear 2010).
While brain volume changes little, major changes are seen in morphology,
neuronal connectivity, receptor subunit compositions and neurotransmitter
levels. This neuronal maturation is ongoing in brain regions that have been
implicated in brain functions such as inhibitory response control, attention and
memory, which in turn may mediate the expression of the behaviour that is
characteristic for adolescence. Interestingly, functional MRI studies during
cognitive performance demonstrate more focal activation of brain regions in
adults as compared to adolescents (Casey et al. 2005; 2008). Therefore, it is
proposed that during adolescence connectivity in the brain is being fine-tuned,
leading to functionally improved and more efficient brain function.
1.5. Consequences of adolescent alcohol-(ab)use
The adolescent brain reacts to alcohol in a unique way. Adolescents seem more
sensitive to the neurotoxic effects of alcohol, while being less sensitive to its
sedative effects. This may allow youngsters to consume larger quantities of
alcohol without being impaired by its intoxicating effects (Nixon and McClain
2010). Furthermore, compared to adults, adolescents demonstrate facilitated
social interaction (Nixon and McClain 2010) and have an increased DA response
to alcohol in the NAc (Philpot et al. 2009). As such, adolescents may perceive
greater reinforcing effects of drinking alcohol.
Several factors may contribute to these ontogenetic differences in drug
response. There is speculation on pharmacokinetic differences between
adolescents and adults (Little et al. 1996), and changes in body composition and
hormones may affect drug metabolism. Also the ontogeny of drug binding
proteins and enzymes for drug metabolism likely plays a role (Spear 2000).
Furthermore the aforementioned neuroanatomical development of the brain may
affect the function of these brain regions and consequently lead to differential
responses between adults and adolescents (Spear 2000). Considering the
interaction of alcohol with the intricate developmental processes of adolescence,
adolescent binge-drinking may induce permanent changes in neurochemistry
and connectivity that lead to long-term behavioural effects (Guerri and Pascual
2010).
1.5.1. Lingering effects of adolescent alcohol-use
The notion that adolescent alcohol exposure may be especially hazardous has
spawned numerous epidemiological investigations. These studies indicate that
adolescent drinking is associated with alcohol-abuse and psychiatric disorders in
later life. Importantly, when alcohol-use is initiated earlier in life, the risk of
developing such disorders seems to increase (Bava and Tapert 2010;DeWit et al.
2000;Grant et al. 2006;McGue et al. 2001a; 2001b). Investigating the long-term
consequences of adolescent alcohol exposure, human studies have identified
possible detrimental effects on different aspects of behaviour, including alcohol
consumption, learning and memory, attention and impulsivity (Tapert 2002;
2004b;Tapert and Brown 1999). Alongside these behavioural deficits, MRI
studies indicate structural and functional changes in the brains of individuals
with a history of heavy adolescent alcohol-use. Grey and white matter integrity
seems to be compromised, hippocampal and PFC volume appear to be
decreased and heavy drinking adolescents show abnormal brain responses in
behavioural tasks (De Bellis et al. 2000; 2005;Medina et al. 2007;Tapert et al.
2004b).
Although these data suggest that adolescent alcohol-use may affect cognition
and neuronal integrity, human studies are unable to determine causality.
Alternatively, it is also possible that a pre-existing condition underlies both
adolescent alcohol-use and cognitive deficits in adulthood. In fact, several
studies indicate, that family relationships, truancy, poor behavioural
control/impulsive behaviour, and other cognitive disabilities are strong
predictors of adolescent alcohol-use (Maggs et al. 2008;McGue et al. 1992;
2001a). Therefore, studies independent from social, environmental and genetic
aspects are required to investigate whether the long-term behavioural changes
are attributable to alcohol-use.
1.6. Animal models
Animal models employed under stable laboratory conditions provide more
control over genetics and environmental factors. These models are simplified
abstractions of the human situation and represent control able aspects of the
more complex situation. For instance, the adolescent rat is often used to study the neurobiology of adolescence, because adolescence in rodents and humans has many parallels (Box 2). Furthermore, in the studies presented in this dissertation extensive use was made of behavioural paradigms to study specific aspects of human behaviour in laboratory rats. Given that these models are abstractions of the original situation, they have specific strengths and weaknesses. Or, as Rosenblueth and Wiener put it: "the best material model for a cat is another, or preferably the same cat" (Rosenblueth and Wiener 1945). In the case of rodent models, they all share the weakness that it is impossible to properly mimic the social, psychological and economic factors that are inextricably linked to the biological factors that drive human behaviour. For example, it wil be difficult to model peer pressure among teenagers, or the social consequences of alcohol-use for AUD patients in a laboratory rat model. Still, in many cases rodent models are the next best option to study the human brain. The translational value of these models can be determined by their relative "face", "construct" and "predictive" validity. This means considering whether the expressed behaviour is adequately homologous between rats and humans, if similar neurobiological processes are involved and whether the model is useful for predicting behavioural outcome of (pharmacological) manipulations in humans (Keeler and Robbins 2011). In the following sections, the strengths and weaknesses of the models relevant to this thesis are reviewed. In addition, it is highlighted how these models have contributed, or may contribute to the study of adolescent alcohol exposure-related behavioural deficits.
Box 2. Translating human adolescence
Compared to epidemiological studies, rodent models offer much better control over the
genetic and environmental influences that confound clinical studies. As such, they
provide an opportunity to investigate the impact of a specific manipulation. However,
one needs to be aware that specific paradigms can only model certain aspects of the
clinical situation. Whether we can model adolescence in rats is therefore a valid
question.
Dr. Linda Spear is one of the pioneers exploring adolescent drug-use in rats and has
written several comprehensive reviews about this unique developmental period (Spear
2000;Spear and Varlinskaya 2005;Spear and Varlinskaya 2010). In short, she
describes that several characteristics of adolescence are seen across different species.
Obviously, the time span of adolescence in rats is much shorter than in humans, and,
alike humans, the exact boundaries of adolescence in rats cannot general y be
delineated, as they differ between individuals. In rats adolescence may extend from
post natal day (PND) 20 to 60. Here it should be noted that adolescence is not the
same as puberty, which is an important part of adolescence and refers to the time
period in which sexual maturity is achieved. Reminiscent to human food-intake during
the growth spurt, adolescent rats display enhanced consummatory behaviour in this
period. Behavioural y, more sophisticated peer interaction, impulsivity and novelty-
seeking are seen in both humans and rats. These transient behavioural changes may
be evolutionary imprinted as they seem essential for becoming independent,
developing adult skil s and move away from the natal family.
Regarding human brain development, rats display analogous brain maturation
patterns, including proliferation, pruning and myelinisation. In contrast, the hormonal
changes seen in humans are less apparent in rats. Whereas the increase in release of
gonadal hormones at puberty is a common characteristic of al mammals, changes in
secretion of androgens is less universal, and not seen in rodents. Psychological
maturation is another aspect of adolescence that cannot be fully model ed.
Nonetheless, rodent models provide a powerful tool to aid in unravel ing the (long-
term) effects of drugs of abuse on the ongoing behavioural and neuronal development
in adolescence.
1.6.1. Alcohol exposure protocols
Thus far, most rodent studies have focussed on acute and short-term effects of
alcohol exposure on behaviour, whereas research on long-term effects is more
restricted and has yielded mixed results. This may be due to differences in
experimental design (e.g. strain, age of exposure, administration route and
exposure pattern), as well as the different behavioural paradigms that were
used to study the consequences of adolescent alcohol exposure. Indeed,
experimental outcome may be dependent on the method of adolescent alcohol
exposure (Maldonado-Devincci et al. 2010a). Careful consideration of a suitable
adolescent alcohol treatment regimen that precedes behavioural testing is
therefore essential. Commonly used procedures include feeding or drinking of
alcohol containing food or liquids, intra-peritoneal or intra-gastric administration,
and housing in alcohol vapour-saturated chambers.
In this thesis two distinct standardized alcohol exposure protocols were used
(Box 3). The first "continuous self-administration" (CSA) was employed to study
the long-term consequences of continuous voluntary consumption of moderate
amounts of alcohol. Rats receiving CSA treatment had continuous free access to
two bottles in the home cages, one containing sweetened alcohol, the other
water. Although adolescent rats will voluntarily drink large amounts of alcohol
(Maldonado-Devincci et al. 2010b), their intake does not reflect the high blood
alcohol levels and intermittent exposure pattern that are often seen in binge
drinking youth (Tapert et al. 2004a;van Laar et al. 2010;WHO 2010). Therefore,
binge drinking was mimicked with a "binge-like injection" (BLI) protocol. Rats
receiving this treatment were intermittently exposed to large quantities of
alcohol through intra-peritoneal injections. In Chapters 2 and 3 the long-term
consequences of CSA or BLI treatment were evaluated by investigating the
performance of rats in different behavioural paradigms five weeks after
treatment had ended.
Box 3. Alcohol treatment
Young rats were treated for ten days, either during (post natal day (PND) 34-43, peri-adolescent group) or directly after adolescence (PND 62-71, post-adolescent group)
Continuous Self-administration (CSA).
Wistar rats had a free choice to drink from two bottles on their home cage during treatment. One always contained water, the other contained either: 1. water (water group), 2. saccharin (0.2%, w/v) (saccharin group) or 3. sweetened alcohol (5-10%, v/v + saccharin 0.2%, w/v) (alcohol group), depending on the test group they were assigned to. During treatment the alcohol concentration was increased over time from 5% (day 1-4), 7.5% (day 5-7), to 10% (day 8-10). The position of the bottles was randomized between subjects and over days.
Binge-like injection (BLI).
Binge-like alcohol-treated animals were given a single intraperitoneal (i.p.) injection of 20% (v/v) alcohol (2.5g/kg) every other day for ten days. This resulted in a total of five injections; peri-adolescent animals were injected on postnatal day (PND) 34, 36, 38, 40 and 42; post-adolescent animals were injected at PND 62, 64, 66, 68 and 70. Control animals received an identical treatment with the exception that they were injected with sterile saline for i.p. injection instead of alcohol. Alcohol treatment resulted in blood alcohol levels exceeding 200
mg/dl during the first h after injections for both peri- and post-adolescent animals.
1.6.2. Modelling alcohol-taking and seeking
Of all behavioural modalities that may be altered by adolescent alcohol
exposure, alcohol-intake has been most widely studied. In line with human data,
several studies indicate that a history of adolescent alcohol consumption
increases alcohol-intake in (young) adult rodents (Maldonado-Devincci et al.
2010a;Pascual et al. 2009;Rodd-Henricks et al. 2002;Siciliano and Smith 2001).
However, several other groups have failed to reproduce these findings
(Siegmund et al. 2005;Slawecki and Betancourt 2002;Vetter et al. 2007). In
view of the variety in treatment protocols used in the abovementioned studies,
in Chapter 2, an operant alcohol Self-Administration-Reinstatement paradigm
(SA) (Box 4) was used to thoroughly dissect whether CSA and/or BLI treatment
affects different aspects of alcohol consumption. The SA paradigm was also
employed to examine the effects of novel treatment strategies for AUD disorders
in Chapters 4 and 5.
In the SA task responses on an "active" operandum (a nose-poke hole or lever)
are reinforced, for instance by delivery of an alcohol solution in a liquid-
receptacle. At the same time, responses at an adjacent "inactive" operandum
are without consequences. Alcohol delivery is coupled to discrete audiovisual
cues. As such, rats acquire alcohol self-administration and form stimulus-reward
associations. The SA paradigm has been widely used to study the neurobiology
of addiction, given that different aspects of (drug) self-administration, such as
acquisition, maintenance, motivation, escalation, extinction and relapse can be
dissected through manipulation of the task parameters (De Vries et al. 2001;De
Vries et al. 2003;Le and Shaham 2002;Shaham et al. 2003).
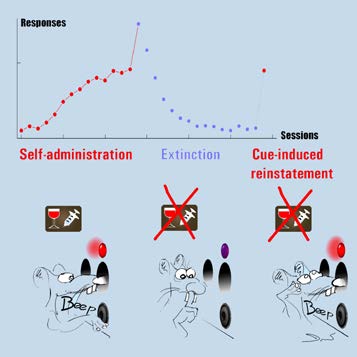

Box 4. Self-administration-reinstatement (SA) paradigm
SA operant chambers were sound-attenuated Skinner boxes with stainless grid floors.
One side of a chamber was equipped with a central liquid receptacle in which an
alcohol solution could be delivered. The receptacle was flanked by two apertures or
nose-poke holes, one active, the other inactive. The nose-poke holes were fitted with
red and white cue lights. A dim red house light and a tone module were built-in to the
opposite wal . In these boxes rats were trained to self-administer alcohol. Nose-pokes
in the active hole were reinforced with an alcohol solution that was always paired to
the presentation of an auditory and a visual cue. Upon acquisition, motivational aspects
of alcohol self-administration were examined by progressively increasing the number of
responses that were required to obtain reinforcement. This procedure is known as a
progressive ratio schedule of reinforcement. To assess whether cues coupled to alcohol
delivery could induce relapse, nose-poking behaviour was first extinguished. Thus,
animals were placed in the training context, but active responses were without
audiovisual cue-exposure or reinforcer delivery. Consequently, animals learn that nose-
poking is no longer reinforced and reduce their active responding. After extinction,
relapse was evaluated by monitoring nose-poke behaviour after presentation of the
cues that were previously paired to reinforcer delivery.
Picture of a SA operant chamber (left panel), Overview SA training (right panel).
Other animal models aimed at investigating aspects of (alcohol) addiction exist, such as conditioned place preference (CPP), two-bottle choice and drug discrimination paradigms. In the former, animals are trained in an apparatus with two distinct compartments. One compartment is paired with drug-administration, whereas the other is associated with placebo-administration. Following a given number of pairings, animals are allowed access to both
compartments without treatment. The animal's choice to spend more time in the drug-associated compartment provides a direct measure of the conditioned rewarding effect of a drug (Shippenberg and Koob 2000). In the two-bottle choice paradigm, an animals' preference for one of two bottles containing either an alcohol solution or water is evaluated by measuring fluid-intake. In the drug discrimination task, pressing one of two levers is reinforced after injection of a drug, whereas the other lever is reinforced after receiving vehicle. Training is continued until the animal learns to select the appropriate lever upon drug or vehicle injection. Once trained, tests of stimulus generalization or antagonism are implemented to determine whether a specific (drug) treatment produces interoceptive stimulus effects qualitatively similar to, or different from that of the training drug (Shippenberg and Koob 2000). For the present studies, the SA model was chosen because it can accurately measure more aspects of drinking than the two-bottle choice paradigm. Furthermore, it is general y regarded to be the most compelling animal model of addiction (Ahmed 2011;O'Connor et al. 2011;Shaham et al. 2003;Sorg 2012). Behaviour in the SA paradigm, as well as (pharmacological) modulation thereof, relates closely to the clinical condition. For instance, there is high concordance between the ability of drugs to support self-administration in rats and their abuse potential in humans (Epstein et al. 2006). A recent study reviewing alcohol and 70 other drugs that have been evaluated in the SA model reported that over 90% of drug cases were concordant with clinical indicators of abuse liability (O'Connor et al. 2011). This may not be surprising, given that the reward circuitry that mediates the reinforcing effects of addictive substances – which includes but is not limited to dopaminergic signalling in the mesolimbic system – is believed to be conserved across mammalian species (Ikemoto 2010). In addition, stimuli that trigger relapse in humans, i.e. (re)exposure to alcohol, alcohol-related cues or stress, reliably reinstate drug-seeking in the SA model (Shaham et al. 2003). Reinstatement also seems to be mediated by a conserved brain circuitry involving dopaminergic and glutamatergic signalling in the prefrontal and orbital frontal cortex, the amygdala, and the striatum (Kalivas et al. 2006). Although the alcohol SA paradigm clearly has predictive value and entails strong parallels with human behaviour and neurobiological processes, there are limitations to its validity. A major criticism is that self-administration does not reflect addiction-like behaviour, given that it is confined to daily sessions in
operant cages. As such, the pattern of intake is different from what is seen in addicts, there are little or no negative consequences to drug-use, and rats have little choice to exert any behaviour other than alcohol self-administration (Ahmed 2010). Considering reinstatement, it should also be acknowledged that the drug-free state preceding relapse occurs for different reasons compared to in humans, and the contingencies involved do not parallel those in human relapse (Epstein et al. 2006). In studying alcohol consumption an additional problem is that acquisition of the task is hampered by the fact that adult rats are less likely to drink alcohol, than for instance to self-administer cocaine. This may be related to the orosensory properties of alcohol that have been reported to induce conditioned taste aversion (Bienkowski et al. 1998). In addition, nose-poking behaviour and the pharmacological effects of alcohol are not tightly coupled in time (Le and Shaham 2002). This skewed temporal relationship is mainly caused by two factors. First, in contrast to most other drugs used in the SA paradigm, a single drop of alcohol earned in the model will not lead to physiologically relevant blood alcohol levels. Instead, it takes the accumulation of several drops of alcohol for a rat to experience its pharmacological effects. Second, it takes time for alcohol to be absorbed in the digestive system, enter the bloodstream and reach the brain. Both taste and delayed pharmacological effects may contribute to difficulty to train rats in the conditioned reinforcement model. Therefore, to facilitate acquisition of alcohol self-administration two distinct approaches were used. In Chapters 4 and 5 animals were trained to consume alcohol in a two-bottle free-access paradigm preceding the SA experiments. Unfortunately, in practice this approach was not applicable in the adolescence studies given that (1) the two-bottle choice paradigm spans more time than the five week period between treatment and testing and (2) an additional six weeks of alcohol exposure following treatment would undoubtedly affect experimental outcome. Therefore, in Chapters 2 and 3 a saccharin-fading procedure was used. Here the rats were given a saccharin sweetened alcohol solution during the first stages of self-administration. When the acquisition curve reached an asymptotic ceiling level, saccharin was slowly faded over time, until the animals responded for an unsweetened alcohol solution. Both methods were successful for acquiring alcohol self-administration, as was evidenced by an asymptotic learning curve and a clear distinction between responding in the active and inactive nose-poke hole.
Altogether, the SA paradigm is considered an animal model that is predictive of
abuse potential and has significantly contributed to the current understanding of
neuronal underpinnings of addiction (O'Connor et al. 2011;Shippenberg and
Koob 2000). In combination with saccharin-fading or the two-bottle choice, it is
well-tailored to study the various aspects of alcohol-taking and seeking
behaviour following adolescent alcohol exposure and to test the efficacy of novel
"anti-relapse" strategies.
1.6.3. Modelling impulsivity & attention
Above, the development of cognitive abilities during adolescence was already
addressed, including development of attentional functions and inhibitory
response control. The latter refers to action restraint and is involved in impulsive
behaviour. Interestingly, impulsivity has often been associated with addiction.
Some researchers propose that heightened levels of impulsive behaviour that
are seen in adolescents can be wired into the brain when alcohol exposure
disturbs brain maturation during adolescence (Crews et al. 2007). In line with
this idea, recent experiments in our laboratory have demonstrated that a history
of adolescent nicotine exposure increases impulsive behaviour in adult rats
(Counotte et al. 2008). Nonetheless, the evidence for an association between
adolescent alcohol exposure and increased impulsivity in later life is inconclusive
and the issue has not been addressed in rodent models.
With respect to attentional functioning, adolescent alcohol-use has been
suggested to ameliorate sustained attention in humans (Tapert and Brown
1999). Although acute attentional effects of alcohol are evident, only one study
has addressed the issue of attention-related long-term consequences of alcohol
exposure in rodents, demonstrating no lingering effects of adolescent alcohol
exposure (Slawecki 2006). Altogether, little is known about the impact of
adolescent alcohol-use on impulsivity and attention. In Chapter 2, the 5-choice
serial reaction time task (5-CSRTT) was employed to further study this (see Box
5). This model was also used in Chapter 4 to study the effects of a putative
novel AUD treatment on attention and impulsivity, given that 5-CSRTT was
initially developed to advance the understanding of medication as well as neural
and neurochemical systems that are involved in attention deficit/hyperactivity
disorder (ADHD) (Robbins 2002).


Box 5. 5-choice serial reaction time task (5-CSRTT)
5-CSRTT operant chambers were sound-attenuated skinner boxes with stainless steel
grid floors. One side of a chamber was equipped with a curved wal that contains five
adjacent apertures, so-cal ed nose-poke holes. The opposite wal was fitted with a food
pel et receptacle. In these boxes rats were trained to detect and respond to a visual
stimulus that appeared in one of the five nose-poke holes to obtain a food pel et (see
schematic overview of the task). By presenting rats with multiple trials per session we
this task was used to measure several behavioural parameters, including: (1)
accuracy, ((number correct trials/(correct + incorrect trials)) 100); (2) premature
responses, i.e. the number of premature responses before the onset of the visual
stimulus, reflecting aspects of loss of inhibitory control (inhibitory response control);
(3) omission errors, i.e. the total number of omitted trials during a session and (4)
correct response latency, i.e. the mean time between stimulus onset and response in
the il uminated aperture.
Picture of a5-CSRTT operant chamber with a food pellet receptacle (left panel),and a curved wal that contains five adjacent nose-poke holes on the right (right panel)
Flowchart 5-CSRTT
In the 5-CSRTT animals are trained to pay sustained attention to a panel of five
apertures and report when and where a stimulus light is presented. By asking
animals to divide their attention over five visual targets, chance performance in
the 5-CSRTT drops to 20%. As such, the 5-CSRTT offers superior effect
sensitivity over the 50% random performance in 2-choice procedures. In
addition, a measure of inhibitory response control can be derived from the
number of inappropriate premature responses that are made during the task
before a visual stimulus is presented. At baseline performance animals show low
within and between subject variance and both improvements and decrements in
performance can be measured. A limitation of the 5-CSRTT is that extensive
daily training (up to three months) is required to reach stable baseline
performance. Furthermore, it requires food restriction to motivate the animals.
The 5-CSRTT is a direct analogue of the human continuous performance task
(CPT), and therefore possesses strong face validity for measuring sustained
attention and inhibitory response control (Carli et al. 1983;Robbins 2002).
Furthermore, drugs that are known to affect impulsive behaviour and attention
in the CPT, also modulate these aspects in the 5-CSRTT. The underlying
neuroanatomical and neurochemical circuitry is thought to largely overlap
between humans and rodents. For instance, the 5-CSRTT engages those neural
systems that are also implicated in ADHD (Dal ey et al. 2008). Similar to the
reward system, these systems involve monoaminergic (i.e. dopaminergic,
noradrenalinergic and serotonergic) signalling in prefrontal and limbic regions
(Winstanley 2011). Over the years, numerous investigators have used the 5-
CSRTT to gain insight in the neurobiology of attention and inhibitory response
control (Pattij and Vanderschuren 2008;Robbins 2002;Winstanley 2011).
1.6.4. Spatial memory
Many psychiatric and neurological disorders, including drug addiction, involve
some aspect of memory impairment. For that reason memory performance has
been widely addressed in preclinical models. There is strong evidence that brain
regions that are known to be involved in memory formation, including the
hippocampus and PFC, are structurally and functionally compromised by
adolescent alcohol exposure (Bava and Tapert 2010;Nixon and McClain 2010).
There is also behavioural data supporting the memory impairing effects of
alcohol exposure (Markwiese et al. 1998;Pascual et al. 2007;Schulteis et al.
2008;Sircar and Sircar 2005), however these impairments may not necessarily
be age-dependent (Acheson et al. 2001;Silvers et al. 2003). In addition, the
long-term memory impairing effects of adolescent alcohol exposure are not well established. Yet, sensitivity to the memory impairing effects of alcohol may be altered by adolescent alcohol exposure (White et al. 2000). Therefore, the effect of adolescent alcohol exposure on spatial memory was examined in Chapter 3, using two different behavioural models (Box 6). The first model employed to study spatial memory was the radial arm maze (RAM) paradigm (Olton 1987;Seamans and Phillips 1994). In the RAM, rats are trained to find food pellets at the end of arms that radiate from a central platform. Depending on the exact test procedures, the RAM allows for measuring enduring or "steady state" differences in spatial memory in allocentric and egocentric settings, i.e. settings where rats are required to navigate according to spatial or bodily cues respectively (Paul et al. 2009). However, the RAM is not well suited for measuring rapid drug effects or to detect specific search strategies. Akin to the 5-CSRTT, the RAM is labour intensive and requires food restriction. As such, performance in the task may be influenced by satiety or nausea, e.g. induced by drugs. Another concern is that while navigating the maze, associative mechanisms may be used to construct cognitive maps of environment. For instance, olfaction or sensory information provided by the rat's vibrissae undoubtedly contribute to navigation, possibly leading to alternative (non-spatial) search strategies. Nonetheless, visual cues seem crucial for spatial learning (Hodges 1996) and the possibility to repeatedly measure performance allows comparison of behavioural performance in different treatment groups. Other maze paradigms can also be used to measure spatial memory, such as the T-maze or the Morris water maze (Hodges 1996;Paul et al. 2009). Here, the RAM was chosen for its positive reinforcement characteristics rather than aversive motivation (as is the case in the Morris water maze) and the flexibility of the paradigm. In addition, an object recognition paradigm was used to evaluate memory processes in rats (Dere et al. 2007). The object recognition model elegantly utilizes the rats' natural preference for novelty. In the object-place recognition (OPR) variant of this paradigm, rats are confronted with two previously encountered, identically shaped objects, one of which has been relocated. Without any training, deprivation or reinforcing stimuli, healthy laboratory rats will spontaneously pay more attention to the relocated object. Expression of this behaviour in rats requires recruitment of the same brain regions that are involved in memory processes in humans, including
Box 6. Spatial memory paradigms
Radial arm maze (RAM)
The RAM consisted of eight arms that radiate evenly from an elevated octagonal central platform. Rats were permitted entry from to the arms via control able Plexiglas guil otine doors that bordered the central platform. The end of each arm contained a sunken food wel . Rats were trained according to a so-
cal ed delayed spatial win-shift paradigm. In this paradigm training trials consisted of a
sample phase and a test phase. In the sample phase animals had to find food pel ets at
the end of four selected arms. The animals were removed and when 5 min had elapsed
the rats were placed back on the maze in order to retrieve food pel ets in the arms that
were not baited during the sample phase. Entering an arm that has been previously
visited, either during the sample phase or in the test phase, was considered a spatial
memory error. The number of errors that were made is compared between test groups
and function as a measure for spatial memory.
Object place recognition (OPR)
OPR was measured in a rectangular arena in which identical objects were placed. Similar to the RAM training OPR training included a sample phase and a test phase that were separated by a delay. In the
sample phase rats were al owed to explore two identical objects placed in two corners of the arena. During the delay one of the objects was shifted to a different corner. In the test phase, the animals were again al owed to explore the objects, one in the old position the other in a novel position. A discrimination index calculated as fol ows (time spent at repositioned - time at familiarly located object)/(time spent at repositioned + time at familiarly located object) revealed to what extent animals displayed OPR.
hippocampus and medial PFC (Dere et al. 2007;Hodges 1996;Olton 1987;Paul et al. 2009). Unfortunately, compared to the RAM the OPR is not as sensitive to changes in performance. In addition, performance may be influenced by olfactory and other sensory information as wells as by innate preference for shape, texture and structure of the objects (Dere et al. 2007). To increase exploration time one may therefore decide to use more complex objects.
To conclude, the OPR provides a relatively easy and time efficient measure of
spatial memory and adds to the possibilities that RAM paradigm offers, because
it is well suited to measure drug effects on spatial memory. Both paradigms
possess translational value, given that both paradigms can be used to predict
how pharmacological agents will affect human behaviour. Furthermore, the RAM
and OPR appear to recruit those neurobiological systems that humans require in
spatial memory tasks (Dere et al. 2007;Hodges 1996;Olton 1987;Paul et al.
2009).
1.7. Aim and outline
In this introduction the complex changes in structure and function that signify
the ongoing remodelling of the adolescent brain were outlined. Exposure to
alcohol - that is known to inhibit neurogenesis and target several of the
neurotransmitter systems that are developing during adolescence - may
therefore be especially hazardous during this period in life. In fact, it is proposed
that through disruption of normal brain maturation adolescent alcohol exposure
may induce long-lasting changes in brain function and behaviour. Indeed,
epidemiological studies have identified that behavioural modalities, such as
motivation, attention, inhibitory response control and spatial memory may be
affected by adolescent alcohol exposure. However, in these studies it is
impossible to discriminate between the "true" long-term effects of alcohol and
the contribution of pre-existing deficits or environmental influences. To fully
comprehend the long-term behavioural effects of adolescent alcohol exposure, it
is therefore important to establish a behavioural profile of adolescent alcohol
exposure that is independent from social, environmental and genetic influences.
Unfortunately, preclinical studies designed for this purpose have yielded mixed
results. As discussed, some studies find long-term behavioural deficits upon
adolescent alcohol exposure, while others do not, or even report improved
function in adolescent alcohol-treated animals. Interpretation of, and
comparison between these studies is hampered by the diversity of exposure
protocols and methodological approaches that have been used. Furthermore,
existing studies have largely focused on consumption and memory, whereas
other cognitive domains, such as attention and inhibitory response control, have
largely been ignored.
This thesis therefore aims to provide a comprehensive behavioural profile
of the long-term consequences of adolescent alcohol exposure,
independent from social, environmental and genetic aspects. To this end, in
Chapter 2 the standardized CSA and BLI treatment protocols (see Box 3) were
employed to systematically explore the consequences of adolescent alcohol
exposure on performance in behavioural models for alcohol-taking and seeking
(SA) and visuospatial attention and inhibitory response control (5-CSRTT). In
Chapter 3, the putative long-term behavioural changes of BLI exposure were
examined in models for spatial memory (RAM and OPR).
The second part of this thesis explores novel approaches in laboratory rats that
aid the development of clinical interventions aiming to reduce alcohol
consumption and prevent relapse in AUD patients. The aim is to identify
putative relapse-preventing properties of varenicline and
reconsolidation blockade using the SA paradigm. Chapter 4 reports on the
dose-dependent effects of varenicline, an α4β2-nicotinic receptor partial agonist,
on alcohol and nicotine-intake, as well as on its relapse-suppressing properties.
Furthermore, the 5-CSRTT was employed to examine the effects of varenicline
on attention and inhibitory response control. The aim of Chapter 5 was to
modulate relapse to alcohol-seeking behaviour by disrupting reconsolidation of
alcohol-related memories using post-reactivation administration of propranolol,
a non-selective β-adrenoreceptor antagonist, or dizocilpine, a non-competitive
NMDA-receptor antagonist. In Chapter 6, the main findings and clinical
consequences of these studies are discussed in a wider context.
Chapter 2
A history of adolescent binge-like alcohol
exposure increases alcohol self-administration
in adulthood, but leaves visuospatial attention
and inhibitory response control unaffected.
J.A. Wouda1,2, Yvar van Mourik1, T. Pattij1, T.J. De Vries1,2,
1Department of Anatomy and Neurosciences, Neuroscience Campus Amsterdam,
VU University medical center and 2Department of Molecular and Cellular
Neurobiology, Center for Neurogenomics and Cognitive Research, Neuroscience
Campus Amsterdam, VU University, Amsterdam, The Netherlands
Our research was supported by a ZON-MW Topgrant 912-06-148.
2.1. Abstract
Rationale. Most people start drinking alcoholic beverages during adolescence.
Although several epidemiological studies indicate that excessive alcohol
consumption during this developmental period may be particularly harmful,
preclinical data on the long-term consequences of adolescent alcohol exposure
remain sparse.
Objectives. We explored the putative long-term effects of adolescent alcohol
exposure on alcohol-taking and seeking as well as on measures of attention and
impulsivity. To this end, peri-adolescent (postnatal day (PND) 34-43) and post-
adolescent (PND 60-69) male Wistar rats were exposed to alcohol using two
distinct standardized protocols; i.e. a continuous self-administration (CSA)
protocol or an intermittent, binge-like injection (BLI) protocol. Depending on the
test group they were assigned to, CSA animals had a free choice between either
(1) two water bottles, (2) water and sweetened water (saccharin, 0.2%) or (3)
water and sweetened alcohol (10%v/v). The BLI groups were injected, every
other day, with either saline or alcohol (2.5 g/kg, i.p., 20%v/v). Five weeks
after treatment, the performance of these animals was examined in either the
operant alcohol self-administration-reinstatement paradigm (SA) or the 5-choice
serial reaction time task (5-CSRTT).
Results. Peri- but not post-adolescence exposure to BLI alcohol-treatment
increased alcohol-intake in adulthood. In contrast, CSA-treated rats acquired
alcohol SA at the same rate as age-matched controls. Both types of treatment
left extinction rates and vulnerability to relapse to alcohol-seeking unaffected.
Neither CSA nor BLI alcohol exposure affected visuospatial attention or inhibitory
response control in the 5-CSRTT. Finally, sensitivity to alcohol challenges in the
5-CSRTT was not altered by any form of alcohol exposure.
Conclusion. Independent from social, environmental and genetic influences
peri-adolescent BLI alcohol exposure, but not CSA alcohol treatment, facilitated
alcohol-intake in later life. Neither form of adolescent alcohol exposure induced
long-term effects on measures of visuospatial attentional or inhibitory response
control. These results are consistent with human data showing that excessive
alcohol-use during adolescence is associated with a higher risk of alcohol-abuse
in later life.
2.2. Introduction
Adolescence, defined as the gradual period of transition from childhood to
adulthood (Spear 2000), is characterized by physical, behavioural, hormonal and
neural maturation. For many people this unique developmental period also
entails the onset of alcohol-use. Adolescents often consume alcohol in an
intermittent pattern of heavy drinking episodes, so-called "binge drinking" (van
Laar et al. 2010). It is generally believed that misuse of alcohol during this
period leads to long lasting behavioural consequences. For instance, early onset
of alcohol-use is a strong predictor of increased alcohol-(ab)use and
psychological disorders in later life (Grant et al. 2006). Furthermore, adolescent
alcohol-use appears to ameliorate sustained attention in humans (Tapert
2004b;Tapert and Brown 1999) and the heightened levels of impulsive
behaviour that are characteristic for adolescence, may not normalize to adult
levels when alcohol exposure disturbs adolescent brain maturation (Crews et al.
2007). Such cognitive deficits may contribute to the aformentioned alcohol-
(ab)use, given that impulsivity is a proposed risk factors for the initiation and
persistence of drug-dependence in general (Pattij and de Vries, 2013).
From these human observations, it is difficult to determine whether behavioural
impairments following adolescent alcohol-use can be solely attributed to the use
of alcohol. Preclinical studies using laboratory animals are more suited to shed
light on this issue, as they offer investigators more control over experimental
conditions and the genetic and environmental influences that confound clinical
studies. Such preclinical studies indicate that there are differences between
adolescent and adult rats with regard to the immediate effects of alcohol.
Adolescent animals seem more tolerant to the sedative effects of alcohol, while
being more vulnerable to neuronal toxicity (e.g. Little et al. 1996;Pascual et al.
2007), for reviews see (Guerri and Pascual 2010;Nixon and McClain 2010;Spear
and Varlinskaya 2010). Less is known about the long-term effects of adolescent
alcohol-use, as the limited number of studies on this issue has yielded mixed
results. This is possibly due to the variety in alcohol-exposure protocols that
have been used, which differ in exposure pattern, dosage and administration
route. Nonetheless, the available data imply that adolescents are particularly
vulnerable to the detrimental effects of alcohol, given that age-dependent
effects of alcohol exposure have been reported on measures of memory,
attention and alcohol-taking and seeking (for reviews see Maldonado-Devincci et
al. 2010b;Nixon and McClain 2010;Spear 2000). Regarding the latter,
intermittent, but not continuous exposure may facilitate intake in later life
(Maldonado-Devincci et al. 2010a). Thus, the exact pattern and conditions of adolescent alcohol exposure possibly determines adult alcohol-intake and may contribute to the mixed results reported in the adolescent alcohol literature. To overcome this problem, we employed two distinct, but standardized alcohol exposure protocols: (1) binge-like injection (BLI) and (2) continuous self-administration (CSA). The first was designed to mimic the intermittent exposure pattern seen in binge drinking youth, the second to model continuous voluntary consumption of moderate amounts of alcohol. Using these protocols we aimed to investigate whether adolescent alcohol exposure induces lingering disturbances in behavioural performance in the absence of social, environmental and genetic influences. First, we examined the consequences of BLI and CSA treatment on different aspects of alcohol-taking and seeking in an operant alcohol self-administration-reinstatement paradigm (SA). Second, we investigated the effects of our standardized treatment on measures of attention and inhibitory response control using the 5-choice serial reaction time task (5-CSRTT). All behavioural testing started five weeks after animals had been exposed to either CSA or BLI treatment, when all animals were in their adulthood.
2.3. Methods
2.3.1. Animals
Time-pregnant Wistar females arrived at five days of gestation (Harlan CPB,
Horst, The Netherlands), and were housed individually in Macrolon cages under
standard conditions and a reversed 12 h light/dark cycle (lights on between 7
pm and 7 am). Upon delivery, litters were culled to eight pups per mother and
preferably consisted of males only, but if necessary were matched with females
(Counotte et al. 2008). At P21, male animals were weaned and housed two per
cage. During the course of behavioural testing in the 5-CSRTT, animals were
food restricted to maintain 85-90% of their free-feeding weight. Water was
available ad libitum. All training and testing sessions were conducted during the
dark phase of the light-dark cycle, at the same time each day. Experimental
procedures were approved by the Animal Ethical Committee of the VU
University, Amsterdam, The Netherlands.
2.3.2. Apparatus and behavioural procedure
Self-administration
Self-administration training and testing was conducted in 32 identical operant
chambers enclosed in sound-attenuating ventilated cubicles (Med Associates
Inc., St. Albans, USA). The operant chambers were equipped with a grid floor,
two nose-poke holes and a central liquid receptacle; a dim red house light and a
tone module (ENV-223AM Med Associates Inc.) were fitted on the opposite wal .
During self-administration, liquid reinforcers were delivered by an infusion pump
(PHM-100, Med Associates Inc.).
Animals were placed in the operant chamber and after 30 s the house lights
were switched on and trials started. Each trial was signalled by illumination of a
red stimulus light located above the active hole, which was extinguished when a
nose-poke was made into this hole. An active nose-poke resulted in the delivery
of 0.2 ml alcohol (10% v/v) in the receptacle and a yellow stimulus light in the
nose-poke hole was illuminated for 5 s combined with a 2-s tone signal (+/- 68
dB, 2900 Hz). After delivery, a time-out period of 15 s commenced during which
all stimulus lights were switched off until the next trial started. Responding
during the time-out period was monitored, but had no programmed
consequences.
5-Choice Serial Reaction Time Task (5-CSRTT)
Experiments were conducted in rat operant chambers with stainless steel grid
floors (MED-NPW-5L; Med Associates Inc.). On-line control of all operant
chambers and data collection were performed using MED-PC version IV (Med
Associates Inc.). Five sessions were scheduled per week from Monday until
Friday, one session per day.
A more detailed description of training in the 5-CSRTT has been reported
previously (van Gaalen et al. 2006). In short, rats were trained to detect and
respond to a 1-s visual stimulus in either one of five apertures, during
presentation of the stimulus or during the 2-s limited hold period, to obtain a
food pellet (45 mg, Formula P; Research Diets Inc., New Brunswick, NJ, USA).
Each session terminated after 100 trials or 30 min, whichever occurred first.
Initially the duration of this stimulus was 32 s and was gradually decreased to
1 s over sessions until animals reached stable baseline performance
(accuracy>80% correct choice and <20% errors of omission). Incorrect,
premature responses (responses during the 5-s inter-trial interval (ITI)) and
errors of omission did not lead to the delivery of a food pellet and resulted in a
5-s time-out period during which the house light was extinguished. Responses
during the time-out period resulted in a new time-out period. Perseverative
responses, i.e. repeated responding into an aperture following correct choice and
before pellet collection were measured but did not have any programmed
consequences. The following behavioural measures are reported: (1) accuracy,
((number correct trials/(correct + incorrect trials)) x 100); (2) premature
responses, i.e. the number of premature responses before the onset of the
visual stimulus, reflecting aspects of loss of inhibitory control (impulsivity); (3)
omission errors, i.e. the total number of omitted trials during a session and (4)
correct response latency, i.e. the mean time between stimulus onset and nose-
poke in the illuminated unit.
2.3.3. Experimental design
Alcohol exposure
Animals were exposed to alcohol for ten days, either during adolescence (PND
34-43, peri-adolescent group) or directly after (PND 62-71, post-adolescent
group).
Continuous Self-administration (CSA). Animals had a free choice to drink from two bottles on their home cage during treatment. One always contained water, the other contained either: (1) water (water group), (2) saccharin (0.2%, w/v) (saccharin group) or (3) sweetened alcohol (5-10%, v/v + saccharin 0.2%, w/v) (alcohol group), depending on the test
group they were assigned to. During treatment the alcohol concentration was increased over time from 5% (day 1-4), 7.5% (day 5-7), to 10% (day 8-10). The position of the bottles was randomized between subjects and over days.
Binge-like injection (BLI). BLI alcohol-treated animals were given a single intra peritoneal (i.p.) injection of 20% (v/v) ethanol (2.5 g/kg) every other day for ten days. This resulted in a total of five injections; peri-adolescent animals were injected on postnatal day (PND) 34, 36, 38, 40 and 42; post-adolescent animals were injected at PND 62, 64, 66, 68 and
70. Control animals received an identical treatment with the exception that they
were injected with in sterile saline for i.p. injection instead of ethanol.
Blood alcohol levels
Using BLI alcohol exposure we aimed to reach blood alcohol levels seen in heavy
drinking adolescents. In a separate pilot study we measured the blood alcohol
levels on the first day of alcohol treatment in both age groups (Figure 5). To this
end, animals were injected with 2.5 g/kg on either PND 34 or 62 and blood
samples were taken from the tail tip 30, 60 and 90 min after injection using
microvette CB300 capillary tubes (Sarstedt, Numbrecht, Germany). The volume
of each sample was approximately 100 µl. Blood samples were spun for 10 min
at 3000 rpm and plasma was stored for blood alcohol analysis. Blood alcohol
levels were determined using an NAD/ADH reagent kit and a standard curve for
quantification (Sigma, Schnelldorf, Germany) (protocol derived from (Lesscher
et al. 2009)).
Blood alcohol levels were not assessed in the CSA group due to difficulties in
pin-pointing the exact timing of drinking bouts in these animals, however
alcohol-intake was monitored by weighing the drinking bottles every 24 h.
Self-administration-reinstatement paradigm (SA)
SA sessions started five weeks following treatment and were held every other
day (three sessions per week) between 12.00 a.m. and 5.00 p.m. Acquisition.
To facilitate acquisition animals were trained to self-administer a 10% (v/v)
alcohol solution that was sweetened with 0.2% saccharin. To familiarize all
animals with this solution, a week before training all animals were given 100 ml
of the sweetened alcohol on the home cages. Saccharin fade. When responding
stabilized on a fixed ratio (FR)1 schedule of reinforcement, the saccharin
concentration was reduced over ten training sessions. Progressive ratio. After
the animals had reached stable responding for a 10% (v/v) alcohol solution we
used a between-session progressive ratio protocol to examine motivational
aspects of alcohol self-administration. Every three to four training sessions the
ratio was increased according to the following schedule (FR:
1,2,3,4,5,7,10,15,20,25,30). Extinction. Subsequently, the animals were trained
until they reached stable responding on FR3 before they underwent context
extinction. Thus, animals were placed in the training context for 15 daily
sessions of one h, without audiovisual cue-exposure or reinforcer delivery.
Relapse. To assess effects on cue-induced relapse, rats were placed in the
training context for a 30 min relapse test. During the relapse test, the houselight
and red cuelight were turned on and nose-poking resulted in the presentation of
the discrete compound audiovisual cues (but no reinforcer delivery) on the same
FR3 schedule that was used during training. To facilitate reinstatement, 0.20 ml
alcohol (12% v/v) was delivered in the receptacle before the session started. We
conducted a series of pilot studies investigating cue-induced alcohol-seeking
under several conditions. Data from these studies, and work by others indicate
that adding a single drop of alcohol, that induces the gustatory, but not the
pharmacological effects of alcohol, results in optimal relapse (Le and Shaham
2002).
5-Choice Serial Reaction Time Task (5-CSRTT)
To examine putative effects of adolescent alcohol exposure on visuospatial
attention and inhibitory response control, five weeks after treatment, rats were
trained in the 5-CSRTT until they reached stable baseline performance on
stimulus duration 1 s (accuracy>80% correct choice and <20% errors of
omission during the last five sessions). After baseline performance was
determined, the ITI was prolonged from 5 to 7 s to provoke an increase in
premature responses. Subsequently the animals were trained for an additional
week and tested under increased attentional demand by reducing the stimulus
duration to either 0.7, 0.5 or 0.3 s using a Latin square design. Next the effects
of alcohol (0, 0.5, 1 and 1.5 g/kg, i.p.) were assessed using a Latin square
design. Tests were conducted twice a week, on Tuesdays and Fridays. On
intermediate days rats were trained under baseline conditions.
2.3.4. Drugs
During treatment all alcohol-treated animals were injected with 20% (v/v)
ethanol (2.5 g/kg). To this end, 96% laboratory alcohol (Interchema, Oosterzee,
The Netherlands) was diluted with sterile saline for i.p. injection appropriate
concentration. The control groups were injected with saline only. For the alcohol
challenge in the 5-CSRTT the same alcohol solution (20% (v/v) ethanol) was
used and injection volumes were adjusted to match the appropriate weight and
dosing.
2.3.5. Statistical analyses
All statistical analyses were performed using the Statistical Package for the
Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA) and all data are
displayed as mean ± SEM. The homogeneity of variance across groups was
determined using Mauchly's tests for equal variances and in case of violation of
homogeneity, corrected Huynh–Feldt degrees of freedom and resulting more
conservative probability values were used for subsequent analyses. In case of
statistically significant main effects, further post hoc comparisons were
conducted using Student–Newman–Keuls tests. The level of probability for
statistically significant effects was set at 0.05. For a clearer representation of the
progressive ratio results, all training days on a specific ratio were collapsed in
one average data point per ratio.
Although both peri- and post-adolescent animals were adult during behavioural
testing, the current and other studies in our laboratory have detected a number
of behavioural differences between the age groups, independent of treatment.
Post-adolescent animals are slightly slower at acquiring cognitive tasks including
the 5-CSRTT and radial arm maze task (Chapter 3). Furthermore, the younger
groups demonstrate elevated object exploration in the object-place recognition
paradigm (Chapter 3), slightly faster response latencies in the 5-CSRTT and
increased response ratio's in the SA. During continuous exposure peri-
adolescent alcohol-treated animals consumed substantially more alcohol than
post-adolescent animals. Therefore, we performed separate analyses for the
different age groups.
For the SA data, the total number of nose-poke responses in the active hole was analyzed using a repeated measures analysis of variance (ANOVA) with treatment as between-subject factor. We separately analyzed the different phases in the experiment (i.e. acquisition, saccharin fade, progressive ratio, extinction and relapse). Data obtained in the 5-CSRTT were subjected to repeated measures ANOVA with treatment as a between-subject factor. We report accuracy, premature responses, omission errors and correct response latency during baseline performance, ITI prolongation, SD reduction and alcohol challenge.
2.4 Results
2.4.1. Adolescent alcohol treatment
Continuous self-administration (CSA) groups
To monitor intake in the CSA groups the two bottles used for treatment were
weighed when they were placed on, or taken from the home cages. These
measurements showed that peri-adolescent animals receiving alcohol treatment
consumed more sweetened alcohol than post-adolescent animals
(F(1,14)=48.456; p<0.001) (Figure 4) and a day * age interaction revealed
differences in intake over time between the age groups (F(1,14)=3,00; p<0.01).
Consumption was significantly elevated at alcohol concentrations of 5% (day 1-
4) (F(1,14)=286.59; p<0.001), 7.5% (day 5-7) (F(1,14)=125.97 and 10% (day
8-10) (F(1,14)=238.64 ; p<0.001). The peri-adolescent animals showed a peak
in their daily intake on day five, when the alcohol concentration was increased
from 5 to 7.5%.
Figure 4. Alcohol-intake during CSA treatment
Binge-like injection (BLI)
Blood alcohol levels following BLI alcohol treatment were determined in a
separate group of animals. Examination of blood samples taken from the tail tip
indicated that alcohol injections during the first day of treatment (2.5 g/kg, i.p.)
resulted in blood alcohol levels over 200 mg/dl during the first h after injections
(Figure 5). The age at which the subjects were exposed to alcohol did not affect
blood alcohol levels.
Blood Alcohol Levels
Figure 5. Blood alcohol levels fol owing BLI alcohol treatment (2.5 g/kg, i.p)
2.4.2. Effects of adolescent alcohol exposure on alcohol-taking and
seeking in adulthood
An overview of the p and F values obtained by statistical analysis of our data
sets is presented in Tables 1-4.
CSA groups
Weight. At the start of operant alcohol self-administration in adulthood, age-
matched animals in the different treatment groups had similar weights (post-
adolescent; water: 459 ± 9 g, saccharin: 463 ± 6 g and alcohol: 452 ± 11 g)
(peri-adolescent; water: 363 ± 7 g, saccharin: 356 ± 6 g and alcohol: 365 ± 6
g). Thus, the weight of alcohol-treated animals did not differ from saccharin- or
water-treated individuals. This suggests that, within each age group, intake was
not influenced by weight differences between treatment groups.
Saccharin fade. We examined the effects of treatment on acquisition, motivation, extinction and reinstatement of alcohol SA (Figure 6). All groups acquired SA of a sweetened alcohol solution at the same pace, as shown by a significant day effect and the lack of a day * treatment interaction or between-subjects drug effects (Table 1; 2-4). When the saccharin concentration in the alcohol solution was slowly faded, a between subject treatment effect was found in the peri-adolescent groups (Table 1; 5-6). Post hoc analyses demonstrated that peri-adolescent saccharin and alcohol groups showed lower response rates as compared to the age-matched water group (p<0.05). By the time that animals were responding on FR1 for 10% alcohol, all groups showed similar levels of responding. Progressive Ratio. To assess the effects of CSA exposure on motivational aspects of alcohol SA we used a between-session progressive ratio paradigm, steadily increasing the fixed ratio schedule of reinforcement from FR1 to FR30. At FR2, FR3, and FR7, a significant between-subject effect combined with post hoc tests (p<0.05) in the peri-adolescent group indicated that alcohol, but not saccharin-treated animals made fewer responses than age-matched water-treated controls (Table 1;7-10). Furthermore, at FR5 peri-adolescent treated with either alcohol or saccharin displayed lower response levels than water controls (Table 1; 11). Extinction. After the progressive ratio phase, all animals underwent context-extinction. Treatment did not affect extinction rates. Relapse. Al groups demonstrated cue-induced reinstatement. A treatment * relapse interaction was found in both peri- and post-adolescent treated groups (Table 1; 14). While alcohol and saccharin exposure facilitated cue-induced relapse (p<0.05) in post-adolescent groups, the same treatment during peri-adolescence attenuated relapse (p<0.05) compared to age-matched water controls.
Figure 6. To the right on page 53 an overview is given of alcohol SA performance of CSA-
treated animals. The panels on the left portray the performance of post-adolescent
animals, the panels on the right the performance of peri-adolescent animals. Total number
of nose-poke responses in the active hole (Mean ± SEM) is depicted. From top to bottom:
acquisition phase and subsequent fading of saccharin in the alcohol solution; between
session progressive ratio; context extinction; cue-induced relapse of alcohol-seeking. *
p<0.05 difference with respect to CSA sweetened alcohol- and CSA water-treated groups.
# p<0.05 difference with respect to CSA water-treated group.
post-adolescent water
peri-adolescent water
post-adolescent alcohol
peri-adolescent alcohol
% saccharin
% saccharin
fixed ratio
fixed ratio
CSA group self-administration
1 start SA F(1,29)<1
F(10,290)=9.211 p<0.001 ε=0.707 F(10,270)=12.637 p<0.001 ε=0.771
3 treatment F(20,290)=1.096 ns
F(20,270)=1.448 ns
4 subject F(2,29)<1
progressive ratio
12 extinction F(42,609)=1.170 ns
F(42, 567)=1.445 ns
14 treatment F(2,29)=8.227 p<0.01
Table 1. An overview of the results from the statistical analysis of the SA data from
animals that received CSA treatment. The table depicts a chronological representation of
the p and F values, the numbers on the left correspond to the in-text references.
BLI groups
Weight. At the start of SA, age-matched saline- and alcohol-treated animals had
similar weights (post-adolescent; saline: 440 ± 7 g and alcohol: 430 ± 7 g)
(peri-adolescent; saline: 360 ± 4 g and alcohol: 364 ± 5 g). This suggests that,
within each age group, intake was not influenced by weight differences between
treatment groups.
Saccharin fade. We studied the effects of treatment on acquisition, motivation,
extinction and reinstatement of alcohol SA (Figure 7). All groups acquired SA of
a sweetened alcohol solution at the same pace, as evidenced by significant day
effect, but no day * treatment interactions or between-subjects drug effects
(Table 2; 16-18). When the saccharin concentration in the alcohol solution was
slowly faded, a between-subject treatment effect just failed to reach significance
in the peri-adolescent groups (Table 2; 19). Post hoc analyses demonstrated
that peri-adolescent animals treated with alcohol tended to earn more
reinforcers than saline-treated age-matched controls (p=0.07). At the end of the
saccharin fade, when animals were responding for an unsweetened 10% alcohol
solution on FR1, individuals that had received peri-adolescent alcohol exposure
earned more reinforcers than saline controls (p<0.05) (Table 2; 20).
Progressive Ratio. To assess the effects of adolescent alcohol exposure on
motivational aspects of alcohol SA we used a between-session progressive ratio
paradigm, steadily increasing the fixed ratio schedule of reinforcement from FR1
to FR30. At FR1 and FR3, a between-subjects effect (Table 2; 20-21) in
combination with post hoc testing (FR1 p<0.05; FR3 p=0.058) revealed that the
peri-adolescent alcohol-treated group responded more than age-matched saline-
treated controls.
Extinction. After the progressive ratio phase, all animals underwent context-
extinction. Treatment did not affect extinction rates.
Relapse. All groups demonstrated cue-induced relapse, but no treatment *
relapse interactions were found.
Figure 7. To the right on page 57 an overview is given of alcohol SA performance in BLI-
treated animals. The panels on the left portray the performance of post-adolescent
animals, the panels on the right the performance of peri-adolescent animals. Total number
of nose-poke responses in the active hole (mean ± SEM) is depicted. From top to bottom:
acquisition phase and subsequent fading of saccharin in the alcohol solution; between
session progressive ratio; context extinction; cue-induced relapse of alcohol-seeking. *
p<0.05 difference between alcohol- and saline-treated groups. # p<0.07 difference
between alcohol- and saline-treated groups.
post-adolescent saline
peri-adolescent saline
post-adolescent alcohol
peri-adolescent alcohol
% saccharin
% saccharin
fixed ratio
fixed ratio
BLI group, Self-administration
15 start SA F(1,41)<1
F(11,451)=22.54 p<0.001 ε=0.639 F(11,440)=15.63 p<0.001 ε=0.801
17 treatment F(11,451)<1
18 subject F(1,41)<1
progressive ratio
22 extinction F(14,574)<1
24 treatment F(1,41)<1
Table 2. An overview of the results from the statistical analysis of the SA data from
animals that received BLI treatment. The table depicts a chronological representation of
the p and F values, the numbers on the left correspond to the in-text references.
2.4.3. 5-Choice serial reaction time task (5-CSRTT)
CSA groups
Baseline performance. We assessed the effects of CSA exposure on baseline
performance (accuracy>80% correct choice and <20% errors of omission during
the last five sessions of training) i.e. on baseline measures of accuracy and
premature responses in the 5-CSRTT (post-adolescents Figure 8; peri-
adolescents Figure 9). All animals reached stable baseline performance on
stimulus duration 1 s. Performance on these measures and control measures
(i.e. omission errors and correct response latency) was stable over five baseline
sessions, except for a slight fluctuation in premature responses. Moreover,
baseline performance did not differ between treatment groups, as a repeated
measures ANOVA did not reveal between-subject treatment effects or within-
subject day * treatment interactions.
ITI increase. After baseline performance was established we investigated
whether the different treatment groups would react differently to manipulations
of task parameters. First, we increased the duration of the ITI from 5 to 7 s to
provoke increased premature responding. We found that ITI manipulation
decreased accuracy. As was expected, prolongation of the ITI increased
premature responding. While the number of omissions made was not affected by
ITI manipulation, ITI prolongation slightly decreased the correct response
latency in the post-adolescent, but not the peri-adolescent treated individuals.
Importantly, performance under increased ITI was not affected by treatment
conditions.
Stimulus duration (SD) reduction. Next, we assessed whether effects of
treatment could be detected under conditions of increased attentional demand.
To this end the SD was shortened from 1 s to 0.7, 0.5 or 0.3 s. As may be
expected, accuracy was reduced when the attentional load was increased (Table
3; 41). Post hoc analyses revealed that compared to SD 1, at SD 0.3 and 0.5
accuracy was reduced in the post-adolescent treated groups (SD0.3, p<0.001;
SD0.5, p<0.001 ), while all SD manipulations reduced accuracy in peri-
adolescent treated individuals (SD0.7, p<0.001; SD0.5, p<0.001; SD0.3,
p<0.001). Reducing SD increased premature responding (Table 3; 42). Post hoc
analyses revealed that this effect was significant at SD0.3 in both post-
adolescent (p<0.01) and peri-adolescent (p<0.01) treated individuals. It may
not be surprising that reducing SD also increased omissions in both post-
Correct Response Latency
post-adolescent waterpost-adolescent saccharinpost-adolescent alcohol
Figure 8. An overview of 5-CSRTT performance of post-adolescent CSA-treated animals.
The fol owing behavioural measures are presented: (accuracy) percentage correct
responses, (impulsivity) number of premature responses,) number of omissions and
latency of correct responses. * p<0.05 difference from baseline.
Correct Response Latency
peri-adolescent waterperi-adolescent saccharinperi-adolescent alcohol
Figure 9. An overview of 5-CSRTT performance of peri-adolescent CSA-treated animals.
The fol owing behavioural measures are presented: (accuracy) percentage correct
responses, (impulsivity) number of premature responses, number of omissions and latency
of correct responses. * p<0.05 difference from baseline. # p<0.05 difference from saline
condition.
CSA group 5-CSRTTnr effect
25 accuracy F(4,132) =2.431 ns
26 responses F(4,132) =3.8 p<0.05 ε=0.795 F(4,132)=4.9
F(4,132) =1.277 ns
28 response F(4,132)<1
Baseline * treatment
29 accuracy F(8,132)<1
30 responses F(8,132)<1
32 response F(8,132)<1
33 accuracy F(1,33) =9.4
34 responses F(1,33) =99.08 p<0.001
36 response F(1,33)=13.59
Table 3. An overview of the results from the statistical analysis of the 5-CSRTT data from
animals that received CSA treatment (continued on page 63). The table depicts a
chronological representation of the p and F values, the numbers on the left correspond to
the in-text references.
CSA group 5-CSRTTnr effect
ITI day * treatment
37 accuracy F(2,33)<1
38 responses F(2,33)<1
40 response F(2,33)=1.03
Stimulus duration
41 accuracy F(3,99) =60.25 p<0.001 ε=0.936 F(3,99) = 123.57 p<0.001
42 responses F(3,99) =8.67 p<0.001 ε=0.876 F(3,99)=8.38
p<0.001 ε=0.889 F(3;99)=37.8
p<0.001 ε=0.809
44 response F(3,99) =38.958 p<0.001
Stimulus duration * treatment
45 accuracy F(6,99)<1
46 responses F(6,99)<1
48 response F(6,99)<1
Alcohol chal enge
49 accuracy F(2,66)<1
50 responses F(2,66)<1
p<0.05 ε=0.844
52 response F2,66)=2.270
Alcohol chal enge* treatment
53 accuracy F(4,66)<1
54 responses F(4,66)=1.786 ns
56 response F(4,66)=4.134
adolescent and peri-adolescent animals (Table 3; 43) at all stimulus durations
(p<0.001). These conditions of increased attentional load caused individuals in
both age groups to respond slightly faster (Table 3; 44) at all three SDs
(p<0.001). No treatment * SD interactions were found, suggesting that
treatment did not affect performance under increased attentional load.
Alcohol challenge. To determine whether treatment affected sensitivity to the
acute effects of alcohol on 5-CSRTT performance animals were injected with
saline, 0.5, 1 or 1.5 g/kg alcohol using a Latin square design. As the 1.5 g/kg
dose induced severe locomotor impairments and therefore high levels of
omissions in most animals, this dose was not included in the analyses.
Acute alcohol affected visuospatial attention in peri-adolescent, but not post-
adolescent treatment groups (Table 3; 49). Post hoc analyses revealed that both
doses of alcohol reduced accuracy in the peri-adolescent treatment groups
(p<0.05). Similar to what was found for accuracy, inhibitory response control
was affected only in individuals treated during adolescence (Table 3; 50). Post
hoc tests revealed that acute alcohol decreased inhibitory response control at 1
g/kg (p<0.05). The alcohol challenge did not affect omissions, and correct
response latency was affected in the peri-adolescent groups only. However, post
hoc analyses did not reveal lingering effects related to treatment.
There were no treatment * drug interactions found for measures of attention
and impulsivity. Although a treatment * drug interaction was detected in for
correct response latency in the post-adolescent groups, post hoc analyses did
not reveal significant effects for individual treatment or alcohol dose. Thus,
alcohol exposure did not alter sensitivity to alcohol challenges in the 5-CSRTT.
BLI Groups
Baseline performance. We assessed the effects of binge-like injection treatment
on baseline performance (accuracy>80% correct choice and <20% errors of
omission during the last five sessions) for accuracy and premature responses in
the 5-CSRTT (post-adolescents Figure 10; peri-adolescents Figure 11). Al
animals reached stable baseline performance on stimulus duration 1 s.
Performance on these measures was stable over five baseline sessions.
Moreover, treatment did not affect baseline performance, as a repeated
measures ANOVA did not reveal between-subject treatment effects or within-
subject day * treatment interactions.
ITI increase. After baseline performance was established we investigated whether the different treatment groups would react differently to manipulations of task parameters. First, we increased the duration of the ITI from 5 to 7 s to provoke increased premature responses. ITI manipulation decreased visuospatial attention while it increased premature responding in both age groups (Table 4; 65-66). The number of omissions was not affected, but ITI prolongation slightly decreased the correct response latency in the post-adolescent, but not the peri-adolescent treated individuals (Table 4; 67-68). Performance under increased ITI was not affected by treatment conditions. Stimulus duration reduction. Next we examined whether effects of treatment could be detected, under conditions of increased attentional demand. To this end, the stimulus duration (SD) was shortened from 1 s to 0.7, 0.5 or 0.3 s. As may be expected accuracy was diminished when attentional load was increased (Table 4; 73). Post hoc analyses revealed that, compared to SD1, at SD0.3 and 0.5 accuracy was reduced in the post-adolescent treated groups (SD0.3, p<0.001; SD0.5, p<0.001), while all manipulations reduced accuracy in peri-adolescent treated individuals (SD0.3, p<0.001; SD0.5, p<0.001; SD0.7, p<0.01). Reducing the stimulus duration increased premature responding (Table 4; 74). Post hoc analyses revealed this effect was significant at SD0.3 in post-adolescent (p<0.01) and at SD0.3 and SD0.5 in peri-adolescent treated individuals (p<0.001). Reducing the stimulus duration increased omissions (Table 4; 75) at SD0.3 and SD0.5 in post-adolescent treated individuals (p<0.001) and at all SDs in the peri-adolescent groups (p<0.001). Under conditions of increased attentional load, individuals in both age groups responded slightly faster (Table 4; 76) at all three SDs (p<0.01). No treatment * SD interactions were found on impulsivity and attentional measures. However, treatment significantly affected correct response latency in the post-adolescent but not in peri-adolescent treatment groups (Table 4; 80). Post hoc analyses did not reveal significant effects of treatment on the different SDs, but post-adolescent alcohol-treated animals seemed more resistant to the effects of SD0.3 (p=0.07) compared to age-matched saline controls. Alcohol challenge. To determine whether treatment affected sensitivity to the acute effects of alcohol on 5-CSRTT performance, animals were injected with saline, 0.5, 1 or 1.5 g/kg alcohol using a Latin square design. As the 1.5 g/kg
Correct Response Latency
post-adolescent salinepost-adolescent alcohol
Figure 10. An overview of 5-CSRTT performance of post-adolescent BLI-treated animals.
The fol owing behavioural measures are presented: (accuracy) percentage correct
responses, (impulsivity) number of premature responses, number of omissions and latency
of correct responses. * p<0.05 difference from baseline. # p<0.05 difference from saline
condition. Τ p=0.07 difference with respect to saline-treatment.
Correct Response Latency
peri-adolescent salineperi-adolescent alcohol
Figure 11. An overview of 5-CSRTT performance of peri-adolescent BLI-treated animals.
The fol owing behavioural measures are presented: (accuracy) percentage correct
responses, (impulsivity) number of premature responses, number of omissions and latency
of correct responses. * p<0.05 difference from baseline.
BLI group 5-CSRTTnr effect
57 accuracy F(4,88) =1.22 ns
58 responses F(4,88) =1.64 ns
60 response F(4,88) =1.24 ns
Baseline * treatment
61 accuracy F(4,88)<1
62 responses F(4,88)<1
64 response F(4,88)<1
65 accuracy F(1,22) =4.85 p<0.05
F(1,22)=39.44 p<0.001
66 responses F(1,22) =56.53 p<0.001
68 response F(1,22) =21.62 p<0.001
Table 4. An overview of the results from the statistical analysis of the 5-CSRTT data from
animals that received BLI treatment (continued on page 63). The table depicts a
chronological representation of the p and F values, the numbers on the left correspond to
the in-text references.
BLI group 5-CSRTTnr
ITI day * treatment
69 accuracy F(1,22)<1
70 responses F(1,22)<1
72 response F(1,22)<1
Stimulus duration
73 accuracy F(3,66) =8.73 p<0.001 ε=0.917 F(3,66) = 9.10 p<0.001 ε=0.738
74 responses F(3,66) =9.50 p<0.001
F(3,66)=4.984 p<0.009 ε=0.728
F(3,66) =1.50 p<0.001 ε=0.602 F(3;66)=52.19 p<0.001 ε=0.723
76 response F(3,66) =22.48 p<0.001
F(3,66)=15.11 p<0.001
Stimulus duration * treatment
77 accuracy F(3,66)<1; ns ns
78 responses F(3,66)<1; ns ns
F(3,66)<1; ns ns
80 response F(3,66) =4.63 p<0.01
Alcohol chal enge
81 accuracy F(2,42)<1
82 responses F(2,44) =3,30 P<0.05
p<0.05 ε=0.620
84 response F(2,42) =3.65 p<0.05
Alcohol chal enge* treatment
85 accuracy F(2,42) =2.09 ns
86 responses F(2,44)<1
88 response F(2,42) =2.80 ns
induced severe locomotor impairments and high levels of omissions in most animals, this dose was omitted from the analyses. Alcohol did not affect visuospatial attention, but inhibitory response control was affected in individuals that were treated directly after adolescence (Table 4; 81-82). Post hoc tests revealed that alcohol decreased inhibitory response control at both 0.5 and 1 g/kg (p<0.05) in the post-adolescent groups. The alcohol challenge affected omissions only in peri-adolescent treated individuals, however, post hoc analyses revealed that there were no performance differences compared to saline treatment. Correct response latency was affected in the post-adolescent groups only (Table 4; 84), post hoc analyses revealed that latency was increased at 1 g/kg in the post-adolescent groups. There were no treatment * drug interactions found for measures of attention and impulsivity. Although the post-adolescent alcohol treatment group seemingly made more omissions than saline controls when challenged with 1 g/kg alcohol, this effect was not significant (Table 4; 87-88).
2.5 Discussion
In this study it was demonstrated that adult rats with a history of peri-
adolescent binge-like injection (BLI) alcohol exposure increased responding for
alcohol on a fixed ratio (FR1) schedule of reinforcement. Importantly, neither
peri-adolescent continuous self-administration (CSA) alcohol treatment nor post-
adolescent CSA or BLI alcohol treatment induced such long-term effects on
operant alcohol-intake. In addition, measures of motivation, extinction and cue-
induced relapse remained unchanged after peri-adolescent or post-adolescent
CSA or BLI alcohol exposure, when compared to age-matched controls. Finally,
visuospatial attention and inhibitory response control were not altered following
CSA or BLI alcohol treatment.
2.5.1. Lingering effects on measures of alcohol-taking and seeking
Our alcohol measures indicate that peri-adolescent BLI alcohol exposure leads to
elevated levels of operant alcohol-intake in adulthood. As is discussed below,
this finding is consistent with most existing data, however preclinical studies on
the subject have yielded mixed outcomes (for an overview see Table 5).
Using a peri-adolescent binge-like injection protocol, comparable to the one
used in the current study, Pascual and colleagues (2009) explored alcohol
consumption more than three weeks after treatment. They report that alcohol-
treated animals voluntarily consumed more alcohol than saline controls in a
limited-access two-bottle choice procedure (Pascual et al. 2009). In another
study, using a binge-like administration protocol, both male and female
Sprague-Dawley rats exposed to intragastrically administered alcohol between
postnatal day (PND) 28 and 45, showed increased voluntary sweetened alcohol-
intake in a limited-access two-bottle choice paradigm, when tested more than
two weeks after treatment (Maldonado-Devincci et al. 2010a).
Several studies investigated adult alcohol-intake using different peri-adolescent
alcohol exposure regimens. Long-Evans hooded rats given a 10% alcohol
solution as sole fluid between PND 21 and 70, drank more than water-treated
controls in a two-bottle preference test, when tested 35 days after treatment
(Siciliano and Smith 2001). Furthermore, peri-adolescent alcohol drinking
increased the likelihood of adult drinking initiation and increased subsequent
extinction resistance and the potential for relapse in female alcohol-preferring
rats that were given 24 h free-choice access to a 15% alcohol solution between
PND 30 and 60 (Rodd-Henricks et al. 2002).
3g/kg/i.p. 29,30;
treated animalsal
Dawley 1.5; 3; 5 28-31; 35-
intake. Males appeared
more vulnerable than
increased preference in
levels at acquisition,
greater resistance to
Rodd-Henricks, prefering access to 30-60
reacquisition compared
to age controls.
Sprague 12 hr/ day
Sprague sweetened
continuo extended
continuo extended
Siegmund, 2005 Wistar
5% or 20% adulthood
Table 5. Preclinical studies on alcohol consumption in rats with a history of adolescent
alcohol exposure.
In contrast, the current study shows that voluntary continuous alcohol exposure during adolescence does not affect acquisition of operant alcohol self-administration. In addition, the number of responses on FR1 and measures of motivation, extinction and relapse did not differ between saccharin and alcohol exposure groups. In line with these results, voluntary alcohol consumption in Sprague-Dawley and Wistar rats exposed continuously to either sweetened or unsweetened alcohol from adolescence onwards was not found be elevated in adulthood (Siegmund et al. 2005;Vetter et al. 2007). Furthermore, Sprague-Dawley rats exposed between PND 30 and 40 for 12 h per day in alcohol vapour chambers did not differ from controls when tested free-access alcohol consumption 52 days after exposure (Siegmund et al. 2005;Slawecki and Betancourt 2002;Vetter et al. 2007). In agreement with human studies indicating that adolescent alcohol-use is associated with alcohol-(ab)use in later life (Brown et al. 2008a;Grant et al. 2006), the studies above collectively indicate that, under certain conditions, adolescent alcohol exposure may have long-term effects on drinking behaviour. Specifically, it seems that alcohol exposure protocols inducing high blood alcohol levels are more likely to result in lingering behavioural effects. Moreover, a binge-like, intermittent pattern of exposure may affect consumption more prominently than continuous exposure (Duka et al. 2004;Maldonado-Devincci et al. 2010a;Pascual et al. 2009). It should be noted that in these animal studies alcohol exposure is the only independent variable. As such, these studies do not model the complex social, genetic and environmental factors that play a role in initiating alcohol-use in youth. Furthermore, the current study did not include protracted alcohol-use after adolescent initiation, which is often seen in humans. These factors may, however, exacerbate the deficits reported here. Thus, while adolescent binge-like alcohol exposure alone only modestly enhanced adult alcohol-intake, it may add to the other vulnerability factors that play an important role in the development of alcohol-use in later life. We found that alcohol exposure affected intake, but not motivation and relapse vulnerability, which is interesting given that these have partially overlapping neurobiology. That is, mesolimbic DA transmission has been implicated in self-administration and the motivation to self-administer drugs of abuse (Di Chiara and Imperato 1988;Everitt et al. 2001) as well as in relapse (Le and Shaham 2002). However, there is a clear difference in the conditions used to measure these behavioural aspects. Compared to self-administration on FR1, on a
progressive ratio schedule only a limited number of responses are reinforced,
the other responses may induce competing extinction-like effects. During
relapse on the other hand, responses are never reinforced. Neurochemically,
relapse may also recruit different neurotransmitter systems, such as the opioid,
glutamate and serotonin systems (Le and Shaham 2002), as wel as additional
brain regions, including the prefrontal and orbital frontal cortex, the amygdala,
and the striatum (Kalivas et al. 2006). Intricate, region specific changes in these
neurobiological mechanisms induced by adolescent alcohol exposure may
therefore differently affect intake, motivation and relapse. Alternatively,
motivation, extinction and relapse were tested in a later stage of the experiment
than intake. As the central nervous system is at least capable of some recovery
from the consequences of peri-adolescent alcohol exposure (Evrard et al. 2006),
it seems possible that any subtle lingering effects on these behavioural facets
may also dissipate over time and therefore could not be detected.
2.5.2. Alcohol exposure protocols
During CSA alcohol exposure, individuals in the peri-adolescent group consumed
more sweetened alcohol than their post-adolescent counterparts. These results
are in line with previous reports showing elevated (sweetened) alcohol
consumption in rats during the adolescent period (Maldonado-Devincci et al.
2010b). Moreover, elevated alcohol-intake during adolescence was also reported
in humans (Spear and Varlinskaya 2010). It is often speculated that
developmental changes in the sensitivity to the pharmacological, motor and
sedative effects of alcohol (Garcia-Burgos et al. 2009;Spear 2000), and
intensive remodelling of the reward pathway during adolescence underlie this
phenomenon (Bava and Tapert 2010;Chambers et al. 2003;Crews et al.
2007;Maldonado-Devincci et al. 2010b). On the other hand differences in
metabolism may play a role. There is some debate about the rate of alcohol
metabolism in adolescent rats which may be faster than in adult rats (Little et al.
1996). This may also contribute to higher intake in adolescents and can possibly
lead to higher peak values and faster clearance of alcohol in adolescent animals.
In view of this putative confounding factor, we measured the blood alcohol
levels after a single alcohol injection on the first day of BLI exposure in a
separate group of animals. Blood alcohol levels peaked over 200 mg/dl in both
post-adolescent and peri-adolescent animals and they remained comparable
during the first 90 min following injection. Thus, during treatment, both age
groups were exposed to similar blood alcohol levels. Furthermore, the blood
alcohol levels established in the BLI groups exceeded a value of 80 mg/dl. In
humans, an intermittent pattern of alcohol-intake that induces blood alcohol
values above 80 mg/dl was dubbed binge-drinking (NIAA, 2004). As such, our
BLI protocol may be considered heavy binge-drinking and results in high blood
alcohol levels that are not seen in animals receiving voluntary alcohol treatment.
A weakness of the BLI treatment is that compared to voluntary intake, forced
administration of addictive drugs may lead to distinct long-term neuroadaptive
and behavioural effects (Jacobs et al. 2003). It is therefore possible that altered
neuroadaptive effects may partially explain the differential outcome.
Alternatively, there is compelling evidence that the adolescent brain is more
sensitive to the neurotoxicity of high blood alcohol concentrations and
subsequent withdrawal effects that are associated with binge-like exposure e.g.
(Crews et al. 2000;Morris et al. 2010;Nixon and Crews 2002;Nixon and McClain
2010;Slawecki 2002;Spear 2000;Swartzwelder et al. 1995). The increased
extent of damage induced by binge-like exposure may not be completely
recovered in later life and/or lead to aberrant brain maturation inducing the
deficits seen here.
2.5.3. Lack of effects on visuospatial attention and inhibitory response
control
In alcohol-dependent subjects impaired performance in cognitive tasks
measuring attention, impulsive action (inhibitory response control), impulsive
choice and reflection-impulsivity alcohol have been reported (Verdejo-Garcia et
al. 2008). Furthermore, impulsivity and attention may be important behavioural
modulators of addiction (Dalley et al. 2008;Scheurich 2005;Sofuoglu 2010;
Pattij and de Vries 2013). Therefore, this study addressed the long-term effects
of alcohol exposure on cognitive performance in the 5-CSRTT. Previous
experiments from our laboratory demonstrated that adolescent nicotine
exposure had long-lasting effects on performance in this task in rats.
Specifically, visuospatial attention and inhibitory response control were affected
when tested more than five weeks after peri-adolescent nicotine exposure
(Counotte et al. 2008; 2011a). Since young adults (17 to 23 years) with a
history of protracted alcohol-use are reported to have attentional deficits (Tapert
and Brown 1999), we hypothesized that peri-adolescent alcohol exposure would
impair visuospatial attention in the 5-CSRTT. In addition, given that impulsive
behaviour is heightened in alcohol-dependent subjects (Verdejo-Garcia et al.
2008) we expected to find reduced inhibitory response control in adolescent
alcohol-treated rats. Conversely, our results demonstrate that neither CSA nor BLI alcohol exposure induced long-term effects on 5-CSRTT performance. As several studies indicate that peri-adolescent alcohol exposure may change the sensitivity to alcohol challenges in later life (Swartzwelder et al. 1998;White et al. 2000; 2002), we subsequently tested the dose-dependent effects of alcohol on 5-CSRTT performance in all treatment groups. Although acute alcohol did affect measures of attention and inhibitory response control, there were no group differences observed, indicating that, in the attentional domain, sensitivity to acute alcohol is not altered by peri- or post-adolescent alcohol exposure. To our knowledge, no other studies have looked into the effects of adolescent alcohol exposure on inhibitory response control and only one other study has looked into sustained attention. In line with our results, this study was unable to detect long-term effects of peri-adolescent alcohol exposure on sustained attention in a 2-choice paradigm (Slawecki 2006). Remarkably, the same study reports that post-adolescent alcohol exposure had a positive effect on sustained attention while increasing the number of omissions, which contradicts the assumption that a history of alcohol exposure would impair attentional performance (Slawecki 2006). No such effect was seen in the current study. Possibly, the more difficult attentional task used in our study or the higher levels of accuracy that were achieved, have prevented detection of facilitating effects of alcohol exposure. In addition, the differences in treatment regimens may have played a role. The continuous, but artificially-induced blood alcohol levels seen in the vapour paradigm used in the Slawecki study exceed those in our CSA groups. The BLI exposure, on the other hand, achieved blood alcohol levels that are similar to those seen in the Slawecki study. However, alcohol-administration in our BLI groups is binge-like rather than continuous and is therefore accompanied by more extensive withdrawal periods. As impaired attention and visuospatial function in humans were associated with the degree of withdrawal experienced in individual subjects (Tapert and Brown 1999) and withdrawal after binge-like alcohol exposure may be especially detrimental (Crews et al. 2000;Duka et al. 2004), this may have counteracted any putative enhancing effects of alcohol exposure in the current study. To summarize, in contrast to human studies that report impairments in the cognitive domain following peri-adolescent alcohol-use, we did not find evidence for such effects. Given that subjects in these human studies showed deficits after protracted alcohol-use (Tapert and Brown 1999), preclinical studies
addressing cognition after extended alcohol exposure starting from adolescence
may yield different effects. Alternatively, the causality issues that classically
hamper the interpretation of epidemiological studies may play a role, i.e. the
attentional deficits seen in the human studies may result from pre-existing
symptomatology in combination with adolescent alcohol-intake.
2.5.4. Conclusions
In the current experiment we have used two different treatment regimens to
investigate the long-term consequences of peri-adolescent alcohol exposure on
alcohol-taking and seeking, as well as on attention and inhibitory response
control. Our findings indicate that independent from social and genetic factors,
delicate long-term effects occur following binge-like forced, but not continuous
volitional self-administration of alcohol. Specifically, a history of peri-adolescent
binge-like alcohol injections increased alcohol-intake during adulthood, but was
not accompanied by changes in visuospatial attention and inhibitory response
control. Although the effects reported here are subtle, the fact that such long-
term consequences were observed after only a limited number of binge-like
alcohol exposures during adolescence is striking. In humans, protracted alcohol-
use, social, environmental and genetic aspects, as well as the acute effects of
alcohol on cognitive development, may to lead to exacerbation of the effects on
alcohol-intake seen here. As such, our results add to the literature showing that
during adolescence individuals may be particularly sensitive to the effects of
alcohol.
2.6. Acknowledgements
We would like to thank Gideon Meerhof for his contribution to the 5-CSRTT
experiments. In addition we would like to acknowledge ZONMW who supported
our research by means of ZONMW Topgrant 912-06-148.
2.7. Disclosure
All authors declare that, except for income received from primary employer, no
financial support or compensation has been received from any individual or
corporate entity over the past three years for research or professional service
and there are no personal financial holdings that could be perceived as
constituting a potential conflict of interest.
Chapter 3
Peri-adolescent alcohol exposure brings on
long-lasting spatial memory deficits.
J.A. Wouda1,2, T. Pattij1 , T.J. De Vries1,2,
1Department of Anatomy and Neurosciences, Neuroscience Campus Amsterdam,
VU University medical center and 2Department of Molecular and Cellular
Neurobiology, Center for Neurogenomics and Cognitive Research, Neuroscience
Campus Amsterdam, VU University, Amsterdam, The Netherlands
Our research was supported by a ZON-MW Top Grant 912-06-148.
3.1. Abstract
Rationale. Adolescence commonly entails the onset of alcohol-use. Several
epidemiological studies indicate that alcohol consumption may be particularly
harmful during this developmental period. For instance, both in humans and in
rats, alcohol exposure has repeatedly been associated with hippocampal
damage. Currently, only limited preclinical data on the long-term behavioural
consequences of adolescent alcohol exposure exist.
Objectives. As the hippocampus is critically involved in spatial learning and
memory, the present experiments addressed the long-term effects of binge-like
alcohol exposure during adolescence on spatial memory performance in the
radial arm maze (RAM) and on object-place recognition (OPR). Male Wistar rats
were subjected to a binge-like injection (BLI) regimen consisting of
intraperitoneal alcohol injections (2.5g/kg) every other day for ten days.
Animals were treated either during adolescence (peri-adolescent group (PND 34-
43)) or directly after adolescence (post-adolescent group (PND 60-69)). Five to
seven weeks after treatment the animals were trained in the RAM or OPR. In the
RAM the number of errors (i.e. the number of entries in unbaited or previously
visited arms) was used as a measure of spatial memory. In the OPR time spent
exploring the relocated object was measured.
Results. Peri-adolescent alcohol treatment resulted in a long-lasting elevation of
errors in the RAM as compared to age-matched saline controls. Such an
elevation was not seen in post-adolescent alcohol-treated animals. Independent
of age, binge-like alcohol treatment abolished OPR, yet, compared to age-
matched saline controls this effect was only significant in the peri-adolescent
group.
Conclusions. In agreement with human studies, the current experiments
suggest that adolescence is a unique developmental period, during which
intermittent alcohol exposure can induce persistent spatial memory
impairments. Given that spatial memory is largely dependent on hippocampal
integrity, it is conceivable that BLI alcohol exposure induces lasting changes in
this brain region. The current behavioural models may be used to test this
hypothesis and further elucidate the neurobiological origins of adolescent alcohol
induced deficits.
3.2. Introduction
Globally, about two billion people drink alcoholic beverages, most of whom
initiated alcohol consumption during adolescence (Grant et al. 2006;WHO 2004).
In this unique developmental period, behavioural changes, such as increased
peer interaction, higher levels of impulsivity and risk-taking (Spear 2000),
coincide with ongoing brain maturation. The latter includes medial prefrontal
cortex development, synaptic pruning, and maturation of neurotransmitter levels
and receptor subunit balances (for reviews and references see (Maldonado-
Devincci et al. 2010b;Nixon and McClain 2010;Spear 2000). Given that alcohol
affects many of these developing neurobiological systems (Chastain 2006),
excessive alcohol exposure during adolescence may cause aberrant brain
maturation and consequently lead to long-term behavioural changes.
Indeed, epidemiological studies indicate that adolescent alcohol consumption is
associated with behavioural dysfunction and drug-abuse in later life. Early
regular alcohol-use predicts later (ab)use of addictive substances including
alcohol (Grant et al. 2006;McGue et al. 2001b). Furthermore, heavy drinking
adolescents show abnormal brain responses in spatial working memory tasks
(Tapert et al. 2004b) and measures of attention (Tapert and Brown 1999).
A brain region that may be particularly sensitive to the effects of adolescent
alcohol is the hippocampus (for review and references see (Chin et al. 2010).
For instance, individuals with a history of adolescent alcohol-use were shown to
have abnormal hippocampal volumes (De Bellis et al. 2000). Accordingly,
preclinical evidence suggests that peri-adolescent alcohol exposure is associated
with hippocampal damage. Adolescent rats exposed to alcohol showed impaired
neurogenesis and proliferation in the hippocampus (Crews et al. 2006;He et al.
2005;Morris et al. 2010). Furthermore, the adolescent hippocampus seems more
sensitive to the LTP suppressive effects of alcohol (Pyapali et al.
1999;Swartzwelder et al. 1995) and alcohol may induce lingering damage in the
form of morphological changes, inflammation and neurodegeneration (Crews et
al. 2000;Evrard et al. 2006;Pascual et al. 2007). How these alcohol-induced
abnormalities affect hippocampus-dependent behaviour is largely unexplored.
Furthermore, there is little understanding of the age-dependency of the long-
term consequences of alcohol exposure. It is generally assumed that adolescents
are more vulnerable in this respect, but empirical evidence is lacking. As it is
almost impossible to disentangle environmental, social and genetic contributions
to the age-related effects of alcohol in human studies, animal models are necessary to isolate these different components. In rodents, maze paradigms are predominantly used to assess spatial memory processes. Over the last decades, different maze types have been developed, such as the Morris water maze, the T- or Y-maze and the eight-arm RAM (Paul et al. 2009). In allocentric versions of these models rats learn to navigate according to spatial cues to find food pellets at the end of arms (T/Y maze and RAM) or to find an escape platform (Morris water maze). A different, but useful model to measure spatial memory is the object recognition paradigm. It takes advantage of the rats' natural tendency to explore novel objects. Several variants of the object recognition paradigm have been developed, tapping into different aspects of memory. The OPR variant was specifically designed to assess spatial memory, but other variants of the object recognition paradigm may also rely on spatial memory to a certain extent (for review see Dere et al. 2007). Rodent studies addressing the effects of adolescent alcohol exposure on spatial memory have yielded mixed results. Oftentimes the interpretation of obtained results in these studies is complex, for instance due to differences in the behavioural models, the treatment window, the administration route, the employed alcohol doses and the exposure and treatment regimens. Regardless of these differences, direct or short-term age-dependent effects of alcohol exposure on spatial memory have been reported by a number of studies. Most of these studies claim that adolescents are more sensitive to the disrupting effects of alcohol (Markwiese et al. 1998;Pascual et al. 2007;Schulteis et al. 2008;Sircar and Sircar 2005). Conversely, others find age independent effects (Acheson et al. 2001;Silvers et al. 2003) and one report indicates that adult animals are more sensitive to the disruptive effects of alcohol on spatial memory (Rajendran and Spear 2004). Collectively, these studies illustrate that spatial memory may be affected in an age-dependent manner, both directly upon alcohol treatment and up to three weeks after treatment is completed. However, whether peri-adolescent alcohol exposure induces effects on spatial memories that extend into adulthood is largely unknown. The purpose of the present study is to further address this issue. As discussed above, the interpretation and integration of results from different studies on the effects of adolescent alcohol exposure is hampered by the wide
variety of treatment and test conditions that are used. In the current experiments, we therefore used a standardized binge-like injection (BLI) treatment that was designed to mimic the intermittent alcohol exposure pattern seen in binge drinking youth (Tapert et al. 2004a;van Laar et al. 2010;WHO 2010). Rats receiving BLI treatment received intermittent intra-peritoneal saline or alcohol injections, either during (peri-adolescent groups) or directly following the adolescent period (post-adolescent groups). Five weeks after BLI treatment, i.e. during adulthood, rats were subjected to behavioural testing. Using this BLI protocol, we previously demonstrated that rats with a history of adolescent alcohol exposure increased alcohol-intake in adulthood, whereas identical treatment did not affect visuospatial attention and inhibitory response control (see Chapter 2). The present work explores the effects of BLI treatment on spatial memory performance in the RAM and OPR paradigm.
3.3. Methods
3.3.1. Animals
Time-pregnant female Wistar rats arrived at five days of gestation (Harlan CPB,
Horst, The Netherlands), and were housed individually in Macrolon cages under
standard conditions and a reversed 12 h light/dark cycle (lights on between 7
pm and 7 am). Upon delivery, litters were culled to eight pups per mother and
preferably consisted of males only, but when necessary were matched with
female pups (Counotte et al. 2008). At P21, male animals were weaned and
housed two per cage. During the course of behavioural testing in the RAM,
animals were food restricted to maintain 85-90% of their free-feeding weight.
Water was available ad libitum. All training and test sessions were conducted
during the dark phase of the light-dark cycle, at the same time each day.
Experimental procedures were approved by the Animal Ethical Committee of the
VU University, Amsterdam, The Netherlands.
3.3.2. Apparatus and behavioural procedures
Radial arm maze. The aluminium eight-arm RAM consisted of an octagonal
central platform (∅ 33 cm) elevated 100 cm from the floor. Eight modular arms
measuring 60 x 12 cm radiated evenly from the central platform. Both arms and
platform were covered with a black PVC anti-slip coating. All arms had
translucent 30 cm high Plexiglas walls. Eight manually controllable Plexiglas
guillotine doors bordered the central platform to contain rats in the central
platform and permit entry to the selected arms. The end of each arm was fitted
with a sunken food well (∅ 2 cm, depth 0.3 cm).
The maze was placed in the center of a laboratory room enriched with distal
visual cues, and a radio constantly broadcasting. The room was dimly lit with red
light and a video camera (Sony, Badhoevedorp, the Netherlands) was mounted
200 cm above the maze.
Object - place recognition. Four identical plastic arena's measuring 79 x 57 x
43 cm (Salma, Ikea) were placed in the centre of the laboratory room described
previously. Arena walls and floors were black and non-transparent. The objects
used for place recognition were aluminium bars (9 x 9 x 30 cm) or cylinders (∅
9cm, 30 cm high). Viewer video tracking software (Biobserve, Bonn, Germany)
was used to analyze behaviour.
3.3.3. Experimental design
Binge-like injection (BLI) treatment.
Animals were treated either during (PND 34-43, peri-adolescent group) or directly after adolescence (PND 62-71, post-adolescent group). BLI alcohol exposure consisted of intraperitoneal (i.p.) injections of 20% (v/v) ethanol (2.5 g/kg) every other day for ten days. This resulted in a total of five injections; peri-adolescent treated animals were injected on
postnatal day (PND) 34, 36, 38, 40 and 42; post-adolescent animals were
injected at PND 62, 64, 66, 68 and 70. Control animals received an identical
treatment with the exception that they were i.p. injected with sterile saline
instead of ethanol. In both peri-and post-adolescent animals this alcohol-
administration protocol resulted in blood alcohol levels over 200 mg/dl during
the first h after injection (Chapter 2).
Radial arm maze (RAM).
Forty-six male animals were used in this experiment, twelve animals per
treatment group, except for the adult saline-treated group that consisted of ten
animals. We used a so-called delayed spatial win-shift paradigm that was freely
adapted from Seamans et al. (Seamans et al. 1995;Seamans and Phillips 1994).
Only twenty-four animals could be trained on a single day, therefore the animals
were trained every other day, four to six days a week. On each training day the
different test groups were equally represented.
Habituation (session 1-2). Training was started five weeks after alcohol
exposure, when all animals were adults. On the first two training days the rats
were allowed to explore the maze for 5 min with no food available. After training
they received 20 food pellets (45 mg, Formula P; Research Diets Inc., New
Brunswick, NJ, USA) in their home cages.
Adaptation (session 3-6). During adaptation all animals received a sample phase
only. Before the sample phase started, a set of four arms was blocked in a semi-
random fashion, i.e. the pattern was such that there were always two adjacent
arms and two non-adjacent arms blocked. The arms that were blocked varied for
a given subject across days and varied among subjects within days. Three food
pellets were placed in the food cups of the four remaining open arms. At the end
of this adaptation period all subjects retrieved all pellets within 5 min and ate all
pellets in the arms in one visit. An arm entry was recorded when a rat moved at least 30 cm into the arm. Training (session 7-41). Training trials consisted of a sample phase and a test phase (Figure 12). A 5-min delay was imposed between the separate phases. During the test phase all arms were open, but only the arms that were blocked during the sample phase were baited. In both the sample and test phase the subjects had a maximum of 5 min to retrieve the food pellets. The order and the total number of arm visits were manually recorded as was the latency to visit all baited arms. During the test phase, entries in previously visited arm were considered errors (i.e. either entries into the unbaited arms that were open during the sample phase or entries in baited arms that were already visited). Animals were removed from the maze after visiting the four baited arms or when 5 min had elapsed. After each test session the maze was thoroughly cleaned with Special Kennel Cleaner (Rogier Bosman Chemie BV, Heijningen, NL).
Figure 12. Experimental design of the
RAM paradigm. During the sample
phase animals are al owed to explore
four open arms that are baited (black
dots). The other arms are blocked by
the guil otine doors (black arms).
Fol owing a 5-min delay the animals
are placed back on the maze. Now al
arms are open, but only the arms that
were blocked during the sample phase
are baited.
Object - place recognition.
A separate group of 48 male animals was treated for this experiment, each
treatment group consisting of twelve animals. Seven weeks after treatment,
when all animals were adult training was started.
Habituation. On day one, to familiarize the animals to the surroundings, each
subject was placed together with their cage-mate into the arena for 10 min. On
day two and three the animals were individually placed in the arena for 10 min.
Test-day. The fourth day all animals were tested for place recognition using four identical copies of either bar shaped or cylindrical objects in each arena. The test-day included a sample phase and a test phase separated by a 5-min delay. Preceding the sample phase the subjects were placed in the arena without objects for 10 min. The animals were removed and returned to their home cages and two identical objects were placed in two corners of the arena (Figure 13). In the sample phase the subjects were placed in the arena and allowed to explore the objects. When 4 min had elapsed the animals were removed from the arena and returned to their home cages for 5 min. During this delay, the objects were replaced by two identically shaped objects, however, one of the objects was shifted to a different corner. Subsequently, the animals were placed in the arena for the test phase and were allowed to explore the objects, one in the old position and one in a novel position, for another 4 min. The time spent exploring the individual objects during the first 60 s of the test phase was recorded. The arenas and objects were thoroughly cleaned with a 70% ethanol solution prior to each test session.
Figure 13. Experimental design of the
OPR paradigm. During the sample
phase animals are al owed to explore
two identical objects. Fol owing a 5-min
delay the animals are placed back in
the arena to explore the objects, one of which has been repositioned.
3.3.4. Drugs
During alcohol treatment animals were injected with, 20% (v/v) ethanol (2.5
g/kg). To this end 96% laboratory alcohol (Interchema, Oosterzee, the
Netherlands) was diluted with sterile saline for i.p. injection appropriate
concentration. The control groups were injected with saline only.
3.3.5. Statistical analyses
All statistical analyses were performed using the Statistical Package for the
Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA) and all data are
displayed as mean ± SEM. The homogeneity of variance across groups was
determined using Mauchly's tests for equal variances and in case of violation of
homogeneity, corrected Huynh–Feldt degrees of freedom and resulting more
conservative probability values were used for subsequent analyses. In case of
statistically significant main effects, further post hoc comparisons were
conducted using Student–Newman–Keuls tests. The level of probability for
statistically significant effects was set at 0.05. For a clearer presentation of the RAM results, the data in the figures are presented in five day bins, i.e. the means of five consecutive days. For the RAM experiment the total number of errors during the test phase is reported. For the OPR the first 60 s of the test phase were analyzed and the following measures are reported: 1) Total exploration time during test phase (time spent at repositioned + time spent at familiarly located object) and 2) Discrimination index (time spent at repositioned - time at familiarly located object)/(time spent at repositioned + time at familiarly located object).
3.4. Results
3.4.1. Radial arm maze
Over time animals of all treatment groups improved their performance during
the test phase of the delayed spatial win-shift paradigm as shown by a
significant day effect on performance (F(34,1428)=11,9; p<0.001, ε=0.689).
Additionally, a day * treatment interaction was observed (F(34,1428)=1,6;
p<0.05 ε=0.689) suggesting a time-dependent effect of treatment on the
number of errors made during the test phase. Furthermore, a between subjects
age * treatment interaction (F(1,42)=4.59; p<0.05) indicated that the
treatment effect differed between age groups. Post hoc analyses showed that
alcohol treatment significantly impaired spatial memory in the peri-adolescent
alcohol-treated group (F(22,495)=1.75; p<0.05), whereas the post-adolescent
treated groups showed equal performance (F(21,423) <1; ns). To examine the
time course of the effect of alcohol exposure on spatial memory we split the
data into seven equal time bins, each containing a block of five training days
(Figure 14). As such, separate univariate ANOVA's per age group for each time
bin determined that the detrimental effect of peri-adolescent alcohol treatment
became apparent in time bins 4-7, while performance was equal in all groups in
time bins 1-3.
# errors test phase post-adolescent
# errors test phase peri-adolescent
Figure 14. The number of errors made during the test phase of the RAM paradigm. The
left panel depicts performance of the post-adolescent groups; peri-adolescent
performance is displayed in the right panel. Each time bin represents five training days. *
p<0.05 difference from saline controls. # p<0.05 difference from saline controls over bins
4-7.
Post-adolescent: bin 1 (F(1,20)=4.1; p=0.06); bin 2 (F(1,20)<1; ns); bin 3
(F(20,1)<1; ns); bin 4 (F(20,1)<1; ns); bin 5 (F(20,1)<1; ns); bin 6
(F(20,1)<1; ns); bin 7 (F(20,1)<1; ns). Peri-adolescent: bin 1 (F(22,1)=1.992;
ns); bin 2 (F(22,1)<1; ns); bin 3 (F(22,1)<1; ns); bin 4 (F(22,1)=5.3; p<0.05);
bin 5 (F(22,1)=3.9; p=0.06); bin 6 (F(22,1)=7.8; p<0.05); bin 7 (F(22,1)=
4.4; p<0.05). These results indicate that both peri-adolescent alcohol and
saline-treated animals initially acquire the task at the same rate, however, after
15 training days the rate of improvement declines more rapidly in the alcohol-
treated group resulting in a long lasting elevation of their error rates compared
to the saline controls.
3.4.2. Place recognition
To ensure that exploration differences did not confine our results, we examined
whether age and/or treatment affected overall exploration (time spent at
repositioned + time spent at familiarly located object) during the first 60 s of the
test phase. Three animals were excluded from the experiment, because they
explored the objects for less than 5 s. A univariate ANOVA revealed a significant
effect of age on exploration levels during the test phase (F(3,40)=4.182;
p<0.05), indicating that older animals show slightly lower exploration time
during first 60 s of the test phase compared to the 30 days younger peri-
adolescent treated groups. Alcohol treatment had no effects on exploration rates
in the separate age groups (F(3,40)=0.40; ns).
To measure the performance of rats during the test period we calculated a
discrimination index (time spent at repositioned - time at familiarly located
object)/(time spent at repositioned + time at familiarly located object). A
positive index indicates a preference for the novel placed object, a negative
score a preference for the familiar object. A score of zero shows that an animal
does not discriminate between the objects. Since we found an age-dependent
effect on total exploration time, we analysed the discrimination index of the
different age groups separately (Figure 15). A univariate ANOVA revealed that
adolescent alcohol-treated animals had a significantly lower recognition index
compared to their age-matched saline controls (F(1,22)=6.45; p<0.05; mean
OS= 0.26 ± 0.08; OA= -0.12 ± 0.13). This effect was specific for the peri-
adolescent treated animals, as post-adolescent alcohol-treated animals did not
differ from their saline controls (F(1,18); p>0.25; mean US=0.3 ± 0.13; mean
UA=0.07 ± 0.13).
To determine whether animals showed preference for the novel or familiar placed object, we calculated the 95% confidence limit for each treatment group. We considered a mean discrimination index with a lower margin of the 95% confidence limit above zero as object recognition. Using this criterion we determined that only the saline-treated groups demonstrated place recognition, whereas the alcohol-treated animals in both age groups, did not. Together, these data demonstrate that both post- and peri-adolescent alcohol-treated animals fail to show OPR seven weeks after treatment. However, the alcohol-effect on OPR is only significant different from age-matched saline-treated animals in the peri-adolescent treated groups.
Figure 15. Performance during the test phase of the OPR paradigm. The left panel
depicts performance of the post-adolescent groups; peri-adolescent performance is
displayed in the right panel. Each panel depicts the average time spent exploring the
objects and the discrimination index of the different treatment groups. * p<0.05 difference
from saline controls. # significant object recognition, i.e. a discrimination index with a
lower margin of the 95% confidence limit above zero.
3.5. Discussion
The present findings show that a history of adolescent binge-like alcohol
exposure results in long-lasting spatial memory impairments in adult rats.
Compared to age-matched controls, peri-adolescent alcohol exposure induced
elevated error rates in a delayed spatial win-shift variant of the RAM. This deficit
that lasted up to six months was not detected in animals that received post-
adolescent alcohol treatment. Furthermore, seven weeks after treatment, OPR
was completely abolished in all alcohol treatment groups, i.e. alcohol-treated
animals could not discriminate between a familiar and a repositioned object in
the OPR task.
Spatial memory is considered to be related to those brain functions that are
involved in recognizing, encoding, storing and recollecting spatial information
about the arrangement of objects or specific routes (Paul et al. 2009). In line
with our results, investigation of these brain functions in humans have revealed
that youth with a history of alcohol-(ab)use have impaired performance in tasks
measuring aspects of spatial memory (Tapert et al. 2004b). Additionally,
preclinical evidence derived from different maze paradigms indicates that spatial
memory performance is uniquely affected in adolescent rats, directly and up to
three weeks after alcohol exposure (Markwiese et al. 1998;Pascual et al.
2007;Rajendran and Spear 2004;Schulteis et al. 2008;Sircar and Sircar 2005).
The long-term effects of peri-adolescent alcohol exposure on spatial memory,
however, have not received intensive investigation.
White and colleagues employed a delayed spatial win-shift RAM protocol similar
to the one used here. They report that intensive binge-like alcohol exposure (5
g/kg, i.p., every other day, post natal day (PND) 30-50 / PND 70-90) did not
affect task acquisition three weeks after treatment. Nonetheless, responsiveness
to the memory-impairing effects of alcohol was increased two months after
exposure selectively in the peri-adolescent group, as was evidenced by an
increased number of errors in the peri-adolescent alcohol group (White et al.
2000). In line with these results, we report no effects of peri-adolescent alcohol
exposure on acquisition of spatial memory during the first month of training (15
training sessions). Through sessions 16-35, however, persistent spatial memory
impairment was seen in individuals that had received peri-adolescent alcohol
treatment. Possibly the specific conditions in our paradigm resulted in a higher
degree of difficulty that allowed us to detect deficits in the peri-adolescent
alcohol-treated group, that would have remained unidentified under less
demanding circumstances. In support of this line of thought, compared to the White study, the error rates reported in our study seem slightly higher in all treatment groups. This may be explained by a number of differences in experimental design, that impact acquisition and performance in the RAM, such as rat strain, daily versus every other day training, treatment intensity and the length of the washout period. Alternatively, the maze in the current study was constructed out of different materials and had different dimensions and distal cues than that used in the White study (2000); all of these factors may have influenced performance. Irrespective of the differences in outcome, similar to our experiment, the White study revealed long-lasting changes in RAM performance following BLI alcohol exposure during adolescence. Our data extend these findings, demonstrating that lingering spatial memory deficits are detectable without the use of additional pharmacological interventions. Taken together, the studies discussed here suggest that, in addition to its unique short term consequences, adolescent alcohol exposure impairs spatial memory even months after adolescent alcohol treatment has ended. The delayed spatial win-shift RAM paradigm is suited for the detection of ‘steady state' deficits in repeated measures designs, but it is labour intensive and not well suited for measuring rapid drug effects (Hodges 1996). The object recognition paradigm, another model used to measure memory in rodents, allows more rapid testing. Object recognition takes advantage of the rats' natural tendency to explore novel objects. Several variants of the paradigm have been developed, tapping into different aspects of memory. By changing the location of objects during the test phase, the OPR variant was designed to specifically assess spatial memory. Nevertheless, all object recognition variants may rely on spatial memory to a certain extent (for review see Dere et al. 2007). The OPR paradigm is less versatile than the RAM and relies on novelty preference rather than goal-oriented navigation; it may therefore engage different ‘spatial' abilities (Dere et al. 2007). Nonetheless, both models employ sample and test phases separated by a delay, may rely on the formation of a cognitive map and are at least partially reliant on hippocampal function (for reviews see Dere et al. 2007;Hodges 1996;Olton 1987). To our knowledge this is the first study to investigate the long-term effects of adolescent binge-like alcohol exposure on performance in the OPR variant of the object recognition paradigm. In correspondence to the deficits seen in RAM performance, we report that individuals receiving binge-like alcohol fail to show
OPR seven weeks after treatment, whereas saline-treated age-matched controls did show a preference for the object in the novel location. Although OPR was impaired seven weeks following alcohol treatment in both peri-adolescent and post-adolescent rats, compared to age-matched saline-treated controls, performance was significantly impaired in peri-adolescents only. This effect was not due to impaired exploration in the alcohol-treated groups, as total time spent exploration exploring the objects did not differ between saline and alcohol-treated individuals. Taken together, these results suggest that peri-adolescent BLI alcohol treatment impairs OPR in adulthood. Our results are consistent with data from previous studies that investigated the effects of alcohol in other variants of the OPR. Using a short delay, novel-object recognition was impaired directly, but not at three weeks after binge-like peri-adolescent alcohol exposure (Pascual et al. 2007). Furthermore, object recognition with a delay of 60 min was impaired at PND 90 and PND 180 with chronic alcohol treatment starting from PND 21 up to the end of the experiment (Garcia-Moreno et al. 2002). Binge-like alcohol treatment in adult animals impaired novel-object recognition one week but not ten weeks after treatment, whereas OPR was disrupted at both the one week and ten week time point (Cippitelli et al. 2010). Thus, a history of binge-like alcohol exposure can deteriorate performance in in different variants of the object recognition paradigm, independent of age of treatment. Nonetheless, the fact that OPR performance was only significantly impaired in peri-adolescent alcohol-treated animals indicates that the extent to which binge-like alcohol treatment affects OPR may be age-dependent. Although the brain regions involved in the detrimental effects of binge-like peri-adolescent alcohol treatment on spatial memory were not studied in closer detail in the current study, specific behavioural tasks we used have repeatedly been associated with hippocampal function. Lesions to the hippocampus, or to its primary input and output pathways, lead to poor performance in spatial tasks in the RAM and impair OPR (Dere et al. 2007;Olton 1987). As several rodent studies report on the harmful effects of alcohol intoxication on neurogenesis and inflammation in the hippocampus (Crews et al. 2006;He et al. 2005;Morris et al. 2010;Pascual et al. 2007), it is possible that the deficits seen here are due to impaired connectivity within the hippocampus and/or between the hippocampus and other brain regions, such as the prefrontal cortex. At the same time, since complex animal behaviour is evaluated in these paradigms other brain regions
are likely to be engaged as well. Research into the molecular and cellular origin
or the neurobiological loci of adolescent alcohol induced spatial memory deficits
is still in its formative years and may therefore profit from the treatment and
behavioural models used in the current study. In fact, we are currently looking
into the proteome changes of hippocampal synapses after adolescent BLI alcohol
exposure.
Taken together, our results demonstrate that binge-like alcohol exposure has
persistent detrimental effects on spatial memory performance, even after
prolonged abstinence extending into adulthood. These effects were apparent
seven weeks following treatment in a one-trial OPR task and up to six months
after treatment in the delay spatial win-shift RAM task. Whereas peri-adolescent
alcohol exposure disrupted performance in both tasks, an age-dependent effect
of alcohol exposure was most apparent in the RAM paradigm. This indicates that
adolescent alcohol-use, in the absence of genetic and environmental influences,
may impair memory performance in later life and thus supports the notion that
adolescence is a unique time window during which alcohol exposure can induce
persistent behavioural abnormalities.
3.6. Acknowledgements
Professor Sabine Spijker and Dr Pieter van Bokhoven your knowledge and
assistance was highly appreciated and indispensable for setting up the OPR
experiments. We would also like to thank Jasper Heinsbroek and Martijn Huits
for their technical assistance during validation of the RAM paradigm. This project
was supported by a ZON-MW Topgrant 912-06-148.
3.7. Disclosure
All authors declare that, except for income received from primary employer, no
financial support or compensation has been received from any individual or
corporate entity over the past three years for research or professional service
and there are no personal financial holdings that could be perceived as
constituting a potential conflict of interest.
Chapter 4
Varenicline attenuates cue-induced relapse to
alcohol-, but not nicotine-seeking, while
reducing inhibitory response control
J.A. Wouda1,2, D. Riga1, W. De Vries1, M. Stegeman1†, Y. van Mourik1, D.
Schetters1, A.N.M. Schoffelmeer1, T. Pattij1, T.J. De Vries1,2
1Department of Anatomy and Neurosciences, Neuroscience Campus Amsterdam,
VU University medical center and 2Department of Molecular and Cellular
Neurobiology, Center for Neurogenomics and Cognitive Research, Neuroscience
Campus Amsterdam, VU University, Amsterdam, The Netherlands
Psychopharmacology (Berl). 2011 Jul;216(2):267-77
4.1. Abstract
Rationale. Treatment of the most widely abused drugs, nicotine and alcohol, is
hampered by high rates of relapse. Varenicline tartrate, an α4β2-nicotinic
receptor partial agonist, is currently prescribed as a smoking cessation aid.
However, there is emerging evidence that it may also modulate alcohol-seeking
and cognitive functioning in rats.
Objectives. As preclinical data on alcohol-taking and relapse are limited, we
used a self-administration-reinstatement model to evaluate the effects of
varenicline on operant responding for alcohol (12% v/v), intravenous nicotine
(40 µg/kg/inf.), sucrose (10% w/v) and on cue-induced relapse to alcohol and
nicotine-seeking in rats. At the cognitive level, we assed varenicline's effects on
5-choice serial reaction time task (5-CSRTT) performance with a focus on
correct responses (a measure for attention), and premature responding
(inhibitory response control or impulsive action) modalities that have previously
been associated with addictive behaviour.
Results. Varenicline, at doses of 1.5 and 2.5 mg/kg reduced alcohol and
nicotine self-administration and enhanced operant responding for sucrose. At
these doses, varenicline reduced cue-induced relapse to alcohol but not nicotine-
seeking. In contrast, at 0.5 mg/kg varenicline facilitated cue-induced nicotine-
seeking. Similar to nicotine, varenicline increased premature responding at low
doses, but had no effect on any of the other behavioural parameters in the 5-
CSRTT.
Conclusions. Our data indicate that varenicline specifically reduced responding
for nicotine and alcohol, but not for natural reinforcers such as sucrose.
Interestingly, varenicline strongly attenuated cue-induced relapse to alcohol-
seeking, but not nicotine-seeking. Varenicline may therefore be a promising aid
in the treatment of alcohol addiction.
4.2. Introduction
Alcohol and nicotine are the two most widely abused addictive substances.
While, several pharmacological treatments for alcohol and nicotine-dependence
are targeted at reducing drug-intake, these treatments generally demonstrate
limited protection against relapse (Anton et al. 2006;Frishman 2009). In this
respect varenicline, a novel agent currently prescribed to aid smoking cessation,
may have a more favourable pharmacological profile than other registered
pharmacotherapeuticals, such as Bupropion (Gonzales et al. 2006;Jorenby et al.
2006). Varenicline is a partial α4β2 nicotinic receptor agonist and was developed
with the assumption that such agents may possibly diminish the consequences
of both nicotine exposure and its absence. Partial α4β2 nicotinic receptor
agonists are postulated to promote smoking cessation by preventing nicotine
from binding to the receptor. At the same time, by partial activation of α4β2
nicotinic receptors, such agents would moderately increase mesolimbic
dopamine release, which is believed to alleviate craving (Coe et al. 2005;Niaura
et al. 2006). Indeed, clinical observations indicate that varenicline is able to 1)
reduce withdrawal symptoms and negative affect during abstinence in treatment
seeking smokers, 2) attenuate the subjective rewarding effects of nicotine
during a scheduled smoking lapse and 3) increase the number of abstinent days
following the smoking lapse in a subgroup of participants (Patterson et al.
2009). Consistent with these clinical findings, preclinical evidence confirms that
varenicline effectively reduces nicotine self-administration in rats (O'Connor et
al. 2010;Rol ema et al. 2007a). Furthermore, in a rat model for relapse,
varenicline attenuated nicotine-primed relapse to nicotine-seeking as wel as
relapse induced by a combination of a nicotine-prime and associated cues. In
contrast, varenicline had no effect on cue-induced relapse alone (O'Connor et al.
2010).
Interestingly, recent findings suggest that varenicline may also modulate
alcohol-seeking and intake in heavy drinking smokers as well as laboratory
animals (McKee et al. 2009;Steensland et al. 2007). Consistent with these
findings, varenicline was shown to counteract alcohols' enhancing effect on
dopamine levels in the nucleus accumbens in rats (Ericson et al. 2009).
Lowering dopamine levels in this region has previously been associated with
reduced alcohol consumption in rats (Ericson et al. 1998, 2000;Soderpalm et al.
2000). Together these findings underline the potential of varenicline as a
treatment for alcohol-use disorders in addition to its efficacy as a smoking
cessation aid.
In humans, abstinence and relapse are associated with cognitive deficits (Scheurich 2005). Therefore, cognitive enhancing agents are pursuit as pharmacotherapy targets for addiction (Sofuoglu 2010). In this respect, nicotine receptor (nAChR) agonists are interesting targets, since acute and chronic administration of nAchR agonists can produce long-lasting cognitive enhancing effects (Buccafusco et al. 2005), such as improvement of working and spatial memory, facilitated associative learning and improved attentional processing (Levin et al. 2006;Rezvani and Levin 2001). For that reason, the putative cognitive enhancing effects of varenicline warrant investigation. In the current study we aim to further elucidate the motivational and cognitive effects of varenicline, with a particular focus on the relapse-preventing properties of this compound in an alcohol-taking and seeking model. To that end, we tested the effects of varenicline on both self-administration and relapse to alcohol- and nicotine-seeking. Moreover, to control for drug specificity, we also evaluated the effects of varenicline on self-administration of the natural reinforcer sucrose. Finally, we employed the 5-choice serial reaction time task (5-CSRTT) to assess possible cognitive enhancing effects of varenicline at the level of visuospatial attention and inhibitory response control (Robbins 2002), the latter being a form of impulsivity that was associated with enhanced motivation to initiate and maintain nicotine self-administration in rats (Diergaarde et al. 2008a).
4.3. Methods
4.3.1. Animals
Male Wistar rats (Harlan CPB, Horst, The Netherlands), weighing 280–320 g
upon arrival were used. They were housed in pairs in a temperature and
humidity controlled room on a 12 h light/dark cycle (lights on between 7 pm and
7 am), with the exception that animals that were implanted with intravenous
silicon catheters were individually housed. Catheters were implanted in the right
jugular vein under gas anaesthesia (Isoflurane) as described before (De Vries et
al. 1999). All training and testing sessions were conducted during the dark
phase of the light-dark cycle, at the same time each day. Experimental
procedures were approved by the Animal Ethical Committee of the VU
University, Amsterdam, The Netherlands.
4.3.2. Apparatus and behavioural procedures
Self-administration
Self-administration training and testing was conducted in 32 identical operant
chambers enclosed in sound-attenuating ventilated cubicles (Med Associates
Inc., St. Albans, USA). The operant chambers were equipped with a grid floor,
two nose-poke holes and a central reinforcer receptacle; a dim red house light
and a tone module (ENV-223AM Med Associates Inc.) were fitted on the opposite
wall. During self-administration, reinforcers were delivered by an infusion pump
(PHM-100, Med Associates Inc.).
Animals were placed in the operant chamber and after 30 s the house light was
switched on and trials started. Each trial was signalled by illumination of a red
stimulus light located above the active hole, which was extinguished when a
nose poke was made into this hole. An active nose-poke resulted either in an
intravenous (i.v.) infusion of nicotine (40 µg/kg), the delivery of 0.2 ml alcohol
(12% v/v) or 0.2 ml 10% sucrose in the receptacle and a yellow stimulus light in
the nose-poke hole was illuminated for 5 s combined with a 2-s tone signal (+/-
68 dB, 2900 Hz). After delivery, a time-out period of 15 s commenced during
which all stimulus lights were switched off until the next trial started.
Responding during time-out was monitored, but had no programmed
consequences.
Two bottle procedure
To facilitate acquisition of operant alcohol self-administration, rats were first
trained to consume alcohol in a two-bottle free-access paradigm. In this
paradigm, upon arrival rats were habituated to two water bottles on their home
cages. Every other day one of the bottles was replaced by a bottle containing a
gradually increasing alcohol solution (from 2% to 12% v/v). In three weeks the
animals reached 12% v/v alcohol and training proceeded to a two bottle limited-
access paradigm, i.e. animals were given access to the 12% solution for 1 h
daily. After ten days of limited-access animals that consumed over 0.35 g/kg
alcohol were selected to enter the operant self-administration phase.
5-choice serial reaction time task (5-CSRTT)
Experiments were conducted in rat operant chambers with stainless steel grid
floors (MED-NPW-5L; Med Associates Inc.). On-line control of all operant
chambers and data collection were performed using MED-PC version IV (Med
Associates Inc.). Five sessions were scheduled per week from Monday until
Friday, one session per day.
A more detailed description of training in the 5-CSRTT has been reported
previously (van Gaalen et al. 2006). In short, rats were trained to detect and
respond to a 1-s visual stimulus in either one of five apertures, during
presentation of the stimulus or during the 2-s limited hold period, to obtain a
food pellet (45 mg, Formula P; Research Diets Inc., New Brunswick, NJ, USA).
Each session terminated after 100 trials or 30 min, whichever occurred first.
Initially the duration of this stimulus was 32 s and was gradually decreased to
1 s over sessions until animals reached stable baseline performance
(accuracy>80% correct choice and <20% errors of omission). Incorrect,
premature responses (responses during the 5-s inter-trial interval (ITI)) and
errors of omission did not lead to the delivery of a food pellet and resulted in a
5-s time-out period during which the house light was extinguished. Responses
during the time-out period resulted in a new time-out period. Perseverative
responses, i.e. repeated responding into an aperture following correct choice and
before pellet collection were measured but did not have any programmed
consequences. The following behavioural measures were recorded: (1) accuracy,
((number correct trials/(correct + incorrect trials)) * 100); (2) latency of correct
responses, i.e. the mean time between stimulus onset and nose-poke in the
illuminated unit; (3) premature responses, i.e. the number of premature
responses before the onset of the visual stimulus, reflecting aspects of loss of
inhibitory control (impulsive action) and (4) perseverative responses after
correct choice, a measure of compulsive behaviour.
4.3.3. Experimental design
Self-administration
In 3 separate experiments we tested the effects of varenicline on nicotine (exp
1), alcohol (exp 2) or sucrose (exp 3) self-administration. Behavioural training
started one week after surgery (nicotine), two-bottle procedure (alcohol) or
arrival (sucrose). Initially, all animals were trained daily in 1 h sessions on a
continuous reinforcement schedule. The fixed ratio was increased during
training, up to FR3 for the nicotine groups and FR4 for alcohol and sucrose
groups. When the levels of responding met predefined selection criteria, the
effect of a graded dose of varenicline on self-administration was tested. Criterion
performance was defined as follows. For the nicotine group animals that
received five or more reinforcers, distributed over the session during the last
three sessions on a FR3 schedule, were selected for testing. In the alcohol group
animals that self-administered over 0.35 g/kg alcohol for three consecutive days
on a FR4 schedule of reinforcement were selected for testing. In the sucrose
group all animals received five or more reinforcers, distributed over the session
during the last three sessions on a FR4 schedule and were selected for testing.
Varenicline tests were conducted twice a week, on Tuesdays and Fridays. On
intermediate days rats were trained to self-administer nicotine, alcohol or
sucrose without being treated with varenicline. To explore the effects of
varenicline on self-administration a within-subject design was used and
treatment was randomized over the subjects using a Latin square design. To
facilitate acquisition the alcohol group (exp 2) was given a single 20-min
habituation session on the first training day, during which only the house light
was illuminated. Nose-poking during this session was without any behavioural
consequences. Furthermore these animals received one free sample of alcohol in
the central receptacle upon the start of the habituation session and the first five
training sessions.
Cue-induced relapse
To assess the effects of varenicline on cue-induced relapse to nicotine or
alcohol-seeking, two separate groups of animals were trained to criterion
performance and subsequently underwent extinction training. Thus, animals
were placed in the training context for 15 daily sessions of 1 h, without
audiovisual cue-exposure or reinforcer delivery.
Relapse: To assess effects of varenicline on cue-induced relapse, rats were
divided into four experimental groups that received either vehicle, 0.5 mg/kg,
1.5 mg/kg or 2.5 mg/kg varenicline. On the test day, all animals were injected
with vehicle or one of three doses 30 min before they were placed in the training
context for a 30 min relapse test. During the relapse test, the houselight and red
cuelight were turned on and nose-poking resulted in the presentation of the
discrete compound audiovisual cues (but no reinforcer delivery) on the FR
schedule used during training. In the alcohol group 0.20 ml alcohol (12% v/v)
was delivered in the receptacle before the session started. We conducted a
series of pilot studies investigating cue-induced alcohol-seeking under several
conditions. Data from these studies, and work by others indicates that adding a
single drop of alcohol, that induces the gustatory, but not the pharmacological
effects of alcohol, results in optimal relapse (Le and Shaham 2002).
5-choice serial reaction time task (5-CSRTT)
To assess putative cognitive enhancing effects of varenicline, rats were trained
in the 5-CSRTT until they reached stable baseline performance on stimulus
duration 1 s (accuracy>80% correct choice and <20% errors of omission during
the last five sessions). Subsequently, the effects of varenicline were assessed
using a Latin square design and tests were conducted twice a week, on
Tuesdays and Fridays. On intermediate days rats were trained normally without
being treated with varenicline. Following the tests with varenicline animals were
trained for an additional week and tested under increased attentional demand by
reducing the stimulus duration to 0.5 s. Subsequently, under these conditions
the effect of 1.5 mg/kg varenicline was tested.
4.3.4. Drugs
Nicotine hydrogen tartrate salt (Sigma, St Louis, MO, USA) was dissolved in
sterile saline and the pH of the solution was adjusted to +/- 7.4 with diluted
NaOH. The nicotine dose is expressed as free base weight. For all alcohol
solutions used, 96% laboratory alcohol (Interchema, Oosterzee, The
Netherlands) was diluted with water to reach the appropriate concentrations.
Varenicline (kindly provided by Solvay Pharmaceuticals, Weesp, The
Netherlands) was suspended in a 1% methylcellulose - 5% mannitol solution
and the pH was adjusted to 7.9. In all studies, varenicline was administered
intraperitoneally with an injection volume of 2 ml/kg, 30 min before testing and
doses were 0.5, 1.5 and 2.5 mg/kg based on previous studies (Rollema et al.
2007a;Steensland et al. 2007).
4.3.5. Statistical analyses
All statistical analyses were performed using the Statistical Package for the
Social Sciences version 15.0 (SPSS Inc., Chicago, IL, USA) and all data are
displayed as mean ± SEM. The homogeneity of variance across groups was
determined using Mauchly's tests for equal variances and in case of violation of
homogeneity, corrected Huynh–Feldt degrees of freedom and resulting more
conservative probability values were used for subsequent analyses. In case of
statistically significant main effects, further post hoc comparisons were
conducted using Student–Newman–Keuls tests. The level of probability for
statistically significant effects was set at 0.05.
For the self-administration data, the dependent variables (i.e. total number of
nose-poke responses in the active and inactive hole and number of reinforcers)
were analyzed using a repeated measures analysis of variance (ANOVA) with
varenicline doses as within-subject factor. For the relapse experiments a
between-subject design was employed, thus the different doses of varenicline
served as between-subject factors. Data obtained in the 5-CSRTT were
subjected to repeated measures ANOVA with varenicline treatment as a within-
subject factor.
4.4. Results
4.4.1. Experiment 1. Nicotine self-administration
To investigate the effects of varenicline on nicotine self-administration, 16 rats
were trained to nose-poke for intravenous nicotine infusions in the presence of
audiovisual cues. Six animals were excluded because they did not meet the
selection criteria or had clogged catheters. Varenicline treatment had an overall
main effect on the total number of active nose-pokes (F(3,27)=36.04, p<0.001)
and further post hoc analyses revealed that 1.5 and 2.5 mg/kg varenicline
significantly reduced nicotine self-administration compared to vehicle (Figure
16a). Varenicline treatment also affected the total number of inactive responses
(F(3,27)=2.98, p<0.05). Post hoc analyses revealed that inactive responding did
not differ from vehicle treatment for any of the varenicline doses, however, the
2.5 mg/kg dose significantly reduced the number of inactive responses
compared to 0.5 mg/kg varenicline.
4.4.2. Experiment 2. Alcohol self-administration
In experiment 1 varenicline was able to dose-dependently reduce nicotine self-
administration. To investigate its effects on alcohol self-administration a
separate group of 16 animals was trained. Twelve animals met our selection
criteria. Varenicline treatment had an overall main effect on operant self-
administration of alcohol (F(3,33)=7.65, p<0.001) and post hoc analyses
revealed that 1.5 and 2.5 mg/kg varenicline significantly reduced alcohol self-
administration compared to vehicle (Figure 16b). In addition, a main effect of
varenicline treatment was also found on the total number of inactive responses
(F(3,33)=3.96, p<0.05). Post hoc analyses revealed that inactive responding did
not differ from saline treatment for any of the varenicline doses, however, both
treatment with 1.5 and 2.5 mg/kg varenicline significantly reduced the number
of inactive responses compared to treatment with 0.5 mg/kg varenicline.
4.4.3. Experiment 3. Sucrose self-administration
To assess whether varenicline treatment selectively affects nicotine and alcohol
self-administration over responding for natural reinforcers, eight rats were
trained to nose-poke for 10% sucrose reinforcers in the presence of audiovisual
cues. All animals acquired stable self-administration of sucrose. Varenicline had
an overall main effect on operant self-administration of sucrose (F(3,21) =6.61,
p<0.01) and post hoc analyses revealed that all varenicline doses significantly
increased sucrose self-administration compared to vehicle (Figure 16c). In
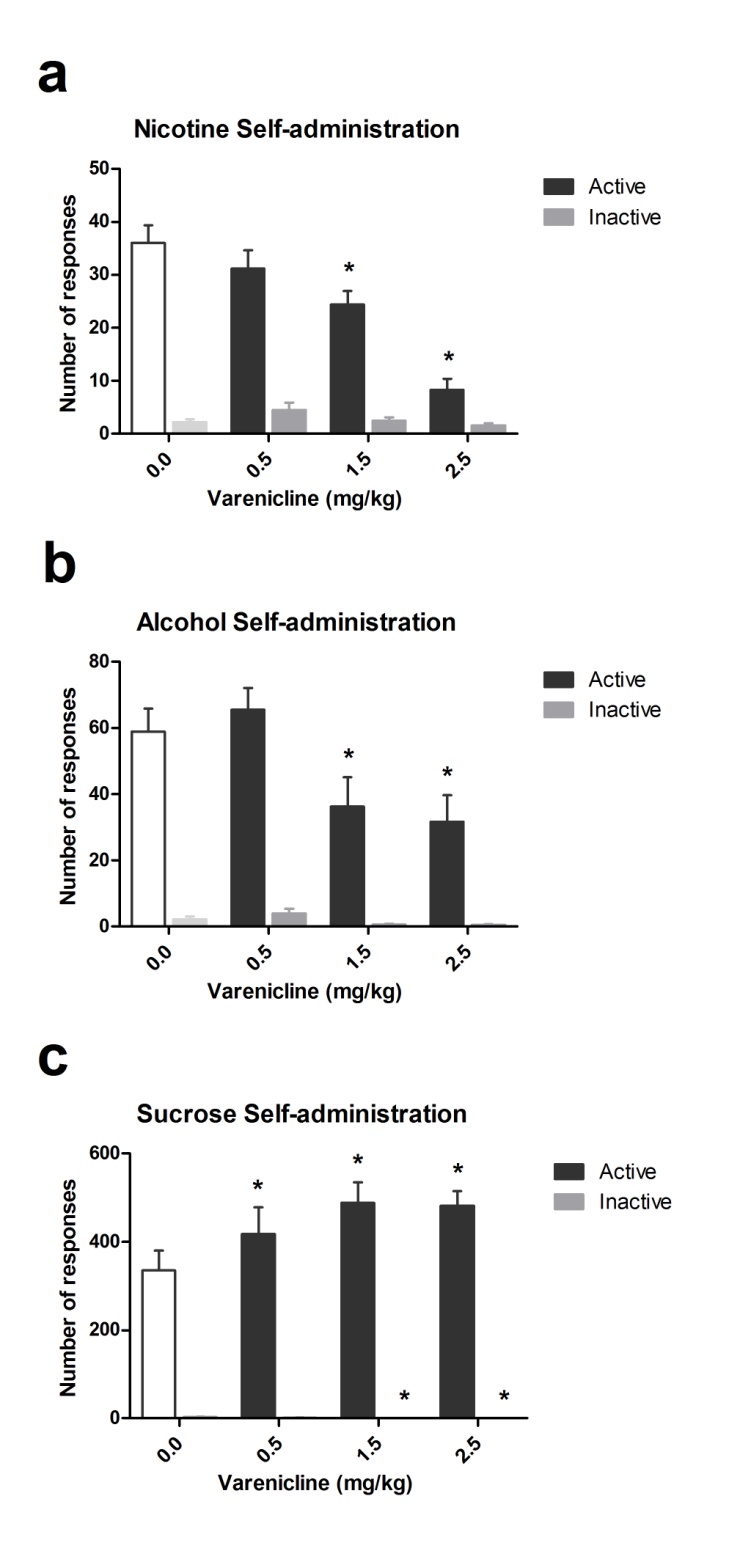
Figure 16. Effects of
varenicline on self-
administration of (a)
nicotine, (b) alcohol and
(c)
sucrose (inactive
responses sucrose self-administration (mean ± SEM): 0.0 g/kg, 3 ± 1.6; 0.5 g/kg, 1.7 ± 0.5; 1.5 g/kg, 0.1 ± 0.1; 2.5 g/kg, 0.1 ± 0.1). * p<0.05 significant difference with respect to 0.0 g/kg group.
addition, a main effect of varenicline treatment was also found for the total
number of inactive responses (F(3,21)=3.49, p<0.05). Post hoc analyses
revealed that only treatment with 2.5 mg/kg varenicline significantly reduced
the number of inactive responses compared to vehicle treatment.
4.4.4. Experiment 4. Nicotine relapse
To assess whether varenicline would be protective against relapse to nicotine-
seeking we investigated its effects on cue-induced relapse after extinction. 54
animals met the selection criteria and were divided into four groups (vehicle
(n=18), 0.5 mg/kg (n=12), 1.5 mg/kg (n=12) or 2.5 mg/kg (n=12)
varenicline). The different experimental groups displayed equivalent levels of
active responding during the last two days of training and extinction (data not
shown). Varenicline had an overal main effect on cue-induced relapse to
nicotine self-administration (F(3,50)=4.28, p<0.01) (Figure 17a). Further
comparisons revealed that the 0.5 mg/kg dose increased responding for nicotine
cues compared to vehicle and 2.5 mg/kg (p<0.05 ). Treatment with 2.5 mg/kg
varenicline significantly reduced the number of active responses compared to
treatment with 0.5 mg/kg varenicline. A treatment effect was also found for
inactive responses (F(3,50)=2.98, p<0.05). Post hoc analyses revealed an
increase in inactive responding in the 0.5 mg/kg varenicline group compared to
all other groups.
Figure 17. Effects of varenicline on cue-induced reinstatement of (a) nicotine- and (b)
alcohol-seeking. * p<0.05 significant difference with respect to 0.0 g/kg group.
4.4.5. Experiment 5. Alcohol relapse
Before the cue-induced relapse test was conducted, the different experimental
groups (vehicle (n=10), 0.5 mg/kg (n=10), 1.5 mg/kg (n=10) or 2.5 mg/kg
(n=10) varenicline) displayed equivalent levels of active responding during the
last two days of training and extinction (data not shown). Varenicline had an
overall main effect on relapse to alcohol-seeking (F(3,36)=13.63, p<0.001) and
post hoc analyses revealed that 1.5 and 2.5 mg/kg varenicline significantly
reduced relapse to alcohol-seeking compared to vehicle, whereas the 0.5 mg/kg
dose seemed to induce a slight, but non-significant increase in alcohol-seeking
(p=0.07) (Figure 17b). Varenicline treatment did not affect inactive responding
(F(3,36)=0.60, p=0.618).
4.4.6. Experiment 6. 5-choice serial reaction time task (5-CSRTT)
To assess putative cognitive-enhancing effects of varenicline, 16 rats were
trained in the 5-CSRTT. Varenicline treatment had a main effect on attention
(F(3,45)=3.92, p<0.05), premature responding (F(3,45)=3.15, p<0.05) and
correct response latency (F(3,45)=2.28, p<0.05), but not on the number of
omissions (F(3,45)=2.28, p=0.1) (Figure 18). Post hoc analyses showed that
the number of premature responses were increased by the 0.5 and 1.5 mg/kg
dose of varenicline (p<0.05), whereas the 2.5 mg/kg dose tended to increase
the number of premature responses (p=0.06). With regard to the effects on
attention and correct response latencies post hoc analyses revealed no
significant effects of any dose compared to vehicle. However, 2.5 mg/kg
varenicline did increase correct response latency compared to the 0.5 and 1.5
mg/kg dose.
To investigate whether varenicline would enhance performance under conditions
of increased attentional load, the effect of 1.5 mg/kg varenicline was tested in
combination with a reduced stimulus duration of 0.5 s. Increasing the attentional
load by reducing the stimulus duration from 1 to 0.5 s reduced accuracy
(F(1,15)=36.94, p<0.001), shortened response latencies (F(1,15)=13.62,
p<0.01) and increased omission rate (F(1,15)=69.40, p<0.001), whereas
premature responding remained unaffected (F(1,15)=2.3, p=0.15) (Figure 19).
In accordance with the aforementioned results, 1.5 mg/kg varenicline increased
the number of premature responses (F(1,15)=27.45, p<0.001), but did not alter
accuracy (F(1,15)=1.42, p=0.25) or correct response latencies (F(1,15)=1.65,
p=0.20). A reduction of omissions was seen after varenicline treatment
(F(1,15)=15.11, p<0.001). Furthermore, a treatment x stimulus duration
interaction effect for correct response latency was found (F(1,15)=6.00,
p<0.05). Post hoc analyses indicated that the reduction of correct response
latencies induced by increased attentional load was less pronounced following
varenicline treatment. None of the other parameters showed a treatment x
stimulus duration interaction (accuracy: F(1,15)<1, p=0.85; omissions: F(1,15)=1.83, p=0.20); premature responding: F(1,15)=1.89, p=0.15).
Figure 18. Effects of varenicline on 5-CSRTT performance. The fol owing behavioural
measures are presented: (a) percentage correct responses (attention), (b) number of
premature responses (impulsive action), (c) number of omissions and (d) latency of
correct responses. * p<0.05 significant difference with respect to 0.0 g/kg group.
Figure 19. Effects of varenicline (1.5 mg/kg) on 5-CSRTT performance under increased
attentional load by reduced stimulus duration. The fol owing behavioural measures are
presented: (a) percentage correct responses (attention), (b) number of premature
responses (impulsive action), (c) number of omissions and (d) latency of correct
responses. *p<0.05 significant difference with respect to 0.0 g/kg group. #p<0.05
significant difference with respect to stimulus duration 1.0 s
4.5. Discussion
In the present study, we evaluated the effects of varenicline on both
motivational processes (nicotine-, alcohol-, and sucrose-seeking) and cognitive
functions (visuospatial attention and inhibitory response control) in rats. We
confirm earlier observations demonstrating that varenicline attenuates nicotine
and alcohol self-administration (O'Connor et al. 2010;Rol ema et al.
2007a;Steensland et al. 2007). In contrast, self-administration of the natural
reinforcer sucrose was increased by varenicline. Importantly, we demonstrate
for the first time that varenicline dose-dependently reduces cue-induced relapse
to alcohol-seeking. Interestingly, cue-induced relapse to nicotine-seeking was
not affected by the higher doses and the lowest dose of varenicline (0.5 mg/kg)
increased responding for nicotine-associated cues. At the cognitive level, we
show that varenicline reduces inhibitory response control without affecting
measures of visuospatial attention in the 5-CSRTT.
In the treatment of drug addiction relapse after abstinence represents a major
problem. Ideally, pharmacotherapies for drug addiction would aid the cessation
of drug-intake and prevent relapse. As both preclinical and clinical data indicate
that varenicline may potentially posses both characteristics (Gonzales et al.
2006;Jorenby et al. 2006;O'Connor et al. 2010;Patterson et al. 2009;Rol ema et
al. 2007a; 2009;Steensland et al. 2007;Tonstad et al. 2006;Tonstad 2006), we
determined the anti-relapse properties of varenicline in a so-called self-
administration-reinstatement model (Shaham et al. 2003). Remarkably, we
found that varenicline strongly attenuated cue-induced alcohol-seeking at the
same doses that reduced alcohol self-administration. This finding suggests that
varenicline has putative protective effects against relapse to alcohol-use, an
important addition to the growing body of evidence that underlines the potential
of varenicline as a treatment for alcohol-use disorders.
How varenicline modulates the neurobiological mechanisms that mediate cue-
induced relapse to alcohol-seeking remains to be unravelled. Pharmacological
studies have identified several neurotransmitter systems that appear to be
involved in alcohol relapse, including the glutamate, opioid, serotonin and
dopamine system. In particular, activation of the mesolimbic dopamine system
and alterations in excitatory amino acid neurotransmission may underlie relapse
to alcohol-seeking (Le and Shaham 2002). Since varenicline was reported to
affect dopamine release in the nucleus accumbens (Coe et al. 2005;Ericson et
al. 2009;Rol ema et al. 2007a), it may alter relapse to alcohol-seeking by modulating dopaminergic neurotransmission in the mesolimbic pathway. In a separate experiment we assessed the effects of varenicline on cue-induced relapse to nicotine-seeking. Remarkably, we found that varenicline, at doses that diminished relapse to alcohol-seeking, did not reduce cue-induced relapse to nicotine-seeking. These results are consistent with recent findings of O'Connor and colleagues (O'Connor et al. 2010). Furthermore, we found enhanced cue-induced relapse at a dose of 0.5 mg/kg. Interestingly, a similar but non-significant (p=0.07) effect was seen on alcohol-seeking. Thus, it seems that varenicline affects cue-induced relapse in a bimodal fashion. A possible explanation may be that the anti-relapse effect is mediated by receptors other than the α4β2-nACh subtype that are activated at higher doses, while at low doses varenicline induces a priming effect via α4β2-nACh-receptors. Indeed, varenicline was shown to be a (partial) agonist for other nACh-receptor subtypes, such as α7- and at higher doses α3β4-, α3β2- and α6-receptors, albeit with much lower affinity (Coe et al. 2005;Mihalak et al. 2006). Hence, varenicline may activate additional nAch receptor subtypes at 1.5 mg/kg and 2.5 mg/kg (Rollema et al. 2009). Regarding varenicline's effects on self-administration our findings confirm that varenicline dose-dependently attenuates nicotine self-administration. Substantial evidence indicates that varenicline may affect nicotine self-administration by reducing nicotine-induced mesolimbic dopamine release (Coe et al. 2005;Rollema et al. 2007a). In agreement with results from Steensland et al. (2007), our data suggest that varenicline has a comparable effect on alcohol self-administration. Similarly, co-administration of varenicline and alcohol counteracted each others' respective enhancing effect on dopamine levels in the nucleus accumbens (Ericson et al. 2009). Nonetheless, it remains to be determined whether varenicline's attenuating effect on alcohol and nicotine self-administration is mediated by the same molecular mechanisms. As mentioned before, varenicline is known to have limited selectivity for different nACh-receptor subtypes (Coe et al. 2005;Mihalak et al. 2006). Some of these receptor subtypes are implicated in the reinforcing effects of alcohol (Chastain 2006;Davis and de Fiebre 2006;Ericson et al. 2009;Jerlhag et al. 2006;Larsson et al. 2004;Lof et al. 2007). Particularly, the α3β4-nAch-receptor seems a likely candidate for the modulation of alcohol-intake (Chatterjee et al. 2010). Therefore, we cannot exclude the notion that varenicline differentially mediates
its effects on nicotine and alcohol self-administration via receptors other than the α4β2-nACh-receptor. To determine whether the attenuating effects of varenicline were selective for alcohol and nicotine and/or due to a reduced ability to make an operant response, we assessed its effects on operant responding for the natural reinforcer sucrose. Interestingly, sucrose self-administration was augmented by varenicline at doses that significantly reduced nicotine and alcohol self-administration. Previous studies investigating intake of natural reinforcers have not reported such effects (O'Connor et al. 2010;Steensland et al. 2007). Varenicline was, however, reported to increase responding for food delivery in rats trained on a variable interval reinforcement schedule (Rollema et al. 2007a). A role for nACh-receptors in sucrose-seeking, however, is not supported by previous data, i.e. nACh-receptor agonists and antagonists have not been reported to attenuate sucrose-intake (Ford et al. 2009;Hendrickson et al. 2009;Nadal et al. 1998;Neugebauer et al. 2006;Steensland et al. 2007). Collectively, these findings imply the following. First, that varenicline selectively attenuates self-administration of alcohol and nicotine, an effect that does not generalize to self-administration of food or sucrose. In fact, our findings suggest that varenicline affects operant responding for natural reinforcers and addictive substances, i.e. nicotine and alcohol, in opposite directions. Second, as varenicline treatment did not impair sucrose self-administration, varenicline's effects on consumption of addictive substances are most probably due to changes in the motivational domain, rather than impaired abilities to perform an operant response. In humans, varenicline was shown to enhance cognitive functioning during abstinence of smoking/drinking. Although the effects were small, varenicline improved sustained attention (as measured by the Penn Continuous Performance Task) and working memory (as measured by the letter –N-back task) (Patterson et al. 2009). Furthermore, recent rodent studies show that varenicline may ameliorate alcohol-induced cognitive deficits in acquisition of contextual and cued associative learning in mice (Gulick and Gould 2008) and improve attentional performance in a sustained attention task with distracter stimuli in rats (Rollema et al. 2009). We used the 5-CSRTT to further explore the effects of varenicline on cognition. Previous research from our and other laboratories revealed that nicotine positively affects cognitive function as
measured in the 5-CSRTT by 1) enhancing sustained attention under certain conditions, 2) decreasing response latency, and 3) reducing the number of omissions. On the other hand, nicotine deteriorates inhibitory control in this task as measured by an increase in premature responding (Blondel et al. 2000;Day et al. 2007;Mirza and Stolerman 1998;van Gaalen et al. 2006). In the current study we found no evidence for cognitive-enhancing effects of varenicline. However, varenicline increased premature responding, albeit to a lesser extent than nicotine (Blondel et al. 2000;Mirza and Stolerman 1998;van Gaalen et al. 2006). This effect may result from enhanced dopaminergic neurotransmission in the mesolimbic pathway induced by nACh receptor activation in the nucleus accumbens or ventral tegmental area (Di Chiara and Imperato 1988;Marshall et al. 1997;Nisell et al. 1994;Wonnacott 1997), that in turn has been demonstrated to modulate premature responding in the 5-CSRTT (Cole and Robbins 1987;Pattij et al. 2007). It should be noted here, that detrimental effects of varenicline on inhibitory response control may be unfavourable during treatment in humans, as previous research from our laboratory has associated poor inhibitory response control with an enhanced motivation to initiate and maintain nicotine self-administration in rats (Diergaarde et al. 2008a). Likewise, in line with these preclinical findings, diminished inhibitory control has been demonstrated to predict unsuccessful smoking cessation in abstinent smokers (Krishnan-Sarin et al. 2007). Interestingly, recent findings from our laboratory suggest, that under comparable conditions, no such association exists between inhibitory response control and alcohol self-administration in rats (Diergaarde, unpublished). The strong relationship between impulsivity and nicotine, but not alcohol-seeking, together with the observation that varenicline diminishes inhibitory control may partly explain the differential effects of this compound on relapse to alcohol and nicotine-seeking. We also tested varenicline in combination with a reduced stimulus duration (0.5 s instead of 1 s), to assess its effects on performance under increased attentional load. Compared to saline treatment, varenicline was unable to improve attentional performance under these challenging conditions. This seems to contradict previous data demonstrating that varenicline significantly improved the capacity to attenuate impaired performance under challenging distracter conditions in a sustained attention task (Rollema et al. 2009). Specific differences between the 5-CSRTT and the sustained attention task may explain this discrepancy. In particular in the sustained attention task an auditory stimulus was used to reduce the discriminability of a visual cue, whereas in the
5-CSRTT the signal duration of a visual cue was reduced. It is possible that
varenicline reduces the impact of a distracter on signal detection, but does not
improve sustained attention. Taken together, varenicline's effects on cognitive
functions as measured in the 5-CSRTT seem limited and less pronounced than
the effects of nicotine, consistent with its lower efficacy at the nACh receptor.
In summary, this is the first report to demonstrate that, in rats, varenicline
attenuates cue-induced alcohol-seeking. Furthermore, at lower doses varenicline
may facilitate nicotine- seeking. As was previously shown, varenicline may
selectively reduce voluntary nicotine and alcohol-intake. Moreover, varenicline
has limited effects on cognitive functioning as measured by the 5-CSRTT,
although varenicline may somewhat reduce inhibitory response control. Such a
behavioural pharmacological profile emphasizes varenicline's potential as a
pharmacotherapeutical agent in the treatment of alcohol-dependence.
4.6. Acknowledgements
Our research was supported by a ZONMW Topgrant 912-06-148.
4.7. Disclosure
All authors declare that, except for income received from primary employer, no
financial support or compensation has been received from any individual or
corporate entity over the past three years for research or professional service
and there are no personal financial holdings that could be perceived as
constituting a potential conflict of interest.
Chapter 5
Disruption of long-term alcohol-related memory
reconsolidation: Role of β-adrenoceptors and NMDA
receptors
J.A. Wouda1,2*, L. Diergaarde1*, D. Riga, Y. van Mourik1, A.N.M. Schoffelmeer1,
T.J. De Vries1,2
1Department of Anatomy and Neurosciences, Neuroscience Campus Amsterdam, VU University medical center and 2Department of Molecular and Cellular Neurobiology, Center for Neurogenomics and Cognitive Research, Neuroscience Campus Amsterdam, VU University, Amsterdam, The Netherlands. * contributed equally Front Behav Neurosci. 2010 Nov 26;4:179
5.1. Abstract
Disrupting reconsolidation of drug-related memories may be effective in
reducing the incidence of relapse. In the current study we examine whether
alcohol-related memories are prone to disruption by the β-adrenergicreceptor
antagonist propranolol (10 mg/kg) and the NMDA receptor antagonist MK801
(0.1 mg/kg) following their reactivation. In operant chambers, male Wistar rats
were trained to self-administer a 12% alcohol solution. After three weeks of
abstinence, the animals were placed in the self-administration cages and were
reexposed to the alcohol-associated cues for a 20-min retrieval period,
immediately followed by a systemic injection of propranolol, MK801 or saline.
Rats were tested for cue-induced alcohol-seeking on the following day. Retrieval
session, injection and test were repeated on two further occasions at weekly
intervals. Both propranolol and MK801 administration upon reactivation did not
reduce alcohol-seeking after the first reactivation test. However, a significant
reduction of alcohol-seeking was observed over three post-training tests in
propranolol-treated animals, and MK801-treated animals showed a strong
tendency towards reduced alcohol-seeking (p=0.06). Our data indicate that
reconsolidation of alcohol-related memories can be disrupted after a long post-
training interval and that particularly β-adrenergic receptors may represent
novel targets for pharmacotherapy of alcoholism, in combination with cue-
exposure therapies.
5.2. Introduction
Alcohol consumption is socially accepted in many cultures. The World Health
Organization estimates that about two billion people regularly drink alcoholic
beverages, 4% of whom have diagnosable alcohol-use disorders. The
economical, health and domestic consequences of excessive alcohol-use are a
burden to society (WHO 2004). Nevertheless, only few pharmacological
treatments of alcohol-dependence are available, and their effectiveness is
limited (Anton et al. 2006). Therefore, development of more effective
treatments is warranted.
In drug addiction, environmental stimuli associated with the effects of self-
administered drugs, including alcohol, are powerful sustainers of addictive
behaviours and can precipitate relapse after prolonged periods of abstinence in
both humans (O'Brien et al. 1998) and laboratory animals (Chaudhri et al.
2008;De Vries et al. 2001). Disrupting drug-associated memories could
therefore be an important new strategy for treating alcoholism and other
addictive behaviours (Diergaarde et al. 2008b;Lee et al. 2005;Miller and
Marshall 2005).
During the last decade, there has been a renewed interest in reconsolidation, a
phenomenon involved in retaining reactivated memories. Memory retrieval is
believed to induce a transient state during which a memory trace becomes liable
to modification, requiring protein synthesis-dependent reconsolidation for the
original memory to be maintained. Memory reconsolidation has been studied in a
number of species, and localized pharmacological manipulations have identified
a variety of brain regions, including basolateral amygdala, hippocampus and
prefrontal cortex, involved in post-reactivation stabilization of memories
(Alberini 2005;Nader et al. 2000;Przybyslawski et al. 1999). The molecular
mechanisms underlying reconsolidation involve both β-adrenergic and
glutamatergic signalling pathways (Debiec and LeDoux 2004;Przybyslawski et al.
1999;Przybyslawski and Sara 1997). For instance, it has been shown that post-
reactivation administration of propranolol, a β-adrenoceptor antagonist, disrupts
reconsolidation of fear-related memories (Alberini 2005;Debiec and LeDoux
2004;Nader et al. 2000;Przybyslawski et al. 1999). Similarly, reconsolidation of
aversive memory is disrupted by post-retrieval injections of NMDA receptor
antagonist MK-801 (Lee et al. 2006b). Collectively, these findings suggest that
both β-adrenergic and glutamatergic signal ing pathways are involved in
aversive memory reconsolidation processes.
Recent evidence of our and other laboratories indicates that β-adrenergic signalling is also involved in reconsolidation of appetitive memories. By employing an instrumental sucrose self-administration paradigm, we established that reactivation of relatively old natural reward-related memories renders them susceptible to interference, i.e. propranolol treatment following reexposure to a sucrose-associated context significantly reduced subsequent sucrose-seeking (Diergaarde et al. 2006). It has also been demonstrated that propranolol (Milton et al. 2008b) and MK801 (Lee and Everitt 2008a) disrupt reconsolidation of associations between sucrose and discrete environmental stimuli. Furthermore, both post- reactivation propranolol, and MK801 administration prior to reactivation reduces subsequent cocaine-seeking (Milton et al. 2008a;Milton et al. 2008b), which implies that β-adrenergic and NMDA receptor mediated signalling is also implicated in reconsolidation of cocaine-related associations. Nonetheless, it was only recently shown that protein synthesis and NMDA receptors are also required for reconsolidation of alcohol-related memories (von der Goltz et al. 2009). The current study extends on these findings using an operant reinstatement procedure that models cue-induced relapse, to examine whether β-adrenergic signalling is also involved in reconsolidation of alcohol-related memories. Thus, we compare the effects of propranolol and MK801 administration following memory reactivation.
5.3. Material and methods
5.3.1. Subjects
Male Wistar rats (Harlan CPB, Horst, The Netherlands), weighing 280-300 g
upon arrival were used. They were housed in pairs in a temperature and
humidity controlled room on a 12 h light/dark cycle (lights on between 7 pm and
7 am). Food and water were available ad libitum. The experiment started two
weeks after the animals had arrived and was conducted during the dark phase of
the light-dark cycle. Experimental procedures were approved by the Animal Care
Committee of the VU University, Amsterdam, The Netherlands.
5.3.2. Apparatus
All experiments were conducted in 16 identical rat operant chambers (TSE, Bad
Homberg, Germany), which were fitted with a dim red house light and two small
holes, one hole in which the rats had to poke in order to obtain a 0.19 ml 12%
alcohol solution (designated ‘nose-poke hole'), and a hole in which the alcohol
was actually delivered (hereby further referred to as ‘receptacle') located on
opposite walls. The nose-poke hole contained a photocell to detect responses.
Red stimulus lights were located above the nose-poke hole and the receptacle,
and yellow stimulus lights were located inside both holes. In addition, each
chamber was equipped with an empty feeder mechanism which was turned on
upon a poke in the nose-poke hole, thereby providing an auditory stimulus. The
stimuli were used to signal alcohol availability or were paired with alcohol
delivery in order to facilitate acquisition of alcohol self-administration (see
below). During reactivation and reconsolidation testing, these cues were present
to study the effect of propranolol or MK801 on cue-induced alcohol-seeking. A
computer interfaced to the chambers was used for equipment operation and
data collection.
5.3.3. Drugs
All drugs were dissolved in sterile saline for intraperitoneal injection (1 ml/kg).
(+/-)-Propranolol hydrochloride (Sigma) was injected at a dose of 10 mg/kg.
(+)-MK-801 hydrogen maleate (Tocris) was injected at a dose of 0.1 mg/kg.
These doses of propranolol and MK801 have previously been reported to disrupt
reconsolidation of different types of memories (Diergaarde et al. 2006;Sadler et
al. 2007)
5.3.4. Procedure
Experiment 1
Two bottle procedure
The rats were daily trained to consume alcohol in a two-bottle free-access
paradigm. Upon arrival they were habituated to two water bottles on their home
cages. Every other day one of the bottles was replaced by bottle containing a
gradual increasing alcohol concentration (2% to 12% v/v). In three weeks the
animals reached 12% v/v alcohol and training proceeded to a two bottle limited-
access paradigm. Animals were given access to the 12% solution for 1 h daily.
After ten days of limited-access animals that consumed > 0.35 g/kg alcohol
were selected to enter the operant self-administration phase. Selected animals
consumed approximately 0.54 g/kg alcohol. Previous work of our laboratory
shows that animals with this amount of alcohol-intake reliably acquire operant
alcohol self-administration and show cue-induced relapse following long-term
extinction (De Vries and Schoffelmeer 2005).
Operant alcohol self-administration
In Figure 21 the experimental setup is depicted. All rats were trained to self-
administer a 12% alcohol solution. On the first day of training they received a
single 20-min habituation session, during which 40 non-contingent rewards were
delivered and only the house light was illuminated. Nose-poking during this
session was without any consequences. On the fol owing days the rats were
trained to self-administer a 12% alcohol solution in 1-h sessions every other
day. A session started with the illumination of the house light. Each trial was
signalled by illumination of a red stimulus light located above the nose-poke
hole, which was extinguished when a nose-poke was made in this hole. Nose-
poking resulted in the immediate delivery of a 0.2 ml alcohol solution (US) in the
receptacle, signalled by the illumination of a white stimulus light in the
receptacle and the sound (click) of the feeder mechanism (compound CS).
Responding was reinforced according to a continuous reinforcement (fixed ratio
1, FR1) schedule. After delivery of the reinforcer, the white stimulus light was
switched off, and a time-out period of 20 s commenced until the next trial
started. Nose-poking behaviour during time-out was monitored, but was without
consequences. After 1 h or when 50 reinforcers had been earned, the house
light was extinguished and the session ended. In order to facilitate acquisition,
the animals received one free sample of alcohol solution in the receptacle upon
the start of habituation and the first five training sessions. When FR1
performance levels stabilized, the training schedule proceeded to FR2 and FR3.
FR3 training continued until stable peak performance levels were achieved,
which indicated that the task was well consolidated. All rats received a total of
19 1-h training sessions.
Alcohol self-administration training was followed by a period of 21 days during
which the rats were kept in their home cages in the colony room, and were
handled weekly. Rats self-administering less than 0.35 g/kg alcohol per session
were excluded from analysis.
Reactivation
Following the 3-week abstinence period the rats were subjected to a reactivation
session, during which the animals were placed in the self-administration cages
for a 20-min period. During the session the house light and the red stimulus
light were presented continuously, the compound CS sound was presented 40
times at variable intervals (VI30, every 25, 30, or 35 s). Thus reactivation
consisted of a combination of the context and cues that were present during
training, yet, in contrast to the training sessions the nose-poke hole as well as
the receptacle were covered during reactivation, and no alcohol was delivered.
Immediately following reactivation rats were transported to the adjacent room
and received an i.p. injection of propranolol (10 mg/kg) or saline. In order to
test whether the effect of propranolol depends on memory retrieval, a separate
group of animals received identical training and abstinence but was not
reactivated. Rats from this no-reactivation experimental group were not placed
in the self-administration cages, but instead, were transported to the adjacent
room where they received a saline or a propranolol injection.
Retention
The day following memory reactivation, the rats were tested for cue-induced
alcohol-seeking in a 1-h session. The session was analogous to FR3 training,
with cue presentation upon every third active nose-poke, however, alcohol was
never delivered. Nose-poking was used as an index of alcohol-seeking
behaviour.
In order to test whether repeated propranolol injections in combination with
reactivation had an additional detrimental effect on alcohol-seeking, retrieval
session, injection and retention test were repeated seven days and 14 days after
the first retrieval test.
Experiment 2
Identical to experiment 1, alcohol self-administration training was followed by a
21-day period during which the rats were kept in their home cages in the colony
room, and were handled weekly. Subsequent reactivation and retention was as
described in experiment 1 with the exception that these animals were injected
with saline or MK801 (0.1 mg/kg) directly after reactivation.
5.3.5. Statistical analysis
SPSS 15.0 for Windows software was used for all statistical analysis and a
significance level of p< 0.05 was selected for all analyses. The reactivation and
no-reactivation groups were tested in separate experiments at different points in
time, and were therefore subjected to separate statistical analysis. An analysis
of variance (ANOVA) in a repeated measures design was used to determine the
effects of propranolol and MK801 on alcohol-related memory reconsolidation
with retention session (session 1-3) as within-subject factor and drug treatment
(propranolol / MK801 vs. saline) as between-subject factors. The same analysis
was used for the no-reactivation groups. Data were checked for sphericity, and a
Huynh-Feldt correction was used if necessary. Active response data failed to
meet homogeneity of variance requirements of ANOVA and were Log10
transformed prior to statistical analyses. Data are presented as mean ± SEM
active nose-poke responses in the Figures.
5.4. Results
5.4.1. Experiment 1: post-reactivation propranolol
All selected rats learned to respond for alcohol (Figure 20) and demonstrated
equivalent levels of active responding during the last two days of the 19-day
training period (Reactivation: F(1,19)<1, p>0.70). At the end of the training,
rats were divided into two groups that showed comparable levels of self-
administration over the last two training sessions (i.e. Reactivated propranolol-
treated (P) rats: 45 +/- 4.4 rewards; reactivated saline (S) rats: 46 +/- 5.6
rewards).
Figure 20. Acquisition of
alcohol self-administration.
Responding was reinforced
according to a continuous
reinforcement (fixed ratio 1,
FR1) schedule. When FR1
performance levels stabilized,
the training schedule
proceeded to FR2 and FR3.
When the animals reached
stable peak performance on
FR3 the animals were divided
into two groups (propranolol
and saline) with similar performance. The effect of reactivation and treatment on behaviour during the retention sessions was tested using a repeated measures analysis. This analysis revealed that three post-reactivation treatments with propranolol reduced alcohol-seeking over all three retention days together as evidenced by a significant drug treatment effect (F(1,19)=7.067, p<0.05) (Figure 21a). No significant within subject interactions of retention session x drug treatment was observed (F(2,38)=2.113, p>0.1). A separate group of animals was trained for the no-reactivation condition. Similar to the animals that were reactivated the no-reactivation groups (i.e. no-reactivation propranolol (NP) and no-reactivation saline (NS)) demonstrated equivalent levels of active responding during the last two days of the 19-day
(A) Memory retention for
alcohol self-administration after
post retrieval manipulation.
Number of active nose-pokes
made during retention test 1, 2
(B) Memory retention for
No - Reactivation
alcohol self-administration
without reactivation. Number of
active nose-pokes made during
retention test 1, 2 and 3.
Figure 21. Experimental setup. After 19 days of alcohol self-administration training, rats
were left undisturbed for 21 days (abstinence). Subsequently, they were exposed to the
self-administration context in which the house light and stimulus light were presented
continuously and the compound cs was presented non-contingently (reactivation). Rats
received a propranolol or saline injection directly after reactivation or received a
propranolol or saline injection without reactivation (no-reactivation). One day later the
animals were tested for memory retention (test). Reactivation and testing was repeated
two times every seven days and 14 days after the first retrieval test.
training period (No-Reactivation: F(1,15)<1, p>0.70) and showed comparable
levels of self-administration (NP: 43 +/- 2.1 rewards; NS: 40.6 +/- 4.2
rewards). In contrast, to what was seen in the reactivated condition repeated
treatment without reactivation did not affect alcohol-seeking (F(1,15)<1,
p>0.80) (Figure 21b), indicating that alcohol-seeking behaviour was not affected
by home cage injections of propranolol.
In all groups, we observed a significant main effect of retention session,
meaning that responding decreased over repeated testing (Reactivation:
F(2,38)=17.596, p<0.001; No-reactivation: F(2,30)=14.781, p<0.001).
5.4.2. Experiment 2: post-reactivation MK801
All groups were trained to stable peak performance and demonstrated
equivalent levels of active responding during the last two days of training
(Reactivation: F(1,14)<1, p>0.65). Reactivated MK801-treated (MK) rats earned
43 +/- 5.3 rewards and reactivated S rats earned 41 +/- 2.5 rewards over the
last two training sessions. These levels of responding were comparable to
responding in experiment 1.
The repeated measures ANOVA just failed to show a significant between subject
effect of treatment on behaviour during the retention sessions (F(1,14)=4.189,
p=0.06) (Figure 22a). This indicates that rats in the MK group had a strong
tendency to show less alcohol-seeking compared to the S group in al three
retention sessions.
A separate group of animals was trained to test the no-reactivation MK801
(NMK) and no-reactivation saline (NS) conditions. Similar to the reactivated
group these animals demonstrated equivalent levels of active responding during
the last two days of the 19-day training period (No-Reactivation: F(1,14)<1,
p>0.50) and earned comparable numbers of rewards (NMK: 45 +/- 4.1 and NS
40.6 +/- 4.2 rewards) during the last two days of training. Repeated treatment
without reactivation did not affect alcohol-seeking (F(1,14)=1.256, p>0.25)
(Figure 22b), however, in contrast to the reactivated condition, NMK animals
tended to increase responding compared to the NS group in al three retention
sessions.
Again a significant main effect of retention session was observed, reduced
responding was seen for all treatments (Reactivation: F(1.7,23)=14.197,
p<0.001; No-reactivation: F(1,14)=9.234, p<0.01).
(A) Memory retention for alcohol
self-administration after post
retrieval manipulation. Number of
active nose-pokes made during
retention test 1, 2 and 3.
ive n
ct 10
(B) Memory retention for alcohol
No - Reactivation
self-administration without
reactivation. Number of active nose-
pokes made during retention test 1,
Figure 22. Experimental setup. After 19 days of alcohol self-administration training, rats
were left undisturbed for 21 days (abstinence). Subsequently, they were exposed to the
self-administration context in which the house light and stimulus light were presented
continuously and the compound cs was presented non-contingently (reactivation). Rats
received a MK801 or saline injection directly after or received a MK801 or saline injection
without reactivation (no-reactivation). One day later the animals were tested for memory
retention (test). Reactivation and testing was repeated two times every seven days and 14
days after the first retrieval test.
5.5. Discussion
Using an operant alcohol self-administration model we demonstrate that (1)
relatively old alcohol-related memories are prone to disruption in a reactivation-
dependent manner and that (2) β-adrenoceptor mediated signalling is critically
involved in reconsolidation of these memories. In addition, animals that received
post-reactivation treatment with NMDA receptor antagonist MK801 showed a
strong tendency to reduce alcohol-seeking (p=0.06). Moreover, we demonstrate
that (3) repeated post-reactivation treatment (repeated reactivation followed by
propranolol injection), but not single treatment, can result in diminished relapse
to alcohol-seeking.
The current experiment adds to our previous findings on reconsolidation of
appetitive memories (Diergaarde et al. 2006), demonstrating that memory
reconsolidation of both sucrose- and alcohol-related memories is dependent, at
least in part, on β-adrenoceptor mediated signalling. Furthermore, these results
complement and extend on recent findings from von der Goltz et al., who show
that single post-reactivation treatment with protein syntheses inhibitor
anisomycin reduces cue-induced alcohol-seeking the following day and seven
days after treatment (von der Goltz et al. 2009). Interestingly, this particular
study reports a transient effect of post- reactivation MK801 treatment; alcohol-
seeking was reduced the following day, but not seven days after treatment. It
should be noted that this study used a saccharin-fading procedure during the
alcohol acquisition phase. This complicates the interpretation of the results, as
recent findings indicate that prior instrumental training for a natural reward
influences reinstatement of drug-seeking behaviour (Clemens et al. 2010). In
other words, environmental cues associated with the natural reinforcer may
have interfered with the memory process. We have shown previously, that
reconsolidation of appetitive (sucrose-related) memories is susceptible to
interference (Diergaarde et al. 2006;Milton et al. 2008b), making it is difficult to
assess whether prior saccharin training may have interfered with the
reconsolidation process in the von der Goltz study. However, our experiments,
that did not involve sweetening to facilitate acquisition of alcohol self-
administration, suggest that alcohol-related memories are prone to disruption in
a reactivation-dependent manner. It should be noted however, that in our study,
the observed effect of MK-801 on memory reconsolidation was only modest.
Although the effect of MK-801 treatment seems more apparent during the first
test session than that of propranolol treatment, it did not quite reach
significance over the three sessions. Thus, it seems that the long term effects of
post reactivation manipulation of the NMDA receptor are less pronounced than the effects of protein synthesis inhibition or blockade of β-adrenoceptors. Recent studies by the Everitt group suggest that this might be due to a time-limited role of NMDA receptors in the reconsolidation process. Thus, in their hands, injections with the NMDA receptor antagonist APV or MK-801 were not effective in reducing instrumental responding for cocaine conditioned reinforcement when given post-retrieval but only when given prior to memory retrieval (Milton et al. 2008a). Similarly, MK-801 impaired conditioned sucrose-seeking when it was administered before, but not after, a memory reactivation session (Lee and Everitt 2008a;Lee and Everitt 2008b;Milton et al. 2008a). Collectively, these studies, in accordance with data presented here, suggest that, in addition to the β-adrenoceptor, the NMDA receptor plays a (time-limited) role in reconsolidation of reward-related memories. Our results are consistent with other studies suggesting that memories related to drugs of abuse are prone to disruption. A recent study addressing this issue in a cocaine self-administration paradigm demonstrated that pre-reactivation antagonism of NMDA receptor reduced the conditioned reinforcing properties of a previously cocaine-paired stimulus (Milton et al. 2008a). In addition several groups investigating drug-related reconsolidation in a conditioned place preference (CPP) paradigm have modulated place preference for cocaine, amphetamine and morphine (Bernardi et al. 2006;Robinson and Franklin 2007;Sadler et al. 2007;Valjent et al. 2006). However, a limitation of these studies is that CPP generally involves relatively new memories, use limited drug pairings and passive rather than active administration of drugs of abuse. In this respect using a reinstatement model designed to mimic aspects of alcohol addiction in humans more closely has several advantages. In particular it involves, (1) alcohol self-administration, (2) repeated exposure to alcohol and related stimuli, (3) alcohol-associated cues that can induce alcohol-seeking and provoke relapse after abstinence and (4) a three week post training interval to model relapse after prolonged period of abstinence. Alternative explanations for our findings need to be considered. Although retention tests were performed under drug-free conditions, it is possible that non-specific or aversive properties of propranolol and MK801 resulted in the reduction of alcohol-seeking that was observed. Given that amnestic effects in this experiment were reactivation-dependent, i.e. they were not found in the no-reactivation groups, non-associative effects of propranolol or MK801 do not
explain our results. This notion is further substantiated by the fact that the doses of propranolol and MK801 used here have not been reported to induce aversive effects (Golden and Houpt 2007;Milton et al. 2008b;Sara et al. 1995). It might be argued that the observed reduction in alcohol-seeking was caused by the fact that pharmacological treatments facilitated extinction rather than disrupting reconsolidation. This possibility, however, seems not very likely. In fact, several studies indicate that pharmacological activation rather than blockade of β-adrenergic or glutamatergic signalling facilitates (extinction) learning (Berman and Dudai 2001;Bevilaqua et al. 2006;Gass and Olive 2008;Popik et al. 2006;Vengeliene et al. 2008), whereas antagonism under certain circumstances impairs extinction learning (Bevilaqua et al. 2006;Hsu and Packard 2008). Moreover, one of the characteristics of drug addiction is that extinguished drug-related memories can reinstate spontaneously, due to drug priming, cue exposure or stress. In contrast, no spontaneous recovery of responding for cocaine-associated cues was reported in a recent study that tested the persistence of memory impairment 15, 22 and 29 days after propranolol treatment (Milton et al. 2008b). Accordingly, cocaine-primed reinstatement was abolished in animals that received repeated post-reactivation propranolol (Fricks-Gleason and Marshall 2008). Together, these observations suggest that a facilitated extinction could not explain our results. In contrast to our findings, many groups investigating reconsolidation report that single treatment is sufficient to induce memory impairment in paradigms for fear conditioning, CPP and operant instrumental learning (Bernardi et al. 2006;Debiec and LeDoux 2004;Diergaarde et al. 2006;Milton et al. 2008b). This discrepancy may be explained by the nature of the memories that are involved in our experiment. Unlike fear-based learning tasks, that allow rapid association of negative emotionally laden stimuli, operant instrumental learning requires multiple training sessions, resulting in many cue-reward pairings. Additional y, our model involves a long post training period. These methodological features are thought to strengthen memory formation and may reduce susceptibility for memory reconsolidation (Diergaarde et al. 2006;Suzuki et al. 2004). Moreover, the addictive nature of alcohol is an important factor influencing memory strength in our paradigm. In addiction, addictive substances, including alcohol, may employ neural systems that are normally involved in memory processes (Robbins et al. 2008). It is believed that drugs of abuse can induce aberrant stimulation of brain structures involved in reward learning, such as the ventral
tegmental area, nucleus accumbens and prefrontal cortex, leading to "overlearning" of drug-related cues (Hyman et al. 2006;Robbins et al. 2008). Alcohol is also known to affect processes that are associated with memory formation. For instance, alcohol exposure affects NMDA-mediated synaptic plasticity and LTP expression (Fadda and Rossetti 1998;Hoffman and Tabakoff 1996;Hyman et al. 2006). These alcohol-related effects, together with the multiple training sessions and the age of the alcohol-related memories in our paradigm, may render memories more resistant to post-reactivation manipulations. Our approach of repeated treatment in order to affect these well established memories related to addictive substances is not unique. In fact, some recent observations indicate that repeated disruption may be more effective than single. Using an amphetamine CPP paradigm Sadler and colleagues showed that only repeated treatment, consisting of memory activation tests followed by systemic MK-801 administration, reduced expression of a well established amphetamine-conditioned place preference (Sadler et al. 2007). Differential effects of single and repeated post-retrieval systemic propranolol injections were also observed in a cocaine CPP procedure (Fricks-Gleason and Marshall 2008). Although both single and repeated propranolol treatment resulted in a reduced place preference compared to the saline-treated groups, repeated treatment but not single treatment abolished cocaine-primed reinstatement. These results indicate that single propranolol treatment did weaken the memory but only repeated treatment disrupted memory for cocaine place preference. On the other hand, several studies, including the von der Goltz study, have observed effects of single treatment (Bernardi et al. 2006;Diergaarde et al. 2006;Itzhak 2008;Kelley et al. 2007;Lee et al. 2006a;Miller and Marshall 2005;Robinson and Franklin 2007;Valjent et al. 2006;von der Goltz et al. 2009;Wang et al. 2008;Zhai et al. 2008) even on reconsolidation of older drug-related memories (Diergaarde et al. 2006;Lee et al. 2006b;von der Goltz et al. 2009). Variation in paradigms, such as species, training schedule, reactivation parameters, post-training interval and extinction sessions, may explain these different observations. Indeed, successful modulation of reconsolidation appears very sensitive to the specific methodological parameters used (Sara 2000;Tronson and Taylor 2007). Nevertheless, collectively, these studies suggest that under certain conditions memories related to drug of abuse undergo reconsolidation. It is therefore of importance to elucidate the signalling pathways mediating NMDA receptor- and β-adrenoceptor-related effects on reconsolidation of drug of abuse-related memories. The basolateral amygdala seems a likely candidate structure to study
these molecular mechanisms, given that it is believed to be involved in associative reward learning and cue-induced relapse (Kalivas and McFarland 2003;Kelley 2004). Moreover, β-adrenoceptor and NMDA receptor antagonism in this region impairs reconsolidation of fear and cocaine self-administration-related memories (Debiec and LeDoux 2004;Milton et al. 2008a). In conclusion we demonstrate in an animal model of relapse to alcohol-seeking, that reconsolidation occurs for old alcohol-related memories upon their reactivation and that particularly noradrenergic transmission plays an important role in this process. These findings suggest that pharmacological intervention in cue-exposure therapies for addictive behaviours may be useful in reducing relapse toward alcohol-use and that repeated treatment could be beneficial.
Chapter 6
General Discussion
6.1. Rationale
The many problems associated with excessive alcohol-use among both
adolescents and adults have for many years driven the scientific investigation of
alcohol-use disorders (AUDs) and putative treatment strategies. As such, it is
striking that the few pharmacotherapies that are currently available to counter
the wide occurrence of (relapse to) alcohol misuse in general have limited
efficacy and that little is known about the impact of teenage alcohol
consumption on well being in later life. Therefore, the work discussed in the
previous chapters explored the behavioural consequences of (excessive)
adolescent alcohol exposure. The performance of rats with a history of
adolescent alcohol exposure was examined in behavioural paradigms designed
to model alcohol-taking and seeking, impulsivity, attention and spatial memory
(Chapters 3 and 4). In parallel experiments, novel treatment options those
suffering from AUD were explored. In these studies, using the same addiction
paradigms, the efficacy of varenicline and reconsolidation blockade as novel
"anti-relapse" strategies were investigated (Chapters 4 and 5).
6.2. Main findings
Studying the long-term consequences of adolescent alcohol exposure, I
observed that binge-like, but not continuous alcohol exposure, resulted in long-
lasting behavioural impairments. Specifically, five weeks after the last alcohol
exposure (Figure 23) animals receiving binge-like alcohol treatment during
adolescence, but not directly after adolescence, increased operant responding
for alcohol and showed impaired spatial memory performance in the radial arm
maze (RAM). Furthermore, binge-like alcohol treatment abolished object-place
recognition (OPR), a spatial memory variant of the object recognition paradigm.
On the other hand, both peri- and post-adolescent binge-like alcohol exposure
left measures of motivation and relapse to alcohol-seeking, as well as measures
for attention and inhibitory response control unaffected.
In a different set of experiments the efficacy of two novel "anti-relapse"
strategies was tested. It was demonstrated that in addition to effectively
reducing alcohol and nicotine self-administration, the partial α4β2-nicotinic
receptor agonist varenicline attenuated cue-induced relapse to alcohol-, but not
to nicotine-seeking. Furthermore, in a protocol aimed at disrupting alcohol-
related memories through targeting reconsolidation, repeated post-reactivation
treatment with the β-adrenergic receptor antagonist propranolol diminished
relapse to alcohol-seeking. Also, animals receiving a similar treatment with
NMDA receptor antagonist dizocilpine (MK801) showed a strong tendency
towards reduced alcohol-seeking.
6.3. Long-term consequences of adolescent alcohol exposure
Chapters 2 and 3 present an extensive profile of the long-term behavioural
consequences of adolescent alcohol exposure. To this end, rats were exposed to
either (1) continuous self-administration (CSA) or (2) binge-like injection (BLI)
treatment during or directly after adolescence (see Figure 23 or Section 1.6.1
Box 3). With CSA treatment, animals had continuous access to a sweetened
Blood Alcohol Levels
Figure 23. BLI alcohol-treated animals were given a single intraperitoneal (i.p.) injection
of 20% (v/v) alcohol (2.5 g/kg) every other day for ten days. This resulted in a total of
five injections; peri-adolescent animals were injected on postnatal day (PND) 34, 36, 38,
40 and 42; post-adolescent animals were injected at PND 62, 64, 66, 68 and 70. Alcohol-
treatment resulted in blood alcohol levels exceeding 200 mg/dl during the first h after
injections for both peri- and post-adolescent animals. Behavioural experiments
commenced five weeks after treatment ended.
alcohol solution. BLI-treated animals received bi-daily alcohol injections (2.5
g/kg). Five weeks after BLI or CSA treatment the performance of rats was
examined in models for alcohol-taking and seeking, attention and inhibitory
response control or spatial memory.
6.3.1. Behavioural effects of binge-like alcohol exposure
Long-lasting behavioural alterations were found in adolescent rats that received
binge-like alcohol treatment, but not in rats that received continuous alcohol
treatment. This result fits in with findings from studies using inbred rat strains
that report that continuous or voluntary alcohol consumption during adolescence
does not affect alcohol-intake in later life (Siegmund et al. 2005;Slawecki et al.
2001;Vetter et al. 2007). It also corresponds to clinical and preclinical evidence
suggesting that the pattern of alternating high blood alcohol concentrations and
subsequent withdrawal periods associated with binge-like alcohol-intake causes
more damage than continuous alcohol consumption (Crews and Boettiger
2009;Duka et al. 2004;Guerri and Pascual 2010).
Adolescent BLI exposure resulted in long-lasting effects on aspects of alcohol-
taking and seeking and aspects of cognition. Adult rats with a history of peri-
adolescent binge-like alcohol exposure increased responding for alcohol on a
fixed ratio (FR)1 schedule of reinforcement in the self-administration-
reinstatement (SA) model. Even though the effects on alcohol-intake were mild,
these results complement previous findings demonstrating that excessive
adolescent alcohol consumption may have long-lasting effects on alcohol-use in
later life in both humans and rats (Grant et al. 2006;Guerri and Pascual
2010;Maldonado-Devincci et al. 2010a). In addition to the effects on alcohol-
taking, detrimental effects of binge-like alcohol treatment on spatial memory
performance were identified. Rats that were exposed to alcohol during
adolescence, but not rats that received alcohol directly after the adolescent
period, displayed a memory impairment in the RAM that extended at least six
months into adulthood. Such lingering effects of alcohol exposure on spatial
memory were also found in the OPR, but these effects were not age-specific.
Not all explored behavioural faculties were affected by peri-adolescent alcohol
exposure. In animals that showed increased SA following adolescent alcohol
treatment no changes were detected on measures of motivation, extinction and
cue-induced relapse. In addition, in the 5-choice serial reaction time task (5-
CSRTT) visuospatial attention and inhibitory response control were not affected.
The latter finding does not exclude the fact that other aspects of impulsivity are
sensitive to adolescent alcohol exposure. For example, impulsive choice, as can
be measured in delay discounting tasks, is affected in alcohol-dependent
subjects (for review see Verdejo-Garcia et al. 2008). As such, it will be
interesting, to study other varieties of impulsivity following adolescent alcohol
exposure.
6.3.2. Neurobiological effects of binge-like alcohol exposure
Several aspects of behaviour are crucially dependent on activity in specific
neuroanatomical loci. As such, the profile put forward in Chapters 2 and 3 may
be related to compromised functioning of these specific brain regions. In view of
its role in the reinforcing effects of addictive substances, it is possible that peri-
adolescent alcohol exposure induced lasting adaptations in the mesolimbic
system. Considering that prefrontal, limbic and striatal processes are also
involved in relapse, impulsivity and attention (Crews and Boettiger 2009;Dalley
et al. 2008;Le and Shaham 2002), further research is required to dissect the
putative dysfunction of the reward circuitry in more detail.
Given that performance in spatial tasks predominantly depends on the
hippocampus and its primary input and output pathways, the results presented
here also strongly point to specific sensitivity of the adolescent hippocampus to
the hazardous effects of alcohol, (for review see Dere et al. 2007;Olton 1987).
This suggestion is supported by several lines of evidence. As was outlined in the
introduction, the hippocampus undergoes extensive structural and
neurochemical remodelling during adolescence and is one of the few sites in the
brain that displays ongoing neurogenesis (Crews et al. 2007). Multiple studies
report that, compared to adult exposure, adolescent alcohol intoxication impairs
neurogenesis in the hippocampus more severely (Crews et al. 2006;He et al.
2005;Morris et al. 2010;Pascual et al. 2007). These findings are in accordance
with human MRI studies indicating that a history of adolescent alcohol-use is
associated with abnormal hippocampal volume (De Bellis et al. 2000).
Furthermore, in a comprehensive review on the acute effects of alcohol on
hippocampal function, Matthews and Silvers argue that acute alcohol-
administration produces dose-dependent memory impairments, similar to the
effects produced by lesions of the hippocampus (Matthews and Silvers 2004).
Through modulation of glutamatergic and GABAergic hippocampal signalling
alcohol affects long-term potentiation (LTP), a long-lasting enhancement of the
effectiveness of synaptic transmission that is considered a cel ular analogue of
memory (Chin et al. 2010;Matthews and Silvers 2004). Accordingly, adolescent
rats are more sensitive to alcohol-induced disruption of LTP than adults (White
and Swartzwelder 2005). As evidence indicates that in the hippocampus both
GABAergic and glutamatergic systems are sensitive to alcohol and undergo
remodelling during adolescence (Chin et al. 2010), it is possible that alcohol-
induced alterations in these hippocampal signalling systems may underlie the
spatial memory impairments observed in our studies.
In light of this, our laboratory recently performed a pilot experiment to explore
whether adolescent alcohol exposure also induces lingering changes in
glutamatergic receptor subunit regulation in the hippocampus. However,
analyzing the hippocampus following adolescent alcohol exposure and OPR
testing did not reveal changes in GluA2, GluA3, NR2A or NR2B protein
expression (Lubbers and Wouda, unpublished observations). Nonetheless,
evidence suggests that alcohol's injurious effects and adolescent brain
development likely converge in the hippocampus. Consequently, deficits in
behaviour requiring hippocampal functioning, such as the impaired spatial
memory reported here may be expected. Even so, prefrontal aberrations
following adolescent alcohol-use have also been reported in epidemiological
studies, such as (gender specific) changes in structure, volume and activity of
the PFC (Caldwell et al. 2005;De Bellis et al. 2005;Medina et al. 2007).
6.3.3. CSA alcohol exposure
As previously stated, no lingering effects of CSA alcohol treatment were
reported. During CSA exposure the animals receiving alcohol could choose
between two bottle bottles; one fil ed with water, the other with a sweetened
alcohol solution. The benefit of this approach is that it is relatively simple to
apply and, analogous to teenage drinking, intake is oral and voluntary.
Furthermore, adolescents initiating alcohol consumption tend to drink sweet
alcoholic beverages (van Laar et al. 2010). A limitation is that voluntary intake
of sweetened alcohol is also a source of individual variability, given that dose,
duration and pattern of intake are determined by the rat. Moreover, in contrast
to teenagers, rodents generally appear to limit their consumption of alcohol to
sub-intoxicating levels. In contrast, for the BLI model it was determined that the
blood alcohol levels on the first day of alcohol exposure peaked over 200 mg/dl
(Figure 23). The latter being well above 80 mg/dl which is considered excessive
alcohol-use in both humans and rats (Bell et al. 2006;Crabbe et al. 2011;NIAAA
2004).
Given that blood alcohol levels were not measured in the CSA-treated animals, an important question is whether these animals were exposed to relevant blood alcohol levels. Measurements of daily consumption during treatment indicate that, in the alcohol-treated groups, alcohol-intake equalled 5 g/kg or more per day in the peri-adolescent animals. In other words, adolescent rats consumed an average of 0.2 g/kg alcohol per h. To put this in perspective, a standard unit (e.g. a glass of beer), contains 10 g of alcohol. Accordingly, a sixteen year old boy weighing 65 kg, would consume 0.15 g/kg per glass. This amount of alcohol would induce mild effects on mood and heart rate. In our laboratory, adult rats that are motivated to work for the ingestion of similar amounts of alcohol, form learned associations between alcohol and environmental stimuli and show cue-induced relapse to alcohol-seeking after abstinence or extinction (see Chapters 4 and 5). In addition, whereas others report that a low dose of alcohol is not sufficient to trigger relapse (Le and Shaham 2002), it may facilitate spatial memory, general activity and social interaction (Acheson et al. 2001;van der Kooy et al. 1983;Varlinskaya et al. 2001;Varlinskaya and Spear 2006). In conclusion, CSA alcohol exposure did not yield long-lasting behavioural deficits, but at 5 g/kg per day, intake most likely brought about behaviourally relevant blood alcohol levels. Although animals receiving CSA alcohol treatment did not show behavioural deficits, the experiment did yield some unexpected results. Whereas animals exposed to saccharin and sweetened alcohol treatment had a comparable performance in the SA paradigm, the peri-adolescent water-treated controls showed distinct behaviour. The latter group earned more reinforcers during the fading procedure, showed an increased response rate during the early phases of between session progressive ratio and demonstrated facilitated cue-induced reinstatement as compared to age-matched saccharin and sweetened alcohol-treated animals. A possible explanation for these findings may be that exposure to a saccharin sweetened solution affected SA performance. A previous study exploring the effects of protracted saccharin sweetened alcohol consumption initiated either in adolescence or in adulthood, however, did not report such changes in adult alcohol-intake (Vetter et al. 2007). Yet, another group recently demonstrated that animals with access to a sucrose solution or to a sucrose sweetened alcohol solution during adolescence demonstrated reduced alcohol-intake during adulthood (Vendruscolo et al. 2010b). Accordingly, the same group concluded in a previous study that sugar over-consumption during
adolescence may reduce subsequent motivation for saccharin by changes in
reward processing (Vendruscolo et al. 2010a). Considering that sweet-alcoholic
beverages have become increasingly popular among adolescents, - 29% of the
drinking adolescents reporting weekly consumption of so-called breezers (van
Laar et al. 2010) – it would be interesting to further explore the long-term
effects of adolescent consumption of sweetened beverages.
6.3.4. The behaviour profile of adolescent alcohol exposure
Together, the results discussed here suggest that binge-like adolescent alcohol
exposure renders rats more vulnerable to increased alcohol-intake and
significantly impairs spatial memory in later life, whereas other aspects of
cognition, i.e. attention and impulsivity, remain unaffected. This behavioural
profile that partially overlaps with that advocated by clinical studies, provides
further support for the idea that alcohol exposure is particularly harmful during
adolescence and induces alterations in alcohol consumption and cognitive
functioning (Grant et al. 2006;Tapert et al. 2004b;Tapert and Brown 1999).
Moreover, the data indicate that these behavioural impairments are long-lasting
and independent from social, environmental and genetic aspects. It should be
noted here that the factors driving adolescent alcohol consumption in humans
are much more complicated than those model ed in rat paradigms. As such,
social (e.g. peer pressure and other psychological aspects), environmental and
genetic aspects, as well as the acute effects of alcohol on cognitive development
and the consequences of protracted alcohol-use, may lead to exacerbation of the
effects on behaviour reported here.
The behavioural profile put forward here is far from complete. Unexplored
behavioural modalities remain, such as stress and anxiety. According to the
tension-reduction hypothesis, alcohol consumption may be a form of self-
medication intended to relieve a state of continuing stress through the anxiolytic
effects of alcohol (Conger 1956). Looking at experimental evidence, however,
the exact relationship between alcohol consumption and stress is not straight
forward. Depending on the type, intensity and frequency of exposure, stress
may increase, decrease or have no effect on alcohol-intake. In addition, stress
may modulate initiation, maintenance and relapse of alcohol self-administration
(Pautassi et al. 2010). Furthermore, it has been suggested that early alcohol
initiation may interact with stress experiences, facilitating initiation or escalation
in alcohol consumption (Pautassi et al. 2010). As such, the influence of stress
and anxiety on adolescent alcohol exposure is worth exploring in additional
studies.
6.3.5. Future directions
Given the detrimental effects of adolescent alcohol-use, development of
prevention and treatment strategies is of importance. As will be discussed in
Section 6.7, ongoing effort is required in order to prevent adolescents from
engaging in excessive (binge-like) alcohol consumption. However, since
excessive alcohol-use among certain groups of adolescents seems to increase
(van der Lely et al. 2011), standardized treatment protocols and behavioural
models, such as the methodology used here, are required to explore the
underlying neurobiological mechanisms that mediate the harmful effects of
adolescent alcohol exposure. These studies will be important to disentangle the
"true" effects of alcohol and the effects of pathology preceding or accompanying
adolescent alcohol exposure, so that treatment can be issued accordingly.
Given that adolescent drug-use often extends beyond drinking alcohol
(discussed in more detail in Section 6.6), an additional topic of investigation is
therefore the putative additive harmful effect of concomitant use of alcohol with
other addictive substances during adolescence. Addressing these issues may
ultimately result in effective treatment for adolescents that are hospitalized
following alcohol intoxication. Furthermore, knowledge on the underlying
mechanisms of the alcohol-induced behavioural deficits seen in later life may
lead to new strategies to rescue this phenotype.
6.4. Novel treatment strategies for alcohol-use disorders
In Chapters 4 and 5 two new exciting approaches that may benefit patients
suffering from AUD were investigated. First the effects of varenicline were
studied in the SA model. This partial α4β2-nicotinic acetylcholine (nACh) agonist
that is currently marketed as a smoking cessation aid is believed to exert a
bimodal activity. Varenicline's strong affinity for the α4β2-nACh receptor has
been suggested to reduce the pleasurable effects of nicotine and alcohol. At the
same time, the partial agonistic properties of varenicline reduce craving and
withdrawal symptoms that are often associated with relapse (Coe et al. 2005).
Here, the putative advantageous effects of varenicline for people suffering from
AUD were investigated.
In agreement with previous studies, varenicline selectively reduced voluntary nicotine and alcohol-intake in rats (Rol ema et al. 2007b;Steensland et al. 2007). Reducing substance-use is only the first step in beating addiction. In view of the high prevalence of relapse in addicts its putative relapse-preventing properties were subsequently investigated. Thus, it was shown for the first time that varenicline attenuates cue-induced relapse to alcohol-seeking, but not to nicotine-seeking. Interestingly, the latter seems to correspond to findings in treatment-seeking smokers. In these subjects cues related to smoking bring forth strong craving responses. Administration of varenicline before a quit attempt did not diminish such cue-specific craving, indicating that the beneficial effects of varenicline in smokers are not mediated by changes in cue-specific craving (Gass 2012). In fact, in our hands cue-induced relapse may actually be facilitated by low dose varenicline. In addition to its relapse-preventing properties, the effects of varenicline on cognitive performance in the 5-CSRTT were investigated. In this task varenicline only had limited effects. Similar to the effects of nicotine it decreased inhibitory response control, albeit to a lesser extent and without affecting visuospatial attention (Blondel et al. 2000;Mirza and Stolerman 1998;van Gaalen et al. 2006). Which mechanisms underlie these effects? Varenicline is known to modulate DA release in the mesolimbic system (Coe et al. 2005;Ericson et al. 2009;Rollema et al. 2007b). As was explained above, this pathway is critically involved in both cue-induced relapse and drug self-administration. As such, mesolimbic dopamine signal ing may be involved in the effects reported here. But it does not explain why varenicline affected cue-induced relapse to alcohol and not to nicotine. Its pharmacological profile may provide an answer. Varenicline was primarily designed as a partial α4β2-nACh receptor agonist, and acts most potently at this receptor (Rollema et al. 2007b). However, varenicline can also bind to other receptors, albeit with much lower affinity. At high dosages varenicline is a full agonist of α7-nACh receptors. Furthermore, it may act as partial agonist of α3β4-, α3β2- and α6-nACh receptors (Coe et al. 2005;Mihalak et al. 2006). Given that nicotine's reinforcing effects are mostly attributed to the α4β2- and α7-receptor (Laviolette and van der Kooy 2004;Mansvelder and McGehee 2002), whereas various nicotinic receptors, including the α3β4-, α4α6β2β3- and α6β2β3-subtypes (Larsson et al. 2004) seem to mediate different behavioural aspects of alcohol (Davis and de Fiebre 2006), it is possible that divergent molecular mechanisms are at play in the behavioural effects of varenicline on alcohol and nicotine-seeking.
6.4.1. Modulating alcohol-related memories
Chapter 5 investigated whether relapse to alcohol-seeking can be modulated by
targeting memories. This study aimed to disrupt reconsolidation of alcohol-
related memories in rats that were trained in the SA paradigm. The results
demonstrated that repeated post-reactivation treatment with the β-adrenergic
receptor antagonist propranolol diminished relapse to alcohol-seeking in rats. A
similar treatment with NMDA glutamate receptor antagonist dizocilpine also
tended to reduce alcohol-seeking.
These data complement recently published findings of Von der Goltz and
colleagues (2009), who report that post-reactivation intra-cerebroventricular
injection of the protein synthesis inhibitor anisomysin reduced alcohol-seeking in
rats. Moreover, in this same study it was shown that dizocilpine treatment after
exposure to alcohol-paired conditioned stimuli reduced alcohol-seeking. The
latter effect was transient, as it was seen one day but not one week after
treatment (von der Goltz et al. 2009). These findings indicate that even
relatively old alcohol-related memories are prone to disruption in a reactivation-
dependent manner. Furthermore they suggest a role for adrenergic and
glutamatergic signalling in this process. Accordingly, post-reactivation β-
adrenergic receptor antagonism was reported to reduce cocaine and morphine
conditioned place preference (Bernardi et al. 2006;Robinson and Franklin 2007),
to modulate the salience of cocaine associated cues in an instrumental model
(Milton et al. 2008b) and to reduce reinstatement of sucrose-seeking in the SA
task (Diergaarde et al., 2006).
The finding that dizocilpine had less pronounced effects than noradrenergic
receptor blockade differs from findings in other behavioural models. For
instance, Milton and co-workers (2008b) were not able to affect cue-induced
cocaine-seeking using post-reactivation propranolol. Furthermore, a recent
report by Lee and colleagues demonstrated dizocilpine- but not propranolol-
induced reactivation-dependent impairment of behavioural responses (Lee and
Everitt 2008b). In addition, NMDA blockade of reconsolidation effectively
reduced amphetamine, morphine and cocaine conditioned place preference
(CPP) (Kelley et al. 2007;Sadler et al. 2007;Zhai et al. 2008). Despite these
mixed results, both β-adrenergic and glutamatergic receptors seem implicated in
reconsolidation. In fact, their downstream effects may partially overlap.
In the last decade, several laboratories have put tremendous effort into
identifying the key molecular mechanisms involved in reconsolidation. An
overview of this literature is beyond the scope of this discussion, but
comprehensive reviews are available elsewhere (eg. Milton and Everitt
2010;Tronson and Taylor 2007). In short, the downstream effects of β-
adrenergic and glutamatergic antagonism possibly converge on downstream
protein kinases and transcription factors, which in turn regulate immediate gene
expression and protein synthesis. However, the exact underpinnings of
reconsolidation remain to be determined and these mechanisms may depend on
the type and strength of memory (e.g. drug- vs. fear-related; repeated vs.
single exposure). Well-established memories may recruit different neuronal
circuits than weaker or newly-formed memories (Robbins et al. 2008) and brain
region-specific differences have been observed between different types of drugs
(Sorg 2012). Further investigation of these matters is required for the
development of effective reconsolidation-based therapeutic strategies in
humans.
6.4.2. Translation to the clinic
Considering all of the above, both the reconsolidation disruption strategy and
varenicline treatment seem to hold promise as future strategies to counter
AUDs. Yet, further investigation is required to evaluate whether these preclinical
approaches are applicable in the clinic. Given that it induces more pronounced
effects, has a relatively easy treatment regimen and is already approved by the
EMEA and FDA as an aid for smoking cessation, varenicline at this moment
seems the most viable new direction for alcohol treatment. Still, clinical trials
investigating the continuous use of varenicline rather than the single exposure
used here, as well as investigation of its long-term efficacy are required.
Furthermore, it will be essential to further examine the putative distinctive
divergent molecular mechanisms that underlie varenicline's effects in alcohol
and nicotine abusers, respectively.
Reconsolidation blockade on the other hand will be more difficult to translate to
clinical settings. With the exception of propranolol, which is already prescribed
to treat hypertension, most agents used to disrupt reconsolidation are toxic
drugs not suitable for use in humans (Monfils et al. 2009). For instance,
systemic injection with dizocilpine, protein kinases or transcription factors may
be accompanied by major side effects, since they are essentially involved in
normal cell functions. As such, systemic administration of propranolol seems the
most viable pharmacological option for reconsolidation blockade in humans.
Interestingly, a recent publication by Xue and colleagues proposes a non-
pharmacological approach to interfere with reconsolidation of drug-memories
(Xue et al. 2012). Their method was based on the observation that rats given an
extinction session directly following reactivation of a fear memory, subsequently
demonstrate attenuated fear expression (Monfils et al. 2009). Xue and
colleagues tested this retrieval-extinction procedure in both rats and humans.
Remarkably, they demonstrated that it not only reduced cocaine and morphine
CPP, as well as drug-priming induced reinstatement of cocaine- and heroin-
seeking in the rodent SA model, but also drug craving in abstinent heroin
addicts (Xue et al. 2012).
6.4.3. Future directions
In view of these exciting developments, it would be interesting to investigate
whether it is also possible to reactivate and disrupt alcohol-related memories in
humans. If reconsolidation blockade upon reactivation using drug-related cues is
successful, many other questions emerge. Does the reconsolidation disruption
approach reduce motivation for drug-use? Are the effects long-lasting? In this
respect, what conditions are optimal for reconsolidation in humans? Is a single
treatment sufficient or (as was the case in the current study) are multiple
reactivations and subsequent reconsolidation disruptions required? Can stress
and drug exposure as well as specific environments still precipitate relapse?
Moreover, reconsolidation disruption upon retrieval using cues in a specific
context does not necessarily mean that other distinctive cues have lost their
power to precipitate relapse in a different context. Cue-reactivation-based
treatments may therefore fail because the alcohol-associated context outside the
clinic may still induce relapse. In addition, in animal studies reconsolidation
blockade seems to effectively weaken drug-related memories, but a certain
amount of instrumental behaviour seems to remain (eg. Chapter 5 or Lee et al.
2006a;Milton et al. 2008a;Milton et al. 2008b). Will these remnants of
instrumental behaviour, e.g. habits or automatic behaviour that is independent
from memory, promote relapse and thus form an obstacle for reconsolidation
disruption approaches in humans?
Regardless of these considerations, promising results from studies of
reconsolidation blockade in healthy volunteers, patients suffering from post-
traumatic stress syndrome and in heroin addicts indicate the feasibility of the
reconsolidation disruption approach (Brunet et al. 2008; Kindt et al. 2009;Xue et
al. 2012). Furthermore, compared to current strategies, modulating
reconsolidation in addiction may have some strong advantages. Whereas current
therapeutic options, including varenicline treatment, seem mostly to suppress
symptoms, reconsolidation therapy may be curative by weakening the memory
traces that drive addictive behaviour. It may also be possible to combine
reconsolidation blockade with other strategies, including extinction therapy or
drugs such as varenicline. In fact, it does not seem likely that a single strategy
will suffice as a treatment for AUD. A combination strategy, on the other hand
may help to overcome potential problems and lead to a true cure rather than
merely suppressing symptoms.
6.5. Consequences of adolescent nicotine- and cannabis-use
Adolescent drug-use is by no means restricted to drinking alcohol. Particularly,
nicotine-use (i.e. smoking tobacco) and cannabis use-are also common among
teenagers (Hibell et al. 2012). On the short-term adolescent smokers show
disturbed working memory processes and attention (Counotte et al.
2011b;Jacobsen et al. 2005). Although data on the long-term effects are limited,
in humans lingering behavioural, cognitive and psychiatric health problems have
been associated with adolescent smoking. These include an increased likelihood
for early adult tobacco and or alcohol-use (Counotte et al. 2011b;Jacobsen et al.
2005;Mathers et al. 2006). Furthermore, similar to their response to alcohol,
adolescent rats exposed to nicotine seem more sensitive to the reinforcing
effects (Shram et al. 2006;Vastola et al. 2002) and may be less sensitive to the
aversive effects of nicotine (O'Dell et al. 2004;Spear and Varlinskaya 2010). In
line with the epidemiological data, rats exposed to nicotine specifically during
peri-adolescence, but not at a later time, display increased intravenous self-
administration of nicotine (Adriani et al. 2003), as well as increased anxiety-
related behaviour (Slawecki et al. 2003;Smith et al. 2006). In addition,
adolescent nicotine exposure increased intravenous cocaine self-administration
and augmented the locomotor response to amphetamine five and 30 days after
treatment. (Collins et al. 2004;McQuown et al. 2007). Regarding cognition,
recent findings from our laboratory indicate that adolescent nicotine induces
persistent deficits in the 5-CSRTT paradigm. In her thesis, Counotte concludes
that peri-adolescent, but not post-adolescent nicotine exposure causes
diminished attentional performance and increments in impulsive action
(premature responses), while leaving impulsive choice intact. Furthermore,
adolescent animals exposed to nicotine showed down-regulation of the metabotropic glutamate receptor (mGluR2) and enhanced DA releasability in the medial PFC, at least up to weeks after exposure. In an elegant follow-up experiment Counotte and colleagues were able rescue this adolescent nicotine-induced attentional deficit by restoring mGluR2 activity (Counotte 2010). Adolescent cannabis abuse may also induce long-lasting behavioural disturbances. Several studies on this subject have emerged from the premise that there is a relation between cannabis-use and schizophrenia. Although a causal link between cannabis-use and schizophrenia remains debatable, it is generally agreed that adolescent cannabis-use exacerbates the symptoms and worsens the schizophrenic prognosis (for review (Malone et al. 2010). These and other studies demonstrate that, akin to what is seen after adolescent alcohol exposure, early-onset cannabis-use in humans has been associated with later abuse and neuropsychiatric disorders in later life (Arseneault et al. 2004;Ehrenreich et al. 1999;Pope, Jr. et al. 2003;Sundram 2006). Adolescent cannabis-use has also been suggested to affect measures of cognition. However, whereas short-term effects seem clear, long-lasting effects are not undisputed (Jager et al. 2010;Schneider 2008). Several studies were unable to detect such changes (Jager et al. 2006;Lyketsos et al. 1999;Pope, Jr. et al. 2001a), while others, particularly those distinguishing between early and late-onset cannabis users report mild disturbances in reaction time, attention, memory and cognitive performance, as well as changes in morphology and cerebral blood flow (Ehrenreich et al. 1999;Fried et al. 2005;Lyketsos et al. 1999;Pope, Jr. et al. 2001b;Wilson et al. 2000). In line with this, several rodent studies report lasting effects of adolescent exposure to cannabinoid receptor agonists, such as WIN, CP55,940 or THC, on sensorimotor gating, object recognition, social recognition and working memory, but not on spatial memory (Cha et al. 2006;O'Shea et al. 2004;Quinn et al. 2008;Schneider et al. 2005;Schneider and Koch 2003; 2005; 2007). In addition, lingering disturbances of tolerance to cannabinoids, morphine, cocaine and amphetamine, consummatory behaviour (i.e., sucrose preference, self-administration of heroin and morphine and food reinforced progressive ratio performance), social behaviour and anxiety-like behaviour have also been reported (Biscaia et al. 2003; 2008;El gren et al. 2007;O'Shea et al. 2004; 2006;Pistis et al. 2004;Quinn et al. 2008;Rubino et al. 2008;Schneider et al. 2005;Schneider and Koch 2003; 2005). Interestingly, a few studies suggest
gender-dependent effects of adolescent cannabis exposure, for instance on social behaviour (O'Shea et al. 2004). Yet, similar to the human studies, not all rodent studies demonstrate lasting behavioural deficits that are specific to adolescent exposure (Biscaia et al. 2008;O'Shea et al. 2006;Rubino and Parolaro 2008). Taken together, studies on adolescent alcohol-, nicotine- and cannabis-use show some remarkable similarities. Yet the evidence available remains inconclusive, as not all animal and human studies demonstrate effects and some behavioural paradigms have gotten more attention than others. Nevertheless, a substantial amount of data indicates a specific vulnerability of the peri-adolescent brain to addictive substances. In particular, the onset of substance abuse early in adolescence has been associated with long-lasting, maybe even permanent disturbances. Behaviourally, adolescent exposure to nicotine, cannabis and alcohol seems to exacerbate substance-use in later life. Furthermore, aspects of cognitive functions, which progressively develop during adolescence, are affected by all three substances. Associated with these behaviours are brain regions, such as the medial prefrontal cortex and the hippocampus. Both show ongoing development during adolescence and particularly the hippocampus seems highly vulnerable to the effects of alcohol and cannabis. There appears to be quite some overlap in the affected behavioural modalities, however the different addictive substances also seem to provoke dissociable behavioural profiles. This is for instance illustrated by the drug specific effects of nicotine on attention and those of alcohol on spatial memory. Considering the partial convergence of neuronal signalling involved in the effects described above, it seems plausible that alcohol, nicotine and cannabis exert their effects via molecular pathways that largely overlap, but to some extent also via distinct mechanisms and brain regions. Even so, the neurobiological mechanisms mediating the specific vulnerability of the adolescent brain to alcohol, nicotine and cannabis are still far from understood. Furthermore it should be noted that other issues may also contribute to the (distinct) behavioural profiles reported within and between the adolescent alcohol, nicotine and cannabis literature. First of all, studies on the effects of adolescent drug exposure are still scarce, and more studies are needed to compose a more complete picture of the consequences of alcohol, nicotine and cannabis-use during adolescence. Second,
the window of exposure, strain, gender and age of "adult controls" differs
drastical y between studies. Nonetheless, considering the strong evidence for the
negative and costly consequences of teenage substance misuse, it will be
necessary to further portray the lingering behavioural consequences of
adolescent alcohol, nicotine or cannabinoid exposure in future experiments. The
treatment protocols and behavioural paradigms used here may provide a basis
for such studies.
6.6. Concomitant use of alcohol and nicotine or cannabis
Adding an additional layer of complexity to the problems associated with
adolescent substance-use, is the frequent concomitant use of alcohol, tobacco
and/or cannabis among adolescents (Agosti et al. 2002;Meyerhoff et al.
2006;Ribeiro-Carvalho et al. 2008). For alcohol and nicotine-dependence there
may be a shared genetic vulnerability (Madden and Heath 2002). Furthermore,
there is a likely convergence of the actions of alcohol and nicotine, given that
they both act on receptors of the nACh receptor family. Also, it is possible that
alcohol can alter the pharmacological binding properties of nicotine, for instance,
by enhancing function of α4β2-, or inhibition of α7-receptors (Davis and de
Fiebre 2006). Interestingly, specific variations of the genomic region containing
the gene cluster coding for α5-, α3- and β4-nAChR subunits may modulate the
readiness to experiment with tobacco and alcohol at an early age (Schlaepfer et
al. 2008).
Irrespective of the underlying mechanisms, several interactions of alcohol and
nicotine have been reported in rodents. For instance, nicotine augments
voluntary alcohol-intake in rats (Le and Shaham 2002). Also, nicotine may
substitute for alcohol in drug discrimination experiments (McMillan et al. 1999)
and cross tolerance has been reported between alcohol and nicotine (Collins et
al. 1988). At sub maximal doses, systemic concomitant application was
demonstrated to have additive effects on accumbal DA release compared to
alcohol or nicotine exposure alone (Tizabi et al. 2007). In accordance, in mice
low doses of nicotine may increase sub maximal alcohol-induced locomotor
stimulation, yet the same dose prevents the maximal stimulatory effect of
alcohol (Soderpalm et al. 2000; 2009). Furthermore, nicotine may ameliorate
the negative effects of alcohol on cognition and coordination (Dar et al.
1994;Meyerhoff et al. 2006). Thus, combining nicotine and alcohol may
potentiate their reinforcing effects, whereas other behavioural measures may be
diminished.
To my knowledge, less is known about putative additive effects of alcohol and
cannabis. However, in peri-adolescent rats activation of CB1 receptors was
suggested to potentiate the toxicity of alcohol (Hansen et al. 2008). As detailed
above, the adolescent hippocampus is suggested to be particularly sensitive to
the neurotoxic effects of both alcohol and cannabis possibly leading to
structural, functional and behavioural abnormalities. Recent studies investigating
the consequences of concomitant alcohol- and cannabis-use during adolescent
revealed a different pattern of hippocampal asymmetry and an aberrant brain
response to a spatial working memory task compared to age matched controls
that only drink heavily (Medina, 2007; Schweinsburg, 2005). Furthermore, a
functional relationship between hippocampal asymmetry and verbal learning was
abnormal among adolescent alcohol or concomitant marijuana and alcohol-users
compared to healthy controls (Medina, 2007). Interestingly, cannabis may also
have neuro-protective effects. Cannabinoids could possibly alleviate
excitotoxicity and oxidative stress that are associated with heavy alcohol
intoxication by inhibiting glutamatergic neurotransmission (Hamelink et al.
2005).
In summary, at present the putative effects of concomitant substance-use in
adolescence remain largely unstudied. However, the studies that exist suggest
that concomitant alcohol and nicotine or cannabis-use may have additive
neurobiological and neurobehavioural effects. In view of the putative
exacerbating consequences of combined-use of alcohol and nicotine or cannabis,
further investigation of both the short- and long-term consequence of
concomitant drug-use during adolescence is necessary.
6.7. Prevention strategies
Given the social, health and economic costs of underage drinking, there is ample
interest in the development of prevention and intervention programs to
minimize this problem. In this regard, it is valuable to discuss what can be
learned from the current experiments.
Prevention and intervention strategies are general y directed at prevention and
harm reduction in whole populations at average risk (e.g. adolescents), unique
subgroups (e.g. children of alcoholics) or specific individuals (e.g. young
addicts). Many prevention strategies are guided by risk factors that have been
identified in clinical studies addressing adolescent alcohol-use. As such, several
prevention programs aim at reducing parental substance-use, delaying initiation
of alcohol-use, and reducing the frequency and amount of alcohol-use (Toumbourou, 2007). As an example, the Dutch government has launched different campaigns addressing under aged drinking in order to educate both parents and adolescents to broaden awareness of the harmful effects of alcohol-use. In view the data presented in this thesis, particularly the vulnerable position of adolescents should become common knowledge. Given that teenagers may be more tolerant to the intoxicating effects of alcohol they should be made aware that this is not a demonstration that one can hold his drink well. Rather it should be regarded as a vulnerability that increases the chance of brain damage. Furthermore, as is underscored by the results presented here, education on adolescent alcohol-use should extend beyond teaching about the well-known immediate risks of alcohol and include the long-term danger of inflicting persistent cognitive deficits. Possibly, a greater public awareness of the harmful effects of alcohol may help to diminish the positive image of alcohol among adolescent youth. Parallel to education, proactive regulatory actions are believed to reduce the chance that adolescents come in contact with alcohol. Reducing alcohol supply and demand, by financial discouragements or by reducing alcohol availability have been shown to be effective to reduce harm among youth. For instance, increasing taxes reduces drinking, also among problem drinkers and adolescents. Also increasing the legal age for the purchase and consumption of alcohol, a means the Dutch government is currently pursuing, has previously been shown to be a cost-effective action to reduce alcohol-related traffic accidents (WHO, 2010). Strict alcohol-related law enforcement, rationing and restrictions on the hours and days of sale were also demonstrated to be successful tools (for review see Toumbourou, 2007). Another potential strategy is to improve conditions for healthy development from birth through adolescence. As was discussed before, adolescent risk-taking and exploring boundaries are often associated with initiation of drug-use. As these are inevitable hallmarks of adolescence, it may not be useful to restrict youth in these activities. Yet, it may be possible to promote a safer, drug-free environment to channel this behaviour (Spear, 2010). In fact, the Netherlands institute for alcohol policy (STAP), has launched the alcohol-free environment project exactly for this purpose (see www.stap.nl).
To conclude, worldwide prevention and intervention programs have adopted a
wide range of tools and targets to reduce adolescent alcohol, including education
of both parents and adolescents, promotion of safer environments and
regulatory interventions. The persistent adolescent alcohol induced behavioural
impairments laid bare in this thesis underscore the continuing need to improve
on these measures and may help guide educational programs.
6.8. Concluding remarks
Currently we are far from fully understanding the long-term consequences of
adolescent alcohol-use. Yet, the work discussed in this thesis demonstrates
long-lasting deficits in consummatory behaviour and memory performance in
rats with a history of adolescent alcohol exposure. As such, it provides a
behavioural profile that is related to adolescent alcohol exposure independent
from social, environmental and genetic aspects. This profile adds to the
accumulating evidence that indicates that excessive adolescent alcohol-use
induces long-lasting behavioural deficits. In view of these harmful consequences,
future studies are required to establish a better understanding of the
neurobehavioural and neurochemical development during adolescence to further
characterize and understand the vulnerabilities seen at this age. Such studies
may be based on the treatment protocol and behavioural paradigms that are
discussed here. Moreover, the progressing insights in the adolescent
vulnerability to alcohol exposure should fuel the development of novel
prevention and treatment strategies, in order to reduce the number of
adolescents that (ab)use alcohol, to treat the teenagers that are hospitalized
with alcohol intoxication and to ameliorate the adolescent alcohol-induced
behavioural deficits seen in later life. In this way the harmful consequences of
excessive adolescent alcohol-use may be diminished for the next generations.
In parallel investigations, preclinical evidence was provided for the efficacy of
two potential new treatments that may aid AUD patients with cessation of
alcohol-use and prevent relapse. It was shown that varenicline may be more
beneficial as cessation aid for people who drink than for smokers, given that
varenicline treatment attenuated alcohol self-administration as well as relapse to
alcohol-seeking in rats. Furthermore, using a reconsolidation disruption
approach it was demonstrated that post-reactivation administration of
propranolol reduced alcohol-seeking in rats. Although clinical trials are required
to further establish the beneficial effects of these two treatments, they provide
putative new therapeutical opportunities for AUD patients, which is of great importance considering the limited efficacy of the current treatment options.
Summary in Dutch, Nederlandse
Samenvatting
7.1 Abstract
Veel mensen genieten graag van een alcoholisch drankje. Helaas, is alcohol
misbruik ook betrokken bij grote sociale, economische en
gezondheidsproblemen. In mijn proefschrift heb ik aspecten van dit laatste
probleem onderzocht met behulp van translationele diermodellen.
Meestal begint men tijdens de adolescentie met het gebruik van alcohol. Er is
echter nog weinig bekend over de lange-termijn gevolgen van alcohol gebruik in
deze periode die gekenmerkt wordt door een variëteit aan neurobiologische- en
gedragsveranderingen. Mijn doel was om een beter beeld te krijgen van lange-
termijn gedragsveranderingen die toe te schrijven zijn aan adolescente alcohol
blootstelling. Ik heb daartoe laten zien dat volwassen ratten die tijdens hun
adolescentie aan alcohol bloot werden gesteld meer alcohol innamen en een
slechter geheugen hadden, terwijl aspecten van aandacht en impulsiviteit
onveranderd bleven. Mijn resultaten bieden een basis voor verdere studies naar
de mechanismen die hieraan ten grondslag liggen en kunnen de ontwikkeling
van nieuwe therapeutische behandelingen stimuleren.
Vervolgens heb ik mijn translationele gedragsmodellen gebruikt om twee nieuwe
strategieën te onderzoeken die gericht zijn op vermindering van alcoholgebruik
en terugval. Ik kon zo aantonen dat het nieuwe "anti-rook" medicijn varenicline
in ratten alcohol zelftoediening en terugval verminderde. Daarnaast heb ik
getracht aan alcohol-geassocieerde geheugensporen te verstoren door het
medicijn propranolol toe te dienen vlak nadat een alcohol-herinnering was
opgehaald. Ratten die deze behandeling kregen vertoonden later minder alcohol-
zoekgedrag. Hoewel er klinische studies nodig zijn om de gunstige effecten van
deze twee benaderingen verder te onderzoeken, leiden ze mogelijk tot nieuwe
behandelingen voor alcohol-afhankelijke patiënten.
7.2 Alcohol studies in translationele modellen: Gedragsmatige
gevolgen van adolescente blootstelling en nieuwe benaderingen
om de neiging tot terugval te verminderen.
"Alcohol studies in translationele modellen" is de wat cryptische titel van dit
proefschrift. Simpel gezegd houdt het in dat ik onderzoek heb gedaan naar
alcohol(gebruik) en dat ik daarvoor niet naar mensen heb gekeken, maar naar
het gedrag van ratten. In hoofdstuk 1, de introductie van mijn proefschrift, leg
ik uit waarom dit onderzoek van belang is. Ik bespreek dat ik, als ik het over
alcohol heb, eigenlijk niet alcohol bedoel, maar ethanol. Dit is de scheikundige
naam voor de verslavende stof die ontstaat bij het vergisten van fruit en
koolhydraten en die al eeuwen over de hele wereld genuttigd wordt in de vorm
van wijn en bier. Vergeleken met andere verslavende stoffen heeft alcohol een
bijzondere werking. Rond eiwitten en celmembranen kan het de plaats van
water in nemen en zo de vorm en functie van andere eiwitten en receptoren
beïnvloeden. Men denkt dat alcohol via deze indirecte modulatie de
communicatie tussen hersencellen verandert en zo de bekende plezierige en
verslavende effecten teweeg brengt. Alcohol wordt door veel wetenschappers
gezien als de meest schadelijke van alle verslavende stoffen, zowel voor de
gebruiker als voor zijn omgeving. Zo is alcoholmisbruik wereldwijd betrokken bij
meer doden dan AIDS; is in Nederland tien procent van de bevolking een stevige
drinker; en kost alcoholgebruik meer dan drie procent van het bruto nationaal
product.
Alcohol misbruik komt niet alleen voor onder volwassenen. Ook pubers en
adolescenten drinken vaak stevig. Zo hoor je in het nieuws regelmatig over
jongeren die "binge-drinken". Hierbij worden niet dagelijks, maar wel regelmatig
(vaak in het weekend) grote hoeveelheden alcohol gedronken in een relatief
korte tijd. Er zijn veel aanwijzingen dat dit drinkpatroon erg schadelijk is voor de
hersenen. Bovendien is binge-drinken voor adolescenten zelfs extra gevaarlijk,
omdat jongeren psychisch en fysiek nog volop in ontwikkeling zijn.
Onderzoekers rapporteren bijvoorbeeld een mogelijke relatie tussen adolescent
alcoholgebruik en verslaving op latere leeftijd. Daarnaast wordt het in verband
gebracht met psychische stoornissen, geheugen- en aandachtsproblemen. In
veel gevallen is het echter niet mogelijk om met zekerheid aan te tonen dat
deze gedragsstoornissen door adolescent alcoholgebruik veroorzaakt zijn. In
epidemiologische studies, waarin onderzoek gedaan wordt met mensen, is het
namelijk niet mogelijk om onderscheid te maken tussen de gevolgen van alcohol
en de invloed van genetische- en omgevingsfactoren. De "translationele
diermodellen" waar ik het in de titel van mijn proefschrift over heb zijn daarom
erg nuttig. Ze bieden onderzoekers de mogelijkheid om deze factoren in de
beheersbare omgeving van het laboratorium op de universiteit beter te
controleren. Daarom heb ik in hoofdstuk 2 en 3 van mijn proefschrift gebruik
gemaakt van de rat als model voor de mens en heb ik het gedrag van volwassen
ratten die tijdens hun adolescentie zijn blootgesteld aan alcohol vergeleken met
het gedrag van ratten die geen alcohol kregen. Op deze wijze kreeg ik een beter
inzicht in de gedragsveranderingen die aan adolescente alcoholblootstelling toe
te schrijven zijn.
Naast de gevolgen van adolescent alcoholgebruik ben ik ook geïnteresseerd in
alcohol misbruik en alcoholafhankelijkheid op latere leeftijd. Een belangrijk
probleem bij het bestrijden van alcohol misbruik is dat patiënten die afhankelijk
zijn geworden van alcohol niet zomaar kunnen stoppen met drinken. Bovendien
vallen zelfs degenen die al voor langere tijd gestopt zijn met drinken, vaak toch
weer terug in alcoholgebruik. Dit kan bijvoorbeeld veroorzaakt worden door
stress of door zogenaamde "cues" die het vroegere alcoholgebruik weer in
herinnering brengen; denk bijvoorbeeld aan geuren, smaak en de aan het
gebruik verwante voorwerpen. De huidige medicatie voor mensen die proberen
te stoppen met drinken ondersteunt vaak wel het stoppen, maar is in de regel
weinig effectief om terugval te voorkomen. Om deze reden heb ik in hoofdstuk 4
en 5 gedragsmodellen in ratten gebruikt om nieuwe methoden te onderzoeken
die alcoholgebruik en terugval kunnen onderdrukken.
7.2.1.Gedragsmatige gevolgen van adolescent alcoholgebruik
In de eerste twee experimentele hoofdstukken van mijn proefschrift wilde ik de
lange termijn gevolgen van adolescente alcohol blootstelling op het gedrag van
ratten uitgebreid in kaart brengen. Om te beginnen moest ik hiervoor een
methode ontwikkelen waarmee ik ratten tijdens hun adolescentie aan alcohol
kon blootstellen. Dit is nog niet gemakkelijk. Hoewel jonge ratten in veel
opzichten op jonge mensen lijken, duurt de adolescente periode bij ratten
bijvoorbeeld maar 10 dagen (van dag 34 na de geboorte tot dag 43). Daarnaast
drinken jonge ratten meer alcohol dan volwassenen, maar zullen ze van nature
geen binge-drink gedrag vertonen. Na een aantal mogelijke vormen van
adolescente alcohol blootstelling te hebben overwogen, heb ik uiteindelijk van
twee strategieën gebruik gemaakt. Bij de eerste strategie heb ik mijn
adolescente ratten de mogelijkheid gegeven om zoete alcohol te drinken, dit
noem ik "continue zelftoediening" (CZT). Bij de tweede methode heb ik mijn ratten om de dag injecties gegeven met een grote hoeveelheid alcohol. Deze laatste methode zorgde voor alcoholbloedwaarden die vergelijkbaar zijn met wat gemeten wordt bij jongeren die stevig binge-drinken, daarom noem ik deze strategie "binge toediening" (BT). Voor de experimenten beschreven in hoofdstuk 2 en 3 zijn mijn ratten op een van deze twee manieren aan alcohol blootgesteld. Omdat het mij ging om de lange-termijn gevolgen van adolescente alcoholblootstelling, onderzocht ik het gedrag van de dieren pas vijf na de behandeling, wanneer de dieren volwassen waren. Zoals ik al eerder even aanstipte zijn er uit klinisch onderzoek sterke aanwijzingen dat adolescent alcoholgebruik de kans op verslaving op latere leeftijd verhoogt. In hoofdstuk 2 heb ik daarom het drinkgedrag van ratten onderzocht nadat ik ze tijdens hun adolescentie aan alcohol had blootgesteld. Hiervoor trainde ik mijn ratten in een zogenaamde zelftoediening taak (ZTT). Deze taak voerde ik uit in speciale "operante" kooien. In de wand van deze kooien zaten twee gaten waarin de ratten met hun neus konden prikken. Boven één van deze gaten brandde een lampje. Als een rat in dit gat zijn neus stak, werd dit door een sensor gedetecteerd en werd de rat beloond met een druppel alcohol. Deze beloning ging gepaard met een geluid en een ander lampje (een audiovisuele cue). Met behulp van de ZTT heb ik laten zien dat volwassen ratten die tijdens hun adolescentie op BT wijze aan alcohol bloot zijn gesteld zichzelf significant meer alcohol toedienden dan hun soortgenoten die een controle behandeling kregen (de controle behandeling bestond óf uit injecties met een zoutoplossing tijdens de adolescentie, óf injecties met alcohol, maar dan vlak na de adolescente periode). Deze gedragstaak heb ik ook gebruikt om de motivatie van ratten om te werken voor alcohol te bepalen en om terugval naar alcoholgebruik te onderzoeken in ratten met een geschiedenis van adolescente alcohol blootstelling. Deze aspecten van drinkgedrag werden niet veranderd door BT blootstelling. In ratten die een CZT alcoholbehandeling hadden gekregen tijdens hun adolescentie, vond ik helemaal geen meetbare lange-termijn gevolgen in deze taak. In hoofdstuk 2 beschrijf ik ook experimenten waarin ik heb gekeken naar de cognitieve vaardigheden aandacht en impulsiviteit. Afwijkingen in deze aspecten van cognitief gedrag worden vaak in verband gebracht met verslaving. Voor dit onderzoek gebruikte ik de vijf keuze seriële reactietijd taak (5KSRTT). Hiervoor werden de ratten ook in operante kooien getraind. In deze kooien zat echter een
wand met vijf gaten waarin een lichtje gepresenteerd kan worden. Een rat wordt
in de 5KSRTT geleerd om te wachten tot in één van de vijf gaten een lampje aan
gaat en vervolgens met zijn snuit in dit gat te prikken. Doet de rat dit goed dan
wordt hij beloond met een voedselbrokje. Steekt een rat zijn neus in een gat
voordat het lichtje is aan gegaan, dan zegt dit iets over impulsiviteit. Reageert
de rat niet, of steekt hij zijn neus in het verkeerde gat wanneer het lichtje
brandt, dan zegt dit iets over de aandacht van het dier. Met mijn experimenten
heb ik laten zien dat beide vormen van adolescente alcohol blootstelling (BT en
CZT) geen meetbare lange termijn gevolgen hadden op de aspecten van
impulsiviteit en aandacht die in deze taak gemeten worden.
Hoofdstuk 3 was gericht op een ander gedragsaspect, namelijk het (ruimtelijk)
geheugen. Bij het ruimtelijk geheugen is de hippocampus betrokken, een
hersenkern waarvan het bekend is dat deze kan beschadigen als gevolg van
stevig alcoholgebruik. Ik heb twee verschillende taken gebruikt om het
ruimtelijk geheugen van mijn ratten te onderzoeken. In de eerste, het radiale
arm doolhof, moesten de ratten onthouden waar ik voedselbrokjes verstopt had
in een doolhof dat bestond uit een centraal platform waar acht armen op
uitkwamen (zie hoofdstuk 1 Box 5). Ratten die tijdens hun adolescentie BT
alcohol hadden gekregen vonden dit moeilijker dan de controle dieren. Ze
maakten meer fouten bij het zoeken naar voedselbrokjes. In de tweede taak, de
object-plaats herkenningstaak, werden mijn ratten in een kooi gezet waarin
twee gelijkvormige objecten stonden. Daar mochten ze even aan snuffelen.
Daarna werden de ratten uit de kooi gehaald en werd een object verplaatst.
Vervolgens werden de ratten na een korte pauze terug gezet en moesten ze
aangeven welk object verplaatst was. Ook in deze taak presteerden de ratten
die BT alcohol hadden gehad minder goed. Echter, controle ratten, die niet
tijdens maar direct na de adolescentie BT alcoholbehandeling kregen, hadden
ook moeite met deze taak. Het alcohol effect in deze taak was dus niet "leeftijd
specifiek", zoals in de doolhof taak wel het geval was.
7.2.2. Beperken van alcoholgebruik en terugval
In het tweede deel van mijn proefschrift heb ik nieuwe methoden onderzocht die
mogelijk alcoholgebruik en terugval kunnen onderdrukken in alcoholafhankelijke
individuen. In hoofdstuk 4 heb ik hiervoor het medicijn varenicline in de eerder
beschreven ZTT getest. Dit nieuwe medicijn wordt momenteel voorgeschreven
aan mensen die willen stoppen met roken. Varenicline heeft een tweeledige
werking. Het verhindert de aangename effecten van nicotine door de receptoren
waarop nicotine in de hersenen aangrijpt af te schermen. Daarnaast stimuleert
varenicline deze nicotine receptoren ook een beetje, zodat het in rokers de
hunkering naar nicotine vermindert. Omdat gebleken is dat rokers die
varenicline gebruiken niet alleen minder gaan roken, maar ook minder gaan
drinken, wilde ik onderzoeken wat de effecten van varenicline zijn op het drink
gedrag van ratten. In mijn experimenten heb ik laten zien dat behandeling met
varenicline er voor zorgt dat ratten zichzelf minder alcohol toedienden.
Bovendien vertoonden de ratten minder terugval dan dieren die geen varenicline
kregen toegediend. Verrassend was, dat dit terugval-onderdrukkende effect niet
aanwezig was in ratten die zichzelf nicotine toedienden in plaats van alcohol.
In hoofdstuk 5 werd een andere strategie om terugval te verminderen
onderzocht, waarbij ik gebruik maakte van geheugen manipulatie. Tot voor kort
veronderstelden wetenschappers dat nieuwe (leer)ervaringen met een actief
proces in het geheugen worden vastgelegd (geconsolideerd) en deze herinnering
vervolgens onveranderbaar is. Echter, er is een nieuwe hypothese die stelt dat
wanneer geconsolideerd geheugen opgehaald of geactiveerd wordt, dat deze
herinnering veranderd kan worden en bovendien opnieuw met een actief proces
moet worden vastgelegd om behouden te blijven. Door dit zogenaamde
"reconsolidatie" proces met bepaalde farmaceutische stoffen te beïnvloeden kan
vastgelegd geheugen worden gemanipuleerd. Zo hebben onderzoekers in ratten
en mensen laten zien dat angst-gerelateerde herinneringen uitgewist kunnen
worden als er na het ophalen van deze herinnering de β-adrenerge receptor
blokker propranolol wordt toegediend. Nu is het zo dat bij verslaving
herinneringen aan de verslavende stof en aan gerelateerde cues een belangrijke
rol spelen bij het terugval en naar het oude middelen misbruik. Daarom heb ik
onderzocht of ik terugval naar alcoholdrinkgedrag kon verminderen door de
reconsolidatie van alcohol-gerelateerde herinneringen te verstoren. Hiervoor heb
ik ratten getraind in de ZTT en ze geleerd om een audiovisuele cue te associëren
met een alcoholbeloning. Vervolgens heb ik na een periode van onthouding hun
alcohol-gerelateerde herinneringen geactiveerd en ze een injectie gegeven met
propranolol. Ratten die deze behandeling kregen vertoonden later minder
terugval naar alcoholzelftoediening dan hun soortgenoten die in plaats van
propranolol met een zoutoplossing waren geïnjecteerd.
7.3. Conclusie
In hoofdstuk 6 vat ik mijn resultaten nogmaals samen en plaats ze in een
bredere context. Met betrekking tot de effecten van adolescente alcohol
blootstelling zijn mijn belangrijkste bevindingen dat BT - maar niet CZT - alcohol
blootstelling resulteert in langdurige gedragsveranderingen. Vijf weken na de laatste alcohol behandeling vertoonden mijn inmiddels volwassen ratten verhoogde alcoholzelftoediening en een verminderd ruimtelijk geheugen. Adolescente alcohol behandeling had geen aantoonbare lange termijn gevolgen voor motivatie en terugval naar alcohol-zoekgedrag of op aandacht en impulsiviteit. Dit gedragsprofiel is een gevolg van de blootstelling aan alcohol en staat los van sociale, milieu en genetische aspecten en past binnen de voortschrijdende inzichten dat adolescenten kwetsbaarder zijn voor alcohol dan volwassenen. Verder maakt het duidelijk dat het binge drinken op jonge leeftijd kan leiden tot permanente veranderingen in geheugenprocessen en alcoholgebruik. In het licht van deze schadelijke gevolgen zijn toekomstige studies nodig om een beter begrip van de neurologische en neurochemische veranderingen te krijgen die adolescent alcoholgebruik veroorzaakt. Dit om kwetsbaarheid op deze leeftijd verder te karakteriseren en de gevolgen ervan beter te kunnen beheersen. Dergelijke studies kunnen gebaseerd worden op het behandelingsprotocol en de gedragstaken die ik in mijn proefschrift heb gebruikt. De resultaten van deze studies kunnen de ontwikkeling van nieuwe preventie- en behandelstrategieën om alcoholmisbruik onder jongeren en de gevolgen er van te verminderen stimuleren. In een tweede reeks van experimenten onderzocht ik de werkzaamheid van twee nieuwe "anti-terugval" strategieën. Ik liet zien dat de partiële α4β2-nicotine receptor agonist varenicline naast effectieve vermindering van alcoholzelftoediening ook cue-geïnduceerde terugval naar alcohol zoekgedrag onderdrukte. Daarnaast heb ik in een geheugen reconsolidatie taak laten zien dat ratten na herhaalde geheugenactivering in combinatie met behandeling met de β-adrenerge receptor antagonist propranolol verminderde terugval naar alcohol zoekgedrag vertoonden. Hoewel er nog klinische studies nodig zijn om de gunstige effecten van deze twee behandelingen in mensen te onderzoeken, bieden mijn resultaten aanknopingspunten voor nieuwe behandelingen voor alcoholafhankelijke patiënten. Dit is belangrijk omdat de werkzaamheid van huidige medicatie tekort schiet terwijl alcohol misbruik grote sociale- en gezondheidsproblemen veroorzaakt.
8. Acknowledgements/ Dankwoord
Mijn dank gaat uit naar al e mensen en dieren die een rol hebben gespeeld bij de
totstandkoming van dit proefschrift.
8.1. De directe begeleiding
Professor de Vries, promotor en leermeester, het heeft even geduurd, maar hier
is ie dan. Ik dank u voor uw wijsheid en geduld. Dank ook voor de vrijheid die u
mij gaf om mijn onderzoek op mijn manier en op mijn tempo uit te voeren. Nog
meer dank voor de momenten dat u mij die vrijheid niet gaf; zonder uw kritiek,
kennis en sturing was dit boekje er niet geweest. Dr. Pattij, ik heb groot respect
voor de wijze waarop u wetenschap bedrijft. Dank voor uw toegankelijkheid, ik
kan alleen maar hopen dat een stukje van uw degelijkheid, integriteit en kennis
op mij is overgedragen.
Ik bof dat ik met allebei mijn begeleiders zo goed kan opschieten. Taco, ik heb
met weinig andere mensen zo slap geouwehoerd, gedronken en het bed
gedeeld. Tommy, van jou misschien geen knuffels, maar bij de familie Pattij
(daarvoor ook dank aan Leontien!) was er altijd een warm welkom. Heren, ik
ben dankbaar voor deze mooie balans van professionaliteit en plezier, en hoop
dat we nog regelmatig zullen proosten op het leven.
8.2. De Yvar
En dan was er nog Yvar, niet mijn begeleider, maar minstens zo belangrijk voor
mijn vorming. Als student, collega en vriend ben je mijn steun en toeverlaat
geweest. Ik kon altijd rekenen op een helpende hand, en toen je eenmaal had
geleerd om niet te hard knijpen kon ik me geen betere analist wensen. Ondanks
een korte periode met een op maat gemaakte Yvar-mepper konden we bijzonder
goed met elkaar overweg. Het was altijd genieten van je vrolijkheid. Yvar, je
bent geweldig!
8.3. De leescommissie
Wim van den Brink, Merel Kindt, Heidi Lesscher, Ton Schoffelmeer, Sabine.
Spijker, Oliver Stiedl en Reinout Wiers, ik dank u hartelijk voor de aandacht,
energie en tijd die u aan mijn proefschrift gegeven heeft. Sabine en Oliver, dank
ook voor jullie constructieve commentaar, het zal de aankomende adolescentie
publicatie sterk verbeteren.
8.3. De collega's van de vierde
Ton, u heeft me steeds heeft gepusht om er het beste uit te halen. Bedankt voor
de hulp bij het voorbereiden van mijn verdediging. Ik heb er van genoten dat ik
mocht werken bij uw vakgroep. Wat een fantastische werksfeer was er op ons
lab en wat hebben we naast het doen van onderzoek tijd gevonden voor leuke
dingen: tafeltennis, Rusty Balls, de blauwe koe, limoncello, road trip!, Brugge,
de Beurs van Berlage, de Wildeman, congressen, labuitjes en natuurlijk een
biertje of whisky als de 5 in de klok zat. Hierdoor heb ik met veel van jullie een
speciale band op kunnen bouwen die verder strekt dan het col egiale. Nog
steeds voelt een bezoekje aan het lab daarom als thuis komen. Mijn dank
daarvoor! Taco, Tommy, Ton, Leontien, Jamie, Annemarie, Micha, Benjamin,
Mieke, Mieke, Marloes, Karlijn, Nathaly, Wendy, Donnee, Rob, Dustin, Yvar,
Mathijs, Halfdan, Kees, François, Aafje, Joost, Nienke, Rolinka, Roeland, John,
John, Haarmans, Meiling, Sandra, Silvie, Jasper, Gideon, Martijn, Nicky, en alle
anderen, ik heb veel dierbare herinneringen aan jullie.
8.3.1. De kamer genoten
Tommy, Mieke, Dustin, Matthijs, Nienke en Roeland, bedankt voor de
gezelligheid. Ik heb veel gehad aan jullie steun en geduld als het tegenzat of de
onzekerheid weer eens toesloeg. Mieke ik vond het erg jammer dat je al na een
jaar richting het zuiden vertrok. Ik waardeerde je zachtaardige manier waarop je
met onze ratjes omging. (hoewel ik zelfs ome Rob wel eens tegen de ratjes heb
horen fluisteren). Dustin, je bent een eigenwijze jokkebrok. Ik weet nog goed
dat we het hele gebouw hebben doorzocht naar mijn sleutels die jij uit de deur
had gehaald. Desondanks, herinner ik me vooral het plezier waarmee we
gewerkt, getafeltennist en gesport hebben. Matthijs, je was een belangrijke bron
van rust in onze kamer, de waardige manier waarop je bent gestorven (je was
bijna even oud als ik!) heeft bij mij een diepe indruk achter gelaten. Nienke het
was gezellig, ik waardeerde het dat we ook privé met elkaar mee konden leven.
Roeland, je was een bron van positieve energie en altijd bereid om te helpen
(natuurlijk ook bedankt voor de gevulde koelkast).
8.3.2 De studenten
Yvar, Silvie, Danai, Gideon, Martijn and Jasper het was bijzonder leuk om met
jullie samen te werken. Danai your unlimited enthousiasm combined with your
scientific curiosity and excessive word-use was inspiring. "Gebroken arm"
Gideon en Jasper met jullie heb ik zoveel uren in het rode licht van de stallen
door gebracht, dat het maar goed is dat we altijd wat boeiends te bespreken
hadden of anders wel naar een mooi liedje op de radio konden luisteren(replay).
Aan jullie allen mijn dank voor de hulp en alles wat ik heb geleerd dankzij jullie
intelligente en kritische vragen.
8.3.3 De afdeling psychiatrie
Altijd fijn om een leuke andere afdeling op dezelfde verdieping te hebben. Om
de een of andere reden zijn wij sneller geïntegreerd dan de anatomie en
neurowetenschappers. Dank voor de prettige samenwerking. Stella en Zsuszika,
jullie waren een prachtige aanvulling op ons lab. Stella bedankt voor onze
kleurrijke gesprekken. "Grupenfuhrer" Zsuzika mijn vaste KIT partner, bedankt
voor de fantastische steun bij het schrijven van dit boekje!
8.3.4. Jamie
Jamie you're a terrific and knowledgeable scientist, but somehow I associate you
with late nights, disappearing acts and lots and lots of fun? You may not be able
to keep much weed in here, but I hope you'll find some for this thesis. Thank
you for trusting me with your dog and your friendship. Will we skype?
8.4. De collega's van MCN
Door het uitblijven van gedragseffecten heb ik minder met jullie samen gewerkt
dan ik vooraf had gepland. Desondanks was voor mijn experimenten de hulp
van Sabine, Guus, Bart, Pieter en Danai en natuurlijk Danielle essentieel. Ik met
plezier met jullie samengewerkt en de kerstborrels en het tripje naar Madrid
waren erg fijn!
8.5. Mijn vrienden
Om jullie hier allemaal toe te spreken gaat te ver. Ik hoop dat jullie weten hoe
belangrijk jullie zijn in mijn dagelijks leven. Toch een paar worden voor de
mensen die wel heel direct hebben bijgedragen aan het proefschrift. Jeff
Glennon, through your intel igence and sparkling enthusiasm for neuroscience
you have thought me the exciting beauty of science. Esger, dank voor je hulp
met de layout, hoe fijn is het dat je met een hoogzwangere vrouw toch tijd kon
maken om me op het laatste moment te helpen. Martijn, dank voor je illustraties
ik ben er blij mee.
8.6. De paranimfen
Tjeerd en Niels, natuurlijk spelen jullie al jaren een belangrijke rol in mijn leven.
Als de nood het hoogst is, kan ik bij jullie altijd raad en rust vinden. Maar voor
jullie volgt natuurlijk nog een belangrijke taak. Bedankt dat ik op jullie mag
leunen op het moment suprême.
8.7. Wiskurkie
Joost: feestcielid, vriend, collega en bijna paranimf. Je draait al weer jaren mee
en hebt vrijwel al es wat ik heb geschreven gelezen en geredigeerd (want er was
nog veel meer tekst dan wat de heren de Vries, Schoffelmeer en Pattij hebben
weggestreept). Je was een fantastisch klankbord en belangrijke stimulans om te
blijven geloven in mijn data (ook al doen ratten natuurlijk maar wat in die
kooitjes van ze ;p). Ik heb veel bewondering voor je diepgaande kennis en
begrip van de neurobiologie van verslaving en impulsiviteit, ik heb er vaak van
kunnen profiteren. Ik hoop dat we ons goede (skype) contact zullen houden.
Bedankt voor alles.
8.8. Mijn bloedverwanten, aangetrouwden en hun nageslacht
Bedankt! Maar in het bijzonder Pap man, Sjoerd en Christel, Tjeerd en
Annemiek. Ik heb geboft dat ik de laatste telg ben in een bijzonder fijn gezin.
Jullie hebben mij de fantastische combinatie van genetische- en
omgevingsfactoren gegeven die ik nodig had om te komen waar ik nu ben (en
nog verder). Dank voor het geloof in mijn kunnen.
8.9. Helena
Mijn vrouw. Van alle bovengenoemden heb jij misschien nog wel het meest
geleden onder mijn strijd met dit proefschrift. Desondanks heb je me altijd
gesteund en (vaak tevergeefs) aangespoord om het boekje nu eindelijk eens af
te ronden. Nu is het dan zo ver. Ik dank je voor je liefde en je eindeloze geduld
met mij. Ik heb je lief!
Zo klaar.
9. References
A
Acheson SK, Ross EL, Swartzwelder HS (2001) Age-independent and dose-response effects of ethanol on spatial memory in rats. Alcohol 23:167-175
Adriani W, Spijker S, Deroche-Gamonet V, Laviola G, Le MM, Smit AB, Piazza PV (2003) Evidence for enhanced neurobehavioral vulnerability to nicotine during periadolescence in rats. J Neurosci 23:4712-4716
Agosti V, Nunes E, Levin F (2002) Rates of psychiatric comorbidity among U.S. residents with lifetime cannabis dependence. Am J Drug Alcohol Abuse 28:643-652
Ahmed SH (2010) Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev 35:172-184
Ahmed SH (2011) The science of making drug-addicted animals. Neuroscience
Alberini CM (2005) Mechanisms of memory stabilization: are consolidation and reconsolidation similar or distinct processes? Trends Neurosci 28:51-56
Anton RF, O'Mal ey SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Mil er WR, Pettinati HM, Randal CL, Swift R, Weiss RD, Wil iams LD, Zweben A (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized control ed trial. JAMA 295:2003-2017
Arseneault L, Cannon M, Witton J, Murray RM (2004) Causal association between cannabis and psychosis: examination of the evidence. Br J Psychiatry 184:110-117
B
Bava S, Tapert SF (2010) Adolescent brain development and the risk for alcohol and other
drug problems. Neuropsychol Rev 20:398-413
Bel RL, Rodd ZA, Lumeng L, Murphy JM, McBride WJ (2006) The alcohol-preferring P rat and animal models of excessive alcohol drinking. Addict Biol 11:270-288
Belue RC, Howlett AC, Westlake TM, Hutchings DE (1995) The ontogeny of cannabinoid receptors in the brain of postnatal and aging rats. Neurotoxicol Teratol 17:25-30
Berman DE, Dudai Y (2001) Memory extinction, learning anew, and learning the new: dissociations in the molecular machinery of learning in cortex. Science 291:2417-2419
Bernardi RE, Lattal KM, Berger SP (2006) Postretrieval propranolol disrupts a cocaine conditioned place preference. Neuroreport 17:1443-1447
Besnard A, Caboche J, Laroche S (2012) Reconsolidation of memory: A decade of debate. Prog Neurobiol
Bevilaqua LR, Bonini JS, Rossato JI, Izquierdo LA, Cammarota M, Izquierdo I (2006) The entorhinal cortex plays a role in extinction. Neurobiol Learn Mem 85:192-197
Bienkowski P, Koros E, Piasecki J, Kostowski W (1998) Prior exposure to MK-801 sensitizes rats to ethanol-induced conditioned taste aversion. Alcohol Alcohol 33:116-120
Biscaia M, Fernandez B, Higuera-Matas A, Miguens M, Viveros MP, Garcia-Lecumberri C, Ambrosio E (2008) Sex-dependent effects of periadolescent exposure to the cannabinoid agonist CP-55,940 on morphine self-administration behaviour and the endogenous opioid system. Neuropharmacology 54:863-873
Biscaia M, Marin S, Fernandez B, Marco EM, Rubio M, Guaza C, Ambrosio E, Viveros MP (2003) Chronic treatment with CP 55,940 during the peri-adolescent period differential y affects the behavioural responses of male and female rats in adulthood. Psychopharmacology (Berl) 170:301-308
Blondel A, Sanger DJ, Moser PC (2000) Characterisation of the effects of nicotine in the five-choice serial reaction time task in rats: antagonist studies. Psychopharmacology (Berl) 149:293-305
Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, Swartzwelder S, Martin C, Chung T, Tapert SF, Sher K, Winters KC, Lowman C, Murphy S (2008a) A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics 121 Suppl 4:S290-S310
Brown TE, Lee BR, Sorg BA (2008b) The NMDA antagonist MK-801 disrupts reconsolidation of a cocaine-associated memory for conditioned place preference but not for self-administration in rats. Learn Mem 15:857-865
Brunet A (2008) Effect of post-retrieval propranolol on psychophysiologic responding during subsequent script-driven traumatic imagery in post-traumatic stress disorder. J Psychiatry Res 42:503-506
Buccafusco JJ, Letchworth SR, Bencherif M, Lippiel o PM (2005) Long-lasting cognitive improvement with nicotinic receptor agonists: mechanisms of pharmacokinetic-pharmacodynamic discordance. Trends Pharmacol Sci 26:352-360
C
Cahil K, Ussher MH (2011) Cannabinoid type 1 receptor antagonists for smoking
cessation. Cochrane Database Syst RevCD005353
Caldwel LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF (2005) Gender and adolescent alcohol use disorders on BOLD (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol 40:194-200
Carli M, Robbins TW, Evenden JL, Everitt BJ (1983) Effects of lesions to ascending noradrenergic neurones on performance of a 5-choice serial reaction task in rats; implications for theories of dorsal noradrenergic bundle function based on selective attention and arousal. Behav Brain Res 9:361-380
Casey BJ, Getz S, Galvan A (2008) The adolescent brain. Dev Rev 28:62-77
Casey BJ, Tottenham N, Liston C, Durston S (2005) Imaging the developing brain: what have we learned about cognitive development? Trends Cogn Sci 9:104-110
Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder HS (2006) Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav 83:448-455
Chambers RA, Taylor JR, Potenza MN (2003) Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 160:1041-1052
Chastain (2006) Alcohol, Neursotransmitter Systems and Behavior. J of Genl Psyc 133:329-335
Chatterjee S, Steensland P, Simms JA, Holgate J, Coe JW, Hurst RS, Shaffer CL, Lowe J, Rol ema H, Bartlett SE (2010) Partial Agonists of the alpha3beta4(*) Neuronal Nicotinic Acetylcholine Receptor Reduce Ethanol Consumption and Seeking in Rats. Neuropsychopharmacology
Chaudhri N, Sahuque LL, Janak PH (2008) Context-induced relapse of conditioned behavioral responding to ethanol cues in rats. Biol Psychiatry 64:203-210
Chin VS, Van Skike CE, Matthews DB (2010) Effects of ethanol on hippocampal function during adolescence: a look at the past and thoughts on the future. Alcohol 44:3-14
Cippitel i A, Zook M, Bel L, Damadzic R, Eskay RL, Schwandt M, Heilig M (2010) Reversibility of object recognition but not spatial memory impairment fol owing binge-like alcohol exposure in rats. Neurobiol Learn Mem 94:538-546
Clemens KJ, Cail e S, Cador M (2010) The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology (Berl)
Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, Shrikhande A, Heym JH, Schaeffer E, Rol ema H, Lu Y, Mansbach RS, Chambers LK, Rovetti CC, Schulz DW, Tingley FD, III, O'Neil BT (2005) Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem 48:3474-3477
Cole BJ, Robbins TW (1987) Amphetamine impairs the discriminative performance of rats with dorsal noradrenergic bundle lesions on a 5-choice serial reaction time task: new evidence for central dopaminergic-noradrenergic interactions. Psychopharmacology (Berl) 91:458-466
Col ins AC, Burch JB, de Fiebre CM, Marks MJ (1988) Tolerance to and cross tolerance between ethanol and nicotine. Pharmacol Biochem Behav 29:365-373
Col ins SL, Montano R, Izenwasser S (2004) Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Brain Res Dev Brain Res 153:175-187
Conger JJ (1956) Alcoholism: theory, problem and chal enge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol 17:296-305
Counotte DS (2010) The neurobiology of adolescent nicotine exposure. PhD Thesis
Counotte DS, Goriounova NA, Li KW, Loos M, van der Schors RC, Schetters D, Schoffelmeer AN, Smit AB, Mansvelder HD, Pattij T, Spijker S (2011a) Lasting synaptic changes underlie attention deficits caused by nicotine exposure during adolescence. Nat Neurosci 14:417-419
Counotte DS, Smit AB, Pattij T, Spijker S (2011b) Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev Cogn Neurosci 1:430-443
Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer AN, De Vries TJ, Smit AB, Pattij T (2008) Long-Lasting Cognitive Deficits Resulting from Adolescent Nicotine Exposure in Rats. Neuropsychopharmacology
Crabbe JC, Harris RA, Koob GF (2011) Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci 1216:24-40
Crews F, He J, Hodge C (2007) Adolescent cortical development: a critical period of vulnerability for addiction. Pharmacol Biochem Behav 86:189-199
Crews FT, Boettiger CA (2009) Impulsivity, frontal lobes and risk for addiction. Pharmacol Biochem Behav 93:237-247
Crews FT, Braun CJ, Hoplight B, Switzer RC, III, Knapp DJ (2000) Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res 24:1712-1723
Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K (2006) Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience 137:437-445
Dal ey JW, Mar AC, Economidou D, Robbins TW (2008) Neurobehavioral mechanisms of impulsivity: fronto-striatal systems and functional neurochemistry. Pharmacol Biochem Behav 90:250-260
Dar MS, Bowman ER, Li C (1994) Intracerebel ar nicotinic-cholinergic participation in the cerebel ar adenosinergic modulation of ethanol-induced motor incoordination in mice. Brain Res 644:117-127
Davis TJ, de Fiebre CM (2006) Alcohol's actions on neuronal nicotinic acetylcholine receptors. Alcohol Res Health 29:179-185
Day M, Pan JB, Buckley MJ, Cronin E, Hol ingsworth PR, Hirst WD, Navarra R, Sullivan JP, Decker MW, Fox GB (2007) Differential effects of ciproxifan and nicotine on impulsivity and attention measures in the 5-choice serial reaction time test. Biochem Pharmacol 73:1123-1134
De Bel is MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hal J, Kersh A, Keshavan MS (2000) Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry 157:737-744
De Bel is MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB (2005) Prefrontal cortex, thalamus, and cerebel ar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol Clin Exp Res 29:1590-1600
De Vries TJ, Schoffelmeer AN (2005) Cannabinoid CB1 receptors control conditioned drug seeking. Trends Pharmacol Sci 26:420-426
De Vries TJ, Schoffelmeer AN, Binnekade R, Vanderschuren LJ (1999) Dopaminergic mechanisms mediating the incentive to seek cocaine and heroin fol owing long-term withdrawal of IV drug self-administration. Psychopharmacology (Berl) 143:254-260
De Vries TJ, Homberg JR, Binnekade R, Raaso H, Schoffelmeer AN (2003) Cannabinoid modulation of the reinforcing and motivational properties of heroin and heroin-associated cues in rats. Psychopharmacology (Berl) 168:164-169
De Vries TJ, Shaham Y, Homberg JR, Crombag H, Schuurman K, Dieben J, Vanderschuren LJ, Schoffelmeer AN (2001) A cannabinoid mechanism in relapse to cocaine seeking. Nat Med 7:1151-1154
Debiec J, LeDoux JE (2004) Disruption of reconsolidation but not consolidation of auditory fear conditioning by noradrenergic blockade in the amygdala. Neuroscience 129:267-272
Dere E, Huston JP, De Souza Silva MA (2007) The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev 31:673-704
DeWit DJ, Adlaf EM, Offord DR, Ogborne AC (2000) Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry 157:745-750
Di Chiara G, Imperato A (1988a) Drugs abused by humans preferential y increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci U S A 85:5274-5278
Diamond I, Gordon AS (1997) Cel ular and molecular neuroscience of alcoholism. Physiol Rev 77:1-20
Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de VW, Schoffelmeer AN, De Vries TJ (2008a) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63:301-308
Diergaarde L, Schoffelmeer AN, De Vries TJ (2006) Beta-adrenoceptor mediated inhibition of long-term reward-related memory reconsolidation. Behav Brain Res 170:333-336
Diergaarde L, Schoffelmeer AN, De Vries TJ (2008b) Pharmacological manipulation of memory reconsolidation: towards a novel treatment of pathogenic memories. Eur J Pharmacol 585:453-457
Duka T, Gentry J, Malcolm R, Ripley TL, Borlikova G, Stephens DN, Veatch LM, Becker HC, Crews FT (2004) Consequences of multiple withdrawals from alcohol. Alcohol Clin Exp Res 28:233-246
Ehrenreich H, Rinn T, Kunert HJ, Moel er MR, Poser W, Schil ing L, Gigerenzer G, Hoehe MR (1999) Specific attentional dysfunction in adults fol owing early start of cannabis use. Psychopharmacology (Berl) 142:295-301
El gren M, Spano SM, Hurd YL (2007) Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32:607-615
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1-16
Ericson M, Blomqvist O, Engel JA, Soderpalm B (1998) Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol 358:189-196
Ericson M, Engel JA, Soderpalm B (2000) Peripheral involvement in nicotine-induced enhancement of ethanol intake. Alcohol 21:37-47
Ericson M, Lof E, Stomberg R, Soderpalm B (2009) The smoking cessation medication varenicline attenuates alcohol and nicotine interactions in the rat mesolimbic dopamine system. J Pharmacol Exp Ther 329:225-230
Everitt BJ, Dickinson A, Robbins TW (2001) The neuropsychological basis of addictive behaviour. Brain Res Brain Res Rev 36:129-138
Evrard SG, Duhalde-Vega M, Tagliaferro P, Mirochnic S, Caltana LR, Brusco A (2006) A low chronic ethanol exposure induces morphological changes in the adolescent rat brain that
are not fully recovered even after a long abstinence: an immunohistochemical study. Exp Neurol 200:438-459
Fadda F, Rossetti ZL (1998) Chronic ethanol consumption: from neuroadaptation to neurodegeneration. Prog Neurobiol 56:385-431
Ford MM, Fretwel AM, Nickel JD, Mark GP, Strong MN, Yoneyama N, Finn DA (2009) The influence of mecamylamine on ethanol and sucrose self-administration. Neuropharmacology 57:250-258
Foulds J (2006) The neurobiological basis for partial agonist treatment of nicotine dependence: varenicline. Int J Clin Pract 60:571-576
Fricks-Gleason AN, Marshal JF (2008) Post-retrieval beta-adrenergic receptor blockade: effects on extinction and reconsolidation of cocaine-cue memories. Learn Mem 15:643-648
Fried PA, Watkinson B, Gray R (2005) Neurocognitive consequences of marihuana--a comparison with pre-drug performance. Neurotoxicol Teratol 27:231-239
Frishman WH (2009) Smoking cessation pharmacotherapy. Ther Adv Cardiovasc Dis 3:287-308
Garcia-Burgos D, Gonzalez F, Manrique T, Gal o M (2009) Patterns of ethanol intake in preadolescent, adolescent, and adult Wistar rats under acquisition, maintenance, and relapse-like conditions. Alcohol Clin Exp Res 33:722-728
Garcia-Moreno LM, Conejo NM, Capil a A, Garcia-Sanchez O, Senderek K, Arias JL (2002) Chronic ethanol intake and object recognition in young and adult rats. Prog Neuropsychopharmacol Biol Psychiatry 26:831-837
Gass JT, Olive MF (2008) Positive Al osteric Modulation of mGluR5 Receptors Facilitates Extinction of a Cocaine Contextual Memory. Biol Psychiatry
Golden GJ, Houpt TA (2007) NMDA receptor in conditioned flavor-taste preference learning: blockade by MK-801 and enhancement by D-cycloserine. Pharmacol Biochem Behav 86:587-596
Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Bil ing CB, Watsky EJ, Gong J, Wil iams KE, Reeves KR (2006) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized control ed trial. JAMA 296:47-55
Grant JD, Scherrer JF, Lynskey MT, Lyons MJ, Eisen SA, Tsuang MT, True WR, Bucholz KK (2006) Adolescent alcohol use is a risk factor for adult alcohol and drug dependence: evidence from a twin design. Psychol Med 36:109-118
Guerri C, Pascual M (2010) Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 44:15-26
Gulick D, Gould TJ (2008) Varenicline ameliorates ethanol-induced deficits in learning in C57BL/6 mice. Neurobiol Learn Mem 90:230-236
Hamelink C, Hampson A, Wink DA, Eiden LE, Eskay RL (2005) Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J Pharmacol Exp Ther 314:780-788
Hansen HH, Krutz B, Sifringer M, Stefovska V, Bittigau P, Pragst F, Marsicano G, Lutz B, Ikonomidou C (2008) Cannabinoids enhance susceptibility of immature brain to ethanol neurotoxicity. Ann Neurol 64:42-52
He J, Nixon K, Shetty AK, Crews FT (2005) Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. Eur J Neurosci 21:2711-2720
Hendrickson LM, Zhao-Shea R, Tapper AR (2009) Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6J mice. Psychopharmacology (Berl) 204:563-572
Hibel B, Guttormsson U, Ahlström S, Balakireva O, Bjarnason T, Kokkevi A, Kraus L (2012) The 2011 ESPAD Report.
Hodges H (1996) Maze procedures: the radial-arm and water maze compared. Brain Res Cogn Brain Res 3:167-181
Hoffman PL, Tabakoff B (1996) Alcohol dependence: a commentary on mechanisms. Alcohol Alcohol 31:333-340
Hsu E, Packard MG (2008) Medial prefrontal cortex infusions of bupivacaine or AP-5 block extinction of amphetamine conditioned place preference. Neurobiol Learn Mem 89:504-512
Hyman SE, Malenka RC, Nestler EJ (2006) Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci 29:565-598
Ikemoto S (2010) Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neurosci Biobehav Rev 35:129-150
Itzhak Y (2008) Role of the NMDA receptor and nitric oxide in memory reconsolidation of cocaine-induced conditioned place preference in mice. Ann N Y Acad Sci 1139:350-357
Itzhak Y, Anderson KL (2007) Memory reconsolidation of cocaine-associated context requires nitric oxide signaling. Synapse 61:1002-1005
Jacobs EH, Smit AB, De Vries TJ, Schoffelmeer AN (2003) Neuroadaptive effects of active versus passive drug administration in addiction research. Trends Pharmacol Sci 24:566-573
Jacobsen LK, Krystal JH, Mencl WE, Westerveld M, Frost SJ, Pugh KR (2005) Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biol Psychiatry 57:56-66
Jager G, Block RI, Luijten M, Ramsey NF (2010) Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J Am Acad Child Adolesc Psychiatry 49:561-72, 572
Jager G, Kahn RS, van den Brink W, van Ree JM, Ramsey NF (2006) Long-term effects of frequent cannabis use on working memory and attention: an fMRI study. Psychopharmacology (Berl) 185:358-368
Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA (2006) Role of the subunit composition of central nicotinic acetylcholine receptors for the stimulatory and dopamine-enhancing effects of ethanol. Alcohol Alcohol 41:486-493
Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Wil iams KE, Bil ing CB, Gong J, Reeves KR (2006) Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized control ed trial. JAMA 296:56-63
Kalivas PW, McFarland K (2003) Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 168:44-56
Kalivas PW, Peters J, Knackstedt L (2006) Animal models and brain circuits in drug addiction. Mol Interv 6:339-344
Kauer JA, Malenka RC (2007) Synaptic plasticity and addiction. Nature Reviews Neuroscience 8, 844-858
Keeler JF, Robbins TW (2011) Translating cognition from animals to humans. Biochem Pharmacol 81:1356-1366
Kel ey AE (2004) Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev 27:765-776
Kel ey JB, Anderson KL, Itzhak Y (2007) Long-term memory of cocaine-associated context: disruption and reinstatement. Neuroreport 18:777-780
Kindt M, Soeter M, Vervliet B (2009) Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci 12:256-258
Klemm WR (1998) Biological water and its role in the effects of alcohol. Alcohol 15:249-267
Krishnan-Sarin S, Reynolds B, Duhig AM, Smith A, Liss T, McFetridge A, Caval o DA, Carrol KM, Potenza MN (2007) Behavioral impulsivity predicts treatment outcome in a smoking cessation program for adolescent smokers. Drug Alcohol Depend 88:79-82
Larsson A, Jerlhag E, Svensson L, Soderpalm B, Engel JA (2004) Is an alpha-conotoxin MII-sensitive mechanism involved in the neurochemical, stimulatory, and rewarding effects of ethanol? Alcohol 34:239-250
Laviolette SR, van der Kooy D (2004) The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nat Rev Neurosci 5:55-65
Le A, Shaham Y (2002) Neurobiology of relapse to alcohol in rats. Pharmacol Ther 94:137-156
Lee JL, Di CP, Thomas KL, Everitt BJ (2005) Disrupting reconsolidation of drug memories reduces cocaine-seeking behavior. Neuron 47:795-801
Lee JL, Everitt BJ (2008a) Appetitive memory reconsolidation depends upon NMDA receptor-mediated neurotransmission. Neurobiol Learn Mem 90:147-154
Lee JL, Everitt BJ (2008b) Reactivation-dependent amnesia in Pavlovian approach and instrumental transfer. Learn Mem 15:597-602
Lee JL, Milton AL, Everitt BJ (2006) Cue-induced cocaine seeking and relapse are reduced by disruption of drug memory reconsolidation. J Neurosci 26:5881-5887
Lee JL, Milton AL, Everitt BJ (2006b) Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci 26:10051-10056
Lesscher HM, Kas MJ, van der ES, van Lith HA, Vanderschuren LJ (2009) A grandparent-influenced locus for alcohol preference on mouse chromosome 2. Pharmacogenet Genomics 19:719-729
Levin ED, McClernon FJ, Rezvani AH (2006) Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology (Berl) 184:523-539
Li TK (2008) Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J Gastroenterol Hepatol 23 Suppl 1:S2-S8
Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS (1996) Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res 20:1346-1351
Lof E, Olausson P, deBejczy A, Stomberg R, McIntosh JM, Taylor JR, Soderpalm B (2007) Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berl) 195:333-343
Lyketsos CG, Garrett E, Liang KY, Anthony JC (1999) Cannabis use and cognitive decline in persons under 65 years of age. Am J Epidemiol 149:794-800
Madden PA, Heath AC (2002) Shared genetic vulnerability in alcohol and cigarette use and dependence. Alcohol Clin Exp Res 26:1919-1921
Maggs JL, Patrick ME, Feinstein L (2008) Childhood and adolescent predictors of alcohol use and problems in adolescence and adulthood in the National Child Development Study. Addiction 103 Suppl 1:7-22
Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL (2010a) Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacol Biochem Behav 96:476-487
Maldonado-Devincci AM, Badanich KA, Kirstein CL (2010b) Alcohol during adolescence selectively alters immediate and long-term behavior and neurochemistry. Alcohol 44:57-66
Malone DT, Hil MN, Rubino T (2010) Adolescent cannabis use and psychosis: epidemiology and neurodevelopmental models. Br J Pharmacol 160:511-522
Mansvelder HD, McGehee DS (2002) Cel ular and synaptic mechanisms of nicotine addiction. J Neurobiol 53:606-617
Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS (1998) Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res 22:416-421
Marshal DL, Redfern PH, Wonnacott S (1997) Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem 68:1511-1519
Mason BJ, Heyser CJ (2010) Acamprosate: a prototypic neuromodulator in the treatment of alcohol dependence. CNS Neurol Disord Drug Targets 9:23-32
Mathers M, Toumbourou JW, Catalano RF, Wil iams J, Patton GC (2006) Consequences of youth tobacco use: a review of prospective behavioural studies. Addiction 101:948-958
Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI (1990) Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346:561-564
Matthews DB, Silvers JR (2004) The use of acute ethanol administration as a tool to investigate multiple memory systems. Neurobiol Learn Mem 82:299-308
McGue M, Iacono WG, Legrand LN, Elkins I (2001a) Origins and consequences of age at first drink. II. Familial risk and heritability. Alcohol Clin Exp Res 25:1166-1173
McGue M, Iacono WG, Legrand LN, Malone S, Elkins I (2001b) Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res 25:1156-1165
McGue M, Pickens RW, Svikis DS (1992) Sex and age effects on the inheritance of alcohol problems: a twin study. J Abnorm Psychol 101:3-17
McKee SA, Harrison EL, O'Mal ey SS, Krishnan-Sarin S, Shi J, Tetrault JM, Picciotto MR, Petrakis IL, Estevez N, Balchunas E (2009) Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol Psychiatry 66:185-190
McLaughlin CR, Martin BR, Compton DR, Abood ME (1994) Cannabinoid receptors in developing rats: detection of mRNA and receptor binding. Drug Alcohol Depend 36:27-31
McMil an DE, Li M, Shide DJ (1999) Differences between alcohol-preferring and alcohol-nonpreferring rats in ethanol generalization. Pharmacol Biochem Behav 64:415-419
McQuown SC, Bel uzzi JD, Leslie FM (2007) Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicol Teratol 29:66-73
Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF (2007) Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol 29:141-152
Meyerhoff DJ, Tizabi Y, Staley JK, Durazzo TC, Glass JM, Nixon SJ (2006) Smoking comorbidity in alcoholism: neurobiological and neurocognitive consequences. Alcohol Clin Exp Res 30:253-264
Mihalak KB, Carrol FI, Luetje CW (2006) Varenicline is a partial agonist at alpha4beta2 and a full agonist at alpha7 neuronal nicotinic receptors. Mol Pharmacol 70:801-805
Mil er CA, Marshal JF (2005) Molecular substrates for retrieval and reconsolidation of cocaine-associated contextual memory. Neuron 47:873-884
Milton AL, Everitt BJ (2010) The psychological and neurochemical mechanisms of drug memory reconsolidation: implications for the treatment of addiction. Eur J Neurosci 31:2308-2319
Milton AL, Lee JL, Butler VJ, Gardner R, Everitt BJ (2008a) Intra-amygdala and systemic antagonism of NMDA receptors prevents the reconsolidation of drug-associated memory and impairs subsequently both novel and previously acquired drug-seeking behaviors. J Neurosci 28:8230-8237
Milton AL, Lee JL, Everitt BJ (2008b) Reconsolidation of appetitive memories for both natural and drug reinforcement is dependent on {beta}-adrenergic receptors. Learn Mem 15:88-92
Mirza NR, Stolerman IP (1998) Nicotine enhances sustained attention in the rat under specific task conditions. Psychopharmacology (Berl) 138:266-274
Misanin JR, Mil er RR, Lewis DJ (1968) Retrograde amnesia produced by electroconvulsive shock after reactivation of a consolidated memory trace. Science 160:554-555
Monfils MH, Cowansage KK, Klann E, LeDoux JE (2009) Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science 324:951-955
Morris RG, Inglis J, Ainge JA, Olverman HJ, Tulloch J, Dudai Y, Kel y PA (2006) Memory reconsolidation: sensitivity of spatial memory to inhibition of protein synthesis in dorsal hippocampus during encoding and retrieval. Neuron 50:479-489
Morris SA, Eaves DW, Smith AR, Nixon K (2010) Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus 20:596-607
Müller GE, Pilzecker A (1900) Experimentel e beitrage zur lehre vom gedachtnis. Z Psychol1-288
Nadal R, Chappel AM, Samson HH (1998) Effects of nicotine and mecamylamine microinjections into the nucleus accumbens on ethanol and sucrose self-administration. Alcohol Clin Exp Res 22:1190-1198
Nader K, Hardt O (2009) A single standard for memory: the case for reconsolidation. Nat Rev Neurosci 10:224-234
Nader K, Schafe GE, Le Doux JE (2000) Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature 406:722-726
Nader K, Einarsson EO (2010) Memory reconsolidation: an update. Ann N Y Acad Sci 1191:27-41
Neugebauer NM, Zhang Z, Crooks PA, Dwoskin LP, Bardo MT (2006) Effect of a novel nicotinic receptor antagonist, N,N'-dodecane-1,12-diyl-bis-3-picolinium dibromide, on nicotine self-administration and hyperactivity in rats. Psychopharmacology (Berl) 184:426-434
NIAAA (2004) National Institute on Alcohol Abuse and Alcoholism Newsletter, Winter 2004, Issue 3.
Niaura R, Jones C, Kirkpatrick P (2006) Varenicline. Nat Rev Drug Discov 5:537-538
Nisel M, Nomikos GG, Svensson TH (1994) Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 16:36-44
Nixon K, Crews FT (2002) Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem 83:1087-1093
Nixon K, McClain JA (2010) Adolescence as a critical window for developing an alcohol use disorder: current findings in neuroscience. Curr Opin Psychiatry
Nutt DJ, King LA, Phil ips LD (2010) Drug harms in the UK: a multicriteria decision analysis. Lancet 376:1558-1565
O'Brien CP, Childress AR, Ehrman R, Robbins SJ (1998) Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol 12:15-22
O'Connor EC, Chapman K, Butler P, Mead AN (2011) The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev 35:912-938
O'Connor EC, Parker D, Rol ema H, Mead AN (2010) The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration fol owing repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berl) 208:365-376
O'Del LE, Roberts AJ, Smith RT, Koob GF (2004) Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res 28:1676-1682
O'Shea M, McGregor IS, Mal et PE (2006) Repeated cannabinoid exposure during perinatal, adolescent or early adult ages produces similar longlasting deficits in object recognition and reduced social interaction in rats. J Psychopharmacol 20:611-621
O'Shea M, Singh ME, McGregor IS, Mal et PE (2004) Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol 18:502-508
Olton DS (1987) The radial arm maze as a tool in behavioral pharmacology. Physiol Behav 40:793-797
Oslin DW, Berrettini WH, O'Brien CP (2006) Targeting treatments for alcohol dependence: the pharmacogenetics of naltrexone. Addict Biol 11:397-403
Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C (2007) Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. Eur J Neurosci 25:541-550
Pascual M, Boix J, Felipo V, Guerri C (2009) Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem 108:920-931
Patrick, Charles H. Alcohol, Culture, and Society. Durham, NC: Duke University Press, 1952. Reprint edition by AMS Press, New York, 1970.
Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, Frey JM, Siegel S, Lerman C (2009) Varenicline improves mood and cognition during smoking abstinence. Biol Psychiatry 65:144-149
Pattij T, Janssen MC, Vanderschuren LJ, Schoffelmeer AN, van Gaalen MM (2007) Involvement of dopamine D1 and D2 receptors in the nucleus accumbens core and shel in inhibitory response control. Psychopharmacology (Berl) 191:587-598
Pattij T, Vanderschuren LJ (2008) The neuropharmacology of impulsive behaviour. Trends Pharmacol Sci 29:192-199
Pattij T, De Vries TJ (2013) The role of impulsivity in relapse vulnerability. Current Opinion in Neurobiology: 23 epub ahead of print
Paul CM, Magda G, Abel S (2009) Spatial memory: Theoretical basis and comparative review on experimental methods in rodents. Behav Brain Res 203:151-164
Paus T, Keshavan M, Giedd JN (2008) Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci 9:947-957
Pautassi RM, Camarini R, Quadros IM, Miczek KA, Israel Y (2010) Genetic and environmental influences on ethanol consumption: perspectives from preclinical research. Alcohol Clin Exp Res 34:976-987
Pedreira ME, Perez-Cuesta LM, Maldonado H (2004) Mismatch between what is expected and what actual y occurs triggers memory reconsolidation or extinction. Learn Mem 11:579-585
Philpot RM, Wecker L, Kirstein CL (2009) Repeated ethanol exposure during adolescence alters the developmental trajectory of dopaminergic output from the nucleus accumbens septi. Int J Dev Neurosci 27:805-815
Pistis M, Perra S, Pil ol a G, Melis M, Muntoni AL, Gessa GL (2004) Adolescent exposure to cannabinoids induces long-lasting changes in the response to drugs of abuse of rat midbrain dopamine neurons. Biol Psychiatry 56:86-94
Pope HG, Jr., Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D (2003) Early-onset cannabis use and cognitive deficits: what is the nature of the association? Drug Alcohol Depend 69:303-310
Pope HG, Jr., Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D (2001a) Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry 58:909-915
Pope HG, Jr., Gruber AJ, Yurgelun-Todd D (2001b) Residual neuropsychologic effects of cannabis. Curr Psychiatry Rep 3:507-512
Popik P, Wrobel M, Bisaga A (2006) Reinstatement of morphine-conditioned reward is blocked by memantine. Neuropsychopharmacology 31:160-170
Przybyslawski J, Roullet P, Sara SJ (1999) Attenuation of emotional and nonemotional memories after their reactivation: role of beta adrenergic receptors. J Neurosci 19:6623-6628
Przybyslawski J, Sara SJ (1997) Reconsolidation of memory after its reactivation. Behav Brain Res 84:241-246
Pyapali GK, Turner DA, Wilson WA, Swartzwelder HS (1999) Age and dose-dependent effects of ethanol on the induction of hippocampal long-term potentiation. Alcohol 19:107-111
Quinn HR, Matsumoto I, Cal aghan PD, Long LE, Arnold JC, Gunasekaran N, Thompson MR, Dawson B, Mal et PE, Kashem MA, Matsuda-Matsumoto H, Iwazaki T, McGregor IS (2008) Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression fol owing exposure. Neuropsychopharmacology 33:1113-1126
Rajendran P, Spear LP (2004) The effects of ethanol on spatial and nonspatial memory in adolescent and adult rats studied using an appetitive paradigm. Ann N Y Acad Sci 1021:441-444
Rezvani AH, Levin ED (2001) Cognitive effects of nicotine. Biol Psychiatry 49:258-267
Ribeiro-Carvalho A, Lima CS, Filgueiras CC, Manhaes AC, Abreu-Vil aca Y (2008) Nicotine and ethanol interact during adolescence: effects on the central cholinergic systems. Brain Res 1232:48-60
RIVM: Rijksinstituut voor Volksgezondheid en Milieu. van Amsterdam JCG, Opperhuizen A, Koeter MWJ, van Aerts LAGJM, van den Brink W (2009) Ranking van Drugs. Een vergelijking van de schadelijkheid van drugs.
Robbins TW (2002) The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 163:362-380
Robbins TW, Ersche KD, Everitt BJ (2008) Drug addiction and the memory systems of the brain. Ann N Y Acad Sci 1141:1-21
Robinson MJ, Franklin KB (2007) Central but not peripheral beta-adrenergic antagonism blocks reconsolidation for a morphine place preference. Behav Brain Res 182:129-134
Rodd-Henricks ZA, Bel RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK (2002) Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res 26:1632-1641
Rol ema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, Sands SB, Schaeffer E, Schulz DW, Tingley FD, III, Wil iams KE (2007a) Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52:985-994
Rol ema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Wil iams KE (2007b) Rationale, pharmacology and clinical efficacy of partial agonists of alpha4beta2 nACh receptors for smoking cessation. Trends Pharmacol Sci 28:316-325
Rol ema H, Hajos M, Seymour PA, Kozak R, Majchrzak MJ, Guanowsky V, Horner WE, Chapin DS, Hoffmann WE, Johnson DE, McLean S, Freeman J, Wil iams KE (2009) Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochem Pharmacol 78:813-824
Rosenblueth A, Wiener N (1945) The Role of Models in Science. Philosophy of Science 12:316-321
Rubino T, Parolaro D (2008) Long lasting consequences of cannabis exposure in adolescence. Mol Cel Endocrinol 286:S108-S113
Rubino T, Vigano' D, Realini N, Guidali C, Braida D, Capurro V, Castiglioni C, Cherubino F, Romualdi P, Candeletti S, Sala M, Parolaro D (2008) Chronic delta 9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33:2760-2771
Sadler R, Herzig V, Schmidt WJ (2007) Repeated treatment with the NMDA antagonist MK-801 disrupts reconsolidation of memory for amphetamine-conditioned place preference. Behav Pharmacol 18:699-703
Samson HH, Tol iver GA, Haraguchi M, Hodge CW (1992) Alcohol self-administration: role of mesolimbic dopamine. Ann N Y Acad Sci 654:242-253
Sara SJ (2000) Retrieval and reconsolidation: toward a neurobiology of remembering. Learn Mem 7:73-84
Sara SJ, Dyon-Laurent C, Herve A (1995) Novelty seeking behavior in the rat is dependent upon the integrity of the noradrenergic system. Brain Res Cogn Brain Res 2:181-187
Scheurich A (2005) Neuropsychological functioning and alcohol dependence. Curr Opin Psychiatry 18:319-323
Schlaepfer IR, Hoft NR, Col ins AC, Corley RP, Hewitt JK, Hopfer CJ, Lessem JM, McQueen MB, Rhee SH, Ehringer MA (2008) The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol Psychiatry 63:1039-1046
Schneider M (2008) Puberty as a highly vulnerable developmental period for the consequences of cannabis exposure. Addict Biol 13:253-263
Schneider M, Drews E, Koch M (2005) Behavioral effects in adult rats of chronic prepubertal treatment with the cannabinoid receptor agonist WIN 55,212-2. Behav Pharmacol 16:447-454
Schneider M, Koch M (2003) Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 28:1760-1769
Schneider M, Koch M (2005) Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology 30:944-957
Schneider M, Koch M (2007) The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. Eur Neuropsychopharmacol 17:180-186
Schulteis G, Archer C, Tapert SF, Frank LR (2008) Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol 42:459-467
Seamans JK, Floresco SB, Phil ips AG (1995) Functional differences between the prelimbic and anterior cingulate regions of the rat prefrontal cortex. Behav Neurosci 109:1063-1073
Seamans JK, Phil ips AG (1994) Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci 108:456-468
Shaham Y, Shalev U, Lu L, De WH, Stewart J (2003) The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 168:3-20
Shippenberg TS, Koob GF (2000) Recent Advances in Animal Models of Drug Addiction. Neuropsychopharmacology: The Fifth Generation of Progress. pp 1381-1392
Shram MJ, Funk D, Li Z, Le AD (2006) Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacology (Berl) 186:201-208
Siciliano D, Smith RF (2001) Periadolescent alcohol alters adult behavioral characteristics in the rat. Physiol Behav 74:637-643
Siegmund S, Vengeliene V, Singer MV, Spanagel R (2005) Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res 29:1139-1145
Silvers JM, Tokunaga S, Berry RB, White AM, Matthews DB (2003) Impairments in spatial learning and memory: ethanol, al opregnanolone, and the hippocampus. Brain Res Brain Res Rev 43:275-284
Simpson H (1997) Homer vs. the Eighteenth Amendment.
Sircar R, Sircar D (2005) Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res 29:1402-1410
Slawecki CJ (2002) Altered EEG responses to ethanol in adult rats exposed to ethanol during adolescence. Alcohol Clin Exp Res 26:246-254
Slawecki CJ (2006) Two-choice reaction time performance in Sprague-Dawley rats exposed to alcohol during adolescence or adulthood. Behav Pharmacol 17:605-614
Slawecki CJ, Betancourt M (2002) Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol 26:23-30
Slawecki CJ, Betancourt M, Cole M, Ehlers CL (2001) Periadolescent alcohol exposure has lasting effects on adult neurophysiological function in rats. Brain Res Dev Brain Res 128:63-72
Slawecki CJ, Gilder A, Roth J, Ehlers CL (2003) Increased anxiety-like behavior in adult rats exposed to nicotine as adolescents. Pharmacol Biochem Behav 75:355-361
Smith LN, McDonald CG, Bergstrom HC, Brielmaier JM, Eppolito AK, Wheeler TL, Falco AM, Smith RF (2006) Long-term changes in fear conditioning and anxiety-like behavior fol owing nicotine exposure in adult versus adolescent rats. Pharmacol Biochem Behav 85:91-97
Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA (2000) Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res 113:85-96
Soderpalm B, Lof E, Ericson M (2009) Mechanistic studies of ethanol's interaction with the mesolimbic dopamine reward system. Pharmacopsychiatry 42 Suppl 1:S87-S94
Sofuoglu M (2010) Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction 105:38-48
Sorg BA (2012) Reconsolidation of drug memories. Neurosci Biobehav Rev 36:1400-1417
Spear LP (2000) The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417-463
Spear LP (2010) The Behavioral Neuroscience of Adolescence
Spear LP, Varlinskaya EI (2010) Sensitivity to ethanol and other hedonic stimuli in an animal model of adolescence: implications for prevention science? Dev Psychobiol 52:236-243
Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE (2007) Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A 104:12518-12523
Sundram S (2006) Cannabis and neurodevelopment: implications for psychiatric disorders. Hum Psychopharmacol 21:245-254
Suzuki A, Josselyn SA, Frankland PW, Masushige S, Silva AJ, Kida S (2004) Memory reconsolidation and extinction have distinct temporal and biochemical signatures. J Neurosci 24:4787-4795
Swartzwelder HS, Richardson RC, Markwiese-Foerch B, Wilson WA, Little PJ (1998) Developmental differences in the acquisition of tolerance to ethanol. Alcohol 15:311-314
Swartzwelder HS, Wilson WA, Tayyeb MI (1995) Age-dependent inhibition of long-term potentiation by ethanol in immature versus mature hippocampus. Alcohol Clin Exp Res 19:1480-1485
Tambour S, Quertemont E (2007) Preclinical and clinical pharmacology of alcohol dependence. Fundam Clin Pharmacol 21:9-28
Tapert SF, Baratta MV, Abrantes AM, Brown SA (2002) Attention dysfunction predicts substance involvement in community youths. J Am Acad Child Adolesc Psychiatry 41:680-686
Tapert SF, Brown SA (1999) Neuropsychological correlates of adolescent substance abuse: four-year outcomes. J Int Neuropsychol Soc 5:481-493
Tapert SF, Caldwel L, Burke C (2004a) Alcohol and the adolescent brain - Human studies. Alcohol Research & Health 28:205-212
Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ (2004b) Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res 28:1577-1586
Tizabi Y, Bai L, Copeland RL, Jr., Taylor RE (2007) Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shel . Alcohol Alcohol 42:413-416
Tonstad S (2006) Smoking cessation efficacy and safety of varenicline, an alpha4beta2 nicotinic receptor partial agonist. J Cardiovasc Nurs 21:433-436
Tonstad S, Tonnesen P, Hajek P, Wil iams KE, Bil ing CB, Reeves KR (2006) Effect of maintenance therapy with varenicline on smoking cessation: a randomized control ed trial. JAMA 296:64-71
Toumbourou JW, Stockwel T, Neighbors C, Marlatt GA, Sturge J, Rehm J (2007) Interventions to reduce harm associated with adolescent substance use. Lancet 369:1391-1401
Tronel S, Sara SJ (2002) Mapping of olfactory memory circuits: region-specific c-fos activation after odor-reward associative learning or after its retrieval. Learn Mem 9:105-111
Tronson NC, Taylor JR (2007) Molecular mechanisms of memory reconsolidation. Nat Rev Neurosci 8:262-275
Valjent E, Corbil e AG, Bertran-Gonzalez J, Herve D, Girault JA (2006) Inhibition of ERK pathway or protein synthesis during reexposure to drugs of abuse erases previously learned place preference. Proc Natl Acad Sci U S A 103:2932-2937
van der Kooy D, O'Shaughnessy M, Mucha RF, Kalant H (1983) Motivational properties of ethanol in naive rats as studied by place conditioning. Pharmacol Biochem Behav 19:441-445
van der Lely N, van Dalen W, Pereira RR, van Hoof J (2011) Alcoholintoxicaties bij jongeren in Nederland: Een onderzoek bij kinderafdelingen in Nederlandse ziekenhuizen.
van Gaalen MM, Brueggeman RJ, Bronius PF, Schoffelmeer AN, Vanderschuren LJ (2006) Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology (Berl) 187:73-85
van Laar MW, Cruts AAN, van Ooyen-Houben MMJ, Meijer RF, Brunt T (2010) Nationale Drug Monitor, Jaarbericht 2009. Trimbos-instituut,
Varlinskaya EI, Spear LP (2006) Differences in the social consequences of ethanol emerge during the course of adolescence in rats: social facilitation, social inhibition, and anxiolysis. Dev Psychobiol 48:146-161
Varlinskaya EI, Spear LP, Spear NE (2001) Acute effects of ethanol on behavior of adolescent rats: role of social context. Alcohol Clin Exp Res 25:377-385
Vastola BJ, Douglas LA, Varlinskaya EI, Spear LP (2002) Nicotine-induced conditioned place preference in adolescent and adult rats. Physiol Behav 77:107-114
Vendruscolo LF, Gueye AB, Darnaudery M, Ahmed SH, Cador M (2010a) Sugar overconsumption during adolescence selectively alters motivation and reward function in adult rats. PLoS One 5:e9296
Vendruscolo LF, Gueye AB, Vendruscolo JC, Clemens KJ, Mormede P, Darnaudery M, Cador M (2010b) Reduced alcohol drinking in adult rats exposed to sucrose during adolescence. Neuropharmacology 59:388-394
Vengeliene V, Kiefer F, Spanagel R (2008) D-cycloserine facilitates extinction of conditioned alcohol-seeking behaviour in rats. Alcohol Alcohol 43:626-629
Verdejo-Garcia A, Lawrence AJ, Clark L (2008) Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev 32:777-810
Vetter CS, Doremus-Fitzwater TL, Spear LP (2007) Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res 31:1159-1168
von der Goltz C, Vengeliene V, Bilbao A, Perreau-Lenz S, Pawlak CR, Kiefer F, Spanagel R (2009a) Cue-induced alcohol-seeking behaviour is reduced by disrupting the reconsolidation of alcohol-related memories. Psychopharmacology (Berl) 205:389-397
Wang XY, Zhao M, Ghitza UE, Li YQ, Lu L (2008) Stress impairs reconsolidation of drug memory via glucocorticoid receptors in the basolateral amygdala. J Neurosci 28:5602-5610
White AM, Ghia AJ, Levin ED, Swartzwelder HS (2000) Binge pattern ethanol exposure in adolescent and adult rats: differential impact on subsequent responsiveness to ethanol. Alcohol Clin Exp Res 24:1251-1256
White AM, Swartzwelder HS (2005) Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev Alcohol 17:161-176
White AM, Truesdale MC, Bae JG, Ahmad S, Wilson WA, Best PJ, Swartzwelder HS (2002) Differential effects of ethanol on motor coordination in adolescent and adult rats. Pharmacol Biochem Behav 73:673-677
WHO (2004) World Health Organisation (WHO), Global Status Report on Alcohol 2004; WHO Department of Mental Health and Substance Abuse (Geneve).
WHO (2010) World Health Organisation (WHO), European Status Report on Alcohol and Health 2010.
Wil iams SH (2005) Medications for treating alcohol dependence. Am Fam Physician 72:1775-1780
Wilson W, Mathew R, Turkington T, Hawk T, Coleman RE, Provenzale J (2000) Brain morphological changes and early marijuana use: a magnetic resonance and positron emission tomography study. J Addict Dis 19:1-22
Winstanley CA (2011) The utility of rat models of impulsivity in developing pharmacotherapies for impulse control disorders. Br J Pharmacol 164:1301-1321
Winters BD, Tucci MC, DaCosta-Furtado M (2009) Older and stronger object memories are selectively destabilized by reactivation in the presence of new information. Learn Mem 16:545-553
Wonnacott S (1997) Presynaptic nicotinic ACh receptors. Trends Neurosci 20:92-98
Wouda JA, Riga D, de VW, Stegeman M, van MY, Schetters D, Schoffelmeer AN, Pattij T, De Vries TJ (2011) Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berl) 216:267-277
Xue YX, Luo YX, Wu P, Shi HS, Xue LF, Chen C, Zhu WL, Ding ZB, Bao YP, Shi J, Epstein DH, Shaham Y, Lu L (2012) A memory retrieval-extinction procedure to prevent drug craving and relapse. Science 336:241-245
Zhai H, Wu P, Chen S, Li F, Liu Y, Lu L (2008) Effects of scopolamine and ketamine on reconsolidation of morphine conditioned place preference in rats. Behav Pharmacol 19:211-216
Source: http://www.stap.nl/content/bestanden/wouda-alcohol-studies-in-translational-models.pdf
Asian Transactions on Basic and Applied Sciences (ATBAS ISSN: 2221-4291) Volume 02 Issue 01 Development of Antidiabetic Active Compounds from Ethyl Acetate Extract of Acorus calamus L. Sri Hartati, Rizna T. Dewi, A. Darmawan and Megawati diabetes mellitus in the World. In the year 2000, there are Abstract— In research development of herbal medicine from
4 CE credits written for orthodontists, dentists, dental hygienists, and assistants. Advances in Orthodontic A Peer-Reviewed Publication Written by Jeremy J. Mao, DDS, PhD and Chung H. Kau, DDS, MScD, MBA, PhD, M Orth, FDS, FFD(Ortho), FAMS(Ortho) This course has been made possible through an unrestricted educational grant. The cost of this CE course is $59.00 for 4 CE credits.










